Abstract
A novel alphavirus was isolated from the louse Lepidophthirus macrorhini, collected from southern elephant seals, Mirounga leonina, on Macquarie Island, Australia. The virus displayed classic alphavirus ultrastructure and appeared to be serologically different from known Australasian alphaviruses. Nearly all Macquarie Island elephant seals tested had neutralizing antibodies against the virus, but no virus-associated pathology has been identified. Antarctic Division personnel who have worked extensively with elephant seals showed no serological evidence of exposure to the virus. Sequence analysis illustrated that the southern elephant seal (SES) virus segregates with the Semliki Forest group of Australasian alphaviruses. Phylogenetic analysis of known alphaviruses suggests that alphaviruses might be grouped according to their enzootic vertebrate host class. The SES virus represents the first arbovirus of marine mammals and illustrates that alphaviruses can inhabit Antarctica and that alphaviruses can be transmitted by lice.
The genus Alphavirus in the family Togoviridae represents a group of enveloped, plus-strand viruses comprising over 40 known members. Alphaviruses are classified as arboviruses since they are maintained in nature by a biological transmission cycle between susceptible vertebrate hosts and hematophagous arthropods, usually ticks or mosquitoes. Alphaviruses have been grouped by geographic distribution into Old and New World viruses (28). The New World alphaviruses, which include Venezuelan equine encephalitis (VEE), eastern equine encephalitis (EEE), and western equine encephalitis (WEE) viruses are pathogenic for humans, horses, and certain bird species (26, 28). The Old World alphaviruses are associated with rheumatic disease in humans and include the Australian Ross River (RR) and Barmah Forest viruses, the Asian/African chikungunya virus, the African o'nyong-nyong virus, and the European Ockelbo virus, which is a subtype of Sindbis virus (28). Weaver et al. (39) hypothesized that alphaviruses may have originated a few thousand years ago in the New World, possibly through recombination with plant viruses (9), and spread to the Old World via bird migration. Recently, two fish alphaviruses have been described, salmon pancreas disease virus (40) and rainbow trout sleeping disease virus (37), suggesting either aquatic origins for alphaviruses or an invasion of the marine environment by terrestrial alphaviruses.
Macquarie Island is located some 2,000 km south (54°30'S, 159°E) off the Australian mainland. The island has been a rich source of tick-borne arboviruses. A flavivirus of penguins (22) has been identified on Macquarie Island, as have flaviviruses, bunyaviruses, and orbiviruses, whose enzootic hosts are also likely to be birds (3, 23, 33). The island is also home to about 12% of the world's population of southern elephant seals (Mirounga leonina), roughly 78,000 animals (16), which are known to be infested with the blood-sucking louse Lepidophthirus macrorhini (25). The Macquarie Island elephant seal population has decreased by ≈50% since 1950 (10) and continues to decline at a rate of ≈1.7% per annum (D. J. Slip and H. R. Burton, unpublished data). The cause of this decline is unknown (12). Viral infections (1, 27) and pollution-induced immunosuppression (36) have been associated with unexpected seal mortality in Europe and Africa, prompting a worldwide search for pathogens of seals (13). Vector-borne pathogens such as arboviruses are a significant cause of infectious disease in mammals (2). However, none have been described for marine mammals, perhaps due to the paucity of hematophagous arthropods of marine mammals (31). One exception is the suborder of echinophthiriid blood-sucking lice found on some species of seals (15). These considerations prompted a search for an arbovirus of seals and resulted in the isolation of a new alphavirus, the southern elephant seal virus (SES virus), from the elephant seal louse, L. macrorhini.
MATERIALS AND METHODS
Collection of lice.
Live lice were collected from male and female southern elephant seals of known age that had returned to Macquarie Island for breeding, the annual molt, and the mid-year haulout. The animals were part of life history studies at Macquarie Island. Prior to examination, the animals were anesthetised with a 1:1 mixture of Tiletamine and Zolazepam as described previously (21). The skin of the seal was searched for lice, which were most often encountered on the hind flippers (24). Lice were removed with a pair of fine forceps, snap frozen, stored at −80°C in glass vials, and shipped to mainland Australia.
Bleeding of seals.
A 10-ml blood sample was collected from anesthetised seals (see above) via the extradural-intravertebral vein in the lower lumbar region, using a 90-mm 18-gauge spinal needle. The blood was left to clot for 30 min and centrifuged for 5 minutes at 3,000 × g. The serum was collected, stored at −80°C, and shipped to mainland Australia.
Virus isolation.
BHK-21 cells (ATCC CCL-10) were grown in medium comprising bicarbonate-buffered RPMI 1640 (Gibco-BRL, Life Technologies, Rockville, Md.) supplemented with 5% fetal bovine serum (JRH Biosciences, Lenexa, Kans.) 2 mM glutamine (Sigma), 100 μg of penicillin per ml, and 100 IU of streptomycin per ml (CSL Ltd., Melbourne, Australia) at 37°C in 5% CO2. Lice were individually chopped aseptically using a scalpel, transferred to 1.5-ml Eppendorf tubes, and ground in 500 μl of medium using a motorized pestle. The debris and contaminations were removed by centrifugation (12,000 × g for 10 min at 4°C), and the supernatant was added undiluted, at 1/10 and 1/100 dilutions, to BHK-21 cells (≈104 cells per well of a 24-well plate containing ≈1 ml of medium). The cultures were maintained at 37°C and 5% CO2 and passaged every 5 to 7 days by transferring ≈100 μl of the supernatant onto fresh BHK-21 cell cultures.
Virus titer determination.
The virus was serially diluted 10-fold in quadruplicate and incubated with Vero cells (ATCC CCL-81), and the virus titer was determined from the resultant cytopathic effect. Virus titers are expressed as a log10 50% cell culture infectious dose (CCID50), as described previously (18).
Electron microscopy.
Vero cells were infected with SES virus (multiplicity of infection, ≈1) and incubated for 48 h before being processed for transmission electron microscopy. Infected cells were scraped, fixed for 40 min in 2.5% (vol/vol) glutaraldehyde in 0.1 M cacodylate phosphate buffer (Sigma) (pH 7.2; 300 mosmol), washed in the same buffer (three times for 30 min), postfixed for 1 h in 1% (wt/vol) osmium tetroxide in cacodylate buffer, rinsed in distilled water (four times for 5 min), dehydrated through graded ethanol (70 to 100%), and infiltrated and embedded in Spurr's epoxy resin (ProSciTech, Thuringowa, Australia). Sections were cut on a Leica-Reichert-Jung Ultracut E microtome and double stained in uranyl acetate and lead citrate. The supernatant from infected cell cultures were stained with 2% phosphotungstic acid (ProSciTech) (pH 6.5). Sections were examined using a Hitachi H7000 scanning-transmission electron microscope at 100 kV, and negative-stained samples were examined at 75 kV. The microscope was calibrated with a 2,160-lines/mm standard (Agar Scientific Ltd., Stansted, United Kingdom).
Preparation of murine anti-SES virus antisera.
BALB/c mice were given two intraperitoneal injections of ≈105 CCID50 of SES virus 5 weeks apart, and serum was collected 2 weeks after the second injection.
Virus neutralization assay by virus dilution and constant serum.
Virus was serially diluted 10-fold in medium, and 50 μl of each dilution was added to a 96-well plate in duplicate. An equal volume of polyclonal antisera diluted 1/10 in medium was added, and the mixture was incubated at 37°C for 90 min. Vero cells (104 in 100 μl of medium) were then added, and the plate was incubated at 37°C in 5% carbon dioxide. After 6 days the cells were simultaneously fixed and stained with phosphate-buffered saline containing 10% paraformaldehyde and 0.05% crystal violet (Sigma). The neutralization index (NI) was calculated as the CCID50 of virus in the absence of sera minus the CCID50 of virus in the presence of a 1/10 dilution of test serum. Sera with an NI greater than 1.5 were considered to have neutralizing activity. All sera were heat inactivated for 30 min at 56°C.
SES virus cDNA preparation.
The virus was passaged a total of four times in BHK-21 cells and once in Vero cells prior to RNA preparation and purification. Near-confluent Vero cells in a T25 flask were infected (multiplicity of infection, ≈1) and cultured for 33 h. Cells were scraped off the flask into medium and pelleted (1,500 × g for 5 min at 4°C). Total RNA isolation reagent (1 ml) (Advanced Biotechnologies Ltd., London, United Kingdom) was added to the pellet, and RNA was prepared as specified by the manufacturer.
First-strand cDNA synthesis was performed in a reaction mixture containing approximately 2 ng of RNA preparation, 1 μl of random hexamer oligonucleotides, 250 mM Tris-HCl (pH 8.3), 375 mM KCl, 15 mM MgCl2, 100 mM dithiothreitol, 10 mM each dATP, dCTP, dGTP, and dTTP, and 200 U of Superscript II (Life Technologies) as specified by the manufacturer.
PCR amplification and cloning of the structural region of the SES virus.
Degenerate oligonucleotides were designed from the most highly conserved region of a nucleotide sequence alignment of the structural region of alphaviruses; they were primer 1 [5′TA(C/T) A(A/G)(C/T) TGG CA(C/T) CA(C/T) GGI GCI GTI 3′] and primer 2 [5′CCI CCC CAC AT(A/G) AAI GG(A/G) TAI ACI CC 3′] (where I represents deoxyinosine). The amplification reaction mixture contained 2 μl of randomly primed cDNA, 1 μM each primer oligonucleotide, 50 mM Tris-HCl (pH 9.0), 1.5 mM MgCl2, 15 mM (NH4)2SO4, 0.1% Triton X-100, 200 μM each dATP, dCTP, dGTP, and dTTP, and 0.4 U of DyNAzyme II DNA polymerase (Finnzymes Oy, Ospoo, Finland) in a 20-μl reaction volume. The cycling conditions were 1 cycle of 94°C for 2 min, followed by 35 cycles of 94°C for 20 s and 50°C for 30 s, and a final 72°C extension for 10 min. PCR products were analyzed by electrophoresis on 1% agarose gels and directly sequenced. The products were ligated into pGEM-T vector (Promega, Madison, Wis.) using 15 ng of PCR product, 50 ng of pGEM-T vector, 2× rapid ligase buffer, and 3 U of T4 DNA ligase (Promega). The ligation reaction mixture was incubated at 15°C for 20 h. Then 2 μl of the ligation mixture was used to transform Escherichia coli XL-10 Blue competent cells (Stratagene, La Jolla, Calif.) as specified by the manufacturer.
DNA sequencing.
PCR products were directly sequenced using primers 1 and 2, and three clones containing the structural protein insert were sequenced with universal M13 forward and reverse primers. Once a specific sequence was generated, primer 3 (5′TAC AGT CGA TGG CTT CAG ACG 3′) and primer 4 (5′ ATA CGC ACT TAC TCC GAA TGC 3′) were synthesized to allow sequencing of both strands of the ≈1.9-kb insert. Sequencing was performed using the BigDye (Applied Biosystems Inc.) fluorescent-chain terminator technology as specified by the manufacturer. The products were analysed on an ABI PRISM 377 DNA sequencer.
Sequence analysis.
The nucleotide sequences were assembled into a single contig of the SES virus sequence using the Staden program gap4 (32) maintained at ANGIS, the Australian National Genomic Information Service (http://www.angis.org.au). The predicted amino acid sequence was determined using the GCG Inc. program Translate, maintained at ANGIS. The SES virus nucleotide sequence was subject to a BLASTX search at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov) against the nonredundant GenBank database. An alignment of alphavirus amino acid sequences was created using the program ClustalX (35) and then edited manually. A phylogeny of the aligned amino acid sequences was constructed using the neighbor-joining algorithm (29), and bootstrap support percentages (4) for each node were obtained from 1,000 resamplings of the original data set as implemented in the program MEGA (14). Any positions that contained gaps or unknown residues were not included in the phylogenetic reconstruction (complete deletion), and three regions that were difficult to align were also removed: positions 38 to 51, positions 102 to 127, and positions 588 to 606. This resulted in a total of 589 aligned amino acid residues available for the phylogenetic reconstruction.
RESULTS
Electron microscopy of the SES virus.
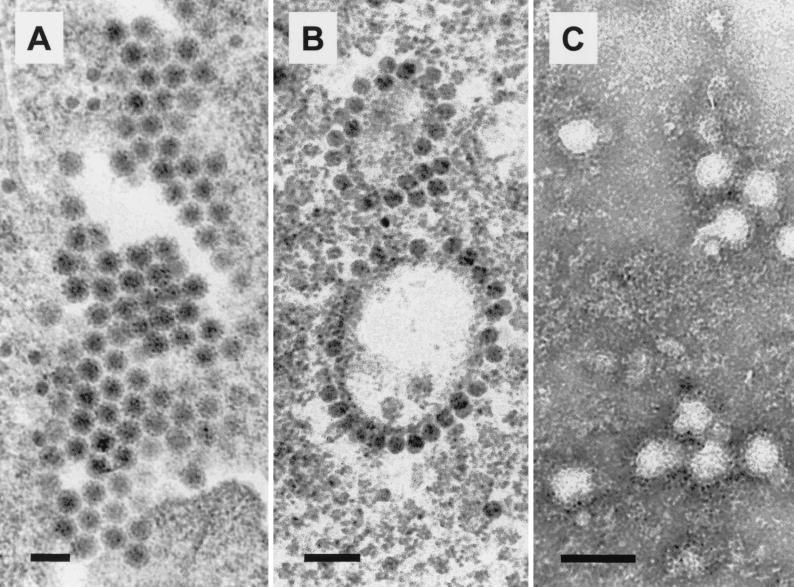
After individual processing of 12 lice, the extracts from two lice produced CPE in BHK-21 cells. One of these was processed for electron microscopy after passage in Vero cells to avoid potentially confounding detection of the endogenous retroviruses in BHK-21 cells. Transmission electron microscopy of Vero cells infected with the SES virus showed extracellular enveloped viruses with a diameter of 55 ± 1 nm (n = 24) (Fig. 1A) and cytoplasmic nucleocapsid particles (29 ± 2 nm; n = 24) frequently associated with vesicular membranes (Fig. 1B). Negative-contrast electron microscopy of the tissue culture supernatants from virus-infected cells showed enveloped spherical particles (65 ± 3 nm; n = 24) (Fig. 1C) with surface projections of 10 ± 1 nm (n = 14). These ultrastructural features are consistent with alphavirus morphology (5, 28).
FIG. 1.
Electron micrographs of SES virus grown in Vero cells. (A) Transmission electron micrograph of extracellular enveloped viruses 24 h postinfection. (B) Transmission electron micrograph of cytoplasmic nucleocapsids associated with cytoplasmic membranes (vacuoles and vesicles) 24 h postinfection. (C) Negative-contrast electron micrograph of virus particles stained with phosphotungstic acid. The envelope and surface projections are apparent. Bars, 100 nm.
SES virus serology.
To determine the seroprevalence of SES virus antibodies in Macquarie Island elephant seals, a panel of elephant seal sera were tested for the ability to neutralize SES virus. Of 11 1-year-old seals, 2 were found to be seropositive, whereas nearly all tested seals older than 2 years were seropositive (Table 1), suggesting that nearly all seals become infected with the SES virus within 2 years of birth.
TABLE 1.
Sera from 60 Maquarie Island elephant seals tested for SES virus-neutralizing activity
| Yr of tagginga | Seala | NIb |
|---|---|---|
| 1998 | P455 | 2.5 |
| P324 | 3.5 | |
| P276 | 0 | |
| P568 | 0.5 | |
| P431 | 1.5 | |
| P734 | 0.5 | |
| E202 | 0.5 | |
| E701 | 0 | |
| E102 | 1.5 | |
| E494 | 1.0 | |
| E614 | 1.0 | |
| 1997 | D126 | 2.5 |
| D826 | 3.0 | |
| D261 | 3.0 | |
| D102 | 2.0 | |
| D799 | 4.5 | |
| D876 | 2.5 | |
| D214 | 2.5 | |
| D197 | 4.0 | |
| D874 | 4.0 | |
| D666 | 4.0 | |
| D483 | 2.0 | |
| D511 | 3.0 | |
| D533 | 2.5 | |
| M689 | 2.5 | |
| M953 | 2.0 | |
| M084 | 3.5 | |
| 1996 | K655 | 3.5 |
| K528 | 2.0 | |
| K733 | 3.5 | |
| K018 | 2.5 | |
| K266 | 2.5 | |
| K869 | 3.0 | |
| K281 | 3.0 | |
| K159 | 3.5 | |
| K147 | 1.5 | |
| N722 | 3.0 | |
| N576 | 3.0 | |
| N040 | 3.0 | |
| N673 | 3.0 | |
| N775 | 3.0 | |
| N613 | 2.5 | |
| N741 | 2.5 | |
| 1995 | J937 | 3.5 |
| J805 | 2.5 | |
| L434 | 3.0 | |
| L204 | 2.5 | |
| 1994 | F286 | 3.0 |
| F579 | 3.0 | |
| H779 | 2.5 | |
| H403 | 2.5 | |
| 1993 | B542 | 2.5 |
| B069 | 2.0 | |
| C209 | 3.5 | |
| 1991 | B4656 | 3. |
All seals were tagged after birth. The year of tagging is indicated above each column; seals whose code numbers begin with P or E were 1 year old in 1999.
The NI was calculated as described in Materials and Methods. NI values above 1.5 indicate the presence of significant neutralizing antibodies and are shown in bold type. Serum was collected in 1999.
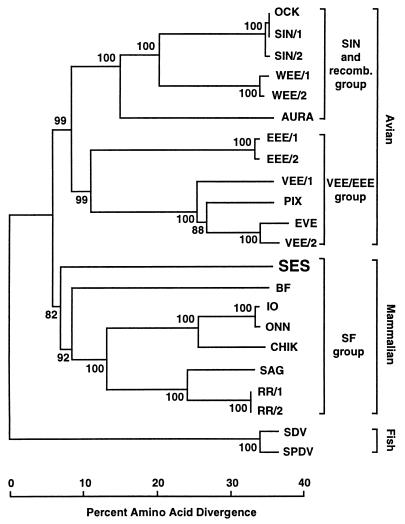
To investigate the serological relationship of SES virus to other alphaviruses, anti-SES virus antisera from SES virus-infected mice was tested against a panel of Australasian alphaviruses in neutralization assays. Inoculation of adult mice with SES virus resulted in a brief asymptomatic low-grade viremia lasting 2 to 3 days (data not shown) and the production of neutralizing antibodies. The anti-SES virus antisera neutralized SES virus but failed to neutralize significantly any of the other alphaviruses tested (Table 2). These results suggest that the SES virus is serologically different from its nearest neighbors in the Semliki Forest (SF) group (see Fig. 3).
TABLE 2.
Ability of murine anti-SES virus antiserum to neutralize a panel of Australasian alphaviruses
| Alphavirus | NIa |
|---|---|
| SES virus | 4.0 |
| Ross River virus (T48) | 0 |
| Barmah Forest virus | 0 |
| Semliki Forest virus | 0.5 |
| Chikungunya virus | 1.0 |
| Getah virus | 0.5 |
| Sindbis virus | 0.5 |
FIG. 3.
Phylogeny of representative alphaviruses derived from 589 residues of structural protein sequence. The percent bootstrap values for each node are shown. The alignment used as input can be downloaded from the EMBL alignment database (ftp://ftp.ebi.ac.uk/pub/databases/embl/align) with accession number DS44746. Prior to constructing the phylogeny, three regions that were difficult to align were removed: positions 38 to 51, positions 102 to 127, and positions 588 to 606. The full names and accession numbers for each sequence are as follows: AURA (Aura virus, AAD13623), BF (Barmah Forest virus, AAB40702), CHIK (chikungunya virus, L37661), EEE/1 (EEE virus, AAA67908), EEE/2 (AAC53760), EVE (Everglades strain, VEE virus, AF075251), IO (Igbo Ora virus, AAC97207), OCK (Ockelbo virus, M69205), ONN (o'nyong-nyong virus, AAC97205), PIX (Pixuna strain, VEE virus, AF075256), RR/1 (Ross River virus, AAA47404), RR/2 (P08491), SAG (Sagiyama virus, BAA92847), SDV (sleeping disease virus, AJ238578), SIN/1 (Sindbis virus, P27285), SIN/2 (AAA86134), SES virus (AF315122), SPDV (salmon pancreas disease virus, AJ012631), VEE/1 (VEE virus, AAD14563), VEE/2 (AAD27803), WEE/1 (WEE virus, AAF60166), WEE/2 (J03854).
Sera from six Antarctic Division personnel who have worked extensively and closely with elephant seals failed to neutralize SES virus (data not shown), indicating that human-seal contact does not readily result in infection of humans with SES virus. It is unclear whether L. macrorhini, a species-specific louse, actually bites humans. Control sera from five Queensland Institute of Medical Research staff members, two of whom were seropositive for RR virus, also failed to neutralize the virus (data not shown).
SES virus sequence.
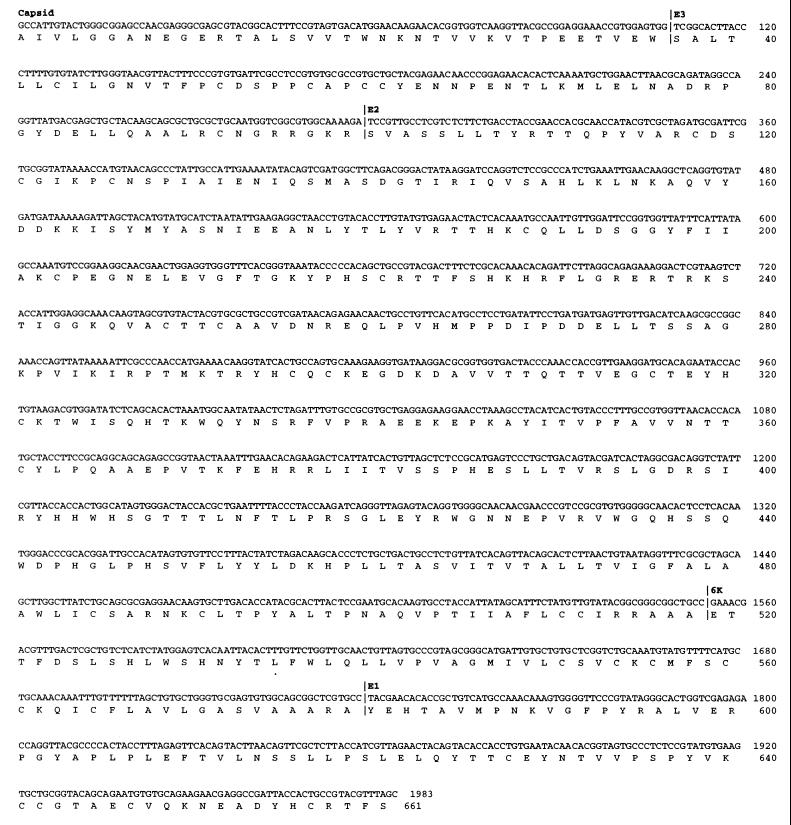
The nucleotide sequence from the structural protein region is shown in Fig. 2 (1,983 bp) and includes part of the capsid protein, all of the small glycoprotein E3, all of the envelope glycoprotein E2, all of the hydrophobic peptide linker 6K, and part of the envelope glycoprotein E1. The 100 best matches from the BLASTX search against GenBank were all alphaviruses, and the top four matches were all viruses from the SF group (34): Igbo Ora virus, o'nyong-nyong virus, Sagiyama virus, and chikungunya virus (data not shown). Phylogenetic reconstruction showed that SES virus clustered with the SF group with a bootstrap support of 82% (Fig. 3). SES virus also clustered with the SF group if the more divergent fish alphavirus sequences were removed (data not shown).
FIG. 2.
Nucleotide sequence and translated amino acid sequence from the structural region of the SES virus. The boundaries of the structural proteins are indicated and were obtained by reference to the sequence of WEE virus (8).
DISCUSSION
This paper describes the first known arbovirus of marine mammals, an alphavirus infecting Macquarie Island elephant seals. The virus was isolated from the blood-sucking elephant seal louse, L. macrorhini, a species-specific louse that infests elephant seals shortly after birth (25). The isolation of SES virus from L. macrorhini and the high SES virus seroprevalence in the elephant seal population strongly suggests that SES virus is transmitted by these lice. No formal proof of the vector competence of L. macrorhini is available, and other insects such as ticks and mosquitoes might represent the true vectors, with lice simply consuming infected blood meals. However, this is unlikely because Macquarie Island has no mosquitoes (7, 30, 38) and ticks are rarely found on elephant seals (25). In addition, the SES virus was isolated from a louse taken from a 5-year-old seal. Such animals are very likely to be seropositive (Table 1), and lice are unlikely to ingest infectious virus from animals with neutralizing antibodies. After infection, the lice (like mosquitoes) probably remain infected for life. The lice can survive the seal's long period at sea and reproduce when the seals haul out twice a year for 3 to 5 weeks to breed and molt (25).
Alphaviruses are generally believed to be transmitted by mosquitoes (class Insecta, order Heteroptera), although Ixodes ticks (class Arachnida) have been implicated in the transmission of VEE and Sindbis viruses (6, 19). The transmission of an alphavirus by sucking lice (class Insecta, order Anoplura) has not been generally reported, although other insects are known to transmit alphaviruses. For instance, the bug Oeciacus vicariu (class Insecta, order Diptera) is believed to transmit Fort Morgan virus (a close relative of WEE virus) between birds (39). Interestingly, the distantly related hematophagous arthropod, the salmon louse Lepeophtheirus salmonis (phylum Arthropoda, subphylum Crustacea), has been implicated as a possible vector for the transmission of the salmon pancreas disease virus (40).
Alphaviruses have so far been found on all continents except Antarctica (34). Macquarie Island is outside the Antarctic Circle; however, elephant seals range extensively within Antarctica. For example, an elephant seal from Macquarie Island has been sighted on Peter 1ØY, which lies within the Antarctic circle some 5,000 km from Macquarie Island and 1,800 km from the South American mainland (11). In addition, a male seal tagged at the Windmill Islands, Antarctica, has also been sighted at Macquarie Island (C. R. McMahon, unpublished observation). Alphaviruses therefore inhabit every continent on the planet. The ability of long-lived lice infected with alphaviruses to be carried such large distances on marine mammals also suggests that alphaviruses can travel between South America and Australasia via Antarctica. Other species of echinophthiriid blood-sucking lice on other species of seal may also carry the SES virus, and such lice may also be hosts for other arboviruses.
Previous sequence analysis has shown that there are three principal genocomplexes within the genus Alphavirus, the VEE-EEE group, the SF group, and the Sindbis group (34) and our analysis shows that the SES virus segregates with the SF group of Australasian and African alphaviruses (Fig. 3). There is also a recombinant group containing members of the WEE complex, the result of recombination between EEE and Sindbis group ancestors (9, 39). The recombination point is somewhere in the E3 protein (8), and since most of our sequence data are downstream of E3 (Fig. 2), we are not able to test if SES virus is a recombinant. However, SES virus is unlikely to be a member of the recombinant group since it segregates with the SF group of Australasian and African alphaviruses with a high bootstrap support of 82% and not with either of the two groups that gave rise to the recombinant viruses (Fig. 3). A grouping of SES virus with the SF group is also observed if the short region of capsid sequence alignment (i.e., upstream of the recombination point) is used to construct a phylogenetic tree (data not shown).
Alphaviruses have been grouped according to their geographic localization in the New World and Old World (34, 39). It has been proposed that alphaviruses originated in the New World several thousand years ago and were then spread by birds to the Old World (17, 39). The VEE-EEE group is found exclusively in the New World, while the SF and Sindbis groups are predominantly Old World viruses. Migratory birds have traditionally been thought to play a significant role in the local dispersion and global distribution of alphaviruses (34). With birds, fish, and now marine mammals potentially being able to transport alphaviruses over large distances, geographic isolation of alphaviruses for extended periods during evolution might be difficult to countenance. An alternative grouping of alphaviruses based on the virus's vertebrate hosts emerges from the phylogenetic analysis (Fig. 3), which illustrates that alphaviruses segregate into viruses that primarily use fish, birds, or mammals as their natural enzootic hosts. The fish alphaviruses (sleeping disease virus and salmon pancreas disease virus) clearly segregate as a separate group. The American encephalitis viruses (VEE-EEE group) and the European Sindbis group can all utilize birds as enzootic hosts (20, 26), although small mammals can also serve as hosts (39) and epizootic infections of humans and horses occur for many of these viruses (34). Finally, the Australasian and African alphaviruses, which include the SES virus, utilize mammals as their favored enzootic hosts (39). The discovery of alphaviruses of fish, birds, and now marine mammals might suggest the existence of amphibian and reptilian alphaviruses.
ACKNOWLEDGMENTS
M. L. Linn, J. Gardner, D. Warrilow, and G. A. Darnell contributed equally to the experimental work and should be considered joint first authors. A. Suhrbier and R. W. Slade contributed equally to the intellectual input and planning and should be considered joint last authors.
This work was funded by Australian Centre for International & Tropical Health & Nutrition, the National Health and Medical Research Council, the Australian Antarctic Division (project 2265), the Seaworld Research and Rescue Foundation, and Tequilla Sunnies Pty Ltd.
We thank A. Rosenstengel (QIMR) and J. MacKenzie, Department of Microbiology and Parasitology, University of Queensland, for their help. Thanks also go to R. E. Shope, WHO Arbovirus Reference Centre, University of Texas, for the kind gift of antibodies.
REFERENCES
- 1.an de Bildt M W, Vedder E J, Martina B E, Sidi B A, Jiddou A B, Ould Barham M E, Androukaki E, Komnenou A, Niesters H G, Osterhaus A D. Morbilliviruses in Mediterranean monk seals. Vet Microbiol. 1999;69:19–21. doi: 10.1016/s0378-1135(99)00082-6. [DOI] [PubMed] [Google Scholar]
- 2.Davis J W, Karstad L H, Trainer O D, editors. Infectious diseases of wild mammals. 2nd ed. Ames: Iowa State University Press; 1981. [Google Scholar]
- 3.Doherty R L, Carley J G, Murray M D, Main A J, Jr, Kay B H, Domrow R. Isolation of arboviruses (Kemerovo group, Sakhalin group) from Ixodes uriaecollected at Macquarie Island, Southern ocean. Am J Trop Med Hyg. 1975;24:521–526. doi: 10.4269/ajtmh.1975.24.521. [DOI] [PubMed] [Google Scholar]
- 4.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 5.Froshauer S, Kartenbeck J, Helenius A. Alphavirus RNA replicase is located on the cytoplasmic surface of endosomes and lysosomes. J Cell Biol. 1988;107:2075–2086. doi: 10.1083/jcb.107.6.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gresikova M, Sekeyova M, Tempera G, Guglielmino S, Castro A. Identification of a Sindbis virus strain isolated from Hyaloma marginatumticks in Sicily. Acta Virol. 1978;22:231–232. [PubMed] [Google Scholar]
- 7.Gressitt J L, Forster R R, Fain A, Smithers C N, Stannard L J, Eastop V F, Alexander C P, Brundin L, Quate L W, Hardy D E, Wirth W W, Dunnet G M, Sabrosky C W, Yoshimoto C M, Common I F B. Insects of Macquarie Island. Pacific Insects. 1962;4:905–978. [Google Scholar]
- 8.Hahn C S, Lustig S, Strauss E G, Strauss J H. Western equine encephalitis virus is a recombinant virus. Proc Natl Acad Sci USA. 1988;85:5997–6001. doi: 10.1073/pnas.85.16.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haseloff J, Goelet P, Zimmern D, Ahlquist P, Dasgupta R, Kaesberg P. Striking similarities in amino acid sequence among nonstructural proteins encoded by RNA viruses that have dissimilar genomic organization. Proc Natl Acad Sci USA. 1984;81:4358–4362. doi: 10.1073/pnas.81.14.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hindell M A, Burton H R. Past and present status of the southern elephant seal (Mirounga leonina) at Macquarie Island. J Zool. 1987;213:365–380. [Google Scholar]
- 11.Hindell M A, McMahon C R. Elephant seals circle the globe: a record movement for a southern elephant seal (Mirounga leonina), from Macquarie Island to Peter 1 O/Y. Mar Mamm Sci. 2000;16:504–507. [Google Scholar]
- 12.Hindell M A, Slip D J, Burton H R. Possible causes of the decline of southern elephant seal populations in the southern Pacific and southern Indian Oceans. In: LeBoeuf B J, Laws R M, editors. Elephant seals: population ecology, behaviour, and physiology. Berkeley: University of California Press; 1994. pp. 66–84. [Google Scholar]
- 13.Kerry K, Riddle M, Clarke J. Diseases of Antarctic wildlife. Report for The Scientific Committee on Antarctic Research and The Council of Managers of National Antarctic Programs. Kingston, Australia: Australian Antarctic Division; 1999. [Google Scholar]
- 14.Kumar S, Tamura K, Nei M. MEGA: Molecular evolutionary genetic analysis, version 1.0. University Park: The Pennsylvania State University; 1993. [Google Scholar]
- 15.Lauckner G. Diseases of mammalia: Pinnipedia. In: Kinne O, editor. Diseases of marine animals. Hamburg, Germany: Biologische Anstalt Helgoland; 1985. pp. 683–793. [Google Scholar]
- 16.Laws R M. History and present status of southern elephant seal populations. In: LeBoeuf B J, Laws R M, editors. Elephant seals: population ecology, behaviour, and physiology. Berkeley: University of California Press; 1994. pp. 49–65. [Google Scholar]
- 17.Levinson R, Strauss J H, Strauss E G. Determination of the complete nucleotide sequence of the genomic RNA of O'Nyong-nyong virus and its use in the construction of phylogenetic trees. Virology. 1990;175:110–123. doi: 10.1016/0042-6822(90)90191-s. [DOI] [PubMed] [Google Scholar]
- 18.Linn M L, Aaskov J, Suhrbier A. Antibody dependent enhancement and persistence in macrophages of an arbovirus associated with arthritis. J Gen Virol. 1996;77:407–412. doi: 10.1099/0022-1317-77-3-407. [DOI] [PubMed] [Google Scholar]
- 19.Linthicum K J, Logan T M. Laboratory transmission of Venezuelan equine encephalomyelitis virus by the tick Hyalomma truncatum. Trans R Soc Trop Med Hyg. 1994;88:126. doi: 10.1016/0035-9203(94)90536-3. [DOI] [PubMed] [Google Scholar]
- 20.Lundstrom J O, Turell M J, Niklasson B. Antibodies to Ockelbo virus in three orders of birds (Anseriformes, Galliformes and Passeriformes) in Sweden. J Wildl Dis. 1992;28:144–147. doi: 10.7589/0090-3558-28.1.144. [DOI] [PubMed] [Google Scholar]
- 21.McMahon C R, Burton H, McLean S, Slip D, Bester M. Field immobilisation of southern elephant seals with intravenous tiletamine and zolazepam. Vet Rec. 2000;146:251–254. doi: 10.1136/vr.146.9.251. [DOI] [PubMed] [Google Scholar]
- 22.Morgan I R, Westbury H A, Campbell J. Viral infections of little blue penguins (Eudyptula minor) along the southern coast of Australia. J Wildl Dis. 1985;21:193–198. doi: 10.7589/0090-3558-21.3.193. [DOI] [PubMed] [Google Scholar]
- 23.Moss S R, Ayres C M, Nuttall P A. The Great Island subgroup of tick-borne orbiviruses represents a single gene pool. J Gen Virol. 1988;69:2721–2727. doi: 10.1099/0022-1317-69-11-2721. [DOI] [PubMed] [Google Scholar]
- 24.Murray M D. Ecology of the louse Lepidophthirus marcrorhini Enderlein 1904 on the elephant seal Mirounga leonina(L.) Nature. 1958;182:404–405. doi: 10.1038/182404b0. [DOI] [PubMed] [Google Scholar]
- 25.Murray M D, Nicholls D G. Studies on the ectoparasites of seals and penguins I. The ecology of the louse Lepidophthirus macrorhini Enderlein on the southern elephant seal Mirounga leonina(L.) Aust J Zool. 1965;13:437–454. [Google Scholar]
- 26.Olsen G H, Turell M J, Pagac B B. Efficacy of eastern equine encephalitis immunization in whooping cranes. J Wildl Dis. 1997;33:312–315. doi: 10.7589/0090-3558-33.2.312. [DOI] [PubMed] [Google Scholar]
- 27.Osterhaus A D, Rimmelzwaan G F, Martina B E, Bestebroer T M, Fouchier R A. Influenza B virus in seals. Science. 2000;288:1051–1053. doi: 10.1126/science.288.5468.1051. [DOI] [PubMed] [Google Scholar]
- 28.Peters C J, Dalrymple J M. Alphaviruses. In: Fields B N, Knipe D M, Chanok R M, et al., editors. Virology. 2nd ed. New York, N.Y: Raven Press; 1990. pp. 713–761. [Google Scholar]
- 29.Saitou N, Nei M. The neighbour-joining method: a new method for constructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 30.Selkirk P M, Seppelt R D, Selkirk D R. Microbiology, parasitology, and terrestrial arthropods. In: Selkirk P M, Seppelt R D, Selkirk D R, editors. Subantarctic Macquarie Island: environment and biology. Cambridge, United Kingdom: Cambridge University Press; 1990. pp. 188–202. [Google Scholar]
- 31.Slade R W. Limited MHC polymorphism in the southern elephant seal: Implications for MHC evolution and marine mammal population biology. Proc R Soc, London Ser B. 1992;249:163–171. doi: 10.1098/rspb.1992.0099. [DOI] [PubMed] [Google Scholar]
- 32.Staden R. The Staden sequence analysis package. Mol Biotechnol. 1996;5:233–241. doi: 10.1007/BF02900361. [DOI] [PubMed] [Google Scholar]
- 33.St. George T D, Doherty R L, Carley J G, Filippich C, Brescia A, Casals J, Kemp D H, Brothers N. The isolation of arboviruses including a new flavivirus and a new Bunyavirus from Ixodes (Ceratixodes) uriae(Ixodoidea: Ixodidae) collected at Macquarie Island, Australia, 1975–1979. Am J Trop Med Hyg. 1985;34:406–412. doi: 10.4269/ajtmh.1985.34.406. [DOI] [PubMed] [Google Scholar]
- 34.Strauss J H, Strauss E G. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Loveren H, Ross P S, Osterhaus A D, Vos J G. Contaminant-induced immunosuppression and mass mortalities among harbor seals. Toxicol Lett. 2000;112–113:319–324. doi: 10.1016/s0378-4274(99)00198-8. [DOI] [PubMed] [Google Scholar]
- 37.Villoing S, Bearzotti M, Chilmonczyk S, Castric J, Bremont M. Rainbow trout sleeping disease virus is an atypical alphavirus. J Virol. 2000;74:173–183. doi: 10.1128/jvi.74.1.173-183.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson K C. ANARE Scientific Report Series, B1. Zoology. Melbourne, Australia: Australian National Antarctic Research Expeditions; 1967. The terrestrial Arthropoda of Macquarie Island; pp. 1–90. [Google Scholar]
- 39.Weaver S C, Kang W, Shirako Y, Rümenapf T, Strauss E G, Strauss J H. Recombinational history and molecular evolution of Western equine encephalitis complex alphaviruses. J Virol. 1997;71:613–623. doi: 10.1128/jvi.71.1.613-623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weston J H, Welsh M D, McLoughlin M F, Todd D. Salmon pancreas disease virus, an alphavirus infecting farmed Atlantic salmon, Salmo salarL. Virology. 1999;256:188–195. doi: 10.1006/viro.1999.9654. [DOI] [PubMed] [Google Scholar]