Abstract
BRCA1 is one of the most frequently-mutated tumor suppressor genes in ovarian and breast cancers. Loss of BRCA1 triggers homologous recombination (HR) repair deficiency, consequently leading to genomic instability and PARP inhibitors (PARPi)-associated synthetic lethality. Although, the roles of BRCA1 in DNA repair and replication have been extensively investigated, its tumor suppressive functions beyond genome safeguard remain poorly understood. Here, we report that BRCA1 promotes ferroptosis susceptibility through catalyzing K6-linked polyubiquitination of GPX4 and subsequently accelerating GPX4 degradation. Depletion of BRCA1 induces ferroptosis resistance in ovarian cancer cells due to elevated GPX4 protein, and silence of GPX4 significantly suppresses the growth of BRCA1-deficient ovarian cancer xenografts. Importantly, we found that PARPi triggers ferroptosis in ovarian cancer cells, inhibition of GPX4 markedly increase PARPi-induced ferroptosis in BRCA1-deficient ovarian cancer cells. Combined treatment of GPX4 inhibitor and PARPi produces synergistic anti-tumor efficacy in BRCA1-deficient ovarian cancer cells, patient derived organoid (PDO) and xenografts. Thus, our study uncovers a novel mechanism via which BRCA1 exerts tumor suppressive function through regulating ferroptosis, and demonstrates the potential of GPX4 as a therapeutic target for BRCA1-mutant cancers.
Keywords: BRCA1, Ferroptosis, PARP inhibitors, GPX4 ubiquitination, Ovarian cancer
Graphical abstract
Highlights
-
•
BRCA1 deficiency leads to increase of GPX4 and ferroptosis resistance.
-
•
BRCA1 catalyzes GPX4 polyubiquitination and promotes its degradation.
-
•
Ferroptosis represents a novel mechanism for BRCA1-mediated tumor suppression.
-
•
GPX4i and PARPi synergistically suppresses BRCA1-deficient cancers.
1. Introduction
As one of the most common mutated genes, loss of function of BRCA1 plays critical roles in tumorigenesis and cancer progression in various cancers, especially in ovarian and breast cancers [1]. Approximately 20–30 % of ovarian or breast cancer patients carry BRCA1 mutations, which are associated with poor overall survival [2]. BRCA1 gene is located on chromosome 17, consists of 22 exons and encodes a protein of 1863 amino acids [3]. BRCA1 protein is the main controller in DNA double-strand break (DSB) repair pathway choice between homologous recombination (HR) and non-homologous end-joining (NHEJ) repair, it accumulates at DNA DSB sites and favors HR repair during S/G2 phase [[4], [5], [6]]. Meanwhile, BRCA1 is also involved in DNA replication fork protection during DNA replication stress [7]. Thus, loss of BRCA1 leads to genomic instability characterized by increased DNA breaks and chromosomal aberrations. As an important cancer susceptibility gene, mutations in BRCA1 confers lifetime risks of breast and ovarian cancer up to 80 % and 50 % respectively [2]. BRCA1-deficient cancers display an autonomous inflammatory state and are intrinsically resistant to immune checkpoint blockade (ICB) and MEK inhibitor [[8], [9], [10]]. Similar with BRCA1, the other breast cancer susceptibility gene, BRCA2 is also involved in regulation of DSBs repair and frequently mutated in ovarian cancer cells. However, only BRCA2 mutations, but not BRCA1 mutations, are associated with improved chemotherapy response compared with wild-type (WT) BRCA1/2 in patients with ovarian cancer [11,12]. In addition, Brca1185stop mutation predisposed mice to mammary tumors, albeit HR repair function is preserved in Brca1185stop cells through alternative translation of RING domain-less BRCA1 protein [13,14]. These distinct features in BRCA1-deficient cancer cells suggest BRCA1 possess other functions beyond DNA repair regulation.
PARP is a group of enzymes catalyzing the poly ADP-ribosylation (PARylation) on substrate proteins. PARP1 is the most abundant and well-investigated PARP family protein, it can rapidly recognize the DNA damage sites and directly participates in DNA single-strand break (SSBs) repair [15]. Inhibition of PARP1 results in accumulation of unrepaired SSBs, which are subsequently converted into DNA DSBs. The DNA DSBs formed after treatment of PARP inhibitors (PARPi) highly rely on HR repair, as a result, PARP inhibitors selectively eliminate HR repair deficient cancer cells, such as the cancer cells with BRCA1/2 mutations [16]. Although, PARPi elicits synthetic lethality and have been approved for treatment of BRCA1/2-mutant cancers [17], differential activity of PARP inhibitors has been observed in BRCA1-mutant versus BRCA2-mutant cancers and BRCA1-mutation carriers responded less favorably to PARP inhibitor than patients with BRCA2-mutations [18]. Thus, the emergence of intrinsic PARP inhibitor resistance in BRCA1-mutant cancer cells prompts the novel strategy to optimize PARP inhibitor therapy in BRCA1-mutant cancers.
Ferroptosis is a form of iron-dependent cell death, which is resulted from lethal lipid peroxidation and characterized by cell shrinkage and increased mitochondrial membrane density [19]. Ferroptosis is controlled by intracellular oxidation and antioxidant systems and the selenium-containing anti-oxidant enzyme glutathione peroxidase 4 (GPX4) is considered as the central repressor of ferroptosis [20]. GPX4 catalyzes the reduction of hydroperoxides of polyunsaturated fatty acids in membranes and inhibits lipid peroxidation [21]. Ferroptosis plays critical roles in various of physiological and pathological processes including neurodegenerative diseases, acute renal failure, heart injury, cancer, and so on [19]. As a regulated-cell death, ferroptosis susceptibility is highly associated with cancer progression and therapeutic resistance [22,23]. In our present study, we showed that BRCA1 ubiquitinates and degrades GPX4 to dictate ferroptosis sensitivity in ovarian cancer cells, which is associated with its tumor suppressive function in vivo. We further found PARP inhibitors, the premier treatment for BRCA1/2-mutant cancers, triggered ferroptosis in ovarian cancer cells, and inhibition of GPX4 greatly promoted PARP inhibitor-induced ferroptosis in BRCA1-deficient ovarian cancer cells. Therefore, our study uncovers that GPX4-mediated ferroptosis susceptibility represents a novel tumor suppressive function of BRCA1, as well as a therapeutic target to optimize PARP inhibitor therapy in BRCA1-deficient cancers.
2. Materials and methods
2.1. Human samples
Ovarian cancer tissues were collected at Nanjing Maternity and Child Health Care Hospital and informed consent was obtained from all patients. The use of all patients’ specimens was approved by the Institutional review board (IRB) of Nanjing Maternity and Child Health Care Hospital.
2.2. Reagents, antibodies and plasmids
Anti-BRCA1 (sc-6954), anti-HA (sc-57592), anti-β-Actin (sc-47778) and anti-Flag (sc-807) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-GPX4 (67763-1-Ig), anti-SLC7A11 (26864-1-AP) anti-myc (60003-2-Ig) antibody were purchased from Proteintech Group Inc. (Chicago, IL, USA). Anti–HO–1 (A1346) and anti-SLC40a1 (A14884)were purchased from ABclonal technology (Wuhan, China). anti-TFR1 (AF8136) were purchased from Beyotime biotechnology(Beijing, China). Anti-Ubiquitin (#3936) were obtained from Cell signaling technology (Danvers, MA). Anti-4-HNE(MAB3249) was purchased from R&D Systems (Minneapolis, MN, USA). Human BRCA1 siRNA (target sequence: #1, 5′-CCA CAC GAT TTG ACG GAA A-3’; #2, 5′-CTA CTC ATG TTG TTA TGA A-3′) were obtained from Ribobio (Guangzhou, China), in vivo cholesterol-modified GPX4 siRNA (target sequence: 5′-GGA GTA ACG AAG AGA TCA A-3′) were purchased from Ribobio (Guangzhou, China). Recombinant GPX4 protein (#26906) was purchased from Cayman Chemical Company (Ann Arbor, USA). Ubiquitin Activating Enzyme E1/UBA1 protein (11990-H20B-50) was purchased from Sino Biological Inc. (Beijing, China). Recombinant human ubiquitin-conjugating enzyme E2 UBCH5C(P00347) was purchased from Solarbio Life Sciences (Beijing, China). Recombinant ubiquitin (20487) was purchased from Cayman Chemical (MI, USA). Flag-GPX4, HA-BRCA1, Myc-BARD1, Myc-BRCA1 plasmids were obtained from Jinbeijin biotechnology (Nanjing, China). GPX4 shRNAs were obtained from MiaoLingBio (Wuhan, China). HA-WT, K6-, K11-, K27-, K29-, K33-, K48-, K63- Ubiquitin plasmids were kindly gifted by Dr. Chen, Yan (Shandong Normal University). Myc-BRCA1 truncates plasmids were generated by Nanjing Genebay Biotech Inc. (Nanjing, China) using Myc tagged full length BRCA1 as a templet.
2.3. Cell culture and transfection
UWB1.289, UWB1.289-BRCA1, A2780, SKOV3, OVCAR3 and HCC1937 cells were obtained from American Type Culture Collection (ATCC). UWB1.289 and UWB1.289-BRCA1 cells were cultured in 50 % RPMI (Roswell Park Memorial Institute)-1640 (Gibco, USA) plus 50 % MEGM (Mammary Epithelial Growth Medium) (#CC-3150, Lonza, NJ) supplemented with 3 % fetal bovine serum (FBS, ExCell Bio, Shanghai, China) and penicillin-streptomycin (100 U/mL, Gibco, USA). A2780, SKOV3, HCC1937 and OVCAR3 were cultured in RPMI-1640 medium supplemented with 10 % FBS and penicillin-streptomycin. Cells were regularly tested for mycoplasma contamination using MycoBlue Mycoplasma Detector (D101-01, Vazyme, Nanjing, China). Cells were transfected with plasmids using ExFect transfection reagent (Vazyme, NJ, China) according to manufacturer's instruction.
2.4. Organoid culture
Patient derived ovarian cancer organoid (PDO) (BRCA1c.3756_3759del, PDO-ID: KOOA-009) model was established by Beijing K2 Oncology Inc. (Beijing, China). This ovarian cancer tissue was collected at People's Hospital of Yixing City and was approved by the Medical Ethical Committee of the People's Hospital of Yixing City. Informed consent was obtained from the patient. The organoids were cultured in ovarian cancer organoids culture medium (#K2O-M-OA, K2 Oncology, Beijing, China). After seeding in 96 wells plates, organoids were treated with serial concentrations of Olaparib, ML210 or their combination for 5 days. Then, organoid viability was determined by the Cell Titer-Glo assay (#G9683, Promega, Madison, WI) and synergistic effects were calculated by ZIP synergy score.
2.5. Ovarian cancer xenograft
All the animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of China Pharmaceutical University. The 6 weeks-old female BALB/c athymic nude mice were purchased from the GemPharmatech (Nanjing, China) and housed in a specific pathogen-free (SPF) facility. 1 × 107 of A2780 parental or BRCA1-KO cells in 100 μl PBS were inoculated subcutaneously into the right flank of mice. Tumor width (W) and length (L) were measured and the tumor volume (V) was calculated by formula V = L × W2/2. When tumor volume reached around 50–100 mm3, mice were randomly grouped and treated respectively. in vivo cholesterol-modified GPX4 siRNA were administrated intratumorally three time per week. For evaluating therapeutic efficacy of ML210/Olaparib combination, mice with BRCA1-KO xenografts were treated with PBS (control), ML210 (intraperitoneal, 30 mg/kg), Olaparib (oral, 100 mg/kg) or their combination. Tumor volumes were measured every three days and tumor growth were monitored.
2.6. Immunoprecipitation (IP)
Cells were collected in EBC buffer (50 mM Tris-HCl pH = 7.6–8.0, 120 mm NaCl, 0.5 % NP-40, 1 mM EDTA, 50 mm NaF, 1 mmNa3VO4 and 1 mM β-mercaptoethanol) supplemented with cocktail protease inhibitor (EMD Millipore, Burlington, MA) and lysed by sonication. After centrifugation, cell lysates were incubated with indicated antibodies and protein A/G agarose beads (sc-2003, Santa Cruz biotechnology, USA) at 4 °C overnight with rotation. For the flag immunoprecipitation, cell lysates were incubated with anti-flag M2 affinity gel (A2220, Sigma-Aldrich, USA) at 4 °C overnight with rotation. After washing 5 times with EBC buffer, beads were boiled in 40 μl of 2 × SDS-PAGE sample buffer for 6min and the proteins in the immunoprecipitation complex were analyzed by Western blot.
2.7. Immunohistochemical (IHC) staining
The ovarian cancer tissue microarray (ZL-OVA961) was purchased from Shanghai Wellbio Biotechnology (Shanghai, China). Firstly, the sections were deparaffinized in 100 % xylene and rehydrated with gradient ethanol (100 %, 90 %, 70 %, 30 % and 0 %). After inactivation of endogenous peroxidase by 3 % hydrogen peroxide and antigen retrieval in citrate buffer by heat, the IHC staining was performed using VECTASTAIN universal Quick kit (PK-8800, Vector Laboratories, USA) according to the manufacturer's instruction. The following primary antibody dilutions were used: anti-GPX4 (67763-1-IG,1:2000), anti-BRCA1 (ab16780,1:1000), anti-4-HNE (MAB3249, 1:500). The semiquantitative determination of GPX4, 4-HNE and BRCA1 were carried out using immunoscore based on the percentage and intensity of positive staining as previous described.
2.8. Generation of knockout (KO) cells
BRCA1 was depleted in A2780 and OVCAR3 cells using CRISPR/Cas9 technique as previously described [24,25]. Briefly, cells were transduced with lentiCRISPRV2-puro carrying BRCA1 sgRNA and then selected with puromycin (2 μg/ml) and seeded for growing single clones. Then, single clones were picked and BRCA1 expression was examined by Western blot. BRCA1 KO A2780 cells were transduced with lentiCRISPRV2-neo-53BP1 sgRNA, selected with G418 (800 μg/ml), and 53BP1 KO was examined by Western blot. The sgRNA sequences targeting BRCA1 or 53BP1 were in Supplemental Table 2.
2.9. Lipid peroxidation assay
Lipid peroxidation was measured by BODIPY 581/591 C11 (Thermo Fisher Scientific, Waltham, MA, USA) staining. Briefly, cells were seeded in 6-well plates at a density of 1 × 106 cells per well and treated with or without indicated agents for 24 h. After treatment, cells were incubated with fresh complete medium containing 10 μM BODIPY 581/591 C11 dye (D3861, Invitrogen) at 37 °C for 1 h. After washing with PBS, lipid peroxidation was then analyzed by flow cytometry (Accuri C6 cytometer, BD Biosciences) using the 488-nm laser for excitation. The relative lipid peroxidation level was indicated by the percentage of cells gated by the black dotted line based on the fluorescence intensity in FL1 channel, and the data were analyzed using the FlowJo 10 software.
2.10. In situ proximity ligation assay (PLA)
Proximity Ligation Assay (PLA) was performed using the Duolink In Situ Red Starter Kit Mouse/Rabbit (DUO92101, MilliporeSigma) according to the manufacturer's protocol (MilliporeSigma, Burlington, MA). Briefly, A2780 cells were fixed with 4 % paraformaldehyde for 15 min and permeabilized with 0.2 % TritonX-100. After blocking with Duolinke blocking solution for 1 h at room temperature, cells were then incubated with primary antibodies overnight at 4 °C. After incubation with PLA probe solution at 37 °C for 1 h, cells were then incubated with amplification solution for 100 min at 37 °C. After mounting with Prolong Gold antifade reagent containing DAPI (Invitrogen, CA), PLA signals were captured by confocal microscope (Lecia SP8, Leica Microsystems Inc.).
2.11. Ubiquitination assay
For the in vivo ubiquitination assay, cells were co-transfected with indicated combination of constructs and treated with MG132 (5 μM) for 6 h before harvesting. Cells were then collected in EBC buffer (50 mM Tris-HCl pH = 7.6–8.0, 120 mM NaCl, 0.5 % NP-40, 1 mM EDTA, 2 mM NaF, 1 μM Na3VO4 and 1 mM β-mercaptoethanol) supplemented with protease inhibitor and lysed by sonication. After centrifugation, the supernatant was incubated with anti-flag affinity gel at 4 °C overnight with rotation. After extensive washing with EBC buffer, the beads were suspended with 2 × SDS sample buffer and boiled at 100 °C for 5 min. The bound proteins were separated on SDS-PAGE and the GPX4 ubiquitination was analyzed by Western blot. For the in vitro ubiquitin ligation assay, the heterodimer of WT or I26A flag-BRCA11−303 and BARD1 were purified from transfected 293T cells by anti-flag affinity chromatography and 3 × FLAG peptide elution. His-GPX4 (1 μg) was incubated in the reaction mixture (50 mM Tris-HCl pH7.5, 120 mM NaCl, 0.5 mM DTT, 5 mM ATP, 5 mM MgCl2, 2 mM NaF and 5 μM ZnCl2) containing 0.1 μg E1, 0.5 μg UBCH5C, 2 μg Ub, 1 μg each of flag-BRCA11−303 and BARD1. After incubation at 37 °C for 3 h, SDS-PAGE sample buffer was added into the reaction and boiled at 100 °C for 5 min. The ubiquitinated GPX4 was detected by Western blot with anti-Ub antibody.
2.12. Mass spectrometry analysis of GPX4 ubiquitination sites
The sites of poly-ubiquitination on GPX4 were identified by mass spectrometry (LC-MS/MS) in PTM-Biolabs Inc. (Hangzhou, China). Briefly, HEK293T cells were co-transfected with flag-GPX4 and HA-ubiquitin, and poly-ubiquitinated flag-GPX4 was purified from cells after MG132 treatment (20 μM, 6 h) by immunoaffinity purification using anti-DYKDDDDK G1 affinity resin (GenScript, Nanjing, China) and 3X flag peptide elution (Beyotime, Shanghai, China). The poly-ubiquitinated GPX4 were then resolved in SDS-PAGE and in-gel tryptic digestion was performed. After extraction and dry, the tryptic peptides were then resuspended in 2 % acetonitrile/0.1 % formic acid and subjected to tandem mass spectrometry (MS/MS) in Q-Exactive plus (Thermo Fisher Scientific, Waltham, MA, USA). The resulting mass spectra data were processed using Proteome Discoverer 2.4. software and searched against human protein database.
2.13. Apoptosis analysis
Cells were treated with indicated concentrations of etoposide for 48 h and cell apoptosis was measured using FITC-Annexin V apoptosis detection kit (556547, BD Biosciences) according to manufacturer's instruction. Briefly, after washing with PBS, cells were then incubated with 200 μl 1xbinding buffer containing annexin V-FITC and propidium iodide (PI) at room temperature in dark for 25 min. Then, 200 μl 1Xbinding buffer was added and cell apoptosis was analyzed by flow cytometry.
2.14. Cell death and viability assay
Cell death was measured by PI (Propidium Iodide) staining as previously descried. After treatment, cells were collected and washed once with PBS, followed by incubation with 50 μg/ml PI in PBS for 15 min. Then, dead cell with PI positive were analyzed by flow cytometry. Cell viability was measured using Cell Counting Kit-8 (CCK-8) (Doijindo, Japan). Cells were seeded into 96-well plate at 5 × 103 cells per well, and were then treated as indicated for 72 h. After treatment, cells were incubated with CCK-8 reagent for 1 h at 37 °C, followed by measurement of the OD value at 450 nm with a microplate reader.
2.15. Colony formation assay
103 of cells were seeded into 6-well plate and grown in presence of indicated concentrations of agents for 7–14 days until colonies were visible. Then, colonies were washed with PBS, and stained with 0.5 % crystal violet in 20 % methanol. Colonies were then counted and the relative colony formation was normalized to un-treated group.
2.16. Quantitative real time PCR (qRT-PCR)
Total mRNA was extracted using TRIzol reagent (Solarbio,Beijing, China) according to the manufacturer's instruction, followed by synthesis of complementary DNA (cDNA) using oligo (dT) primers by a reverse transcriptase kit (Vazyme, Nanjing, China). Then, qPCR was performed using SYBR Green Master Mix wiper (Vazyme, Nanjing, China) on Bio-Rad Real-Time PCR instruments. The relative mRNA levels were calculated by the 2–ΔΔCT method and normalized to β-Actin. The specific primers were: BRCA1 p1: 5′-GAA ACC GTG CCA AAA GAC TTC-3’; BRCA1 p2: 5′-CCA AGG TTA GAG AGT TGG ACA C-3’; GPX4 p1: 5′-GAG GCA AGA CCG AAG TAA ACT AC-3’; GPX4 p2:5′-CCG AAC TGG TTA CAC GGG AA-3’; GAPDH p1: 5′-GGA GCG AGA TCC CTC CAA AAT-3’; GAPDH p2: 5′-GGC TGT TGT CAT ACT TCT CAT GG-3’.
2.17. Subcellular fractionation
Nuclear and cytosolic fractionation assay was performed using cytoplasmic extraction Kit (P0028, Beyotime, Beijing, China) according to manufacturer's instructions. Briefly, 5 × 106 of cells were suspended in cytoplasmic extraction reagent. After incubation in ice for 15 min, followed by centrifugation at 14000 rpm × 10 min, the supernatant cytoplasmic protein was collected. And the pellet was then suspended in nuclear extraction reagent and incubated in ice for 30 min to extract the nuclear proteins after centrifugation.
2.18. Label free quantitative proteomics analysis
Quantitative proteomics was performed by Shanghai Applied Protein Technology Co., Ltd (Shanghai, China). Briefly, UWB1.289 and UWB1.289-BRCA1 cells were collected and lysed in SDT (4%SDS,100 mM Tris-HCl pH7.6) buffer. 20 μg of protein for each sample was resolved in SDS-PAGE and followed by trypsin digestion according to filter-aided sample preparation (FASP) procedure. The digested peptides were then desalted and reconstituted in 40 μl of 0.1 % (v/v) formic acid. LC-MS/MS analysis was performed on a Q Exactive mass spectrometer (Thermo Scientific) that was coupled to Easy nLC (Proxeon Biosystems, now Thermo Fisher Scientific). The MS raw data for each sample were combined and searched using the MaxQuant 1.6.14 software for identification and quantitation analysis.
2.19. Statistical analysis
Data were shown from one representative experiment of at least three independent replicates and presented as mean ± standard deviation (SD). A two-sided t-test was used to analyze the statistical significance of differences between groups. Multiple comparisons for more than two groups were analyzed by two-way ANOVA with Tukey's test. p < 0.05 was considered statistically significant.
3. Results
3.1. BRCA1-deficiency confers ferroptosis resistance in ovarian cancer cells
As a commonly mutated tumor suppressor, BRCA1 maintains genomic integrity to restrain cancer development [26]. The specific mechanism of tumor suppression beyond DNA repair remains unclear. To explore the anti-tumor functions of BRCA1, BRCA1-null cell line UWB1.289 and UWB1.289 transduced with BRCA1 (UWB-BRCA1) were subjected to quantitative proteomics analysis (Fig. 1A). We found 214 proteins were significantly increased and 187 proteins were decreased in UWB.1289 cells compared with UWB-BRCA1 (Supplemental Table 1). Notably, among these differentially expressed proteins, GPX4 was remarkedly increased in BRCA1-deficient UWB1.289 cells (Fig. 1B), and gene ontology (GO) enrichment revealed that cell response to lipid pathway was significantly affected (Fig. 1C). Thus, we speculated that BRCA1 might affect cell vulnerability to lipid peroxidation. To test our hypothesis, we treated cells with ferroptosis inducer RSL3 and Erastin, we interestingly found that BRCA1 expression rendered UWB1.289 cells susceptible to RSL3 or Erastin induced-cell death (Supplemental Fig. 1A). Meanwhile, iron chelating agent deferoxamine (DFO) reversed RSL3-induced cell death in UWB-BRCA1 cells (Fig. 1D), suggesting BRCA1 regulates ferroptosis sensitivity. In contrast, silence of BRCA1 protected BRCA1-proficient A2780 cells from RSL3-induced cell death (Fig. 1E–G). Meanwhile, we did not detect significant change of etoposide induced-apoptotic cell death in BRCA1-expressing UWB1.289 or BRCA1-silenced A2780 cells (Figs. S1B–D). And, ferroptosis inhibitor ferrostatin-1 (fer-1) completely reversed RSL3-induced cell death in both control or BRCA1-knockdown cells (Fig. S1E).
Fig. 1.
BRCA1 promotes ferroptosis susceptibility in ovarian cancers. (A) Western blot analysis of BRCA1 protein expression in UWB1.289 and UWB1.289+BRCA1(UWB-BRCA1) cells. (B) Volcano plot of quantitative proteomics displays the differentially expressed proteins between UWB1.289 and UWB-BRCA1 cells. (C) Gene Ontology (GO) pathway enrichment analysis of significantly changed proteins. (D) Indicated cells were treated with increasing concentrations of RSL3 for 24 h in presence or absence of 100 μM DFO (desferoxamine), dead cells were measured by PI staining. Data were presented as mean ± standard deviation (SD), n = 3. (E) Western blot analysis of BRCA1 expression in A2780 cells transfected with control (Ctrl) or BRCA1 siRNA. (F–G) Control (Ctrl) or BRCA1 siRNA transfected A2780 cells were treated with indicated dosages of RSL3 for 24 h, followed by analysis of cell viability (F) and PI staining (G). (H–J) UWB1.289/UWB-BRCA1 cells (H), Ctrl/BRCA1 siRNA transfected A2780 cells (I), BRCA1 small-guide RNA (sgRNA) mediated- A2780 knockout (KO) cells (J) were treated with or without 0.5 μM RSL3 for 24 h, lipid peroxidation was detected by BODIY 581/591 C11 labeling. The representative flow cytometry profile (left) and quantification of lipid peroxidation (right) was shown. The depletion of BRCA1 in A2780 BRCA1 KO cells were confirmed by Western blot (upper right, J). Data were presented as mean ± SD from three replicates. (K) Immunohistochemistry (IHC) analysis of 4-HNE(4-Hydroxynonenal) in wild-type (WT) or BRCA1-mutant ovarian cancer tissues. The presentative staining (left) or quantification of staining score (right) was shown. Data were presented as mean ± standard deviation (SD), n = 9. **p < 0.01 and ***p < 0.001 by 2-tailed t-test.
Lipid peroxidation is a hallmark of ferroptosis [19]. Consistently, we clearly detected lipid peroxidation in UWB-BRCA1 cells, but not in UWB1.289 cells after RSL3 treatment (Fig. 1H). In contrast, silence of BRCA1 attenuated RSL3-induced lipid peroxidation in A2780 cells (Fig. 1I). Similar observation of decreased lipid peroxidation was also detected in A2780-BRCA1 knockout (KO) cells after RSL3 treatment (Fig. 1J). Treatment of fer-1 completely abolished RSL3-induced lipid peroxidation in both BRCA1-profienct and deficient cells (Figs. S1F–G).To further confirm the ferroptosis regulation by BRCA1 in vivo, we analyzed the level of 4-hydroxynonenal (4-HNE), a reactive aldehyde product and a biomarker of lipid peroxidation [27], in BRCA1 wild-type (WT) or mutant ovarian cancer tissues. As expected, significant decrease of 4-HNE were detected in BRCA1-mutant cancer tissues (Fig. 1K). These results reveal that BRCA1-deficiency in ovarian cancer cells promotes ferroptosis resistance.
3.2. BRCA1 promotes ferroptosis through degrading GPX4
To validate the decrease of GPX4 in BRCA1-expressing UWB1.289 cells, we then examined the levels of GPX4 protein along with several common ferroptosis regulators in UWB1.289 and UWB-BRCA1 cells, as well as A2780 cells with or without BRCA1 knockdown by Western blot. As expected, we observed that BRCA1-expression reduced GPX4 expression in UWB1.289 cells, while BRCA1-silence increased GPX4 protein level in A2780 cells (Fig. 2A). Although, the levels of SLC40A1, HO-1 and SLC7A11 were changed in UWB-BRCA1 cells, we failed to detect similar changes in BRCA1-silenced A2780 cells (Fig. 2A). Similar observations of increased GPX4 were also detected in A2780 BRCA1-KO cells and BRCA1-silenced SKOV3 or OVCAR-3 cells. (Fig. 2B–C). Whereas, we did not detect significant changes of GPX4 mRNA levels in BRCA1-expressed UWB1.289 cells or BRCA1-silenced ovarian cancer cells (Fig. 2D–E), suggesting that BRCA1 post-transcriptionally regulates GPX4 expression.
Fig. 2.
BRCA1 negatively regulates GPX4 protein stability. (A) Western blot analysis of indicated protein levels in UWB1.289, UWB1.289 re-expressing BRCA1(UWB-BRCA1), control (ctrl) or BRCA1 siRNA transfected-A2780 cells. (B–C) Western blot analysis of GPX4 protein levels in Ctrl/BRCA1-KO A2780 cells (B), SKOV3 and OVCAR-3 cells transfected with ctrl or BRCA1 siRNA(C). (D–E) Relative mRNA levels of BRCA1 and GPX4 were quantified in UWB1.289/UWB-BRCA1 cells (D), or BRCA1-silenced A2780, SKOV3 and OVCAR-3 cells (E). (F) UWB1.289 and UWB-BRCA1 cells were treated with 100 μg/ml cycloheximide (CHX) for 0–8 h, followed by Western blot analysis of indicated proteins. (G) A2780 cells were transfected with ctrl or BRCA1 siRNA, 48 h after transfection, cells were treated with 100 μg/ml cycloheximide (CHX) for 0–8 h, then indicated proteins were assessed by Western blot. (H–I) IHC(H) and Western blot (I) analysis of GPX4 expression in WT or BRCA1-mutant ovarian cancer tissues. (J–K) UWB1.289, UWB-BRCA1 or UWB-BRCA1 cells stably expressing flag-GPX4 were treated with or without 0.5 μM RSL3 for 24 h, followed by analysis of lipid peroxidation using BODIY 581/591 C11 labeling (J) and cell death using PI staining (K). The GPX4 expressions in above cells were confirmed by Western blot. Data were presented as mean ± standard deviation (SD). *p < 0.05, **p < 0.01 and ***p < 0.001 by two-way ANOVA test. n.s. stands for no significance.
We then examined whether BRCA1 affects GPX4 degradation through cycloheximide (CHX) chase assay, As shown in Fig. 2F, BRCA1-restoration markedly accelerated GPX4 degradation. Similarly, BRCA1-silence delayed GPX4 degradation in A2780 cells (Fig. 2G). Besides, treatment of proteasome inhibitor MG-132 significantly increased GPX4 protein level in UWB-BRCA1 cells (Fig. S2A). To further confirm the regulation of GPX4 by BRCA1 in vivo, we analyzed GPX4 protein level in BRCA1-WT or mutant ovarian cancer tissues. Consistently, we detected significant higher level of GPX4 in BRCA1-mutant cancer tissues compared with BRCA1-WT cancer (Fig. 2H–I). These results demonstrate that BRCA1 promoting GPX4 degradation. Notably, forced expression of GPX4 significantly reversed RSL3 induced-cell death and lipid peroxidation in UWB-BRCA1 cells (Fig. 2J–K). Thus, these results suggest BRCA1 promotes ferroptosis through the down-regulation of GPX4.
3.3. BRCA1 interacts with GPX4 via RING domain
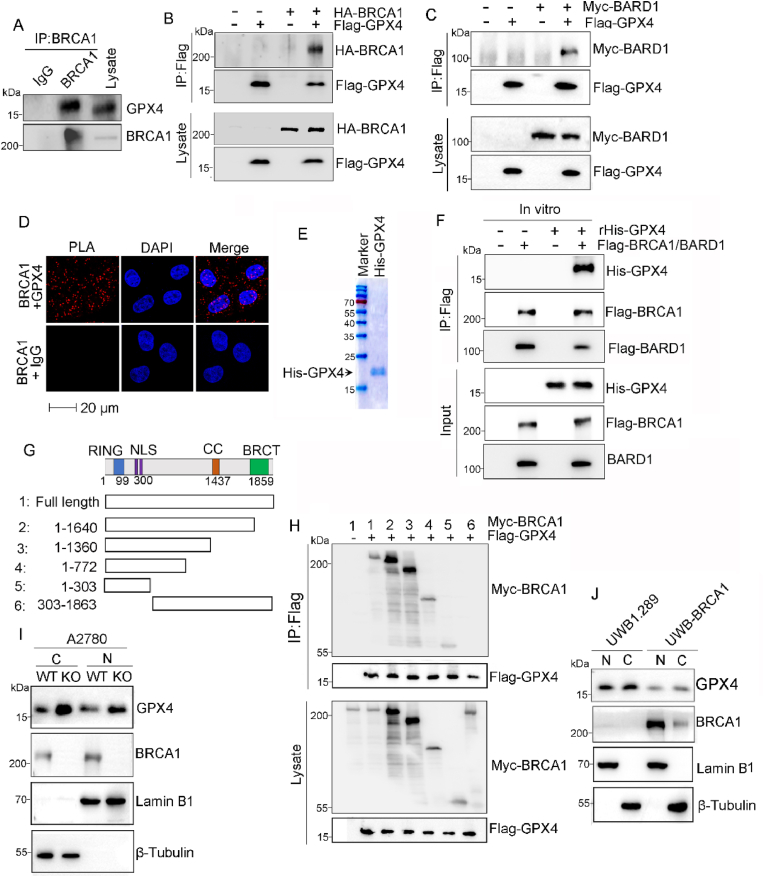
To investigate the underlying mechanism by which BRCA1 destabilizes GPX4, we performed immunoprecipitation (IP) analysis to examine whether BRCA1 physically interacts GPX4, and BRCA1/GPX4 interaction was detected in A2780 cells (Fig. 3A). BRCA1/GPX4 interaction was also observed in A2780 cells exogenously co-expressed with Flag-GPX4 and HA-BRCA1 (Fig. 3B and Supplemental Fig. 2B). Meanwhile, we also found GPX4 interacted with BARD1, the heterodimeric binding partner of BRCA1 (Fig. 3C). Then, we employed proximity ligation assay (PLA) to further confirm the BRCA1/GPX4 association. Consistently, PLA signals of BRCA1/GPX4 interaction was clearly detected, and their interaction was observed in both cytoplasm and nuclear of A2780 cells (Fig. 3D), which is consistent with previous reports that both GPX4 and BRCA1 have been observed in cytoplasm and nucleus [28,29]. Next, we prepared purified his6-GPX4 to determine whether BRCA1/BARD1 directly interacts with GPX4 (Fig. 3E). The in vitro IP assay confirmed that recombinant his6-GPX4 directly interacts with flag-BRCA1/BARD1 (Fig. 3F). BRCA1 is a large protein comprises multiple functional domains including a RING finger domain, a pair of nuclear localization signals (NLS), a coiled-coil (CC) domain and a pair of BRCT domain from N- to C-terminus [26]. To map the GPX4 binding site on BRCA1 protein, we generated five distinct BRCA1 deletion mutants (Fig. 3G). Flag-GPX4 was co-expressed with full length or distinct truncated Myc-BRCA1 in A2780 cells, and the IP assay was carried out with anti-flag affinity gel and the result showed that removal of N-terminal 303 amino acids abolished the interaction between BRCA1 and GPX4, while the BRCA1 truncations with N-terminal 303 amino acids maintained GPX4 interaction (Fig. 3H). This result indicating BRCA1 interacts with GPX4 through its N-terminus that contains RING domain. Given that GPX4 is localized in cytoplasm and nucleus [30]. We thus performed subcellular fractionation assay to examine BRCA1-mediated GPX4 regulation. As shown in Fig. 3I–J, elevation of both cytosolic and nuclear GPX4 were observed in BRCA1-deficient cells, which is consistent with BRCA1/GPX4 association in cytoplasm and nucleus.
Fig. 3.
BRCA1 interacts with GPX4 via RING domain. (A) Co-immunoprecipitation (co-IP) analysis of BRCA1/GPX4 interaction from lysates derived from A2780 cells. (B–C) A2780 cells were co-transfected with indicated plasmids, followed by IP analysis of BRCA1/GPX4 association using anti-flag affinity resin. (D) Proximity ligation assay (PLA) for the interaction between BRCA1 and GPX4 in A2780 cells. (E) The Coomassie blue staining of purified His-GPX4 proteins. (F) 100 ng of recombinant(r) his-GPX4 was incubated with or without purified flag-BRCA1/BARD1 protein in EBC buffer (50 mm Tris-HCl pH 7.6–8.0, 120 mm NaCl, 0.5 % NP-40, 1 mm EDTA, 1 mm β-mercaptoethanol, 50 mm NaF, and 1 mmNa3VO4) for 20 min, followed by IP with anti-flag affinity resin. (G) Schematic diagram of BRCA1 protein and various myc tagged BRCA1 deletion mutants. (H) A2780 cells were co-transfected with Flag-GPX4 along with indicated myc tagged BRCA1 truncations, followed by IP with anti-flag affinity resin and Western blot with indicated antibodies. (I–J) Nuclear(N) and cytoplasmic (C) Fractions were isolated from UWB1.289/UWB-BRCA1 cells (I), parental or BRCA1-KO A2780 (J) cells, and subjected to Western blot analysis with indicated antibodies. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
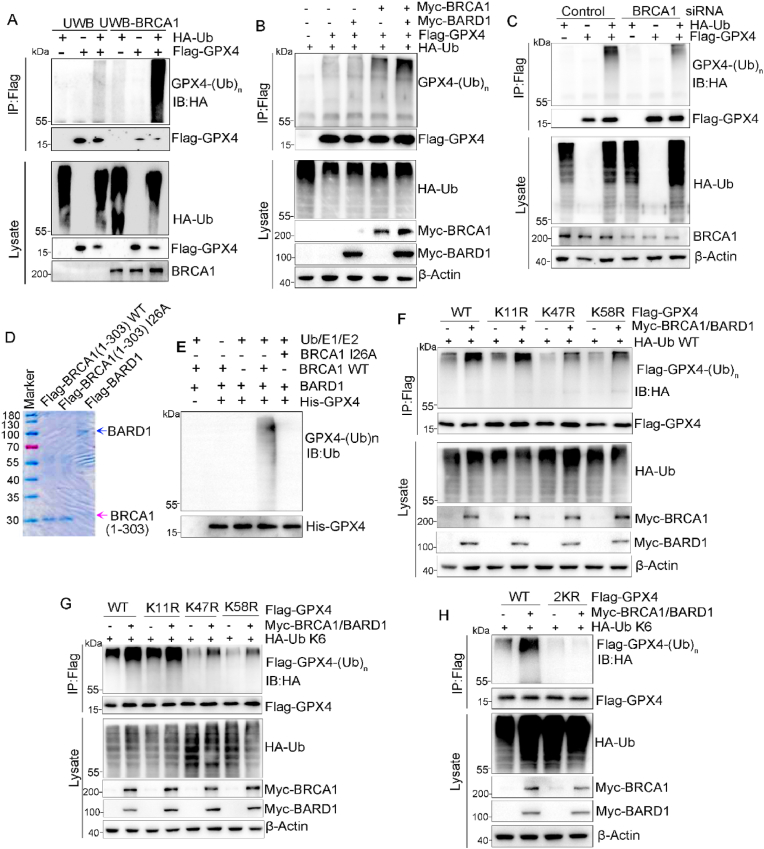
3.4. BRCA1 catalyzes K6-polyubiquitination on GPX4 lysine 47 and 58
Given that the N-terminal RING domain of BRCA1 confers E3 ligase activity when bound with BARD1 [31] and we found BRCA1 promoted GPX4 degradation (Fig. 2). Thus, we hypothesized that BRCA1 ubiquitinates and targets GPX4 for proteasomal degradation. To test this hypothesis, we analyzed GPX4 polyubiquitination levels in UWB1.289 and UWB-BRCA1 cells through co-transfection of flag-GPX4 and HA-ubiquitin (HA-Ub). As expected, we detected higher level of GPX4 ubiquitination in UWB-BRCA1 cells compared with UWB.1289 cells (Fig. 4A). Similarly, overexpression of BRCA1/BARD1 markedly increased GPX4 polyubiquitination in A2780 cells (Fig. 4B). In contrast, silence of BRCA1 resulted in reduced GPX4 polyubiquitination (Fig. 4C). Then, we tested whether BRCA1/BARD1 directly catalyzes GPX4 polyubiquitination through in vitro ubiquitination assay using purified WT, E2-binding deficient mutant I26A flag-BRCA1 1-303, as well as flag-BARD1 protein (Fig. 4D). We found that WT BRCA11−303/BARD1 complex, but not I26A BRCA1 1-303/BARD1 complex catalyzed GPX4 polyubiquitination in presence of E1/E2/Ub (Fig. 4E), demonstrating that BRCA1 is a GPX4 E3 ligase. Next, we generated A2780-BRCA1 KO cells stably expressing flag tagged WT or I26A-mutant BRCA1, and I26A mutation of BRCA1 increased GPX4 protein level in A2780 cells (Fig. S3A). We also detected reduction of GPX4 degradation and its polyubiquitination in A2780 cells expressing I26A-mutant BRCA1 (Figs. S3B–C). As a result, decreased lipid peroxidation was observed in A2780 cells carrying I26A-BRCA1 upon RSL3 treatment (Fig. S3D). And, BRCA1 I26A mutation accelerated tumor growth in in vivo (Figs. S3E–F).
Fig. 4.
BRCA1/BARD1 catalyzes K6-linked polyubiquitination of GPX4 on lysine 47 and 58. (A) Flag-GPX4 was co-transfected with or without HA-ubiquitin (Ub) into UWB1.289/UWB-BRCA1 cells, and then treated with 5 μM MG132 for 6 h before harvesting for analysis of flag-GPX4 ubiquitination. (B) A2780 cells were co-transfected with indicated plasmids, IP was performed using anti-flag affinity resin after MG132 treatment for 6 h and ubiquitination of flag-GPX4 was analyzed by Western blot. (C) A2780 cells with or without BRCA1 knockdown were co-transfected with indicated plasmids, ubiquitination of flag-GPX4 was analyzed by IP with anti-flag affinity resin after MG132 treatment. (D–E) Purified His-GPX4 was incubated in presence of Ub/E1/E2, purified flag-BARD1, and either WT or I26A mutant flag-BRCA1 1-303 protein, then his-GPX4 ubiquitination was analyzed. (F–G) WT, K11R, K47R or K58R flag-GPX4 was co-transfected with WT (F) or K6-specfic(G) HA-ubiquitin, along with or without myc-BRCA1/BARD1 into A2780 cells, then flag-GPX4 ubiquitination was analyzed after MG132 treatment. (H) WT or K47RK58R (2 KR) flag-GPX4 was co-transfected with HA-Ub and myc-BRCA1/BARD1 complex, followed by analysis of flag-GPX4 ubiquitination after MG132 treatment.
Ubiquitin molecule contains seven lysine(K) residues including K6, K11, K27, K29, K33, K48 and K63 [32]. Any one of these K residues could be linked to form poly-ubiquitin chain. To determine the linkage type of polyubiquitin chain on GPX4 catalyzed by BRCA1, we first analyzed the overall ubiquitination patterns on GPX4 in A2780 cells through co-transfection of flag-GPX4 with HA tagged wild-type (WT) or distinct linkage-specific ubiquitin, and we observed WT, K6-, K11- and K48-linked ubiquitination on GPX4 (Fig. S4A). And forced expression of Myc-BRCA1 and Myc-BARD1 significantly increased K6-linked polyubiquitination of GPX4, but did not obviously affect K11- and K48-linked GPX4 polyubiquitination (Fig. S4B), suggesting that BRCA1/BARD1 catalyzes K6-polyubiquitination on GPX4. By mass spectrometry analysis, we further found GPX4 were K6-ubiquitinated at three sites including K11, K47 and K58 (Fig. S4C). To determine which ubiquitination sites are catalyzed by BRCA1/BARD1, lysine(K) to arginine (R) mutants (K11R, K47R and K58R) were generated. Mutation of either K11, K47 and K58 resulted in reduction of K6-polyubiquitination of GPX4 (Fig. S4D), which validated polyubiquitination of GPX4 on these three sites in vivo. Meanwhile, K47R or K58R mutation, but not K11R mutation reduced Myc-BRCA1/BARD1-induced WT or K6-linked poly-ubiquitination on GPX4 (Fig. 4F–G). And compound mutation of K47R/K58R (2 KR) abolished K6-linked poly-ubiquitination on GPX4 in presence of Myc-BRCA1/BARD1 overexpression (Fig. 4H), indicating BRCA1/BARD1 catalyzes K6-polyubiquitination of GPX4 on K47 and K58.
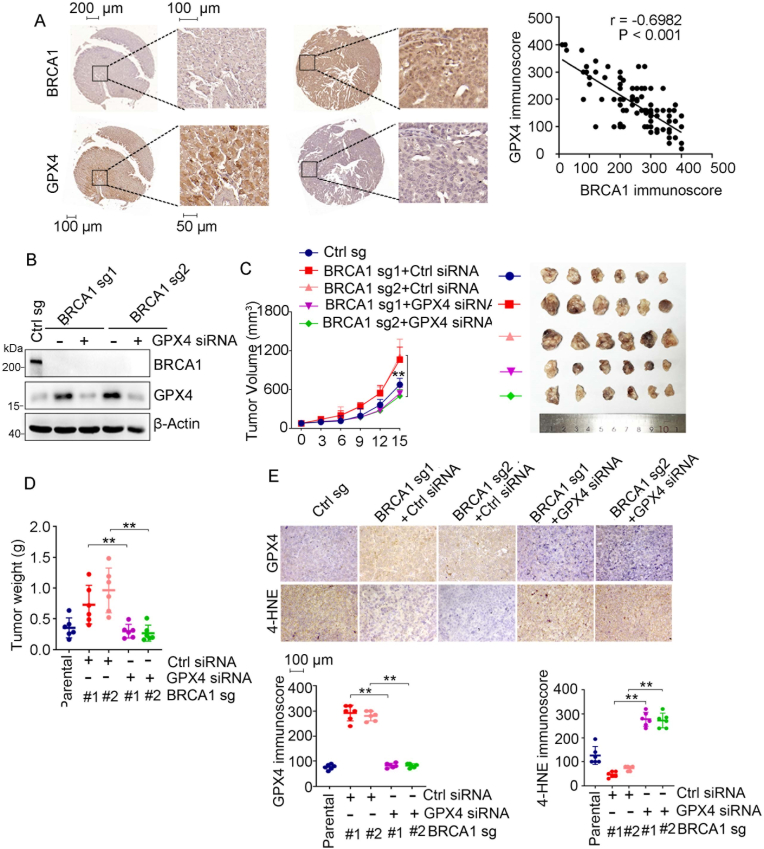
3.5. GPX4 is critical for tumor growth in BRCA1-deficient ovarian cancer
In light of its ferroptosis suppressive function, GPX4 is involved in cancer progression and chemoresistance. To study the potential relevance of GPX4 in BRCA1-related ovarian cancer, we first examined the protein levels of GPX4 and BRCA1 in 96 cases of ovarian cancer tissues. In consistent with that BRCA1/BARD1 promotes GPX4 degradation (Fig. 2), we found that GPX4 protein level is negatively corelated to BRCA1 protein level in ovarian cancer tissues (Fig. 5A). We then investigated the role of GPX4 in progression of BRCA1-deficient ovarian cancer cells. Silence of GPX4 did not obviously affect in vitro proliferation of BRCA1-deficient ovarian cancer cells (Fig. 5B and Fig. S5A). Loss of BRCA1 accelerated A2780 xenograft tumor growth in vivo, which is consistent with previous studies [33,34], intriguingly, we found that treatment of cholesterol-modified in vivo siRNA targeting GPX4 significantly suppressed growth of BRCA1-deficient A2780 xenografts (Fig. 5C–D). GPX4 knockdown by in vivo siRNA were validated by IHC (Fig. 5E). Significant decrease of 4-HNE were observed in BRCA1-KO tumors, which were reversed by GPX4 silence (Fig. 5E), however, we did not detect obvious change of ferroptosis regulator SLC7A11 and FSP1 in above tumors (Fig. S5B). While, treatment of ferroptosis inhibitor ferrostatin-1 significantly reversed GPX4 knockdown induced tumor growth suppression (Figs. S5C–D). Thus, our results suggest that GPX4 promotes BRCA1-deficient tumor growth likely through suppressing ferroptosis.
Fig. 5.
GPX4 promotes growth in BRCA1-deficient ovarian cancer. (A) IHC analysis of BRCA1 and GPX4 protein expression in ovarian cancer tissue. The representative IHC staining (left) and correlation analysis of GPX4 immunoscore and BRCA1 immunoscore (right) were shown. N = 96. (B) BRCA1-KO A2780 cells were transfected with or without cholesterol-modified in vivo GPX4 siRNA, followed by Western blot analysis of indicated proteins. (C–D) Tumor growth curve(C) and tumor weights (D) of indicated A2780 xenografts treated with control (ctrl) or GPX4 siRNA. (E) IHC analysis of GPX4 and 4-HNE levels in indicated xenograft tumors. The representative IHC staining (left) and their quantification (right) were shown. Data were presented as mean ± standard deviation (SD), n = 6. **p < 0.01by two-way ANOVA test.
3.6. GPX4 silence enhances PARP inhibitors triggered-ferroptosis
PARP inhibitors elicit synthetic lethality in BRCA1-mutant tumors and are the most common treatment for BRCA-related cancers [17]. To test whether BRCA1-regulated ferroptosis susceptibility is involved in PARP inhibitor sensitivity, we firstly treated BRCA1-proficient A2780 cells and BRCA1-deficient UWB1.289 cells with increasing concentrations of Olaparib. Interestingly, we found that Olaparib dose-dependently elicited lipid peroxidation in both A2780 and UWB1.289 cells (Fig. 6A and Fig. S6A). Olaparib-triggered lipid peroxidation markedly occurred at 24–48 h after treatment (Figs. S6B–C).
Fig. 6.
Inhibition of GPX4 potentiates therapeutic efficacy of PARP inhibitor in BRCA1-deficient ovarian cancers. (A) UWB1.289 cells were treated increasing concentrations of Olaparib (Ola) for 24 h, and the lipid peroxidation was analyzed by BODIY 581/591 C11. The representative flow cytometry profiles (left) and quantification of lipid peroxidation (right) were shown. (B) Western blot analysis of indicated proteins in UWB1.289 cells stably expressing control (ctrl) or GPX4 shRNA. (C–D) Ctrl or GPX4 shRNA transfected UWB.1289 cell were treated with or without 100 μM Olaparib (Ola) for 48 h, and lipid peroxidation (C) and cell death (D) were analyzed by BODIY 581/591 C11 labeling and PI staining respectively. (E) UWB1.289 cells expressing ctrl or GPX4 shRNA were grown in presence or absence of 2 μM Olaparib for 7–10 days, followed by colony formation analysis. (F) Western blot analysis of GPX4 expression in control or BRCA1-KO A2780 cells expressing ctrl or GPX4 shRNA. (G) BRCA1-KO A2780 cells with or without GPX4 knockdown were grown in presence or absence of 2 μM Olaparib for 7–10 days, followed by colony formation analysis. (H–I) BRCA1-KO A2780 cells expressing ctrl or GPX4 shRNA were treated with or without 100 μM Olaparib (Ola) for 48 h, and lipid peroxidation (H) and cell death (I) were analyzed by BODIY 581/591 C11 labeling and PI staining respectively. Data represents the mean ± SD from at least three independent replicates, *p < 0.01, and ***p < 0.001 by two-way ANOVA test.
Meanwhile, another PARP inhibitor talzazoparib also induced lipid peroxidation in A2780 cells (Fig. S6D). In addition, we collected a recurrent ovarian cancer sample after PARP inhibitor niraparib treatment and analyzed 4-HNE level in cancer tissues before and after niraparib therapy. Consistently, an obviously elevated 4-HNE was detected in patient's cancer tissue after niraparib treatment (Fig. S6E). These results suggest PARP inhibition triggers ferroptosis in ovarian cancer cells.
Given that BRCA1-deficiency increased GPX4 and resulted in ferroptosis resistance (Fig. 1, Fig. 2), we hypothesized that the inhibition GPX4 would render BRCA1-mutant cancer cells sensitive to PARP inhibitor-induced ferroptosis. As expected, C11-BODIPY (581/591) staining assay showed that silence of GPX4 markedly increased Olaparib-induced lipid peroxidation in UWB1.289 cells (Fig. 6B–C). Consequently, knockdown of GPX4 resulted in increase of cell death (Fig. 6D), as well as decrease of clonogenic survival in presence of Olaparib treatment in UWB1.289 cells (Fig. 6E). Similar results were observed in HCC1937 cells, a different BRCA1-deficient cell line (Figs. S7A–C). Then, we silenced GPX4 in BRCA1-KO A2780 cells to further confirm whether inhibition of GPX4 could sensitize BRCA1-deficient cancer cells to PARP inhibitors (Fig. 6F). As expected, increased Olaparib sensitivity was observed in BRCA1-depleted A2780 cells compared with parental cells, while, GPX4-silence led to greater sensitivity to Olaparib in BRCA1-KO A2780 cells (Fig. 6G). Meanwhile, GPX4-silence greatly enhanced Olaparib-induced lipid peroxidation and cell death in BRCA1-KO A2780 cells (Fig. 6H–I). Similar observations were obtained in BRCA1-KO OVCAR433 cells (Figs. S7D–G). Meanwhile, ferrostatin-1 treatment significantly reversed GPX4 knockdown-induced Olaparib hypersensitivity (Fig. S8). Therefore, these results demonstrate the inhibition of GPX4 reverse ferroptosis resistance and confers hypersensitivity to PARP inhibition in BRCA1-deficient cancer cells.
3.7. Combination treatment with PARP and GPX4 inhibitors synergistically inhibits growth of BRCA1-deficient cancer cells
To test the therapeutic efficacy of PARP inhibitor in combination with GPX4 inhibitor in BRCA1-deficient cancers, UWB1.289 and HCC1937 cells were treated with increasing concentrations of Olaparib, GPX4 covalent inhibitor ML210, or their combination. Cell growth analysis showed that Olaparib and ML210 synergistically inhibited growth of UWB.1289 and HCC1937 cells (Fig. 7A). Colony formation assay confirmed that dual treatment of Olaparib and ML210 induced greater inhibition on clonogenic survival compared with Olaparib or ML210 alone (Fig. 7B). Meanwhile, we detected dramatic increase of cell death and lipid peroxidation in Olaparib/ML210 co-treated UWB1.289 and HCC1937 cells (Fig. 7C–D, Figs. S9A–B). Since, PARPi elicits ferroptosis in both BRCA1/2 proficient and deficient cells (Fig. S6). We thus examined the clonogenic survival of BRCA1/2 WT and BRCA1/53BP1 double knockout (DKO) cells after treatment of Olaparib, ML210 or their combination. As shown in Figs. S9C–E, enhanced anti-tumor effects of Olaparib/ML210 combination were also observed in BRCA1/53BP1 DKO and BRCA1/2 WT ovarian cancer cells.
Fig. 7.
Combination of PARP and GPX4 inhibitors synergistically inhibits BRCA1-deficient cancers. (A) UWB1.289 or HCC1937 cells were combined treated with serial dosages of Olaparib and ML210 for three days, followed by Bliss synergy analysis. (B) Colony formation analysis of UWB1.289 or HCC1937 cells treated with 0.5 μM Olaparib, 0.5 μm ML210 or their combination. (C–D) UWB.1289 cells were treated with 100 μM Olaparib, 2 μM ML210, or their combination for 48 h, and lipid peroxidation (C) and PI staining (D) assays were performed. (E–F) BRCA1-mutant ovarian cancer organoids (PDO) were treated with serial concentrations of olaparib, ML210, or their combination for 5 days, then the organoid viability was determined. The representative organoid after treatment (E) The ZIP synergy score (F) were shown. (G) BRCA1-mutant ovarian cancer organoids were treated with Olaparib (50 μM), ML210 (5 μM), ferrostatin-1(Fer-1) (10 μM) or their combination as indicated for 5 days, and organoid viability was measured. (H–I) BRCA1-KO A2780 xenograft bearing-mice were administrated with PBS (ctrl), olaparib (100 mg/kg), ML210 (30 mg/kg) or their combination, tumor volumes were measured and tumor growth curve (H) and tumor wights (I) were shown. (J) IHC analysis of 4-HNE in indicating drug-treated xenograft tumor tissues. The representative IHC staining (left) and quantification of IHC staining (right) were shown. Data were presented as means ± SD, n = 6. *P < 0.05, **P < 0.01, and ***P < 0.001 by two-way ANOVA test.
We next employed patient-derived ovarian cancer organoids with BRCA1-mutation to further evaluate the therapeutic efficacy of Olaparib in combination with ML210. Consistent with the observation in BRCA1-mutant cells, combined treatment of Olaparib and ML210 produced synergistic anti-tumor effects in BRCA1-mutant ovarian cancer organoids (Fig. 7E–F). The synergistic effect of ML210 and Olaparib on organoid viability (Fig. 7G) and ferroptosis marker malondialdehyde (MDA) production were reversed by ferrostatin-1 (Fig. S9F), suggesting ML210 sensitizes BRCA1-mutant cancer cells to PARPi by ferroptosis induction. Then, we measured the anti-tumor effects of Olaparib, ML210 and their combination in BRCA1-KO A2780 xenograft tumors. As shown in Fig. 7H–J, dual treatment of ML210 and Olaparib produced enhanced anti-tumor efficacy compared with ML210 or Olaparib alone. We also observed a higher level of 4-HNE in ML210/Olaparib co-treated tumors (Fig. 7I). However, we did not detect obvious toxicity including body weight loss, tissue damages and blood biochemical parameters in mice received combination treatment (Fig. S10).
4. Discussion
BRCA1 is a large protein with 1863 amino acids that contains multiple functional domains including RING domain, nuclear localization signal (NLS), coiled-coil domain (CC) and BRCT domain [35]. It is well established that BRCA1 maintains genome integrity through BRCT domain-mediated DNA repair and replication function [7]. The accumulation of several HR repair proteins including CtIP, RAP80 and PALB2 at DNA damage sites depends on their interaction with BRCA1 on BRCT domain [36]. Cells with BRCA1S1598F mutation that ablate BRCT phospho-recognition are deficient in HR repair and hypersensitive to genotoxic stress. Apart from HR repair [36], BRCA1 is also reported to be involved in reprogramming transcription and metabolism [37,38], which may also contribute to its tumor suppressive roles. In this study, we uncovered an unexpected role of BRCA1 in regulation of cell death, a hallmark of cancer cells. We demonstrate that BRCA1 deficiency confers ferroptosis resistance in ovarian cancer cells, which promotes tumor growth in vivo. BRCA1 promotes ferroptosis through catalyzing polyubiquitination of ferroptosis repressor GPX4, and subsequently leads to GPX4 degradation. This pro-ferroptosis function of BRCA1 relies on its RING domain that binds to BARD1 protein and forms a BRCA1/BARD1 heterodimer to exert ubiquitin E3 ligase activity. RING domain contained N-terminus of BRCA1 is sufficient to bind and ubiquitinate GPX4. In agreement with our study, mice carrying Brca1185stop mutation, which expressed a RING-less BRCA1 preserved its HR repair functions, developed mammary tumors [13]. Our observation also supports the fact that the C61G mutation of BRCA1, which disrupts BRCA1/BARD1 heterodimerization and abrogates its ubiquitin ligase activity, is a common pathogenic missense mutation in breast cancer patients [39]. A recent report reveals that BRCA1 directly associates with and drives GPX4 transcription [40]. As a result, BRCA1 deficiency creates a vulnerability to ferroptosis when PARP and GPX4 are co-inhibited [40]. In line with this recent study, our data also demonstrated that the combination of GPX4i and PARPi treatment produced synergistic effects in BRCA1-deficient cancers. However, our study uncovered a different mechanism: BRCA1 directly ubiquitinates GPX4. Therefore, our study provides novel evidence to support RING-domain is essential for tumor suppressive function of BRCA1.
The SLC7A11-GSH-GPX4 axis is a well-defined antioxidant system and plays a key role against ferroptosis [19]. As an important repressor in ferroptosis, GPX4 has emerged as an attractive target for cancer therapies [28]. The regulatory mechanisms that control GPX4 protein expression have been widely investigated. Several transcription factors including CREB, VDR, NRF2 have been reported to drive GPX4 transcription [41]. Importantly, GPX4 protein level or enzymatic activity are also regulated by succination, ubiquitination or phosphorylation mediated-post-translational modifications (PTM) [42]. GPX4 protein level is controlled by ubiquitination-mediated proteasomal degradation and E3 ligase HOIP catalyzes linear ubiquitination of GPX4 [43]. In present study, we identified BRCA1 is a novel E3 ubiquitin ligase that attaches K6-linked polyubiquitin chain to GPX4. Depletion of BRCA1 resulted in elevated GPX4 protein level and ferroptosis resistance. BRCA1 has been previously reported to be in involved in oxidative stress regulation. BRCA1 upregulates NRF2 expression, an antioxidant protein, via binding to its promoter [44]. BRCA1 could also physically interacts with NRF2 and modulates its activity [45]. The regulatory network of BRCA1-mediated GPX4 and NRF2 in oxidative stress regulation are needed to be further investigated.
PARP inhibitors, the first synthetic lethal therapy, are widely employed to managing cancer patients with BRCA1/2 mutations [17]. The treatment of PARP inhibitors triggers unrepairable DNA DSBs in BRCA1/2-mutated cancer cells due to defect in HR repair [17]. As a result, PARP inhibitor sensitivity primarily relies on HR repair activity and the accumulation of DNA DSBs in cancer cells. However, previous study along with our present data found that PARP inhibitors induce ferroptosis in ovarian cancer cells regardless of BRCA1/2 mutation [46], suggesting that ferroptosis is another anti-cancer mechanism of PARP inhibitors beyond DNA damage. Unlike compromised HR repair in BRCA1-deficient cancer cells that confers sensitivity to PARP inhibitors, our observation showed that BRCA1-deficient cancer cells are resistant to ferroptosis due to GPX4 elevation. This intrinsic resistance to ferroptosis in BRCA1-mutated cancer cells may provide an explanation for the clinical observation that BRCA1-altered cancer patients responded worse to PARP inhibitors compared with BRCA2-altered cancer patients [12,18], even though both BRCA1-and BRCA2-altered cancer cells contains defective HR repair. Our data revealed that PARP inhibitors elicit ferroptosis in both BRCA1-proficient or deficient ovarian cancer cells. Thus, our results imply PARP inhibitors kill cancer cells through two independent mechanisms: ferroptosis and DSBs. Although HR repair is incompetent in BRCA1/2-deficient cancer cells, ferroptosis-related factors including GPX4 are also involved in regulation of PARPi-sensitivity. In present study, we further tested and validated that inhibition of GPX4 sensitize BRCA1-deficient ovarian cancer cells to PARPi-induced ferroptosis. Combination treatment of GPX4 and PARP inhibitor produced synergistic anti-tumor efficacy in BRCA1-deficient ovarian cancers. Thus, we present study provides a novel strategy to optimize PARP inhibitor therapy in BRCA1-mutant cancer patients.
Ethical compliance
The use of all patients’ specimens was approved by the Institutional review board (IRB) of Nanjing Maternity and Child Health Care Hospital [file Number: 2019) NFKSL-068]. Animal experiments were approved by IACUC of China Pharmaceutical University under protocol number: 2022-01-029.
CRediT authorship contribution statement
Xuexia Xie: Writing – original draft, Data curation. Congcong Chen: Supervision, Conceptualization. Cong Wang: Methodology. Yongjian Guo: Software, Formal analysis. Binghe Sun: Data curation. Jiaxin Tian: Methodology. Jin Yan: Resources. Dake Li: Funding acquisition. Guo Chen: Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by National Natural Science Foundation of China (82073042, 82372973 to G. Chen; 82272692 to DK. Li), Innovation and Entrepreneurship (Shuangchuang) Program of Jiangsu Province (JSSCTD202350 to G. Chen) and Guangdong Basic and Applied Basic Research Foundation (2022B1515020105 to G. Chen). We would like to thank Dr. Yan Chen (Shandong Normal University) for kindly providing us with the HA-Ubiquitin plasmids. We also acknowledge the public platform of Animal Experimental Center and Mr. Shuoshuo Hou for the use of SPF facility.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2024.103350.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Krais J.J., Johnson N. BRCA1 mutations in cancer: coordinating deficiencies in homologous recombination with tumorigenesis. Cancer Res. 2020;(80):4601–4609. doi: 10.1158/0008-5472.CAN-20-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li S., Silvestri V., Leslie G., Rebbeck T.R., Neuhausen S.L., Hopper J.L., et al. Cancer risks associated with BRCA1 and BRCA2 pathogenic variants. J. Clin. Oncol. 2022;40:1529–1541. doi: 10.1200/JCO.21.02112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu X., Tan W., Song Q., Pei H., Li J. BRCA1 and breast cancer: molecular mechanisms and therapeutic strategies. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.813457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escribano-Diaz C., Orthwein A., Fradet-Turcotte A., Xing M., Young J.T., Tkac J., et al. A cell cycle-dependent regulatory circuit composed of 53BP1-RIF1 and BRCA1-CtIP controls DNA repair pathway choice. Mol. Cell. 2013;49:872–883. doi: 10.1016/j.molcel.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Foo T.K., Xia B. BRCA1-Dependent and independent recruitment of PALB2-BRCA2-RAD51 in the DNA damage response and cancer. Cancer Res. 2022;82:3191–3197. doi: 10.1158/0008-5472.CAN-22-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isono M., Niimi A., Oike T., Hagiwara Y., Sato H., Sekine R., et al. BRCA1 directs the repair pathway to homologous recombination by promoting 53BP1 dephosphorylation. Cell Rep. 2017;(18):520–532. doi: 10.1016/j.celrep.2016.12.042. [DOI] [PubMed] [Google Scholar]
- 7.Tarsounas M., Sung P. The antitumorigenic roles of BRCA1-BARD1 in DNA repair and replication. Nat. Rev. Mol. Cell Biol. 2020;21:284–299. doi: 10.1038/s41580-020-0218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruand M., Barras D., Mina M., Ghisoni E., Morotti M., Lanitis E., et al. Cell-autonomous inflammation of BRCA1-deficient ovarian cancers drives both tumor-intrinsic immunoreactivity and immune resistance via STING. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu Y., Helenius M., Vaananen K., Bulanova D., Saarela J., Sokolenko A., et al. BRCA1-deficient breast cancer cell lines are resistant to MEK inhibitors and show distinct sensitivities to 6-thioguanine. Sci. Rep. 2016;(6) doi: 10.1038/srep28217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J., Shu X., Xu J., Su S.M., Chan U.I., Mo L., et al. S100A9-CXCL12 activation in BRCA1-mutant breast cancer promotes an immunosuppressive microenvironment associated with resistance to immunotherapy. Nat. Commun. 2022;(13):1481. doi: 10.1038/s41467-022-29151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolton K.L., Chenevix-Trench G., Goh C., Sadetzki S., Ramus S.J., Karlan B.Y., et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307:382–390. doi: 10.1001/jama.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang D., Khan S., Sun Y., Hess K., Shmulevich I., Sood A.K., et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306:1557–1565. doi: 10.1001/jama.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drost R., Dhillon K.K., van der Gulden H., van der Heijden I., Brandsma I., Cruz C., et al. BRCA1185delAG tumors may acquire therapy resistance through expression of RING-less BRCA1. J. Clin. Invest. 2016;126:2903–2918. doi: 10.1172/JCI70196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y., Krais J.J., Bernhardy A.J., Nicolas E., Cai K.Q., Harrell M.I., et al. RING domain-deficient BRCA1 promotes PARP inhibitor and platinum resistance. J. Clin. Invest. 2016;126:3145–3157. doi: 10.1172/JCI87033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demeny M.A., Virag L. The PARP enzyme family and the hallmarks of cancer Part 1. Cell intrinsic hallmarks. Cancers. 2021;13 doi: 10.3390/cancers13092042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lord C.J., Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J.M., Ledermann J.A., Kohn E.C. PARP Inhibitors for BRCA1/2 mutation-associated and BRCA-like malignancies. Ann. Oncol. 2014;25:32–40. doi: 10.1093/annonc/mdt384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taza F., Holler A.E., Fu W., Wang H., Adra N., Albany C., et al. Differential activity of PARP inhibitors in BRCA1- versus BRCA2-altered metastatic castration-resistant prostate cancer. JCO Precis Oncol. 2021;5 doi: 10.1200/PO.21.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang X., Stockwell B.R., Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021;22:266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu K., Yan M., Liu T., Wang Z., Duan Y., Xia Y., et al. Creatine kinase B suppresses ferroptosis by phosphorylating GPX4 through a moonlighting function. Nat. Cell Biol. 2023;25:714–725. doi: 10.1038/s41556-023-01133-9. [DOI] [PubMed] [Google Scholar]
- 21.Liang D., Feng Y., Zandkarimi F., Wang H., Zhang Z., Kim J., et al. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell. 2023 doi: 10.1016/j.cell.2023.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X., Kang R., Kroemer G., Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021;18:280–296. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 23.Lei G., Zhuang L., Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer. 2022;(22):381–396. doi: 10.1038/s41568-022-00459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen B., Ge T., Jian M., Chen L., Fang Z., He Z., et al. Transmembrane nuclease NUMEN/ENDOD1 regulates DNA repair pathway choice at the nuclear periphery. Nat. Cell Biol. 2023;25:1004–1016. doi: 10.1038/s41556-023-01165-1. [DOI] [PubMed] [Google Scholar]
- 25.Lim K.S., Li H., Roberts E.A., Gaudiano E.F., Clairmont C., Sambel L.A., et al. USP1 is required for replication fork protection in BRCA1-deficient tumors. Mol. Cell. 2018;72:925–941 e924. doi: 10.1016/j.molcel.2018.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takaoka M., Miki Y. BRCA1 gene: function and deficiency. Int. J. Clin. Oncol. 2018;(23):36–44. doi: 10.1007/s10147-017-1182-2. [DOI] [PubMed] [Google Scholar]
- 27.Brown C.W., Chhoy P., Mukhopadhyay D., Karner E.R., Mercurio A.M. Targeting prominin2 transcription to overcome ferroptosis resistance in cancer. EMBO Mol. Med. 2021;13 doi: 10.15252/emmm.202013792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J., Roh J.L. Targeting GPX4 in human cancer: implications of ferroptosis induction for tackling cancer resilience. Cancer Lett. 2023;559 doi: 10.1016/j.canlet.2023.216119. [DOI] [PubMed] [Google Scholar]
- 29.Santivasi W.L., Wang H., Wang T., Yang Q., Mo X., Brogi E., et al. Association between cytosolic expression of BRCA1 and metastatic risk in breast cancer. Br. J. Cancer. 2015;113:453–459. doi: 10.1038/bjc.2015.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savaskan N.E., Ufer C., Kuhn H., Borchert A. Molecular biology of glutathione peroxidase 4: from genomic structure to developmental expression and neural function. Biol. Chem. 2007;388:1007–1017. doi: 10.1515/BC.2007.126. [DOI] [PubMed] [Google Scholar]
- 31.Xiang T., Ohashi A., Huang Y., Pandita T.K., Ludwig T., Powell S.N., et al. Negative regulation of AKT activation by BRCA1. Cancer Res. 2008;(68):10040–10044. doi: 10.1158/0008-5472.CAN-08-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damgaard R.B. The ubiquitin system: from cell signalling to disease biology and new therapeutic opportunities. Cell Death Differ. 2021;28:423–426. doi: 10.1038/s41418-020-00703-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibnat N., Chowdhury E.H. Retarding breast tumor growth with nanoparticle-facilitated intravenous delivery of BRCA1 and BRCA2 tumor suppressor genes. Sci. Rep. 2023;13:536. doi: 10.1038/s41598-022-25511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., Wang Z., Qi Z., Yin S., Zhang N., Liu Y., et al. The negative interplay between Aurora A/B and BRCA1/2 controls cancer cell growth and tumorigenesis via distinct regulation of cell cycle progression, cytokinesis, and tetraploidy. Mol. Cancer. 2014;(13):94. doi: 10.1186/1476-4598-13-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J., Lu L.Y., Yu X. The role of BRCA1 in DNA damage response. Protein Cell. 2010;1:117–123. doi: 10.1007/s13238-010-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Billing D., Horiguchi M., Wu-Baer F., Taglialatela A., Leuzzi G., Nanez S.A., et al. The BRCT domains of the BRCA1 and BARD1 tumor suppressors differentially regulate homology-directed repair and stalled fork protection. Mol. Cell. 2018;72:127–139 e128. doi: 10.1016/j.molcel.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart M.D., Zelin E., Dhall A., Walsh T., Upadhyay E., Corn J.E., et al. BARD1 is necessary for ubiquitylation of nucleosomal histone H2A and for transcriptional regulation of estrogen metabolism genes. Proc. Natl. Acad. Sci. U. S. A. 2018;A(115):1316–1321. doi: 10.1073/pnas.1715467115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanakkanthara A., Kurmi K., Ekstrom T.L., Hou X., Purfeerst E.R., Heinzen E.P., et al. BRCA1 deficiency upregulates NNMT, which reprograms metabolism and sensitizes ovarian cancer cells to mitochondrial metabolic targeting agents. Cancer Res. 2019;(79):5920–5929. doi: 10.1158/0008-5472.CAN-19-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drost R., Bouwman P., Rottenberg S., Boon U., Schut E., Klarenbeek S., et al. BRCA1 RING function is essential for tumor suppression but dispensable for therapy resistance. Cancer Cell. 2011;20:797–809. doi: 10.1016/j.ccr.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 40.Lei G., Mao C., Horbath A.D., Yan Y., Cai S., Yao J., et al. BRCA1-Mediated dual regulation of ferroptosis exposes a vulnerability to GPX4 and PARP Co-inhibition in BRCA1-deficient cancers. Cancer Discov. 2024;(14):1476–1495. doi: 10.1158/2159-8290.CD-23-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai C., Chen X., Li J., Comish P., Kang R., Tang D. Transcription factors in ferroptotic cell death. Cancer Gene Ther. 2020;(27):645–656. doi: 10.1038/s41417-020-0170-2. [DOI] [PubMed] [Google Scholar]
- 42.Cui C., Yang F., Li Q. Post-translational modification of GPX4 is a promising target for treating ferroptosis-related diseases. Front. Mol. Biosci. 2022;(9) doi: 10.3389/fmolb.2022.901565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong K., Wei R., Jin T., Zhang M., Shen J., Xiang H., et al. HOIP modulates the stability of GPx4 by linear ubiquitination. Proc. Natl. Acad. Sci. U. S. A. 2022;(119) doi: 10.1073/pnas.2214227119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu P., Liu Q., Xie Y., Shi X., Li Y., Peng M., et al. Breast cancer susceptibility protein 1 (BRCA1) rescues neurons from cerebral ischemia/reperfusion injury through NRF2-mediated antioxidant pathway. Redox Biol. 2018;(18):158–172. doi: 10.1016/j.redox.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorrini C., Baniasadi P.S., Harris I.S., Silvester J., Inoue S., Snow B., et al. BRCA1 interacts with Nrf2 to regulate antioxidant signaling and cell survival. J. Exp. Med. 2013;210:1529–1544. doi: 10.1084/jem.20121337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong T., Lei G., Chen X., Li H., Zhang X., Wu N., et al. PARP inhibition promotes ferroptosis via repressing SLC7A11 and synergizes with ferroptosis inducers in BRCA-proficient ovarian cancer. Redox Biol. 2021;(42) doi: 10.1016/j.redox.2021.101928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.