Abstract
The CTC series of cobalt chelates display in vitro and in vivo activity against herpes simplex virus types 1 and 2 (HSV-1 and HSV-2). The experiments described here identify the stage in the virus life cycle where CTC-96 acts and demonstrate that the drug inhibits infection of susceptible cells. CTC-96 at 50 μg/ml has no effect on adsorption of virions to Vero cell monolayers. Penetration assays reveal that CTC-96 inhibits entry of the virus independent of gC and cellular entry receptors. This observation was supported by the failure to detect the accumulation of virus-specified proteins and α mRNA transcripts when CTC-96 is present at the onset of infection. Moreover, virion-associated αTIF does not accumulate in the nucleus of cells infected in the presence of CTC-96. CTC-96 targets the initial fusion event between the virus and the cell and also inhibits cell-to-cell spread and syncytium formation. Furthermore, CTC-96 inhibits plaque formation by varicella-zoster virus and vesicular stomatitis virus as efficiently as by HSV-1. Collectively, these experiments suggest that CTC-96 is a broad-spectrum inhibitor of infection by enveloped viruses and that it inhibits HSV-1 infection at the point of membrane fusion independent of the type of virus and cellular receptors present.
Infection by the alphaherpesviruses herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) results in a variety of viral diseases including oral and genital epithelial lesions, encephalitis, and ocular keratitis (15, 16, 20, 96, 103). Among these, herpetic ocular infection is the leading infectious cause of blindness in developed countries (48, 55, 56, 96). Herpesvirus infections are characterized by their ability to establish latency and reactivate from the latent state (80). In immunocompetent and immunocompromised patients herpesvirus infections are among the most frequent causes of viral disease (78, 93, 104). Both primary and recrudescent infections in immunocompromised patients are life threatening (78, 93, 104). Thus, there exists considerable interest in developing treatments for preventing infection and reducing the pathogenesis of primary and recurrent infections by HSV.
Several nucleoside analogs are approved for use in the treatment of herpesvirus infections (e.g., acyclovir, penciclovir, valaciclovir, and famciclovir), and derivatives of these are being developed and/or are undergoing clinical trials (2, 5, 17). These drugs are activated by the HSV thymidine kinase, and thus their primary target is virus DNA synthesis (26, 32). Not surprisingly, drug-resistant strains are appearing with increasing frequency (13, 14, 17, 28, 29, 54, 70, 85). Resistance arises from mutations in the TK gene (18, 27) or mutations in the gene encoding DNA polymerase (13, 14, 47, 70, 85). Therefore, new drugs need to be developed that target other aspects of the virus life cycle in order to find more effective treatments against the existing drug-resistant strains as well as all the known herpesviruses.
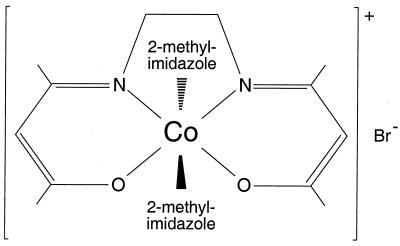
The CTC series of cobalt-containing compounds possess anti-inflammatory (105) and antiviral (3, 22, 24, 97) activity. Several CTC complexes have moderate activity in vitro and in vivo against HSV-1 and 2, varicella-zoster virus (VZV), cytomegalovirus (CMV), and Epstein-Barr virus (EBV) (3, 22, 24, 97). However, the data for the inhibitory effects against VZV, CMV, and EBV are anecdotal (22, 24, 97). Previous studies showed that CTC-96 (Fig. 1), a derivative of CTC-23 (3, 22, 24, 97, 105), is the least cytotoxic and most effective of these compounds against HSV-1 and HSV-2 (3, 22). CTC-96 is also effective in inhibiting HSV-1 replication in tissue culture (3). In a rabbit eye model, CTC-96 is able to reduce the corneal surface HSV-1 titer and facilitate recovery from dendritic keratitis (3, 22). It has been suggested that the anti-inflammatory properties of the CTC complexes may aid in recovery from ocular disease (3). However, CTC-96 is not a global virus inhibitor since it is ineffective in the cottontail rabbit papillomavirus model (72).
FIG. 1.
Chemical structure of CTC-96.
The antiherpetic activity of the CTC series has been known for many years. However, neither the mechanism by which, nor the stage of the virus life cycle at which, CTC-96 exerts its inhibitory action on HSV-1 is known. The body of evidence provided here demonstrates that while virus can attach to cells it cannot enter in the presence of CTC-96. The implications of this inhibition for the use of CTC-96 as a tool for analyzing the biology of HSV-1 entry and as an antiviral therapy are discussed. Furthermore, CTC-96 severely reduces the plaquing efficiency of VZV and vesicular stomatitits virus (VSV).
MATERIALS AND METHODS
Cells and viruses.
Vero cells were maintained in Dulbecco's minimal essential medium (DMEM; Gibco BRL, Grand Island, N.Y.) supplemented with 5% bovine calf serum (BCS; HyClone Laboratories Inc., Logan, Utah). Human fetal lung-Chang (Helf) cells (BioWhittaker, Inc., Walkersville, Md.) were grown in DMEM supplemented with 10% fetal bovine serum (FBS; HyClone Laboratories Inc.). The Chinese hamster ovary (CHO) cell lines C8 (95), CHO-HveC-1 (34), IEβ8 (67, 95), and IEβ8/HveA (67, 95) (provided by P. Spear, Northwestern University) were maintained as described previously. Unless otherwise indicated, all media used, including overlay media, contained 100 U of penicillin per ml and 100 μg of streptomycin per ml (Gibco BRL).
The wild-type herpesvirus used was HSV-1 Glasgow strain 17 (8). vJSsyn− is described below. vBSΔ27, a lacZ-containing ICP27 deletion virus, was described previously (89). The Ellen strain of VZV was provided by P. Annunziato (Columbia University). VSV was provided by V. Racaniello (Columbia University). HS1, a glycoprotein C− (gC) HSV-1 (KOS) virus, was described previously (73).
HSV-1 preparation. (i) Cell-associated HSV-1.
Vero cell monolayers were infected at low multiplicities of infection (MOIs) and incubated at 37°C for 2 to 3 days. Infected cells were scraped into the medium and pelleted by low-speed centrifugation. The infected cell pellet was washed with phosphate-buffered saline (PBS) (2.7 mM KCl, 1.2 mM KH2PO4, 138 mM NaCl, 8.1 mM Na2HPO4 · 7H2O), resuspended in DMEM containing 1% BCS, and subjected to five freeze-thaw cycles. The virus was then titrated on Vero cells.
(ii) Partially purified HSV-1.
Vero cells were infected at a low MOI and incubated at 37°C for 2 to 3 days. Infected cells were scraped into the medium, centrifuged at 900 × g for 5 min at 4°C, washed with PBS, and centrifuged again. The infected cell pellet was resuspended in PBS-ABC (PBS containing 5 mM MgCl2 and 7 mM CaCl2) and incubated on ice for 15 min. The cell suspension was disrupted by 15 strokes in a sterile Wheaton Dounce homogenizer using pestle B. The nuclei were pelleted at 3,000 × g for 5 min at 4°C. The virions in the supernatant were pelleted by centrifugation at 20,000 × g for 75 to 90 min at 4°C, and the virus pellet was resuspended in PBS-ABC-ICS-glu (PBS-ABC containing 1% inactivated BCS and 0.1% glucose). The resuspended virus was pelleted through a 3-ml sucrose cushion (30% sucrose in 50 mM NaCl–10 mM Tris [pH 7.8]) for 2 h at 188,000 × g in a Beckman SW41 rotor (6, 25). The virus pellet was resuspended in PBS-ABS-ICS-glu and then titrated on Vero cells.
(iii) 35S-labeled HSV-1.
Vero cells were infected at a MOI of 10. Then 500 μCi of Tran35S-label (1,175 Ci/mmol; ICN Biomedicals, Inc., Costa Mesa, Calif.) per ml of methionine-free DMEM (Specialty Media Inc., Lavalette, N.J.) containing 5% dialyzed FBS and 10% normal DMEM (6, 25) was added to the infected cells at 2 h postinfection (p.i.), and the cells were incubated at 37°C for 26 h. 35S-labeled virions were partially purified from the infected cells as described above. The specific activity of the 35S-labeled virus was 5 × 10−4 cpm per PFU.
(iv) vJSsyn− purification.
Vero cells were infected at a low MOI. Syncytial (syn−) plaques were isolated, and the virus in them was plaque purified two additional times. Cell-associated syn− virus (vJSsyn−) was prepared as described above.
Plaque assays. (i) HSV-1.
HSV-1 was preincubated with the indicated concentrations of CTC-96 (REDOX Pharmaceutical Corp., Greenvale, N.Y.) on ice for several minutes. The pretreated virus suspension was then diluted to or maintained at the indicated concentrations of CTC-96. For pretreatment of Vero cell monolayers, 50 μg of CTC-96 per ml was added to the medium, and where indicated the cells were then washed in fresh medium with no drug for the indicated times prior to infection with untreated virus. Cells were infected in the presence of various concentrations of CTC-96 or in its absence. After 1 h of adsorption at 37°C in DMEM supplemented with 1% BCS with and without CTC-96, methylcellulose overlay medium (DMEM containing 1.5% methylcellulose and 1% BCS) containing the indicated amount of CTC-96 was added to the infected cell monolayers. The plates were incubated at 37°C for several days and fixed with methanol. The cell monolayers were stained with 0.1% crystal violet, and plaques were counted.
(ii) VSV.
Virus was diluted in PBS with 0.2% BCS and adsorbed to Vero cell monolayers as described above. After adsorption, infected cells were overlaid with methylcellulose overlay medium and incubated at 37°C for 2 days. Monolayers were fixed and stained as described above for HSV-1.
Adsorption assay.
Adsorption of 35S-labeled HSV-1 and detection of bound virus were performed as described previously (6). Briefly, 35S-labeled HSV-1 was adsorbed for 1 h at 4°C to Vero cell monolayers at a MOI of 0.1, 1, or 10 in DMEM containing 1% BCS with or without 50 μg of CTC-96 per ml. The plates were washed four times with 500 μl of ice-cold PBS at 4°C on ice. Each plate was incubated for 10 min in 500 μl of ice-cold radioimmunoprecipitation assay buffer (10 mM NaPO4 [pH 7.2], 150 mM NaCl, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM NaF, 2 mM EDTA) at 4°C. The cpm associated with 100 μl of each lysate was measured by liquid scintillation spectrometry (Wallac Inc., Gaithersburg, Md.). Experiments for each condition were performed in triplicate.
RT-PCR.
RNAs were extracted from infected Vero cell monolayers, and the accumulation of α4 and α27 mRNAs was determined by coupled reverse transcription (RT) and PCR using the commercial kit, EZ rTth RNA PCR kit (Perkin-Elmer, Foster City, Calif.). The primers used during RT were 4-2 and ICP27-L-RT (58). Following RT, the secondary primers, 4-1 and ICP27-U-RT, were added for PCR (58). These primers result in amplification of a 100-bp fragment and a 220-bp fragment from the α4 and α27 RNAs, respectively.
Western blot analysis.
Vero cell monolayers were infected at a MOI of 5 in the presence or absence of 50 μg of CTC-96 per ml. Protein preparation and Western blot analysis were performed as previously described (57). Immunodetection of proteins was performed using the following antibodies: ICP0, rabbit polyclonal antibody CLU7 (57); ICP27, rabbit polyclonal antibody CLU38 (57); glycoprotein B (gB), rabbit polyclonal antibody R69 (provided by G. Cohen, University of Pennsylvania); and αTIF, rabbit polyclonal antibody anti-VP16 (Clontech Laboratories Inc., Palo Alto, Calif.). The secondary antibodies used were goat anti-rabbit and goat anti-mouse immunoglobulin G (IgG) conjugated to horseradish peroxidase (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.). Immunoblots were developed as previously described (58).
αTIF immunofluorescence.
Vero cell monolayers seeded onto coverslips were infected at a MOI of 100 on ice at 4°C for 45 min. Prewarmed (37°C) DMEM containing 1% BCS was added to each plate, and the plates were incubated at 37°C for the indicated times and then washed twice with ice-cold PBS on ice. The infected cells were fixed in 3.7% formaldehyde in PBS for 30 min and washed with PBS. The fixed monolayers were permeabilized in −20°C acetone for 10 min, and then the cells were washed sequentially with water and PBS. Fixed cells were incubated for 20 min in 10% normal goat serum (NGS; Roche, Indianapolis, Ind.) in PBS containing 0.1% Tween 20 (PBST) and washed twice with PBST. The coverslips were next incubated for 30 min in PBST containing 1% NGS and a 1:200 dilution of rabbit polyclonal anti-VP16 antibody (Clontech Laboratories, Inc.) and washed six times with PBST. The infected cell monolayers were then incubated for 30 min in PBST containing 1% NGS and a 1:200 dilution of goat anti-rabbit IgG antibody conjugated to fluorescein isothiocyanate (FITC; Kirkegaard & Perry Laboratories, Inc.) and washed six times with PBST. The coverslips were then mounted on slides in Biomeda gel/mount solution (Fisher Scientific, Springfield, N.J.) and viewed with a 100× lens of a Zeiss LSM 4100 confocal laser-scanning system attached to a Zeiss Axiovert 100TV inverted microscope. Each image is a composite of 1-μm serial sections.
Penetration assays. (i) Plaque assays.
HSV-1 or HS1 (a gC− virus) were diluted in PBS with or without 100 μg of heparin sodium salt (Sigma, St. Louis, Mo.) per ml and/or 50 μg of CTC-96 per ml. The diluted virus was then adsorbed to Vero or A549 cell monolayers for 1 h at 4°C on ice and shifted to 37°C for an additional hour. The cells were washed with PBS, citrate buffer (135 mM NaCl, 10 mM KCl, 40 mM citric acid [pH 3.0]) (39, 45), or 48% (wt/wt) polyethylene glycol (PEG) 8000 in PBS (82, 83). The wash buffer was immediately removed, and the cells were carefully washed twice with PBS. Then the infected monolayers were overlaid with methylcellulose overlay medium and incubated at 37°C for several days. Plaques were detected as described above.
(ii) Liquid β-galactosidase assays.
Three to four days after seeding, CHO cells were infected with wild-type HSV-1 or vBSΔ27 and washed as described above for the plaque penetration assay. After being washed, the cells were overlaid with the appropriate medium and incubated at 37°C for 30 h as described previously (67, 95). The infected cells were washed twice with PBS and lysed in 1× passive lysis buffer (Promega Corp., Madison, Wis.) containing 10 μg of soybean trypsin inhibitor per ml, 5 μg of leupeptin per ml, 1 mM phenylmethylsulfonyl fluoride, 0.1 mM l-1-chloro-3-(4-tosylamido)-7-amino-2-heptanone, and 0.1 mM l-1-chloro-3-(4-tosylamido)-4-phenyl-2-butanone. The protein concentration was measured using protein assay dye reagent (Bio-Rad Laboratories, Hercules, Calif.) as specified by the manufacturer. The β-galactosidase activity in the lysates was measured as described previously (84), and β-galactosidase activity was calculated using the following equation: [optical density at 420 nm/(milliliters of lysate) (time at 37°C)] × 1,000.
Cell-to-cell spread and syncytium formation.
Vero cell monolayers were seeded onto coverslips and infected at a MOI of 0.01 in DMEM supplemented with 1% BCS. At 8 h p.i., the medium was replaced with DMEM containing 1% BCS with or without 50 μg of CTC-96 per ml and/or a 1:100 dilution of pooled anti-HSV human sera. At 8 or 16 h p.i., the infected monolayers were washed with PBS, fixed, permeabilized, and examined as described above for αTIF. The primary antibody used was the rabbit polyclonal antibody CLU38 (anti-ICP27) (57), while the secondary antibody was goat anti-rabbit IgG conjugated to rhodamine or FITC (Kirkegaard & Perry Laboratories, Inc.). The slides were viewed with a Leitz Dialux microscope with optical systems for the selective visualization of rhodamine or FITC.
VZV immunohistochemistry.
Helf cell monolayers on two-chambered slides were infected with 20 μl of cell-associated VZV in DMEM containing 2% FBS with and without 50 μg of CTC-96 per ml. At 28 h p.i., the slides were washed, fixed, and permeabilized as for αTIF with the exception that the slides were washed two additional times with Tris-buffered saline (TBS) (25 mM Tris, 137 mM NaCl, 3 mM KCl) prior to permeabilization. The slides were then washed twice with TBS, blocked for 20 min in TBS containing 1% goat serum (Sigma), and incubated for 30 min in 200 μl of TBS containing 1% goat serum and a 1:200 dilution of rabbit polyclonal anti-ORF 29 (60). The slides were washed three times with TBS for 5 min each, incubated for 30 min in 200 μl of TBS containing 1% goat serum and a 1:200 dilution of goat anti-rabbit IgG conjugated to alkaline phosphatase (Kirkegaard & Perry Laboratories, Inc.), and then washed three additional times. The reaction was developed for 5 min using a commercial kit, alkaline phosphatase substrate kit III (Vector Laboratories, Inc., Burlingame, Calif.), as specified by the manufacturer and then washed several times with water. The slides were viewed with a Leitz Dialux microscope.
RESULTS
CTC-96 inhibits HSV-1 replication in tissue culture.
The antiviral activity of the CTC complexes against several herpesviruses has been described previously (3, 22, 24, 97). The majority of these studies have been in vivo protocols that addressed the efficacy of the CTC series of compounds against herpesviruses (3, 22, 24, 97). Comparison of several CTC complexes showed that CTC-96 was the most potent inhibitor of HSV-1 in tissue culture and in a rabbit eye model (3). However, the mechanism(s) of action of these drugs is unknown.
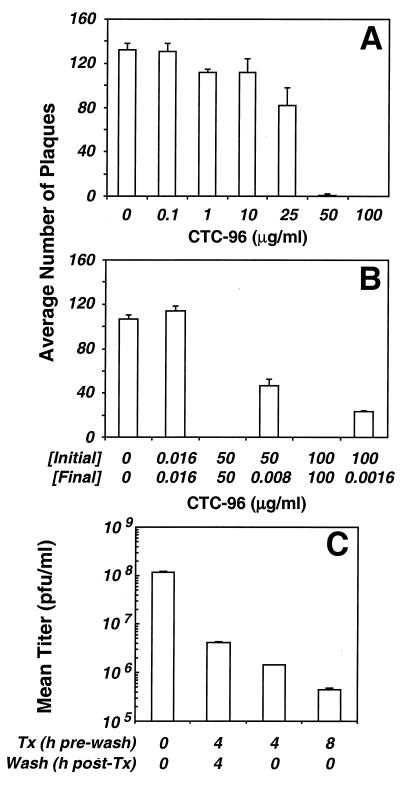
Plaque assays were performed to determine the MIC of CTC-96 for HSV-1. CTC-96 at 25 μg/ml prevented the formation of approximately 30% of HSV-1 plaques. By comparison, ≥50 μg of CTC-96 per ml completely inhibited plaque formation (Fig. 2A). This nonlinear inhibitory profile suggests that 25 μg/ml is not sufficient to saturate its target. Furthermore, CTC-96 must be present throughout the initial stages of infection since removal of drug by dilution before adsorption of the virus only partially inhibited the formation of plaques (Fig. 2B). It was unclear whether this partial blockage was the result of a lag in initiation of infection or if the drug affected an aspect of the virus and/or cellular machinery necessary for efficient production of HSV-1 plaques. However, prior incubation of Vero cell monolayers for 4 or 8 h with 50 μg of CTC-96 per ml resulted in a marked decrease in virus yield (Fig. 2C). This decrease in yield was partially reversed if the drug-treated cells were washed before infection (Fig. 2C). These results suggest that short-term treatment with CTC-96 does not irreversibly alter the infectivity of HSV-1 virions. Similarly, while cells exposed to drug for 4 h were severely incapacitated for their ability to support virus replication (≥99%), they produced four- to fivefold more virus following reversal of drug (Fig. 2C). We note that extended exposure (>2 to 3 days) to 50 μg of CTC-96 per ml results in cell death.
FIG. 2.
Effect of CTC-96 on HSV-1 plaque formation in tissue culture. (A) Vero cell monolayers were infected with HSV-1 in the presence of the indicated concentrations of CTC-96. After several days at 37°C, the infected monolayers were fixed and stained and the number of plaques was determined. Data represent the average number of plaques formed from four experiments. (B) HSV-1 was preincubated with the indicated concentrations of CTC-96 [Initial]. Immediately prior to adsorption, CTC-96 was diluted to the indicated final concentrations [Final]. Plaque assays were performed as described in panel A. Data represent the average number of plaques from two experiments. (C) Vero cell monolayers were preincubated with 50 μg of CTC-96 per ml for the indicated times (Tx). CTC-96 was removed, and fresh medium was added to the cells for the indicated times (Wash). The cells were infected with untreated HSV-1, and virus yields were determined 16 h p.i. A representative experiment performed in duplicate is shown.
CTC-96 has no effect on attachment of HSV-1 to Vero cell monolayers.
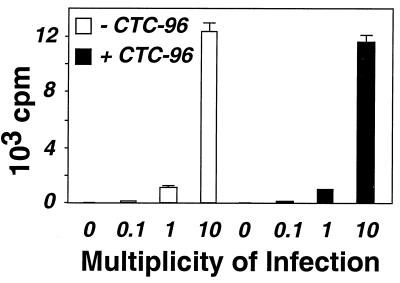
CTC-96 inhibits HSV-1 plaque formation (Fig. 2). However, it is not apparent by what mechanism(s) it achieves this inhibition. A prerequisite for HSV-1 infection is binding of the virion envelope glycoproteins to cell surface receptors (90–92; also see reference 77 and references therein). For instance, gC and gB bind to heparan sulfate (38, 39, 53, 86, 106) while glycoprotein D (gD) attaches to HveA, a herpesvirus entry mediator receptor (67). Suramin was recently shown to block attachment of HSV virions to their cellular receptors (1); therefore, it was possible that CTC-96 inhibited binding to the cell surface. Accordingly, we asked whether HSV-1 was able to bind to Vero cells in the presence of 50 μg of CTC-96 per ml. Partially purified 35S-labeled virions were adsorbed to Vero cell monolayers at 4°C, and the amount of radioactivity that remained cell associated after several washes was measured. The amount of virus bound to cells increased linearly with the MOI from 0.1 to 10 PFU per cell and was unaffected by CTC-96 (Fig. 3). Thus, we reasoned that CTC-96 must inhibit a postattachment phase of infection.
FIG. 3.
HSV-1 attachment in the presence of CTC-96. 35S-labeled HSV-1 was adsorbed to Vero cell monolayers at a MOI of 0.1, 1 or 10 on ice for 45 min in the presence (solid bars) or absence (empty bars) of 50 μg/ml of CTC-96. The infected cells were washed several times and the amount of bound virus was quantitated (see Materials and Methods). The data presented are the average cell-associated counts per minute (cpm) and were performed in triplicate.
Virus proteins do not accumulate when CTC-96 is present during infection.
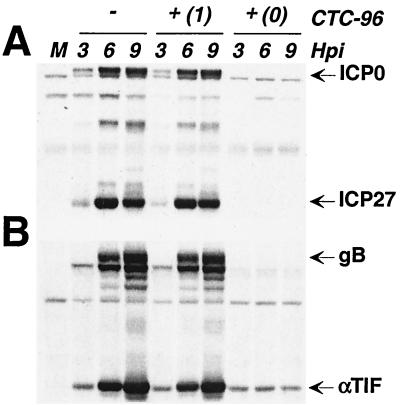
The expression of HSV-1 genes and their gene products occurs in a temporal fashion and is classified into three kinetic classes (43, 44). The production of proteins from all of these classes is required to produce infectious progeny (43, 44). To determine whether the inhibitory action of CTC-96 on plaque formation results from a delay in the temporal order of HSV-1 infection, we examined the accumulation of virus proteins in the presence of 50 μg of CTC-96 per ml. CTC-96 prevented the accumulation of gene products from all kinetic classes when present from the onset of infection (Fig. 4). The appearance of bands reactive with αTIF and gB antibodies, when CTC-96 was present from the initiation of infection, does not result from de novo synthesis of these β/γ proteins (Fig. 4B) (37, 42). Rather, these bands represent the proteins associated with the infecting virions (Fig. 4B) since both αTIF and gB are present in the virion (11, 61–63, 108). If, however, CTC-96 was added after the initiation of infection, there was little or no effect on the accumulation of the α gene products, ICP0 and ICP27 (Fig. 4A), or on the β/γ gene products, αTIF and gB (Fig. 4B). Thus, once the cascade of protein synthesis was initiated, CTC-96 had no significant effect on accumulation of virus proteins (Fig. 4). These results suggest that CTC-96 exerts its inhibitory effect(s) on the HSV-1 life cycle after attachment but at or before the synthesis of virus-specified proteins.
FIG. 4.
Accumulation of virus-specified proteins in the presence of CTC-96. Vero cell monolayers were either mock infected (M) or infected with HSV-1 at a MOI of 5 in the presence (+) or absence (−) of 50 μgl of CTC-96 per ml. CTC-96 was added either at the onset of infection (0) or at 1 h p.i. (1). Infected cell extracts were harvested at the indicated hour postinfection (Hpi). Western blot analysis was performed using the rabbit polyclonal antibodies CLU7 (anti-ICP0) and CLU38 (anti-ICP27) (A) or R69 (anti-gB) and anti-VP16 (anti-αTIF) (B).
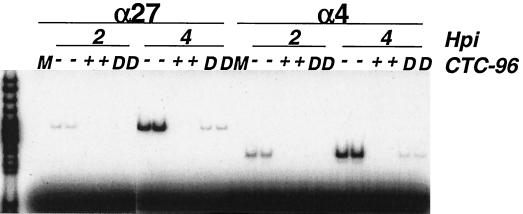
α mRNAs do not accumulate if CTC-96 is present throughout infection.
Initiation of HSV-1 immediate-early gene expression does not require de novo protein synthesis (30, 94, 100). Transcription of the α genes is initiated by the virion-associated protein αTIF in concert with the cellular transcription apparatus (10, 36, 71, 75). The protein products of the α4 and α27 genes are essential for the subsequent transcription of β and γ genes (21, 23, 66, 76, 79). Therefore, using RT-PCR analysis, we examined whether the α4 and α27 mRNAs accumulated in the presence of CTC-96. Neither of these mRNAs was detected when CTC-96 was present from the onset of infection (Fig. 5). However, after a short lag period, α4 and α27 mRNAs began to accumulate if CTC-96 was diluted to 0.83 μg/ml before adsorption (Fig. 5). This finding supports the data in Fig. 2 suggesting that the inhibitory effect(s) of CTC-96 is reversible. The lack of accumulation of α mRNAs in the presence of drug does not distinguish between whether CTC-96 acts at the level of α mRNA transcription or at a stage before transcription of α genes. However, the results of this and the preceding experiment suggest that the site of action of CTC-96 must be before virus DNA is transcribed.
FIG. 5.
Effect of CTC-96 on the accumulation of α RNAs in HSV-1-infected cells. Vero cell monolayers were either mock infected (M) or infected with HSV-1 at a MOI of 5 in the presence (+) or absence (−) of 50 μg/ml of CTC-96. HSV-1 pretreated with 50 μg of CTC-96 per ml was diluted (D) to a final CTC-96 concentration of 0.83 μg/ml before adsorption. Total infected cell RNA was prepared at 2 and 4 h p.i. The α4 and α27 mRNAs were amplified by RT-PCR in the presence of [α-32P]dCTP under linear amplification conditions (see Materials and Methods). The amplimers were electrophoretically separated through polyacrylamide gels and visualized by autoradiography. The leftmost lane represents relative size markers. Experiments for each condition were performed in duplicate.
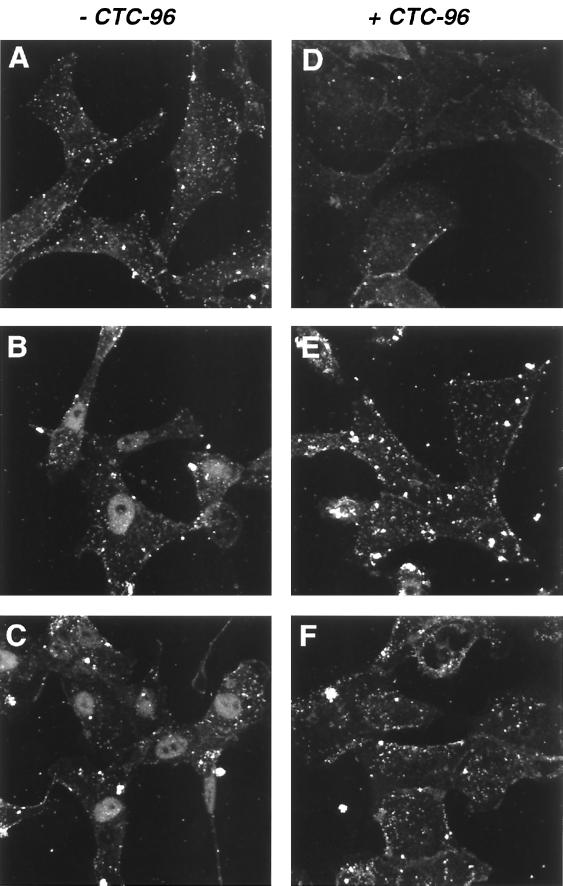
αTIF is absent from the nuclei of cells infected in the presence of CTC-96.
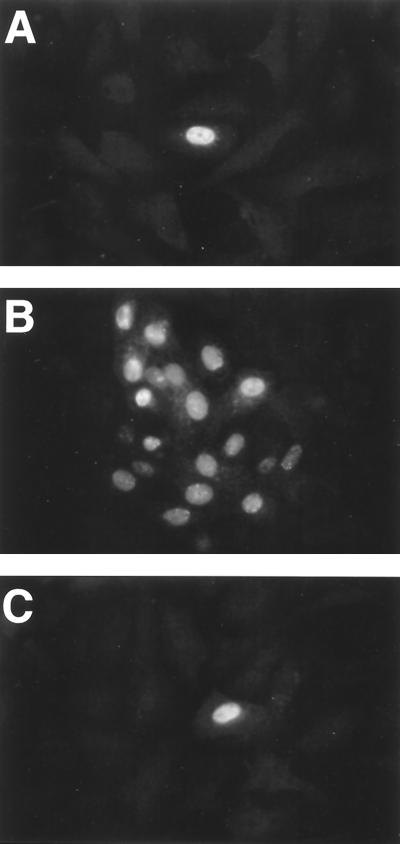
After entry and uncoating, the HSV capsid moves through the cytoplasm to the nuclear pores (88). αTIF, a tegument protein, also translocates to the nucleus after associating with host cell factor, a cellular protein (51). In addition, αTIF appears to remain associated with the virus capsid in the cytoplasm (107). We therefore determined whether αTIF is present in the nuclei of cells infected in the presence of CTC-96 since this protein is required for transcription of immediate-early genes such as α27 and α4. Indirect immunofluorescence analysis of untreated cells showed that αTIF could be detected in the nucleus by 30 min postinfection (Fig. 6B). Nuclear accumulation was not the result of de novo synthesis, since synthesis of αTIF was not detected until 2 h p.i. under these same conditions (data not shown). When CTC-96 was present, αTIF was not detected in the nucleus at 1 h p.i. (Fig. 6F). In addition, we found that input HSV-1 genomic DNA does not accumulate in the nuclei of cells infected with HSV-1 in the presence of CTC-96 (data not shown). These results lead us to postulate that the introduction of capsid-associated proteins and DNA does not occur in the presence of CTC-96. Thus, while virus can bind in the presence of CTC-96, the drug blocks a step between binding and introduction of the capsid and therefore infection.
FIG. 6.
Accumulation of αTIF in the nucleus of cells infected with HSV-1. HSV-1 was adsorbed to Vero cell monolayers at a MOI of 100 for 45 min at 4°C in the presence (D to F) or absence (A to C) of 50 μg of CTC-96 per ml. Infected monolayers were then warmed to 37°C for 5 min (A and D), 30 min (B and E), and 60 min (C and F), after which they were fixed and permeabilized. The location of αTIF was ascertained using a rabbit polyclonal antibody to αTIF and a goat anti-rabbit IgG antibody conjugated to FITC. The immunofluorescence signal was visualized by confocal microscopy. Each image is a composite of 1-μm serial sections.
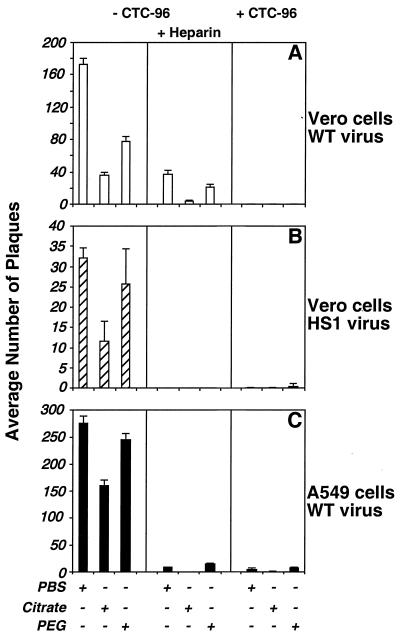
CTC-96 inhibits penetration of HSV-1 in a variety of cell types independent of gC and the type of entry receptor.
HSV-1 entry is composed of several distinct steps that can be differentiated by their susceptibility to specific washes. The initial stage of attachment involves binding of the viral glycoproteins, gB and/or gC, to cell surface heparan sulfate proteoglycans (38, 39, 86, 106). After binding, the virus becomes resistant to removal from the cell surface by PBS (39–41, 45, 64). However, at this point bound virus is sensitive to elution by heparin and inactivation by low-pH citrate buffer (39–41, 45, 64, 73). After the initial binding event, virus attachment becomes more stable as gD and possibly other virus glycoproteins bind to their cell surface receptor(s). gD binds to several different cellular receptors (HveA to HveD) (12, 35, 50, 67, 99, 102). Binding of gD to its entry receptor appears to play two roles, the first of which is in securing the attachment event and the second is in facilitating or initiating penetration of the virus into the cell. The initial binding of gD to its receptor renders the virus-cell interaction resistant to elution by heparin but sensitive to citrate (39–41, 45, 64, 73). Once the fusion phase of entry is initiated, the bound virions are no longer sensitive to inactivation by citrate buffer (39–41, 45, 64, 73). Penetration assays (39–41, 45, 64) can then be implemented to address the stage affected by CTC-96 by assaying the sensitivity of each phase of entry to specific buffers.
A plaque assay was used to assess whether HSV-1 was able to penetrate cells in the presence of CTC-96 (Fig. 7). To assess whether CTC-96 inhibits entry at a point downstream of the initial gC binding event, a penetration assay using HS1, a gC− virus, was also performed (73). The absence of gC had no effect on the ability of CTC-96 to inhibit penetration, suggesting that the drug affects an event downstream of gC attachment (Fig. 7B). This result is consistent with the data in Fig. 3 and supports our contention that the block occurs after attachment. It seemed plausible that CTC-96 was having a specific effect on a gD binding or fusion event. Therefore, we assessed whether there was a cell type-dependent difference in the ability of CTC-96 to inhibit virus entry (Fig. 7A and C). CTC-96 inhibited the penetration of wild-type virus in the presence (data not shown) and absence (Fig. 7A and C, right panels) of heparin in the nonhuman primate Vero cell line (Fig. 7A) and in the human cell line A549 (Fig. 7C). Thus, both CTC-96 and heparin, a competitive inhibitor of the first step in virus attachment, block infection.
FIG. 7.
Effect of CTC-96 on penetration of HSV-1 into monkey and human cells. Vero (A and B) or A549 (C) monolayers were infected with wild-type (WT) (A and C) or gC− (HS1) (B) HSV-1 on ice for 1 h and then shifted to 37°C for an additional 1 h in the presence (+) or absence (−) of 50 μg of CTC-96 per ml and/or 100 μg of heparin per ml. Infected cells were washed with either PBS, citrate buffer, or PEG 8000 as indicated, and infection was monitored by a plaque assay. Although not shown, no plaques were formed on monolayers infected in the presence of CTC-96 and heparin with either type of cells or viruses. Data denote the average number of plaques from one representative experiment performed in triplicate.
As described previously, PEG was able to induce fusion of the virus envelope with the plasma membrane (Fig. 7, left and middle panels) (31, 82, 83). However, CTC-96 blocked PEG-mediated fusion (Fig. 7, right panels). While this was surprising, we subsequently demonstrated that CTC-96 also inhibited PEG-induced cell fusion (data not shown). These results suggest that CTC-96 nonspecifically inhibits membrane fusion.
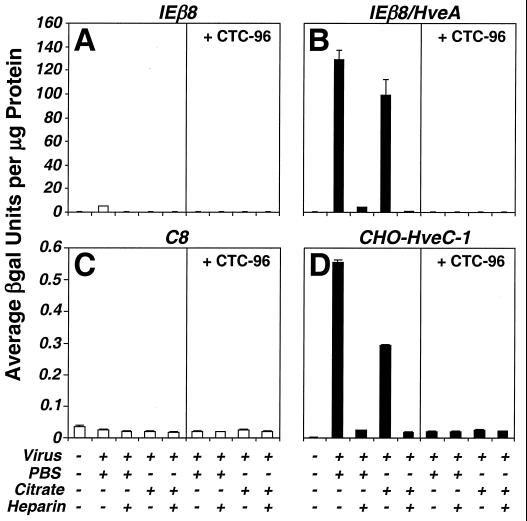
The penetration data do not differentiate between whether the block results from a nonspecific effect on membranes or whether it specifically affects a gD-dependent event. Therefore, to differentiate between these possibilities, penetration assays in CHO cells expressing either HveA or HveC were performed (Fig. 8). CHO cells are inherently resistant to infection by HSV because they lack the appropriate entry receptors (35, 86). Since both HveA and HveC allow entry by most strains of HSV-1 (35, 50, 67, 99, 102), we asked if CTC-96 inhibited entry in CHO cells expressing HveA or HveC. Penetration was assayed by measuring the level of β-galactosidase activity in CHO cells transformed with an αTIF-inducible lacZ reporter construct (Fig. 8A and B) or by infection with vBSΔ27, a virus with an αTIF-responsive lacZ reporter that does not replicate in noncomplementing cells (Fig. 8C and D). CTC-96 and heparin inhibited HveA-mediated (Fig. 8B) and HveC-mediated (Fig. 8D) entry compared to the no-drug controls (Fig. 8B and D, left panels, respectively) and the untreated CHO cell lines (Fig. 8A and C), which do not express either receptor. Thus, CTC-96 prevents infection of cells and the block to HSV-1 entry is independent of the type of entry receptor used.
FIG. 8.
Assay for virus penetration into CHO cells expressing HveA or HveC. Penetration assays using CHO cells expressing HveA or HveC were performed as in the experiment in Fig. 7, with the exception that penetration was assayed at 30 h p.i. by measuring β-galactosidase (βgal) activity. (A and B) IEβ8 (A) and IEβ8/HveA (B) cells, containing an αTIF-inducible lacZ construct, were infected with wild-type HSV-1 virus. (C and D) C8 (C) and CHO-HveC-1 (D) cells were infected with vBSΔ27. Data are presented as the average amount of β-galactosidase activity per microgram of protein of infected cell lysate. Each data set was obtained in triplicate.
CTC-96 blocks cell-to-cell spread of wild-type and syn− HSV-1.
CTC-96 prevents virus macromolecular synthesis (Fig. 4 and 5), the appearance of αTIF (Fig. 6) and the virus genome in the nuclei of infected cells, and the activation of reporter constructs in CHO cells genetically engineered to be infected by HSV-1 (Fig. 8). The virus glycoproteins (gB, gD, and gH-gL) and cellular receptors (HveA, HveB, and HveC), that are important for fusion of the HSV-1 envelope with the cell membrane are also involved in virus-induced cell fusion and cell-to-cell spread of virus (9, 19, 68, 69, 95). Therefore, we asked whether CTC-96 was able to inhibit cell-to-cell spread of HSV-1 in tissue culture. Vero cell monolayers were infected at a low MOI in the absence of drug. At 8 h p.i., pooled anti-HSV human sera and/or CTC-96 was added to the infected monolayers. In the presence and absence of neutralizing antibody, HSV-1 was able to spread to adjacent cells, as demonstrated by the spread of the immunofluorescence signal from single cells at 8 h p.i. to surrounding cells at 16 h p.i. (Fig. 9, compare panels A and B). However, after addition of CTC-96 at 8 h p.i., we were unable to demonstrate the development of multicelled foci at 16 h p.i. (Fig. 9C). The ability of CTC-96 to inhibit the spread of wild-type virus was quantified (Table 1). These results demonstrate that CTC-96 added at 8 h p.i. inhibited the spread of virus to adjacent cells. Similar results were obtained with human A549 cells (data not shown).
FIG. 9.
Cell-to-cell spread of HSV-1 in the presence of CTC-96. Vero cell monolayers were infected at a MOI of 0.01. At 8 h p.i., the medium was replaced with medium supplemented with a 1:100 dilution of pooled human anti-HSV sera with (C) or without (B) 50 μg of CTC-96 per ml. Infected monolayers were fixed at 8 h p.i. (A) or 16 h p.i. (B and C) and stained for ICP27 with a rabbit polyclonal antibody, CLU38, and goat anti-rabbit IgG conjugated to rhodamine.
TABLE 1.
The spread of wild-type and syncytium-forming viruses is inhibited by CTC-96
| Virus | Time (h) p.i. | HSV antibody | CTC-96 (50 μg/ml) | % of infected cells with HSV-1-positive nuclei
|
|
|---|---|---|---|---|---|
| Single cell | Plaquea | ||||
| Wild type | 8 | − | − | 91 | 9 |
| 16 | − | − | 12 | 88 | |
| 16 | + | − | 13 | 87 | |
| 16 | − | + | 84 | 16 | |
| 16 | + | + | 84 | 16 | |
| vJSsyn− | 8 | − | − | 76 | 24 |
| 16 | − | − | 44 | 56 | |
| 16 | + | − | 48 | 52 | |
| 16 | − | + | 92 | 8 | |
| 16 | + | + | 86 | 14 | |
A plaque is defined as a cluster of cells containing two or more HSV-1-positive nuclei as assayed by indirect immunofluorescence of ICP27.
While cell-to-cell spread and syncytium formation may share some essential factors, previous studies suggest that they are distinct events (12, 35, 77, 90). To determine if CTC-96 inhibits the development of multinucleated foci (syncytia) by syn− viruses, the drug was added at 8 h p.i. and the formation of syncytia was monitored. CTC-96 inhibited the formation of syncytia in Vero cells infected with vJSsyn− (Table 1) and MP (data not shown). These data also demonstrate that the block by CTC-96 occurs in a gK-independent manner as vJSsyn− is defective in gK, and not gB, as determined by its sensitivity to mellitin (4) and cyclosporin A (references 65 and 98 and data not shown). Moreover, when virions labeled in their membranes with the lipophilic fluorescent dye octadecyl rhodamine B chloride are bound to cells in the presence of drug, they fail to dequench (data not shown), supporting our contention that CTC-96 inhibits membrane fusion events. Therefore, CTC-96 inhibits all of the major fusion events in the HSV-1 life cycle, suggesting that it is a nonspecific inhibitor of fusion.
CTC-96 nonspecifically inhibits infection by enveloped viruses.
It has been suggested that CTC compounds inhibit infection by other herpesviruses such as VZV, CMV and EBV (22, 24, 97). We confirmed that plaque formation by VZV was inhibited by 50 μg of CTC-96 per ml (Table 2). To ascertain if this inhibition was specific for the herpesvirus family, we asked if 50 μg of CTC-96 per ml inhibited plaque formation by a rhabdovirus, VSV. Table 2 demonstrates that CTC-96 inhibited plaque formation by VSV as efficiently as it inhibited plaque formation by HSV-1 and VZV. Inhibition of VSV plaque formation did not result from a block in endocytosis, since CTC-96 does not inhibit the uptake of Lysotracker, a fluorescent endocytic marker (data not shown). Thus, CTC-96 does not inhibit VSV infection by interfering with endocytosis. These results suggest that CTC-96 inhibits infection by HSV-1, VZV, and VSV, perhaps by targeting a common cellular mechanism that is required for entry by these enveloped viruses.
TABLE 2.
CTC-96 is a broad-spectrum antiviral drug
Data are shown as percent inhibition compared to the no-drug control.
Data were obtained by plaque assays.
Virus infection was monitored by immunohistochemical analysis using antibody to ORF 29.
Approximately 2% of the drug-treated infected monolayer appeared positive because cell-associated VZV containing cellular debris was used to initiate the infection.
DISCUSSION
The way in which CTC-96 inhibits HSV-1 infection in tissue culture was studied using assays that probe virus processes that are essential for productive infection. Consistent with a previous report (3), we found that concentrations of CTC-96 of ≥50 μg/ml completely inhibited plaque formation (Fig. 2A and B). Prior incubation of either HSV-1 or cell monolayers with CTC-96 reduced the infectivity of HSV-1 (Fig. 2B) and the ability of cell monolayers to support virus growth (Fig. 2C) in a partially reversible manner. CTC-96 did not affect adsorption of HSV-1 to Vero cell monolayers (Fig. 3). However, because virus does not penetrate cells infected in the presence of drug, no de novo-synthesized virus-specific proteins (Fig. 4), RNA (Fig. 5), or DNA (data not shown) is detected under these conditions. Furthermore, in the presence of CTC-96, virion-associated αTIF (Fig. 6) and HSV-1 DNA (data not shown) also do not accumulate in the nucleus.
Penetration assays demonstrated that CTC-96 was blocking virus entry (Fig. 7 and 8). HSV-1 was unable to penetrate either Vero or A549 cells as determined by plaque formation assays (Fig. 7A and C). This effect was independent of gC, since HS1, a gC− virus, was also inhibited by CTC-96 (Fig. 7B). These observations suggested that an event downstream of attachment was the target for the drug. We therefore assayed the ability of HSV-1 to enter CHO cells expressing either HveA or HveC to determine if this effect was specific for one or more essential gD interactions (Fig. 8). HSV-1 was unable to penetrate CHO cells expressing either entry receptor in the presence of CTC-96 (Fig. 8B and D). Therefore, unlike the antiviral drugs currently approved for treatment of HSV-1, which act on virus-specified enzymes to inhibit replication, CTC-96 prevents the entry of virus into cells.
While our data reveal that CTC-96 inhibits membrane fusion events, they do not provide insight into the exact mechanism of inhibition. CTC-96 may alter the structure of proteins required for membrane fusion by preventing the conformational change of virus glycoproteins and/or cellular receptors that are believed to be important for fusion initiation and completion. CTC compounds selectively unfold proteins in vitro (7). Accordingly, if the cell and virus fusogenic proteins require precise conformations to function, CTC-96 may inhibit their function by preventing protein-protein and/or protein-membrane interactions required for membrane fusion.
Several virus glycoproteins that play a role in the fusion of the virus envelope with the plasma membrane are also involved in cell-to-cell spread and cell fusion. Therefore, to gain further insight into the mode of action of CTC-96, we examined whether virus was able to spread to adjacent cells and/or form syncytia. Addition of CTC-96 to cells previously infected with HSV-1 rendered them unable to form multicelled foci (Fig. 9 and Table 1). Thus, CTC-96 inhibits cell-to-cell spread regardless of whether the virus infects adjacent cells by direct contact with the plasma membranes or via the interstitial space. Furthermore, a syncytium-forming virus, vJSsyn−, was unable to form syncytia in the presence of CTC-96 (Table 1). These data do not exclude a specific effect against all the virus and cellular proteins involved in entry, cell-to-cell spread, and virus-induced cell fusion. However, the inhibition of PEG-induced virus-cell (Fig. 7) and cell-cell (data not shown) fusion and the inhibition of infection by other enveloped viruses (Table 2) suggest that CTC-96 exerts its effects nonspecifically. Therefore, the inhibition of the fusion processes involved in HSV-1 infection by CTC-96 suggests that there may be a general mechanism of fusion shared by these processes.
The CTC compounds were shown to irreversibly bind to and specifically inhibit Sp1, a DNA binding Zn finger protein in vitro (59). Based on this observation, it was postulated that the antiviral activity of the CTC compounds could inhibit human immunodeficiency virus type 1 (HIV-1) by binding to Zn finger containing nucleocapsid proteins as well as to Sp1, which may be important for HIV-1 virus transcription (59). Furthermore, the cytotoxic effects of CTC-96 may result from in vivo inhibition of cellular and viral Zn finger-containing proteins. The HSV-1 immediate-early gene promoters contain numerous Sp1 sites (49) that might provide secondary targets for CTC-96 if it enters cells. However, our results suggest that these are not alternative targets of CTC-96. It seems likely that CTC-96 does not enter cells efficiently because of the temporal requirement for addition of CTC-96 to inhibit virus replication (Fig. 2 and 5). This is further supported by our observations that virus-specified protein synthesis continues unabated when drug is added at 1 h p.i. (Fig. 4) and that there is only a negligible decrease in virus titers when CTC-96 is added at 16 h p.i. and virus is harvested 4 h later (data not shown). Hence, CTC-96 does not appear to act intracellularly. Therefore, based on our demonstration that CTC-96 inhibits virus-mediated cell fusion, the long-term cytotoxic effects of CTC-96 may result from inhibition of global and/or local membrane dynamics, a vital cellular process. Despite its toxic effect on cells in culture, 50 μg of CTC-96 per ml does not appear to be toxic in animal models (3, 22, 24, 97).
We have been unable to isolate CTC-96-resistant viruses. This suggests that either CTC-96 targets one or more essential cellular or virus components or it affects a global process such as membrane dynamics. This latter theory is supported by the demonstration that CTC-96 affects both virus and cellular targets (Fig. 2). Furthermore, this effect may not be specific, since the drug also inhibits PEG-induced cell-to-virus (Fig. 7) and cell-to-cell (data not shown) fusion. If membrane fluidity was altered by CTC-96, it would need to be partially reversible, since infection, which is partially inhibited by preincubation of cells or virus with CTC-96, is restored on dilution of the drug. This could reflect de novo synthesis resulting in turnover of the target. Thus, inhibition of virus entry by CTC-96 and its effect on cell viability have implications for a global inhibitory mechanism of membrane coalescence.
The appearance of acyclovir-resistant herpesviruses in patients significantly intensified the effort to develop drugs that inhibit another aspect of the virus life cycle. One attractive target for antiherpetic drugs is the initial stage of infection (i.e., entry and uncoating). Several drugs exist that inhibit HSV infection by blocking attachment or fusion of the virus envelope with the plasma membrane. Heparin, a polysulfonate complex, can block the attachment via gC and or gB through competitive binding for heparan sulfate proteoglycans (64, 86, 106). Sumarin, a derivative of urea, is also able to block HSV attachment but, unlike heparin, is also able to inhibit cell-to-cell spread (1). Unlike heparin and sumarin, n-docosanol, a saturated primary alcohol, does not inhibit virus binding; rather, it inhibits fusion of the virus envelope with the plasma membrane of the cell (74). Therefore, it has a broad spectrum and is effective against other enveloped viruses including influenza A virus (74).
In view of a previous report that CTC-96 inhibits infection by other herpesviruses (97), it is likely that the mechanism of inhibition is similar for these viruses. Consistent with previous reports (97), we observed inhibition of VZV and VSV plaque formation by CTC-96 (Table 2). Inhibition of VZV by CTC-96 suggests that the mechanism of action is not specific for gD, since VZV is the only alphaherpesvirus without a known gD homologue (81). Our hypothesis that CTC-96 nonspecifically targets an essential fusion event is further supported by its ability to inhibit plaque formation by VSV. Despite differences in the fusogenic apparatus at the atomic level, it has been proposed that several enveloped viruses share an analogous process of membrane fusion (33, 46, 52, 87, 101). Analysis of the efficacy of CTC-96 against other enveloped viruses could reveal a common mechanism(s) of membrane fusion between viruses and cells.
ACKNOWLEDGMENTS
We thank Patricia Spear and Gary Cohen for helpful discussions; O. Lungu, C. Waldburger, B. McDermott, and T. Swayne for technical assistance; and Xiaoshan Wen, Avery Mathhews, and Tarik Soliman for performing some of the experiments described here.
This study was supported in part by funds from Columbia Innovation Enterprises and REDOX Pharmaceutical Corporation and by grants RR10506 (Shared Instrumentation Grant), CA13696 (Herbert Irving Cancer Center), and AI-33952 (to S.J.S.) from the Public Health Service.
REFERENCES
- 1.Aguilar J S, Rice M, Wagner E K. The polysulfonated compound suramin blocks adsorption and lateral diffusion of herpes simplex virus type-1 in Vero cells. Virology. 1999;258:141–151. doi: 10.1006/viro.1999.9723. [DOI] [PubMed] [Google Scholar]
- 2.Alrabiah F A, Sacks S L. New antiherpesvirus agents: their targets and therapeutic potential. Drugs. 1996;52:17–32. doi: 10.2165/00003495-199652010-00002. [DOI] [PubMed] [Google Scholar]
- 3.Asbell P A, Epstein S P, Wallace J A, Epstein D, Stewart C C, Burger R M. Efficacy of cobalt chelates in the rabbit eye model for epithelial herpetic keratitis. Cornea. 1998;17:550–557. doi: 10.1097/00003226-199809000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Baghian A, Kousoulas K G. Role of the Na+, K+ pump in herpes simplex virus type 1-induced cell fusion: mellitin causes specific reversion of syncytial mutants with the Syn1 mutation to Syn+ (wild-type) phenotype. Virology. 1993;196:548–556. doi: 10.1006/viro.1993.1510. [DOI] [PubMed] [Google Scholar]
- 5.Balfour H H. Antiviral drugs. N Engl J Med. 1999;340:1255–1268. doi: 10.1056/NEJM199904223401608. [DOI] [PubMed] [Google Scholar]
- 6.Banfield B W, Leduc Y, Esford L, Schubert K, Tufaro F. Sequential isolation of proteoglycan synthesis mutants by using herpes simplex virus as a selective agent: evidence for a proteoglycan-independent virus entry pathway. J Virol. 1995;69:3290–3298. doi: 10.1128/jvi.69.6.3290-3298.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blum O, Haiek A, Cwikel D, Dori Z, Meade T, Gray H B. Isolation of a myoglobin molten globule by selective cobalt(III)-induced unfolding. Proc Natl Acad Sci USA. 1998;95:6659–6662. doi: 10.1073/pnas.95.12.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown S, Ritchie D A, Subak-Sharpe J H. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J Gen Virol. 1973;18:329–346. doi: 10.1099/0022-1317-18-3-329. [DOI] [PubMed] [Google Scholar]
- 9.Cai W, Gu B, Person S. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J Virol. 1988;62:2596–2604. doi: 10.1128/jvi.62.8.2596-2604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell M E M, Palfreyman J W, Preston C M. Identification of herpes simplex virus DNA sequences which encode a trans-acting polypeptide responsible for stimulation of immediate early transcription. J Mol Biol. 1984;180:1–19. doi: 10.1016/0022-2836(84)90427-3. [DOI] [PubMed] [Google Scholar]
- 11.Claesson-Welsh L, Spear P G. Oligomerization of herpes simplex virus glycoprotein B. J Virol. 1986;60:803–806. doi: 10.1128/jvi.60.2.803-806.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attribute of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J Virol. 1998;72:9992–10002. doi: 10.1128/jvi.72.12.9992-10002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coen D M, Schaffer P A. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc Natl Acad Sci USA. 1980;77:2265–2269. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins P, Larder B A, Oliver N M, Kemp S, Smith I W, Darby G. Characterization of a DNA polymerase mutant of herpes simplex virus from a severely immunocompromised patient receiving acyclovir. J Gen Virol. 1989;70:375–382. doi: 10.1099/0022-1317-70-2-375. [DOI] [PubMed] [Google Scholar]
- 15.Corey L, Spear P. Infections with herpes simplex viruses. N Engl J Med. 1986;314:686–691. doi: 10.1056/NEJM198603133141105. [DOI] [PubMed] [Google Scholar]
- 16.Corey L, Spear P. Infections with herpes simplex viruses. N Engl J Med. 1986;314:749–757. doi: 10.1056/NEJM198603203141205. [DOI] [PubMed] [Google Scholar]
- 17.Crumpacker C S. Molecular targets of antiviral therapy. N Engl J Med. 1989;321:163–172. doi: 10.1056/NEJM198907203210306. [DOI] [PubMed] [Google Scholar]
- 18.Darby G, Field H J, Salisbury S A. Altered substrate specificity of herpes simplex virus thymidine kinase confers acyclovir-resistance. Nature. 1981;289:81–83. doi: 10.1038/289081a0. [DOI] [PubMed] [Google Scholar]
- 19.Davis-Poynter N, Bell S, Minson T, Browne H. Analysis of the contributions of herpes simplex virus type 1 membrane proteins to the induction of cell-cell fusion. J Virol. 1994;68:7586–7590. doi: 10.1128/jvi.68.11.7586-7590.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Schryver A, Meheus A. Epidemiology of sexually transmitted diseases: the global picture. Bull W H O. 1990;68:639–654. [PMC free article] [PubMed] [Google Scholar]
- 21.DeLuca N A, McCarthy A M, Schaffer P A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate early regulatory protein ICP4. J Virol. 1985;56:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devlin H, Geary P, Pavan-Langston D, Dori Z, Dunkel E C. Efficacy of CTC topical therapy during HSV-1-induced epithelial and stromal keratitis in the rabbit. Investig Ophthalmol Visual Sci. 1993;34:1348. [Google Scholar]
- 23.Dixon R F, Schaffer P A. Fine structure mapping and functional analysis of temperature-sensitive mutants in the gene encoding the herpes simplex virus type 1 immediate early protein VP175. J Virol. 1980;36:189–203. doi: 10.1128/jvi.36.1.189-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunkel E C, Geary P A, Brooks J, Pavan-Langston D. CTC 23 efficacy in vitro and on HSV-1-induced ocular epithelial and stromal disease in the rabbit. Antiviral Res Suppl. 1991;1:135. [Google Scholar]
- 25.Dyer A P, Banfield B W, Martindale D, Spannier D-M, Tufaro F. Dextran sulfate can act as an artificial receptor to mediate a type-specific herpes simplex virus infection via glycoprotein B. J Virol. 1997;71:191–198. doi: 10.1128/jvi.71.1.191-198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elion G B, Furman P A, Fyfe J A, de Mirnada P, Beauchamp L, Schaeffer H J. Selectivity of action of an antiherpetic agent 9-(2-hydroxyethoxymethyl)guanine. Proc Natl Acad Sci USA. 1977;74:5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis M N, Keller P M, Fyfe J A, Martin J L, Rooney J F, Straus S E, Lehrman S N, Barry D W. Clinical isolates of herpes simplex virus type 2 that induce a thymidine kinase with altered substrate specificity. Antimicrob Agents Chemother. 1987;31:1117–1125. doi: 10.1128/aac.31.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erice A, Gil-Roda C, Perez J-L, Balfour H H, Jr, Sannerud K J, Hanson M N, Boivin G, Chou S. Antiviral susceptibilities and analysis of UL97 and DNA polymerase sequences of clinical cytomegalovirus isolates from immunocompromised patients. J Infect Dis. 1997;17S:1087–1092. doi: 10.1086/516446. [DOI] [PubMed] [Google Scholar]
- 29.Erlich K S, Mills L, Chatis P, Mertz G J, Busch D F, Follansbee S E, Grant R M, Crumpacker C S. Acyclovir-resistant herpes simplex virus infections in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1989;320:293–296. doi: 10.1056/NEJM198902023200506. [DOI] [PubMed] [Google Scholar]
- 30.Frenkel N, Silverstein S, Cassai E, Roizman B. RNA synthesis in cells productively infected with herpes simplex virus. VIII. Control of transcription and of transcript abundancies of unique and common sequences of herpes simplex virus 1 and 2. J Virol. 1973;11:886–892. doi: 10.1128/jvi.11.6.886-892.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuller A O, Spear P G. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc Natl Acad Sci USA. 1987;84:5454–5458. doi: 10.1073/pnas.84.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fyfe J A, Keller P M, Furman P A, Miller R L, Elion G B. Thymidine kinase from herpes simplex virus phosphorylates the antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978;253:8721–8727. [PubMed] [Google Scholar]
- 33.Gaudin Y, Tuffereau C, Durrer P, Brunner J, Flamand A, Ruigrok R. Rabies virus-induced membrane fusion. Mol Membr Biol. 1999;16:21–31. doi: 10.1080/096876899294724. [DOI] [PubMed] [Google Scholar]
- 34.Geraghty R J, Jogger C R, Spear P G. Cellular expression of alphaherpesvirus gD interferes with entry of homologous and heterologous alphaherpesviruses by blocking access to a shared gD receptor. Virology. 2000;268:147–158. doi: 10.1006/viro.1999.0157. [DOI] [PubMed] [Google Scholar]
- 35.Geraghty R J, Krummenacher C, Cohen G H, Eisenberg R J, Spear P G. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 36.Gerster T, Roeder R G. A herpesvirus trans-activating protein interacts with transcription factor OTF-1 and other cellular proteins. Proc Natl Acad Sci USA. 1988;85:6347–6351. doi: 10.1073/pnas.85.17.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall L M, Draper K G, Frink R J, Costa R H, Wagner E K. Herpes simplex virus mRNA species mapping in EcoRI F fragment. J Virol. 1982;43:594–609. doi: 10.1128/jvi.43.2.594-607.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herold B C, Visalli R J, Susmarski N, Brandt C R, Spear P G. Glycoprotein C-independent binding of herpes simplex virus to cells requires cell surface heparan sulphate and glycoprotein B. J Gen Virol. 1994;75:1211–1222. doi: 10.1099/0022-1317-75-6-1211. [DOI] [PubMed] [Google Scholar]
- 39.Herold B C, WuDunn D, Soltys N, Spear P G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Highlander S L, Cai W H, Person S, Levine M, Glorioso J C. Monoclonal antibodies define a domain on herpes simplex virus glycoprotein B involved in virus penetration. J Virol. 1988;62:1881–1888. doi: 10.1128/jvi.62.6.1881-1888.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Highlander S L, Sutherland S L, Gage P J, Johnson D C, Levine M, Glorioso J C. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J Virol. 1987;61:3356–3364. doi: 10.1128/jvi.61.11.3356-3364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holland L E, Sandri-Goldin R M, Goldin A L, Glorioso J C, Levine M. Transcriptional and genetic analyses of the herpes simplex virus type 1 genome: coordinates 0.29 to 0.45. J Virol. 1984;49:947–959. doi: 10.1128/jvi.49.3.947-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci USA. 1975;72:1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang A S, Wagner R R. Penetration of herpes simplex virus into human epidermoid cells. Proc Soc Exp Biol Med. 1964;116:863–869. doi: 10.3181/00379727-116-29392. [DOI] [PubMed] [Google Scholar]
- 46.Hughson F M. Enveloped viruses: a common mode of membrane fusion? Curr Biol. 1997;7:R565–R569. doi: 10.1016/s0960-9822(06)00283-1. [DOI] [PubMed] [Google Scholar]
- 47.Hwang C B C, Ruffner K L, Coen D M. A point mutation within a distinct conserved region of the herpes simplex virus DNA polymerase gene confers drug resistance. J Virol. 1992;66:1774–1776. doi: 10.1128/jvi.66.3.1774-1776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hyndiuk R A, Glasser D B. Herpes simplex keratitis. In: Tabbarra K, Hyndiuk R A, editors. Infections of the eye. Diagnosis and management. Boston, Mass: Little, Brown, & Co.; 1986. pp. 343–368. [Google Scholar]
- 49.Jones K A, Tjian R. Sp1 binds to promoter sequences and activates herpes simplex virus “immediate-early” gene transcription in vitro. Nature. 1985;317:179–182. doi: 10.1038/317179a0. [DOI] [PubMed] [Google Scholar]
- 50.Krummenacher C, Nicola A V, Whitbeck J C, Lou H, Hou W, Lambris J D, Geraghty R J, Spear P G, Cohen G H, Eisenberg R J. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein or herpes virus entry mediator, two structurally unrelated mediators of virus entry. J Virol. 1998;72:7064–7074. doi: 10.1128/jvi.72.9.7064-7074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.La Boissiere S, Hughes T, O'Hare P. HCF-dependent nuclear import of VP16. EMBO J. 1999;18:480–489. doi: 10.1093/emboj/18.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamb R A, Joshi S B, Dutch R E. The paramyxovirus fusion protein forms an extremely stable core trimer: structural parallels to influenza virus haemagglutinin and HIV-1 gp41. Mol Membr Biol. 1999;16:11–19. doi: 10.1080/096876899294715. [DOI] [PubMed] [Google Scholar]
- 53.Laquerre S, Argnani R, Anderson D B, Zucchini S, Manservigi R, Glorioso J C. Heparan sulfate proteoglycan binding by herpes simplex virus type 1 glycoproteins B and C, which differ in their contributions to virus attachment, penetration, and cell-to-cell spread. J Virol. 1998;72:6119–6130. doi: 10.1128/jvi.72.7.6119-6130.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laughlin C A, Black R J, Feinberg J, Freeman D J, Ramsey J, Ussery M A, Whitley R J. Resistance to antiviral drugs: although relatively new and poorly understood, viral resistance to drugs is an increasingly significant clinical issue. ASM News. 1991;57:514–517. [Google Scholar]
- 55.Liesegang T J. Epidemiology of ocular herpes simplex. Natural history in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107:1160–1165. doi: 10.1001/archopht.1989.01070020226030. [DOI] [PubMed] [Google Scholar]
- 56.Liesegang T J, Melton L J, Daly P J, Ilstrup D M. Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107:1155–1159. doi: 10.1001/archopht.1989.01070020221029. [DOI] [PubMed] [Google Scholar]
- 57.Lium E K, Panagiotidis C A, Wen X, Silverstein S J. Repression of the α0 gene by ICP4 during a productive herpes simplex virus infection. J Virol. 1996;70:3488–3496. doi: 10.1128/jvi.70.6.3488-3496.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lium E K, Silverstein S J. Mutational analysis of the herpes simplex virus type 1 ICP0 C3HC4 zinc ring finger reveals a requirement for ICP0 in the expression of the essential α27 gene. J Virol. 1997;71:8602–8614. doi: 10.1128/jvi.71.11.8602-8614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Louie A Y, Meade T J. A cobalt complex that selectively disrupts the structure and function of zinc fingers. Proc Natl Acad Sci USA. 1998;95:6663–6668. doi: 10.1073/pnas.95.12.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lungu O, Panagiotidis C A, Annunziato P W, Gershon A A, Silverstein S J. Aberrant intracellular localization of varicella zoster virus regulatory proteins during latency. Proc Natl Acad Sci USA. 1998;95:7080–7085. doi: 10.1073/pnas.95.12.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mackem S, Roizman B. Differentiation between α promoter and regulator regions of herpes simplex virus 1: the functional domains and sequence of a movable α regulator. Proc Natl Acad Sci USA. 1982;79:4917–4921. doi: 10.1073/pnas.79.16.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mackem S, Roizman B. Regulation of α genes of herpes simplex virus: the α27 promoter-thymidine kinase chimera is positively regulated in converted L cells. J Virol. 1982;43:1015–1023. doi: 10.1128/jvi.43.3.1015-1023.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mackem S, Roizman B. Structural features of the herpes simplex virus alpha-gene 4, 0, and 27 promoter-regulatory sequences which confer alpha-regulation on chimeric thymidine kinase genes. J Virol. 1982;44:939–946. doi: 10.1128/jvi.44.3.939-949.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McClain D S, Fuller A O. Cell-specific kinetics and efficiency of herpes simplex virus type 1 entry are determined by two distinct phases of attachment. Virology. 1994;198:690–702. doi: 10.1006/viro.1994.1081. [DOI] [PubMed] [Google Scholar]
- 65.McKenzie R C, Epand R M, Johnson D C. Cyclosporine A inhibits herpes simplex virus-induced cell fusion but not virus penetration into cells. Virology. 1987;159:1–9. doi: 10.1016/0042-6822(87)90341-2. [DOI] [PubMed] [Google Scholar]
- 66.McMahan L, Schaffer P A. Repressing and enhancing functions of the herpes simplex virus regulatory protein ICP27 map to C-terminal regions and are required to modulate viral gene expression very early in infection. J Virol. 1990;64:3471–3485. doi: 10.1128/jvi.64.7.3471-3485.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 68.Navarro D, Paz P, Pereira L. Domains of herpes simplex virus 1 glycoprotein B that function in virus penetration, cell-to-cell spread, and cell fusion. Virology. 1992;186:99–112. doi: 10.1016/0042-6822(92)90064-v. [DOI] [PubMed] [Google Scholar]
- 69.Novotny M J, Parish M L, Spear P G. Variability of herpes simplex virus gL and anti-gL antibodies that inhibit cell fusion but not viral infectivity. Virology. 1996;221:1–13. doi: 10.1006/viro.1996.0347. [DOI] [PubMed] [Google Scholar]
- 70.Nugier F, Colin J N, Aymard M, Langlois M. Occurrence and characterization of acyclovir-resistant herpes simplex virus isolates: report on a two-year sensitivity screening survey. J Med Virol. 1992;36:1–12. doi: 10.1002/jmv.1890360102. [DOI] [PubMed] [Google Scholar]
- 71.O'Hare P, Goding C R, Haigh A. Direct combinatorial interaction between a herpes simplex virus regulatory protein and a cellular octamer-binding factor mediates specific induction of virus immediate-early gene expression. EMBO J. 1988;7:4231–4238. doi: 10.1002/j.1460-2075.1988.tb03320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ostrow R S, Coughlin S, McGlennen R C, Liu Z, Zelterman D, Faras A J. Topical CTC-96 accelerates wart growth in rabbits infected with cottontail rabbit papillomavirus. Antiviral Res. 1994;24:27–35. doi: 10.1016/0166-3542(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 73.Pertel P E, Spear P G. Modified entry and syncytium formation by herpes simplex virus type 1 mutants selected for resistance to heparin inhibition. Virology. 1996;226:22–33. doi: 10.1006/viro.1996.0624. [DOI] [PubMed] [Google Scholar]
- 74.Pope L E, Marcelletti J F, Katz L R, Lin J Y, Katz D H, Parish M L, Spear P G. The anti-herpes simplex virus activity of n-docosanol includes inhibition of the viral entry process. Antiviral Res. 1998;40:85–94. doi: 10.1016/s0166-3542(98)00048-5. [DOI] [PubMed] [Google Scholar]
- 75.Post L E, Mackem S, Roizman B. Regulation of alpha genes of HSV: expression of chimeric genes produced by fusion of thymidine kinase with alpha gene promoters. Cell. 1981;24:555–565. doi: 10.1016/0092-8674(81)90346-9. [DOI] [PubMed] [Google Scholar]
- 76.Preston C M. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J Virol. 1979;29:275–284. doi: 10.1128/jvi.29.1.275-284.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rajcani J, Vojvodova A. The role of herpes simplex virus glycoproteins in the virus replication cycle. Acta Virol. 1998;42:103–118. [PubMed] [Google Scholar]
- 78.Reusser P. Current concepts and challenges in the prevention and treatment of viral infections in immunocompromised cancer patients. Support Care Cancer. 1998;6:39–45. doi: 10.1007/s005200050130. [DOI] [PubMed] [Google Scholar]
- 79.Rice S A, Knipe D M. Gene-specific trans-activation by the herpes simplex virus type 1 alpha protein ICP27. J Virol. 1988;62:3814–3823. doi: 10.1128/jvi.62.10.3814-3823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roizman B. An inquiry into the mechanisms of recurrent herpes infections in man. In: Pollard M, editor. Perspectives in virology. Vol. 4. New York, N.Y: Harper & Row; 1968. p. 283. [Google Scholar]
- 81.Roizman B, Sears A K. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2231–2295. [Google Scholar]
- 82.Roller R J, Herold B C. Characterization of a BHK(TK−) cell clone resistant to postattachment entry by herpes simplex virus types 1 and 2. J Virol. 1997;71:5805–5813. doi: 10.1128/jvi.71.8.5805-5813.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roller R J, Roizman B. A herpes simplex virus Us11-expressing cell line is resistant to herpes simplex virus infection at a step in viral entry mediated by glycoprotein D. J Virol. 1994;68:2830–2839. doi: 10.1128/jvi.68.5.2830-2839.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Vol. 3. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 85.Schnipper L E, Crumpacker C S. Resistance of herpes simplex virus to acycloguanosine: role of thymidine kinase and DNA polymerase loci. Proc Natl Acad Sci USA. 1980;77:2270–2273. doi: 10.1073/pnas.77.4.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shieh M-T, WuDunn D, Montgomery R I, Esko J D, Spear P G. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J Cell Biol. 1992;116:1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Skehel J J, Wiley D C. Coiled coils in both intracellular vesicle and viral membrane fusion. Cell. 1998;95:871–874. doi: 10.1016/s0092-8674(00)81710-9. [DOI] [PubMed] [Google Scholar]
- 88.Sodeik B, Ebersold M W, Helenius A. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J Cell Biol. 1997;136:1007–1021. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Soliman T, Sandri-Goldin R, Silverstein S. Shuttling between the nucleus and cytoplasm of the herpes simplex virus type 1 regulatory protein ICP27 mediates the expression of late proteins. J Virol. 1997;71:9188–9197. doi: 10.1128/jvi.71.12.9188-9197.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spear P G. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 91.Spear P G. Glycoproteins specified by herpes simplex virus. In: Roizman B, editor. The Herpesviruses. Vol. 3. New York, N.Y: Plenum Press; 1985. pp. 315–356. [Google Scholar]
- 92.Spear P G, Eisenberg R I, Cohen G H. Three classes of cell surface receptors for alphaherpesvirus entry. Virology. 2000;275:1–8. doi: 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- 93.Stewart J A, Reff S E, Pellet P E, Corey L, Whitely R J. Herpesvirus infection in persons infected with human immunodeficiency virus. Clin Infect Dis. 1995;21(Suppl. 1):S114–120. doi: 10.1093/clinids/21.supplement_1.s114. [DOI] [PubMed] [Google Scholar]
- 94.Swanstrom R I, Pivo K, Wagner E K. Restricted transcription of the herpes simplex virus genome occurring early after infection and in the presence of metabolic inhibitors. Virology. 1974;66:140–150. doi: 10.1016/0042-6822(75)90185-3. [DOI] [PubMed] [Google Scholar]
- 95.Terry-Allison T, Montgomery R, Whitbeck J C, Xu R, Cohen G H, Eisenberg R J, Spear P G. HveA (herpesvirus entry mediator A), a coreceptor for herpes simplex virus entry, also participates in virus-induced cell fusion. J Virol. 1998;72:5802–5810. doi: 10.1128/jvi.72.7.5802-5810.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thomas J, Rouse B T. Immunopathogenesis of herpetic ocular disease. Immunol Res. 1997;16:375–386. doi: 10.1007/BF02786400. [DOI] [PubMed] [Google Scholar]
- 97.Vogt P E, Hartline C B, Gerchow T P, Kern E R. Antiviral activity of a series of cobalt containing complexes against herpesvirus infection in vitro and in vivo. Antiviral Res. 1992;17S:114. [Google Scholar]
- 98.Walev I, Lingen M, Lazzaro M, Weise K, Falke D. Cyclosporine A resistance of herpes simplex virus-induce “fusion from within” as a phenotypical marker of mutations in the syn 3 locus of the glycoprotein B gene. Virus Genes. 1994;8:83–86. doi: 10.1007/BF01703606. [DOI] [PubMed] [Google Scholar]
- 99.Warner M S, Geraghty R J, Martinez W M, Montgomery R I, Whitbeck J C, Xu R L, Eisenberg R J, Cohen G H, Spear P G. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and psuedorabies virus. Virology. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 100.Watson R J, Clements J B. Characterization of transcription-deficient temperature-sensitive mutants of herpes simplex virus. Virology. 1978;91:364–379. doi: 10.1016/0042-6822(78)90384-7. [DOI] [PubMed] [Google Scholar]
- 101.Weissenhorn W, Dessen A, Calder L J, Harrison S C, Skehel J J, Wiley D C. Structural basis for membrane fusion by enveloped viruses. Mol Membr Biol. 1999;16:3–9. doi: 10.1080/096876899294706. [DOI] [PubMed] [Google Scholar]
- 102.Whitbeck J C P, Peng C, Lou H, Xu R, Willis S H, Ponce de Leon M, Peng T, Nicola A V, Montgomery R I, Warner M S, Sooulika A M, Spruce L A, Moore W T, Lambris J D, Spear P G, Cohen G H, Eisenberg R J. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol. 1997;71:6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Whitley R J. Herpes simplex viruses. In: Fields B N, Knipe D M, editors. Virology. 2nd ed. New York, N.Y: Raven Press, Ltd.; 1990. pp. 1843–1887. [Google Scholar]
- 104.Wood M J. Antivirals in the context of HIV disease. J Antimicrob Chemother. 1996;37(Suppl. B):97–112. doi: 10.1093/jac/37.suppl_b.97. [DOI] [PubMed] [Google Scholar]
- 105.Wooley P H, Whalen J D. The influence of superoxide scavenging compound CTC 23 on type II collagen-induced arthritis in mice. Agents Actions. 1992;35:273–279. doi: 10.1007/BF01997511. [DOI] [PubMed] [Google Scholar]
- 106.WuDunn D, Spear P G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou Z H, Chen D H, Jakana J, Rixon F J, Chiu W. Visualization of tegument-capsid interactions and DNA in intact herpes simplex virus type 1 virions. J Virol. 1999;73:3210–3218. doi: 10.1128/jvi.73.4.3210-3218.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu Q, Courtney R J. Chemical crosslinking of glycoproteins on the envelope of herpes simplex virus. Virology. 1988;167:377–384. [PubMed] [Google Scholar]