Abstract
Background
The incidence of muscle atrophy or sports injuries is increasing with time and population aging, thereby attracting considerable attention to muscle generation research. Muscle satellite cells, which play an important role in this process, lack comprehensive literature regarding their use for muscle regeneration. Hence, this study aimed to analyze the hotspots and trends in satellite cell research from 2010 to 2023, providing a reference for muscle regeneration research.
Methods
Studies on satellite cells’ role in muscle regeneration from 2010 to 2023 were retrieved from the Web of Science Core Collection. Using CiteSpace and VOSviewer, we analyzed annual publications, authors and co-citing authors, countries and institutions, journals and co-citing journals, co-citing references, and keywords.
Results
From 2010 to 2023, 1468 papers were retrieved, indicating an overall increasing trend in the number of annual publications related to satellite cells in muscle regeneration. The United States had the highest number of publications, while the Institut National de la Santé et de la Recherche Médicale was the institution with the most publications. Among journals, " PloS One" had the highest number of published papers, and "Cell" emerged as the most co-cited journal. A total of 7425 authors were involved, with Michael A. Rudnicki being the author with the highest number of publications and the most co-cited author. The most cited reference was "Satellite cells and the muscle stem cell niche." Among keywords, “satellite cells” was the most common, with "heterogeneity" having the highest centrality. Frontier themes included "Duchenne muscular dystrophy,” "skeletal muscle,” "in-vivo,” "muscle regeneration,” "mice," "muscle atrophy,” "muscle fibers,” "inflammation,” " mesenchymal stem cells,” and "satellite cell."
Conclusion
This study presents the current status and trends in satellite cell research on muscle regeneration from 2010 to 2023 using bibliometric analyses, providing valuable insights into numerous future research directions.
Keywords: Muscle regeneration, Satellite cells, Bibliometrics, Muscle atrophy, Muscle injury
Highlights
-
•
Visual analysis of muscle regeneration and satellite cells literature (2010–2023).
-
•
The United States leads in research volume, with Italy, China, and Japan emerging.
-
•
Michael A. Rudnicki excels in muscle regeneration and satellite cell research.
-
•
Duchenne muscular dystrophy and inflammation emerge as key research focuses.
1. Introduction
Sarcopenia, initially described by Rosenberg [1] as a disease primarily affecting older adults and characterized by a loss of skeletal muscle mass and muscle function [2], is a growing global health concern. It is estimated that 10–16 % of older people worldwide suffer from sarcopenia [3]. Several factors, such as lack of exercise, hormonal and cytokine imbalances, protein synthesis, and remodeling of regenerative motor units, contribute to the development of sarcopenia [4].
As research on muscle atrophy increases, the concept of muscle regeneration is gaining public attention. Muscles undergo repair and regeneration, termed muscle regeneration, when subjected to external forces or intrinsic damage [5]. Muscle regeneration is a complex process involving the regulation of various cells, growth factors, and signaling pathways. Following muscle injury, a large number of inflammatory factors accumulate, and necrotic tissue appears, activating the proliferation, differentiation, and fusion of satellite cells. This process, in turn, generates new muscle fibers for the reconstruction of muscle function [6]. Dysregulation of muscle regeneration may contribute to the development of sarcopenia [7].
Muscle regeneration primarily relies on satellite cells located between the plasma and basement membranes of muscle fibers [8]. These cells, also known as myosatellite cells, were discovered by American scientist Mauro as early as 1961 [9]. They are pluripotent stem cells present in skeletal muscle tissues. These cells play essential roles in the growth, development, repair [10], aging [11], and maintenance of homeostasis [12] of skeletal muscles. They also serve as the main source of muscle regenerative repair [13], producing differentiated cells and self-renewing stem cells through asymmetric division [14] when muscle damage requires repair.
Bibliometrics is the analysis of published information (e.g., books and journal articles) and their associated data (e.g., abstracts and keywords) using statistics to describe or express the relationships between published works [15]. Currently, the study of muscle regeneration has attracted significant attention, with many cell types implicated, including macrophages [6,16], adipogenic progenitors [17], and myosatellite cells [18]. Satellite cells, in particular, serve as the main drivers of muscle regeneration and provide important support for this process [19]. However, there exists a gap in the literature regarding the use of satellite cells for muscle regeneration. This study aimed to explore the current global research status and development trend in satellite cells’ involvement in muscle regeneration over the past decade. We analyzed the interrelationship between muscle regeneration and myosatellite cells, along with identifying current research hotspots in this field. Network mapping was employed to visualize these hotspots, providing a comprehensive reference for future research endeavors. We believe these comprehensive analyses provide new insights into the mechanisms underlying muscle regeneration and lay the foundation for the development of innovative therapeutic approaches.
2. Materials and methods
2.1. Data source
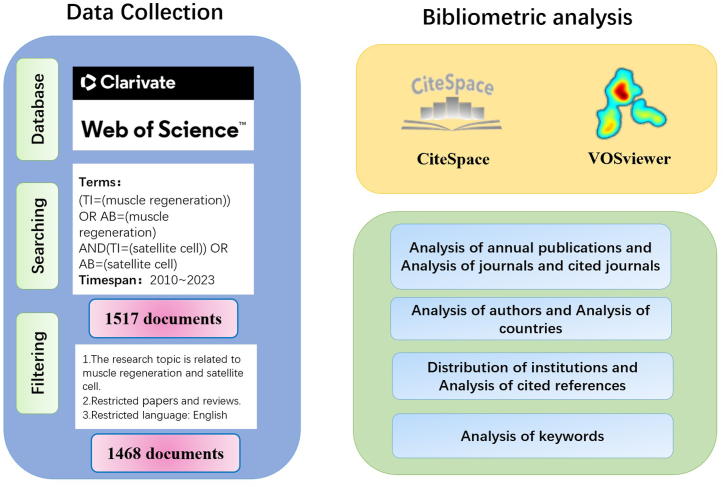
We selected the Science Citation Index Expanded from the Web of Science Core Collection (WOSCC) database, which is the preferred database for bibliometrics research [20]. The search process is illustrated in Fig. 1.
Fig. 1.
The search process.
2.2. Search strategy
On March 14, 2024, we conducted a search in the WOSCC using the following search strategy: (TI=(muscle regeneration)) OR AB=(muscle regeneration) AND (TI=(satellite cell)) OR AB=(satellite cell). The search was limited to publications from January 2010 to December 2023.
2.3. Inclusion and exclusion criteria
Two reviewers simultaneously screened the literature based on the following criteria: (1) the literature mainly focused on muscle regeneration and satellite cells, and its content was accessible; (2) the literature consisted of reviews and articles; (3) the language of the articles was English; (4) the topics of the articles were related to muscle regeneration and satellite cells; and (5) duplicate literature was excluded.
2.4. Bibliometric analysis
After searching the WOSCC, we reviewed the titles and abstracts of the articles, screened them according to the inclusion and exclusion criteria, and finally extracted 1354 articles from the complete record text files in the WOS marked with citation references. We used CiteSpace 6.2.R4 [21] and VOSviewer 1.6.19 [22] for bibliometric analysis and visualization of the network diagram to better understand and interpret the development trends of the topic. Concurrently, we used Excel tables for a descriptive statistical analysis of publication countries, years, journals, and other parameters, and VOSviewer to visualize author, institution, and country distributions. The sizes of the nodes in the figure represent their frequency of occurrence. For example, if the frequency of an institution's appearance is proportional to the size of the node in the figure, the node connection represents the relationship between the cooperation of the institutions. We used CiteSpace to analyze the reference literature and keywords. CiteSpace, developed by Chaomei Chen [21], is a Java-based application that visualizes the relationship between data based on the circumstances of the article. Generally, the size of a node represents the frequency of the data. The higher the frequency, the larger the displayed node. The connection between nodes represents a mutual relationship, cooperation, or common citation relationship between the data.
3. Results
3.1. Analysis of annual publications
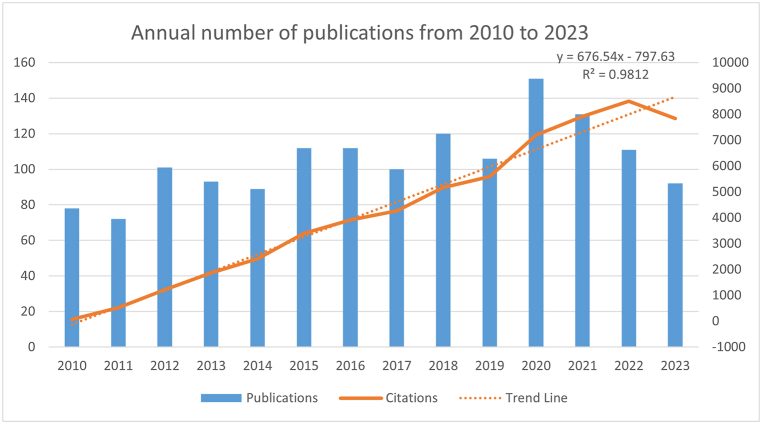
We searched for 1468 articles on muscle regeneration and myosatellite cells in the WOSCC published between 2010 and 2024. Sorted by year (Fig. 2 and Table S1), it can be observed from the figure that the number of studies on myosatellite cells in muscle regeneration has steadily increased since 2010. The growth trend is most prominent from 2020 to 2021, with a slight decrease noted in the last two years, 2022 and 2023, although the overall trend remains upward. This suggests a growing interest in myosatellite cells in muscle regeneration. Additionally, both the number of publications and citations have shown a gradual increase. Articles on myosatellite cells in muscle regeneration accumulated a total of 28,922 citations, with an average of 41.42 citations per paper and an H-index of 105 (as of March 14, 2024).
Fig. 2.
Annual number of published articles from 2010 to 2023.
3.2. Analysis of countries
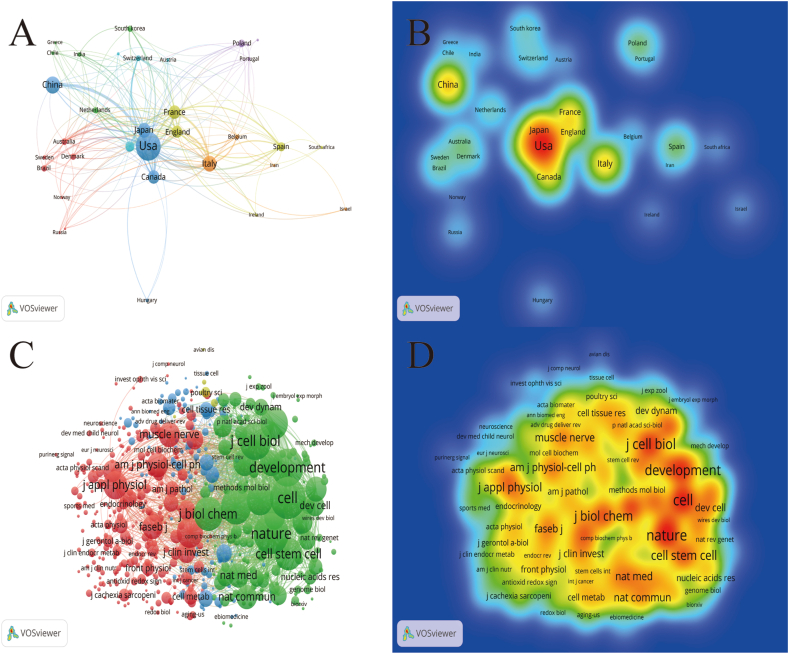
In the field of muscle regeneration and satellite cell research, an analysis was conducted on 1468 articles published from 56 different countries. Using VOSviewer for analysis and setting a minimum literature quantity of 5, 34 countries were selected. Fig. 3A and B shows network and density views of the connections between countries. The top ten countries in terms of publication quantity are listed in Table S2. Notably, the United States (520 articles, 25770 citations, total link strength 285) accounts for approximately one-third of the total article quantity, making it the country with the highest publication volume. China (199 articles), Italy (173 articles), Japan (155 articles), and Canada (130 articles) follow closely behind, rounding out the top five contributors to this field. Therefore, the United States, China, and Italy are at the forefront of global research on muscle regeneration and satellite cells.
Fig. 3.
Distribution of muscle regeneration and myosatellite cells countries and cited journals from 2010 to 2023 (A. Network view B. Density view C. Network view D. Density view).
3.3. Journals and Co-cited journals
All the articles were published in a total of 445 different journals. Listing the top five journals in the field of muscle regeneration and myosatellite cell research with the highest number of articles (Table S3), "PloS One" (52 articles) stands out among them, publishing the highest number of articles. "Stem Cells" and "Skeletal Muscle" are specifically dedicated to stem cells and muscle research, respectively. FASEB Journal is a general journal for life science research, while Nature Communications is a sub-journal of the renowned and influential [23]. When analyzing the co-cited journal views (Fig. 3C and D), it was observed that the network view had the largest nodes with the most links, and the darkest density view featured prominent journals such as Cell, Nature, and Journal of Cell Biology (listed in Table S3). This indicates that the study of satellite cells in muscle regeneration has received extensive attention, and many well-known journals have also explored this area in depth.
3.4. Institutional distribution
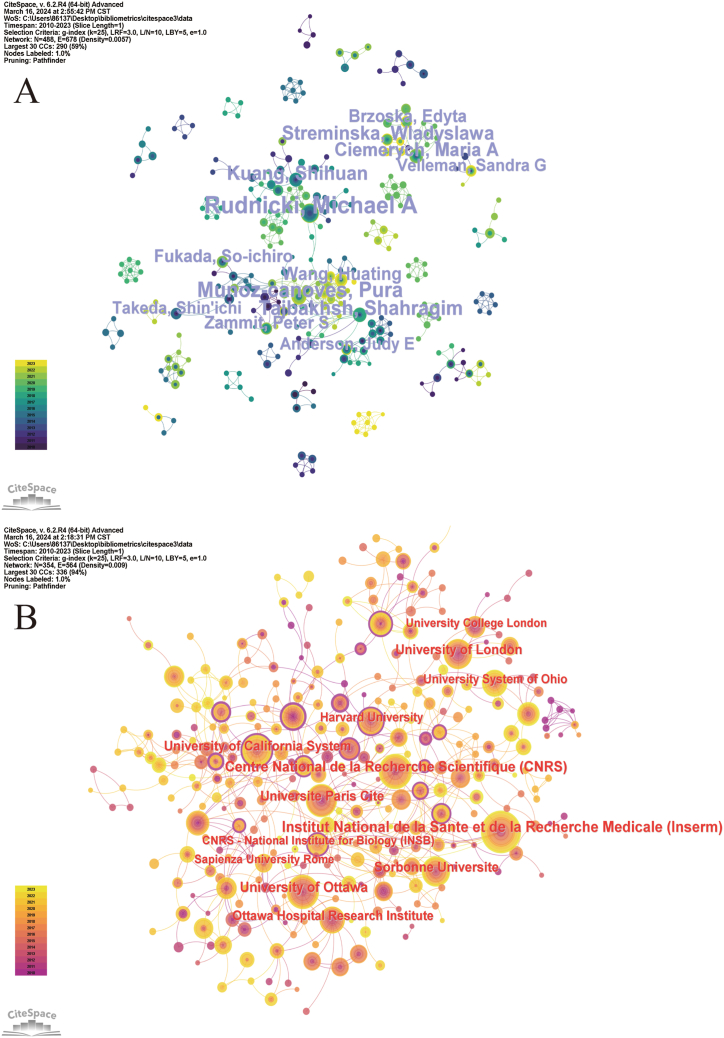
The top five institutions in the field of muscle regeneration and satellite cell research based on publication volume are presented in Table 1 and Fig. 4B. The institution with the highest publication volume was the Institut National de la Santé et de la Recherche Médicale (Inserm) (89 articles), followed by the Centre National de la Recherche Scientifique (CNRS) (68articles) and the University of Ottawa (57 articles). The top five institutions based on centrality are listed in Table 1, with the University of Texas System, University of California San Francisco, and the National Institutes of Health (NIH), USA, having the highest centrality (0.21). Although the University of Texas System had only published eight articles, it had the highest centrality, indicating its core position in the collaborative network. Most top-ranking institutions and universities are located in Europe and North America, indicating that these regions are more active in muscle regeneration and satellite cell research.
Table 1.
Top 5 institutions in terms of publications and centrality.
| Rank | Frequency | Organization | Rank | Centrality | Frequency | Organization |
|---|---|---|---|---|---|---|
| 1 | 89 | Institution National de la Santé et de la Recherche Médicale (Inserm) | 1 | 0.21 | 8 | University of Texas System |
| 2 | 68 | Centre National de la Recherche Scientifique (CNRS) | 2 | 0.21 | 7 | University of California San Francisco |
| 3 | 57 | University of Ottawa | 3 | 0.21 | 10 | National Institutes of Health (NIH) - USA |
| 4 | 51 | University of London | 4 | 0.2 | 14 | CNRS - National Institute for Biology (INSB) |
| 5 | 51 | Université Paris Cité | 5 | 0.2 | 8 | Boston Children's Hospital |
Fig. 4.
Institutional branch network and Co-author network map.
3.5. Authors and Co-cited authors
A total of 7425 authors have contributed to the field of muscle regeneration and satellite cell research. The top five contributors (as shown in Table S4) are Rudnicki, Michael A.; Munoz-canoves, Pura; Tajbakhsh, Shahragim; Kuang, Shihuan; and Streminska, Wladyslawa. According to the co-authorship network and co-cited author density views (Fig. 4A), Rudnicki Michael A. had the highest publication volumes. In an article co-authored by Rudnicki, Michael A. and Tajbakhsh, Shahragim, it was mentioned that under normal conditions, satellite cells in the muscle are in a quiescent state, but once the muscle is damaged, the satellite cells are activated, promoting muscle regeneration [24]. The authors studied the mechanisms and functions of the interconnections between muscle regeneration and satellite cells from different perspectives.
3.6. Citation analysis of reference literature
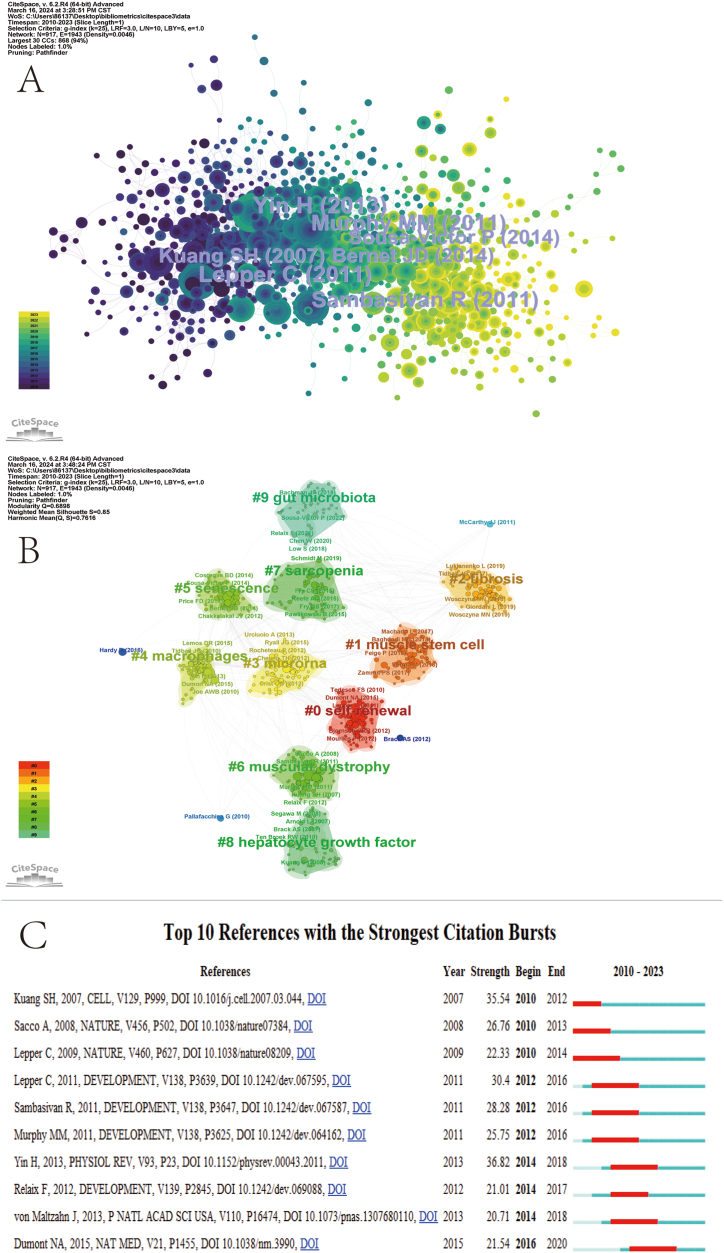
Frequently cited references are documents cited in one or more publications within a series of publications. Literature with numerous citations is usually influential and highly credible in the field. A network diagram of the field of muscle regeneration and satellite cells was generated using CiteSpace (Fig. 5A). Based on the citation count (Table 2), four publications had more than 100 citations. The top-ranked literature is "Satellite Cells and the muscle stem cell niche” by Yin H (2013), which discusses the ecological niche of satellite cells and muscle stem cells [25]. This is followed by “Absolute requirement of Pax7-positive satellite cells for acute injury-induced skeletal muscle regeneration” by Lepper C (2011) [8] and “Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration” by Sambasivan R (2011) [26], both published in 2011. These studies have elaborated on the role of satellite cells in muscle regeneration and the underlying mechanisms. According to the order of centrality (as shown in Table 2), the literature with the highest centrality is Fiore D (2016) [27], "Pharmacological blockage of fibro/adipogenic progenitor expansion and suppression of regenerative fibrogenesis is associated with impaired skeletal muscle regeneration," which elucidates the relationship between fibro/adipogenic progenitor cells and muscle regeneration after muscle injury, along with the potential mechanisms involved.
Fig. 5.
cited references map. (A. Network view B. Reference clustering view C. Top 10 cited references).
Table 2.
Top 5 references for frequency and Top 5 references for centrality.
| Rank | Frequency | Co-cited reference | Rank | Centrality | Co-cited reference |
|---|---|---|---|---|---|
| 1 | 134 | Yin H (2013) | 1 | 0.12 | Fiore D (2016) |
| 2 | 117 | Lepper C (2011) | 2 | 0.09 | Bentzinger CF (2013) |
| 3 | 112 | Sambasivan R (2011) | 3 | 0.09 | Baghdadi MB (2018) |
| 4 | 103 | Murphy MM (2011) | 4 | 0.09 | Lemos DR (2015) |
| 5 | 74 | Kuang SH (2007) | 5 | 0.08 | Crist CG (2012) |
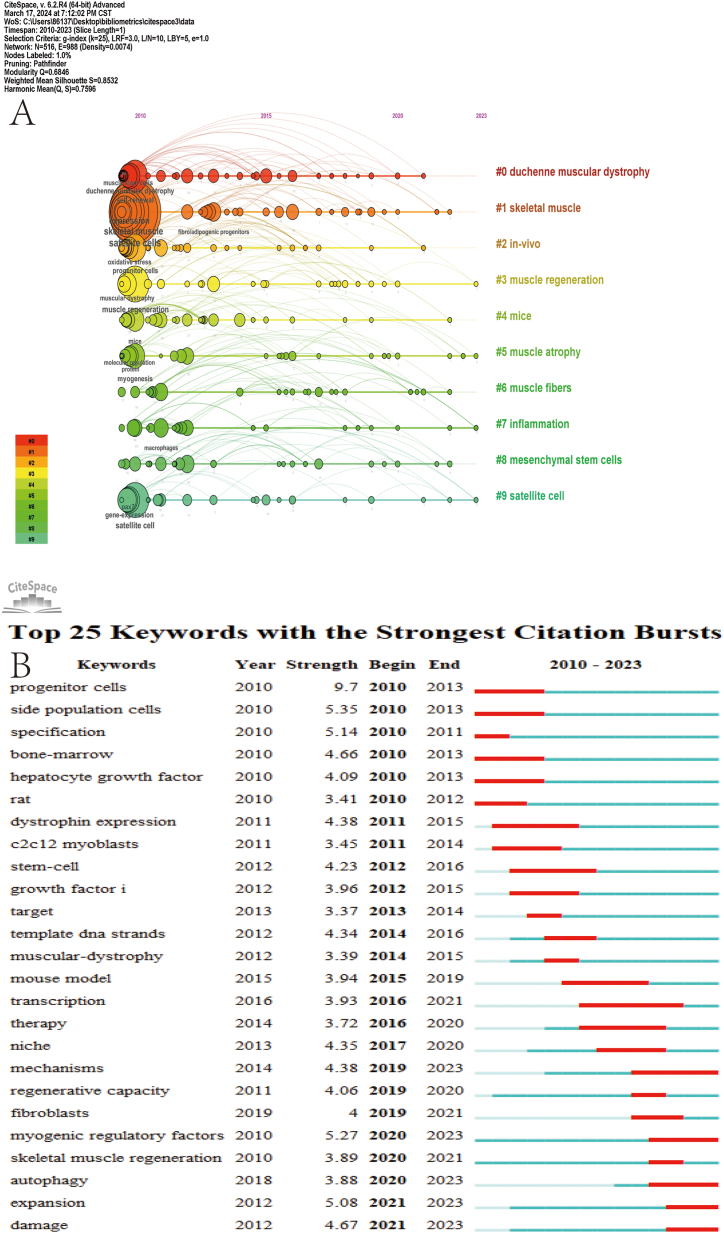
Reference clustering analyses were performed using CiteSpace, generating ten panels (Fig. 5B). A Q value of 0.6898 and an S value of 0.85 indicate that the cluster structure is stable, plausible, and persuasive. As can be seen from the figure, the largest cluster is #0, labeled "self-renewal,” followed by "muscle stem cells" (cluster #1), "fibrosis" (cluster #2), and "microRNA" (cluster #3). Other important clusters include "macrophages,” "senescence,” "muscle atrophy,” "sarcopenia,” "Hepatocyte Growth Factor," and "Gut Flora.” This shows that the current field favorites are still mainly around the research of muscle stem cells [28,29], followed by research on how to restore the regenerative function of muscle after muscle fibrosis [30,31], and the relationship between microRNA and muscle regeneration [32]. The reference timeline graph allows for analyzing the current research trends in a field (Fig. 7A). From the figure, it can be seen that the self-renewal of muscle regeneration was the most popular as early as 2005. By 2010, the hotspots of research gradually shifted towards "sarcopenia" and "microRNA.” In 2015, scholars also conducted in-depth research on the intestinal flora. As can also be seen from the reference explosion chart (Fig. 5C), the second most explosive is "Asymmetric self-renewal and commitment of satellite stem cells in muscle," published in the famous journal ''Cell'' as early as 2007. This article introduces in detail the dominant role of myosatellite cells in muscle regeneration. The self-renewal of satellite cells is regulated by various factors, which lays a solid foundation for the better study of satellite cells in the future [33].
Fig. 7.
Visualization map of top 25 keyword with the strongest citation bursts and visualization map of timeline viewer (A. Top 25 keywords B. Timeline graph).
3.7. Keyword analysis
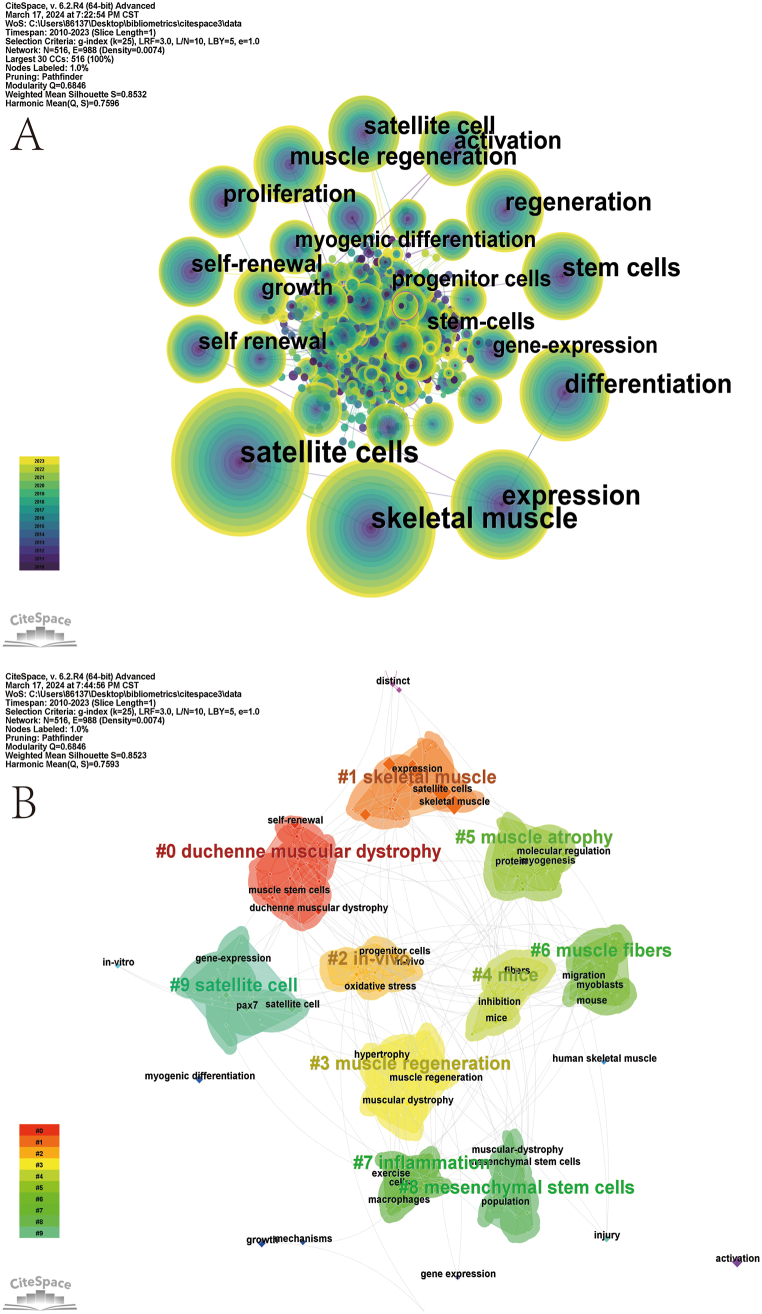
The keywords of an article reflect its main content and the research hotspots in the field. Therefore, we used CiteSpace to generate a keyword network diagram (as shown in Fig. 6A). In total, 516 keywords were identified, with 17 keywords appearing more than 100 times. The top five keywords by frequency (as shown in Table 3) were "satellite cells,” "skeletal muscle,” "expression,” "differentiation,” and "stem cells.” According to the degree of centrality, the top ones are "heterogeneity,” "repair,” "migration,” "muscle,” and "connective tissue fibroblasts,” with "heterogeneity" having the highest centrality (0.21). This suggests that "heterogeneity" may be the research hotspot in recent years. A cluster analysis of keywords can better illustrate the current major research themes in this field. The Q-value of the clustering result was 0.6846, and the S-value was 0.8523, indicating that the clustering effect was significant and reasonably efficient(Fig. 6B). A total of six clusters were generated to illustrate the research trends in the field. These clusters include "Duchenne muscular dystrophy,” "skeletal muscle,” "in-vivo,” "muscle regeneration,” "mice" and "muscle atrophy,” "muscle fibers,” "inflammation,” "mesenchymal stem cells,” "satellite cell.” Among these, "Duchenne muscular dystrophy" ranked the highest, indicating that muscle regeneration in Duchenne muscular dystrophy has been increasingly studied in recent years.
Fig. 6.
Keywords Network map and clustering map (A. Network view B. Clustering view).
Table 3.
Top 5 for keyword centrality and frequency.
| Rank | Centrality | keyword | Rank | frequency | keyword |
|---|---|---|---|---|---|
| 1 | 0.21 | heterogeneity | 1 | 669 | satellite cells |
| 2 | 0.18 | repair | 2 | 593 | skeletal muscle |
| 3 | 0.17 | migration | 3 | 386 | expression |
| 4 | 0.14 | muscle | 4 | 293 | differentiation |
| 5 | 0.12 | connective tissue fibroblasts | 5 | 249 | stem cells |
The research hotspots and trends in this field can be observed from keyword bursts. The blue line indicates the time interval, and the red line indicates the keyword burst period. By detecting keyword bursts, the analysis identified 25 keywords with the strongest bursts. As can be seen from (Fig. 7B), the keyword with the strongest burst strength is "progenitor cells" (9.76), which may be crucial for studying the regeneration of myosatellite cells. In recent years, the most popular area of research is "expansion,” and the expansion of satellite cells is regarded as the cornerstone of muscle regeneration [34].
4. Discussion
This is the first visual analysis of the literature related to muscle regeneration and satellite cells over the past 13 years. Bibliometric analyses of studies related to muscle regeneration and satellite cells between 2010 and 2023 were conducted using CiteSpace and VOSviewer. The analysis covered publication volume, co-authors, institutions, countries, references, and keywords, shedding light on the current status and future research trends. With advancements in muscle regeneration research, satellite cells have gradually become a research hotspot.
4.1. General information of main Findings
We analyzed 1468 papers on muscle regeneration and satellite cells in WOSCC. The results show that, from 2010 to 2023, the annual number of papers generally increased, with a significant rise after 2020. The United States played an important role in promoting research on muscle regeneration and satellite cells, contributing the highest number of published papers. In recent years, countries such as Italy, China, and Japan have experienced rapid growth. However, there is still a need to strengthen international collaboration and academic impact. Therefore, the current research distribution is geographically unbalanced. Additionally, three of the top five institutions in terms of publications are from France: Inserm, CNRS, and Université Paris Cité. This highlights the increasing importance of research on satellite cells for muscle regeneration in France in recent years. Furthermore, compared to other regions, the University of Texas System, University of California San Francisco, and NIH, USA ranked higher in terms of centrality. This suggests a greater focus on mutual collaborative research among different regions.
The impact factor (IF) is a measure of the frequency with which an academic journal's published articles are cited by other academic journals within a specific period and is widely considered an important indicator of a journal's quality and influence in the field of medicine [35]. Among the top five journals in terms of publication volume, "Nature Communications" had the highest impact factor (16.6). Established in 2010, "Nature Communications" primarily publishes high-quality research in biology and other related fields [23]. It is a Q1 journal of the Chinese Academy of Sciences and has published numerous high-quality articles on muscle regeneration and satellite cells.
Rudnicki, Michael A was identified as the author with the highest number of publications in the field of muscle regeneration and satellite cells. He has long been conducting research in stem cell biology and muscle regeneration medicine. His laboratory is dedicated to exploring the role of satellite cells in muscle regeneration and repair processes [18] as well as how to use stem cells to treat diseases such as muscle atrophy [36]. His research also involves the differentiation [37], proliferation, and self-renewal of muscle stem cells [33], as well as the regulatory factors and signaling pathways involved in muscle regeneration [38]. The top five centralities and most cited references underscore the importance of satellite cells in muscle regeneration, mainly because satellite cells are a major component of muscles. With increasing evidence on the role of myosatellite cells in muscles [26], attention has been focused on their role in muscle repair [39]. According to the ten most cited references (Fig. 5C), it can be observed that initially, researchers focused on the properties of satellite cells themselves [33]. However, with the publication of "Self-renewal and expansion of single transplanted muscle stem cells" in 2010, research began to explore how satellite cells can be used to treat [40]and improve the regenerative function of muscle [26] as well as the role satellite cells play in other muscle disorders [8].
4.2. Research trends and hotspots of myosatellite cells in muscle regeneration
Keywords often reflect research hotspots in a field. By conducting a cluster analysis of the literature, research hotspots related to satellite cells and muscle regeneration were identified. The top cluster is "Duchenne muscular dystrophy." It is well known that "Duchenne muscular dystrophy" is a progressive muscle disease caused by a lack of dystrophin protein, resulting in skeletal muscle dysfunction [41,42]. Researchers are continuously searching for effective treatment methods. Studies have shown that Duchenne muscular dystrophy can be improved by autologous and allogeneic stem cell transplantation [43], which restores the function of satellite cells and improves muscle atrophy in patients [[44], [45], [46]]. Stem cell therapy is an emerging method expected to become an effective means of treating Duchenne muscular dystrophy in the future [47]. Future research on muscle regeneration should focus on Duchenne muscular dystrophy. Researchers plan to use gene editing technology [48]and stem cell therapy to correct genetic defects in patients with Duchenne muscular dystrophy, promoting muscle regeneration and functional recovery [49,50].
In recent years, a growing body of research has shown that muscle regeneration is inextricably associated with inflammation. Although inflammation is often regarded as harmful, it is crucial for repairing muscle damage [51]. Skeletal muscle regeneration is regulated by various inflammatory responses, and immune cells play a key role in muscle regeneration [52]. Macrophage regulation is thought to influence the function of satellite cells in muscle regeneration, highlighting the importance of the immune system in muscle repair [53,54]. In the future, as research on inflammation and muscle regeneration intensifies, we expect that more studies will be dedicated to optimizing the inflammatory microenvironment to enhance muscle regeneration.
One of the key components is "mesenchymal stem cells" (MSCs), which are pluripotent cells present in the adult bone marrow that can be derived or differentiated into other cell lines [55,56]. They can also be derived from myosatellite cells and can be used to treat diseases by secreting relevant chemicals. Studies have shown that they can improve acute alcoholic liver injury [57,58]. Studies have also shown that injecting MSCs into the muscles of mice activates skeletal turnover and metabolic breakdown [59,60].
In the future, more research will be devoted to combining stem cells with muscle regeneration, which will help develop new treatments to facilitate the repair of muscle damage and the treatment of muscle diseases.
Muscle regeneration and satellite cell research are rapidly evolving and becoming increasingly concentrated areas of research. This field covers a wide range of topics published in journals across multiple disciplines, demonstrating the researchers’ interest in different fields of muscle regeneration mechanisms and therapeutic approaches. In addition, satellite cells have great potential for applications in muscle regeneration and play an important role in the treatment of muscle injuries and diseases. As satellite cell research continues to deepen, breakthroughs and innovations are expected, bringing new hope to the field of muscle regenerative medicine.
4.3. Limitations
This study analyzed research trends in the field using bibliometric tools. Unlike traditional reviews, the data analyses in this study are relatively comprehensive and objective, providing readers with deeper insights. It is important to note, however, that because of the limitations of the software and research methodology, the selected articles were limited to those in the WOSCC database; articles from other databases were not included. Therefore, 1468 articles may not be fully representative of all the available information in the field, which is an important limitation of this study.
5. Conclusions
Satellite cells hold significant research value for muscle regeneration. Visual analysis revealed a growing number of papers annually, with an increased presence in core international journals. Enhanced cooperation and communication among countries, institutions, and authors are imperative. Current research focuses on growth, skeletal muscle, extracellular matrix, hepatocyte growth factor, and muscle wasting, offering promising advances in regeneration strategies.
Funding
This work was supported by the Ganzhou Municipal Health Commission (2022-2-088) ,Jiangxi Provincial Administration of Traditional Chinese Medicine (2022A264), Health Commission of Jiangxi Province (202,310,737) ,Ganzhou guiding science and Technology Plan (GZ2023ZSF131).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
CRediT authorship contribution statement
Nan Huang: Writing – review & editing, Methodology, Investigation, Conceptualization. Kang Zou: Writing – review & editing, Methodology, Investigation, Conceptualization. Yanbiao Zhong: Writing – review & editing, Visualization, Validation, Supervision, Software, Resources, Project administration. Yun Luo: Writing – review & editing, Visualization, Validation, Supervision, Software, Resources, Project administration. Maoyuan Wang: Writing – review & editing, Visualization, Validation, Supervision, Software, Resources, Project administration. Li Xiao: Writing – original draft, Formal analysis, Data curation.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Li Xiao reports financial support was provided by Ganzhou Municipal Health Commission (2022-2-088). Li Xiao reports financial support was provided by Jiangxi Provincial Administration of Traditional Chinese Medicine (2022A264). Li Xiao reports financial support was provided by Health Commission of Jiangxi Province (202,310,737). Li Xiao reports financial support was provided by Ganzhou guiding science and Technology Plan (GZ2023ZSF131). If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank VOSviewer, Cite Space for their support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e37529.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Rosenberg I.H. Sarcopenia: origins and clinical relevance. J. Nutr. 1997;127(5 Suppl):990s–991s. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 2.Binazzi M., Simonetti S. Granuloma annulare, necrobiosis lipoidica, and diabetic disease. Int. J. Dermatol. 1988;27(8):576–579. doi: 10.1111/j.1365-4362.1988.tb02408.x. [DOI] [PubMed] [Google Scholar]
- 3.Yuan S., Larsson S.C. Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabolism. 2023;144 doi: 10.1016/j.metabol.2023.155533. [DOI] [PubMed] [Google Scholar]
- 4.Dhillon R.J., Hasni S. Pathogenesis and management of sarcopenia. Clin. Geriatr. Med. 2017;33(1):17–26. doi: 10.1016/j.cger.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chargé S.B., Rudnicki M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004;84(1):209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 6.Chen B., Shan T. The role of satellite and other functional cell types in muscle repair and regeneration. J. Muscle Res. Cell Motil. 2019;40(1):1–8. doi: 10.1007/s10974-019-09511-3. [DOI] [PubMed] [Google Scholar]
- 7.Brzeszczyńska J., et al. Alterations in the in vitro and in vivo regulation of muscle regeneration in healthy ageing and the influence of sarcopenia. J Cachexia Sarcopenia Muscle. 2018;9(1):93–105. doi: 10.1002/jcsm.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lepper C., Partridge T.A., Fan C.M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138(17):3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauro A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961;9(2):493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bischoff R. Proliferation of muscle satellite cells on intact myofibers in culture. Dev. Biol. 1986;115(1):129–139. doi: 10.1016/0012-1606(86)90234-4. [DOI] [PubMed] [Google Scholar]
- 11.Suetta C., et al. Ageing is associated with diminished muscle re-growth and myogenic precursor cell expansion early after immobility-induced atrophy in human skeletal muscle. J Physiol. 2013;591(15):3789–3804. doi: 10.1113/jphysiol.2013.257121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zammit P.S., et al. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J. Cell Biol. 2004;166(3):347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whalen R.G., et al. Expression of myosin isoforms during notexin-induced regeneration of rat soleus muscles. Dev. Biol. 1990;141(1):24–40. doi: 10.1016/0012-1606(90)90099-5. [DOI] [PubMed] [Google Scholar]
- 14.Le Grand F., Rudnicki M.A. Skeletal muscle satellite cells and adult myogenesis. Curr. Opin. Cell Biol. 2007;19(6):628–633. doi: 10.1016/j.ceb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broadus R.N. Toward a definition of “bibliometrics”. Scientometrics. 1987;12:373–379. doi: 10.1007/BF02016680. [DOI] [Google Scholar]
- 16.Shang M., et al. Macrophage-derived glutamine boosts satellite cells and muscle regeneration. Nature. 2020;587(7835):626–631. doi: 10.1038/s41586-020-2857-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giuliani G., Rosina M., Reggio A. Signaling pathways regulating the fate of fibro/adipogenic progenitors (FAPs) in skeletal muscle regeneration and disease. FEBS J. 2022;289(21):6484–6517. doi: 10.1111/febs.16080. [DOI] [PubMed] [Google Scholar]
- 18.Dumont N.A., et al. Satellite cells and skeletal muscle regeneration. Compr. Physiol. 2015;5(3):1027–1059. doi: 10.1002/cphy.c140068. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt M., et al. Adult stem cells at work: regenerating skeletal muscle. Cell. Mol. Life Sci. 2019;76(13):2559–2570. doi: 10.1007/s00018-019-03093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mongeon P., Paul-Hus A. The journal coverage of Web of Science and Scopus: a comparative analysis. Scientometrics. 2015;106(1):213–228. doi: 10.1007/s11192-015-1765-5. [DOI] [Google Scholar]
- 21.Chen C. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 2006;57(3):359–377. doi: 10.1002/asi.20317. [DOI] [Google Scholar]
- 22.van Eck N.J., Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–538. doi: 10.1007/s11192-009-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nature communications at ten. Nat. Commun. 2020;11(1):1812. doi: 10.1038/s41467-020-15592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bar-Nur O., et al. Direct reprogramming of mouse fibroblasts into functional skeletal muscle progenitors. Stem Cell Rep. 2018;10(5):1505–1521. doi: 10.1016/j.stemcr.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin H., Price F., Rudnicki M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013;93(1):23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambasivan R., et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138(17):3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- 27.Fiore D., et al. Pharmacological blockage of fibro/adipogenic progenitor expansion and suppression of regenerative fibrogenesis is associated with impaired skeletal muscle regeneration. Stem Cell Res. 2016;17(1):161–169. doi: 10.1016/j.scr.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Fang J., et al. Skeletal muscle regeneration via the chemical induction and expansion of myogenic stem cells in situ or in vitro. Nat. Biomed. Eng. 2021;5(8):864–879. doi: 10.1038/s41551-021-00696-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molina T., Fabre P., Dumont N.A. Fibro-adipogenic progenitors in skeletal muscle homeostasis, regeneration and diseases. Open Biol. 2021;11(12) doi: 10.1098/rsob.210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moiseeva V., et al. Senescence atlas reveals an aged-like inflamed niche that blunts muscle regeneration. Nature. 2023;613(7942):169–178. doi: 10.1038/s41586-022-05535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahdy M.A.A. Skeletal muscle fibrosis: an overview. Cell Tissue Res. 2019;375(3):575–588. doi: 10.1007/s00441-018-2955-2. [DOI] [PubMed] [Google Scholar]
- 32.Dey P., Soyer M.A., Dey B.K. MicroRNA-24-3p promotes skeletal muscle differentiation and regeneration by regulating HMGA1. Cell. Mol. Life Sci. 2022;79(3):170. doi: 10.1007/s00018-022-04168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuang S., et al. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129(5):999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tierney M.T., et al. STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat Med. 2014;20(10):1182–1186. doi: 10.1038/nm.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garfield E. How can impact factors be improved? Bmj. 1996;313(7054):411–413. doi: 10.1136/bmj.313.7054.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hekmatnejad B., Rudnicki M.A. Transplantation to study satellite cell heterogeneity in skeletal muscle. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.902225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuang S., Rudnicki M.A. The emerging biology of satellite cells and their therapeutic potential. Trends Mol. Med. 2008;14(2):82–91. doi: 10.1016/j.molmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Feige P., Rudnicki M.A. Muscle stem cells. Curr. Biol. 2018;28(10):R589–r590. doi: 10.1016/j.cub.2018.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy M.M., et al. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138(17):3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sacco A., et al. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456(7221):502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sussman M. Duchenne muscular dystrophy. J. Am. Acad. Orthop. Surg. 2002;10(2):138–151. doi: 10.5435/00124635-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Duan D., et al. Duchenne muscular dystrophy. Nat Rev Dis Primers. 2021;7(1):13. doi: 10.1038/s41572-021-00248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato M., et al. A new immunodeficient Duchenne muscular dystrophy rat model to evaluate engraftment after human cell transplantation. Front. Physiol. 2023;14 doi: 10.3389/fphys.2023.1094359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sienkiewicz D., et al. Duchenne muscular dystrophy: current cell therapies. Ther Adv Neurol Disord. 2015;8(4):166–177. doi: 10.1177/1756285615586123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang M., et al. Duchenne muscular dystrophy: pathogenesis and promising therapies. J. Neurol. 2023;270(8):3733–3749. doi: 10.1007/s00415-023-11796-x. [DOI] [PubMed] [Google Scholar]
- 46.Boyer O., et al. Myogenic cell transplantation in genetic and acquired diseases of skeletal muscle. Front. Genet. 2021;12 doi: 10.3389/fgene.2021.702547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun C., et al. Stem cell-based therapies for Duchenne muscular dystrophy. Exp. Neurol. 2020;323 doi: 10.1016/j.expneurol.2019.113086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elangkovan N., Dickson G. Gene therapy for Duchenne muscular dystrophy. J. Neuromuscul. Dis. 2021;8(s2):S303–s316. doi: 10.3233/jnd-210678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun C., et al. Therapeutic strategies for Duchenne muscular dystrophy: an update. Genes. 2020;11(8) doi: 10.3390/genes11080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Markati T., et al. Emerging therapies for Duchenne muscular dystrophy. Lancet Neurol. 2022;21(9):814–829. doi: 10.1016/s1474-4422(22)00125-9. [DOI] [PubMed] [Google Scholar]
- 51.Chazaud B. Inflammation and skeletal muscle regeneration: leave it to the macrophages. Trends Immunol. 2020;41(6):481–492. doi: 10.1016/j.it.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Yang W., Hu P. Skeletal muscle regeneration is modulated by inflammation. J Orthop Translat. 2018;13:25–32. doi: 10.1016/j.jot.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nawaz A., et al. Depletion of CD206(+) M2-like macrophages induces fibro-adipogenic progenitors activation and muscle regeneration. Nat. Commun. 2022;13(1):7058. doi: 10.1038/s41467-022-34191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang C., et al. Age-related decline of interferon-gamma responses in macrophage impairs satellite cell proliferation and regeneration. J Cachexia Sarcopenia Muscle. 2020;11(5):1291–1305. doi: 10.1002/jcsm.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pittenger M.F., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 56.de Morree A., Rando T.A. Regulation of adult stem cell quiescence and its functions in the maintenance of tissue integrity. Nat. Rev. Mol. Cell Biol. 2023;24(5):334–354. doi: 10.1038/s41580-022-00568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chung J.S., et al. Skeletal muscle satellite cell-derived mesenchymal stem cells ameliorate acute alcohol-induced liver injury. Int. J. Med. Sci. 2022;19(2):353–363. doi: 10.7150/ijms.68971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Didamoony M.A., et al. Innovative preconditioning strategies for improving the therapeutic efficacy of extracellular vesicles derived from mesenchymal stem cells in gastrointestinal diseases. Inflammopharmacology. 2023;31(6):2973–2993. doi: 10.1007/s10787-023-01350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takegaki J., et al. Intramuscular injection of mesenchymal stem cells activates anabolic and catabolic systems in mouse skeletal muscle. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-00627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y.H., et al. The application of bone marrow mesenchymal stem cells and biomaterials in skeletal muscle regeneration. Regen Ther. 2020;15:285–294. doi: 10.1016/j.reth.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.