Abstract
Background
The planning and implementation of intervention measures against schistosomiasis, particularly mass administration, require knowledge of the current status of the infection. This is important for monitoring the impact of the intervention on disease indicators such as a decline in infection prevalence, intensity of infection, and urogenital morbidities. Following repeated rounds of mass treatment in northwestern Tanzania, the epidemiology of urogenital schistosomiasis has changed; thus, for the effective planning and allocation of resources, it is important to understand the current status of the disease in the targeted groups. Therefore, the objective of the current study was to determine the prevalence, intensity, and associated factors of Schistosoma haematobium infection and urinary tract morbidities in school-aged children from northwestern Tanzania.
Materials and methods
An analytical cross-sectional study was conducted among schoolchildren aged 5–17 years between November and December 2022. A single urine sample was collected from each child and examined for the presence of S. haematobium eggs and microhaematuria using a urine filtration technique and a urine reagent dipstick. Each child underwent an ultrasonographic examination of the urinary tract according to the World Health Organization standards (Niamey protocol) to detect S. haematobium-related morbidities.
Results
Of the 3225 participants, 54.2 % were female, and the mean age was 10.9 (±1.89) years. The overall prevalence of S. haematobium was 17.7 % (95 % CI: 16.4–19.1, 572/3225). Of the 572 infected children, 81.8 % (95 % CI: 78.4–84.9, 468/572) had light-intensity infections, and 18.2 % (95 % CI: 14.9–21.4, 104/572) had heavy-intensity infections. The prevalence of macro- and microhaematuria was 2.4 % (95 % CI: 1.9–3) and 18.5 % (95 % CI: 17.2–19.8), respectively. Age (aOR: 1.2, 95 % CI: 1.0–1.5), district of residence (aOR: 2.1, 95 % CI: 1.7–2.7) and history of schistosomiasis (aOR: 2.5, 95 % CI: 1.9–3.2) were significantly associated with urinary schistosomiasis infection. However, swallowing praziquantel during the last mass drug administration was protective (aOR 0.6, 95 % CI: 0.4–0.8). The overall prevalence of ultrasound-detectable urinary tract abnormalities was 9.9 % (95 % CI: 8.9–11.1, 299/2994) and included urinary bladder abnormalities in 9.9 % (95 % CI: 8.8–11, 297/2994), ureter abnormalities in 0.2 % (95 % CI: 0.07–0.4, 6/2994), and kidney abnormalities in 0.2 % (95 % CI: 0.09–0.4, 7/2994). Calcification of the urinary bladder was observed in 0.9 % (95 % CI: 0.6–1.3, 29/2994) of the examined children.
Conclusions
Schistosoma haematobium infection is still prevalent among schoolchildren in the study setting, and it causes substantial morbidity at an early age. Transmission is driven by the age of the child, district of residence, and history of schistosomiasis. However, swallowing praziquantel in rounds of mass drug administration reduces transmission. Urogenital schistosomiasis infection is associated with haematuria and ultrasound-detectable morbidities. In S. haematobium endemic areas, routine ultrasound screening for urinary tract morbidities could be considered in annual mass treatment programmes for early management. Special attention should be given to children with proteinuria, microhaematuria, and heavy infection intensities.
Keywords: Prevalence, Schoolchildren, Schistosoma haematobium, Ultrasound, Urinary tract morbidities, Tanzania
1. Introduction
Schistosomiasis is a neglected tropical disease of significant public health concern, particularly in the sub-Saharan African region, which accounts for 90 % of the over 251 million cases occurring worldwide (Mazigo et al., 2021). Within this region, Tanzania has the second-highest prevalence of schistosomiasis, following Nigeria. Approximately 52 % of the Tanzanian population is either exposed or lives in areas with a high risk of exposure (Mazigo et al., 2022a). Two-thirds of these cases in Tanzania are due to the Schistosoma haematobium parasite, which is endemic throughout the country, but its transmission is focal and varied (Mwanga, 2020). The S. haematobium parasite, which resides in the urinary venous plexus, leads to conditions such as haematuria and dysuria (Knopp et al., 2018). Chronic infections increase the risk of HIV and anaemia and can cause squamous cell carcinoma in the urinary bladder, as well as deformities in the ureters and kidneys, known as hydronephrosis (Ng'weng'weta and Tarimo, 2017). These infections have a particularly negative impact on children, resulting in stunted growth, cognitive impairment, and delayed development (Malibiche et al., 2023). Despite being preventable and treatable, schistosomiasis causes significant morbidity and mortality, with kidney failure due to S. haematobium infection resulting in approximately 150,000 deaths annually in Africa (Mnkugwe et al., 2019). Disease transmission becomes prevalent in impoverished areas with inadequate safe water and limited access to sanitation facilities (Alade et al., 2023; El-Zeiny et al., 2021; Green et al., 2021; Nazareth et al., 2022).
In Tanzania, urogenital schistosomiasis is widely distributed but more endemic along the eastern and southeastern coasts, the islands of Unguja and Pemba in Zanzibar, and the hinterland areas of the northwestern zones of the country (Mushi et al., 2022a; Pennance et al., 2018).
The World Health Organization (WHO) 2021–2030 Roadmap has supported more ambitious public health targets, including a goal for the elimination of schistosomiasis as a public health problem (currently defined as <1 % proportion of heavy egg patent intensity Schistosoma infections) (WHO, 2021). Multiple rounds of annual mass drug administration (MDA) with praziquantel (PZQ) have been carried out in the country for more than 15 years (Mazigo et al., 2022a; Pennance et al., 2016). Despite repeated rounds of treatment, some areas remain with a high prevalence of the disease (Kittur et al., 2017; Mazigo et al., 2022a). As a result, before planning and conducting the next MDA rounds, it is critical to re-evaluate the levels of S. haematobium infection in endemic areas. The re-evaluation will detect persisting pockets of the disease as well as the geographical location of the at-risk population. This would enable data-driven improvements to MDA approaches in areas where prevalence remains high, even distribution of the few available resources to support preventive mass chemotherapy in the country (Mazigo et al., 2022a). Although there are studies that have reported on the prevalence of this disease in different settings, data on the severity of urinary tract morbidities are scarce. This study, therefore, sought to investigate the prevalence of S. haematobium, the intensity of the infection, its associated factors, and the scale of related urinary tract morbidities among schoolchildren in the Simiyu region of Tanzania. The understanding gained from these epidemiological data will inform the planning and implementation of targeted, data-driven mass treatment.
2. Materials and methods
2.1. Study area and demographics
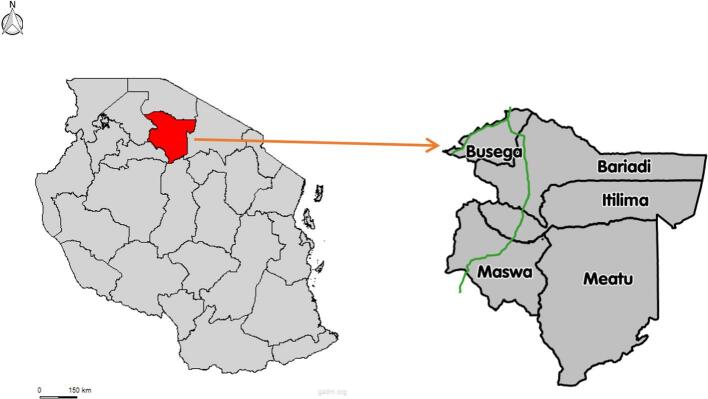
The Simiyu region is located between 3°08′17.88”S and 34°17′16.08″E. It is situated at the southern end of Lake Victoria in northwest Tanzania and spans 25,212 km2 with five districts: Maswa, Itilima, Busega, Bariadi, and Meatu. It is bordered by the Mara region to the north, the Shinyanga and Singida regions to the south, the Mwanza region to the west via Lake Victoria, and the Arusha region to the east. The inland areas of the Maswa and Meatu districts feature seasonal rivers and streams flowing into various water bodies. These water bodies facilitate the transmission of S. haematobium, especially at the end of the rainy season or during the dry season. According to previous data, the prevalence of schistosomiasis in these districts varies between 3.3 % and 58.3 % (Mazigo et al., 2022b). The last round of school MDA in these districts was conducted in 2020. According to the 2022 census, the region's population stands at 2,140,497, with 62 % aged between 0 and 19 years, forming a large group of school-aged children (National Bureau of Statistics (NBS). The United Republic of Tanzania., 2022). The region experiences an annual rainfall of 700 mm to 1000 mm, contributing to the filling of seasonal and temporary water bodies. The average annual temperature ranges from 18 °C to 31 °C, creating a favourable climate for the breeding of Bulinus snails. The inhabitants primarily engage in agriculture and small businesses, with major crops including maize, rice/paddy, beans, millet, sweet potatoes, and cotton. Livestock keeping is also prevalent, with animals such as cattle, donkeys, goats, sheep, dogs, cats, ducks, and poultry being commonly kept (National Bureau of Statistics (NBS). The United Republic of Tanzania., 2022). Fig. 1.
Fig 1.
A map of Tanzania showing the position of Simiyu region and administrative districts.
(credit to https://gadm.org/maps/TZA/simiyu.html And https://commons.wikimedia.org/wiki/File:Simiyu-Region.svg)
2.2. Study design, population, inclusion criteria, and exclusion criteria
A school-based analytical cross-sectional study was conducted to determine the prevalence, intensity, associated factors, and patterns of S. haematobium-related urinary tract morbidities among school children in northwestern Tanzania.
The study included primary school children aged 5–17 years from selected schools in the Simiyu region, specifically those residing in the Maswa and Meatu districts. These children had no history of using PZQ in the past six months, according to the MDA reports available at their schools. Their parents or guardians signed a consent form, and the children agreed to participate by signing an assent form. Children who failed to provide urine samples or who were absent on the day of data collection were excluded from the study.
2.3. Sample size determination and sampling
The sample size was calculated by using Charan's formula, which is used for estimating a proportion in cross-sectional studies (Charan et al., 2021), given by
where Z1-α/2 is the standard normal variate (1.96 at 95 % confidence level), P is the expected prevalence of S. haematobium in the population 52.7 % (Mushi et al., 2022a), and d is the desired margin of error (relative precision, assumed to be 3 %). Given that we employed a two-stage cluster sampling method, we considered a design effect of 2 and a nonresponse rate of 33 %. As a result, the study required a minimum sample size of 3177. Consequently, we enrolled a total of 3225 participants.
The recruitment of participants for this study was conducted between November and December 2022. The study involved 2 districts from 29 that participated in the precision mapping of schistosomiasis and soil-transmitted helminths in northwestern Tanzania (Mazigo et al., 2022b). The Maswa and Meatu districts were randomly selected to participate in this study. A two-stage cluster sampling technique was employed. This was done as follows: In the first stage, from the list of all 264 primary schools present in the two districts, 16 primary schools were selected randomly as the clusters. In the second stage, at each cluster (school), a simple random sampling technique using the lottery method (drawing numbers in a random fashion) was used to select a minimum of 200 students (100 boys and 100 girls) from class one to class six from each school. The WHO recommends the selection of a total of 200–250 schoolchildren for each ecologically homogenous area to evaluate the prevalence and intensity of infection for surveys aimed at assessing the need for control measures (Cha et al., 2019; Montresor et al., 1998).
2.4. Data collection tools and techniques
2.4.1. Questionnaire interviews
A face-to-face interview was conducted with each selected participant using a pretested questionnaire. The questionnaire was self-constructed based on the available literature and was administered in the Kiswahili language. The questionnaire collected information on sociodemographic information, history of anti-helminthic treatment, general sanitation, hygiene practices, and water contact behaviours of the school children.
2.4.2. Parasitological examination of S. haematobium eggs using a urine filtration technique
At each school, the children were provided with a clean, dry, and well-labelled urine container. They were instructed to collect a fresh urine sample in the full volume of the container for laboratory investigation. A single urine sample was collected from each child between 10:00 am and 2:00 pm after exercising. S. haematobium eggs were quantified using the urine filtration technique. The collected urine samples were vigorously shaken, and 10 ml of urine from each sample was drawn and passed through a polycarbonate filter. Two medical laboratory technicians microscopically examined each urine filter for the presence of S. haematobium eggs after staining them with a drop of iodine. The S. haematobium eggs on each slide were counted, and the data were documented in the laboratory form. Twenty percent of the positive and negative microscopic slides were reexamined in the field by a third laboratory technician for quality assurance. This method has been described elsewhere (Mazigo et al., 2022a; Mushi et al., 2022b; WHO, 2002).
2.4.3. Examination of macro- and micro haematuria using urine dipstick reagents
All collected urine samples were visually examined for the presence of macrohaematuria using a color chart. Urine reagent strips were used for the examination of microhaematuria. The results were recorded as positive or negative.
2.4.4. Ultrasonographical examination of urinary tract morbidities
Ultrasonography was performed using a portable ultrasonography device with a 5 MHz curved array transducer, according to WHO standard guidelines (Richter et al., 2000). Examinations were performed by two senior sonographers with extensive experience in S. haematobium-related urogenital ultrasonography-detectable morbidities. Recruited study participants were prepared by drinking fluids 30 min to one hour before examination to ensure that they had a full bladder before being examined. Pathological urogenital lesions associated with S. haematobium infection in the lower and upper urinary tract were recorded according to the Niamey guidelines (Richter et al., 2000). In the case of a textured pattern suggestive of obvious lower and upper urinary tract pathological lesions related to S. haematobium, a picture was taken, and corresponding urogenital lesions were recorded. For quality assurance, the two sonographers discussed the pictures that were taken with indications of urinary tract pathology. A radiologist examined 20 % of the participants for quality control of the ultrasound results from the two sonographers.
2.5. Data management and analysis
The data were double-entered in Microsoft Excel for cleaning, consistency checking, and error correction before being imported into Stata version 15 (Stata Corp, College Station, Texas, USA) for analysis. Descriptive statistics were used to obtain frequency tables and proportions. Categorical data were compared using the chi-square test, while continuous data were compared using means or medians with their respective measures of dispersion. Infection intensities were classified into two categories: light infections (1–49 eggs/10 ml) and heavy infections (≥50 eggs/10 ml). The geometrical mean egg output was calculated based on infected children. The risk factors for urogenital schistosomiasis in children were evaluated using univariate and multivariate logistic regression. Multivariate analysis was performed on all independent variables with a p value less than 0.25 in the univariate analysis. The regression analysis used S. haematobium infection as the dependent variable. Statistical significance was set at a p value of <0.05. Infected children with at least one point according to the WHO score were considered to have urinary tract abnormalities, and participants with at least one point in the upper urinary tract were considered to have upper urinary tract abnormalities for successive analysis, which was conducted based on the Niamey criteria (Richter et al., 2000).
2.6. Ethical considerations
The study obtained ethical approval from the National Institute for Medical Research of Tanzania (MR/53/100/718) and the Bugando Medical Centre/Catholic University of Health and Allied Sciences Institution Review Board (CREC/667/2023). Permission to conduct the study was obtained from the regional and district administrative authorities. Written informed consent forms were obtained from the schoolteachers on behalf of the parents/guardians who permitted teachers to consent for their children to participate in the study. Assent forms were available for the children to read and sign before they participated in the study. All S. haematobium-infected children were treated with 40 mg/kg body weight PZQ.
3. Results
3.1. Sociodemographic characteristics of the study participants
A total of 3225 schoolchildren who were enrolled in 16 primary schools from the Maswa and Meatu districts of the Simiyu region were involved in the study. More than half (54.3 %) of the participants were females. The mean age of the participants was 10.9 ± 1.9 years. Table 1.
Table 1.
Sociodemographic characteristics of the school children (n = 3225).
| Variable | Category | n (%) | 95 % CI |
|---|---|---|---|
| Sex | Female | 1752 (54.3) | 52.5–56.1 |
| Male | 1473 (45.7) | 43.9–47.4 | |
| Age (mean ± SD) | 10.9 ± 1.9 | ||
| ≤10 | 1294 (40.1) | 38.4–41.8 | |
| >10 | 1931 (59.9) | 58.1–61.6 | |
| Residency | ≤ 2 years | 110 (3.4) | 2.8–4.0 |
| >2 years | 3115 (96.6) | 95.9–97.2 | |
| District | Maswa | 2075 (64.3) | 62.7–65.9 |
| Meatu | 1150 (35.7) | 34.0–37.3 |
3.2. Prevalence and intensity of S. haematobium infection among school children
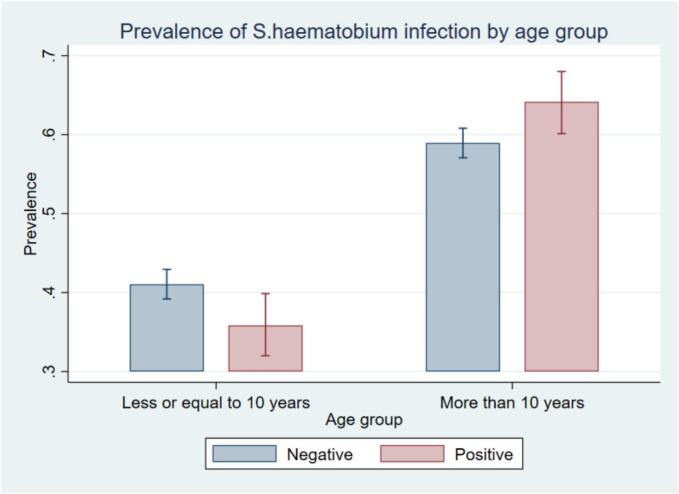
The overall prevalence of S. haematobium infection was 17.7 % (95 % CI: 16.4–19.1). The prevalence of S. haematobium infection was greater among males (20 %, 95 % CI: 18.0–22.1), children aged above 10 years (19 %, 95 % CI: 17.3–20.9), and children from the Meatu district (26.5 %, 95 % CI, 23.9–29.2). There was a statistically significant difference in the prevalence of infection across different age groups (p = 0.021), between sexes (p = 0.002), and among districts (p < 0.001). (Table 2, Fig 2, Fig 3).
Table 2.
Prevalence of macrohaematuria, microhaematuria, and S. haematobium infection stratified by sociodemographic characteristics of the school children (n = 3225).
| Variable | Category | Total |
S. haematobium infection n (%) |
Chi-square, P value |
Macrohaematuria n (%) |
Chi-square, P value | Microhaematuria n (%) |
Chi-square, p value |
|---|---|---|---|---|---|---|---|---|
| Sex | Female | 1752 | 277 (15.8) | χ2(1) = 9.7514, P = 0.002⁎ |
32 (1.8) | χ2(1) =4.6984 P = 0.017⁎ |
286 (16.3) | χ2(1) =11.840, P = 0.001⁎ |
| Male | 1473 | 295 (20.0) | 46 (3.1) | 310 (21.1) | ||||
| Age | ≤10 | 1294 | 205 (15.8) | χ2(1) = 5.3138, P = 0.021⁎ |
29 (2.2) | χ2(1) = 0.2885, P = 0.591 | 217(16.8) | χ2(1) = 4.1991 P = 0.040⁎ |
| >10 | 1931 | 367 (19.0) | 49 (2.5) | 379 (19.6) | ||||
| Residency | ≤ 2 years | 110 | 20 (18.2) | χ2(1) =0.0155, P = 0.901 |
3 (2.7) | χ2(1) = 0.046, P = 0.830 | 25 (22.3) | χ2(1) = 1.3633, P = 0.243 |
| >2 years | 3115 | 552 (17.7) | 75 (2.4) | 571 (18.3) | ||||
| District | Maswa | 2075 | 267 (12.9) | χ2(1) =94.547, P < 0.001⁎ |
9 (0.4) | χ2(1) = 97.1364 P < 0.001⁎ |
304 (14.7) | χ2(1) = 56.6597, P < 0.001⁎ |
| Meatu | 1150 | 305 (26.5) | 69 (6.0) | 292 (25.4) | ||||
| Overall | 3225 | 572 (17.7) | 78 (2.4) | 596 (18.5) |
Statistically significant (p < 0.05).
Fig 2.
Prevalence of S. haematobium infection by age group.
Fig 3.
Prevalence of S. haematobium infection by sex.
Among all S. haematobium-infected children, 81.9 % (95 % CI: 78.5–85, 469/572) had a light intensity of infection, and 18 % (95 % CI: 14.9–21.4, 103/572) had a heavy intensity of infection. The mean number of eggs per 10 ml of urine was 55.2 ± 31.98 and ranged from 1 to 1768 eggs. A high proportion of males had a heavy infection intensity of 20.3 % (95 % CI: 15.8–25.3), and 19.7 % of the children resided in the Meatu district (95 % CI: 15.3–24.5). Table 3.
Table 3.
The infection intensity classification according to the school children's socio-demographic characteristics (n = 572).
| Variable |
Category |
Total |
S. haematobium intensity |
Chi-square |
|
|---|---|---|---|---|---|
| Light n (%) | Heavy n (%) | P value | |||
| Sex | Males | 295 | 235 (79.7) | 60 (20.3) | χ2(1) = 1.9055 |
| Females | 277 | 233 (84.1) | 44 (15.9) | P = 0.167 | |
| Age | ≤10 years | 205 | 167 (81.5) | 38 (18.5) | χ2(1) = 0.0270 |
| >10 years | 367 | 301 (82.0) | 66 (17.9) | P = 0.869 | |
| District | Maswa | 267 | 223 (83.5) | 44 (16.5) | χ2(1) = 0.9756 |
| Meatu | 305 | 245 (80.3) | 60 (19.7) | P = 0.323 | |
| Residency | ≤ 2 years | 20 | 16(80.0) | 4 (20.0) | χ2(1) = 0.0461 |
| >2 years | 552 | 452 (81.9) | 100 (18.1) | P = 0.830 | |
| Overall | 572 (100) | 468 (81.8) | 104 (18.2) | ||
3.2.1. Prevalence of macro- and microhaematuria among school children
The prevalence of macrohaematuria and microhaematuria was 2.4 % (95 % CI: 1.9–3) and 18.5 % (95 % CI: 17.2–19.8), respectively. A significant association was observed between the presence of macrohaematuria and sex, with males exhibiting a greater likelihood of having macrohaematuria than females (χ2 = 4.6984, P = 0.017). The district of residence also showed a significant association with macrohaematuria, with children from the Meatu district being more likely to have macrohaematuria than those from Maswa (χ2 = 94.547, P < 0.001). A significant association was observed between the presence of macrohaematuria and infection with S. haematobium (χ2 = 347.9882, P < 0.001). Out of 572 children infected with S. haematobium, 483 (75.2 %) exhibited microhaematuria, while out of the 2653 children who tested negative for S. haematobium, 166 (6.3 %) showed signs of microhaematuria. Table 2.
3.3. Factors associated with S. haematobium infection among school children
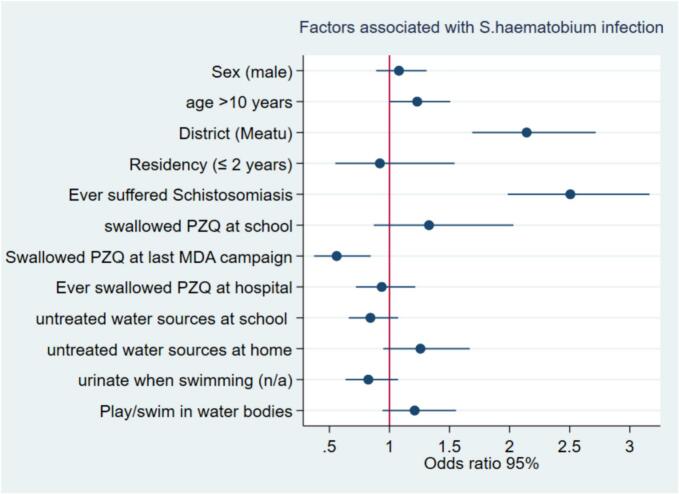
Based on multivariate analysis, only age, district, history of suffering from schistosomiasis, and reported swallowing PZQ at the last MDA campaign remained significantly associated with S. haematobium infection among school children. Children aged more than ten years were more likely to have S. haematobium infection than were those aged less than ten years (aOR: 1.2, 95 % CI: 1.0–1.5). Children who lived in the Meatu district were more likely to have S. haematobium infection than were those who lived in Maswa (aOR 2.1, 95 % CI: 1.7–2.7). Children who reported ever suffering from schistosomiasis were more likely to be infected with S. haematobium than were those who had never suffered from schistosomiasis (aOR 2.5, 95 % CI: 1.9–3.2). Children who swallowed PZQ during the last round of the MDA campaign were less likely to have S. haematobium infection (aOR: 0.6, 95 % CI: 0.4–0.8) than were those who did not swallow PZQ during the last round of the MDA campaign. (Table 4, Fig. 4).
Table 4.
Univariate and multivariate analyses of factors associated with S. haematobium infection among school children (n = 3225).
|
Univariate analysis |
Multivariable analysis |
|||||
|---|---|---|---|---|---|---|
| Variable | Category | Frequency n (%) | cOR (95 % CI) | P value | aOR (95 % CI) | P value |
| Sex | Female | 1752 (54.3) | 1 | |||
| Male | 1473 (45.7) | 1.3 (1.1–1.6) | 0.002⁎ | 1.1 (0.9–1.3) | 0.442 | |
| Age | ≤10 | 1294 (40.1) | 1 | |||
| >10 | 1931 (59.9) | 1.2 (1.0–1.5) | 0.021⁎ | 1.2 (1.0–1.5) | 0.044⁎ | |
| District | Maswa | 2075 (64.3) | 1 | |||
| Meatu | 1150 (35.7) | 2.4(2.0–2.9) | <0.001⁎ | 2.1 (1.7–2.7) | <0.001⁎ | |
| Residency | > 2 years | 3115 (96.6) | 1 | |||
| ≤ 2 years | 110 (3.4) | 1.0 (0.6–1.7) | 0.901 | 0.9 (0.5–1.5) | 0.753 | |
| Ever suffered Schistosomiasis | No | 710 (22.4) | 1 | |||
| Yes | 2460 (77.6) | 2.5 (2.1–3.0) | <0.001⁎ | 2.5 (1.9–3.2) | <0.001⁎ | |
| Ever swallowed PZQ at school | No | 941 (29.7) | 1 | |||
| Yes | 2229 (70.3) | 0.8 (0.6–0.9) | 0.010⁎ | 1.3 (0.9–2.0) | 0.189 | |
| Swallowed PZQ at the last MDA campaign | No | 144 (4.5) | 1 | |||
| Yes | 2085 (65.8) | 0.5 (0.4–0.8) | 0.001⁎ | 0.6 (0.4–0.8) | 0.006⁎ | |
| Ever swallowed PZQ at the hospital | No | 2636 (83.2) | 1 | |||
| Yes | 534(16.9) | 1.7 (1.4–2.1) | <0.001⁎ | 0.9 (0.7–1.2) | 0.617 | |
| Water source at school | Treated | 1356 (42.8) | 1 | |||
| Untreated | 1814 (57.2) | 1.5 (1.2–1.8) | <0.001⁎ | 0.8 (0.7–1.1) | 0.163 | |
| Water source at home | Treated | 817 (25.8) | 1 | |||
| Untreated | 2353 (74.2) | 1.8 (1.5–2.3) | <0.001⁎ | 1.3 (0.9–1.7) | 0.112 | |
| Urinate when swimming | No | 802 (25.3) | 1 | |||
| Yes | 1090(34.4) | 1.6 (1.2–1.9) | <0.001⁎ | 1.2 (0.9–1.6) | 0.137 | |
Statistically significant (p < 0.05).
Fig 4.
Factors associated with Schistosoma haematobium infection among school children.
3.4. Prevalence of urinary tract morbidities among study participants
The overall prevalence of ultrasound-detectable urinary tract abnormalities was 9.9 % (95 % CI: 8.9–11.1), which included urinary bladder abnormalities at 9.9 % (95 % CI: 8.8–11), ureter abnormalities at 0.2 % (95 % CI: 0.07–0.4), kidney abnormalities at 0.2 % (95 % CI: 0.09–0.4) and calcification of the urinary bladder at 0.9 % (95 % CI: 0.6–1.3).
There was a significant association between urinary tract morbidities and sex, with males being more likely to have urinary tract morbidities than females (χ2 = 47.8306, P < 0.001). There was also a significant association between urinary tract morbidities and age and district. Children aged above 10 years were more likely to have urinary tract morbidities than were those aged younger than 10 years (χ2 = 7.9796, P = 0.01). Children who lived in the Meatu district were more likely to develop urinary tract morbidities than those who lived in Maswa (χ2 = 38.6456, P < 0.001). (Table 5).
Table 5.
Univariate and multivariate analyses of factors associated with urinary tract morbidities among school children (n = 2994).
|
Univariate analysis |
Multivariable analysis |
||||||
|---|---|---|---|---|---|---|---|
| Variable | Category | n (%) | UT morbidity positive n (%) |
COR (95 % CI) |
P value |
aOR (95 % CI) |
P value |
| Sex | Female | 1637 (54.7) | 107 (6.5) | 1 (Ref) | |||
| Male | 1357 (45.3) | 192 (14.2) | 2.4 (1.8–3.0) | 0.000⁎ | 1.8 (1.2–2.7) | 0.003⁎ | |
| Age | ≤10 years | 1209(40.4) | 98 (8.1) | 1 (Ref) | |||
| >10 years | 1785 (59.6) | 201 (11.2) | 1.4 (1.1–1.9) | 0.005⁎ | 1.0 (0.7–1.6) | 0.837 | |
| District | Maswa | 2047 (68.4) | 157 (7.7) | 1 (Ref) | |||
| Meatu | 947(31.6) | 142 (14.9) | 2.1 (1.7–2.7) | 0.000⁎ | 1.4 (0.9–2.2) | 0.118 | |
| Residency | >2 years | 2896 (96.7) | 288 (9.9) | 1 (Ref) | |||
| ≤ 2 years | 98 (3.3) | 11 (11.2) | 1.1 (0.6–2.2) | 0.678 | 1.2 (0.4–3.5) | 0.776 | |
|
Macro haematuria |
Negative | 2933 (97.9) | 273(9.3) | ||||
| Positive | 61 (2.0) | 26 (8.7) | 7.2 (4.3–12.2) | 0.000⁎ | 0.6 (0.3–1.1) | 0.134 | |
| Micro haematuria | Negative | 2462 (82.2) | 105 (4.3) | ||||
| positive | 532(17.8) | 194 (36.5) | 12.9 (9.9–16.8) | 0.000⁎ | 3.7 (2.0–6.6) | 0.000⁎ | |
| Proteinuria | Negative | 2488 (83.1) | 147 (5.9) | ||||
| Positive | 506 (16.9) | 152 (30.0) | 6.8 (5.3–8.8) | 0.000⁎ | 1.3 (0.8–1.9) | 0.233 | |
| Infection intensity | Light | 400 (81.5) | 126 (31.5) | 1 (Ref) | |||
| Heavy | 91 (18.5) | 48 (52.8) | 2.4 (1.5–3.9) | < 0.000⁎ | 2.5 (1.4–4.4) | 0.002⁎ | |
Statistically significant (p < 0.05).
3.5. Factors associated with urinary tract morbidities among school children
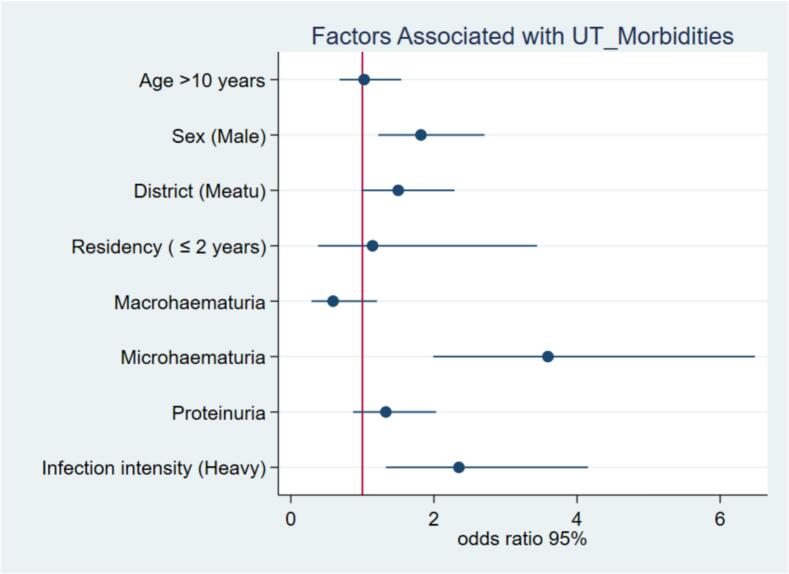
On multivariable analysis, only sex, microhaematuria, and infection intensity remained significantly associated with urinary tract morbidities. Males were two times more likely to develop urinary tract morbidities than females were (aOR: 1.8, 95 % CI: 1.2–2.7, p = 0.003). Children who had microhaematuria were four times more likely to have urinary tract morbidities (aOR: 3.7, 95 % CI: 2.0–6.6, p > 0.001) than were those who had no microhaematuria. Children who had severe S. haematobium infection were three times more likely to develop urinary tract morbidities than were those with mild infection (aOR: 2.5, 95 % CI: 1.4–4.4, p = 0.002). (Table 5, Fig. 5).
Fig 5.
Factors associated with urinary tract morbidities among school children.
3.6. Patterns of urinary tract morbidities among study participants
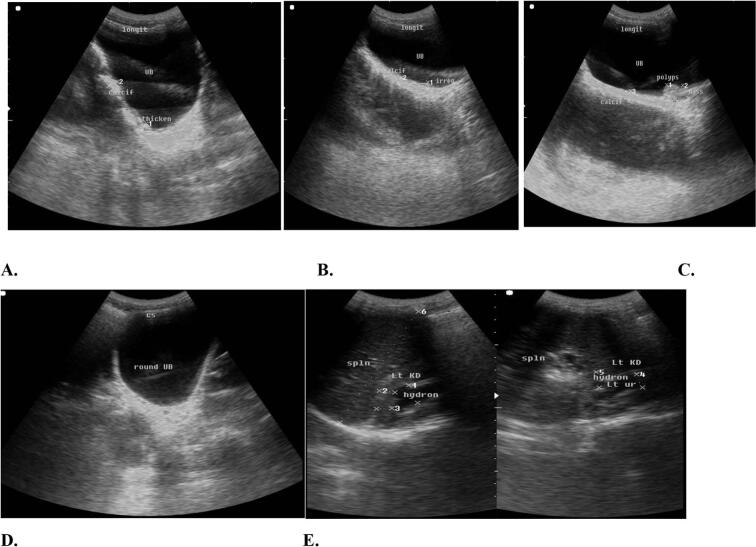
Ultrasound findings revealed pathological changes in 9.9 % (95 % CI: 8.9–11.1) of the children who participated in the study. The urinary bladder was the common site of alteration and was abnormal in 10 % (95 % CI: 8.8–11) of the school-aged children. Ureteral abnormalities were detected in 0.2 % (95 % CI: 0.1–0.4) of the participating children, and kidney abnormalities were detected in 0.2 % (95 % CI: 0.1–0.4) of the children. Calcification of the urinary bladder was observed in 0.9 % (95 % CI: 0.6–1.3) of the children. Fig. 6.
Fig. 6.
Images showing A. Bladder calcification, thickening. B. Bladder wall irregularities, C. Mass, pseudo polyps D. Bladder shape (round/distorted) and E. Hydronephrosis.
4. Discussion
This study provides epidemiological data on the prevalence, intensity, and associated factors of Schistosoma haematobium infection and urinary tract morbidities in school-aged children from northwestern Tanzania. The overall prevalence of S. haematobium was 17.7 %. The majority of participants had light-intensity infections. The study revealed that the age of the child above ten years, the district of residence, and the history of suffering from schistosomiasis were associated with S. haematobium infection among school-aged children. Moreover, swallowing PZQ during the MDA campaign was protective. In general, 9.9 % of the participants had urinary tract morbidities, with the urinary bladder accounting for the majority of alterations in the majority of children. Male sex, microhaematuria, and heavy infection intensity were the factors significantly associated with urinary tract morbidity among school children.
4.1. Prevalence and intensity of S. haematobium infection among school children
Overall, this study demonstrated that S. haematobium is still a public health problem in Tanzania. The overall prevalence and intensity of infection in this study indicate a moderate-risk community according to the WHO categorization of endemic communities (Crompton and World Health Organization, 2006). Moreover, the infection prevalence was higher among males and increased with age, similar to findings from studies conducted in other settings of Tanzania and Sudan (Mushi et al., 2022a; Senkwe et al., 2022). The overall prevalence is high in this setting compared to that in four districts of northwestern (Mazigo et al., 2022a) and southern Tanzania (Malibiche et al., 2023). Although all districts are in the annual MDA program, the higher prevalence in this setting could be attributed mostly to environmental factors. The Maswa and Meatu districts are characterized by black soil with multiple inland water bodies that serve as sources of water for domestic, agricultural, and animal use, contributing to continuous human-water contact throughout the year. Studies have shown that the presence of potential inland water bodies accessible by households is a predictor of S. haematobium infection (Mazigo et al., 2021). Evidence from parallel malacological surveys conducted in this setting revealed a high prevalence of cercarial shedding of Bulinus, which could add evidence to the observed high prevalence in school children. The competence of intermediate host snails has been described as a factor in the transmission of S. haematobium infection (Mazigo et al., 2021).
The (WHO) 2021–2030 Roadmap for Neglected Tropical Diseases (NTDs) has set a goal for the elimination of schistosomiasis as a public health problem, which has been defined as <1 % of heavy egg patent intensity Schistosoma infections (WHO, 2021). However, 18 % of infected children had heavy infection intensities in this study. These findings corroborate the findings of Itilima, Lindi, and Zanzibar (Knopp et al., 2018; Maseke et al., 2022; Mushi et al., 2022a). The observed proportion of heavy intensities indicates the existence of significant S. haematobium-related morbidities in this area. In endemic areas where children are exposed to infections throughout the year, the rates of schistosomiasis reinfection remain elevated even after treatment (Mazigo et al., 2022a). This highlights the urgency for consistent use of PZQ to achieve sustained morbidity control via MDA. Additionally, annual treatment reduces heavy infections and thereby diminishes environmental contamination with Schistosoma eggs. This is important to align with the World Health Organization's ambitious objective of transitioning towards the elimination phase of schistosomiasis.
4.2. Factors associated with S. haematobium infection among school children
Multiple factors have been described to be associated with S. haematobium infections. In our current study, the following factors remained independently associated with S. haematobium infections: age, district of residence, history of suffering from schistosomiasis, and reported swallowing of PZQ at the last MDA campaign.
Children over ten years old were more likely to have S. haematobium infection, similar to findings from Ethiopia (Deribew et al., 2022). This can be explained by their active lifestyles and frequent contact with contaminated water bodies through activities such as swimming, playing, agriculture, and animal grazing (Chala and Torben, 2018; Sacolo et al., 2018). In regions such as Sub-Saharan Africa, the infection starts at a young age and intensifies as the child grows (Mazigo et al., 2021). In endemic areas, tolerance to parasite loads can be observed (Mushi et al., 2022a).
The area of residence has been associated with S. haematobium transmission in other studies from Tanzania (Mazigo et al., 2022a). Although these two districts have many geographical and environmental features in common, a greater percentage of the children from Maswa district reported having access to treated water sources than did those from Meatu. This indicates that the majority of school children in Meatu have limited access to safe water sources and utilize unsafe water sources, which exposes them to infection.
Children who had ever suffered from schistosomiasis were more likely to have S. haematobium infection. This indicates possible ongoing reinfections in the study area, similar to findings from the Lindi region (Mushi et al., 2022a). Children who are continuously exposed to infested water become infected again after they are treated. On the other hand, heavily infected children could remain excreting eggs in urine for a few weeks after treatment. This is because PZQ is effective for adult worms but does not kill juvenile parasites (Senghor et al., 2015). Hence, after adult worms are killed, the juvenile parasites will mature and continue laying eggs.
Our findings also showed that children who swallowed PZQ in the last round were less likely to have S. haematobium infection than were those who did not swallow PZQ. This finding supports the role of school MDA in schistosomiasis control. Before the study in these districts, the MDA was provided in 2020, which is two years before conducting this study. Therefore, those children who missed the opportunity in the last MDA campaign were more likely to suffer from infections than those who were treated. Moreover, the observed infections in these districts may be attributed to the absence of MDA during the previous year, a consequence of the COVID-19 pandemic. Repeated treatment with PZQ has been proven to reduce schistosomiasis morbidity and interrupt transmission (Houmsou et al., 2018; Senghor et al., 2015). Studies have also demonstrated that S. haematobium transmission is focused and heterogeneous. The epidemiology of the disease has also changed as a result of repeated rounds of PZQ treatment. As a result, despite annual MDA, some areas still have pockets of infection (Mazigo et al., 2022a; Mushi et al., 2022b). Thus, there is a need for integrated control between MDA and environmental and targeted snail control efforts to achieve sustained schistosomiasis control. However, 4.5 % of the children reported that they did not swallow the PZQ during the last round. The main reasons reported were fear of side effects, school absenteeism, and parents' prohibition. In most schistosomiasis endemic areas, little motivation and engagement in schistosomiasis control strategies, including negative attitudes towards MDA, has been reported (Mushi et al., 2022b; Mazigo et al., 2022a, Mazigo et al., 2022b).
Prevalence of urinary tract morbidities and associated factors among school children.
Ultrasound findings revealed greater morbidity among males and those older than ten years. The prevalence of urogenital morbidities is lower in this setting than in Angola and Ivory Coast (Barda et al., 2017; Bocanegra García et al., 2018). The observed higher prevalence of morbidities in children older than ten years could be due to their exposure at a young age, as Schistosoma morbidities take a long time to develop. Generally, the overall lower prevalence of morbidities in our study could be attributed to the deworming programs that have been conducted in recent years in the study area, which have significantly reduced the number of morbidities. Studies have shown that PZQ has an effect on reducing S. haematobium-related urogenital morbidities, which are observed after six months posttreatment (Bocanegra García et al., 2018).
Bladder morbidity was more prevalent than upper urinary tract morbidities, a finding that aligns with previous studies (Bocanegra García et al., 2018; Cozzi et al., 2020; Wiegand et al., 2021). Similarly, lower morbidity rates were observed in the upper urinary tract than in the lower urinary tract, which is consistent with the results of other studies(Agniwo et al., 2023; Barda et al., 2017; van der Werf et al., 2003; Wami et al., 2015). Adult schistosomes lay eggs in the venous plexus of the urinary bladder, leading to the deposition and trapping of the eggs in the bladder tissue. Eggs trapped in tissues also take approximately 14 to 21 days to find their way to the urinary bladder before being excreted in urine. Thus, the urinary bladder is more exposed than other organs. We observed bladder calcification in a small percentage of children, which suggests chronic infection with S. haematobium. This infection, which begins at an early age, along with factors such as immune response and chronic inflammation, could increase the risk of squamous cell carcinoma of the urinary bladder in these younger children. (Yohana et al., 2023).
Male children were more prone to urinary tract morbidities, a pattern consistent with findings reported by Angola (Bocanegra García et al., 2018). This finding corroborates the observed higher prevalence of infection in males. The heavy S. haematobium intensities indicate that a greater number of eggs are excreted in the urinary bladder, which contributes to severe pathology of the urinary bladder in males.
Microhaematuria was a significant predictor of urinary tract morbidities, which is consistent with findings from other studies (Morenikeji et al., 2014; Salawu and Odaibo, 2014; Vester et al., 1997). However, these findings differ from those in Angola (Bocanegra García et al., 2018). The observed difference could be explained by the large sample size used in our study compared to only 157 children who were screened in Angola. Surprisingly, we observed that 6.3 % of the S. haematobium egg-negative children had microhaematuria. This indicates the challenge of improving the sensitivity of the urine filtration technique. The urine filtration test for S. haematobium, while commonly used, has been found to have varying sensitivity (Deribew et al., 2022). This means that it may not detect all cases of infection, particularly in instances of light infection of ≤5 eggs per 10 ml of urine (Knopp et al., 2018). Therefore, it is possible that some of the children who tested negative for S. haematobium using this method were actually infected. This finding indicates the potential for the use of highly sensitive techniques such as molecular methods for detecting S. haematobium infection in future surveys (Sow et al., 2023). On the other hand, microhaematuria, or microscopic traces of blood in the urine, can occur for many reasons, including urinary tract infections. Other causes include menstruation, certain medications, vigorous exercise, kidney stones and bladder stones, kidney disease, urethral strictures, and cancer.
In all age groups, proteinuria is a good indicator of kidney and urinary tract abnormalities (Brouwer et al., 2004; Morenikeji et al., 2014; Odetunde et al., 2011). Persistent proteinuria in childhood may cause a hypercoagulable state, dysregulation of fluid, electrolyte imbalance, susceptibility to infections, and a tendency towards anaemia and malnutrition, contributing to the early consequences of schistosomiasis, such as nutritional and cognitive impairment or stunting (Bocanegra García et al., 2018). However, some levels of proteinuria are virtually universal in children with urinary schistosomiasis as a consequence of the damage caused by the passage of eggs through the bladder wall. This has been clearly shown in an experimental model in an animal host, and it is one of the few causes of proteinuria with a nonrenal origin (Bocanegra García et al., 2018). However, during multivariable analysis, proteinuria was not statistically significant.
In this study, heavy infection intensity was significantly associated with urinary tract morbidities. These findings are consistent with the findings of other studies (Brouwer et al., 2004; Njaanake et al., 2023). Heavy infection with S. haematobium has been reported to be an indicator of severe urinary tract morbidities since approximately 50 % of the excreted eggs are observed in urine (Shams et al., 2022). This shows that a greater number of eggs remain lodged in the vasculature of the urogenital system, which accounts for severe pathology in the host.
The current MDA strategies are designed to reduce infection intensities to less than 1 %, with a focus on reducing morbidities related to schistosomiasis (Lo et al., 2022). In public health interventions, the most common criteria used include measuring egg counts and analysing urine for haematuria and proteinuria. These are considered indirect indicators of urinary tract impairment (Bocanegra García et al., 2018). However, a more precise and specific assessment of organ pathology should ideally be pursued. Ultrasound can detect damage to the bladder wall and genitourinary tract. When combined with parasitological results and urine analyses, these findings provide a comprehensive indication of the effects of chronic infection (Barda et al., 2017). Therefore, incorporating ultrasound screening in schistosomiasis surveys is crucial and can serve as an indicator of the need for additional clinical management procedures.
4.3. Study limitations
Given the cross-sectional design of this study, causality cannot be established. However, the information gathered on urinary morbidities, despite not establishing causality, is a strength of our study. These data will contribute valuable evidence for planning control programs. We mitigated this limitation by demonstrating associations and eliminating potential confounders through multivariate analysis using a logistic regression model. The use of a single urine specimen could have underestimated the prevalence of S. haematobium infection, as it does not take into account the day-to-day variation in egg excretion compared to whether duplicate samples were collected on different days. However, this was mitigated by ensuring that the children exercised before sample collection and sample collection between 10:00 am and 2:00 pm, which is the optimum time for laying eggs. The use of two to three consecutive samples could be a recommendation for our next studies.
5. Conclusion and recommendations
The findings from this study indicate that S. haematobium is still a public health problem in the study setting. The transmission of schistosomiasis in children is driven by the age of the child being older than ten years, district of residence, and history of suffering from schistosomiasis. Moreover, swallowing PZQ during the MDA campaign reduces transmission. Urinary tract morbidities are prevalent among male partcipants, participant with microhaematuria, and those with a heavy intensity of infection.
The prevalence and heavy intensity of >10 % in the study area indicate the need for annual preventive chemotherapy with a single dose of PZQ at ≥75 % treatment coverage. This should include all age groups from 2 years and above to control schistosomiasis-related morbidities according to the WHO schistosomiasis guidelines of 2022 (Lo et al., 2022; WHO, 2022). Furthermore, it is crucial to routinely screen for urinary tract morbidities for early intervention in schistosomiasis-related morbidities in school-aged children. This might require modifying treatment strategies, particularly for children showing early signs of urinary lesions, and integrating additional management procedures alongside MDA. Special attention should be given to males, children with microhaematuria, and those with heavy infection intensities, as these factors may indicate severe urinary tract morbidities.
Ethical approval and consent to participate
The study obtained ethical approval from the National Institute for Medical Research of Tanzania (MR/53/100/718) and the Bugando Medical Centre/Catholic University of Health and Allied Sciences Institution Review Board (CREC/667/2023). The regional and district administrative authorities granted permission for the study. School teachers, acting on behalf of parents/guardians, provided written informed consent for the children's participation.
Funding
This study of Nyanda C. Justine was funded by the Else Kröner-Fresenius Foundation in the framework of the Else Kröner Center Würzburg-Mwanza (2018_HA10SP). The funders had no role in the study design; collection, analysis, or interpretation of the data; writing of the manuscript; or the decision to submit the article for publication.
Availability of data and materials
The data used in the analysis of this study are available upon reasonable request.
Author contributions
Conceptualization: NCJ, HDM. Data curation: NCJ. Formal analysis: NCJ. Funding acquisition: HDM, AM, NCJ. Investigation: NCJ. Methodology: HDM, AM, NCJ. Project administration: HDM, NCJ. Supervision: HDM, AM, KB. Validation: NCJ. Visualization: NCJ, TRL. Writing – original draft: NCJ. Writing – review & editing: HDM, AM, AF, TRL. The final, submitted version of the manuscript has been read and approved by all the authors.
CRediT authorship contribution statement
Nyanda C. Justine: Writing – original draft, Visualization, Validation, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Titus R. Leeyio: Writing – review & editing, Visualization. Antje Fuss: Writing – review & editing, Supervision. Klaus Brehm: Supervision. Humphrey D. Mazigo: Writing – review & editing, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization. Andreas Mueller: Writing – review & editing, Supervision, Methodology, Funding acquisition.
Declaration of competing interest
The authors declare that no competing interests exist.
Acknowledgements
The authors would like to thank the school children, their parents, and school teachers for their participation in the study. We would like to thank the support from Simiyu regional leaders, district leaders, and NTD coordinators. The Catholic University of Health and Allied Sciences and EKC for supporting the study, the supervisors, the board of advisors, and all research team members for their profound support throughout the study. Special thanks to the entire research team, laboratory technicians, scientists, nurses, and research assistants for their assistance during the fieldwork.
References
- Ng’weng’weta S.B., Tarimo D.S. Urinary schistosomiasis among preschool-age children in an endemic area of Kinondoni municipality, Dar Es Salaam, Tanzania 2016. Asian Pac. J. Trop. Dis. 2017;7:162–168. doi: 10.12980/apjtd.7.2017D6-359. [DOI] [Google Scholar]
- Agniwo P., Sidibé B., Diakité A., Niaré S.D., Guindo H., Akplogan A., Ibikounlé M., Boissier J., Dabo A. Ultrasound aspects and risk factors associated with urogenital schistosomiasis among primary school children in Mali. Infect. Dis. Poverty. 2023;12:40. doi: 10.1186/s40249-023-01071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alade T., Ta-Tang T.-H., Nassar S.A., Akindele A.A., Capote-Morales R., Omobami T.B., Berzosa P. Prevalence of Schistosoma haematobium and intestinal helminth infections among Nigerian school children. Diagnostics. 2023;13:759. doi: 10.3390/diagnostics13040759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barda B., Coulibaly J.T., Hatz C., Keiser J. Ultrasonographic evaluation of urinary tract morbidity in school-aged and preschool-aged children infected with Schistosoma haematobium and its evolution after praziquantel treatment : a randomized controlled trial. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocanegra García C., Pintar Z., Serres X., Mendioroz J., Moreno M., Gallego S., López T., Soriano-Arandes A., Aznar M.L., Sikaleta N., Gil E., Salvador F., Molina I. Ultrasound findings and associated factors to morbidity in Schistosoma haematobium infection in a highly endemic setting. Trop. Med. Int. Health. 2018;23:221–228. doi: 10.1111/tmi.13020. [DOI] [PubMed] [Google Scholar]
- Brouwer K.C., Munatsi A., Ndhlovu P.D., Wagatsuma Y., Shiff C.J. Urinary schistosomiasis in Zimbabwean school children: predictors of morbidity. Afr. Health Sci. 2004;4:115–118. [PMC free article] [PubMed] [Google Scholar]
- Cha S., Elhag M.S., Lee Y.-H., Cho D.-S., Ismail H.A.H.A., Hong S.-T. Epidemiological findings and policy implications from the nationwide schistosomiasis and intestinal helminthiasis survey in Sudan. Parasit. Vectors. 2019;12:429. doi: 10.1186/s13071-019-3689-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chala B., Torben W. An epidemiological trend of urogenital schistosomiasis in Ethiopia. Front. Public Health. 2018;6:1–9. doi: 10.3389/fpubh.2018.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charan J., Kaur R., Bhardwaj P., Singh K., Ambwani S.R., Misra S. Sample size calculation in medical research: a primer. Ann. Natl. Acad. Med. Sci. India. 2021;57:074–080. doi: 10.1055/s-0040-1722104. [DOI] [Google Scholar]
- Cozzi D., Bertelli E., Savi E., Verna S., Zammarchi L., Tilli M., Rinaldi F., Pradella S., Agostini S., Miele V. Ultrasound findings in urogenital schistosomiasis: a pictorial essay. J. Ultrasound. 2020;23:195–205. doi: 10.1007/s40477-019-00405-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton, D.W.T., World Health Organization Preventive chemotherapy in human helminthiasis : coordinated use of anthelminthic drugs in control interventions : a manual for health professionals and programme managers. Chim. Helminthiases Chez Homme Util. Coord. Médicam. Anthelminthiques Pour Interv. Lutte Man. À Intent. Prof. Santé Adm. Programme. 2006;62 [Google Scholar]
- Deribew K., Yewhalaw D., Erko B., Mekonnen Z. Urogenital schistosomiasis prevalence and diagnostic performance of urine filtration and urinalysis reagent strip in schoolchildren. Ethiopia. PloS One. 2022;17 doi: 10.1371/journal.pone.0271569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Zeiny M.E., Ghoneim A.M., Abu Samak O.A., Khidr A.A. Abundance and annual distribution of freshwater snails and some trematode Cercariae at Damietta governorate. Egypt. Helminthologia. 2021;58:233–247. doi: 10.2478/helm-2021-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A.E., Anchang-Kimbi J.K., Wepnje G.B., Ndassi V.D., Kimbi H.K. Distribution and factors associated with urogenital schistosomiasis in the Tiko Health District, a semiurban setting, south west region. Cameroon. Infect. Dis. Poverty. 2021;10:1–15. doi: 10.1186/s40249-021-00827-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmsou R.S., Wama B.E., Agere H., Uniga J.A., Amuta E.U., Kela S.L. High efficacy of Praziquantel in Schistosoma haematobium-infected children in Taraba state, Northeast Nigeria: a follow-up study. Sultan Qaboos Univ. Med. J. SQUMJ. 2018;18:304. doi: 10.18295/squmj.2018.18.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittur N., Binder S., Campbell C.H., King C.H., Kinung’Hi S., Olsen A., Magnussen P., Colley D.G. Defining persistent hotspots: areas that fail to decrease meaningfully in prevalence after multiple years of mass drug administration with Praziquantel for control of schistosomiasis. Am. J. Trop. Med. Hyg. 2017;97:1810. doi: 10.4269/AJTMH.17-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp S., Ame S.M., Hattendorf J., Ali S.M., Khamis I.S., Bakar F., Khamis M.A., Person B., Kabole F., Rollinson D. Urogenital schistosomiasis elimination in Zanzibar: accuracy of urine filtration and haematuria reagent strips for diagnosing light intensity Schistosoma haematobium infections. Parasit. Vectors. 2018;11:552. doi: 10.1186/s13071-018-3136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo N.C., Schemelzer F., Bezerra M., Colley D.G., Fleming F.M., Homeida M., Kabatereine N., Kabole F.M., Traore M.S., Webster J.P., Utzinger J., Zhou X., Danso-appiah A., Eusebi P., Loker E.S. Review of 2022 WHO guidelines on the control and elimination of schistosomiasis. Lancet Infect. Dis. 2022;11:e327–e335. doi: 10.1016/S1473-3099(22)00221-3. [DOI] [PubMed] [Google Scholar]
- Malibiche D., Mushi V., Justine N.C., Silvestri V., Mhamilawa L.E., Tarimo D. Prevalence and factors associated with ongoing transmission of Schistosoma haematobium after 12 rounds of Praziquantel mass drug administration among school age children in southern Tanzania. Parasite Epidemiol. Control. 2023;23 doi: 10.1016/j.parepi.2023.e00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maseke L.S., Mushi V., Tarimo D., Kwesigabo G., Mazigo H. Adolescents and young adults excluded from preventive chemotherapy for schistosomiasis control in northern Tanzania: are they at risk and reservoirs of infection? Prevalence and determinants of transmission in northern Tanzania. IJID Reg. 2022;4:111–119. doi: 10.1016/j.ijregi.2022.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazigo H.D., Uisso C., Kazyoba P., Nshala A., Mwingira U.J. Prevalence, infection intensity and geographical distribution of schistosomiasis among preschool and school aged children in villages surrounding Lake Nyasa. Tanzania. Sci. Rep. 2021;11:1–11. doi: 10.1038/s41598-020-80317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazigo H.D., Mwingira U.J., Zinga M.M., Uisso C., Kazyoba P.E., Kinunghi S.M., Mutapi F. Urogenital schistosomiasis among preschool and school aged children in four districts of north western Tanzania after 15 years of mass drug administration: geographical prevalence, risk factors and performance of haematuria reagent strips. PLoS Negl. Trop. Dis. 2022;16 doi: 10.1371/journal.pntd.0010834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazigo H.D., Zinga M.M., Kepha S., Yard E., McRee-Mckee K., Kabona G., Ngoma D.D., Nshala A. Precision and geographical prevalence mapping of schistosomiasis and soil-transmitted helminthiasis among school-aged children in selected districts of northwestern Tanzania. Parasit. Vectors. 2022;15:492. doi: 10.1186/s13071-022-05547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnkugwe R.H., Minzi O.S., Kinung’hi S.M., Kamuhabwa A.A., Aklillu E. Efficacy and safety of Praziquantel for treatment of Schistosoma mansoni infection among school children in Tanzania. Pathogens. 2019;9:28. doi: 10.3390/pathogens9010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montresor, A, D.W.T.C., A. Hall, D.A.P.B. and L, S., 1998. Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level: A guide for managers of control programmes, geneva:world health organization,1–49.
- Morenikeji O., Quazim J., Omoregie C., Hassan A., Nwuba R., Anumudu C., Adejuwon S., Salawu O., Jegede A., Odaibo A. A cross-sectional study on urogenital schistosomiasis in children; haematuria and proteinuria as diagnostic indicators in an endemic rural area of Nigeria. Afr. Health Sci. 2014;14:390. doi: 10.4314/ahs.v14i2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushi V., Zacharia A., Shao M., Mubi M., Tarimo D. Persistence of Schistosoma haematobium transmission among school children and its implication for the control of urogenital schistosomiasis in Lindi. Tanzania. PLOS ONE. 2022;17 doi: 10.1371/journal.pone.0263929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushi V., Zacharia A., Shao M., Mubi M., Tarimo D. Prevalence and risk factors for urogenital schistosomiasis among underfives in Mtama District in the Lindi region of Tanzania. PLoS Negl. Trop. Dis. 2022;16:1–23. doi: 10.1371/journal.pntd.0010381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwanga J.R., Kinung’hi S.M., Mosha J., Angelo T., Maganga J., Campbell C.H. Village response to mass drug Administration for Schistosomiasis in Mwanza region, northwestern Tanzania: are we missing socioeconomic, cultural, and political dimensions? Am. J. Trop. Med. Hyg. 2020;103:1969–1977. doi: 10.4269/ajtmh.19-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Bureau of Statistics (NBS). The United Republic of Tanzania . Finance; Ministry of: 2022. Population and Housing Census. [Google Scholar]
- Nazareth L.C., Lupenza E.T., Zacharia A., Ngasala B.E. Urogenital schistosomiasis prevalence, knowledge, practices and compliance to MDA among school-age children in an endemic district, southern East Tanzania. Parasite Epidemiol. Control. 2022;18 doi: 10.1016/j.parepi.2022.e00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njaanake K.H., Omondi J., Mwangi I., Jaoko W.G., Anzala O. Urinary interleukins (IL)-6 and IL-10 in schoolchildren from an area with low prevalence of Schistosoma haematobium infections in coastal Kenya. PLOS Glob. Public Health. 2023;3 doi: 10.1371/journal.pgph.0001726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odetunde O., Aderibigbe A.O.S., Njeze N., Achigbu K.I., Chinawa J.M., Odetunde O.A., Okafor H.U. Renal sonographic findings in Nigerian pre- school children with asymptomatic proteinuria. Indian J. Appl. Res. 2011;4:1–4. doi: 10.15373/2249555x/July2014/193. [DOI] [Google Scholar]
- Pennance T., Person B., Muhsin M.A., Khamis A.N., Muhsin J., Khamis I.S., Mohammed K.A., Kabole F., Rollinson D., Knopp S. Urogenital schistosomiasis transmission on Unguja Island, Zanzibar: characterization of persistent hot-spots. Parasit. Vectors. 2016;9:1–13. doi: 10.1186/S13071-016-1847-0/TABLES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennance T., Ame S.M., Amour A.K., Suleiman K.R., Allan F., Rollinson D., Webster B.L. Occurrence of Schistosoma bovis on Pemba Island, Zanzibar: implications for urogenital schistosomiasis transmission monitoring. Parasitology. 2018;145:1727–1731. doi: 10.1017/S0031182018001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J., Hatz C.G., Campagne N.R., Bergquist J.J. Word Health Organ; 2000. Ultrasound in Schistosomiasis;A Practical Guide to the Standardized Use of Ultrasonography for the Assessment of Schistosomiasis-related Morbidity; pp. 1–48. [Google Scholar]
- Sacolo H., Chimbari M., Kalinda C. Knowledge, attitudes and practices on schistosomiasis in sub-Saharan Africa: a systematic review. BMC Infect. Dis. 2018;18 doi: 10.1186/s12879-017-2923-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salawu O.T., Odaibo A.B. Urogenital schistosomiasis and urological assessment of hematuria in preschool-aged children in rural communities of Nigeria. J. Pediatr. Urol. 2014;10:88–93. doi: 10.1016/j.jpurol.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Senghor B., Diaw O.T., Doucoure S., Sylla S.N., Seye M., Talla I., Bâ C.T., Diallo A., Sokhna C. Efficacy of praziquantel against urinary schistosomiasis and reinfection in Senegalese school children where there is a single well-defined transmission period. Parasit. Vectors. 2015;8:362. doi: 10.1186/s13071-015-0980-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkwe M.N., Berta K.K., Yibi S.M., Sube J., Bidali A., Abe A., Onyeze A., Ajo J.P.H., Pascale J.R., Ndenzako F., Olu O.O. Prevalence and factors associated with transmission of schistosomiasis in school-aged children in South Sudan: a cross-sectional study. Pan Afr. Med. J. 2022;42 doi: 10.11604/pamj.supp.2022.42.1.34006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams M., Khazaei S., Ghasemi E., Nazari N., Javanmardi E., Majidiani H., Bahadory S., Anvari D., Fatollahzadeh M., Nemati T., Asghari A. Prevalence of urinary schistosomiasis in women: a systematic review and meta-analysis of recently published literature (2016–2020) Trop. Med. Health. 2022;50:12. doi: 10.1186/s41182-022-00402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sow D., Sylla K., Dieng N.M., Senghor B., Gaye P.M., Fall C.B., Goumballa N., Diallo A., Ndiaye J.L.A., Parola P., Sokhna C., Doucouré S., Faye B. Molecular diagnosis of urogenital schistosomiasis in preschool children, school-aged children and women of reproductive age at community level in Central Senegal. Parasit. Vectors. 2023;16:43. doi: 10.1186/s13071-023-05671-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werf M.J., van der Werf M.J., de Vlas S.J., Brooker S., Looman C.W.N., Nagelkerke N., Nagelkerke N., Habbema J.D.F., Engels D. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–139. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Vester U., Kardorff R., Traoré M., Traoré H.A., Fongoro S., Juchem C., Franke D., Korte R., Gryseels B., Ehrich J.H.H., Doehring E. Urinary tract morbidity due to Schistosoma haematobium infection in Mali. Kidney Int. 1997;52:478–481. doi: 10.1038/ki.1997.356. [DOI] [PubMed] [Google Scholar]
- Wami W.M., Nausch N., Midzi N., Midzi N., Gwisai R., Mduluza T., Woolhouse M.E.J., Mutapi F. Identifying and evaluating field indicators of urogenital schistosomiasis-related morbidity in preschool-aged children. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Prevention and control of schistosomiasis and soil-transmittedhelminthiasis: report of a WHO expert Committee. WHO Tech. Rep. Ser. 2002:912. [PubMed] [Google Scholar]
- WHO . 2021. Ending the neglect to attain the Sustainable Development Goals A road map for neglected tropical diseases 2021–2030. [Google Scholar]
- WHO . World Health Organization; Geneva: 2022. Guidelines on Control and Elimination of Human Schistosomiasis. [PubMed] [Google Scholar]
- Wiegand R.E., Secor W.E., Fleming F.M., French M.D., King C.H., Deol A.K., Montgomery S.P., Evans D., Utzinger J., Vounatsou P., de Vlas S.J. Associations between infection intensity categories and morbidity prevalence in school-age children are much stronger for Schistosoma haematobium than for S. Mansoni. PLoS Negl. Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohana C., Bakuza J.S., Kinung’hi S.M., Nyundo B.A., Rambau P.F. The trend of schistosomiasis related bladder cancer in the lake zone, Tanzania: a retrospective review over 10 years period. Infect. Agent. Cancer. 2023;18:10. doi: 10.1186/s13027-023-00491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in the analysis of this study are available upon reasonable request.