Abstract
Paraneoplastic cast nephropathy, a rare cause of acute kidney injury, is most commonly observed in cases of multiple myeloma and is characterized by the formation of intratubular casts composed of monoclonal light chains. Nonmonoclonal paraneoplastic cast nephropathy has also been reported in patients with pancreatic acinar cell carcinoma or prolactinoma. In this case report, we present a case of polyclonal cast nephropathy in a patient with metastatic acinar cell carcinoma. We aim to emphasize the significance of recognizing this uncommon complication in patients with solid tumors and to discuss the diagnostic challenges and potential pathophysiology of this unique condition.
Index Words: Acinar cell carcinoma, kidney biopsy, kidney pathology, paraneoplastic cast nephropathy, onconephrology
Acute kidney injury (AKI) is common in patients with malignancy,1, 2, 3 most frequently associated with hypoperfusion, urinary tract obstruction, tumor lysis syndrome, thrombotic microangiopathy, or drug nephrotoxicity.4 AKI can also occur in the presence of intratubular casts, causing damage directly to the tubular cells. The most common malignancy associated form is myeloma cast nephropathy, which is characterized by the formation of intratubular, hard, fractured, periodic acid Schiff (PAS) negative casts composed of monoclonal light chains.5,6 Intratubular casts with similar morphologic characteristics, but composed by polyclonal light chains, have been reported in patients without multiple myeloma and specifically as a paraneoplastic syndrome in patients with pancreatic acinar cell carcinoma or prolactinoma.7, 8, 9, 10, 11
In this case report, we present the clinical course and kidney biopsy findings of a 64-year-old man with metastatic acinar cell carcinoma who developed polyclonal paraneoplastic cast nephropathy. We aim to highlight the importance of considering this rare complication in patients with solid tumor malignancies and discuss the diagnostic challenges and pathophysiology for this condition.
Case Report
Clinical History
A White man in his 60s with a history of type 2 diabetes, aortic atherosclerosis, and cerebrovascular disease, presented with a 6-month history of diarrhea, abdominal pain, early satiety, and weight loss. Magnetic resonance imaging of the abdomen demonstrated peritoneal carcinomatosis. Biopsy of a peritoneal mass showed cytopathologic features consistent with an acinar cell carcinoma of the pancreas. Initial chemotherapy included 5-fluorouracil, oxaliplatin, irinotecan, and trastuzumab. Shortly after starting chemotherapy, the patient had progressive worsening of kidney function, with serum creatinine levels increasing from a baseline of 0.98 mg/dL to 5.4 mg/dL. There were no episodes of hypotension documented and no recent use of nonsteroidal anti-inflammatories. His serum creatinine levels failed to improve despite several attempts of intravenous fluid resuscitation.
Initial Laboratory Data
Initial diagnostic work-up included a urinalysis, which showed proteinuria (1+ to 2+), no microscopic hematuria or pyuria, and no evidence of crystals or cellular casts. His urine albumin-creatinine ratio was 83 mg/g, and his urine protein-creatinine ratio was 2,292 mg/g. A kidney ultrasound showed ∼12 cm kidneys, with normal echogenicity and cortical thickness, and he was negative for hydronephrosis. C3 levels were normal, and C4 levels were slightly elevated at 55 (reference 13-39 mg/dL). Serum protein electrophoresis and immunofixation did not show any monoclonal proteins, and both serum kappa and lambda free light chains were elevated (5.9 mg/dL and 4.14 mg/dL, respectively), with a normal kappa:lambda ratio (1.43). Serologic work-up was otherwise negative for antinuclear antibodies, anti-double stranded DNA antibodies, anti-neutrophil cytoplasmic antibodies, and hepatitis B or C infection.
Kidney Biopsy
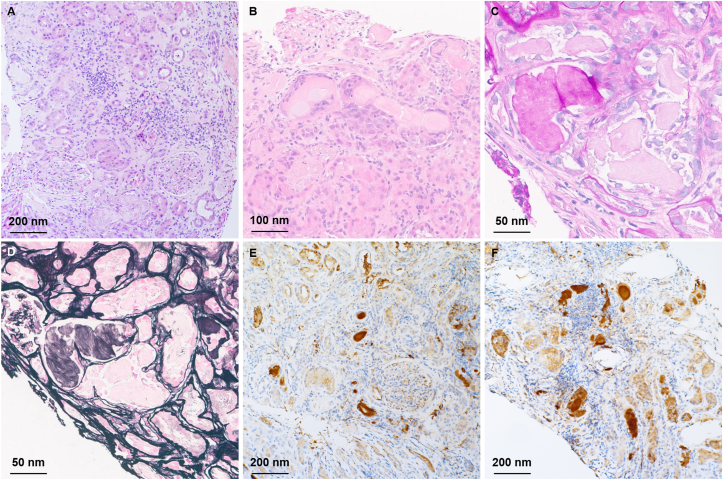
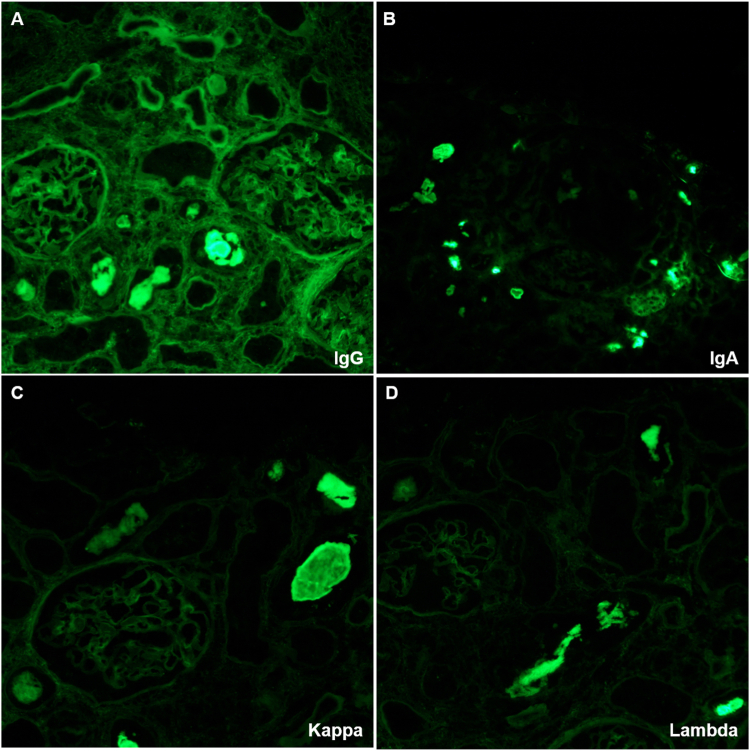
A kidney biopsy was performed. On light microscopy, the glomeruli and vasculature were unremarkable. There was a diffuse mild and focally dense interstitial inflammation of lymphocytes, mononuclear cells, rare plasma cells, and rare eosinophils (Fig 1A). There was a diffuse mild and focally severe acute tubular injury with numerous granular and hard fractured intratubular casts with occasional giant cell reactions (Fig 1B). Some of the intratubular casts were positive based on PAS staining but the majority were negative (Fig 1C, D). On immunofluorescence analysis, no significant reactivity for any of the antibodies used (IgG, IgA, IgM, C3, C1q, albumin, fibrinogen, and kappa and lambda light chains) was detected in glomeruli, vessels, or interstitium. Intratubular casts were strongly positive for IgG, IgA, kappa, and lambda light chains (Fig 2). Immunostaining for kappa and lambda light chain was repeated on paraffin sections to confirm the findings and revealed strong reactivity for both kappa and lambda light chains on immunohistochemistry (Fig 1E, F).
Figure 1.
(A) Periodic acid Schiff showing overall disruption of the kidney normal architecture, tubular injury, and interstitial inflammation. (B) Hematoxylin and eosin showing numerous granular and hard fractured intratubular casts with associated giant cell reactions. (C, D) Some of the intratubular casts are positive on periodic acid Schiff and silver stains indicating Tamm-Horsfall casts, whereas the paraneoplastic casts are negative. Immunostaining for kappa (E) and lambda (F) light chains reveals strong reactivity in the paraneoplastic casts and lack of immunoreactivity in the nonparaneoplastic casts.
Figure 2.
Immunofluorescent staining showed the intratubular casts were strongly positive for IgG (A), IgA (B), and kappa (C) and lambda (D) light chains.
Diagnosis
The overall findings are consistent with paraneoplastic cast nephropathy superimposed with moderately severe acute interstitial nephritis.
Clinical Follow-up
For acute interstitial nephritis, he was treated with 60 mg prednisone daily for 2 weeks followed by a 6-week taper. The duration of steroid treatment was limited because of side effects including blurry vision. Unfortunately, his kidney function progressively worsened, and he was started on hemodialysis. Two months later, the patient died because of cancer progression.
Discussion
Paraneoplastic cast nephropathy is an uncommon kidney manifestation of solid tumors.7 Cast nephropathies can occur in association with a variety of conditions, neoplastic and non-neoplastic, and manifest clinically with AKI. The most common non-neoplastic forms are attributed to the intratubular accumulation of tubular cell debris, red blood cells, myoglobin, hemoglobin, bile, or Tamm-Horsfall proteins, whereas the most common form associated with cancer is myeloma cast nephropathy.12, 13, 14, 15, 16 The morphologic and chromatic characteristics of the intratubular casts and immunoreactivity with specific antibodies, are key to the diagnosis (Table 1). For example, red blood cell, myoglobin and hemoglobin casts are granular, strongly eosinophilic, and red on trichrome stain, although myoglobin casts and hemoglobin casts stain positive with antibodies against myoglobin and hemoglobin, respectively.15,16 Bile casts are finely granular and brownish in color and react to antibodies against bile.14 Granular and Tamm-Horsfall casts are nonspecific manifestation of tubular injury.17 They are both eosinophilic and do not react with any of the antibodies that would detect the other type of casts. However, granular casts are usually PAS negative, whereas Tamm-Horsfall casts are strongly PAS positive.18 The rarity of paraneoplastic cast nephropathy and the morphologic similarities with myeloma cast nephropathy account for the diagnostic challenges that pathologists can encounter. In both conditions, intratubular casts are strongly eosinophilic but PAS negative; they appear hard and fractured and are associated with an inflammatory reaction. The differential diagnosis is therefore based on the clinical history and immunoreactivity: monoclonality of the myeloma casts versus polyclonality of the paraneoplastic casts in our case (both kappa and lambda light chains are positive on immunofluorescence and immunohistochemistry analysis). It is notable that Tamm-Horsfall casts, reflecting tubular injury, can account for a considerable percent of intratubular casts in both conditions, potentially masking the diagnostic casts. Another important clinical clue in our case is the discordance between urine albumin-creatinine ratio (83 mg/g) and urine protein-creatinine ratio (2292 mg/g), which is highly suggestive of the presence of a paraprotein in the urine.
Table 1.
Key characteristics of tubular casts
| Casts | H&E | PAS | Trichrome | IF | Others |
|---|---|---|---|---|---|
| Tamm-Horsfall | Pink and homogenous | Bright pink | Pale blue | Nonspecific | Uromodulin IHC positive |
| Granular | Pink and granular | Pale | Pale | Nonspecific | N/A |
| Light chain | Pink and fractured | Pale | Metachromatic | Monotypic light chain | Discordant UACR and UPCR |
| Hemoglobin | Red globular | Light to dark pink | Bright red | Nonspecific | Hemoglobin IHC positive |
| Myoglobin | Red-brown globular | Light to dark pink | Bright red | Nonspecific | Myoglobin IHC positive |
| Bile | Yellow-green to red | Red to dark red | Red to green | Nonspecific | Bilirubin stain positive |
| Solid tumor associated | Pink and fractured | Pale | Metachromatic | Polytypic light chain or negative | Discordant UACR and UPCR |
Abbreviations: H&E, Hematoxylin and eosin; IHC, immunohistochemistry; IF, immunofluorescence; UACR, urine albumin-creatinine ratio; UPCR, urine protein-creatinine ratio.
There have been several other reported cases of paraneoplastic cast nephropathy associated with pancreatic cancer7, 8, 9,11 and one case associated with prolactinoma10 as listed in Table 2.7, 8, 9, 10, 11 One notable finding in our case is that the intratubular casts stained positive for both kappa and lambda light chains. Out of the 5 previously published cases, only 1 showed similar double positivity, whereas 3 reported negative stains for both light chains. The significance of the IgG and IgA positivity is unclear, as only 1 prior case reported these staining results, which were notably negative. The exact proteomic contents of the casts in our case were not studied, but prior studies have shown that these casts may contain tumor-specific proteins such as REG1α and CPA1.7,8
Table 2.
Comparison of reported cases of paraneoplastic cast nephropathy associated with solid tumors
| Case Report | Cancer Diagnosis | Cancer Extent | Age | Sex | Procedure | Giant Cell Reaction | PAS of Casts | Kappa/lambda | Heavy Chain | IHC | Proteomics |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Current case | Pancreatic acinar cell carcinoma | Metastatic | 60s | Male | Biopsy | Yes | Negative | Both positive | IgG, IgA | Kappa, lambda | Not reported |
| Nasr et al7 (2019) | Pancreatic mixed acinar neuroendocrine carcinoma | Not reported | 64 | Male | Biopsy | Yes | Negative | Negative | Not reported | REG1A, CPA1 | REG1A, REG1B, CPA1 |
| Nasr et al8 (2021) | Pancreatic mixed acinar neuroendocrine carcinoma | Metastatic | 38 | Male | Autopsy | Yes | Negative | Negative | Not reported | REG1A, CPA1 | REG1A, REG1B, REG1C |
| Min et al9 (1976) | Pancreatic acinic cell adenocarcinoma | Metastatic | 54 | Male | Autopsy | Yes | Negative | Not reported | Not reported | Not reported | Not reported |
| Reducka et al11(1988) | Pancreatic acinar cell carcinoma | Metastatic | 52 | Male | Autopsy | Yes | Negative | Both positive | Negative | Not reported | Not reported |
| Mohamed et al10 (2022) | Malignant prolactinoma | Metastatic | 54 | Male | Biopsy | Yes | Negative | Negative | Not reported | Prolactin | Not reported |
Abbreviations: IHC, immunohistochemistry.
Given the rarity of this disease, the pathophysiology of solid tumor-associated paraneoplastic cast nephropathy is not well understood. In myeloma cast nephropathy, excess monoclonal free light chains produced by neoplastic plasma cells are freely filtered by glomeruli and overwhelm the reabsorption capacity of proximal tubules. The free light chains, with a strong affinity for Tamm-Horsfall protein, form casts with Tamm-Horsfall protein and obstruct the distal tubules, causing tubule rupture and a giant cell inflammatory reaction, which eventually leads to interstitial fibrosis and tubular atrophy.12,19 A similar mechanism could be postulated for solid tumor-associated paraneoplastic cast nephropathy. The proteomic analysis by Nasr et al7,8 demonstrated that the tubular casts were mainly composed of regenerating protein 1 alpha (REG1a), which is normally produced by pancreatic acinar cells. Although we were not able to perform proteomic studies ourselves, it is highly likely the same protein can be found in the tubular casts in our case. The low molecular weight (19 kDa) and the aggregating properties of REG1a make it possible to be freely filtered by glomeruli and form casts within the tubular lumen.8
The management for paraneoplastic cast nephropathy is generally challenging, mostly because of the advanced stage of the neoplastic process. Interstitial inflammation is not uncommon in the setting of neoplastic or paraneoplastic cast nephropathy. Although it could represent a reactive process to the nephrotoxic casts, it could also be the manifestation of a superimposed drug-induced process, which may have contributed to his acute kidney failure. Despite treatment with steroids, the patient’s kidney function continued to worsen, necessitating hemodialysis.
In conclusion, paraneoplastic cast nephropathy is an extremely rare but important cause of AKI in patients with solid tumors. Clinicians should maintain a high index of suspicion for this condition in patients with malignancies and unexplained deterioration of kidney function, especially with discordant urine albumin-creatinine ratio and urine protein-creatinine ratio. Obtaining a kidney biopsy in these patients is of upmost importance because it would help us identify the etiology of the AKI, understand underlying pathophysiology, determine prognostic factors, and promptly initiate the most appropriate disease management strategies. Further studies are required to better comprehend the proteomic contents of the casts and to optimize diagnostic and therapeutic approaches.
Article Information
Authors’ Full Names and Academic Degrees
Bangchen Wang, MD, PhD, Micah Schub, MD, David I. Ortiz-Melo, MD, and Laura Barisoni, MD
Support
None.
Financial Disclosure
Dr Barisoni is a consultant at Verex, Sangamo, and Protalix, a member of the scientific advisory board at Nephcure, and a member of the steering committee and co-chair of the Clinical Trial Committee at International Society of Glomerular Diseases.
Patient Protections
This study protocol has been granted an exemption from requiring ethics approval by the Institutional Review Board at Duke University. No written informed consent was required or obtained for this case report.
Peer Review
Received November 17, 2023. Evaluated by 1 external peer reviewer, with direct editorial input from an Associate Editor and the Editor-in-Chief. Accepted in revised form June 18, 2024.
Footnotes
Complete author and article information provided before references.
References
- 1.Benoit D.D., Hoste E.A. Acute kidney injury in critically ill patients with cancer. Crit Care Clin. 2010;26:151–179. doi: 10.1016/j.ccc.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Kemlin D., Biard L., Kerhuel L., et al. Acute kidney injury in critically ill patients with solid tumours. Nephrol Dial Transplant. 2018;33(11):1997–2005. doi: 10.1093/ndt/gfy051. [DOI] [PubMed] [Google Scholar]
- 3.Kitchlu A., McArthur E., Amir E., et al. Acute kidney injury in patients receiving systemic treatment for cancer: a population-based cohort study. J Natl Cancer Inst. 2019;111:727–736. doi: 10.1093/jnci/djy167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosner M.H., Jhaveri K.D., McMahon B.A., Perazella M.A. Onconephrology: the intersections between the kidney and cancer. CA Cancer J Clin. 2021;71:47–77. doi: 10.3322/caac.21636. [DOI] [PubMed] [Google Scholar]
- 5.Stokes M.B., Valeri A.M., Herlitz L., et al. Light chain proximal tubulopathy: clinical and pathologic characteristics in the modern treatment era. J Am Soc Nephrol. 2016;27:1555–1565. doi: 10.1681/ASN.2015020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korbet S.M., Schwartz M.M. Multiple myeloma. J Am Soc Nephrol. 2006;17:2533–2545. doi: 10.1681/ASN.2006020139. [DOI] [PubMed] [Google Scholar]
- 7.Nasr S.H., Wehbe E., Said S.M., Dasari S., Quoc T., Kurtin P.J. Paraneoplastic cast nephropathy associated with pancreatic mixed acinar-neuroendocrine carcinoma: a case report. Am J Kidney Dis. 2019;74:558–562. doi: 10.1053/j.ajkd.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Nasr S.H., Dasari S., Theis J.D., et al. Paraneoplastic REG1alpha cast nephropathy associated with mixed acinar-neuroendocrine carcinoma. Kidney Int Rep. 2021;6:1178–1182. doi: 10.1016/j.ekir.2021.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Min K., Cain G.D., Gyorkey P., Gyorkey F. Myeloma-like lesions of the kidney. Occurrence in a case of acinic cell adenocarcinoma of the pancreas. Arch Intern Med. 1976;136:1299–1302. [PubMed] [Google Scholar]
- 10.Mohamed M., Brown A., Wood K., Wong E. Paraneoplastic cast nephropathy associated with malignant prolactinoma: a case report and literature review. Clin Nephrol. 2022;98:49–53. doi: 10.5414/CN110503. [DOI] [PubMed] [Google Scholar]
- 11.Reducka K., Gardiner G.W., Sweet J., Vandenbroucke A., Bear R. Myeloma-like cast nephropathy associated with acinar cell carcinoma of the pancreas. Am J Nephrol. 1988;8:421–424. doi: 10.1159/000167629. [DOI] [PubMed] [Google Scholar]
- 12.Sathick I.J., Drosou M.E., Leung N. Myeloma light chain cast nephropathy, a review. J Nephrol. 2019;32:189–198. doi: 10.1007/s40620-018-0492-4. [DOI] [PubMed] [Google Scholar]
- 13.Stokes M.B. Vancomycin in the kidney-a novel cast nephropathy. J Am Soc Nephrol. 2017;28:1669–1670. doi: 10.1681/ASN.2017010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Somagutta M.R., Jain M.S., Pormento M.K.L., et al. Bile cast nephropathy: a comprehensive review. Cureus. 2022;14 doi: 10.7759/cureus.23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dvanajscak Z., Walker P.D., Cossey L.N., et al. Hemolysis-associated hemoglobin cast nephropathy results from a range of clinicopathologic disorders. Kidney Int. 2019;96:1400–1407. doi: 10.1016/j.kint.2019.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Najafian B., Fogo A.B., Lusco M.A., Alpers C.E. AJKD atlas of renal pathology: myoglobin cast nephropathy. Am J Kidney Dis. 2017;69:e7–e8. doi: 10.1053/j.ajkd.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Lameire N., Van Biesen W., Vanholder R. Acute renal failure. Lancet. 2005;365:417–430. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 18.Dvanajscak Z., Cossey L.N., Larsen C.P. A practical approach to the pathology of renal intratubular casts. Semin Diagn Pathol. 2020;37:127–134. doi: 10.1053/j.semdp.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Doshi M., Lahoti A., Danesh F.R., et al. Paraprotein-related kidney disease: kidney injury from paraproteins-what determines the site of injury? Clin J Am Soc Nephrol. 2016;11:2288–2294. doi: 10.2215/CJN.02560316. [DOI] [PMC free article] [PubMed] [Google Scholar]