Abstract
In some cell types the paramyxovirus simian virus 5 (SV5) causes little cytopathic effect (CPE) and infection continues productively for long periods of time; e.g., SV5 can be produced from MDBK cells for up to 40 days with little CPE. SV5 differs from most paramyxoviruses in that it encodes a small (44-amino-acid) hydrophobic integral membrane protein (SH). When MDBK cells were infected with a recombinant SV5 containing a deletion of the SH gene (rSV5ΔSH), the MDBK cells exhibited an increase in CPE compared to cells infected with wild-type SV5 (recovered from cDNA; rSV5). The increased CPE correlated with an increase in apoptosis in rSV5ΔSH-infected cells over mock-infected and rSV5-infected cells when assayed for annexin V binding, DNA content (propidium iodide staining), and DNA fragmentation (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling assay). In rSV5ΔSH-infected MDBK cells an increase in caspase-2 and caspase-3 activities was observed. By using peptide inhibitors of individual caspases it was found that caspase-2 and caspase-3 were activated separately in rSV5ΔSH-infected cells. Expression of caspase-2 and -3 in rSV5ΔSH-infected MDBK cells appeared not to require STAT1 protein, as STAT1 protein could not be detected in SV5-infected MDBK cells. When mutant mice homologous for a targeted disruption of STAT1 were used as a model animal system and infected with the viruses it was found that rSV5ΔSH caused less mortality than wild-type rSV5, consistent with the notion of clearance of apoptotic cells in a host species.
Apoptosis, or programmed cell death, is the physiological process by which unwanted cells undergo morphologic changes, protease activation, chromosomal DNA fragmentation, and eventually cell death. This process is important for normal development, tissue homeostasis, immune modulation, and host defense against viral infection (reviewed in reference 13). The caspases (cysteine aspartate-specific proteases) play important roles in regulating different apoptotic pathways (39). Caspases can be roughly divided into initiator and effector caspases. Initiator caspases are involved in upstream regulatory events, and effector caspases are directly responsible for proteolytic cleavages that lead to cell death. Known initiator caspases include caspase-8 and -9, and known effector caspases include caspase-3, -6, and -7. Some caspases, such as caspase-2, can be both initiator and effector caspases. Viral infections can activate a variety of cellular pathways that lead to apoptosis. For example, interferons produced in response to viral infections activate pathways leading to activation of caspase-1, -3, and -8 and subsequent apoptosis (6, 36). Among the negative-stranded enveloped RNA viruses, influenza virus, vesicular stomatitis virus, rabies virus, Sendai virus, and Newcastle disease virus (NDV) are known to induce apoptosis in tissue culture cells (reviewed in reference 33). In a natural infection, the infected host organisms are thought to inhibit and eliminate viral infection by sacrificing virus-infected cells through apoptosis. However, many viruses have also developed means to delay and inhibit apoptosis to avoid being eliminated along with their host cells. For example, cowpox virus encodes a viral protein, CrmA, that blocks apoptosis by inhibiting caspase-1 and caspase-3 (37, 42), and Epstein-Barr virus, adenovirus, and herpes simplex virus express multiple viral proteins that inhibit apoptosis at different steps of apoptotic cascades (15, 33).
Simian virus 5 (SV5) is a member of the Rubulavirus genus of the family Paramyxoviridae, a genus which includes many important human and animal pathogens, such as mumps virus, human parainfluenza virus types 2 and 4 and NDV. Although SV5 was originally isolated from cultured primary monkey cells, its natural host is the dog, in which it causes kennel cough (26). Other members of the Paramyxoviridae include Sendai virus, human parainfluenza virus type 3, measles virus, canine distemper virus, rinderpest virus, and respiratory syncytial (RS) virus. SV5 contains a negative-sense single-stranded RNA of 15,246 nucleotides and encodes eight known viral proteins: nucleocapsid protein (N), V protein, phosphoprotein (P), matrix protein (M), fusion protein (F), small hydrophobic integral membrane protein (SH), hemagglutinin-neuraminidase (HN), and polymerase protein (L). The P protein mRNA is synthesized through a cotranscriptional RNA editing process in which two nontemplated G residues are inserted into the templated mRNA transcript (38). The N, P, V, and L proteins are associated with the RNA genome to form the nucleocapsid core; minimally, N, P, and L form the viral transcription and replication complex. The SV5 V protein appears to be a multifunctional protein, as it is also involved in regulating the SV5-induced interferon response. It has been found that the V protein mediates the degradation of signal transducer and activation of transcription (STAT1) (9), a transcription factor required for the interferon response (7). The V protein also interacts with the cellular protein DDB1 (24), and this interaction may be involved in the known effect of V protein slowing the progression of the cell cycle in virus-infected cells (23). The M protein is a peripheral membrane protein, and the SH, F, and HN proteins are integral membrane proteins. F and HN are involved in viral entry into cells, and HN is important for virus release from cells (reviewed in reference 22).
The SV5 SH integral membrane protein is a minor component of virions (17). The SH protein contains 44 amino acid residues with a predicted C-terminal ectodomain of 5 residues, a transmembrane domain of 23 residues, and an N-terminal cytoplasmic tail of 16 residues. By using reverse genetic procedures for SV5 (18), it was found that an SH-deletion-containing SV5 (rSV5ΔSH) could be recovered, indicating that the SH protein was not essential for virus viability in tissue culture. The growth rates, infectivities, and plaque sizes of wild-type SV5 recovered from cDNA (rSV5) and rSV5ΔSH were found to be very similar. In addition, in a quantitative assay for the ability of rSV5 and rSV5ΔSH to cause cell-cell fusion, no difference was observed (17).
The SH gene is not common to all members of the Paramyxoviridae, and the only other virus that contains an analogous gene located between the genes for F and HN is the closely related Rubulavirus, mumps virus (11, 34). In the Pneumovirus RS virus, a gene encoding a third integral membrane protein, designated SH, has been identified (5, 28). However, the RS virus SH protein is considerably larger than that of SV5, and it is not known if the RS virus SH protein is a functional counterpart of the SV5 SH protein. A recombinant RS virus with the SH gene deleted is also viable in tissue culture (3).
A defining characteristic of SV5 is that the virus can grow in MDBK cells for up to 40 days with little observable cytopathic effect (CPE) (4). We report here that MDBK cells infected with rSV5ΔSH exhibit extensive CPE in contrast to wild-type rSV5-infected MDBK cells and this CPE is due to induction of caspase-dependent apoptosis.
MATERIALS AND METHODS
Viruses and cells.
HeLa T4, A549, L929, MDBK, and MDCK cells were maintained in Dulbecco modified Eagle medium (DMEM) with 10% fetal calf serum (FCS). BHK 21-F cells were maintained in DMEM with 10% tryptose phosphate broth and 10% FCS. Cell lines previously contaminated with mycoplasma were cured using ciprofloxacin (10 μg/ml) (Bayer AG, West Haven, Conn.). All cells used were determined to be free of mycoplasma contamination by using a PCR-based assay and specific DNA primers (Boehringer-Mannheim, Indianapolis, Ind.).
rSV5ΔSH was generated by using a reverse genetics method from an infectious clone of SV5 (plasmid pBH 276) (18) from which the SH gene was deleted (plasmid pBH324) (17). rSV5 and rSV5ΔSH were grown in MDBK cells and harvested 5 to 7 days postinfection (p.i.) as described previously (29). Virus stocks were mycoplasma free. Virus titers were determined by plaque assay using BHK 21-F cells (30). For virus infection, cell monolayers were washed with phosphate-buffered saline (PBS) and then infected with viruses in DMEM–1% bovine serum albumin (BSA) at a multiplicity of infection (MOI) of 0. 1 to 10 PFU/cell for 1 to 2 h at 37°C. The monolayers were then washed and incubated with DMEM containing 2% FCS at 37°C.
Apoptosis assays: annexin V binding, propidium iodide staining of DNA, and TUNEL assay.
Confluent MDBK cells in 6-cm plates were infected with SV5 at an MOI of 10 PFU/cell. One or three days p.i. the monolayers were trypsinized and combined with the floating cells in the media. The harvested cells were then pelleted by centrifugation at 250 × g for 10 min at 4°C and washed with PBS. For annexin V binding, the cells were incubated with fluorescein isothiocyanate (FITC)-labeled annexin (annexin-V-FLUOS) for 15 min at room temperature according to the manufacturer's protocol (Roche Diagnostics Corp, Mannheim, Germany). The fluorescence of 20,000 cells was examined by using a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, Calif.). For propidium iodide staining, infected cells were harvested as described above and then fixed with 0.25% formaldehyde for 2 h at 4°C and washed in PBS. The fixed cells were resuspended in 1 ml 50%–DMEM–50% FCS, permeabilized by adding 3 ml of 70% ethanol, and incubated at 4°C for at least 2 h and up to 3 days. The permeabilized cells were incubated with monoclonal antibody (MAb) P-k (specific for V and P proteins) (32) (0.5 ml of a 1:500 dilution in PBS–1% BSA) at 4°C for 1 h, washed extensively with PBS, and then incubated with FITC-labeled anti-mouse secondary antibody (Organon-Teknika Corp., Charlotte, N.C.) (0.5 ml at 1:1,000 in PBS–1% BSA) for 1 h at 4°C. The cells were finally incubated with 500 μl of 50-μg/ml propidium iodide (Sigma-Aldrich, St. Louis, Mo.) for 1 h at 4°C. The cells were then analyzed on a FACSCalibur flow cytometer. Infected cells were selected by plotting FL2-A (DNA content) versus FL1-H (V and P expression).
For terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assays, the cells were harvested and permeabilized as described above for propidium iodide staining. The cells were then incubated with 50 μl of TUNEL reaction mixture (in situ cell death detection kit; Roche Diagnostics Corp.) for 2 to 3 h in the dark at 37°C. Phycoerythrin-labeled secondary antibody was used, and the cells were analyzed by flow cytometry. For TUNEL assays of nuclei, the cells were incubated with 500 μl of hypotonic buffer (20 mM Tris-Cl [pH 7.8], 5 mM MgCl2, 0.2 mM EDTA, 1 mM dithiothreitol, 5 mM β-glycerolphosphate, 0.5 mM phenylmethylsulfonyl fluoride, and 10 μg of pepstatin, aprotinin, and leupeptin/ml) on ice for 15 min to release nuclei. The nuclei were pelleted by centrifugation at 1,000 rpm for 10 min using a tabletop centrifuge and resuspended in PBS. The nuclei were then incubated with 50 μl of TUNEL reaction mixture containing FITC-labeled dUTP for 2 to 3 h at 37°C. The fluorescence intensity of the nuclei was analyzed by flow cytometry. For in situ TUNEL assays, cells grown on coverslips were fixed with 0.5% formaldehyde for 1 h and then permeabilized with 50% ethanol–50% PBS with 1% BSA for 1 h at 4°C. The cells were first incubated with MAb P-k and then with Texas red-labeled secondary antibody. Subsequently, the cells were incubated with 50 μl of TUNEL reagent for 2 h at 37°C in a humidified incubator. The cells were examined using an LSM 410 confocal microscope (Zeiss Inc., Thornwood, N.Y.). To quantify in situ TUNEL assay fluorescence, 10 random fields were chosen and numbers of fluorescent nuclei were counted.
Single-step growth rate.
Monolayers of HeLa T4 and MDBK cells in 35-mm plates were washed with PBS and then infected with rSV5 or rSV5ΔSH in DMEM–1% BSA at an MOI of 10 PFU/cell for 1 to 2 h at 37°C. The cells were then washed with PBS and maintained in DMEM–2% FCS. Viruses released into media were collected at 0, 6, 12, 18, 36, 72, and 144 h p.i. The titers of viruses were determined by plaque assay on BHK 21F cells.
Caspase activity assay.
Assay kits for assaying caspase activity were purchased from Chemicon International, Inc. (Temecula, Calif.). Assays were carried out using the manufacturer's protocol, and released chromophore was quantified using a microplate reader with a 405-nm filter (Bio-Tek Instruments, Inc. Winooski, Vt.).
Inhibition of apoptosis with caspase inhibitors.
All caspase inhibitors were purchased from Enzyme Systems (Livermore, Calif.) and dissolved in dimethyl sulfoxide. After infection of MDBK cells with rSV5 and rSVΔSH, the cells were maintained in DMEM–2% FCS containing the caspase inhibitors. Peptide caspase inhibitors were dissolved in dimethyl sulfoxide; the general caspase inhibitor Z-VAD-FMK was used at a final concentration of 200 μM, the caspase-2 inhibitor Z-VAVAD-FMK was used at 50 μM, and the caspase-3 inhibitor Z-DEVD-FMK was used at 100 μM.
Immunoblotting.
MDBK cells in 6-cm plates were infected with rSV5ΔSH or rSV5, and 2 and 4 days p.i., cells were lysed in 0.5 ml of protein lysis buffer (2% sodium dodecyl sulfate [SDS], 62.5 mM Tris-HCl [pH 6.8], 2% dithiothreitol) and lysates were sonicated briefly to shear DNA. Lysate (80 μl) was subjected to SDS-polyacrylamide gel electrophoresis using a 10% gel (30). Polypeptides were transferred to Immobilon-P membrane (Millipore Corp., Bedford, Mass.) using a wet-gel transfer apparatus (Bio-Rad, Hercules, Calif.). The membrane was incubated first with primary antibodies against STAT1 (a mixture of A-2, C-136, and E-23; Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) and then with a mixture of anti-mouse and anti-rabbit immunoglobulin secondary antibodies conjugated to horseradish peroxidase. The proteins on the membrane were detected using the ECL+ kit (Amersham Pharmacia, Piscataway, N.J.), and chemiluminescence was detected using a Storm System PhosphorImager (Molecular Dynamics Inc., Sunnyvale, Calif.).
RESULTS
rSV5ΔSH causes CPE in MDBK cells.
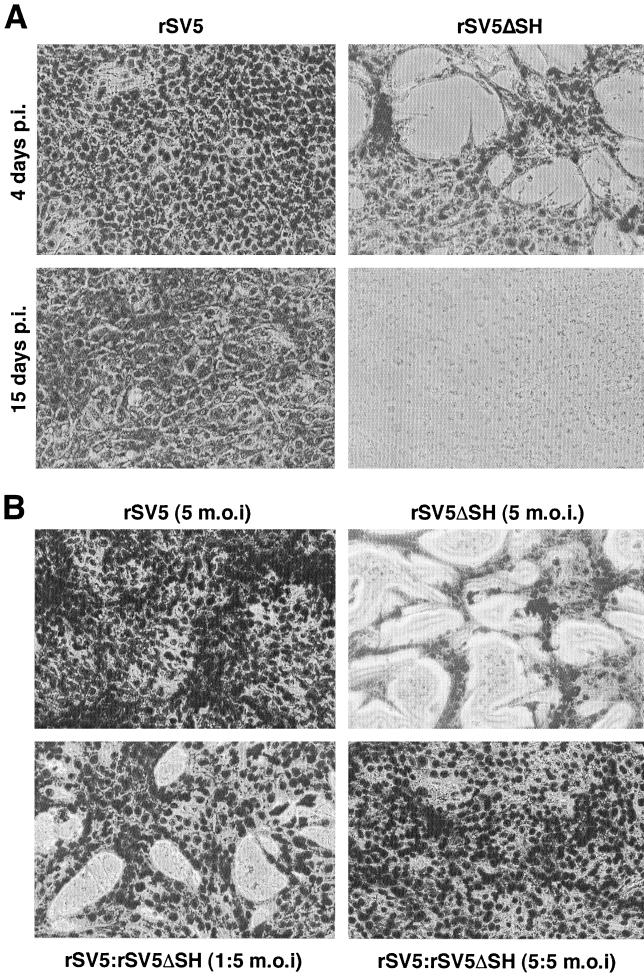
SV5 replicates in MDBK cells for many days with minimal CPE (4) (Fig. 1A). In contrast, on careful examination of MDBK cells infected with rSV5ΔSH (a recombinant SV5 that lacks the SH gene), it was observed that by 4 days p.i. there was extensive cell loss from the monolayers and by 15 days p.i. the monolayers were lost completely (Fig. 1A). However, when MDBK cells were coinfected with rSV5ΔSH and wild-type rSV5 at equal multiplicities, the CPE was not observed (Fig. 1B). Furthermore, when wild-type rSV5 was used to superinfect rSV5ΔSH-infected MDBK cells, 24 h after the first infection, CPE was inhibited (data not shown). These data suggest that expression of the SH protein confers an effect that prevents CPE in MDBK cells.
FIG. 1.
Infection of MDBK cells with rSV5ΔSH induces a CPE that is overcome by coinfection with rSV5. (A) MDBK cells in 12-well plates were infected with rSV5 or rSV5ΔSH at an MOI of 10 PFU/cell. At 4 and 15 days p.i. the cells were stained using HEMA3 and photographed at a magnification of ×200. (B) MDBK cells in six-well plates were infected with rSV5 or rSV5ΔSH, or coinfected with both viruses. At 4 days p.i. the cells were stained and photographed.
rSV5ΔSH induces apoptosis in MDBK cells.
To investigate whether the observed CPE in rSV5ΔSH-infected MDBK cells was due to apoptosis, we used three different measurements of apoptosis that when taken together are strong indicators of apoptosis: (i) increase in annexin V binding due to loss of asymmetry in cell membrane phospholipids, (ii) DNA content decrease, measured using propidium iodine staining, and (iii) increase in DNA fragmentation, assayed using the TUNEL assay.
Apoptotic cells undergo a loss of asymmetry in cell membrane phospholipids with an increase of phosphatidyl serine on the outer leaflet. Annexin V, a calcium-dependent phospholipid-binding protein, has a high affinity for phosphatidyl serine, and it can be used to monitor changes in phosphatidyl serine localization. When mock-infected, rSV5-infected, or rSV5ΔSH-infected MDCK cells at 3 days p.i. were incubated with FITC-labeled annexin V and the fluorescent cells were examined by flow cytometry, a considerable increase in annexin V binding was observed in rSV5ΔSH-infected cells (binding level [mean ± standard error of the mean] of 38.88% ± 0.46%) compared to mock-infected (10.66% ± 0.25%) or rSV5-infected (13.58% ± 1.02%) cells.
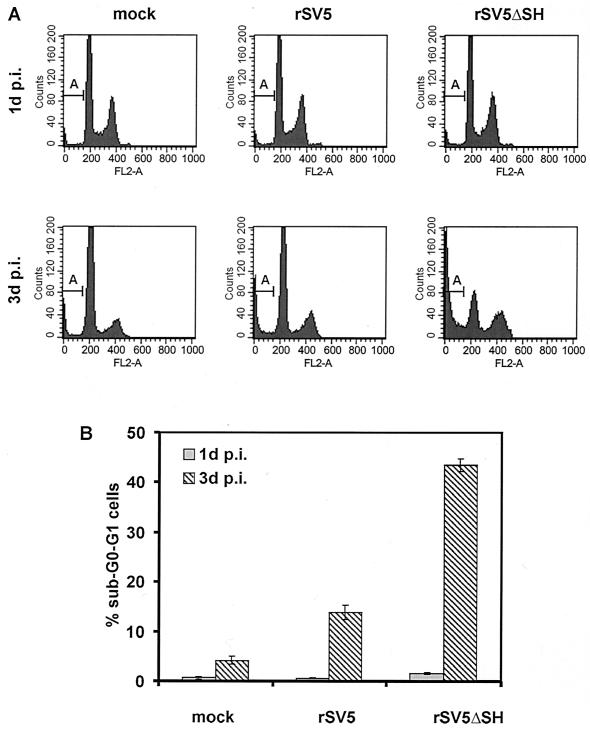
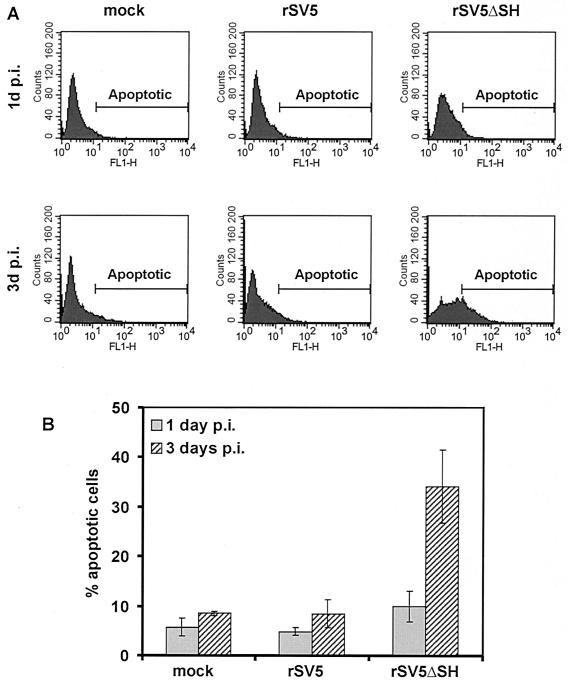
For propidium iodide staining, MDBK cells were mock infected, rSV5 infected, or rSV5ΔSH infected, and at 1 or 3 days p.i., the cells (floating and attached) were harvested, permeabilized, and incubated with propidium iodide. Analysis of the cells by flow cytometry showed that at 1 day p.i. in either rSV5- or rSV5ΔSH-infected cells very few cells had reduced DNA staining below that of G0-G1 (sub-G0-G1) cells, the usual criterion for apoptotic cells (27). However, by 3 days p.i. about 40% of rSV5ΔSH-infected MDBK cells had a sub-G0-G1 DNA staining level, compared to 13% for rSV5-infected cells and 4% for mock-infected cells (Fig. 2). To check viral expression levels in rSV5- and rSV5ΔSH-infected cells, cells were also stained with MAb P-k (32), which is specific for the P and V proteins. Fluorescence was measured by flow cytometry, and the P and V expression levels in rSV5- and rSV5ΔSH-infected cells were found to be equivalent (data not shown). To confirm that the rSV5ΔSH-infected MDBK cells undergo a greater extent of apoptosis than rSV5-infected MDBK cells, the extent of DNA fragmentation was examined by an in situ fluorescent TUNEL assay, and at 1 day and 3 days p.i. cells were analyzed by flow cytometry. As shown in Fig. 3, by 3 days p.i. ∼35% of rSV5ΔSH-infected MDBK cells were apoptotic whereas only a small fraction of rSV5-infected or mock-infected MDBK cells were apoptotic.
FIG. 2.
Detection of DNA content of rSV5- and rSV5ΔSH-infected MDBK cells using propidium iodide staining. MDBK cells in 6-cm plates were infected and processed for propidium iodide staining of DNA 1 or 3 days p.i. as described in Materials and Methods. (A) DNA staining of 10,000 cells was detected by flow cytometry. “FL2-A” indicates DNA content; “A” indicates sub-G0-G1 DNA content, which is considered apoptotic. (B) Histogram of data computed from three samples like those shown in panel A for each time point and virus.
FIG. 3.
TUNEL assay of rSV5- and rSV5ΔSH-infected MDBK cells. MDBK cells were mock infected or infected with rSV5 or rSV5ΔSH and at 1 or 3 days p.i. were processed for the TUNEL assay as described in Materials and Methods. (A) Detection by flow cytometry of fluorescently labeled DNA. The area marked “Apoptotic” indicates fluorescence intensity of cells considered positive for the TUNEL assay and hence apoptotic. (B) Histogram of three independent experiments as in panel A at each time point.
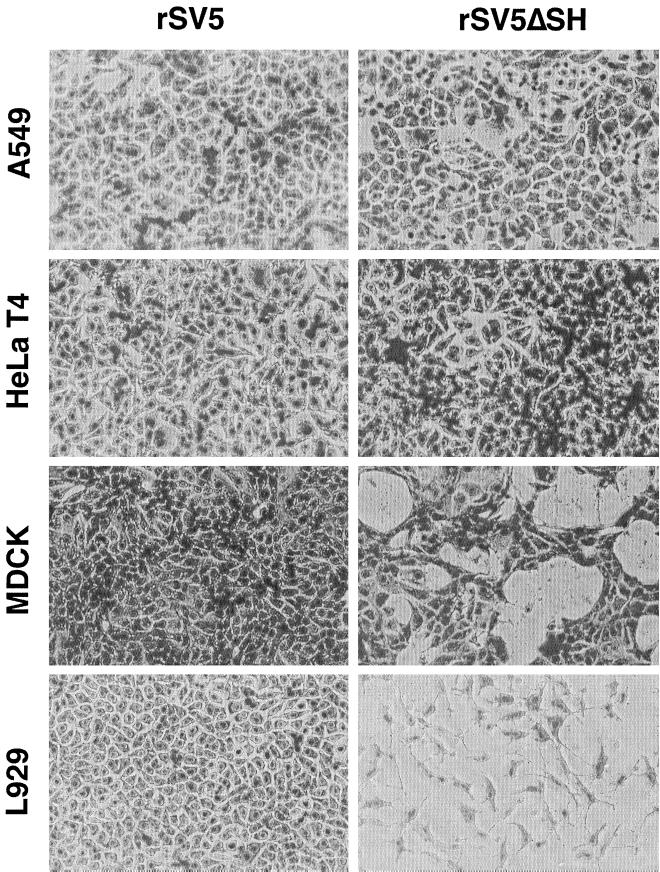
To determine if rSV5ΔSH caused CPE and apoptosis in cell lines other than MDBK (bovine) cells, L929 (mouse) cells, MDCK (canine) cells, HeLa T4 (human) cells, and A549 (human) cells were infected with rSV5 and rSV5ΔSH and examined for CPE at various times p.i. The cell types infected with rSV5 did not show evidence of severe CPE (Fig. 4). However, for rSV5ΔSH-infected cells, severe CPE was observed in L929 cells 2 to 3 days p.i. and in MDCK cells at 6 to 7 days p.i. In contrast, rSV5ΔSH-infected HeLa T4 cells and A549 cells showed little CPE even after 7 days p.i. (Fig. 4). The extent of apoptosis was then quantified by using a TUNEL assay, and as shown in Table 1, the CPE observed in rSV5ΔSH-infected L929 and MDCK cells correlated with induction of apoptosis. None of the infected cell types showed evidence of large syncytia, although rSV5-infected HeLa cells did show an occasional multinucleated cell. CV-1 (monkey) and BHK (hamster) cells were not used in this study of rSV5ΔSH-induced apoptosis because on SV5 infection they exhibit extensive syncytium formation.
FIG. 4.
CPE in human, canine, and mouse cells infected with rSV5ΔSH. A549, HeLa T4, MDCK, and L929 cells were infected at an MOI of 10 PFU per cell. At various times cells were stained with HEMA3. A549 cells and HeLa T4 cells were stained 7 days p.i., MDCK cells were stained 6 days p.i., and L929 cells were stained 3 days p.i.
TABLE 1.
Induction of apoptosis by rSV5ΔSH infectiona
| Virus | % Apoptotic cells
|
|||
|---|---|---|---|---|
| A549 | HeLa T4 | MDCK | L929 | |
| None (mock infection) | 0.57 | 0.01 | 5.96 | 1.41 |
| rSV5 | 0.70 | 2.44 | 6.90 | 1.93 |
| rSV5ΔSH | 1.52 | 1.76 | 12.58 | 45.07 |
The cells in 6-cm plates were infected at an MOI of 10 PFU per cell. A549 cells and HeLa T4 cells were harvested 7 days p.i.; MDCK cells were collected 6 days p.i.; L929 cells were collected 3 days p.i. The collected cells were treated for the TUNEL assay and analyzed by flow cytometry as described in Materials and Methods. Values are averages for three samples.
Apoptosis does not confer a growth advantage in tissue culture cells.
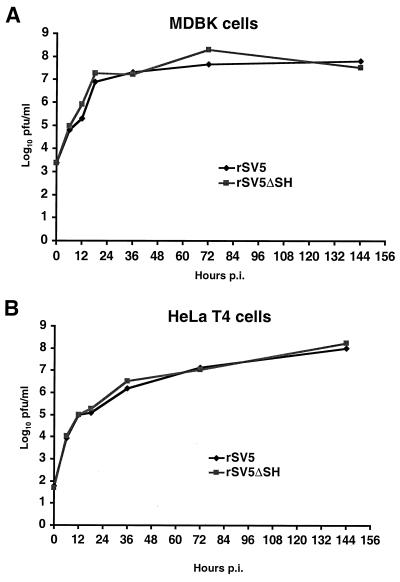
It is usually thought that programmed cell death of virus-infected cells is beneficial for the host organism, as death of infected cells provide a means of inhibiting or eliminating virus spread. However, some viruses take advantage of apoptosis as a means of releasing or spreading virions more efficiently. For example, human immunodeficiency virus is reported to have an increased release of virions from apoptotic cells (1). To determine if apoptosis induced in rSV5ΔSH-infected cells facilitated virus growth, the single-step rates of growth of rSV5 and rSV5ΔSH were determined in MDBK and HeLa T4 cells. As shown in Fig. 5, the growth curves of rSV5 and rSV5ΔSH in MDBK cells were similar, as were the growth curves in HeLa T4 cells.
FIG. 5.
Single-step growth rate of rSV5ΔSH, compared to rSV5, is not affected by apoptosis. Confluent MDBK (A) or HeLa (B) cells in 35-mm plates were infected with rSV5 or rSV5ΔSH at an MOI of 10 PFU per cell. Media were collected 0, 6, 12, 18, 36, 72, and 144 h p.i. The viral titers were determined by plaque assay using BHK 21F cells.
rSV5ΔSH-induced apoptosis in MDBK cells involves the caspase pathway.
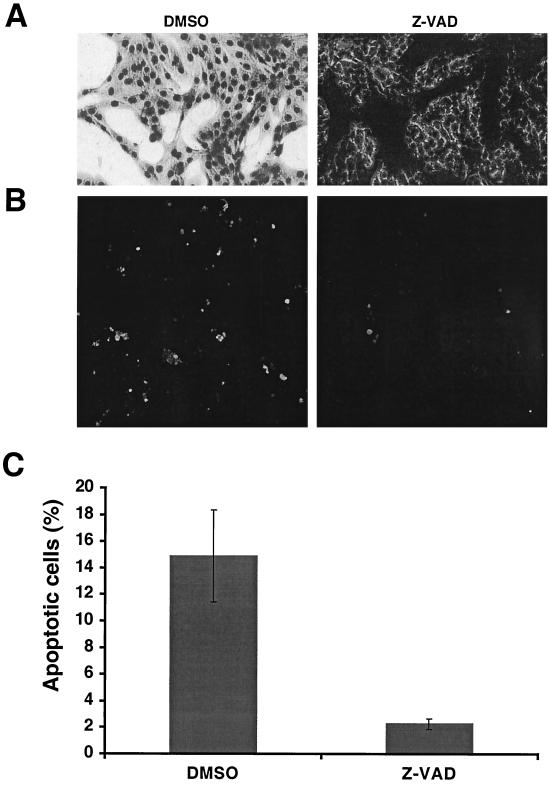
Although there are a variety of pathways leading to apoptosis, the majority, but not all, of these pathways culminate in the activation of caspases, the major effectors of programmed cell death. To investigate the role of caspases in rSV5ΔSH-induced apoptosis, a general caspase peptide inhibitor, Z-VAD-FMK, was used. As shown in Fig. 6A, addition of Z-VAD-FMK to rSV5ΔSH-infected MDBK cells largely prevented the CPE observed in mock-treated cells. An in situ TUNEL assay was performed on cells grown on coverslips, and it was found that addition of Z-VAD-FMK to rSV5ΔSH-infected MDBK cells reduced the number of apoptotic cells (Fig. 6B). To quantify the TUNEL assay, Z-VAD-FMK-treated or mock-treated cells (attached cells and released cells in supernatant) were harvested, nuclei were prepared and subjected to a TUNEL assay protocol, and fluorescence was analyzed by flow cytometry. As shown in Fig. 6C, Z-VAD-FMK treatment reduced the number of rSV5ΔSH-infected MDBK cells from ∼15% to 2%. Importantly for the interpretation of these experiments, Z-VAD-FMK did not affect the accumulation of SV5-specific protein in cells, as determined by immunoblotting of treated and mock-treated infected-cell lysates (data not shown).
FIG. 6.
Apoptosis in rSV5ΔSH-infected MDBK cells is inhibited by a general caspase inhibitor, Z-VAD-FMK. MDBK cells were infected with rSV5ΔSH and treated with Z-VAD-FMK (200 μM) for 3 days. (A) Cells were stained with HEMA3 and photographed. (B) In situ TUNEL assay showing FITC staining. All FITC-stained cells were infected with SV5, as shown by Texas red staining for the P- and V-specific MAb. (C) Quantification of TUNEL assay. The cells were harvested and treated with hypotonic buffer to release nuclei. The nuclei were treated with TUNEL assay reagent and analyzed by flow cytometry. The averages of three separate experiments are shown.
rSV5ΔSH-induced apoptosis in MDBK cells involves caspase-2 and caspase-3.
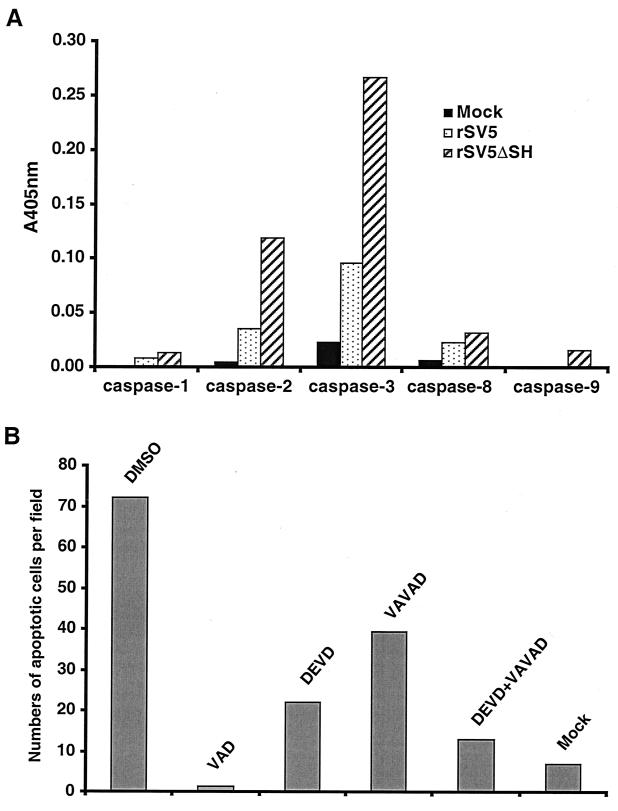
The caspases are synthesized as inactive procaspase precursors that are converted to the active form by proteolytic cleavage, catalyzed by other caspases. Regulation of caspase activation is thus critical to cell survival. To investigate which caspases are activated in rSV5ΔSH-infected MDBK cells at 3 days p.i., cell lysates were prepared and assayed for specific caspases using a colorimetric assay (see Materials and Methods). As shown in Fig. 7A, caspase-2 and caspase-3 activities were increased in rSV5ΔSH-infected MDBK cells compared to those in mock-infected cells. It was also observed that caspase-2 and caspase-3 activities were increased in rSV5-infected cells compared to those in mock-infected cells but not to the extent observed in rSV5ΔSH-infected cells. In contrast to the increased activities of caspase-2 and caspase-3, the activities of caspase-1, caspase-8, and caspase-9 were only very slightly increased in rSV5ΔSH-infected MDBK cells compared to activities in mock-infected cells.
FIG. 7.
Caspase activities of rSV5ΔSH- and SV5-infected MDBK cells. (A) Activities of caspase-1, -2, -3, -8, and -9. MDBK cells were infected with rSV5ΔSH or rSV5, and 3 days p.i. the cells were lysed. Caspase activities of cytosolic extracts were measured as described in Materials and Methods. (B) Inhibition of apoptosis by caspase-2 and caspase-3 inhibitors. MDBK cells on coverslips were infected with rSV5ΔSH in the presence of caspase inhibitors (Z-VAVAD-FMK, 50 μM; Z-DEVD-FMK, 100 μM) and processed for the TUNEL assay as described in Materials and Methods. Fluorescence was visualized using a Zeiss LSM 410 confocal microscope. Average numbers of apoptotic nuclei from 10 randomly chosen fields are shown.
To determine whether the increases in caspase-2 and caspase-3 activity found in rSV5ΔSH-infected MDBK cells are important for rSV5ΔSH-mediated apoptosis, rSV5ΔSH-infected MDBK cells on coverslips were treated with the specific caspase-2 inhibitor Z-VAVAD-FMK and/or the specific caspase-3 inhibitor Z-DEVD-FMK, and an in situ TUNEL assay was performed. As shown in Fig. 7B, it was found that the individual caspase-2 and caspase-3 inhibitors partially blocked apoptosis in rSV5ΔSH-infected MDBK cells, but when added in combination, they largely inhibited apoptosis. Thus, the data suggest that both caspase-2- and caspase-3-like activities contribute independently to apoptosis in rSV5ΔSH-infected MDBK cells.
STAT1 is not required for activation of caspase-2 and caspase-3.
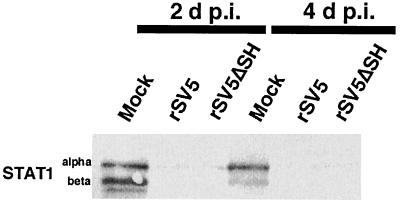
Interferons are produced in response to many viral infections. Interferons activate, through receptor-mediated signal transduction and activation of the transcription factor STAT1, pathways leading to activation of caspase-1, -3, and -8 and subsequent apoptosis (6, 36). Thus, it was important to know whether the apoptosis induced in rSV5ΔSH-infected MDCK cells was due to an altered STAT1 response. In human 2fTGH cells infected with SV5, the viral V protein mediates the degradation of STAT1, hence ablating interferon signaling (9). However, in mouse cells infected with SV5, STAT1 is not degraded and the viral infection is limited by the interferon response (8). As shown above, infection of mouse L929 cells with rSV5ΔSH caused severe CPE and apoptosis whereas CPE was not observed in rSV5ΔSH-infected cells of human origin (HeLa T4 and A549 cells). To examine the status of STAT1 in rSV5- and rSV5ΔSH-infected MDBK cells, lysates were prepared at 2 and 4 days p.i. and subjected to Western blotting using antibodies against STAT1 protein. As shown in Fig. 8, STAT1α and -β were detected in mock-infected MDBK cells but not in rSV5- or rSV5ΔSH-infected cells. Thus, these data suggest that in rSV5ΔSH-infected MDBK cells, as in human 2fTGH cells, STAT1 is absent and that expression of caspase-2 and caspase-3 does not depend on STAT1 protein and pathways associated with STAT1.
FIG. 8.
Activation of caspase-2 and -3 does not require STAT1. MDBK cells infected with rSV5ΔSH or rSV5 were lysed 2 and 4 days p.i. Lysates were subjected to SDS-polyacrylamide gel electrophoresis on 10% gels and immunoblotted using antibodies specific for STAT1.
rSV5ΔSH is less pathogenic in STAT1−/− mice than wild-type rSV5.
To examine the pathogenicity of rSV5ΔSH in vivo, a small animal model system is needed. However, SV5 infection of laboratory strains of inbred mice does not cause morbidity or mortality. Recently, we established a small animal model system for studying SV5 pathogenesis using BALB/c mutant mice homozygous for a targeted disruption of STAT1. The STAT1−/− BALB/c mice are the ninth backcross generation of 129 × C57BL/6 STAT1−/− mice (10) to BALB/c. A full description of this model system will be published elsewhere. Mice were inoculated intranasally with rSV5ΔSH or rSV5. As shown in Table 2, all the mice survived infection by rSV5ΔSH at a dosage of 105 PFU and five out of six mice survived infection by rSV5ΔSH at a dosage of 106 PFU. In contrast, wild-type rSV5 infection of STAT1−/− mice resulted in significant mortality (six out of six died after infection with 106 PFU, and three out of six died after infection with 105 PFU). To show that the viruses replicated in the mouse lung, at 4 days p.i. two groups of three mice inoculated with 106 PFU virus were sacrificed and lungs were homogenized. rSV5ΔSH and rSV5 were found to grow to average titers of 9.5 × 105 and 5.8 × 106 PFU/g of lung tissue, respectively. These data suggest that rSV5ΔSH is less pathogenic in vivo than wild-type rSV5, even though it causes vastly greater CPE in mouse, bovine, and canine cell types, a finding consistent with the notion of clearance of apoptotic cells in a host species.
TABLE 2.
Survival of STAT1−/− mice following SV5 infectiona
| Virus | Dosage (PFU) | Survivalb at day p.i.
|
|||||
|---|---|---|---|---|---|---|---|
| 2 | 7 | 10 | 13 | 15 | 22 | ||
| rSV5 | 105 | 6/6 | 6/6 | 5/6 | 4/6 | 3/6 | 3/6 |
| 106 | 5/6 | 4/6 | 3/6 | 0/6 | 0/6 | 0/6 | |
| rSV5ΔSH | 105 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 |
| 106 | 6/6 | 6/6 | 6/6 | 6/6 | 5/6 | 5/6 | |
Four- to six-week-old STAT1−/− mice were inoculated intranasally under anesthesia using 50 μl of PBS containing different dosages of viruses. Animals were monitored daily and sacrificed when observed to be in extremis. All procedures were in accordance with NIH guidelines on the care and use of laboratory animals.
Number of STAT1−/− mice surviving/number tested.
DISCUSSION
Many members of the Paramyxoviridae have been found to cause severe CPE and apoptosis. Sendai virus causes apoptosis by activating caspase-3 and caspase-8 (2, 16, 19). Measles virus not only induces apoptosis in the cells it infects (12) but also induces apoptosis of uninfected activated T lymphocytes, possibly by producing functional TRAIL from measles virus-infected dendritic cells (14, 40). NDV causes apoptosis by eliciting interferon- and tumor necrosis factor-mediated responses (25, 43). RS virus is thought to enhance neutrophil apoptosis in vivo (41), but it does not cause apoptosis in tissue-cultured cells, even though RS virus infection results in severe CPE (35). In contrast, SV5 multiplies for long periods with minimal CPE in many cell types (4).
Initial examination of a recombinant SV5 lacking its SH gene indicated that the recovered rSV5ΔSH virus was indistinguishable from rSV5 in terms of growth rate, plaque size, virus yield, and fusion activity (17). Further investigation of properties of rSV5ΔSH indicated that it was able to induce severe CPE and apoptosis in L929, MDCK, and MDBK cells but little detectable CPE and apoptosis in A549 and HeLa T4 cells (Fig. 4 and Table 1). As coinfection of rSV5ΔSH with rSV5 did not result in CPE, this observation lends support to the notion that expression of the SH protein is the key factor in preventing induction of apoptosis. The time required for CPE to be observed was also different in different cell lines. Mouse L929 cells took the least time (2 to 3 days) to exhibit massive CPE, while MDCK cells took 5 to 7 days p.i. The observation that rSV5ΔSH can grow efficiently in HeLa T4 cells without causing severe CPE (Fig. 5) indicates that there may be another viral protein(s) that prevents CPE and apoptosis in HeLa cells. It has been reported that the deletion of the V gene from Sendai virus resulted in a virus that causes more CPE in some but not all cell lines (20). The SV5 V protein has been found to bind to zinc, associate with the cellular protein DDB1, cause a delay in progression through the cell cycle, and cause degradation of STAT1 protein in human cells but not mouse cells (9, 23, 24, 31). Whether the V protein of SV5 is involved in blocking CPE and/or apoptosis in the human cells is not clear. We are currently attempting to generate a SV5 without the V gene to explore the possibility that V may be involved in blocking apoptosis in HeLa T4 and A549 cells. Although rSV5ΔSH causes vastly greater CPE in mouse, bovine, and canine cell types than wild-type rSV5, it was found that rSV5ΔSH was less pathogenic than wild-type rSV5 in STAT1−/− mice, the only available small animal model for studying the pathogenesis of SV5. This finding is consistent with the notion of clearance of apoptotic cells in a host species.
To investigate the cellular processes utilized to cause apoptosis in the absence of expression of the SH protein, a general caspase inhibitor, Z-VAD-FMK, was used, and it was found to block apoptosis in rSV5ΔSH-infected MDBK cells. The observed increased activities of caspase-2 and caspase-3 in rSV5ΔSH-infected MDBK cells suggest that SH is required to block upstream signaling of caspase-2 and caspase-3 activation. We do not know whether the moderate increase of caspase-3 activity in SV5-infected MDBK cells is significant (Fig. 6A), but wild-type-rSV5-infected cells also had a slightly higher number of apoptotic cells than mock-infected cells (Fig. 3B). Thus, SV5 infection of MDBK cells may not result in a complete inhibition of apoptosis.
The pathway by which apoptosis occurs in rSV5ΔSH-infected cells together with the mechanism by which SH protein interferes with the apoptotic pathway remains to be determined. It seems likely that the SH cytoplasmic tail interacts with cellular proteins that are involved in apoptotic signaling, and this also remains to be determined. The data indicate that caspase-2 and caspase-3, but not caspase-1, -8, and -9, are activated in rSV5ΔSH-infected MDBK cells, suggesting that the latter caspases are not activators of caspase-2 and -3 in the rSV5ΔSH-infected cells. The fact that the inhibitor of caspase-2 did not block apoptosis completely suggests that activation of caspase-3 does not depend on caspase-2. Previously, it has been found that STAT1 protein is required for expression of caspase-2 and caspase-3 (21). However, STAT1 protein was not detected in rSV5ΔSH-infected MDBK cells, possibly because STAT1 protein underwent SV5 V protein-mediated degradation, as occurs in SV5-infected human cells (9). Thus, it is unlikely that activation of caspase-2 and caspase-3 in rSV5ΔSH-infected MDBK cells depends on STAT1 protein or interferon responses.
ACKNOWLEDGMENTS
We thank Anthony P. Schmitt and Reay G. Paterson and many other members of the Lamb lab for helpful discussions.
This research was supported in part by Public Health Service Research Award AI-23173 from the National Institute of Allergy and Infectious Diseases. G.Y.L. was supported by NIH Medical Scientist Training Program grant T32 GM-08152. B.H. is an Associate and R.A.L. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Antoni B A, Sabbatini P, Rabson A B, White E. Inhibition of apoptosis in human immunodeficiency virus-infected cells enhances virus production and facilitates persistent infection. J Virol. 1995;69:2384–2392. doi: 10.1128/jvi.69.4.2384-2392.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bitzer M, Prinz F, Bauer M, Spiegel M, Neubert W J, Gregor M, Schulze-Osthoff K, Lauer U. Sendai virus infection induces apoptosis through activation of caspase-8 (FLICE) and caspase-3 (CPP32) J Virol. 1999;73:702–708. doi: 10.1128/jvi.73.1.702-708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukreyev A, Whitehead S S, Murphy B R, Collins P L. Recombinant respiratory syncytial virus from which the entire SH gene has been deleted grows efficiently in cell culture and exhibits site-specific attenuation in the respiratory tract of the mouse. J Virol. 1997;71:8973–8982. doi: 10.1128/jvi.71.12.8973-8982.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choppin P W. Multiplication of a myxovirus (SV5) with minimal cytopathic effects and without interference. Virology. 1964;23:224–233. doi: 10.1016/0042-6822(64)90286-7. [DOI] [PubMed] [Google Scholar]
- 5.Collins P L, Wertz G W. Nucleotide sequences of the 1B and 1C nonstructural protein mRNAs of human respiratory syncytial virus. Virology. 1985;143:442–451. doi: 10.1016/0042-6822(85)90384-8. [DOI] [PubMed] [Google Scholar]
- 6.Dai C, Krantz S B. Interferon gamma induces upregulation and activation of caspases 1, 3, and 8 to produce apoptosis in human erythroid progenitor cells. Blood. 1999;93:3309–3316. [PubMed] [Google Scholar]
- 7.Darnell J E., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 8.Didcock L, Young D F, Goodbourn S, Randall R E. Sendai virus and simian virus 5 block activation of interferon-responsive genes: importance for virus pathogenesis. J Virol. 1999;73:3125–3133. doi: 10.1128/jvi.73.4.3125-3133.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Didcock L, Young D F, Goodbourn S, Randall R E. The V protein of simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation. J Virol. 1999;73:9928–9933. doi: 10.1128/jvi.73.12.9928-9933.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durbin J E, Hackenmiller R, Simon M C, Levy D E. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 11.Elango N, Kovamees J, Varsanyi T M, Norrby E. mRNA sequence and deduced amino acid sequence of the mumps virus small hydrophobic protein gene. J Virol. 1989;63:1413–1415. doi: 10.1128/jvi.63.3.1413-1415.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esolen L M, Park S W, Hardwick J M, Griffin D E. Apoptosis as a cause of death in measles virus-infected cells. J Virol. 1995;69:3955–3958. doi: 10.1128/jvi.69.6.3955-3958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evan G, Littlewood T. A matter of life and cell death. Science. 1998;281:1317–1322. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 14.Fugier-Vivier I, Servet-Delprat C, Rivailler P, Rissoan M C, Liu Y J, Rabourdin-Combe C. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med. 1997;186:813–823. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galvan V, Roizman B. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc Natl Acad Sci USA. 1998;95:3931–3936. doi: 10.1073/pnas.95.7.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcin D, Taylor G, Tanebayashi K, Compans R, Kolakofsky D. The short Sendai virus leader region controls induction of programmed cell death. Virology. 1998;243:340–353. doi: 10.1006/viro.1998.9063. [DOI] [PubMed] [Google Scholar]
- 17.He B, Leser G P, Paterson R G, Lamb R A. The paramyxovirus SV5 small hydrophobic (SH) protein is not essential for virus growth in tissue culture cells. Virology. 1998;250:30–40. doi: 10.1006/viro.1998.9354. [DOI] [PubMed] [Google Scholar]
- 18.He B, Paterson R G, Ward C D, Lamb R A. Recovery of infectious SV5 from cloned DNA and expression of a foreign gene. Virology. 1997;237:249–260. doi: 10.1006/viro.1997.8801. [DOI] [PubMed] [Google Scholar]
- 19.Itoh M, Hotta H, Homma M. Increased induction of apoptosis by a Sendai virus mutant is associated with attenuation of mouse pathogenicity. J Virol. 1998;72:2927–2934. doi: 10.1128/jvi.72.4.2927-2934.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato A, Kiyotani K, Sakai Y, Yoshida T, Nagai Y. The paramyxovirus, Sendai virus, V protein encodes a luxury function required for viral pathogenesis. EMBO J. 1997;16:578–587. doi: 10.1093/emboj/16.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A, Commane M, Flickinger T W, Horvath C M, Stark G R. Defective TNF-alpha-induced apoptosis in STAT1-null cells due to low constitutive levels of caspases. Science. 1997;278:1630–1632. doi: 10.1126/science.278.5343.1630. [DOI] [PubMed] [Google Scholar]
- 22.Lamb R A, Kolakofsky D. Paramyxoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1177–1204. [Google Scholar]
- 23.Lin G Y, Lamb R A. The V protein of the paramyxovirus simian parainfluenza virus 5 (SV5) slows progression of the cell cycle. J Virol. 2000;74:9152–9166. doi: 10.1128/jvi.74.19.9152-9166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin G Y, Paterson R G, Richardson C D, Lamb R A. The V protein of the paramyxovirus SV5 interacts with damage-specific DNA binding protein. Virology. 1998;249:189–200. doi: 10.1006/viro.1998.9317. [DOI] [PubMed] [Google Scholar]
- 25.Lorence R M, Rood P A, Kelley K W. Newcastle disease virus as an antineoplastic agent: induction of tumor necrosis factor-alpha and augmentation of its cytotoxicity. J Natl Cancer Inst. 1988;80:1305–1312. doi: 10.1093/jnci/80.16.1305. [DOI] [PubMed] [Google Scholar]
- 26.McCandlish I A, Thompson H, Cornwell H J, Wright N G. A study of dogs with kennel cough. Vet Rec. 1978;102:293–301. doi: 10.1136/vr.102.14.293. [DOI] [PubMed] [Google Scholar]
- 27.Nicoletti I, Migliorati G, Pagliacci M C, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 28.Olmsted R A, Collins P L. The 1A protein of respiratory syncytial virus is an integral membrane protein present as multiple, structurally distinct species. J Virol. 1989;63:2019–2029. doi: 10.1128/jvi.63.5.2019-2029.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paterson R G, Harris T J R, Lamb R A. Analysis and gene assignment of mRNAs of a paramyxovirus, simian virus 5. Virology. 1984;138:310–323. doi: 10.1016/0042-6822(84)90354-4. [DOI] [PubMed] [Google Scholar]
- 30.Paterson R G, Lamb R A. The molecular biology of influenza viruses and paramyxoviruses. In: Davidson A, Elliott R M, editors. Molecular virology: a practical approach. Oxford, United Kingdom: IRL Oxford University Press; 1993. pp. 35–73. [Google Scholar]
- 31.Paterson R G, Leser G P, Shaughnessy M A, Lamb R A. The paramyxovirus SV5 V protein binds two atoms of zinc and is a structural component of virions. Virology. 1995;208:121–131. doi: 10.1006/viro.1995.1135. [DOI] [PubMed] [Google Scholar]
- 32.Randall R E, Young D F, Goswami K K A, Russell W C. Isolation and characterization of monoclonal antibodies to simian virus 5 and their use in revealing antigenic differences between human, canine and simian isolates. J Gen Virol. 1987;68:2769–2780. doi: 10.1099/0022-1317-68-11-2769. [DOI] [PubMed] [Google Scholar]
- 33.Roulston A, Marcellus R C, Branton P E. Viruses and apoptosis. Annu Rev Microbiol. 1999;53:577–628. doi: 10.1146/annurev.micro.53.1.577. [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi K, Tanabayashi K, Hishiyama M, Yamada A. The mumps virus SH protein is a membrane protein and not essential for virus growth. Virology. 1996;225:156–162. doi: 10.1006/viro.1996.0583. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi R, Tsutsumi H, Osaki M, Haseyama K, Mizue N, Chiba S. Respiratory syncytial virus infection of human alveolar epithelial cells enhances interferon regulatory factor 1 and interleukin-1β-converting enzyme gene expression but does not cause apoptosis. J Virol. 1998;72:4498–4502. doi: 10.1128/jvi.72.5.4498-4502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka N, Sato M, Lamphier M S, Nozawa H, Oda E, Noguchi S, Schreiber R D, Tsujimoto Y, Taniguchi T. Type I interferons are essential mediators of apoptotic death in virally infected cells. Genes Cells. 1998;3:29–37. doi: 10.1046/j.1365-2443.1998.00164.x. [DOI] [PubMed] [Google Scholar]
- 37.Tewari M, Telford W G, Miller R A, Dixit V M. CrmA, a poxvirus-encoded serpin, inhibits cytotoxic T-lymphocyte-mediated apoptosis. J Biol Chem. 1995;270:22705–22708. doi: 10.1074/jbc.270.39.22705. [DOI] [PubMed] [Google Scholar]
- 38.Thomas S M, Lamb R A, Paterson R G. Two mRNAs that differ by two nontemplated nucleotides encode the amino coterminal proteins P and V of the paramyxovirus SV5. Cell. 1988;54:891–902. doi: 10.1016/S0092-8674(88)91285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thornberry N A, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 40.Vidalain P O, Azocar O, Lamouille B, Astier A, Rabourdin-Combe C, Servet-Delprat C. Measles virus induces functional TRAIL production by human dendritic cells. J Virol. 2000;74:556–559. doi: 10.1128/jvi.74.1.556-559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S Z, Smith P K, Lovejoy M, Bowden J J, Alpers J H, Forsyth K D. The apoptosis of neutrophils is accelerated in respiratory syncytial virus (RSV)-induced bronchiolitis. Clin Exp Immunol. 1998;114:49–54. doi: 10.1046/j.1365-2249.1998.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Q, Snipas S, Orth K, Muzio M, Dixit V M, Salvesen G S. Target protease specificity of the viral serpin CrmA. Analysis of five caspases. J Biol Chem. 1997;272:7797–8800. doi: 10.1074/jbc.272.12.7797. [DOI] [PubMed] [Google Scholar]
- 43.Zorn U, Dallmann I, Grosse J, Kirchner H, Poliwoda H, Atzpodien J. Induction of cytokines and cytotoxicity against tumor cells by Newcastle disease virus. Cancer Biother. 1994;9:225–235. doi: 10.1089/cbr.1994.9.225. [DOI] [PubMed] [Google Scholar]