Abstract
Purpose
The impact of dietary counseling on body composition in early breast cancer patients (EBC) treated with aromatase inhibitors (AIs) is uncertain. The aim of this study was to assess the effects of a diet counseling program on weight, BMI, total and regional body composition in patients treated with AIs.
Methods
This observational study involved 194 EBC patients, of which 97 attended a 6-month personalized counseling program, based on Mediterranean diet principles (cohort A) and 97 did not (cohort B). Dual-energy X-ray absorptiometry (DXA) scan was used to measure the total and regional fat and lean body mass, before (baseline) and after at least 18 months of AI-therapy.
Results
Weight and BMI increased significantly, on the average, in cohort B, but not in cohort A. In the cohorts A and B, fat mass increased by 10 % and 7.7 % respectively, while lean mass decreased by 3.3 % and 2.6 % from before to after AI therapy, without statistically significant differences between them using the Mann-Whitney test. The changes in body composition were greater in premenopausal than in postmenopausal women at cancer diagnosis. The proportion of patients with sarcopenia, obesity and sarcopenic obesity increased from before to after AI therapy, similarly in both cohorts.
Conclusions
Patients treated with AIs reported an increase in fat mass and a decrease in lean mass, and consequently an increase in sarcopenia and obesity, regardless of the participation in a dietary counseling program. A combined dietary counseling and physical exercise program may be necessary for preventing these unfavourable changes in these patients.
Keywords: Body composition, Fat mass, Lean mass, Sarcopenia, Obesity, Diet, Physical exercise
Highlights
-
•
Lifestyle is still considered the keystone for preventing obesity and sarcopenia.
-
•
Patients treated with AIs reported an increase in fat mass and a decrease in lean mass.
-
•
Dietary counseling with physical exercise program could prevent these unfavourable changes.
1. Introduction
More than a half of patients with early breast cancer (EBC) experience significant weight gain, particularly in body fat, after receiving antineoplastic treatment [1]. Unhealthy dietary habits and lack of physical activity are the main causes, with hormone therapy and chemotherapy as favoring factors [[1], [2], [3], [4]]. The increase in body fat, particularly visceral fat, is associated with an increased risk of chronic diseases such as type 2 diabetes and cardiovascular diseases [[5], [6], [7]]. Moreover, several EBC women experience a loss of lean body mass after therapy, which can lead to sarcopenia and sarcopenic obesity [8,9]. Sarcopenia and sarcopenic obesity are an emerging public health problem in the elderly worldwide, due to substantial adverse effects on mental and physical health, and on all-cause mortality [10,11]. Sarcopenic EBC patients are more prone to experiencing drug-induced side effects, are more at risk of disease relapse or progression, and have a decreased survival rate than women who are not sarcopenic [8,9,12]. Sarcopenic obesity has been considered a possible side‐effect of breast cancer treatment [13,14], although the few studies on the effects of the hormonal derangement induced by recent aromatase inhibitor (AI) on body composition reported inconsistent results [[15], [16], [17]]. Most studies on weight gain in EBC patients assessed body weight as the outcome, which however has low sensitivity for detecting both obesity and sarcopenia, and cannot differentiate between fat mass and lean mass, since a concurrent increase in fat mass and decrease in lean mass produces substantial changes in body composition without variation in body weight [13,18,19]. Dual-energy X-ray absorptiometry (DXA), bioelectrical impedance analyses or computed tomography are the recommended tools for the assessment of total and regional body composition at present [11,[18], [19], [20]]. Lifestyle is still considered the keystone for preventing obesity and sarcopenia, and several randomized controlled trials (RCTs) showed that, in EBC patients, dietary and physical activity interventions can be effective in reducing weight, body mass index (BMI) and waist circumference in overweight or obese women, and in preventing weight gain in normal weight women [21,22]. However, RCTs yielded less consistent results as regards the effects of interventions on body composition, particularly fat mass and fat distribution, and muscle mass [21], and few data are available on the impact of routinely applied, real world, lifestyle interventions on body composition in EBC patients after adjuvant hormone therapy. In the present study we aimed to assess the changes over time of body weight, BMI, fat body mass and lean body mass, in the whole body and in different body districts, in EBC patients given hormone therapy with AIs, according to women's participation in a dietary counseling program.
2. Patients and methods
2.1. Study design
This is an observational, single-centre cohort study, conducted at the Medical Oncology Unit and Breast Unit of Azienda Socio Sanitaria Territoriale (ASST) Spedali Civili of Brescia (Italy), from 2018 to 2020. This is a secondary analysis of a prospective study (internal protocol No 3270, registered in August 2014) that involved additional retrospective collection of data and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline [23].
Each EBC patient was offered to participate in a dietary counseling program, provided by a trained dietitian, before starting AI therapy. The patients who attended at least 6 months of the dietary counseling were considered candidate for the present study, and assigned to cohort A if eligible, whereas patients who did not participate in the program were candidate for the cohort B.
Patients of the two cohorts were paired individually in a 1:1 ratio, using the propensity score method.
2.2. Participants
Patients who had histologically confirmed EBC (stage I-III) and completed surgery, chemotherapy, and received at least 18 months of AI therapy were eligible. It was necessary for the patients to have two DXA scans, one before starting AI therapy and the other after completing at least 18 months of AI therapy. Patients who had poor performance status (ECOG >2), metastatic disease, or had previously received treatment for other tumors were excluded from the study.
2.3. Dietary counseling program
Each patient assigned to cohort A had monthly meetings with a trained dietician, where they received a meal plan with daily energy intake and macronutrient composition tailored to their dietary habits and nutritional status. The dietary advice considered each patient's illness and reported symptoms, including constipation, nausea, meteorism, and fatigue. The principles of the Mediterranean diet were followed, focussed on reduced consumption of simple sugars, sweets, meat and poultry, salt, milk and dairy products, and ultra-processed foods, and on increased consumption of vegetables and fruits, nuts, whole grains, legumes and water. The dietician emphasized the patient's commitment to meal planning by reviewing the patient's seven-day food diary each session. Moreover, the dietician encouraged each patient to increase their physical activity at any time.
Patients assigned to cohort B, who did not participate in the dietary education program were only given general advice on healthy eating habits.
Both dietary counseling for cohort A and general advice for cohort B were based on the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) guideline recommendations [[24], [25], [26]] and on the Italian Dietary Guidelines on recommended levels of energy and nutrients intake [27,28].
2.4. Demographics, lifestyle and diet assessment
The age at EBC diagnosis was recorded. Working activity was categorized as employed or unemployed and tobacco use as previous/current smoker vs. never smoked.
Physical activity was evaluated using the International Physical Activity Questionnaire (IPAQ) [29], which assesses specific types of activity, i.e. walking, moderate-intensity and vigorous-intensity activities and calculates the Metabolic Equivalent of Tasks (METs). A total score was calculated adding the scores of walking and moderate and vigorous activities, and subjects were classified into 3 categories: low (<600 METs), moderate (600–2,999 METs), and high (≥3,000 METs) physical activity.
Assessment of anthropometric parameters was done following standardized procedures, with subjects only wearing light clothes and without shoes. Height was measured using a wall-mounted stadiometer. Weight was measured using a calibrated digital scale. BMI was calculated by dividing body weight (kilograms) by height squared (meters).
2.5. Body composition assessment
The body composition was assessed with body dual-energy x-ray absorptiometry using Hologic QDR-4500W instrumentation (DXA; Hologic Corporation, Waltham, Massachusetts, software version 9.03) [30]. DXA scan was performed in all patients of the two cohorts before (baseline) and after at least 18 months of AI-therapy. The DXA analysis was used to calculate:
-
1)
total fat mass, expressed in kg and in percentage, and total lean mass, in kg, in the whole body and in the body districts: right and left arms, right and left legs, trunk, and head;
-
2)
the following ratios: trunk to appendicular fat; total fat mass (kg) to square of height (meters) (fat mass index, FMI); total lean mass (kg) to square of height (meters) (lean mass index, LMI); the sum of arms and legs lean mass (kg) to square of height (meters) (appendicular lean mass index, ALMI).
Sarcopenic obesity was defined as the co-existence of excess adiposity and low muscle mass according to the criteria proposed by the European Society for Clinical Nutrition and Metabolism (ESPEN) [20] and the European Association for the Study of Obesity (EASO) [20] and by the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) [31]. In agreement with previous studies based on DXA measurements [20,31], the following cut-off points for obesity, sarcopenia, and sarcopenic obesity were established: total fat mass percentage >40.8 % and ALMI <5.5 kg/m2.
3. Statistical analyses
Categorical variables were expressed as frequencies and percentages, and means with their 95 % confidence intervals (95 % CI) or medians and inter quartile ranges for continuous variables, according to their distribution. To obtain a similar set of participants in both cohort A and B, a propensity scores matching procedure was calculated using a logistic regression model based on age, BMI, pT and pN at the baseline as covariates. According to this procedure, the candidate subjects of the two groups were matched through the algorithm of nearest neighbor matching without substitution, matching each patient receiving the dietary counseling (cohort A) with a patient not receiving the dietary counseling (cohort B), to minimize the absolute distance in terms of propensity score, with a maximum acceptable threshold of 0.01 [32]. The comparisons of weight, BMI, and body composition parameters between the two cohorts were performed using the nonparametric Mann-Whitney U test, due to non-normal, skewed, distribution of the continuous variables. For the same variables, the differences between before and after AI therapy were tested with the Wilcoxon signed-rank test for paired data, for each cohort. The effects of the dietary counseling program on the before-after AI therapy changes in body parameters were evaluated with the difference-in-differences approach, using the non-parametric Mann-Whitney U test for the comparison of the before-after AI therapy differences between the two cohorts. Furthermore, the analysis of covariance (ANACOVA) was used to evaluate the association of each body parameter value after AI therapy with the cohort type (independent variable), with each parameter value at baseline and age at diagnosis as covariates.
The comparisons of proportions between the two cohorts were performed using the chi square and the exact test, whereas the intra-cohort comparisons of the proportion of women with sarcopenia, obesity and sarcopenic obesity from before to after AI therapy were done using the McNemar exact test for paired data, for each cohort. Logistic regression models were also fitted to assess the association of sarcopenia, obesity and sarcopenic obesity after AI therapy with demographic, clinical and body composition parameters before AI therapy (baseline). Separate analyses by menopausal status were also done to assess the possible impact of treatment-induced menopause on body composition changes from before to after AI therapy. All the statistical tests were two-sided with a type I error of 5 %. We did all the analyses with SPSS (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.) and Stata Statistical Software: Release 18. (College Station, TX: StataCorp LLC. 2023).
4. Results
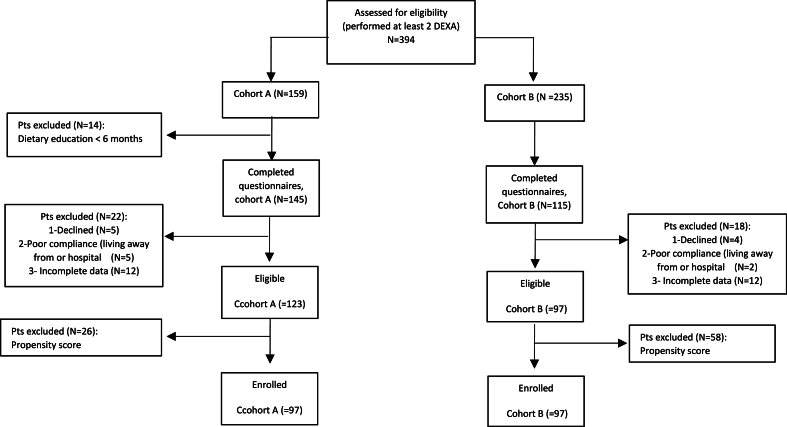
Three-hundred and ninety-four women treated with AIs and followed at the Breast Unit of the ASST-Spedali Civili of Brescia from 2018 to 2020 were selected as candidate (Fig. 1): 159 received the dietary counseling (cohort A) and 235 did not (cohort B). In the cohort A, 14 patients were excluded because attended the dietary counseling program for less than 6 months Additionally, 22 patients in cohort A and 18 in cohort B were excluded due to refusal or ineligibility (poor compliance or incomplete questionnaires provided), and 26 and 58 patients in cohorts A and B, respectively, because of the propensity score procedure. Finally, 97 patients in the cohort A and 97 in the cohort B were included in the study.
Fig. 1.
Consort diagram.
4.1. Patients’ characteristics
Table 1 details the characteristics of the 194 patients who participated in the study. The two cohorts were similar regarding age, weight, BMI, working activity and smoking habits, and for the following tumor characteristics: pT and pN stage, histological type, Her-2 amplification, and expression of estrogen and progesterone receptors. Patients in cohort A displayed a greater proportion of grade 3, high proliferative tumors, and therefore received adjuvant chemotherapy more frequently than cohort B. Most women were postmenopausal when received BC treatment in both groups, without significant differences between them. Among premenopausal patients, 20 of 33 (60.6 %) and 20 of 22 (90.9 %) received ovarian suppression in cohorts A and B, respectively. The majority of women practiced low physical activity, more in the cohort B than cohort A (76.3 % and 54.6 %, respectively).
Table 1.
Characteristics of the patients in a dietary counseling group (cohort A) and in a control group (cohort B) before starting aromatase inhibitors (AI) therapy (before).
| Characteristic | Cohort A (n = 97) n (%) | Cohort B (n = 97) n (%) | Pa |
|---|---|---|---|
| Age (years), median (range) | 59 (33–80) | 63 (40–83) | 0.14 |
| Menopausal status | |||
| Pre-menopause | 33 (35.1 %) | 22 (23.7 %) | 0.09 |
| Post-menopause | 61 (64.9 %) | 71 (76.3 %) | |
| Weight (kg), median (IQR) | 65 (57–73) | 63 (57–72) | 0.52 |
| BMI (kg/m2), median, IQR | 24.6 (21.5–28.7) | 24.2 (21.5–26.7) | 0.58 |
| Working activity | |||
| unemployed | 33 (34.1 %) | 43 (44.4 %) | 0.14 |
| employed | 64 (65.9 %) | 54 (55.6 %) | |
| Physical activityb | |||
| Low | 53 (54.6 %) | 74 (76.3 %) | 0.002 |
| Moderate | 39 (40.2 %) | 23 (23.7 %) | |
| High | 5 (5.1 %) | 0 | |
| Smoking habitsc | |||
| No | 76 (81.7 %) | 68 (77.3 %) | 0.45 |
| Yes | 17 (17.5 %) | 20 (20.6 %) | |
| pTd | |||
| 1 | 53 (54.6 %) | 60 (61.9 %) | 0.39 |
| ≥2 | 42 (45.4 %) | 37 (38.1 %) | |
| pNd | |||
| 0 | 40 (42.1 %) | 52 (53.6 %) | 0.11 |
| ≥1 | 55 (57.8 %) | 45 (46.4 %) | |
| Histological type | |||
| No Special Type (NST) | 75 (77.3 %) | 68 (70.1 %) | 0.25 |
| Other | 22 (22.7 %) | 29 (29.9 %) | |
| Gradinge | |||
| G1 o G2 | 29 (29.9 %) | 58 (59.7 %) | <0.001 |
| G3 | 68 (70.1 %) | 39 (40.3 %) | |
| HER 2 | |||
| Negative | 70 (72.2 %) | 78 (80.4 %) | 0.18 |
| Positive | 27 (27.8 %) | 19 (19.6 %) | |
| Chemotherapy | |||
| No | 20 (20.6 %) | 62 (63.9 %) | <0.001 |
| Yes | 77 (79.4 %) | 35 (36.1 %) | |
| Duration of therapy (months), mean (SD) | 25.5 (7.2) | 28.5 (9.4) | 0.016 |
| ER (%), median (IQR) | 100 (93–100) | 100 (95–100) | 0.97 |
| PgR (%), median (IQR) | 80 (25–95) | 62 (15–95) | 0.47 |
| Ki67 (%), median (IQR) | 26 (15–36.5) | 18 (12.5–27) | 0.0001 |
Cohort A: participants in a dietary counseling program; Cohort B: receiving only general advice on healthy diet guidelines. pT: pathological tumor stage; pN: pathological nodal status; IQR: interquartile range; G1: well-differentiated tumour; G2 moderately differentiated tumour; G3: undifferentiated tumour; HER2: Human epidermal growth factor receptor; ER: estrogen receptor; PgR: progesterone receptor.
Mann-Whitney and chi-square test for unpaired data for continuous and categorical variables, respectively.
Physical activity: low: <600, moderate: 600–2999, and high: ≥3000 MET-min/week.
Data for 93 and 88 patients in cohort A and B, respectively.
Data for 95 patients in cohort A.
Data for 93 and 95 patients in cohort A and B, respectively.
The cohort A patients participated at all the monthly meetings and the counseling sessions.
The mean duration of AI treatment was 25.5 (SD 7.2) months and 28.5 (SD 9.4) months, in cohorts A and B, respectively (p = 0.016). Postmenopausal patients received letrozole, while premenopausal patients received exemestane and ovarian function suppression.
4.2. Changes in weight, BMI and body composition parameters
Table 2 shows the changes in anthropometric parameters and body composition parameters before and after AIs treatment in cohort A and B. Body weight and BMI remained stable during treatment in cohort A and showed a slight, statistically significant, increase in cohort B (+1.9 %, 95 % CI 0.8–3.1). The lean mass decreased significantly in both cohorts (−3.3 % and −2.6 % in cohorts A and B, respectively), as well as LMI and ALMI (−3.3 % for both measures in cohort A −2.6 % and −1.1 %, respectively, in cohort B). On the opposite, the fat mass increased, in absolute values (+10.1 % and +7.7 % in cohorts A and B, respectively) and in percentages of body weight (+9.0 % and +5.5 %, respectively). Accordingly, FMI and trunk/appendicular fat ratio increased in cohort A (+10.6 %% and +8.1 %, respectively) and B (+7.4 % for both indices). The difference-in-differences analysis showed no statistically significant differences in the before-after changes between the two cohorts for each parameter, using ANACOVA with the baseline measure of each parameter and age as covariates. Only the baseline value of each body parameter and age at diagnosis were predictive of the percent change in lean and fat mass from before to after AI therapy by ANACOVA. Percent change in fat mass decreased by increasing age (Suppl. Fig. 1) and by increasing baseline fat mass (Suppl. Fig. 2); and percent change in ALMI decreased by increasing baseline lean mass (Suppl. Fig. 3) and was not related with age, fitting quadratic regression models. Similar inverse relationships with baseline values were found for the other body composition indices.
Table 2.
Body weight, body mass index (BMI), and lean and fat mass before and after at least 18 months of aromatase inhibitors (AI) therapy in a dietary counseling (cohort A) and in a control cohort (cohort B).
| Parameter | Cohort A |
Cohort B |
Pa | ||
|---|---|---|---|---|---|
| Mean (95 % CI) | % change from before to after Mean (95 % CI) | Mean (CI 95 %) | % change from before to after Mean (95 % CI) | ||
| Weight (kg) | |||||
| Before AI therapy | 66.40 (63.94–68.86) | +0.8 | 65.27 (62.80–67.74) | +1.9 | 0.242 |
| After AI therapy | 66.76 (64.28–69.24) | (-0.7 to 2.3) | 66.37 (63.94–68.80) | (0.8–3.1) | |
| Pb | 0.645 | < 0.001 | |||
| BMI (kg/m2) | |||||
| Before AI therapy | 25.14 (24.18–26.09) | +0.8 | 24.85 (23.92–25.77) | +1.9 | 0.242 |
| After AI therapy | 25.25 (24.31–26.18) | (-0.7 to 2.3) | 25.26 (24.36–26.16) | (0.8–3.1) | |
| pb | 0.788 | 0.272 | 0.001 | ||
| Lean mass (kg) | |||||
| Before AI therapy | 40.49 (39.45–41.52) | −3.3 | 39.39 (38.43–40.35) | −2.6 | 0.478 |
| After AI therapy | 39.06 (38.04–40.07) | (-4.8 to −1.8) | 38.33 (37.34–39.32) | (-3.8 to −1.4) | |
| pb | < 0.001 | < 0.001 | |||
| LMI (kg/m2) | |||||
| Before AI therapy | 15.29 (14.90; 15.68) | −3.3 | 14.95 (14.60; 15.30) | −2.6 | 0.223 |
| After AI therapy | 14.71 (14.33; 15.09) | (-4.8 to −1.8) | 14.59 (14.22; 14.97) | (-3.8 to −1.4) | |
| pb | < 0.001 | 0.001 | |||
| ALMI (kg/m2) | |||||
| Before AI therapy | 6.44 (6.03–6.85) | −3.3 | 6.02 (5.86; 6.18) | −1.1 | 0.114 |
| After AI therapy | 6.09 (5.90; 6.27) | (-5.4 to −1.2) | 5.96 (5.77; 6.14) | (-2.9 to 0.8) | |
| pb | <0.001 | 0.320 | |||
| Fat mass (kg) | |||||
| Before AI therapy | 24.54 (22.87–26.22) | 10.1 | 23.90 (22.26–25.54) | 7.7 | 0.302 |
| After AI therapy | 26.50 (24.79–28.22) | (6.6–13.7) | 25.44 (2379–27.09) | (4.8–10.7) | |
| pb | < 0.001 | < 0.001 | |||
| Fat mass % | |||||
| Before AI therapy | 35.8 % (34.4–37.1) | 9.0 | 35.8 % (34.4–37.1) | 5.5 | 0.06 |
| After AI therapy | 38.4 % (37.1–39.7) | (6.3–11.6) | 37.8 % (36.4–39.1) | (3.3–7.8) | |
| pb | < 0.0001 | < 0.0001 | |||
| FMI (kg/m2) | |||||
| Before AI therapy | 9.25 (8.60; 9.89) | 10.6 | 9.11 (8.46; 9.77) | 7.4 | 0.165 |
| After AI therapy | 10.01 (9.37; 10.66) | (7.1–14.1) | 9.66 (9.01; 10.30) | (4.4–10.4) | |
| pb | < 0.001 | < 0.001 | |||
| Trunk/Appendicular fat ratio | |||||
| Before AI therapy | 0.92 (0.87–0.97) | 8.1 | 0.95 (0.90; 1) | 7.4 | 0.687 |
| After AI therapy | 0.99 (0.93–1.04) | (5.5–10.7) | 1.02 (0.96; 1) | (4.8–10.0) | |
| pb | < 0.0001 | < 0.0001 | |||
CI: Confidence interval; BMI: body mass index; ht: height in meters; ALMI: appendicular lean mass index; FMI: fat mass index.
Mann-Whitney U test for the comparison of the before-after differences between the two cohorts.
Wilcoxon signed-rank test for the before-after comparison for each cohort.
4.3. Changes in weight, BMI and body composition parameters according to menopausal status at EBC diagnosis
The mean age of premenopausal and postmenopausal women at EBC diagnosis was 50.7 (SD 0.9) and 65.9 (SD 0.7) years, respectively (p < 0.0001), without statistically significant differences between the two cohorts by menopausal status. Among premenopausal women, 67.3 % received chemotherapy (72.7 % and 59.1 % in cohorts A and B, respectively, p = 0.3), whereas among postmenopausal women 52.3 % received chemotherapy (82.0 % and 26.8 % in cohorts A and B, respectively, p < 0.001).
The before-after mean changes in body parameters in women who were in premenopausal or postmenopausal at EBC diagnosis are shown in Suppl. Table 1. Body weight and BMI increased more in premenopausal (+1.7 %, p = 0.3, and +2.7 %, p = 0.02, in cohort A and B, respectively) than in postmenopausal women (+0.1 % and +1.7 % in cohort A and B). Lean mass decreased more in premenopausal (−4.2 % and −3.3 % in cohort A and B) than in postmenopausal women (−2.8 % and −2.6 %% in cohort A and B). Similar trends were observed for LMI and ALMI. Fat mass increased more in premenopausal (+15.4 % and +9.2 % in cohort A and B), than in postmenopausal women (+6.9 % and +7.4 % in cohort A and B). Similar trends were found for fat mass %, FMI and trunk/appendicular fat ratio. However, ANACOVA models including baseline values of each parameter and age at diagnosis showed that the before-after changes in body composition parameters were not associated with postmenopausal status and cohort type (p > 0.1 for each parameter).
4.4. Changes in body composition parameters of different body districts
The changes in body composition parameters of different districts (trunk, upper and lower limbs, head) from before to after AI therapy in the two cohorts are shown in Table 3. The lean mass decreased in all body districts, more in cohort A than B for left arm (−5.2 % and −1.0 %, respectively; p = 0.004), left leg (−3.1 % and −0.3 %; p = 0.027) and right leg (−2.8 % and −0.5 %; p = 0.17). Fat mass increased from before to after AI therapy in all districts (arms, legs, trunk), without statistically significant differences between the cohorts.
Table 3.
Lean and fat mass of the regional districts, before and after at least 18 months of aromatase inhibitors (AI) therapy, in a dietary counseling (cohort A) and in a control cohort (cohort B).
| Parameter | Cohort A |
Cohort B |
Pa | ||
|---|---|---|---|---|---|
| Mean (95 % CI) | % change from before Mean (95 % CI) | Mean (CI 95 %) | % change from before Mean (95 % CI) | ||
| Trunk lean mass (kg) | |||||
| Before AI therapy | 20.71 (20.18–21.24) | −3.4 | 20.43 (19.89–20.97) | −3.1 | 0.779 |
| After AI therapy | 20.07 (19.44–20.50) | (-5.1 to −1.6) | 19.78 (19.25–20.31) | (-4.5 to −1.7) | |
| Pb | < 0.001 | < 0.001 | |||
| Left arm lean mass (kg) | |||||
| Before AI therapy | 1.91 (1.84–1.97) | −5.2 | 1.84 (1.78–1.90) | −1.0 | 0.004 |
| After AI therapy | 1.80 (1.74–1.87) | (-7.1 to −3.3) | 1.83 (1.77–1.88) | (-3.1 to 1.2) | |
| pb | < 0.001 | 0.061 | |||
| Right arm lean mass (kg) | |||||
| Before AI therapy | 1.99 (1.92–2.06) | 0.1 | 1.97 (1.91–2.02) | −0.04 | 0.796 |
| After AI therapy | 1.97 (1.90–2.04) | (-2.6 to 2.8) | 1.96 (1.90–2.02) | (-2.7 to 2.0) | |
| pb | 0.474 | 0.686 | |||
| Left leg lean mass (kg) | |||||
| Before AI therapy | 6.31 (6.11–6.51) | −3.1 | 5.96 (5.77–6.16) | −0.3 | 0.027 |
| After AI therapy | 6.11 (5.91–6.32) | (-5.0 to −1.2) | 5.94 (5.75–6.14) | (-1.9 to 1.3) | |
| pb | <0.001 | 0.320 | |||
| Right leg lean mass (kg) | |||||
| Before AI therapy | 6.78 (5.91–7.66) | −2.8 | 6.07 (5.88–6.26) | −0.5 | 0.176 |
| After AI therapy | 6.23 (6.03–6.43) | (-5.4 to −0.2) | 6.03 (5.83–6.22) | (-2.5 to 1.6) | |
| pb | 0.010 | 0.550 | |||
| Head lean mass (kg) | |||||
| Before AI therapy | 3.04 (2.96–3.12) | −6.1 | 3.02 (2.96–3.09) | −6.3 | 0.862 |
| After AI therapy | 2.86 (2.80–2.92) | (-8.2 to −4.0) | 2.82 (2.77–2.86) | (-8.1 to −4.5) | |
| p | < 0.001 | < 0.001 | |||
| Trunk fat mass (kg) | |||||
| Before AI therapy | 11.49 (10.51–12.47) | 15.7 | 11.36 (10.33–12.40) | 12.0 | 0.250 |
| After AI therapy | 12.69 (11.70–13.67) | (10.8–20.6) | 12.45 (11.48–13.43) | (8.0–16.0) | |
| pb | < 0.001 | < 0.001 | |||
| Left arm fat mass (kg) | |||||
| Before AI therapy | 1.54 (1.42–1.66) | 11.7 | 1.46 (1.34–1.58) | 6.4 | 0.124 |
| After AI therapy | 1.65 (1.53–1.78) | (0.9–16.4) | 1.52 (1.40–1.63) | (1.6–11.2) | |
| pb | < 0.001 | 0.184 | |||
| Right arm fat mass (kg) | |||||
| Before AI therapy | 1.55 (1.4–1.68) | 7.6 | 1.48 (1.36–1.60) | 4.5 | 0.314 |
| After AI therapy | 1.62 (1.50–1.75) | (3.6–11.6) | 1.49 (1.39–1.59) | (0.1–9.0) | |
| pb | 0.010 | 0.445 | |||
| Left leg fat mass (kg) | |||||
| Before AI therapy | 4.38 (4.10–4.66) | 8.2 | 4.28 (4.00–4.57) | 4.6 | 0.113 |
| After AI therapy | 4.69 (4.38–5.00) | (4.8–11.6) | 4.44 (4.14–4.74) | (1.6–7.6) | |
| pb | < 0.001 | 0.012 | |||
| Right leg fat mass (kg) | |||||
| Before AI therapy | 4.66 (4.36–4.95) | 5.5 | 4.45. (4.16–4.75) | 4.8 | 0.787 |
| After AI therapy | 4.88 (4.56–5.20) | (2.6–8.3) | 4.60 (4.29–4.90) | (1.1–8.6) | |
| pb | 0.002 | 0.009 | |||
| Head fat mass (kg) | |||||
| Before AI therapy | 0.90 (0.88–0.93) | 2.3 | 0.89 (090–0.91) | 0.3 | 0.254 |
| After AI therapy | 0.92 (0.90–0.94) | (-0.2 to 4.8) | 0.90 (0.87–0.90) | (-2.0 to 2.7) | |
| Pb | 0.092 | 0.327 | |||
CI: Confidence interval.
Mann-Whitney U test for the comparison of the before-after differences between the two cohorts.
Wilcoxon signed-rank test for the before-after comparison for each cohort.
4.5. Changes in the prevalence of sarcopenia, obesity, and sarcopenic obesity
The percentage of women with sarcopenia, obesity, and sarcopenic obesity before and after AI therapy in each cohort is shown in Table 4. The proportion of women with sarcopenia increased slightly, more in cohort A (from 15.5 % to 28.9 %) than in cohort B (from 23.7 % to 26.8 %), without statistically significant difference between the two cohorts before and after AI-therapy. The proportion of obesity increased similarly in both cohorts (from 23.7 % to 37.1 % and from 23.7 % to 34.0 % in cohort A and B, respectively). As a consequence, the proportion of sarcopenic obesity was small at baseline, but increased more than two times from before to after therapy in both cohorts (from 2.0 % to 7.2 %, and from 3.0 % to 8.3 %, in cohorts A and B, respectively).
Table 4.
Sarcopenia, obesity and sarcopenic obesity according to DXA assessment of body composition before and after at least 18 months of aromatase inhibitors (AI) therapy, in a dietary counseling (cohort A) and in a control cohort (cohort B).
| Parameter | Cohort A |
Cohort B |
||
|---|---|---|---|---|
| N (%) | % increase from before to after AI therapy | N (%) | % increase from before to after AI therapy | |
| Sarcopenia | ||||
| Before AI therapy | 15 (15.5) | 86.7 % | 23 (23.7) | 13.0 % |
| After AI therapy | 28 (28.9) | 26 (26.8) | ||
| Pa | 0.004 | 0.55 | ||
| Obesity | ||||
| Before AI therapy | 23 (23.7) | 56.5 % | 23 (23.7) | 43.5 % |
| After AI therapy | 36 (37.1) | 33 (34.0) | ||
| pa | 0.007 | 0.02 | ||
| Sarcopenic obesity | ||||
| Before AI therapy | 2 (2.0) | 250 % | 3 (3.0) | 167 % |
| After AI therapy | 7 (7.2) | 8 (8.3) | ||
| pa | 0.18 | 0.13 | ||
CI: Confidence interval.
McNemar exact test for the comparison of the before-after proportions for each cohort.
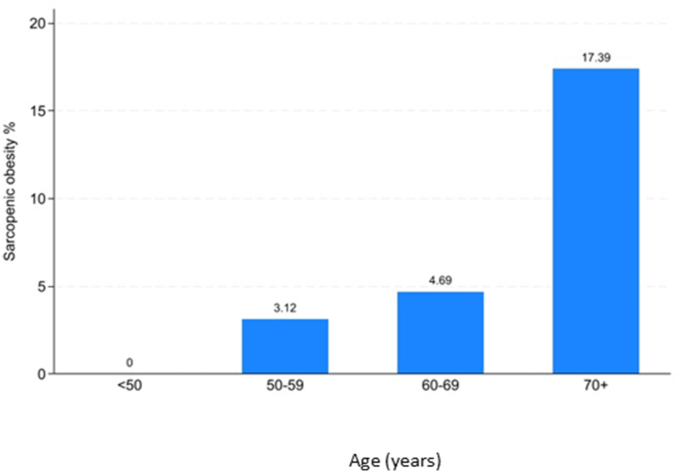
Overall, 15 of 194 women (7.7 %) had sarcopenic obesity after AI therapy, with a substantial increase with increasing age, from none in women aged <50 years to 17.4 % in those aged 70 years and over at diagnosis (Fig. 2).
Fig. 2.
Percentage of women with sarcopenic obesity after therapy with aromatase inhibitors according to age.
Women who already had sarcopenia, obesity, and sarcopenic obesity before AI therapy were more likely to experience these conditions after AI therapy. More than 80 % of women who were sarcopenic or obese before AI therapy were still sarcopenic or obese after AI therapy, in both cohorts (Suppl. Table 2). Sarcopenic obesity after AIs was more prevalent among women with this condition prior to AI therapy in cohort B, while there were no women with this condition in cohort A.
The associations between sarcopenia, obesity, and sarcopenic obesity after AI therapy as dependent variables and age and body composition indices at baseline as independent variables were assessed using multiple logistic regression analysis (Suppl. Table 3). Sarcopenia was inversely associated with baseline ALMI (OR = 0.034; 95 % CI 0.012–0.10), obesity was positively associated with baseline FBM% (OR = 1.48; 1.32–1.65), and sarcopenic obesity was associated with baseline ALMI (OR = 0.16; 95 % CI 0.06–0.46), FBM% (OR = 1.21; 95 % CI 1.06–1.37) and age (OR = 1.09; 95 % CI 1.02–1.18). No associations were found between the cohort type (A vs B) and clinical variables and sarcopenia, obesity or sarcopenic obesity, when also taking account of baseline values, by logistic regression analysis.
5. Discussion
The main findings of this study are that there was a decrease in lean mass and a rise in fat mass, despite no significant body weight changes, from before to after at least 18 months of AI treatment in both EBC patients who attended, and in those who did not attend, a dietary counseling program. Accordingly, sarcopenia, obesity and sarcopenic obesity increased from before to after AI therapy in both groups. Premenopausal women experienced larger body composition changes than postmenopausal women at diagnosis; although menopausal status had no statistically significant impact on changes in body composition parameters before and after AI therapy when taking account of before therapy (baseline) values. The presence of sarcopenia, obesity and sarcopenic obesity at baseline was highly predictive of the presence of the same condition after AI therapy.
The changes in body composition observed in this study are in line with the increase in fat mass (+7.2 %) and decrease in lean mass (−3.1 %) observed after 18 months of AI treatment in a recently published series of 428 EBC patients who attended our department in 2014–2022 [17]. Some, though not all, observational studies reported similar changes in body composition in EBC survivors, from before to after chemotherapy and hormone therapy, with wide differences between them [13,14,16,33]. The prevalence of sarcopenia after AI therapy (27.9 % in both cohorts combined) was in agreement with 33 % of BC patients with sarcopenia in two recent meta-analyses [9,12]. However, a high degree of heterogeneity was found in the prevalence of sarcopenia among the studies, with a range of 12 %–73 %, due to significant differences in demographics and clinical characteristics, time (before, during or after therapy), method of assessment, and cut-off for diagnosis [9,12].
The reason for changes in body composition in women with EBC who undergo adjuvant therapy is still questioned. On the one hand, the rapid transition to menopause in premenopausal BC patients with adjuvant therapy leads to an increase in body fat and decrease in lean mass, similarly to what naturally occurs in early premenopausal healthy women [13]. Indeed, we found larger body composition changes in premenopausal than postmenopausal BC women in both cohorts during the study period. When considering the two cohorts together, we found larger changes in lean mass (−3.9 %), and fat mass (+12.9 %) in premenopausal EBC patients who underwent AI treatment, than the annual changes observed in lean mass (−0.2 %) and fat mass (+2.7 %) in healthy White women during the menopause transition in a USA community-based cohort study [34]. On the other hand, the body composition changes in postmenopausal BC patients may partly reflect the natural increases in fat mass and decreases in lean mass in healthy aging [13]. The body composition mean values of EBC patients aged 50 years and over of the two cohorts together at baseline were similar to the mean or median values reported in cross-sectional studies performed in healthy Caucasian women of the same age, in the Italian and other Western populations (Suppl. Table 4) [[35], [36], [37], [38], [39], [40]]. However, the changes observed in EBC patients aged 50 years and over from before to after AI therapy, in about 2 years, were relevant (−2.7 % in LMI, +7.6 % in FMI and +6.1 % in FM%) and much bigger than the differences observed between each 10-year age category and the subsequent one in most studies on the general population. Therefore, EBC women undergoing AI therapy seem to have an accelerated aging process of their body composition compared to healthy women of the same age. Indeed a prospective study revealed that EBC patients in the first year after chemotherapy gained weight more rapidly than women from the same risk cohort [41] who were cancer-free, age- and menopausal-status-matched. However, a recent RCT testing AI treatment vs placebo in postmenopausal women at increased risk of developing BC found no association between AI treatment and body composition changes at 9–18 months [42]. Anyway, there are no studies on body composition changes in BC patients undergoing adjuvant treatment that included also “control” women without BC, and therefore no definite conclusion on this point can be done at present [13]. Our dietary counseling program consists in regular, personalized, advice provided by an expert dietician, based on the principles of the Mediterranean diet, according to the guidelines of the Italian Society of Human Nutrition for the general population [27,28], and of international Agencies and Scientific Associations for cancer survivors [24,43]. Adherence to Mediterranean diet was associated with reduced all-cause mortality in cancer patients in various studies, and this diet is recommended for cancer survivors [24,44,45]. However, our dietary counseling program had no effects on body composition changes in EBC patients from before to after AI therapy. These results suggest that the goal of contrasting the unhealthy body composition changes in BC patients may be difficult to achieve. Indeed, among behavioral interventions on women treated for BC cancer, about half showed positive effect on preventing or reducing obesity but very few showed positive effects on contrasting sarcopenia and sarcopenic obesity [13]. The reasons for the unsatisfactory results of our dietary counseling program are unclear, since we found that women who participated in the program modified their dietary habits accordingly, and that the program prevented weight gain and favoured weight loss in overweight and obese women, from before to after chemotherapy [46]. The risk of gaining weight was higher in women who received hormone treatment and declined with increasing adherence to the Mediterranean diet [[46], [47], [48]]. Our counseling program included only general recommendations to practice physical activity and did not provide supervised exercise training directly. The proportion of women who claimed to practice moderate physical activity was approximately twice in the intervention than in the control group. However, most EBC patients said to practice null or low physical activity also in the intervention group, similarly to findings from another Italian study on women with BC [47]. Various RCTs have shown that intervention programs based on dietary advice alone can be effective to reduce weight, BMI, waist circumference and body fat mass, but they also result in lean mass decrease, though smaller than that observed in body fat mass [13,22]. A recent review of RCTs on combined dietary and exercise interventions on body composition showed a reduction of both fat body mass (−2 kg) but also of lean body mass, although of lower size (−0.4 kg) [49]. A combination of caloric restriction plus structured aerobic and resistance exercise training reduced fat mass and attenuated the loss of lean mass in cancer patients [[49], [50], [51]]. The most effective interventions to improve body composition in BC women seem to be resistance training for preserving and improving lean mass, and caloric restriction in combination with resistance and aerobic exercise to reduce fat mass in BC patients [51]. The largest effects on lean body mass were observed when resistance training was included as part of the exercise intervention, both during and after adjuvant treatment [52]. Resistance training may also improve muscle strength, fatigue, pain, and overall quality of life in BC patients [53]. The use of DXA as a method to analyse the whole and regional body composition, the inclusion of a control group using proximity score for matching and the blinded evaluation of patients’ DXA by radiologists are the major strengths of this study. Its main weaknesses are the observational, retrospective design, and the absence of one arm with a physical activity program.
In conclusion, our study shows that dietetic counseling alone may be not effective to contrast the unhealthy body composition changes in BC patients receiving AI therapy, and this implies the need to implement also a supervised physical exercise, including resistance exercise training, in these women.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki.
Approval was granted by the Ethics Committee of ASST Spedali Civili of Brescia, Italy.
Informed consent Informed consent was obtained from all individual participants included in the study.
Funding
The authors declare that no founds, grants, or other support were received during the preparation of this manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from Rebecca Pedersini on reasonable request.
CRediT authorship contribution statement
Rebecca Pedersini: Supervision, Formal analysis, Conceptualization. Greta Schivardi: Writing – original draft, Data curation. Marta Laganà: Validation. Lara Laini: Data curation, Investigation, Writing – original draft. Pierluigi di Mauro: Writing – review & editing, Formal analysis, Conceptualization. Manuel Zamparini: Methodology, Formal analysis. Vito Amoroso: Writing – review & editing, Validation. Alessia Bonalumi: Formal analysis, Data curation. Sara Bosio: Investigation, Data curation. Barbara Zanini: Formal analysis, Data curation, Conceptualization. Chiara Buizza: Formal analysis, Data curation. Nicole Villa: Resources, Formal analysis, Data curation. Marco Ravanelli: Validation, Supervision, Formal analysis. Luca Rinaudo: Validation, Conceptualization. Salvatore Grisanti: Writing – review & editing, Supervision. Davide Farina: Writing – review & editing, Investigation, Data curation. Alfredo Berruti: Writing – review & editing, Resources, Conceptualization. Francesco Donato: Validation, Formal analysis. Deborah Cosentini: Writing – original draft, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
A heartfelt thanks to the association of patients ESA (Educazione alla Salute Attiva), FIRM Onlus (Fondazione Inter nazionale di Ricerca in Medicina) and Beretta foundation for their constant support to the Medical Oncology and Breast Unit of the ASST Spedali Civili of Brescia.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2024.103794.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Vance V., Mourtzakis M., Mccargar L., Hanning R. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev. 2011;12:282–294. doi: 10.1111/j.1467-789X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 2.Berg M.M.G.A., Winkels R.M., Kruif J.T.C.M., Laarhoven H.W.M., Visser M., Vries J.H.M., et al. Weight change during chemotherapy in breast cancer patients: a meta-analysis. BMC Cancer. 2017 doi: 10.1186/s12885-017-3242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandhi A., Copson E., Eccles D., Durcan L., Howell A., Morris J., et al. Predictors of weight gain in a cohort of premenopausal early breast cancer patients receiving chemotherapy. Breast. 2019;45:1–6. doi: 10.1016/j.breast.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Goyal A., Milner G.E., Cimino-Mathews A., Visvanathan K., Wolff A.C., Sharma D., et al. Weight gain after hormone receptor-positive breast cancer. Curr Oncol. 2022 doi: 10.3390/curroncol29060326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu H jie, Ho M., Liu X., Yang J., Chau P.H., Fong D.Y.T. Incidence and temporal trends in type 2 diabetes by weight status: a systematic review and meta-analysis of prospective cohort studies. J Glob Health. 2023 doi: 10.7189/JOGH.13.04088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogers R.P., Bemelmans W.J.E., Hoogenveen R.T., Boshuizen H.C., Woodward M., Knekt P., et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med. 2007 doi: 10.1001/archinte.167.16.1720. [DOI] [PubMed] [Google Scholar]
- 7.Oikonomou E.K., Antoniades C. The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol. 2019 doi: 10.1038/s41569-018-0097-6. [DOI] [PubMed] [Google Scholar]
- 8.Chan D.S.M., Vieira R., Abar L., Aune D., Balducci K., Cariolou M., et al. Postdiagnosis body fatness, weight change and breast cancer prognosis: global Cancer Update Program (CUP global) systematic literature review and meta-analysis. Int J Cancer. 2023 doi: 10.1002/ijc.34322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberto M., Barchiesi G., Resuli B., Verrico M., Speranza I., Cristofani L., et al. Sarcopenia in breast cancer patients: a systematic review and meta-analysis. Cancers. 2024 doi: 10.3390/cancers16030596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prado C.M., Batsis J.A., Donini L.M., Gonzalez M.C., Siervo M. Sarcopenic obesity in older adults: a clinical overview. Nat Rev Endocrinol. 2024 doi: 10.1038/s41574-023-00943-z. [DOI] [PubMed] [Google Scholar]
- 11.Ji T., Li Y., Ma L. Sarcopenic obesity: an emerging public health problem. Aging Dis. 2022 doi: 10.14336/AD.2021.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang M.K., Park S., Raszewski R., Park C.G., Doorenbos A.Z., Kim S. Prevalence and clinical implications of sarcopenia in breast cancer: a systematic review and meta-analysis. Support Care Cancer. 2024;32:1–12. doi: 10.1007/s00520-024-08532-0. [DOI] [PubMed] [Google Scholar]
- 13.Sheean P.M., Hoskins K., Stolley M. Body composition changes in females treated for breast cancer: a review of the evidence. Breast Cancer Res Treat. 2012 doi: 10.1007/s10549-012-2200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gadéa E., Thivat E., Planchat E., Morio B., Durando X. Importance of metabolic changes induced by chemotherapy on prognosis of early-stage breast cancer patients: a review of potential mechanisms. Obes Rev. 2012 doi: 10.1111/j.1467-789X.2011.00957.x. [DOI] [PubMed] [Google Scholar]
- 15.Van Londen G.J., Perera S., Vujevich K., Rastogi P., Lembersky B., Brufsky A., et al. The impact of an aromatase inhibitor on body composition and gonadal hormone levels in women with breast cancer. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-010-1223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Battisti S., Guida F.M., Coppa F., Vaccaro D.M., Santini D., Tonini G., et al. Modification of abdominal fat distribution after aromatase inhibitor therapy in breast cancer patients visualized using 3-D computed tomography volumetry. Clin Breast Cancer. 2014 doi: 10.1016/j.clbc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Pedersini R., Schivardi G., Laini L., Zamparini M., Bonalumi A., di Mauro P., et al. Changes in body composition in early breast cancer patients treated with aromatase inhibitors. J Endocrinol Invest. 2024 doi: 10.1007/s40618-024-02401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okorodudu D.O., Jumean M.F., Montori V.M., Romero-Corral A., Somers V.K., Erwin P.J., et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes. 2010 doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 19.Romero-Corral A., Lopez-Jimenez F., Sierra-Johnson J., Somers V.K. Differentiating between body fat and lean mass - how should we measure obesity? Nat Clin Pract Endocrinol Metabol. 2008 doi: 10.1038/ncpendmet0809. [DOI] [PubMed] [Google Scholar]
- 20.Donini L.M., Busetto L., Bischoff S.C., Cederholm T., Ballesteros-Pomar M.D., Batsis J.A., et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Obes Facts. 2022 doi: 10.1159/000521241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lake B., Damery S., Jolly K. Effectiveness of weight loss interventions in breast cancer survivors: a systematic review of reviews. BMJ Open. 2022 doi: 10.1136/bmjopen-2022-062288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raji Lahiji M., Vafa S., de Souza R.J., Zarrati M., Sajadian A., Razmpoosh E., et al. Effect of dietary-based lifestyle modification approaches on anthropometric indices and dietary intake parameters in women with breast cancer: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2022 doi: 10.1093/advances/nmac062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014 doi: 10.1016/j.ijsu.2014.07.013. [DOI] [Google Scholar]
- 24.Clinton SK, Giovannucci EL, Hursting SD. The world cancer research fund/American institute for cancer research third expert report on diet, nutrition, physical activity, and cancer: impact and future directions. J Nutr. 2020 Apr 1;150(4):663–671. doi: 10.1093/jn/nxz268. PMID: 31758189; PMCID: PMC7317613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pekmezi D.W., Demark-Wahnefried W. Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol (Madr) 2011;50:167–178. doi: 10.3109/0284186X.2010.529822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavalette C., Adjibade M., Srour B., Sellem L., Fiolet T., Hercberg S., et al. Cancer-specific and general nutritional scores and cancer risk: results from the prospective NutriNet-Santé cohort. Cancer Res. 2018;78:4427–4435. doi: 10.1158/0008-5472.CAN-18-0155. [DOI] [PubMed] [Google Scholar]
- 27.Rossi L., Berni Canani S., Censi L., Gennaro L., Leclercq C., Scognamiglio U., et al. The 2018 revision of Italian dietary guidelines: development process, novelties, main recommendations, and policy implications. Front Nutr. 2022 doi: 10.3389/fnut.2022.861526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CREA . 2019. Linee guida per la sana alimentazone (Revisione 2018) [Google Scholar]
- 29.Ipaq Research Committee Guidelines for data processing and analysis of the international physical activity questionnaire (IPAQ) – short and long forms. Ipaq. 2005 [Google Scholar]
- 30.Kendler D.L., Borges J.L.C., Fielding R.A., Itabashi A., Krueger D., Mulligan K., et al. The official positions of the international society for clinical densitometry: indications of use and reporting of DXA for body composition. J Clin Densitom. 2013 doi: 10.1016/j.jocd.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 31.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austin P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011 doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Londen G.J., Perera S., Vujevich K., Rastogi P., Lembersky B., Brufsky A., et al. The impact of an aromatase inhibitor on body composition and gonadal hormone levels in women with breast cancer. Breast Cancer Res Treat. 2011;125:441–446. doi: 10.1007/s10549-010-1223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greendale G.A., Sternfeld B., Huang M.H., Han W., Karvonen-Gutierrez C., Ruppert K., et al. Changes in body composition and weight during the menopause transition. JCI Insight. 2019 doi: 10.1172/jci.insight.124865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santoro A., Bazzocchi A., Guidarelli G., Ostan R., Giampieri E., Mercatelli D., et al. A cross-sectional analysis of body composition among healthy elderly from the European NU-AGE study: sex and country specific features. Front Physiol. 2018 doi: 10.3389/fphys.2018.01693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ofenheimer A., Breyer-Kohansal R., Hartl S., Burghuber O.C., Krach F., Schrott A., et al. Reference values of body composition parameters and visceral adipose tissue (VAT) by DXA in adults aged 18–81 years—results from the LEAD cohort. Eur J Clin Nutr. 2020 doi: 10.1038/s41430-020-0596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly T.L., Wilson K.E., Heymsfield S.B. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS One. 2009 doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Mesquita Barros Almeida Leite C., Di Renzo L., Salimei P.S., Gualtieri P., Schieferdecker M.E.M., Vilela R.M., et al. Lean body mass: reference values for Italian population between 18 to 88 years old. Eur Rev Med Pharmacol Sci. 2018 doi: 10.26355/eurrev-201811-16415. [DOI] [PubMed] [Google Scholar]
- 39.Imboden M.T., Swartz A.M., Finch H.W., Harber M.P., Kaminsky L.A. Reference standards for lean mass measures using GE dual energy x-ray absorptiometry in Caucasian adults. PLoS One. 2017 doi: 10.1371/journal.pone.0176161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imboden M.T., Welch W.A., Swartz A.M., Montoye A.H.K., Finch H.W., Harber M.P., et al. Reference standards for body fat measures using GE dual energy x-ray absorptiometry in Caucasian adults. PLoS One. 2017 doi: 10.1371/journal.pone.0175110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gross A.L., May B.J., Axilbund J.E., Armstrong D.K., Roden R.B.S., Visvanathan K. Weight change in breast cancer survivors compared to cancer-free women: a prospective study in women at familial risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2015 doi: 10.1158/1055-9965.EPI-15-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pegington M., Zhen Tam H., Brentnall A., Sestak I., Adams J., Blake G.M., et al. Body composition changes during breast cancer preventive treatment with anastrozole: findings from the IBIS-II trial. Prev Med Reports. 2024;38 doi: 10.1016/j.pmedr.2024.102620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rock C.L., Thomson C.A., Sullivan K.R., Howe C.L., Kushi L.H., Caan B.J., et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA A Cancer J Clin. 2022 doi: 10.3322/caac.21719. [DOI] [PubMed] [Google Scholar]
- 44.Chen G., Leary S., Niu J., Perry R., Papadaki A. The role of the mediterranean diet in breast cancer survivorship: a systematic review and meta-analysis of observational studies and randomised controlled trials. Nutrients. 2023 doi: 10.3390/nu15092099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morze J., Danielewicz A., Przybyłowicz K., Zeng H., Hoffmann G., Schwingshackl L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur J Nutr. 2021 doi: 10.1007/s00394-020-02346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pedersini R., Laganà M., Bosio S., Zanini B., Cosentini D., di Mauro P., et al. Is weight gain preventable in women with early breast cancer undergoing chemotherapy? A real-world study on dietary pattern, physical activity, and body weight before and after chemotherapy. Breast Cancer Res Treat. 2023;202:461–471. doi: 10.1007/s10549-023-07095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pedersini R., di Mauro P., Bosio S., Zanini B., Zanini A., Amoroso V., et al. Changes in eating habits and food preferences in breast cancer patients undergoing adjuvant chemotherapy. Sci Rep. 2021 doi: 10.1038/s41598-021-92138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magno S., Rossi M.M., Filippone A., Rossi C., Guarino D., Maggiore C., et al. Screening for physical activity levels in non-metastatic breast cancer patients undergoing surgery: an observational study. Integr Cancer Ther. 2022 doi: 10.1177/15347354221140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baguley B.J., Dalla Via J., Fraser S.F., Daly R.M., Kiss N. Effectiveness of combined nutrition and exercise interventions on body weight, lean mass, and fat mass in adults diagnosed with cancer: a systematic review and meta-analysis. Nutr Rev. 2023 doi: 10.1093/nutrit/nuac079. [DOI] [PubMed] [Google Scholar]
- 50.Barnes O., Wilson R.L., Gonzalo‐encabo P., Kang D.W., Christopher C.N., Bentley T., et al. The effect of exercise and nutritional interventions on body composition in patients with advanced or metastatic cancer: a systematic review. Nutrients. 2022 doi: 10.3390/nu14102110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kudiarasu C., Lopez P., Galvão D.A., Newton R.U., Taaffe D.R., Mansell L., et al. What are the most effective exercise, physical activity and dietary interventions to improve body composition in women diagnosed with or at high-risk of breast cancer? A systematic review and network meta-analysis. Cancer. 2023;129:3697–3712. doi: 10.1002/cncr.35043. [DOI] [PubMed] [Google Scholar]
- 52.Fraser S.F., Gardner J.R., Dalla Via J., Daly R.M. The effect of exercise training on lean body mass in breast cancer patients: a systematic review and meta-analysis. Med Sci Sports Exerc. 2022 doi: 10.1249/MSS.0000000000002792. [DOI] [PubMed] [Google Scholar]
- 53.Montaño-Rojas L.S., Romero-Pérez E.M., Medina-Pérez C., Reguera-García M., de Paz J.A. Resistance training in breast cancer survivors: a systematic review of exercise programs. Int J Environ Res Publ Health. 2020 doi: 10.3390/ijerph17186511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from Rebecca Pedersini on reasonable request.