Abstract
Background:
Bovine respiratory disease (BRD) is a complex illness that impacts the respiratory system of domestic cattle, resulting in significant financial losses for the agriculture industry. Inactivated or modified live (MLV) pathogen vaccines are often used as a management tool to prevent and control BRD effectively.
Aim:
The purpose of this study is to assess the cell-mediated immune response (CMI) induced by two commercially available polyvalent vaccines, namely the MLV (cattle master gold FP) and the inactivated (CATTLEWIN-5K) vaccine.
Methods:
A total of 20 seronegative heifers against 4 BRD viruses, bovine alphaherpisvirus-1 (BoAHV-1), bovine viral diarrhea virus (BVDV BVDV-1: Pesti virus A; BVDV-2: Pesti virus B), bovine respiratory syncytial virus (BRSV) and bovine parainfluenza virus-3 (BPIV3) were chosen for this study. The heifers were divided into three groups. The first group (n = 6) received no vaccination and was kept as a control. The second and third groups (seven heifers each) were vaccinated twice with either an MLV or inactivated vaccine. The gene expression level of interleukin-6 (IL-6) and interferon-gamma (INF-γ) was measured using real-time quantitative polymerase chain reaction on the 7th, 14th, 21st, 28th, and 60th days post-vaccination. The results were compared with the control group to study the effectiveness of the vaccines.
Results:
There was an upregulation in the expression level of IL-6 and INF-γ in both MLV and inactivated vaccinated groups. The level of IL-6 mRNA expression was statistically increased from the 14th and 28th days post-vaccination in MLV and inactivated vaccine groups, respectively. The expression level of INF-γ increased significantly from the 2nd and 4th weeks post-vaccination in the MLV and inactivated vaccine groups, respectively. The mean expression level of IL-6 and INF-γ mRNAs was significantly higher in the MLV vaccine group than in the inactivated vaccine group at each examination time.
Conclusion:
Both investigated vaccines are efficient in stimulating CMI, particularly with the MLV vaccine showing a higher preponderance in IL-6 and INF-γ.
Keywords: BRD, cell mediated immunity, IL-6, INF-γ, vaccine
Introduction
Respiratory tract infections are prevalent in animal livestock, and bovine respiratory disease (BRD) is a general term for many respiratory disorders that affect the upper and lower respiratory tracts of cattle and buffaloes. BRD is a widespread issue in the beef and dairy industries, causing significant economic losses, including decreased growth performance, treatment costs, and reduced milk yield (Johnson and Pendell, 2017). BRD is a multifactorial disorder that results from a combination of various infectious agents and environmental stress factors such as transportation, high animal density, changes in diet, and unsanitary hygienic conditions (Nagy et al., 2022). Moreover, the problem of the life-long latency of some viral causes of BRD impedes the efficiency of control programs (Jones and Chowdhury, 2007). Nowadays, several bacterial and viral pathogens are proven to be involved in the disease initiation and progression. The clinical presentation of BRD infection usually starts with a common cold-like upper respiratory tract infection that either results in a full recovery or develops into life-threatening pneumonia in cases of secondary bacterial infection (Kamel et al., 2024). The most commonly found bacterial agents in clinically infected cattle with BRD include Pasteurella multocida, Mannehemia hymolytica, and Histophilus somni (Centeno-Martinez et al., 2022). Other viral agents that can initiate BRD include, Bovine alphaherpesvirus-1 (BoAHV-1), bovine viral diarrhea virus (BVDV), bovine respiratory syncytial virus (BRSV), and bovine parainfluenza virus-3 (BPIV3). BoAHV-1 infection can spread intracellularly, which allows the virus to evade circulating antibodies (Jones et al., 2011). Therefore, the evolution of cell-mediated immunity (CMI) is crucial to control the spread of the virus and the recurrence of infection, besides humeral immunity. Interferon-gamma (INF-γ) is a soluble protein that is produced mainly by T lymphocytes and natural killer cells (NK), which promotes the activation of macrophages and the destruction of virus-infected cells (Schroder et al., 2004). The expression level of INF-γ is used mainly to monitor the development of the T helper 1 (Th1) response after immunization (Zhang et al., 2011). During a natural infection or after vaccination, the body releases various cytokines, including pro-inflammatory and anti-inflammatory ones such as IL-1, IL-6, IL-8, and tumor necrosis factor (TNF). These cytokines play a crucial role in recruiting neutrophils and monocytes to the site of infection and stimulating the synthesis of acute-phase proteins, which are important during acute inflammation (McGill and Sacco, 2020). To prevent and manage BRD, vaccines made from inactivated or modified live (MLV) pathogens are commonly used. Generally, animal vaccination triggers humoral and CMI responses (Righi et al., 2023). By identifying the most effective vaccines, farmers can take proactive measures to protect their herds, minimize the risk of infection, and ensure the long-term health and productivity of their cattle.
In this study, the difference in the expression level of IL-6 and INF-γ was evaluated after immunization with either MLV or inactivated vaccines against BRD. The objective of this study is to evaluate the cellular immunity produced by two commercially inactivated and MLV vaccines that are traditionally used in Egypt.
Materials and Methods
Animals
An experimental study was conducted on 20 Holstein heifers aged 10–15 months from January 2024 to March 2024. All the heifers were raised on a private dairy farm in the Gharbia Governorate, Egypt. This farm was chosen as none of the animals had been previously vaccinated against BRD. Before the experiment began, all the animals were tested to determine their serological reactions to four BRD viruses (BoAHV-1: Abou Hammad strain, BVDV-1: Iman strain, BVDV-2: strain 125, BRSV: strain 375L and BPIV3: strain 45) using a virus neutralization test, following the OIE (2014) guidelines. All the heifers were fed a total mixed ration comprising silage, corn, soybeans, and flax seeds.
Clinical observation of animals
The heifers were clinically monitored by a veterinarian three days before the start of the experiment and up until the end of the first week after vaccination. Their body temperature was checked daily, and the injection site was inspected for any signs of inflammation.
Study design and vaccination
Seronegative heifers (n = 20) were randomized into three groups. The first one (n = 6) was injected with normal saline and kept as a control. The second group (n = 7) was inoculated with the Cattle Master Gold FP vaccine (a freeze-dried preparation of chemically altered IBR and BPIV-3 viruses, MLV BRSV, and a liquid adjuvant preparation of inactivated BVDV (BVDV-1: Pesti virus A; BVDV-2: Pesti virus B). They were injected with 5 ml subcutaneously, and boosters were administered on the 28th day according to the manufacturer’s recommendations. The third group (n = 7) was inoculated with CATTLEWIN-5K (an adjuvant vaccine of inactivated BPIV-3, IBR, BRSV, and BVDV (BVDV-1: Pesti virus A; BVDV-2: Pesti virus B). They were injected with 2 ml intramuscularly, and boosters were administered on the 28th day according to the manufacturer’s recommendations.
Sample collection
Blood samples of 5 ml were collected from both control and vaccinated animals’ jugular veins. This was done using a sterile syringe and collected into sterile vacutainer tubes without anticoagulants for serum separation. The samples were collected on five different occasions, i.e., on the 7th, 14th, 21st, 28th, and 60th days post-vaccination, to monitor the animals’ response to the vaccine.
Evaluation of CMI by analysis of cytokine-related genes by real-time Polymerase chain reaction (PCR)
Ribonucleic acid (RNA) extraction
An aliquot of 200 µl of each serum sample was mixed with 600 µl of RLT buffer and incubated at room temperature for 10 minutes. The total RNA was then extracted and purified using the QIAamp RNeasy Mini kit (Qiagen, Germany, GmbH) as per the manufacturer’s recommendations. The purity and of extracted RNA was assessed using a NanoDrop ND-1000 spectrophotometer using OD260/OD280 ratio with an average range for all collected samples was 1.35-1.7 and OD260/OD230 ratio with an average range for all collected samples was 2.18-2.12. the average concentration of extracted RNA from all samples was 130-180 ng/ul.
Quantification of cytokines expression by real-time PCR
Step-one real-time qPCR was used to measure the expression profile of INF-γ and IL-6 cytokine genes using TranScript Green One-Step qRT-PCR Super Mix. The oligonucleotide primers for IL-6, INF-γ genes, and the B-actin gene as an internal control were provided by Metabion (Germany). The amplification reaction of RT-qPCR was carried out in a final volume of 25 µl containing 10 µl of the 2x HERA SYBR® Green RT-qPCR Master Mix (Willowfort, UK), 1 µl of RT Enzyme Mix (20X), 0.5 µl of each primer of 20 pmol concentration, 5 µl of water, and 3 µl of RNA template and performed in a step-one real-time PCR machine. The cycling conditions of Step One qPCR and target gene primers are listed in Table 1. The melting curves of the final PCR products were analyzed to determine contamination and primer dimers. The mRNA levels of each cytokine were normalized against the amount of B-actin mRNA. The mean thermal cycle values (CT) were calculated using the comparative CT method “ΔΔCt,” which involved the ratio (2^-ΔΔCt) as described by Yuan et al. (2006). The gene expression of each cytokine was then calculated as the fold change between the vaccinated and control groups. The fold change of the control group was calculated as (1 ± 0.0).
Table 1. Target genes, primer sequences, and cycling condition of real-time qPCR.
| Target genes | Primers sequences sequence (5′→3′) | Reverse transcription | Primary denaturation | Amplification (40 cycles) | References | ||
|---|---|---|---|---|---|---|---|
| Secondary denaturation | Annealing (optics on) | Extension | |||||
| B-actin | F: CGTGGGCCGCCCTAGG CACCA |
50˚C 30 minutes |
94˚C 15 minutes |
94˚C 15 seconds |
55˚C 30 seconds |
72˚C 30 seconds |
Fitzpatrick et al. (2002) |
| R:GGGGGCCTCGGTCAGC AGCAC | |||||||
| IL-6 | F: CCAGCCACAAACACTG ACCT |
Wooldridge and Ealy (2019) | |||||
| R:TAGCTCTCAGGCTGAA CTGC | |||||||
| INF-γ | F: TGATTCAAATTCCGGTG GATG |
Shu et al. (2011) | |||||
| R:TTCATTGATGGCTTT GCGC | |||||||
Statistical analysis
All statistical analyses were done using R (version 4.3.1, R Foundation for Statistical Computing, Vienna, Austria). The test results for all animals were recorded in a Microsoft Excel sheet. All data were tested for their normality of distribution using the Shapiro-Wilk test (a p-value > 0.05 assumes a normal distribution of data). Kruskal Wallis test was used to evaluate the statistical difference in gene expression level at different examination times in each group (a p-value < 0.05 is considered significant). Post-hoc Dunn’s test was used to evaluate the statistical difference in gene expression level at each examination time (post-vaccination) compared to the gene expression level in the control animals in each group (a P-adjusted value < 0.05 is considered significant). An independent student t-test was used to evaluate the statistical difference in the mean cytokines’ expression level between MLV and inactivated vaccinated groups at each examination time.
Ethical approval
The animal handling protocol was approved by the Institutional Animal Care and Use Committee at Zagazig University (approval number: ZU-IACUC/2/F/47/2024).
Results
Clinical observation of the vaccinated animals
During the experimental period, the vaccinated animals were observed clinically, and no abnormal observations were noted. The rectal temperature of all animals remained within the normal physiological range of 38°C–39°C, and there were no signs of inflammation at the injection site.
Evaluation of CMI by assessment of the expression profiles of the INF-γ and IL-6 genes
Blood samples were taken from all vaccinated heifers and analyzed for mRNA expression of the INF-γ and IL-6 genes. As shown in (Fig. 1), mRNA expression of both genes is upregulated in MLV and inactivated vaccine groups. The gene expression level of IL-6 and INF-γ increased gradually in the inactivated vaccinated group, although it elevated abruptly from the first week in the MLV vaccinated group. For the MLV-vaccinated group, the vaccinated animals expressed INF-γ mRNA 5.47, 6.42, 6.48, 6.47, and 6.57fold more than the control group at 7th, 14th, 21st, 28th, and 60th days post-vaccination, respectively (Table 2). These changes were found to be statistically different (p-value < 0.05). Post-hoc statistical analysis revealed a significant increase in INF-γ on the 14th day post-vaccination compared with the control group (P adj value < 0.05). At the same time, gene expression levels were highly significant at the 21st, 28th, and 60th days compared with control animals (p adj value < 0.01). Additionally, this vaccinated group revealed a significant difference in the mean expression level of IL-6 mRNA between different examination times (p-value < 0.05). The post-hoc multiple comparisons showed a significantly high expression of IL-6 mRNA in the 14th and 21st days post-vaccination (p adj value < 0.05). Meanwhile, the level of gene expression was highly significant at the 28th and 60th days compared with the control group (p adj value < 0.01) (Table 2).
Fig. 1. Fold change (FC) of mRNA expression of IL-6 and INF-γ in MLV and inactivated vaccinated groups at each examination point compared with control animals. (A) FC of IL-6 in the MLV vaccine group. (B) FC of IL-6 in the inactivated vaccine group. (C) FC of INF-γ in the MLV vaccine group. (D) FC of INF-γ in the inactivated vaccine group.
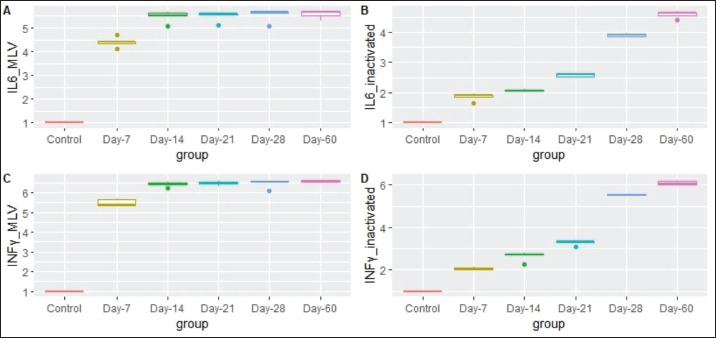
Table 2. Gene expression profiles of IL-6 and INF-γ in MLV and killed vaccinated groups compared with control animals (fold change ± SD).
| MLV vaccine (FC ± SD) | Inactivated vaccine (FC ± SD) | Control (FC ± SD) | ||||
|---|---|---|---|---|---|---|
| IL-6 | INF-γ | IL-6 | INF-γ | IL-6 | INF-γ | |
| 7 | 4.393 ± 0.184 | 5.474 ± 0.169 | 1.854 ± 0.117 | 2.062 ± 0.054 | 1 ± 0.0 | 1 ± 0.0 |
| 14 | 5.468 ± 0.211* | 6.427 ± 0.106* | 2.059 ± 0.030 | 2.650 ± 0.193 | 1 ± 0.0 | 1 ± 0.0 |
| 21 | 5.482± 0.193* | 6.480 ± 0.092** | 2.578 ± 0.054 | 3.286 ± 0.100 | 1 ± 0.0 | 1 ± 0.0 |
| 28 | 5.538 ± 0.238** | 6.473 ± 0.185** | 3.907±0.050** | 5.525± 0.030** | 1 ± 0.0 | 1 ± 0.0 |
| 60 | 5.565 ± 0.160** | 6.579 ± 0.053** | 4.583 ± 0.096** | 6.097 ± 0.098** | 1 ± 0.0 | 1 ± 0.0 |
(FC ±SD): fold change ± standard deviation; (IL-6): Interleukin 6; (INF-γ): Interferon gamma; (*): P-adj value< 0.05; (**): P-adj value< 0.01.
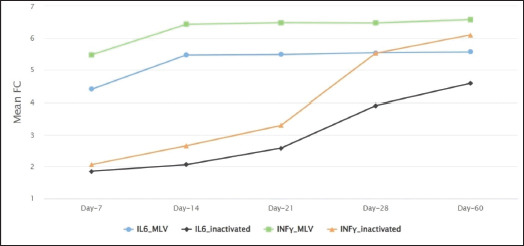
Regarding the inactivated vaccine group, the mean expression level of INF-γ increased from 2.06 to 6.09fold between the 7th and 60th days post-vaccination compared to the control group. The mean difference between these fold changes was statistically significant (p-value < 0.05). The post-hoc Dunn’s test revealed a significant elevation of INF-γ expression levels at the 28th and 60th days compared with the control group (P-adj value < 0.01). The expression level of IL-6 increased from 1.85 to 4.58 fold between the 7th and 60th days post-vaccination compared to their control counterparts. The mean difference between these fold changes was statistically significant (p-value < 0.05). The post-hoc Dunn’s test revealed a highly significant elevation of IL-6 expression level at the 28th and 60th days compared with control animals (P adj value < 0.01) (Table 2). As shown in (Fig. 2), the mean expression level of IL-6 and INF-γ mRNAs was significantly higher in the MLV vaccine group than in the inactivated vaccine group at each examination time.
Fig. 2. Comparison of the mean fold change of IL-6 and INF-γ between MLV and inactivated vaccinated groups at each examination point.
Discussion
BRD is a serious affliction that has a devastating impact on the health and well-being of Holstein cattle. That’s why it’s essential to understand the effectiveness of different commercial vaccines in combating this disease. Cell-mediated immunity is substantial for protection against BRD infection, especially BoAHV-1. This is due to the ability of this virus to elude humeral immunity. This study unequivocally demonstrates that both MLV and inactivated vaccine groups showed an upregulation in the expression levels of IL-6 and INF-γ. The study findings indicate that the MLV vaccine group had a significant and early increase in the expression of IL-6 mRNA from the 14th day following vaccination. On the other hand, the inactivated vaccine group exhibited a substantial increase on the 28th day post-vaccination after booster revaccination. This result came in accordance with Ridpath et al. (2010) who reported that the inactivated bovine respiratory vaccines induce less CMI than MLV vaccines and require booster revaccination to achieve a protective level of immunization. Furthermore, the mean fold change of INF-γ was significantly increased in both the MLV and inactivated vaccine groups from the second and fourth week’s post-vaccination, respectively, indicating that both vaccines are highly effective in initiating CMI through the stimulation of T helper 1 cells to produce INF-γ, importantly, the MLV vaccine displayed greater efficacy in this regard.
Our findings agreed with those of Platt et al. (2006), who evaluated the CMI against pentavalent- MLV vaccines containing (BoAHV-1, BVD type-1, BVD type-2, BRSV, and BPIV3). They found that the mean of expressed INF-γ from peripheral blood mononuclear cells was highly significant in the vaccinated group compared to the control group from 4 to 8 weeks after vaccination. Moreover, Van Anne et al. (2018) conducted an experimental study to evaluate the cellular immunity in vaccinated calves using a multivalent killed adjuvant vaccine for five viruses. They found that the average concentration of extracellular INF-γ in the vaccinated group was not different from that of the control group until day 28. However, it was significantly increased in the vaccinated group after 28th day till the end of the study. Our results are consistent with those of Woolum et al. (2003), who found a significant increase in INF-γ production in vaccinated calves with the MLV Bovi Shield vaccine compared to the control group. Moreover, Kornuta et al. (2022) and Zhang et al. (2024) reported a significant increase in the production of INF-γ, IL-4, and TNF-α after immunization with inactivated and MLV vaccines against BoAHV-1, respectively.
On the other hand, our findings did not agree with those of Aganja et al. (2021), who reported a down regulation in IL-6 gene expression after vaccination with an inactivated vaccine (Barvac Elite 4SH), and no significant difference in INF-γ expression was observed between the control and vaccinated groups. This difference may be due to the sampling time, as they measured cytokine levels on the 14th day after vaccination. Palomares et al. (2021) conducted a study on the effect of an MLV Bovi Shield Gold vaccine on the expression levels of INF-γ or IL-10. They found that there was no significant change in the expression levels of these cytokines after administering the vaccine. Similarly, Kerkhofs et al. (2004) investigated the gamma-interferon response after using two different vaccines. The first vaccine contained inactivated BRSV and BPIV3, while the second contained MLV BRSV and BVDV. They reported that administering both vaccines did not result in a significant change in the INF-γ response.
Throughout the study, the mean fold change of IL-6 and INF-γ was significantly higher in the group vaccinated with MLV than in the inactivated vaccine group. This is consistent with previous studies that have also reported the superior effectiveness of MLV vaccines in inducing cellular immunity (Endsley et al., 2002). Despite the rapprochement of the mean expression level of IL-6 and INF-γ between both groups by the end of the experiment, the statistical difference was still significant. This could be attributed to the small number of animals in each group. Thus, further studies on larger animal samples and for longer periods are needed to confirm these findings. According to a study conducted by Reber et al. (2006), MLV and inactivated BVDV were tested to evaluate their impact on cellular immunity stimulation. Three different vaccination protocols containing MLV BoAHV-1/BPIV3 viruses were used, but the results contradict our findings. The study found no significant change in INF-γ, IL-12, and IL-4 mRNA production between the control and all vaccinated groups. This could be attributed to the vaccines’ inability to stimulate the production of an adequate number of T-helper type 1 cells, as suggested by Charleston et al. (2002).
Conclusion
Based on our investigation, we have found that both vaccines under study are effective in stimulating CMI through the production of IL-6 and INF-γ. The results indicate that the MLV vaccine (cattle master gold) is superior in stimulating CMI from the first week of the experiment. However, further studies are necessary to fully demonstrate the safety aspects, such as the latency and shedding of BoAHV-1. Moreover, further studies are recommended for in vitro assessment of cytokines release following peripheral blood mononuclear cells restimulation by vaccine strains. By better understanding the impact of these vaccines on the immune system, we can develop more effective strategies for preventing and treating this common and costly disease.
Acknowledgment
The authors are grateful to the animal owners of the private dairy farm in Gharbia Governorate, Egypt for subjecting their animals to the study.
Conflict of interest
The authors declare that there is no conflict of interest.
Authors’ contributions
Conceptualization, MIE, NZA, MFE, and MIA; methodology, MEE, and MIA. SGY carried out the statistical analysis and interpreted the results. SGY and EMB wrote the first draft of the manuscript. MIE, NZA, MFE, and MIA revised and edited the initial manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research received no external funding.
Data availability
All data findings during our investigation are available within the manuscript.
References
- Aganja R, Seo K, Ha S, Yi Y, Lee S. Evaluation of immune responses in dairy cows immunized with an inactivated vaccine for bovine respiratory disease. Korean J. Agric. Sci. 2021;48:251–264. [Google Scholar]
- Centeno-Martinez R.E, Glidden N, Mohan S, Davidson J.L, Fernández-Juricic E, Boerman J.P, Schoonmaker J, Pillai D, Koziol J, Ault A, Verma M, Johnson T. Identification of bovine respiratory disease through the nasal microbiome. Anim. Microbiome. 2022;4(1):15. doi: 10.1186/s42523-022-00167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charleston B, Brackenbury L.S, Carr B.V, Fray M.D, Hope J.C, Howard. C.J. and Morison W.I. Alpha/beta and gamma interferons are induced by infection with noncytopathic bovine viral diarrhea virus in vivo. J. Virol. 2002;76:923–927. doi: 10.1128/JVI.76.2.923-927.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endsley J.J, Quade M.J, Terhaar B, Roth J.A. BHV-1-Specific CD4+, CD8+, and gammadelta T cells in calves vaccinated with one dose of a modified live BHV-1 vaccine. Viral Immunol. 2002;15:385–393. doi: 10.1089/08828240260066305. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R, Casey O.M, Morris D, Smith T, Powell R, Sreenan J.M. Postmortem stability of RNA isolated from bovine reproductive tissues. Biochim Biophys Acta (BBA)-Gene Struct Exp. 2002;1574:10–14. doi: 10.1016/s0167-4781(01)00322-0. [DOI] [PubMed] [Google Scholar]
- Johnson K.K, Pendell D.L. Market impacts of reducing the prevalence of bovine respiratory disease in United States beef cattle feedlots. Front. Vet. Sci., 2017;4:189. doi: 10.3389/fvets.2017.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, da Silva L.F, Sinani D. Regulation of the latency-reactivation cycle by products encoded by the bovine herpesvirus 1 (BHV-1) latency-related gene. J. Neurovirol. 2011;17:535–545. doi: 10.1007/s13365-011-0060-3. [DOI] [PubMed] [Google Scholar]
- Jones C, Chowdhury S. A review of the biology of bovine herpesvirus type 1 (BHV-1), its role as a cofactor in the bovine respiratory disease complex and development of improved vaccines. Anim. Health Res. Rev. 2007;8:187–205. doi: 10.1017/S146625230700134X. [DOI] [PubMed] [Google Scholar]
- Kamel M.S, Davidson J.L, Verma M.S. Strategies for bovine respiratory disease (BRD) diagnosis and prognosis: a comprehensive overview. Animals. 2024;14:627. doi: 10.3390/ani14040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhofs P, Tignon M, Petry H, Mawhinney I, Sustronck B. Immune responses to bovine respiratory syncytial virus (BRSV) following use of an inactivated BRSV-PI3-Mannheimia haemolytica vaccine and a modified live BRSV-BVDV vaccine. Vet. J. 2004;167:208–210. doi: 10.1016/S1090-0233(03)00078-9. [DOI] [PubMed] [Google Scholar]
- Kornuta C.A, Cheuquepán F, Bidart J.E, Soria I, Gammella M, Quattrocchi V, Hecker Y. P, Moore D.P, Romera S.A, Marin M.S, Zamorano P.I, Langellotti C.A. TLR activation, immune response and viral protection elicited in cattle by a commercial vaccine against Bovine Herpesvirus-1. Virology. 2022;566:98–105. doi: 10.1016/j.virol.2021.11.014. [DOI] [PubMed] [Google Scholar]
- McGill J.L, Sacco R.E. The Immunology of bovine respiratory disease: recent advancements. Vet. Clin. North. Am. Food. Anim. Pract. 2020;36:333–348. doi: 10.1016/j.cvfa.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Abdallah F, El Damaty H.M, Tariq A, Merwad A.M.A, Alhatlani B.Y, Elsohaby I. Genetic characterization of upper respiratory tract virome from nonvaccinated Egyptian cow-calf operations. PLoS One. 2022;17:e0267036. doi: 10.1371/journal.pone.0267036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE. Manual of diagnostic tests and vaccines for terrestrial animals 2014. 2014. [13 April 2023]. Available via http://www.oie.int/en/international-standard-setting/terrestrial-manual/access-online/
- Palomares R.A, Bittar J.H, Woolums A.R, Hoyos-Jaramillo A, Hurley D.J, Saliki J.T, Ferrer M.S, Bullington A.C, Rodriguez A, Murray T, Thoresen M, Jones K, Stoskute A. Comparison of the immune response following subcutaneous versus intranasal modified-live virus booster vaccination against bovine respiratory disease in pre-weaning beef calves that had received primary vaccination by the intranasal route. Vet. Immunol. Immunopathol. 2021;237:110254. doi: 10.1016/j.vetimm.2021.110254. [DOI] [PubMed] [Google Scholar]
- Platt R, Burdett W, Roth J. A. Induction of antigen-specific T-cell subset activation to bovine respiratory disease viruses by a modified-live virus vaccine. Am. J. Vet. Res. 2006;67:1179–1184. doi: 10.2460/ajvr.67.7.1179. [DOI] [PubMed] [Google Scholar]
- Ridpath J.F, Dominowski P, Mannan R, Yancey R.J, Jackson J.A, Taylor L, Mediratta S, Eversole R, Mackenzie C.D, Neill J.D. Evaluation of three experimental bovine viral diarrhea virus killed vaccines adjuvanted with combinations of Quil A cholesterol and dimethyldioctadecylammonium (DDA) bromide. Vet. Res. Commun. 2010;34:691–702. doi: 10.1007/s11259-010-9442-x. [DOI] [PubMed] [Google Scholar]
- Reber A.J, Tanner M. Okinaga T, Woolums A.R, Williams S, Ensley D.T, Hurley D.J. Evaluation of multiple immune parameters after vaccination with modified live or killed bovine viral diarrhea virus vaccines. Comp. Immunol. Microbiol. Infect. Dis. 2006;29:61–77. doi: 10.1016/j.cimid.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Righi C, Franzoni G, Feliziani F, Jones C, Petrini S. The cell-mediated immune response against Bovine alphaherpesvirus 1 (BoHV-1) infection and vaccination. Vaccines (Basel) 2023;11:785. doi: 10.3390/vaccines11040785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Hertzog P.J, Ravasi T, Hume D.A. Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Shu D, Subharat S, Wedlock D.N, Luo D, de Lisle G.W, Buddle B.M. Diverse cytokine profile from mesenteric lymph node cells of cull cows severely affected with Johne’s disease. Clin. Vaccine Immunol. 2011;18:1467–1476. doi: 10.1128/CVI.05201-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Anne T.R, Rinehart C.L, Buterbaugh R.E, Bauer M.J, Young A.J, Blaha M.L, Klein A.L, Chase C.C.L. Cell-mediated and humoral immune responses to bovine herpesvirus type 1 and bovine viral diarrhea virus in calves following administration of a killed-virus vaccine and bovine herpesvirus type 1 challenge. Am. J. Vet. Res. . 2018;79:1166–1178. doi: 10.2460/ajvr.79.11.1166. [DOI] [PubMed] [Google Scholar]
- Wooldridge L. K, Ealy A. D. Interleukin-6 increases inner cell mass numbers in bovine embryos. BMC Dev. Biol. 2019;19:1–11. doi: 10.1186/s12861-019-0182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolums A.R, Siger L, Johnson S, Gallo G, Conlon J. Rapid onset of protection following vaccination of calves with multivalent vaccines containing modified-live or modified-live and killed BHV-1 is associated with virus-specific interferon gamma production. Vaccine. 2003;21:1158–1164. doi: 10.1016/s0264-410x(02)00560-1. [DOI] [PubMed] [Google Scholar]
- Yuan J.S, Reed A, Chen F, Stewart C.N. Statistical analysis of real-time PCR data. BMC Bioinf. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Fu S, Deng M, Xie Q, Xu H, Liu Z, Hu C, Chen H, Guo A. Attenuation of bovine herpesvirus type 1 by deletion of its glycoprotein G and tk genes and protection against virulent viral challenge. Vaccine. 2011;29:8943–8950. doi: 10.1016/j.vaccine.2011.09.050. [DOI] [PubMed] [Google Scholar]
- Zhang S, Liu G, Zhang Y, Wang C, Xu X, Zhao Y, Xiang Z, Wu W, Yang L, Chen J, Guo A, Chen Y. Investigation of the safety and protective efficacy of an attenuated and marker M. bovis-BoHV-1 combined vaccine in bovines. Front. Immunol. 2024;15:1367253. doi: 10.3389/fimmu.2024.1367253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data findings during our investigation are available within the manuscript.


