Highlights
-
•
Dose-escalated hypofractionated radiation therapy (HFRT) for patients with unresectable or bulky metastatic melanoma was evaluated.
-
•
Forty-nine patients received treatment to 53 targets with 12-17 fractions of HFRT between 2015-2022.
-
•
HFRT provided durable local control and improved tumor-associated symptoms in patients with limited toxicity.
-
•
HFRT is an effective form of local control for advanced melanoma patients with limited overall disease progression not amenable to SBRT.
Abstract
Background and purpose
As patients with advanced melanoma live longer in the context of systemic therapy advancements, better strategies for durable control of bulky tumors are needed. In this study, we evaluated if dose-escalated hypofractionated radiation therapy (HFRT) can provide durable local control and improve tumor-associated symptoms in patients with unresectable or bulky metastatic melanoma for whom stereotactic ablative radiotherapy (RT) approaches are not feasible due to tumor size or location.
Materials and methods
We retrospectively reviewed 49 patients with unresectable or bulky metastatic melanoma who were treated to a total of 53 tumor targets with 12–17 fractions HFRT at our institution between 2015–2022. Clinical scenarios included: unresectable, locoregional only disease (26 %); oligometastatic disease (<3 total sites, 17 %); oligoprogressive disease (<3 sites progressing, 17 %); and aggressive palliation (>5 known sites of disease or with at least 3 sites progressing, 40 %).
Results
Of the 53 HFRT targets, 91 % (n = 48) had radiographic evidence of response as defined by either stabilization (6 %, n = 3), decreased size (74 %, n = 39), or decreased FDG avidity (11 %, n = 6). Of the 43 symptomatic patients, 98 % (n = 42) had symptomatic improvement. One −year local control was 79 %, with 2-year progression-free and overall survival of 33 % and 39 % respectively. The most common acute toxicities were radiation dermatitis (16 %, n = 8) or a pain flare (14 %, n = 7). Late toxicities were uncommon and typically grade 1.
Conclusion
HFRT provides favorable local control and symptomatic relief with limited toxicity in tumors not amenable to surgical resection or stereotactic ablative RT.
Introduction
Treatment for patients with advanced and/or metastatic melanoma has improved dramatically in the past two decades with advances in systemic therapy. In this context, the median survival of patients with metastatic disease has increased from approximately six months to nearly six years [1].
While some patients with advanced disease achieve long-term remission, it is not uncommon for many patients to survive years with isolated sites of disease progression [2], [3]. When this occurs, options include the initiation of a new systemic therapy regimen and/or focusing on promoting disease control at the progressing site(s). Local therapy to a limited volume of unresectable or progressing sites of disease can alleviate symptoms and/or allow patients to continue an otherwise effective systemic therapy course (or continue off therapy) [4]. As such, local therapy continues to play an important role in the treatment of patients with unresectable and/or metastatic melanoma.
The optimal local therapy approach for patients with a limited burden of progressing unresectable locoregional and/or metastatic disease is unknown. Surgery is often considered but depending on the tumor size and/or anatomic region involved, resection may be unacceptably morbid or require prolonged systemic therapy delays [5]. Other local therapy options include intralesional therapy (for easily accessible targets) or interventions like cryoablation (for well-selected patients) [6], [7].
Radiotherapy (RT) is a commonly implemented local therapy option for melanoma progression. Historically, conventionally palliative RT was considered standard-of-care for the treatment of patients with advanced melanoma causing symptoms at sites of disease progression. However, given melanoma’s relative radioresistance, standard palliative RT may have less durable disease control. As such, melanoma has historically been excluded from studies investigating outcomes using regimens such as 8 Gy x1 fraction, which delivers approximately half of the biologic dose compared to other palliative RT regimens [8], [9], [10].
More recently, for patients with limited disease progression, ablative techniques such as stereotactic body radiation therapy (SBRT) have become more commonly applied, as they allow for biological dose escalation and have shown impressive, durable local control rates. Additionally, SBRT has the potential for favorable synergy with commonly used systemic therapies such as immune checkpoint inhibitors [11], [12], [13]. SBRT has been shown to be particularly valuable in promoting progression-free survival in the context of oligometastatic disease, which has been helpful subdivided into states of oligorecurrence, oligoprogression, and oligopersistence by the recent ESTRO/EORTC guidelines [14].
Unfortunately, many metastatic lesions are not amenable to ablative approaches due to target size or anatomic considerations. Thus, we sought to evaluate the efficacy and toxicity of a moderately dose-escalated, hypofractionated radiotherapy (HFRT) regimen in patients with unresectable/metastatic melanoma and life expectancy >6 months. We hypothesize that HFRT can produce favorable rates of durable local control with limited toxicity and decreased burden of disease-associated symptoms such as pain, neurologic deficits or respiratory symptoms.
Material and methods
Clinical data collection
Forty-nine patients with unresectable locoregional and/or metastatic melanoma who were treated to a total of 53 lesions with 12–17 fractions of HFRT (dose/fraction ≥2.5 Gy/fx) at our institution between 2015–2022 were retrospectively identified from institutional databases. Institutional review board approval was obtained before reviewing patients’ medical records. Treatment decisions were based on consensus recommendations from a multidisciplinary melanoma team. Tumor size was assessed based on the largest diameter at the time of CT simulation.
The reason for use of HFRT was categorized into 4 clinical scenarios including: (1) unresectable, locoregional only disease; (2) oligometastatic disease; (3) oligoprogressive disease; or (4) aggressive palliation in the context of further systemic therapy thought to have a high likelihood of efficacy with specific disease sites growing at a disproportionate rate or causing symptoms. Oligometastatic disease was defined as <3 total sites of metastases based on imaging. Oligoprogressive disease was defined as limited sites (<3) of progressive disease in patients with otherwise stable metastatic disease. Aggressive palliation was defined as >5 known sites of disease with at least 3 sites progressing. Typically, 1–2 sites being treated with aggressive palliation had a disproportionately high rate of growth relative to the overall burden of disease.
Radiation treatment parameters
Dose-fractionation was determined by the treating radiation oncologist. A number of factors were used to guide RT prescriptions including target size, anatomic location, and treatment intent. Neighboring Organs at Risk (OARs) were contoured with constraints for planning delineated based on conventional OAR metrics using appropriate α/β-defined EQD2 calculations for the tissue in question. Suggested planning constraints for some frequently encountered OARs are illustrated in Supplemental Table 1. All patients were treated with IMRT or VMAT-based planning approaches.
Follow-up
Local disease response was assessed by cross-sectional imaging after RT and categorized as a decrease in size, a decrease in enhancement or FDG-avidity with stable size, having stable size and enhancement (typically in the absence of PET imaging), or as having progression. Once radiographic reports annotated tumor growth or increasing enhancement/FDG-avidity, if that trend continued at subsequent imaging, the date of the initial imaging suggesting progression was annotated as the time of local disease progression. Symptom relief was assessed by patient reports to their clinical teams as documented in the medical record before and after HFRT.
Statistical analysis
Descriptive statistics were used to evaluate baseline patient, tumor, and treatment characteristics. The Kaplan-Meier method was used to estimate actuarial rates of local control (LC), progression free-survival (PFS), and overall survival (OS), with survival times calculated from the completion of RT. Log rank tests were used to assess the impact of baseline variables on outcomes.
Results
Patient and HFRT target characteristics
Patient and HFRT tumor target characteristics are described in Table 1. The median age was 62 years (interquartile range, [IQR], 48–73 years), and 55 % (n = 27) were female. Twenty-two percent (n = 11) of patients treated had a mucosal primary melanoma while the remaining had a cutaneous primary melanoma. Mutational status was evaluated in 90 % of patients (n = 44); BRAF V600 mutations were present in 41 %.
Table 1.
Patient and tumor characteristics.
| Patient and HFRT Tumor Target Characteristics (n = 49, except as annotated) | ||||
|---|---|---|---|---|
| Median age at HFRT (IQR) | 62 (48–73) | |||
| Sex | n | % | ||
| Male | 22 | 45 % | ||
| Female | 27 | 55 % | ||
| White race | 43 | 88 % | ||
| Melanoma subtype | n | % | ||
| Mucosal | 11 | 22 % | ||
| Cutaneous | 38 | 78 % | ||
| Mutation status | ||||
| BRAF (n = 44 evaluated) | 18 | 41 % | ||
| NRAS (n = 42 evaluated) | 6 | 14 % | ||
| cKIT (n = 42 evaluated) | 4 | 10 % | ||
| ECOG status at time of HFRT (n = 51) | ||||
| PS 0 | 15 | 29 % | ||
| PS 1 | 22 | 43 % | ||
| PS 2 | 10 | 20 % | ||
| PS 3 | 4 | 8 % | ||
| HFRT tumor target anatomic site (n = 53) | ||||
| Head and neck | 6 | 11 % | ||
| Trunk | 30 | 57 % | ||
| Extremities | 7 | 13 % | ||
| Mucosal | 10 | 19 % | ||
| Median tumor target size (cm, IQR) | 6 (3.8–10) | |||
ECOG: Eastern Cooperative Oncology Group, HFRT: hypofractionated radiation therapy, IQR: interquartile range, n = number of patients.
Four patients received HFRT to two independent tumor targets with two of those patients receiving synchronous HFRT to two sites and two patients receiving metachronous HFRT. This translated to 51 episodes of HFRT (in the context of 2 patients receiving metachronous courses to 2 different sites of disease). At the time of HFRT, 73 % (n = 37) had an ECOG performance status 0–1.
Of the 53 HFRT targets, 6 (11 %) were in the head and neck, 30 (57 %) were located on the trunk, 7 (13 %) were located on the upper or lower extremities, and 10 (19 %) were located on a mucosal surface. The mucosal sites were head and neck (n = 5), gynecologic (n = 4), and anorectal (n = 1). The median HFRT tumor target size was 6 cm (IQR 3.8–10 cm). Of note, the largest treated tumor was 23 cm with extension through the axilla, chest wall and supraclavicular area.
Treatment
With consensus after multidisciplinary discussion, the rationale for HFRT was: (1) unresectable locoregional only disease (26 %, n = 14), (2) oligometastatic disease (17 %, n = 9), (3) oligoprogressive disease (17 %, n = 9), and (4) aggressive palliation (40 %, n = 21). Eighty-one percent (n = 43) of HFRT tumor targets were symptomatic with 60 % (n = 32) having pain and 15 % (n = 8) having neurologic symptoms.
Table 2 illustrates the overall disease context in which HFRT was delivered. The median time from diagnosis to HFRT was 1 year (IQR 0–5.5). All except three patients had received systemic therapy at any time (median 2 lines, IQR 1–4). Fourteen patients (26 %) received prior local therapy to the HFRT target site. Twelve of these patients had prior surgery and experienced local recurrence.
Table 2.
Disease context at treatment.
| Disease Context at HFRT (n = 49, except as annotated) | |||
|---|---|---|---|
| Median years from diagnosis to HFRT (IQR) | 1 (0–5.5) | ||
| Prior lines of systemic therapy (med, IQR) | 2 (1–4) | ||
| Class of systemic therapy immediately prior to HFRT (n = 51) | n | % | |
| None | 13 | 25 % | |
| Immune checkpoint inhibitor | 32 | 63 % | |
| Targeted therapy | 5 | 10 % | |
| Chemotherapy | 1 | 2 % | |
| Previous local therapy to tumor target site (n = 53) | 14 | 26 % | |
| Clinical scenario for HFRT (n = 53) | |||
| Unresectable (locoregional disease only) | 14 | 26 % | |
| Oligometastasis (<3 known sites) | 9 | 17 % | |
| Oligoprogression (<3 progressing sites) | 9 | 17 % | |
| Palliation (≥3 progressing sites) | 21 | 40 % | |
| Symptoms related to HFRT target (n = 53) | |||
| None | 10 | 19 % | |
| Pain | 28 | 53 % | |
| Neurologic | 4 | 8 % | |
| Pain + Neurologic | 4 | 8 % | |
| Other | 7 | 13 % | |
HFRT: hypofractionated radiation therapy, IQR: interquartile range, n = number of patients.
All patients received an HFRT regimen of 12–17 fractions with a median of 15 fractions (Table 3). The median GTV dose was 45 Gy (range 36–52.5 Gy). Seventy-four percent of patients (n = 39) received a simultaneous integrated boost (SIB) to either the GTV or a contracted volume from the GTV (median SIB 52.5 Gy, IQR 49.5–52.5 Gy). The median PTV dose was 37.5 Gy (IQR 37.5–37.5 Gy).
Table 3.
Treatment details.
| Radiation therapy details (n = 53) | |||
|---|---|---|---|
| Dose-fractionation schema to GTV | n | % | |
| 36 Gy/12–14 fx | 4 | 8 % | |
| 37.5 Gy/15 fx | 5 | 9 % | |
| 39 Gy/12–13 fx | 2 | 4 % | |
| 41–42 Gy/12–15 fx | 4 | 8 % | |
| 42.5 Gy/17 fx | 4 | 8 % | |
| 45 Gy/14–15 fx | 22 | 42 % | |
| 45.5 Gy/14 fx | 1 | 2 % | |
| 48 Gy/15 fx | 1 | 2 % | |
| 52.5 Gy/15 fx | 10 | 19 % | |
| Dose-fractionation schema to PTV | |||
| 33–36 Gy/12–17 fx | 7 | 13 % | |
| 37.5 Gy/14–15 fx | 33 | 62 % | |
| 39–42 Gy/12–15 fx | 4 | 8 % | |
| 42.5 Gy/17 fx | 3 | 6 % | |
| 45 Gy/15 fx | 6 | 11 % | |
| Simultaneous integrated boost | 39 | 74 % | |
| Targets for which EQD2α/β=3 ≥ 54 Gy | 34 | 64 % | |
EQD2α/β=3 ≥ 54 Gy: Prescription dose to GTV was equal to or greater than 54 Gy, fx: fraction, GTV: gross tumor volume, n = number of patients, PTV: planning target volume.
Response to therapy and outcomes
Of the 53 HFRT targets, 91 % (n = 48) had either stable disease (6 %, n = 3) or tumor response manifest as decrease in size (74 %, n = 39) or decrease in FDG avidity on PET (11 %, n = 6). Of the 43 patients symptomatic from the HFRT tumor target, 98 % (n = 42) had symptomatic improvement after treatment. Of the 32 patients with a prescription pain medication requirement, 19 % (n = 6) had evidence in the electronic medical record of decreasing need for prescription pain medication (Table 4).
Table 4.
Outcomes.
| Outcomes | |||
|---|---|---|---|
| Response to HFRT | n | % | |
| Best radiographic response (n = 53) | |||
| Decreased size | 39 | 74 % | |
| Decreased FDG avidity (stable size) | 6 | 11 % | |
| Stable disease through follow-up | 3 | 6 % | |
| Progressive disease | 5 | 9 % | |
| Symptomatic improvement following RT (n = 43) | 42 | 98 % | |
| Pain medication requirement decrease (n = 32) | 6 | 19 % | |
| Systemic therapy within 1 month after HFRT (n = 51) | |||
| None | 7 | 14 % | |
| Continued | 26 | 51 % | |
| Stopped | 1 | 2 % | |
| New agent | 17 | 33 % | |
| Treatment associated toxicity | |||
| Grade 2acute toxicity (n = 51) | 20 | 39 % | |
| Fatigue | 4 | 8 % | |
| Pain Flare | 7 | 14 % | |
| Dermatitis | 8 | 16 % | |
| Mucositis/Esophagitis | 4 | 8 % | |
| Nausea, vomiting or diarrhea | 3 | 6 % | |
| Grade 3 acute toxicity (n = 51) | 1 | 2 % | |
| Mucositis | 1 | 2 % | |
| Late toxicity (n = 51) | 7 | 14 % | |
| Skin-related | 3 | 6 % | |
| Pulmonary | 2 | 4 % | |
| Neurologic | 1 | 2 % | |
| Dry Mouth | 1 | 2 % | |
| Disease progression during follow-up (median 26 mo) | |||
| Local progression at HFRT target site (n = 53) | 8 | 15 % | |
| In-field | 7 | 13 % | |
| Marginal | 1 | 2 % | |
| Subsequent distant progression (n = 51) | 31 | 61 % | |
| Status at last follow up (n = 49) | |||
| Alive | 23 | 47 % | |
| Deceased | 26 | 53 % | |
HFRT: hypofractionated radiation therapy, IQR: interquartile range, n = number of patients.
Most patients receiving HFRT continued the same systemic therapy they had been taking prior to HFRT (51 %, n = 26), continued off therapy (14 %, n = 7) or discontinued systemic therapy (2 %, n = 1). Thirty-three percent (n = 17) started a new systemic therapy agent within 1 month of completing HFRT.
With a median follow-up of 26 months (IQR 12.5–46 months) for patients alive at last follow-up, 15 % of patients (n = 8) had local progression of the HFRT tumor target. The median GTV dose of those progressing was 43.75 Gy in 15 fractions. One progression event was marginal to the HFRT field while the rest were within the PTV target volume. At the time of local progression, the patient with a marginal recurrence received further RT while the remaining 7 patients were managed with systemic therapy. Sixty-one percent of patients (n = 31) experienced subsequent disease progression outside the HFRT field.
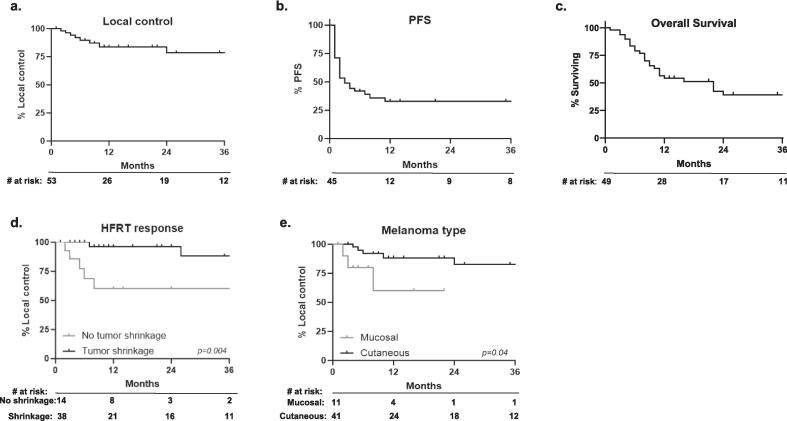
Local control at 1-year (for the 53 treated tumors) was 84 % and at 2-years was 79 %. However, in the context of frequent disease progression elsewhere, 1- and 2-year PFS were both 33 %. Fifty-three percent of patients (n = 26) died during follow-up with a median overall survival of 11.5 months (IQR 6–24.5) (Fig. 1a–c).
Fig. 1.
Patient outcomes after HFRT: (a) Local control of HFRT target. (b) Progression-free survival. (c) Overall survival. Variables associated with local control after HFRT: (d) Decrease in tumor size is associated with local control. (e) Cutaneous melanoma is associated with local control.
Predictors of local control
Table 5 illustrates the association of various disease and treatment factors with local control (LC) Patients with a decrease in size of the HFRT target lesion had significantly better long-term local control than those with no decrease in size (2-year LC 93 % vs. 60 %, p = 0.004, Fig. 1d). Notably, even without shrinkage, more than half of patients never developed in-field disease progression. Patients with a cutaneous melanoma primary were found to have better LC than those with a mucosal melanoma primary (2-year LC 82 % vs. 60 %, p = 0.04, Fig. 1e). A patient’s overall disease context did not appear to influence the efficacy of HFRT given that oligometastatic and oligoprogressive disease were similarly well controlled with HFRT, as were HFRT targets in the context of more widely progressing disease. In addition, prior local therapy to the HFRT target did not influence the likelihood of HFRT efficacy. Higher EQD2 was also not associated with a statistically significant improvement in LC.
Table 5.
Predictors of outcomes.
| 2 yr LC | p-value | ||
|---|---|---|---|
| Sex | |||
| Male (n = 22) | 90 % | 0.136 | |
| Female (n = 27) | 62 % | ||
| Age at RT, yrs | |||
| <60 (n = 22) | 74 % | 0.818 | |
| ≥60 (n = 27) | 82 % | ||
| Primary mucosal melanoma | |||
| No (n = 38) | 82 % | 0.04 | |
| Yes (n = 11) | 60 % | ||
| BRAF mutation | |||
| Absent (n = 26) | 80 % | 0.394 | |
| Present (n = 18) | 75 % | ||
| Prior 2+ lines systemic therapy | |||
| No (n = 20) | 81 % | 0.618 | |
| Yes (n = 29) | 70 % | ||
| Prior local therapy to target site | |||
| No (n = 39) | 85 % | 0.289 | |
| Yes (n = 14) | 62 % | ||
| RT target was unresectable or oligometastatic | |||
| No (n = 30) | 69 % | 0.563 | |
| Yes (n = 23) | 85 % | ||
| Target size | |||
| ≤5 cm (n = 24) | 69 % | 0.478 | |
| >5 cm (n = 29) | 88 % | ||
| EQD2α/β=3 54 Gy+ | |||
| No (n = 19) | 67 % | 0.26 | |
| Yes (n = 34) | 88 % | ||
| Tumor shrinkage after RT | |||
| No (n = 14) | 60 % | 0.004 | |
| Yes (n = 39) | 93 % | ||
EQD2α/β=354 Gy+: Prescription dose to GTV was equal to or greater than 54 Gy, n = number of patients, RT: radiation therapy.
HFRT-associated toxicity
Approximately half of patients had a grade 2 acute toxicity with RT (41 %, n = 21). The most common acute toxicities overall were radiation dermatitis (16 %, n = 8) or a pain flare (14 %, n = 7). Mucositis and/or esophagitis was seen in 10 % of patients (n = 5) and fatigue was experienced by 8 % (n = 4). A single patient receiving head and neck HFRT developed grade 3 mucositis.
Late toxicity was uncommon and typically grade 1. Changes to skin and subcutaneous tissue including fibrosis or pigmentation changes were seen in 3 patients. Two patients had pulmonary toxicities. This included one patient with pneumonitis not requiring steroids, and one with a persistent dry cough.
One patient with sinonasal melanoma receiving urgent simultaneous initiation of ipilimumab and nivolumab with skull base-directed RT developed bilateral vision loss 10 months after HFRT completion. With a prescription of 42.5 Gy in 17 fractions the Dmax of the optic chiasm was only 39.5 Gy (EQD2α/β=2 = 42 Gy) but MRI at the time of progressive vision loss showed bilateral prechiasmatic optic nerve enhancement within the HFRT field which was thought related to treatment. While receiving RT, she had significant improvement in facial numbness and never subsequently progressed locally. After combination PI3K inhibitor and anti-PD1 therapy, the patient now has stable overall disease and is doing well with stable vision loss 2 years later.
Discussion
Patients with advanced or unresectable melanoma are living longer than ever before. However, it is common for patients to develop sites of progression during their disease course. There are many options for how to address these sites of progression including changing/adding systemic therapy as well as considering local therapy options. SBRT has been shown to provide durable local control in patients with metastatic melanoma [15]. Unfortunately, this approach is not always possible in the context of challenging anatomic locations and larger tumors. This study supports HFRT as an alternative approach to control discrete tumor targets in clinical contexts not amenable to SBRT. Specifically, we show that HFRT is a safe and effective local therapy option that provides durable local control (2-year LC 79 %) with limited toxicity. Most patients (74 %) experienced tumor shrinkage on imaging after HFRT. Of the 9 patients with unchanged tumor size, 67 % (n = 6) had decrease in FDG avidity.
The majority of HFRT targets were causing significant symptoms at the time of treatment (43 of 53 tumors, 81 %). The most common symptom was pain, with or without neurologic symptoms (32 of 43 tumors, 74 %). Other tumor-related symptoms included neurologic symptoms alone, respiratory symptoms, bleeding, urinary symptoms and bowel symptoms. Notably, HFRT improved tumor-related symptoms in 98 % of patients. We also found documented evidence of decreasing pain medication requirement in 6 of 32 patients who were on prescription pain medication prior to HFRT. Thus, our data suggests that HFRT provides excellent palliation.
In the absence of effective local therapy options, most patients with even limited sites of progressive disease require changing or adding systemic therapy. In our patient cohort, 67 % of patients (34 of 51 treatment courses) were able to continue off systemic therapy, continue on their existing systemic therapy, or stop systemic therapy after HFRT. By avoiding a requirement for new lines of systemic therapy, a patient can continue an otherwise effective treatment course or potentially avoid the toxicities of systemic therapy for a longer period. Together with symptom palliation, this finding suggests that HFRT can serve as an important tool to mitigate tumor-associated symptoms that can adversely affect quality of life.
The radiation planning details of HFRT are critical to its safety and efficacy. Using IMRT or VMAT approaches allows the implementation of a simultaneous integrated boost technique for most patients, whereby it is possible to dose-escalate gross tumor while providing a lower dose to areas of potential microscopic disease, immediately adjacent to the main tumor volume. Depending on the clinical scenario, we often further dose-escalate a contracted tumor volume with the goal of balancing the risk of toxicity while maximizing disease control. Of note, we always prioritize organ-at-risk constraints to critical structures. Our definition of HFRT is broad and includes regimens between 12–17 fractions with prescription doses to the GTV varying from 36 Gy to 52.5 Gy. Notably, the prescription dose to the PTV is often lower in the context of most patients being treated with a simultaneous integrated boost.
Our study also provides some insight into which patients are particularly well suited for HFRT. Seventy-three percent of patients had an ECOG performance status of 0–1, with a median time from melanoma diagnosis of 1 year. These factors speak to our selection bias in reserving this treatment approach for those with sufficient life expectancy to potentially benefit from greater durability of local control than might be expected with conventional palliative RT regimens. However, patients were typically not treatment-naive and had a median of 2 prior lines of systemic therapy with 63 % receiving immune checkpoint inhibition up to the time of HFRT. It was not uncommon for patients to have received prior local therapy to the HFRT target, with prior surgery being the most common.
The strongest predictor of durable disease control in our HFRT cohort was evidence of radiographic shrinkage (2-year LC 93 % vs. 60 %, p = 0.004). Mucosal melanoma appeared to have lower rates of durable local control in comparison to the cutaneous (2-year LC 60 % vs. 82 %, p = 0.04). However, it is notable that 55 % (6 of 11) of mucosal melanoma patients were treated to their primary site, in comparison to 21 % of cutaneous melanoma patients (8 of 38). One could hypothesize that the radiation sensitivity of mucosal structures limited the aggressiveness of the HFRT dose and field. While there was no statistically significant association between GTV prescription and the likelihood of disease control, patients who received EQD2α/β=3 > 54 Gy, had a numerically higher 2-year local control (88 % vs. 67 %).
HFRT delivered in approximately 15 fractions has a prior track record of efficacy in tumor histologies with known relative radiation resistance. A recent study from our group focused on patients with unresectable or metastatic sarcoma, showed that a similar approach produced a 73 % 1-year local control rate and provided palliative relief to 95 % of those with symptomatic disease [16]. Doses up to 67.5 Gy in 15 fractions for unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma have been found to produce excellent 2-year local control of 94–95 % [17]. A prospective study of 60 Gy in 15 fractions for non-small cell lung cancer patients also found similar local control as conventional definitive RT [18]. Notably, as the total dose increases, the potential risk of toxicity likely rises as well, such that the optimal RT dose to appropriately balance the risk/benefit in patients with advanced disease requires careful thought.
HFRT may not be an optimal treatment approach for all patients with metastatic or unresectable melanoma. This approach typically uses radiation planning techniques (primarily IMRT or VMAT) that require greater time from CT simulation to treatment start than conventional palliative RT. As such, patients with severe symptoms or those with imminent risk of injury to critical structures (e.g. spinal cord) are not good candidates. For such patients, conventional palliative RT continues to play a critical role. However, in thinking about the durability of conventional palliation it is important to acknowledge that many studies investigating the role of various palliative RT regimens specifically excluded melanoma, due to its relative radioresistance [8], [9], [10].
Ultimately, while this study provides support for the use of HFRT in selected melanoma patients with metastatic or unresectable disease who are not amenable to SBRT, there remain unanswered questions about specifically who is most likely to benefit from this treatment approach. Given the retrospective nature of this study, there is intentional selection bias in who received the HFRT regimen and what dose fractionation was chosen. We would propose considering this treatment regimen for melanoma patients who do not require urgent RT initiation and have >6-month life expectancy while considering the availability of future systemic therapy options. While we provide evidence that HFRT can be an important tool in the management of individual progressing sites of disease for melanoma patients, there remains significant room for improvement in overall disease control.
CRediT authorship contribution statement
Sydney A. Keatts: Investigation, Formal analysis, Writing – original draft, Visualization. Aya F. Salem: Data curation, Writing – original draft. David M. Swanson: Formal analysis, Writing – review & editing. Ahsan S. Farooqi: Conceptualization, Resources, Writing – review & editing. Andrew J. Bishop: Conceptualization, Resources, Writing – review & editing. Rodabe N. Amaria: Resources, Writing – review & editing. Jennifer L. McQuade: Resources, Writing – review & editing. Isabella C. Glitza Oliva: Resources, Writing – review & editing. Adi Diab: Resources, Writing – review & editing. Roi Weiser: Resources, Writing – review & editing. Sarah B. Fisher: Resources, Writing – review & editing. Ryan P. Goepfert: Resources, Writing – review & editing. Merrick I. Ross: Resources, Writing – review & editing. B. Ashleigh Guadagnolo: Supervision, Conceptualization, Resources, Writing – review & editing. Devarati Mitra: Supervision, Conceptualization, Investigation, Data curation, Formal analysis, Writing – original draft, Visualization, Funding acquisition.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Amaria reported receiving research funding from Obsidain and OnKure pharma companies outside the scope of the submitted work. Dr. McQuade reported receiving consultation fees from Merck and honoraria from Bristol Myers Squibb and Roche outside the scope of the submitted work. Dr. Glitza reported receiving consultation fees from Biodexa, Everclear and Midatech in addition to speaker fees from Novartis andPfizer. She also reported receiving research support from BMS, MERCK and Pfizer. And she serves on the advisory board of BMS, Novartis, and Pfizer outside the scope of the submitted work. Dr. Diab reported receiving compensation from Memgan for advisory board membership outside the scope of the submitted work. Dr. Ross reported receiving research funding from AMGEN Pharma and travel expenses from Merck Pharma outside the scope of the submitted work. No other disclosures were reported.
Acknowledgment
This research was made possible by grant support to Dr. Mitra from NCATS (KL2TR003168) and RSNA (RSCH2215). The overall effort was also supported by NCI through the Cancer Center Support (Core) Grant (CA016672) to the University of Texas MD Anderson Cancer Center.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2024.100856.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Knight A., Karapetyan L., Kirkwood J.M. Immunotherapy in melanoma: recent advances and future directions. Cancers (Basel) 2023;15(4):1106. doi: 10.3390/cancers15041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Schuckmann L.A., Hughes M.C.B., Ghiasvand R., et al. Risk of melanoma recurrence after diagnosis of a high-risk primary tumor. JAMA Dermatol. 2019;155(6):688–693. doi: 10.1001/jamadermatol.2019.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong X.D., Tyler D., Johnson J.L., DeMatos P., Seigler H.F. Analysis of prognosis and disease progression after local recurrence of melanoma. Cancer. 2000;88(5):1063–1071. doi: 10.1002/(SICI)1097-0142(20000301)88:5<1063::AID-CNCR17>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 4.Comito F., Leslie I., Boos L., et al. Oligoprogression after checkpoint inhibition in metastatic melanoma treated with locoregional therapy: a single-center retrospective analysis. J Immunother. 2020;43(8):250. doi: 10.1097/CJI.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 5.Testori A.A.E., Blankenstein S.A., van Akkooi A.C.J. Surgery for metastatic melanoma: an evolving concept. Curr Oncol Rep. 2019;21(11):98. doi: 10.1007/s11912-019-0847-6. [DOI] [PubMed] [Google Scholar]
- 6.Middleton M.R., Hoeller C., Michielin O., et al. Intratumoural immunotherapies for unresectable and metastatic melanoma: current status and future perspectives. Br J Cancer. 2020;123(6):885–897. doi: 10.1038/s41416-020-0994-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen L., Qi H., Chen S., et al. Cryoablation combined with transarterial infusion of pembrolizumab (CATAP) for liver metastases of melanoma: an ambispective, proof-of-concept cohort study. Cancer Immunol Immunother. 2020;69(9):1713–1724. doi: 10.1007/s00262-020-02566-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shuja M, Elghazaly AA, Iqbal A, et al. Efficacy of 8 Gy single fraction palliative radiation therapy in painful bone metastases: A single institution experience. Cureus. 10(1):e2036. doi:10.7759/cureus.2036. [DOI] [PMC free article] [PubMed]
- 9.Yarnold J.R. 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: randomised comparison with a multifraction schedule over 12 months of patient follow-upOn behalf of the Bone Pain Trial Working Party. Radiother Oncol. 1999;52(2):111–121. doi: 10.1016/S0167-8140(99)00097-3. [DOI] [PubMed] [Google Scholar]
- 10.Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. JNCI: J Natl Cancer Inst 2005;97(11):798-804. doi:10.1093/jnci/dji139. [DOI] [PubMed]
- 11.Rodríguez Plá M., Dualde Beltrán D., Ferrer A.E. Immune checkpoints inhibitors and SRS/SBRT synergy in metastatic non-small-cell lung cancer and melanoma: A systematic review. Int J Mol Sci. 2021;22(21):11621. doi: 10.3390/ijms222111621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Youland R.S., Packard A.T., Blanchard M.J., et al. 18F-FDG PET response and clinical outcomes after stereotactic body radiation therapy for metastatic melanoma. Adv Radiat Oncol. 2017;2(2):204–210. doi: 10.1016/j.adro.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi W. Radiation Therapy for Melanoma. In: Ward WH, Farma JM, eds. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017. Accessed April 10, 2024. http://www.ncbi.nlm.nih.gov/books/NBK481863/.
- 14.Guckenberger M., Lievens Y., Bouma A.B., et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21(1):e18–e28. doi: 10.1016/S1470-2045(19)30718-1. [DOI] [PubMed] [Google Scholar]
- 15.Stinauer M.A., Kavanagh B.D., Schefter T.E., et al. Stereotactic body radiation therapy for melanoma and renal cell carcinoma: impact of single fraction equivalent dose on local control. Radiat Oncol. 2011;6:34. doi: 10.1186/1748-717X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyce-Fappiano D., Damron E.P., Farooqi A., et al. Hypofractionated radiation therapy for unresectable or metastatic sarcoma lesions. Adv Radiat Oncol. 2022;7(3) doi: 10.1016/j.adro.2022.100913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong T.S., Wo J.Y., Yeap B.Y., et al. Multi-institutional phase ii study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. JCO. 2016;34(5):460–468. doi: 10.1200/JCO.2015.64.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iyengar P., Zhang-Velten E., Court L., et al. Accelerated hypofractionated image-guided vs conventional radiotherapy for patients with stage II/III non-small cell lung cancer and poor performance status: A randomized clinical trial. JAMA Oncol. 2021;7(10):1497–1505. doi: 10.1001/jamaoncol.2021.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.