Abstract
Contamination of agricultural products with Cadmium (Cd) is a global problem that should be considered for minimizing the risks to human health. Considering the potential effects of SiNPs in decreasing abiotic stress, a study was conducted to investigate the effect of SiNPs in the reduction of Cd stress on Solanum lycopersicum. SiNPs was used at 0, 25, 50 and 100 mg/l and CdCl2 at 0, 100 and 200 µM concentrations. The results showed that Cd stress caused a significant decrease in dry weight, content of GSH, ASA, significently increasing the activity of GR, APX, GST, SOD, as well as content of H2O2, MDA, proline, and GABA in shoots and roots compared to the control. SiNPs significantly increased shoot and root dry weight compared to the control. As a coenzyme, SiNPs induced the activity of antioxidant enzymes and significantly increased GST and GR gene expression compared to the control. SiNPs also caused a substantial increase in the content of ASA, GSH, proline and GABA compared to the control. By inducing the activity of antioxidant enzymes and metabolites of the ascorbate-glutathione (ASA-GSH) cycle, SiNPs removed a large content of H2O2 and significantly reduced the MDA content, and as a result led to the stability of cell membrane under Cd stress. Induction of ASA-GSH, GABA and SOD cycle by SiNPs clearly showed that SiNPs could be a potential tool to alleviate Cd stress in plants cultivated in areas contaminated with this heavy metal.
Keywords: Ascorbate, Cd tolerance, Glutathione, Oxidative stress, Silicon nanoparticles
Subject terms: Plant cell cycle, Physiology
Introduction
Silicon (Si) is the 8th most abundant element in nature and the 2nd most abundant element in the earth’s crust (after oxygen)1. The concentration of Si in the soil is relatively low, namely 0.1–0.6 mM. The level of Si varies from 0.1 to 10% of dry weight of the plant2,3. The mitigating effects of Si on Cd toxicity have been reported in several plant species such as rice4, wheat5 and maize6. Under exposure to Cd, excessive accumulation of Cd is the source of toxicity in plants7. In tomato, Zhang et al. (2021) showed that SiNPs play an essential role in reducing Cd absorption in root cells and also trap a high concentration of Cd in root cells, which leads to a decrease in Cd transfer to shoots8. Si increases the thickness of cell wall and because of its presence in different parts of the cell wall, it may create an apoplastic barrier leading to the deposition of Cd due to the chemical reactions between Si and Cd, which decreases the bioavailability of Cd7. It has also been reported that Si regulates the development of suberin endoderm and thus reduces the apoplastic transport of Cd in wheat5. Sabir et al. (2024) found that the application of Si in maize increases the biomass and chlorophyll content and also has an essential role in reducing the oxidative stress due to Cd by inducing the activity of superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD)6. Ur Rahman et al. (2021) reported that Si helps wheat to absorb mineral nutrients in a stressful environment and it plays an essential role in alleviating Cd stress via inducing the activity of enzymatic and non-enzymatic antioxidant system7. Several studies showed the beneficial effects of Si on plant growth, chlorophyll content, nutrient absorption and ROS removal mechanism through enzymatic and non-enzymatic antioxidant system under stress conditions5–7. However, little is known regarding the potential benefits of SiNPs on heavy metals, especially Cd stress mitigation. Ghouri et (2024) al also reported that SiNPs has a significant role on xylem loading in reducing Pb concentration in rice cells and preventing its transfer from root to shoot2. In rice, it has been reported that the use of SiNPs regulates the expression of genes responsible for Cd transport and cell wall components, thereby reducing Cd accumulation in rice cells4. Yan et al. (2023) also showed in tomato that SiNPs are more effective than Si in reducing Cd absorption and lead to the reduction in Cd transport to shoots9. SiNPs has unique physical properties that enable easy transfer of it into cells and affect plant metabolic activities10. SiNPs performs better than their bulk materials in mitigating abiotic stresses9. The application of SiNPs spray is relatively new and SiNPs with a size of 20–40 nm is often used for foliar spraying11. The stomatal pathway is the dominant pathway in the absorption of SiNPs, and the size of stomatal pores is 20–500 nm3. Considering that the SiNPs of the present study has a size range of 20–30 nm, they can easily pass through the stomata and enter the plant. When the nanoparticles are absorbed by the plant, redox and other reactions may occur, leading to changes in the morphology of nanoparticles12. Most nanoparticles are soluble and can release ions2,12. There may be proteins that help transport the nanoparticles inside the plant3. Nanoparticles entered into the leaves are transferred to different parts of the plant through apoplastic and symplastic pathways12. The xylem and phloem play an important role in the transport of nanoparticles, while the cell wall is involved in the accumulation of nanoparticles11. SiNPs can also accumulate in the cell wall and increase the mechanical resistance and integrity of the cell wall13. Few studies have addressed the beneficial effects of SiNPs spray on physiological and molecular mechanisms in tomatoes under Cd stress. Tomato, which is one of the most popular and widely consumed products in the world, is rich in useful compounds14. On an industrial scale, tomato is the raw material for a wide range of products such as preserves, concentrates, sauces, ketchup and pastes15. Therefore, there is a strong incentive to minimize the toxic and harmful effects of Cd and thereby produce a high quality product16.
Cd is one of the main pollutants and ranks seventh among the 20 dangerous toxic metals, and it is the first heavy metal in terms of carcinogenicity17. The entry of Cd through mining waste, industry and agricultural applications (especially phosphate fertilizers containing Cd as contaminant) has caused serious threats to sustainable agricultural production and food safety7. Cd has potential adverse effects on the health of animals and humans if it gets close to the food chain18. Heavy metals even at low levels (5-100 µM) cause toxicity of plant products2. The World Health Organization (WHO) guideline for drinking water quality has described the permitted level of Cd as 3 µg/l. Cd concentration in groundwater is up to 1 µg/l, and it is present in the soil at a concentration of 0.01-1 mg/l with a global average of 0.36 mg/kg19. Cd in the rhizosphere prevents root growth and excessive accumulation of Cd in tomato roots is the source of Cd toxicity7. In addition, a considerable amount of Cd is transferred to the upper soil parts, causing disturbances in the photosynthetic apparatus and in other vital reactions of tomatoes8. When Cd enters the plant through the roots, it leads to the production of reactive oxygen species (ROS)7. To neutralize ROS, plants possess an enzymatic antioxidant system (CAT, ascorbate peroxidase (APX), SOD, Glutathione reductase (GR)) along with non-enzymatic molecules (glutathione (GSH), ascorbate (ASA)) and osmotic protectors (proline and sugars)20. Plants also take advantage of Mehler reaction and ASA-GSH cycle for this purpose20. On the other hand, abiotic stresses lead to the induction of γ-aminobutyric acid (GABA) synthesis, which plays an important role in the tolerance to stress conditions21.
To overcome the adverse effects of stress, various methods have been investigated by researchers. In this regard, Si has a high potential in controlling the harmful effects of biotic and abiotic stresses such as Cd stress and can increase stress tolerance by activating physical and biochemical defense reactions5. The role of Si in reducing Cd toxicity has been well studied; however, there are few studies on the impact of SiNPs on mitigating Cd stress, especially at molecular level in Solanum lycopersicum. Based on the existing gaps, this study aimed to investigate the role of SiNPs in decreasing Cd stress in Solanum lycopersicum by evaluation of growth, oxidative stress, ASA-GSH cycle, proline and GABA content. Afterwards, the expression of GR and GST genes as well as the content of GABA was examined. The purpose of this research was to investigate the way SiNPs affects the reduction of Cd stress in Solanum lycopersicum.
Materials and methods
Plant growth conditions and treatments
Tomato seeds (Solanum lycopersicum L. cv. Calj-N3) were purchased from Tehran National Seed Company, which has a license to collect and prepare seeds. All procedures were conducted in accordance with the guidelines. The seeds were first surface sterilized using 0.1% sodium hypochlorite for 10 min, and then they were rinsed with double distilled water and placed on wet filter paper in Petri dishes for germination (10 seeds per Petri dish). They were placed in the dark at 25 °C for 72 h to produce roots. Afterwards, the seedlings were transferred to plastic pots containing perlite. One plant was grown in each pot, and half-strength Hoagland nutrient solution (pH = 5.7 ± 0.1) was used for watering and feeding. The plants were kept in the greenhouse with 16 h of light and 8 h of darkness, 25 °C temperature during the day and 20 °C at night, relative humidity of 70% and a photon flux density (PFD) of 230 µmol photons m−2 s−1. We used 12 treatment groups with 6 replicates for each treatment (3 replicates for measuring biochemical parameters and 3 replicates for measuring dry weight). The 21-day-old seedlings with uniform size were selected and divided into four groups for SiNPs treatment at concentrations of 0, 25, 50 and 100 mg/l. SiNPs were ultrasonicated for 45 min and immediately sprayed on the plants once a day for four days. The presence of SiNPs was revealed by XRD (Fig. 1a), the size and shape of SiNPs were determined by electron microscopy (Day Petronic, Tehran, Iran) (Fig. 1b). The characteristics of SiNPs are shown in Fig. 1. The 25-day-old seedlings were divided into three groups for Cd treatment, and one day after the SiNPs treatment was completed, the seedlings were irrigated with half-strength Hoagland solution containing CdCl2 in concentrations of 0, 100 and 200 µM. Cd treatment continued for 7 days because tomatoes under Cd stress showed a significant phenotypic change on the 6th day, which intensified on the 7th day. Plant height and internode length were measured using a ruler on the sixth day and showed a remarkable decrease, and the plants were harvested on the seventh day. The plants were divided into two groups. One group was placed in an oven at 70 °C for 72 h to measure dry weight (g/plant), and the other group was immediately frozen in liquid nitrogen and kept at -80 °C for further testing.
Fig. 1.
Properties of nano-SiO2 by techniques XRD pattern (a) and SEM micrograph (b).
The concentrations of CdCl2 and SiNPs were optimized through preliminary tests. In the initial experiment for SiNPs treatment, when the plants reached the four-leaf stage (21-day-old), we sprayed SiNPs with concentrations of 0, 25, 50, 100, 150 and 200 mg/l on tomato leaves once a day for 4 days. Seven days after finishing the treatment, we measured the length and weight of shoot and root. The results showed that the 50 mg/l has the greatest effect on increasing plant growth and that the high concentration of 150 and 200 mg/l has no significant effect on plant growth parameters. Therefore, to save the consumption of SiNPs in the main experiment, we used 0, 25, 50 and 100 mg/l. Besides, to finally choose the right concentration, we reviewed various articles. In the initial experiment for Cd stress, when the plants reached the four-leaf stage (21-day-old), we used 0, 100, 200, 300, and 500 µM CdCl2, which were added to Hoagland solution (half strength) and applied to the plants for 7 days. One week after finishing the treatment, we measured the length and weight of shoot and root. We concluded that the plants showed severe signs of stress at 300 and 500 µM; the weight and length of the plant were greatly reduced, and we found that these two concentrations were unbearable for the plant. Therefore, we omitted these two concentrations and used 0, 100 and 200 µM in the main experiment. Furthermore, to finally choose the right concentration, we reviewed various articles.
Determination of biochemical parameters
To measure the hydrogen peroxide (H2O2) level, 500 mg of plant tissue was ground with 0.1% trichloroacetic acid (0.1%). The plant extract was centrifuged using a refrigerated centrifuge for 15 min. Afterward, 0.5 ml of the supernatant solution was added to 0.5 ml of 10 mM potassium phosphate buffer and 1 ml of 1 M potassium iodide, and the absorbance of solutions was read at 390 nm and reported in µM/g fw23.To measure proline content, 300 mg of plant sample was ground in 3% sulfuric acid. Subsequently, 2 ml ninhydrin and glacial acetic acid were added and placed in a hot water bath for one hour and then immediately in a cold water bath. After adding toluene, the absorbance of the upper layer was read at 520 nm and reported in mg/g fw24. Malondialdehyde (MDA) and aldehydes levels were determined according to Heath and Packer (1969). Plant tissues were homogenized using 10% TCA and 0.65% 2-thiobarbituric acid (TBA), which were then centrifuged and the supernatant absorbance was read at 532 and 600 nm and reported in nM/gr fw25.
Plant samples were prepared for ASC, dehydroascorbate (DHA), reduced glutathione (GSH), glutathione oxide (GSSG) analysis by homogenizing 1 g of plant tissue in 10 ml of 5% sulfosalicylic acid. After centrifugation at 22,000×g for 15 min, the supernatant was used for analysis. Briefly, total ASC was determined after reducing DHA to ASC using dithiothreitol (DTT). DHA content was estimated from the difference between total ascorbate pool (ASC + DHA) and ASC. finally reported in µg/g fw26. To measure total glutathione content, 1.5 ml of 0.5 M phosphate buffer (pH = 7.5) and 50 µl of H2O were added to 1 ml of cell extract. GSH content was estimated from the difference between total glutathione and GSSG, which has been clearly explained by Zhang and Kirkham (1996). finally reported in µM/g fw.
GABA content was measured according to Baum et al. method (1996) using HPLC Agilent 1100 (Australia). A total of 0.5 g of the sample was ground with 1 ml of water: chloroform: methanol solution (12:5:3 ratio) and centrifuged for 2 min at 10, 000×g at 4 °C. The supernatant was mixed with 150 µl of borax buffer (pH = 8) and 250 µl of hydroxynaphthaldehyde derivatizing reagent (0.3% w/v in methanol), and the final volume of the solution reached 1 ml with methanol. The sample injection volume was 20 µl, the flow rate was 0.5 ml/min, the mobile phase was methanol: water (38:62 V/V), and detection was done using a UV detector at 330 nm. The retention time for GABA was 12 min, and the total washing time was 20 min. Quantification was done using the curve obtained by the area under peak of different GABA concentrations as the standard. Finally reported in µM/g fw27.
Extraction and determination of enzyme activity
To extract proteins, plant samples were ground in 50 mM potassium phosphate buffer (pH = 7) containing 1 mM phenylmethane sulfonyl fluoride (PMSF), 1 mM sodium ethylene diaminetetraacetic acid (Na2EDTA) and 1% (m/v) polyvinylpyrrolidone (PVP) and centrifuged at 4 °C for 15 min. Supernatants were utilized for measuring enzyme activity28. All spectrophotometric analyzes were performed in a final volume of 3 ml using a Cary 50 UV/visible spectrophotometer. SOD (EC 1.15.1.1) activity was measured based on the inhibition percentage of nitrobuterazolium (NBT) reduction to diformazan by the enzyme present in the extract. The reaction mixture consisted of 50 mM phosphate buffer (pH = 7.0), 0.075 µl NBT, 0.1 mM Na-EDTA, 75 µl riboflavin, 13 mM methionine, and 50 µl enzyme extract .Enzyme activity reported in U/mg Protein. One enzyme unit of SOD is the amount of enzyme causing 50% inhibition of NBT photoregeneration compared to control samples29. APX (EC 1.11.1.11) activity was measured following the decrease in absorbance at 290 nm as a result of ASA oxidation. The reaction mixture contained 50 mM potassium phosphate (pH = 7.0), 10 mM ethylenediaminetetra acetic acid (EDTA), 15 mM H2O2, 0.5 mM ASA, and plant extract. Enzyme activity reported in U/mg Protein30. GR (EC 1.6.4.2) activity was determined following the decrease in absorbance at 340 nm in connection with nicotinamide adenine dinucleotide phosphate (NADPH) oxidation. The reaction mixture included 50 mM tris hydrochloride (Tris-HCl, pH = 7.8), 150 µM NADPH, 500 µM GSSG and 0.05 ml of enzyme extract. Enzyme activity reported in U/mg Protein31.To evaluate glutathione S-transferase (GST, EC 2.5.1.18) activity, the reaction mixture contained 900 µl of 100 mM phosphate buffer (pH = 7.4), 450 µl of reduced 3.5 mM glutathione, 1000 µM of 30 mM 1-Chloro-2,4-dinitrobenzene and 100 µl of the enzyme extract. The absorbance changes of the sample at 340 nm were recorded within one minute. Enzyme activity reported in U/mg Protein32.
Gene expression
Total RNA was extracted using an RNA extraction kit (USA, Fermentase) according to instructions of the kit. The quality and quantity of RNA were determined by spectrophotometry at a wavelength of 260 nm on 2% agarose gel. A total of 1 µg of RNA was treated with Dnase I (Fermentase, USA), and cDNA synthesis was performed using poly T primer [Oligo (dt)] according to the instructions of cDNA synthesis kit (USA, Fermentase). Actin gene primers were taken from Goupil et al. article (2009)33 and those of GR and GST from Kısa article ( 2017)34 (Table 1). Accession numbers are as follows: GR: NM_001321393.1, GST: XM_019212803.2 and ACT: NM_001308447.1, which were extracted from National Center for Biotechnology Information (NCBI) database. Real-time polymerase chain reaction was performed using Rotor gene RG-3000 (Australia Corbett Research) and SYBR Green kit (RealQ Plus 2x Master Mix Green, Ampliqon). Quantitative evaluation of gene expression was done in a volume of 10 µl containing 5 µl of SYBR Green mixture, 0.5 µl of forward and reverse primers and 0.5 µl of cDNA. qRT-PCR reactions were performed with a temperature program of 15 min at 95 °C for the first denaturation, 45 repetitions of a temperature cycle of 15 s at 95 °C, 60 s at 60 °C and 20 s at 72 °C. Actin gene was used as the internal gene as well as for normalization and quantification of gene expression. Before analyzing the data, a melting curve was obtained for each gene, and by checking these curves, the correctness of the peak corresponding to the desired gene and the absence of primer dimer were confirmed.
Table 1.
Gene-specific primers used for qRT-PCR analysis.
| Gene | Sequence for prime | Length (pb) | Tm (ºC) | Gen bank accession number |
|---|---|---|---|---|
| GR | F : TCCCATCGGCTCTGAAGTTAGTGGG | 25 | 69 ºC | NM_001321393.1 |
| R : TCTTTGCATCCTCCAGTTCTGGCCC | 69 ºC | |||
| GST | F : ACTCGTTTTTGGGCTCGTTT | 20 | 49.7 ºC | XM_019212803.2 |
| R : CGATTCAACTCCCTCTGCTT | 51.8 ºC | |||
| ACT | F : GGGATGGAGAAGTTTGGTGGTGG | 23 | 66 ºC | NM_001308447.1 |
| R : CTTCGACCAAGGGATGGTGTAGC | 66 ºC |
Statistical analysis
All treatments were performed in three replicates and the results presented as mean ± SD. Statistical differences were evaluated by Microsoft Excel and two-way ANOVA followed by Duncan’s multiple range test. P < 0.05 was considered to indicate a significant difference.
Results
SiNPs improves Solanum lycopersicum dry weight under cd stress
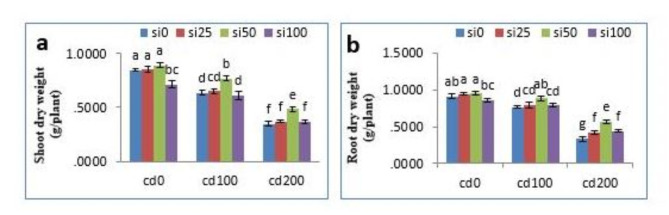
The dry weight of shoots and roots under Cd stress decreased significantly with increasing Cd concentration. Thus, 200µM CdCl2 had the greatest effect on dry weight of the plant, decreasing dry weight of shoot and root by 59.5% and 63.7% compared to control plants, respectively. SiNPs treatment, especially at 50 mg/l concentration, moderated this reduction under stress conditions; 200 µM CdCl2 + 50 mg/l SiNPs caused a significant decrease in dry weight by 42.8% and 38.4% in shoot and root compared to the control, respectively (P < 0.05) (Fig. 2).
Fig. 2.
Effects of different concentrations of CdCl2 and nano-SiO2 on shoot and root dry weight. Mean values followed by similar letters did not differ significantly when determined by the Duncan test.
SiNPs regulates the biochemical responses of Solanum lycopersicumunder Cd stress.
Effect of SiNPs on H2O2, proline, MDA and aldehydes content under cd stress
Table 2 shows that H2O2, proline, MDA and aldehydes content in shoot and root of plants under Cd stress increased significantly relative to control plants. The greatest rise was observed in 200 µM CdCl2, which increased 116.4% and 118.3% the content of H2O2, 504.2% and 464.9% the content of proline 283.7% and 302% the content of MDA and 375% and 237.5% the content of aldehydes in shoots and roots compared to the control, respectively. The SiNPs treatment improved all these parameters, so that 200 µM CdCl2 + 50 mg/l SiNPs significantly increased content of H2O2 by 55.6% and 65%, proline by 284.1% and 260.7%, MDA by 106.4% and 179.1%, aldehydes by 275% and 150% in shoot and root compared to the control, respectively (P.< 0.05) (Tables 2 and 3).
Table 2.
Effects of different concentrations of CdCl2 and nano-SiO2 on shoot and root H2O2, proline, ASC, DHA, GSH and GSSG content.
| CdCl2 (µM) | Nano-SiO2 (mg/l) | Shoot H2O2 (µM/g fw) | Root H2O2 (µM/g fw) | Shoot proline (mg/g fw) | Root proline (mg/g fw) | Shoot ASA (µg/g fw) | Root ASA (µg/g fw) | Shoot DHA (µg/g fw) | Root DHA (µg/g fw) | Shoot GSH (µM/g fw) | Root GSH (µM/g fw) | Shoot GSSG (µM/g fw) | Root GSSG (µM/g fw) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 20.35 ± .82e | 34.84 ± .30e | 25.57 ± .241f | 12.08 ± 1.36f | 1.53 ± .07cd | 1.65 ± .04b | 0.27 ± .02f | 0.36 ± 0.03ef | 3.93 ± .07e | 2.46 ± .22bc | 0.29 ± 0.05def | 0.44 ± 0.03fgh |
| 25 | 19.88 ± 1.03e | 33.39 ± .36e | 27.80 ± 4.03f | 15.66 ± 1.72ef | 1.86 ± .06ab | 1.73 ± .07ab | 0.25 ± .02f | 0.35 ± 0.02ef | 4.50 ± .22cd | 2.64 ± .04bc | 0.24 ± 0.02ef | 0.40 ± 0.05gh | |
| 50 | 18.94 ± .75e | 32.69 ± .98e | 34.74 ± 2.16f | 16.08 ± 2.92ef | 1.96 ± .06a | 1.93 ± .03a | 0.23 ± .03f | 0.33 ± 0.05f | 5.06 ± .10ab | 2.66 ± .10bc | 0.21 ± 0.01f | 0.35 ± 0.02h | |
| 100 | 20.25 ± .42e | 34.10 ± .99e | 40.76 ± 1.77f | 16.83 ± 1.15ef | 1.52 ± .05cd | 1.61 ± .11b | 0.27 ± .02f | 0.36 ± 0.05ef | 4.16 ± .20de | 2.36 ± .17c | 0.25 ± 0.02ef | 0.38 ± 0.04h | |
| 100 | 0 | 29.55 ± 1.00cd | 58.90 ± 2.37c | 35.05 ± 2.69f | 25.91 ± 2.66d | 1.25 ± .12d | 1.05 ± .05e | 0.59 ± .01c | 0.64 ± 0.02d | 4.60 ± .18bcd | 3.06 ± .12ab | 0.38 ± 0.01d | 0.58 ± 0.05e |
| 25 | 26.78 ± 2.06d | 44.40 ± 2.80d | 38.98 ± 3.07f | 20.50 ± 1.66de | 1.53 ± .09cd | 1.21 ± .06de | 0.38 ± .01e | 0.61 ± 0.01d | 4.89 ± .16abc | 3.26 ± .10a | 0.33 ± 0.02de | 0.52 ± 0.01efg | |
| 50 | 20.96 ± 1.06e | 38.30 ± 4.50d | 31.43 ± 3.35f | 14.00 ± 1.88ef | 1.65 ± .12bc | 1.50 ± .06bc | 0.37 ± .02e | 0.45 ± 0.02e | 5.35 ± .18a | 3.43 ± .18a | 0.25 ± 0.02ef | 0.45 ± 0.02fgh | |
| 100 | 28.21 ± 2.06c | 55.97 ± 2.39c | 55.70 ± 2.08e | 27.75 ± 1.95d | 1.35 ± .16cd | 1.33 ± .13c | 0.40 ± .02e | 0.57 ± 0.04d | 4.69 ± .19bcd | 3.42 ± .20a | 0.31 ± 0.01de | 0.53 ± 0.03ef | |
| 200 | 0 | 44.04 ± 1.20a | 76.09 ± 3.45a | 154.51 ± 7.34a | 68.25 ± 3.16a | 0.57 ± .05f | 0.46 ± .07g | 0.78 ± .01a | 1.30 ± 0.05a | 2.10 ± .18g | 1.06 ± .11e | 0.75 ± 0.02a | 1.20 ± 0.05a |
| 25 | 39.28 ± 1.96b | 74.61 ± 2.13a | 137.99 ± 11.96b | 60.08 ± 2.45b | 0.64 ± .11f | 0.53 ± .07g | 0.67 ± .04b | 0.95 ± 0.02b | 2.33 ± .15g | 1.45 ± .07e | 0.65 ± 0.02b | 0.91 ± 0.04c | |
| 50 | 31.67 ± 1.96c | 57.52 ± 3.44c | 98.22 ± 4.82d | 43.58 ± 3.72c | 0.96 ± .05e | 0.81 ± .03f | 0.48 ± .01d | 0.78 ± 0.01c | 3.02 ± .09f | 1.93 ± .10d | 0.53 ± 0.03c | 0.77 ± 0.01d | |
| 100 | 38.80 ± 1.42b | 66.30 ± 1.71b | 122.39 ± 4.45c | 63.16 ± 3.74ab | 0.57 ± .04f | 0.50 ± .06g | 0.59 ± .01c | 0.92 ± 0.02b | 2.10 ± .20g | 1.29 ± .09e | 0.63 ± 0.04d | 1.05 ± 0.04b |
Mean values followed by similar letters did not differ significantly when determined by the Duncan test.
Table 3.
Effects of different concentrations of CdCl2 and nano-SiO2 on shoot and root MDA and aldehydes.
| CdCl2 (µM) | Nano-SiO2 (mg/l) | Shoot MDA (nM/gr FW) | Shoot aldehydes (nM/gr FW) | Root MDA (nM/gr FW) | Root aldehydes (nM/gr FW) |
|---|---|---|---|---|---|
| 0 | 0 | 0.31 ± .03ef | 0.04 ± .006d | 0.48 ± .05d | 0.08 ± .004f |
| 25 | 0.27 ± .03f | 0.04 ± .005d | 0.45 ± .02d | 0.08 ± .005f | |
| 50 | 0.26 ± .02f | 0.03 ± .006d | 0.49 ± .09d | 0.07 ± .005f | |
| 100 | 0.34 ± .02ef | 0.03 ± .006d | 0.48 ± .04d | 0.07 ± .007f | |
| 100 | 0 | 0.48 ± .01d | 0.08 ± .007c | 0.96 ± .03c | 0.13 ± .006d |
| 25 | 0.37 ± .01e | 0.07 ± .007c | 0.89 ± .07c | 0.12 ± .008de | |
| 50 | 0.27 ± .01f | 0.07 ± .002c | 0.80 ± .04c | 0.11 ± .005e | |
| 100 | 0.35 ± .02ef | 0.08 ± .003c | 0.96 ± .03c | 0.11 ± .006de | |
| 200 | 0 | 1.19 ± .02a | 0.19 ± .010a | 1.93 ± .08a | 0.27 ± .012a |
| 25 | 0.82 ± .03b | 0.18 ± .006a | 1.88 ± .12a | 0.25 ± .006ab | |
| 50 | 0.64 ± .03c | 0.15 ± .002b | 1.34 ± .07b | 0.20 ± .005c | |
| 100 | 0.84 ± .03b | 0.18 ± .004a | 1.80 ± .05a | 0.24 ± .004b |
Mean values followed by similar letters did not differ significantly when determined by the Duncan test.
Effect of SiNPs on AsA, DHA, GSH and GSSG under cd stress
Cd stress (200 µM) significantly decreased the content of ASA and GSH and increased the content of DHA and GSH. This reduction was 62.7% and 72.1% for ASA and 46.5 and 56.9% for GSH compared to the control in shoots and roots, respectively. 200 µM Cd caused a significant increase in DHA content by 188.8% and 261.1% and GSSG content by 158.6% and 172.7% in shoots and roots compared to the control. The addition of 50 mg/l SiNPs under 200 µM CdCl2 improved the content of ASA and GSH, so that this reduction reached 37.2% and 50.9% fold for ASA content and 23.1% and 21.5% for GSH content in shoots and roots compared to the control. A total of 200 µM CdCl2 + 50 mg/l SiNPs caused a significant increase in DHA content by 77.7% and 116.6% and GSSG content by 82.7% and 75% in shoots and roots compared to the control, respectively. The results showed that in Cd stress conditions, the addition of 50 mg/l SiNPs restores the redox status of ASA and GSH and thus protects the cell against oxidative stress (P < 0.05) (Table 2).
Effect of SiNPs on GABA content under cd stress
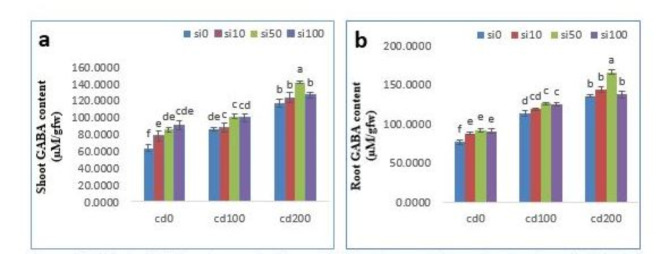
The content of GABA in shoot and root of Solanum lycopersicum under CdCl2 and SiNPs treatment is shown in Fig. 3. Cd stress, especially at 200 µM, caused a significant increase in GABA content by 85.4% and 77% in shoots and roots compared to the control, respectively. Pretreatment of plants with 50 mg/l SiNPs led to a significant increase in GABA content under stress conditions. The maximum content of GABA was observed under treatment with 200 µM CdCl2 + 50 mg/l SiNPs, which was 125.2% and 116.3% compared to the control, respectively (Fig. 3).
Fig. 3.
Effects of different concentrations of CdCl2 and nano-SiO2 on shoot and root GABA. Mean values followed by similar letters did not differ significantly when determined by the Duncan test.
SiNPs regulates the activity of antioxidant enzymes under cd stress
The activities of SOD, APX, GST and GR enzymes under CdCl2 and SiNPs treatment are shown in Table 4. Cd stress alone caused a significant increase in the activity of these enzymes compared to the control, but the treatment with 50 mg/l SiNPs caused a further increase in these enzymes, such that 200 µM CdCl2 + 50 mg/l SiNPs caused a significant rise in the activities of SOD, APX, GR and GST by 273.9%, 207.1%, 93.5%, 86%, 94.4%, 106.6%, 245.4% and 188.2% in shoots and roots compared to control, respectively (P < 0.05) (Table 4).
Table 4.
Effects of different concentrations of CdCl2 and nano-SiO2 on shoot and root SOD, APX, GR and GST activity.
| CdCl2 (µM) | Nano-SiO2 (mg/l) | Shoot SOD (U/mg Protein) | Root SOD (U/mg Protein) | Shoot APX (U/mg Protein) | Root APX (U/mg Protein) | Shoot GR (U/mg Protein) | Root GR (U/mg Protein) | Shoot GST (U/mg Protein) | Root GST (U/mg Protein) |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 1.23 ± .12h | 1.53 ± .03e | 0.93 ± .06de | 1.43 ± .03i | 2.33 ± .08g | 2.40 ± 1.00g | 1.10 ± .057h | 1.70 ± .05f |
| 25 | 1.50 ± .05g | 1.60 ± .05e | 1.00 ± .17cde | 1.70 ± .05fgh | 2.43 ± .12fg | 2.60 ± .05f | 1.23 ± .03h | 1.80 ± .17f | |
| 50 | 1.60 ± .05g | 1.73 ± .12e | 1.03 ± .08cde | 1.86 ± .06def | 2.70 ± .05ef | 2.80 ± .05f | 1.26 ± .06h | 1.90 ± .05f | |
| 100 | 1.23 ± .12h | 1.53 ± .08e | 0.90 ± .17e | 1.93 ± .08de | 2.36 ± .13g | 2.30 ± .10h | 1.16 ± .12h | 1.80 ± .05f | |
| 100 | 0 | 2.03 ± .08f | 2.26 ± .14d | 1.23 ± .03bcd | 1.80 ± .05efg | 2.70 ± .05ef | 2.90 ± .05e | 1.73 ± .03g | 2.30 ± .05e |
| 25 | 2.40 ± .05e | 2.43 ± .12d | 1.20 ± .05bcde | 1.50 ± .05hi | 3.00 ± .05de | 3.20 ± .05cd | 1.93 ± .03f | 2.40 ± .05de | |
| 50 | 2.90 ± .05d | 3.03 ± .08c | 1.30 ± .05bc | 1.93 ± .03de | 3.30 ± .11cd | 3.40 ± .15c | 2.30 ± .05e | 2.60 ± .05d | |
| 100 | 2.26 ± .14f | 2.23 ± .14d | 1.23 ± .08bcd | 1.60 ± .05ghi | 3.00 ± .05de | 3.00 ± .05de | 1.96 ± .06f | 2.40 ± .10de | |
| 200 | 0 | 3.70 ± .05c | 3.73 ± .14b | 1.50 ± .05b | 2.26 ± .13b | 3.30 ± .15cd | 4.10 ± .05b | 2.53 ± .03d | 4.43 ± .06b |
| 25 | 3.86 ± .08bc | 3.90 ± .05b | 1.20 ± .05bcde | 2.20 ± .05bc | 3.56 ± .13c | 4.20 ± .05b | 2.76 ± .08c | 4.53 ± .03b | |
| 50 | 4.60 ± .05a | 4.70 ± .05a | 1.80 ± .05a | 2.66 ± .06a | 4.53 ± .13a | 4.96 ± .08a | 3.80 ± .05a | 4.90 ± .05a | |
| 100 | 4.06 ± .12b | 3.86 ± .08b | 1.40 ± .05b | 2.03 ± .08cd | 4.06 ± .06b | 4.26 ± .12b | 3.03 ± .08b | 4.10 ± .05c |
Mean values followed by similar letters did not differ significantly when determined by the Duncan test.
SiNPs increases GR and GST genes expression under Cd stress
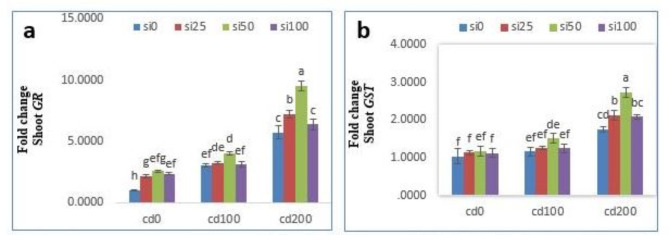
The transcript levels of GR and GST genes also changed in response to SiNPs, Cd stress and their combined treatment (Fig. 4). In general, Cd stress at 200 µM caused a significant increase in transcripts of GR and GST genes by 473% and 69.9% compared to the control, respectively. The expression of transcripts was further elevated in the treatment of 200 µM CdCl2 + 50 mg/l SiNPs and increased 852% and 166% compared to the control, respectively (P < 0.05) (Fig. 4).
Fig. 4.
Effects of different concentrations of CdCl2 and nano-SiO2 on relative gene expression of GR (a) and GST (b) in leaf Solanum lycopersicum. Mean values followed by similar letters did not differ significantly when determined by the Duncan test.
Discussion
One of the most obvious effects of Cd stress in the present study was the decrease in Solanum lycopersicum growth and biomass (Fig. 1). We found that Cd stress has a greater effect on root dry weight than on shoot, and in our previous researches, we recorded more accumulation of Cd in Solanum lycopersicum roots35,36. In the present study, Solanum lycopersicum roots under Cd stress were weak and short, and it was observed that SiNPs led to a deeper and stronger structure by enhancing root growth and biomass. Especially, treatment with 50 mg/l SiNPs significantly increased the dry weight of shoots and roots under Cd stress, and also SiNPs had a greater effect on the dry weight of roots (Fig. 1). In confirmation of our results, Ahmed et al. (2023) reported that the application of SiNPs causes a significant increase in root volume and increases the ratio of root to shoot in maize under Cd stress, which may play a role in improving water and nutrient uptake37 and facilitate the movement of water and nutrients38. The function of root structure can improve the hydraulic effectiveness of the plant in releasing water to the leaves, and SiNPs has a significant role in the hydraulic conductivity of the roots38. In our previous research, we observed an increase in the content of useful nutrients due to the use of SiNPs in Solanum lycopersicum under Cd stress35. Under stress conditions, plant growth and performance are strongly influenced by the absorption and accumulation of nutrients by the roots7. Mineral nutrients that are essential for enzymes, as well as vitamins, pigments and other biological molecules play a vital role in plant growth and provide biochemical and mechanical stability by maintaining cell integrity1. Table 2 shows that Cd stress compromises the plant’s antioxidant defense system due to a significant decrease in the content of antioxidant compounds such as ASA and GSH and reduces the plant’s ability to eliminate H2O2, as a result of which less H2O2 is removed, indicating tendency toward oxidative stress that is reflected in the damage to the cell membrane. As a result, the treatment of SiNPs under Cd stress in the present study greatly reduced MDA content by removing a large content of H2O2 in shoots and roots and led to the stability and integrity of the cell membrane, which is necessary to support selective permeability in plants. In agreement with our results, Thind et al. (2021) reported that Cd stress increases H2O2 and MDA content and that SiNPs treatment decreases their content39. Acila et al. (2024) also reported a decrease in GSH content under Cd stress in Cucurbita pepo because of GSH oxidation through ROS and phytochelatin (PCs) biosynthesis40. This also supports our findings where we observed a decrease in GSH concentration under Cd stress (Table 2). However, the use of 50 mg/l SiNPs in the present study increased the concentration of GSH compared to its control (Cd alone). GSH can form a complex with Cd and detoxify by sequestrating it into the vacuole2. Therefore, the high content of GSH helped detoxify more Cd in the present study (Table 2). GSH is required for many cell functions41. As an antioxidant, GSH can directly react with ROS, and GSH is able to help break the peroxidation chain in plants by stabilizing cell membrane lipids41. Therefore, it is necessary to regenerate GSH by GR41. Cd stress increased the expression and activity of GR gene in the present study (Fig. 4a; Table 4). However, this increase was not enough to remove ROS. We observed that 50 mg/l SiNPs further increased GR gene expression and activity. Most likely, the release of Si ions from SiNPs has increased the GR gene expression pattern under Cd stress2. Consistent with our results, Ashraf et al. (2024) reported that in rice under Cd stress, the use of SiNPs increased gene expression and GR activity, which also augmented GSH content42. Rising GR activity maintains the optimal concentration of NADP+ for electron transfer and protects the photosynthetic machinery against toxic effects of superoxide radicals, as well as keeping the ratio of GSH/GSSG for normal cell function43; therefore, SiNPs further improve the expression and activity of GR. Besides, GSH plays a role in the regeneration of ASA in ASA-GSH cycle41. Hence, in addition to the sufficient content of ASA in the plant, there is a need for a suitable reserve of GSH for better performance of this cycle to reduce oxidative stress41. Among non-enzymatic antioxidants, ASA and GSH are the most important antioxidants with low molecular weight, which act as redox buffer agents, prevent cell membrane oxidation and donate electrons for APX and GPX enzymes1. As the front line of non-enzymatic antioxidant defense, ASA plays a major role in the degradation of H2O244. In our study, the content of ASA decreased under Cd stress, but 50 mg/l SiNPs increased the content of ASA, indicating that SiNPs reduces the destructive effect of Cd (Table 2). By increasing the activity of dehydroascorbate reductase (DHAR) and monodehydroascorbate reductase (MDHAR), SiNPs lead to an increase of ASA content in rice under oxidative stress, which indicate the role of SiNPs as a coenzyme for the activation of these enzymes1. Gharib and Ahmed (2023) reported that Cd stress decreased the content of ASA in rosemary45. Alves et al. (2023) reported that the application of SiNPs in eggplant increased ASA content46, which is in agreement with the results of the present study. The present study showed that Cd stress increases APX activity in Solanum lycopersicum. In agreement with our results, Thind et al. (2021) also reported that Cd stress increases APX activity39, and it was found that 50 mg/l SiNPs treatment led to a further increase in APX activity, which indicates the role of SiNPs as a coenzyme in further activation of APX (Table 4). This APX-induced increase by SiNPs indicates the decrease of H2O2 levels in Solanum lycopersicum and shows an effective reduction of oxidative stress as a result of Cd exposure, which is consistent with the report of Thind et al. (2021) in wheat39. In addition to the role of ASA in antioxidant defense system of the cell, it has been reported that ASA is an important coenzyme for some enzymes involved in the biosynthesis of hormones such as gibberellin47, which can be the reason for the improvement of growth and biomass in plants treated with SiNPs under Cd stress in the present study. SOD, peroxidases and ASA-GSH cycle enzymes constitute the main enzyme network inactivating ROS20. While SOD catalyzes the conversion of superoxide anion to H2O2, the APX reduces H2O2 to H2O48. The ASA-GSH cycle, which consists of four enzymes (APX, MDHAR, DHAR and GR) and two metabolites (ASA and GSH), acts in coordination for H2O2 metabolism and determines whether it acts as a signaling or damaging molecule20. In addition to its role in controlling H2O2 levels, this cycle maintains the oxidation and reduction buffer by regulating the level of ASA and GSH and the redox ratio between them49. In the present study, Cd toxicity caused an increase in SOD activity in Solanum lycopersicum (Table 4), and the plant raised SOD activity to improve its performance, but this increase was not efficient enough to neutralize the superoxide anion. SiNPs acts as a coenzyme inducing more SOD activity in Solanum lycopersicum under Cd stress. This SiNPs-induced increase in SOD activity has a potential mitigating effect on Cd-induced oxidative stress and helps the plant cell manage and neutralize the harmful effects of superoxide anion. Similarly, the positive effects of SiNPs in inducing SOD activity in wheat39 under Cd stress have been reported. Table 2 shows that H2O2 content increases under Cd stress conditions. H2O2 is an activator of the GST gene promoter50,51. Therefore, this enzyme is upregulated under different stresses, including Cd stress52. In the present study, we observed an increase in the expression and activity of GST enzyme’s gene under Cd stress, but SiNPs treatment caused a further increase in the expression and activity of GST under Cd stress (Fig. 4b; Table 4). It is likely that the release of Si ions from SiNPs increased the pattern of GST gene expression under Cd stress2. The increase in the expression and activity of GST under SiNPs not only destroys harmful toxic compounds resulting from fatty acid peroxidation53, but also catalyzes the binding of GSH with toxic derivatives produced by the oxidation of other biomolecules54. In agreement with the present study, Srivastava et al. (2019) reported that in transgenic Arabidopsis, gene expression and higher activity of GST caused tolerance to heavy metal stress55. Kar et al. (2023) reported a decrease in ROS-induced lipid peroxidation in Phaseolus vulgaris along with an increase in GST56. Furthermore, they suggested the genes related to these enzymes as suitable candidates for creating tolerance to stress56. Considering the key role of GST in dealing with oxidative stress and neutralizing toxic substances, it seems that SiNPs managed to alleviate the harmful effects of Cd stress through increasing the activity and expression of this enzyme in the present study, which indicates that SiNPs create an environment with lower oxidative stress. The results of our research showed that SiNPs significantly increase the level of GST and GR transcripts compared to the control along with augmenting the activity of antioxidant enzymes, which indicates that SiNPs usually activate antioxidant enzymes at the level of gene expression. Interestingly, we report for the first time that the application of SiNPs, especially at 50 mg/l, increases the transcripts of GST and GR genes in Solanum lycopersicum under Cd stress. The increase in the activity of antioxidant enzymes that was induced by Cd in the present study (Table 4) indicates that the activity of enzymes at this level may not be efficient enough to neutralize ROS in Solanum lycopersicum under Cd stress. Table 2 shows show that the content of H2O2 is high even after increasing activity of antioxidant enzymes, suggesting that the accumulation of H2O2 exceeds the potential of ROS removal in plants under Cd stress and causes oxidative stress due to the inability to create a balance between the production and removal of ROS. Table 2 also shows that the content of GSH and ASA decreases under Cd stress. The decreasing availability of GSH and ASA redox buffers, which directly and indirectly control the level of ROS, leads to irreversible cell damage due to the excessive accumulation of H2O2 since H2O2 production overcomes the activity of its degrading enzymes. The decrease in GSH and ASA levels in this study is one of the most important factors of GSH-ASA cycle insufficiency, which shows that despite the increase in gene expression and the activity of GSH-ASA cycle enzymes, the cycle is executed inefficiently given the insufficient amount of GSH and ASA, and thereby the plant’s defense mechanisms are not able to deal with the destructive effects of ROS. Nevertheless, SiNPs as a coenzyme inducing gene expression and the activity of enzymes of this cycle increases the content of GSH and ASA and therefore improves the redox status of cells under Cd stress, and it seems that SiNPs contribute to significant reduction of H2O2 content in cells, remarkably decrease the content of H2O2 in 50 mg/l SiNPs + 200µM CdCl2 compared to the control (200 µMCdCl2), create an environment with lower oxidative stress and help maintain the integrity and stability of the cell membrane, which is evident in the reduction of lipid peroxidation (Tables 2, 3 and 4; Fig. 4). In addition, proline metabolism has a regulatory function in oxidation-reduction homeostasis processes, which affects the survival of plants under variable environmental conditions1,7. By stabilizing the redox status of plant cells, proline plays an essential role in stress tolerance1. Proline is considered as an antioxidant to eliminate ROS and maintain the intercellular reservoir, namely a redox buffer for cells7. In our study, proline content increased under Cd stress (Table 2). Similarly, proline content was higher in wheat under Cd stress7. Stress conditions induce pyrroline-5-carboxylate synthetase (P5CS) activity, which leads to further proline accumulation1. The treatment of 50 mg/l SiNPs under Cd stress caused a further increase of proline in the present study, which reveals the role of SiNPs in maintaining the intercellular reservoir to stabilize the redox status of cells. Similarly, Memari-Tabrizi et al. (2021) reported that Cd treatment increases proline content in Satureja hortensis L. grown under Cd stress and that the application of SiNPs leads to a further increase in proline57. Our results showed that Cd stress increases GABA content and that SiNPs treatment leads to a further increase in GABA content (Fig. 3). GABA, which is a type of messenger, plays its role in various physiological processes by increasing the level of hormones, stimulating the activity of antioxidant enzymes (such as CAT, SOD, APX) and protecting the plant against oxidative stress58. Moreover, GABA increases the activity of P5CS, which in turn leads to proline accumulation in plants59,60. On the other hand, stress conditions cause glutamate, a precursor of chlorophyll and proline, to be directed towards proline biosynthesis, as a result of which proline accumulates under stress conditions61. Our previous study showed that Cd stress decreases the amount of chlorophyll35, and in the present study, we observed an increase of proline content under Cd stress, so that SiNPs caused a greater increase in GABA content and more proline accumulation (Table 2; Fig. 3). Proline is one of the most important osmolytes that increases tolerance to stress and leads to the stability of proteins, enzymes and cell membrane, scavenges ROS and protects the cell against oxidative stress1,7.
Interestingly, one of the important aspects of SiNPs in reduction of stress conditions is plant growth, as well as its strong effect in delaying senescence62. de Faria Melo et al. (2023) reported that SiNPs could affect leaf senescence by increasing nutrient efficiency as well as blocking senescence-related genes62. In parallel, another benefit of SiNPs in plants is related to the incorporation of silicon in the cell wall, which is metabolized at a low energy cost compared to the synthesis of structural carbonaceous compounds such as lignin, and this may benefit plant growth under stress conditions46,62. hence, SiNPs guarantees structural resistance and reduces the need for lignin synthesis, which requires plenty of energy that could be used in the synthesis of plant defense organic compounds against stress46,62.
Conclusion
generally, we concluded that the use of SiNPs is an approach for successful tolerate of Solanum lycopersicum under Cd stress based on the following points: (1) It stimulates the expression of GR and GST genes, which was reported for the first time in the present study; (2) It induces the activity of enzymes and metabolites of the ASA-GSH cycle; (3) It increases proline level by increasing the GABA content. In addition, SiNPs can play a role in improving plant growth and performance, increasing the absorption of useful nutrients and decreasing the absorption of Cd, which is followed by reducing oxidative stress. SiNPs can act as a source for safe food production to overcome food security challenges, especially in lands contaminated with the heavy metal Cd, and this technique can be a good alternative to traditional methods. However, field-level studies are needed to better understand and confirm these findings.
Acknowledgements
This study was conducted at department of biology, Urmia University.
Author contributions
Author contributionsR.R., R.J. and M.j.A. designed the experiment. R.R. conducted the test. R.J. and M.J.A. analysed the data. R.R. wrote the manuscript. All authors edited the manuscript and approved the manuscript prior to submission.
Funding
This work was supported by Vice chancellor for research, Urmia University.
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tripthi, D. K. et al. Silicon tackles butachlor toxicity in rice seedlings by regulating anatomical characteristics, ascorbate-glutathione cycle, proline metabolism and levels of nutrients. Sci. Rep.10, 14078 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghouri, F. et al. Silicon and iron nanoparticles protect rice against lead (pb) stress by improving oxidative tolerance and minimizing pb uptake. Sci. Rep.14, 5986 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan, G. et al. Silicon nanoparticles in sustainable agriculture: Synthesis, absorption, and plant stress alleviation. Front. Plant. Sci.15, 1393458 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui, J. et al. Silica nanoparticles alleviate cadmium toxicity in rice cells: Mechanisms and size effects. Environ. Pollut. 228, 363–369 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Wu, J., Mock, H. P., Giehl, R. F. H., Pitann, B. & Mühling, K. H. Silicon decreases cadmium concentrations by modulating root endodermal suberin development in wheat plants. J. Hazard. Mater.364, 581–590 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Sabir, A. et al. Silicon-mediated improvement in Maize (Zea mays L.) resilience: Unrevealing morpho-physiological, biochemical, and root attributes against cadmium and drought stress. Silicon 1–15 (2024).
- 7.Ur Rahman, S. et al. Alleviatory effects of Silicon on the morphology, physiology, and antioxidative mechanisms of wheat (Triticum aestivum L.) roots under cadmium stress in acidic nutrient solutions. Sci. Rep.11, 1–12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang, Z., Wang, L., Lei, X., Tang, L. & Li, Y. Effects of silicon on the growth, nutrient uptake and cadmium accumulation of tomato seedlings. Int. J. Environ. Anal. Chem.105(5), 963–976 (2021). [Google Scholar]
- 9.Yan, G. et al. Comparative effects of silicon and silicon nanoparticles on the antioxidant system and cadmium uptake in tomato under cadmium stress. Sci. Total Environ.904, 166819 (2023). [DOI] [PubMed] [Google Scholar]
- 10.Rastogi, A. et al. Application of silicon nanoparticles in agriculture. 3 Biotech.9, 1–11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laane, H. M. The effects of foliar sprays with different silicon compounds. Plants. 7 (2), 45 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong, J. et al. Foliar application of nanoparticles: Mechanisms of absorption, transfer, and multiple impacts. Environ. Sci. Nano. 8, 1196–1210 (2021). [Google Scholar]
- 13.David, O. A. et al. Complexation and immobilization of arsenic in maize using green synthesized silicon nanoparticles (SiNPs). Sci. Rep.14, 6176 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sayed, E. G. et al. The effective role of nano-silicon application in improving the productivity and quality of grafted tomato grown under salinity stress. Horticulturae. 8, 293 (2022). [Google Scholar]
- 15.Geetha, P. & Rani, I. Post harvest technology and value addition of tomatoes. Food Sci. Res. J.11, 217–229 (2020). [Google Scholar]
- 16.Shi, X. et al. Improvement of Tomato Fruit Quality and Soil nutrients through Foliar spraying fulvic acid under stress of copper and cadmium. Agronomy. 13, 275 (2023). [Google Scholar]
- 17.Zulfiqar, U. et al. Cadmium toxicity in plants: Recent progress on morpho-physiological effects and remediation strategies. J. Soil. Sci. Plant. Nutr.22(1), 212–269 (2022). [Google Scholar]
- 18.Zhao, H. et al. Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci. Rep.11, 9913 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubier, A., Wilkin, R. T. & Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem.108, 104388 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarker, U. & Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep.8, 16496 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan, D. et al. GABA metabolism, transport and their roles and mechanisms in the regulation of abiotic stress (Hypoxia, Salt, Drought) resistance in plants. Metabolites. 13, 347 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoagland, D. R. & Arnon, D. I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn.347, (1950).
- 23.Velikova, V., Yordanov, I. & Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant. Sci.151, 59–66 (2000). [Google Scholar]
- 24.Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant. Soil.39, 205–207 (1973). [Google Scholar]
- 25.Heath, R. L. & Packer, L. Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys.125, 189–198 (1968). [DOI] [PubMed] [Google Scholar]
- 26.Zhang, J. & Kirkham, M. B. Antioxidant responses to drought in sunflower and sorghum seedlings. New. Phytol. 132, 361–373 (1996). [DOI] [PubMed] [Google Scholar]
- 27.Baum, G. et al. Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO J.15, 2988–2996 (1996). [PMC free article] [PubMed] [Google Scholar]
- 28.Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.72, 248–254 (1976). [DOI] [PubMed] [Google Scholar]
- 29.Giannopolitis, C. N. & Ries, S. K. Superoxide dismutases: I. Occurrence in higher plants. Plant. Physiol.59, 309–314 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant. cell. Physiol.22, 867–880 (1981). [Google Scholar]
- 31.Schaedle, M. & Bassham, J. A. Chloroplast glutathione reductase. Plant. Physiol.59, 1011–1012 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carmagnol, F., oise, Sinet, P. M., Rapin, J. & Jerome, H. Glutathione-S-transferase of human red blood cells; assay, values in normal subjects and in two pathological circumstances: Hyperbilirubinemia and impaired renal function. Clin. Chim. Acta. 117, 209–217 (1981). [DOI] [PubMed] [Google Scholar]
- 33.Goupil, P. et al. Expression of stress-related genes in tomato plants exposed to arsenic and chromium in nutrient solution. J. Plant. Physiol.166, 1446–1452 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Kısa, D. Expressions of glutathione-related genes and activities of their corresponding enzymes in leaves of tomato exposed to heavy metal. Russ J. Plant. Physiol.64, 876–882 (2017). [Google Scholar]
- 35.Rahmatizadeh, R., Jamei, R., Arvin, M. J. & Rezanejad, F. Upregulation of LeNRAMP3 and LeFER genes in Solanum lycopersicum confers its cadmium tolerance. Russ J. Plant. Physiol.68, S92–S102 (2021). [Google Scholar]
- 36.Rahmatizadeh, R., Arvin, S. M. J., Jamei, R. & Mozaffari, H. Reza Nejhad, F. Response of tomato plants to interaction effects of magnetic (Fe3O4) nanoparticles and cadmium stress. J. Plant. Interact.14, 474–481 (2019). [Google Scholar]
- 37.Ahmed, S. et al. Foliar application of silicon-based nanoparticles improve the adaptability of maize (Zea mays L.) in cadmium contaminated soils. Environ. Sci. Pollut Res.30, 41002–41013 (2023). [DOI] [PubMed] [Google Scholar]
- 38.Khan, Z. S. et al. Effects of silicon nanoparticles on growth and physiology of wheat in cadmium contaminated soil under different soil moisture levels. Environ. Sci. Pollut Res.27, 4958–4968 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Thind, S. et al. Alleviation of cadmium stress by silicon nanoparticles during different phenological stages of Ujala wheat variety. Arab. J. Geosci.14, 1028 (2021). [Google Scholar]
- 40.Acila, S., Derouiche, S. & Allioui, N. Embryo growth alteration and oxidative stress responses in germinating Cucurbita pepo seeds exposed to cadmium and copper toxicity. Sci. Rep.14, 8608 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasanuzzaman, M., Nahar, K., Anee, T. I. & Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants. 23, 249–268 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashraf, H. et al. Silicon Dioxide nanoparticles-based amelioration of cd toxicity by regulating antioxidant activity and photosynthetic parameters in a line developed from wild rice. Plants. 13, 1715 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ji, D. et al. NADP+ supply adjusts the synthesis of photosystem I in Arabidopsis chloroplasts. Plant. Physiol.189, 2128–2143 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuo, E. Y., Cai, M. S. & Lee, T. M. Ascorbate peroxidase 4 plays a role in the tolerance of Chlamydomonas reinhardtii to photo-oxidative stress. Sci. Rep.10, 13287 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gharib, F. A. E. L. & Ahmed, E. Z. Spirulina platensis improves growth, oil content, and antioxidant activitiy of rosemary plant under cadmium and lead stress. Sci. Rep.13, 8008 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alves, D. M. R., de Oliveira, J. N., de Mello Prado, R. & Ferreira, P. M. Silicon in the form of nanosilica mitigates P toxicity in scarlet eggplant. Sci. Rep.13, 9190 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Aguiar, É. S. et al. Genome and transcriptome analyses of genes involved in Ascorbate Biosynthesis in Pepper Indicate Key genes related to Fruit Development, stresses, and Phytohormone exposures. Plants. 12, 3367 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gill, S. et al. Effect of silicon nanoparticle-based biochar on wheat growth, antioxidants and nutrients concentration under salinity stress. Sci. Rep.14, 6380 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmad, P. et al. Exogenous application of calcium to 24-epibrassinosteroid pre-treated tomato seedlings mitigates NaCl toxicity by modifying ascorbate–glutathione cycle and secondary metabolites. Sci. Rep.8, 1–15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gullner, G., Komives, T., Király, L. & Schröder, P. Glutathione S-transferase enzymes in plant-pathogen interactions. Front. Plant. Sci.9, 1836 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polidoros, A. N. & Scandalios, J. G. Role of hydrogen peroxide and different classes of antioxidants in the regulation of catalase and glutathione S-transferase gene expression in maize (Zea mays L). Physiol. Plant.106, 112–120 (1999). [Google Scholar]
- 52.Li, J. et al. Molecular Cloning of a TCHQD class glutathione S-Transferase and GST function in response to GABA Induction of Melon Seedlings under Root hypoxic stress. Horticulturae. 8, 446 (2022). [Google Scholar]
- 53.Gao, J. et al. Identification and characterization of the glutathione S-Transferase (GST) family in radish reveals a likely role in anthocyanin biosynthesis and heavy metal stress tolerance. Gene. 743, 144484 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Cao, Q. et al. Genome-wide identification of glutathione S-transferase gene family members in tea plant (Camellia sinensis) and their response to environmental stress. Int. J. Biol. Macromol.205, 749–760 (2022). [DOI] [PubMed] [Google Scholar]
- 55.Srivastava, D., Verma, G., Chauhan, A. S., Pande, V. & Chakrabarty, D. Rice (Oryza sativa L.) tau class glutathione S-transferase (OsGSTU30) overexpression in Arabidopsis thaliana modulates a regulatory network leading to heavy metal and drought stress tolerance. Metallomics. 11, 375–389 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Kar, M. & Öztürk, Ş. Analysis of Phaseolus vulgaris gene expression related to oxidative stress response under short-term cadmium stress and relationship to cellular H 2 O 2. Biol. (Bratisl). 75, 1009–1016 (2020). [Google Scholar]
- 57.Memari-Tabrizi, E. F., Yousefpour-Dokhanieh, A. & Babashpour-Asl, M. Foliar-applied silicon nanoparticles mitigate cadmium stress through physio-chemical changes to improve growth, antioxidant capacity, and essential oil profile of summer savory (Satureja hortensis L). Plant. Physiol. Biochem.165, 71–79 (2021). [DOI] [PubMed] [Google Scholar]
- 58.Dabravolski, S. A. & Isayenkov, S. V. The role of the γ-Aminobutyric acid (GABA) in plant salt stress tolerance. Horticulturae. 9, 230 (2023). [Google Scholar]
- 59.Yong, B. et al. Exogenous application of GABA improves PEG-induced drought tolerance positively associated with GABA-shunt, polyamines, and proline metabolism in white clover. Front. Physiol.8, 1107 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ullah, A. et al. Exogenous γ-aminobutyric acid (GABA) mitigated salinity-induced impairments in mungbean plants by regulating their nitrogen metabolism and antioxidant potential. Front. Plant. Sci.13, 1081188 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiong, J. L., Wang, H. C., Tan, X. Y., Zhang, C. L. & Naeem, M. 5-aminolevulinic acid improves salt tolerance mediated by regulation of tetrapyrrole and proline metabolism in Brassica napus L. seedlings under NaCl stress. Plant. Physiol. Biochem.124, 88–99 (2018). [DOI] [PubMed] [Google Scholar]
- 62.de Faria Melo, C. C. et al. Nanosilica modulates C: N: P stoichiometry attenuating phosphorus toxicity more than deficiency in Megathyrsus maximus cultivated in an Oxisol and Entisol. Sci. Rep.13, 10284 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.