Abstract
Background
Urinary incontinence (UI) is a prevalent, health-threatening condition that causes isolation and psychological strain, leading to significant personal distress. The connection between the triglyceride glucose body mass index (TyG-BMI) and UI remains elusive. The purpose of the current research was to investigate any possible relationships between raised TyG-BMI levels and a higher likelihood of UI.
Methods
For a thorough examination, adults 20 years and older with UI were included in cross-sectional research using the data obtained from the National Health and Nutrition Examination Survey (NHANES) from 2001 to 2018. Our investigation centred on three of the significant varieties of UI: Urgent Urinary Incontinence (UUI), Mixed Urinary Incontinence (MUI), and Stress Urinary Incontinence (SUI), employing weighted multivariate logistic regression models for an in-depth evaluation. The TyG-BMI, a possible biomarker, was arranged in increasing order among participants and then assessed with a trend test (P for trend). Moreover, this investigation delved into the non-linear relationships using advanced smoothed curve fitting techniques. Meticulous subgroup analyses were executed to verify the uniformity of the UI and TyG-BMI relationship across diverse demographic groups.
Results
A thorough investigation was conducted with 18,751 subjects to analyze the prevalence and types of UI, showing that 23.59% of individuals suffered from SUI, 19.42% from UUI, and 9.32% from MUI. Considering all possible confounding variables, Multivariate logistic regression analysis showed a substantial relationship between elevated TyG-BMI values and a greater likelihood across all UI categories. Specifically, stratifying the TyG-BMI into quartiles revealed a pronounced positive correlation in the top quartile relative to the bottom, reflected in increased odds ratios for SUI, UUI, and MUI (SUI: OR = 2.36, 95% CI 2.03–2.78, P < 0.0001; UUI: OR = 1.86, 95% CI 1.65–2.09, P < 0.0001; MUI: OR = 2.07, 95% CI 1.71–2.51, P < 0.0001).
Conclusions
Among US adults, an association has been observed wherein increased TyG-BMI values correlate with a higher chance of UI. This suggests that TyG-BMI might be a helpful marker for identifying individuals at risk of UI, providing novel insights into its assessment and management.
Keywords: Triglyceride glucose body mass index, Urinary incontinence, Insulin resistance, Cross-sectional study, NHANES
Background
The condition known as urinary incontinence, distinguished by involuntary urinary outflow, constitutes a widespread medical condition predominantly affecting women [1]. It may pose challenges to physical, social, mental, and emotional well-being [2]. The three types of UI, symptomatically classified, are mixed (MUI), stress (SUI), and urge (UUI). Global statistics show a yearly rise in the overall incidence of UI in females, with rates of occurrence varying from 25 to 45% [3]. Similarly, research on male prevalence in the US demonstrates that UI prevalence escalates from 11% in males aged 60–64 to 31% among individuals aged 85 and older [4]. Numerous individuals remain unaware of this global health challenge, resulting in delayed diagnosis and treatment. UI can stem from a variety of factors, among which metabolic syndrome (METS) is directly linked to its onset and progression [5].
Dyslipidemia, obesity, hypertension, and insulin resistance are the clinical conditions that define METS [6]. However, the most significant contributor to METS's progression is insulin resistance [7]. In a study including 184 female patients, Uzun H et al. [8] identified a correlation between insulin resistance (IR) and overactive bladder syndrome (OAB), which leads to an increased incidence of UI. Due to their complexity and challenging implementation, conventional IR metrics such as the homeostasis model assessment (HOMA-IR) and the hyperinsulinemic-euglycemic clamp (HIEC) are impractical for clinical application [9]. The utilization of TyG-BMI is suggested to be an efficient and convenient substitute that may indicate the presence of IR [10]. Comprehensive research has confirmed that diabetes, hypertension, stroke, heart disease, and fatty liver disease, among others, exhibit a correlation with TyG-BMI [11–17].
Present research often overlooks the combined impacts of various metabolic variables to concentrate solely on a single element, such as the relationship between diabetes or obesity and UI. Studies on UI conducted so far have mainly examined anatomical, physiological, and neural factors; however, a thorough examination of the impact of metabolic parameters, particularly comprehensive metabolic indicators, has yet to be thoroughly discussed. This study examines the connection between UI and TyG-BMI for the first time. TyG-BMI could be linked to a higher incidence of UI.
Methods
Data source and participants
The data utilized in this cross-sectional study were obtained from the National Health and Nutrition Examination Survey (NHANES), a dataset specifically developed to evaluate the health conditions and nutritional status of the general population of the United States. NHANES gathers information on various aspects such as population characteristics, financial status, and public health to assess the overall well-being of the population [18]. At the https://www.cdc.gov/nchs/nhanes/ , the public can access the exhaustive NHANES dataset and research methodology. The present investigation followed the cross-sectional research guidelines established by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [19]. Data spanning nine cycles (2001–2018) from the NHANES database were analyzed. To deal with missing values in the data, we used multiple imputation method. Initially, 91,351 individuals were recruited. Exclusions included participants under 20 years (n = 41,150), those with incomplete UI data (n = 6,365), those lacking BMI, triglyceride, and fasting glucose data (n = 24,593), and pregnant individuals (n = 492). Ultimately, the study comprised 18,751 participants (Fig. 1). The NHANES data used in this study were collected in strict accordance with standard operating procedures. Intra-assay variability was minimized by performing repeated measurements under the same conditions, while inter-assay variability was reduced through standardized procedures and equipment calibration across cycles. We also applied sample weighting and multiple imputation methods in our analysis to further enhance the reliability of the results.
Fig. 1.
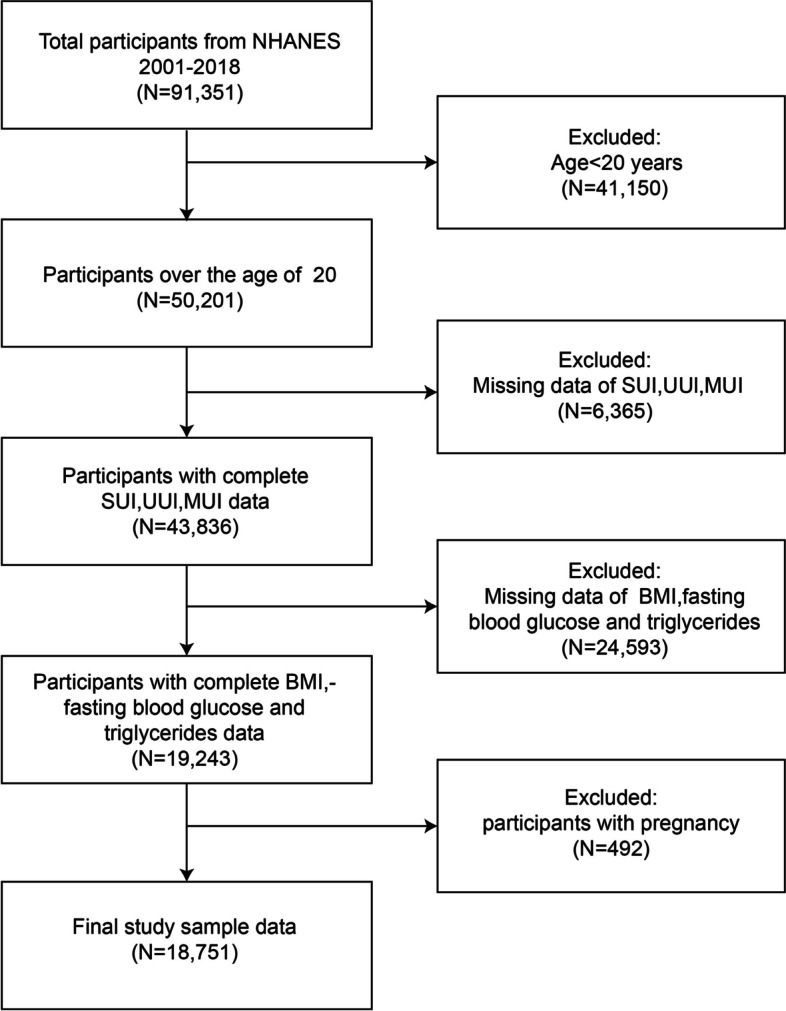
Flowchart of participants selection from NHANES 2001–2018
Exposure and outcome definitions
The exposure variable was defined as TyG-BMI, which was computed by the following formula: Ln [triglycerides (mg/dl) * fasting blood glucose (mg/dl)/2] * BMI (kg/m2) [17]. Triglycerides were enzymatically measured using the spectrophotometric method (Roche Cobas 6000 chemical analyzer), while plasma glucose concentrations were determined using the glucose-oxidase method (Roche/Hitachi Cobas C 501 chemical analyzer).
By assessing the NHANES data, the incidence of UI was determined in the current research, focusing on two questionnaire items related to bladder function. SUI was identified in participants reporting involuntary urine leakage in small amounts due to physical activities, including coughing or exercise, within the past year. UUI was distinguished by the concurrent presence of involuntary urine discharge and an urgent, compelling urge to urinate during the same period. Individuals reporting symptoms of both SUI and UUI were categorized as having MUI, offering a comprehensive classification of UI subtypes in the cohort.
Assessment of covariates of interest
This research assessed vital variables such as age, gender, ethnicity, educational attainment (divided into under high school, high school, and above high school), household income (as determined by the ratio of family income to poverty, which is classified as follows: < 1.3, 1.3–3.5, and > 3.5), the calculation of family poverty ratios was based on the Family Income Poverty Guidelines provided by the US Department of Health and Human Services (DHHS) [20], marital status (with classifications of single, married, living together, widowed, divorced, or separated), smoking status (non-smokers, current smokers, and individuals who have inhaled at least 100 cigarettes in their lifetime but do not smoke at present), alcohol consumption (categorized into ≥ 12 alcoholic beverages annually and non-consumers), diabetes mellitus (identified by physician diagnosis, use of anti-diabetic medication, or an HbA1c > 6.5%), hypertension (defined by physician diagnosis, medication usage, or blood pressure readings > 140/90 mmHg), and high cholesterol. High cholesterol was determined if a physician diagnosed participants used hypercholesterolemia medication, or had a total cholesterol measurement of ≥ 240 mg/dL. Participants were asked if they had health insurance or a different healthcare plan to determine their coverage.
Statistical analysis
Statistical analyses followed CDC guidelines and included NHANES sample weights. Consequently, logistic regression was employed to extrapolate categorical parameters to proportions, while linear regression facilitated the generalization of continuous variables to means with accompanying standard deviations (SD). This study utilized three distinct multivariate logistic regression models to elucidate the connection between TyG-BMI and UI. The models varied in covariate adjustments: Model 1 did not include any covariate modifications, while Model 2 included age, gender, and race. In contrast, Model 3 integrated covariates, including age, gender, education level, alcohol use, family poverty ratio, race/ethnicity, vigorous activity, moderate activity, marital status, smoking status, diabetes, high cholesterol, hypertension and health insurance coverage.
Additionally, TyG-BMI was categorized into quartiles from a continuous measure, enabling a trend analysis to identify potential correlations with UI. Smooth curve fitting methods were used to assess the non-linear correlation. A stratified subgroup analysis, employing hierarchical multiple regression analysis with an interaction term, was conducted to explore heterogeneity among subgroups. By analyzing receiver-operating characteristic (ROC) curves and computing the area under the curve (AUC), it was possible to evaluate TyG-BMI's discriminatory ability in predicting the three kinds of UI. Statistical analyses were performed using R software version 3.4.3 from The R Foundation and Empower software (X&Y Solutions, Inc., Boston, MA, USA). A p-value of less than 0.05 was considered statistically significant.
Results
Characteristics of the participants
The demographic characteristics and other factors are shown in Table 1, categorized by TyG-BMI quartiles. The study included 18,751 participants, with an average age of 50.30 ± 17.80 years, split almost evenly between females (49.45%) and males (50.55%) based on weighted proportions.
Table 1.
Basic characteristics of participants by TyG-BMI among U.S. adults
| TyG-BMI | Overall | Q1 | Q2 | Q3 | Q4 | P-value |
|---|---|---|---|---|---|---|
| N = 18,751 | (112.58–204.87) | (204.87–242.54) | (242.54–287.23) | (287.23–679.46) | ||
| N = 4688 | N = 4687 | N = 4688 | N = 4688 | |||
| Age(year), Mean ± SD | 50.30 ± 17.80 | 45.37 ± 19.16 | 52.24 ± 17.96 | 52.37 ± 17.16 | 51.24 ± 15.82 | < 0.001 |
| Gender, n(%) | < 0.001 | |||||
| Male | 9478 (50.55%) | 2120 (45.22%) | 2620 (55.90%) | 2597 (55.40%) | 2141 (45.67%) | |
| Female | 9273 (49.45%) | 2568 (54.78%) | 2067 (44.10%) | 2091 (44.60%) | 2547 (54.33%) | |
| Race, n(%) | < 0.001 | |||||
| Mexican American | 3148 (16.79%) | 492 (10.49%) | 797 (17.00%) | 938 (20.01%) | 921 (19.65%) | |
| Other Hispanic | 1619 (8.63%) | 322 (6.87%) | 439 (9.37%) | 451 (9.62%) | 407 (8.68%) | |
| Non-Hispanic White | 8597 (45.85%) | 2299 (49.04%) | 2149 (45.85%) | 2045 (43.62%) | 2104 (44.88%) | |
| Non-Hispanic Black | 3697 (19.72%) | 893 (19.05%) | 840 (17.92%) | 924 (19.71%) | 1040 (22.18%) | |
| Other Races | 1690 (9.01%) | 682 (14.55%) | 462 (9.86%) | 330 (7.04%) | 216 (4.61%) | |
| Education level, n(%) | < 0.001 | |||||
| Less than high school | 4787 (25.55%) | 965 (20.60%) | 1208 (25.81%) | 1286 (27.46%) | 1328 (28.34%) | |
| High school or GED | 4375 (23.35%) | 1019 (21.75%) | 1061 (22.67%) | 1165 (24.88%) | 1130 (24.11%) | |
| Above high school | 9572 (51.09%) | 2701 (57.65%) | 2411 (51.52%) | 2232 (47.66%) | 2228 (47.55%) | |
| Marital status, n(%) | < 0.001 | |||||
| Married or living with partners | 11,394 (60.79%) | 2587 (55.20%) | 2930 (62.53%) | 3017 (64.38%) | 2860 (61.05%) | |
| Widowed, divorced, or separated | 4137 (22.07%) | 874 (18.65%) | 1072 (22.88%) | 1060 (22.62%) | 1131 (24.14%) | |
| Never married | 3213 (17.14%) | 1226 (26.16%) | 684 (14.60%) | 609 (13.00%) | 694 (14.81%) | |
| Family poverty ratio, n(%) | < 0.001 | |||||
| < 1.3 | 5078 (29.39%) | 1226 (28.39%) | 1196 (27.63%) | 1217 (28.22%) | 1439 (33.33%) | |
| 1.3–3.5 | 6717 (38.88%) | 1600 (37.05%) | 1714 (39.59%) | 1723 (39.95%) | 1680 (38.91%) | |
| ≥ 3.5 | 5483 (31.73%) | 1492 (34.55%) | 1419 (32.78%) | 1373 (31.83%) | 1199 (27.77%) | |
| Alcohol use, n(%) | < 0.001 | |||||
| Yes | 13,793 (73.76%) | 3505 (74.91%) | 3539 (75.81%) | 3474 (74.33%) | 3275 (69.99%) | |
| No | 4907 (26.24%) | 1174 (25.09%) | 1129 (24.19%) | 1200 (25.67%) | 1404 (30.01%) | |
| Smoking status, n(%) | < 0.001 | |||||
| Never | 9953 (53.12%) | 2600 (55.50%) | 2463 (52.62%) | 2453 (52.35%) | 2437 (52.03%) | |
| Past | 4825 (25.75%) | 893 (19.06%) | 1244 (26.58%) | 1336 (28.51%) | 1352 (28.86%) | |
| Current | 3958 (21.13%) | 1192 (25.44%) | 974 (20.81%) | 897 (19.14%) | 895 (19.11%) | |
| Hypertension, n(%) | < 0.001 | |||||
| Yes | 8182 (43.64%) | 1214 (25.90%) | 1893 (40.41%) | 2253 (48.07%) | 2822 (60.20%) | |
| No | 10,565 (56.36%) | 3473 (74.10%) | 2792 (59.59%) | 2434 (51.93%) | 1866 (39.80%) | |
| Diabetes, n(%) | < 0.001 | |||||
| Yes | 2900 (15.47%) | 179 (3.82%) | 494 (10.54%) | 761 (16.24%) | 1466 (31.27%) | |
| No | 15,850 (84.53%) | 4509 (96.18%) | 4193 (89.46%) | 3926 (83.76%) | 3222 (68.73%) | |
| High cholesterol, n(%) | < 0.001 | |||||
| Yes | 7659 (40.85%) | 1193 (25.45%) | 1936 (41.31%) | 2164 (46.16%) | 2366 (50.47%) | |
| No | 11,092 (59.15%) | 3495 (74.55%) | 2751 (58.69%) | 2524 (53.84%) | 2322 (49.53%) | |
| Health insurance coverage, n(%) | 0.003 | |||||
| Yes | 14,710 (78.66%) | 3615 (77.34%) | 3680 (78.75%) | 3653 (78.16%) | 3762 (80.38%) | |
| No | 3991 (21.34%) | 1059 (22.66%) | 993 (21.25%) | 1021 (21.84%) | 918 (19.62%) | |
| Vigorous physical activity, n(%) | < 0.001 | |||||
| Yes | 4530 (24.16%) | 1554 (33.15%) | 1204 (25.69%) | 1047 (22.34%) | 725 (15.47%) | |
| No | 14,219 (75.84%) | 3134 (66.85%) | 3482 (74.31%) | 3640 (77.66%) | 3963 (84.53%) | |
| Moderate physical activity, n(%) | < 0.001 | |||||
| Yes | 8131 (43.38%) | 2282 (48.68%) | 2099 (44.83%) | 1986 (42.38%) | 1764 (37.63%) | |
| No | 10,613 (56.62%) | 2406 (51.32%) | 2583 (55.17%) | 2700 (57.62%) | 2924 (62.37%) | |
| TG (mg/dl), Mean ± SD | 132.44 ± 120.73 | 79.33 ± 39.48 | 112.88 ± 63.48 | 146.64 ± 88.18 | 190.89 ± 195.25 | < 0.001 |
| FBG (mg/dl), Mean ± SD | 109.15 ± 36.22 | 95.99 ± 15.92 | 104.09 ± 26.67 | 110.25 ± 33.16 | 126.26 ± 51.87 | < 0.001 |
| BMI (kg/m2), Mean ± SD | 28.95 ± 6.66 | 22.05 ± 2.18 | 26.28 ± 1.85 | 29.92 ± 2.27 | 37.55 ± 5.86 | < 0.001 |
| SUI, n(%) | < 0.001 | |||||
| No | 14,399 (76.79%) | 3851 (82.15%) | 3717 (79.30%) | 3628 (77.39%) | 3203 (68.32%) | |
| Yes | 4352 (23.21%) | 837 (17.85%) | 970 (20.70%) | 1060 (22.61%) | 1485 (31.68%) | |
| UUI, n(%) | < 0.001 | |||||
| No | 14,487 (77.26%) | 3906 (83.32%) | 3717 (79.30%) | 3616 (77.13%) | 3248 (69.28%) | |
| Yes | 4264 (22.74%) | 782 (16.68%) | 970 (20.70%) | 1072 (22.87%) | 1440 (30.72%) | |
| MUI, n(%) | < 0.001 | |||||
| No | 16,781 (89.49%) | 4380 (93.43%) | 4269 (91.08%) | 4200 (89.59%) | 3932 (83.87%) | |
| Yes | 1970 (10.51%) | 308 (6.57%) | 418 (8.92%) | 488 (10.41%) | 756 (16.13%) |
Mean ± SD for continuous variables: the P value was calculated by the weighted linear regression model; n (%) for categorical variables: the P value was calculated by the weighted chi-square test
Abbreviation: TyG-BMI Triglyceride glucose body mass index, TG triglyceride, FBG fasting blood glucose, BMI body mass index, SUI Stress Urinary incontinence, UUI Urge urinary incontinence, MUI Mixed urinary incontinence
Among the participants, 23.21% self-reported previous SUI, 22.74% reported UUI, and 10.51% reported MUI. The designated quartiles for triglyceride-glucose body mass index were Q1 (112.58–204.87), Q2 (204.87–242.54), Q3 (242.54–287.23), and Q4 (287.23–679.46). A substantial rise in the incidence of UI was linked to higher TyG-BMI categories. Additionally, individuals in the upper TyG-BMI quartile were more inclined to be women, white, possess advanced education, be married or cohabiting, experience middle household poverty rates, consume alcohol, have elevated blood pressure, and engage in lower levels of physical activity.
Correlation of TyG-BMI with the likelihood of urinary incontinence (SUI, UUI, MUI)
The correlation between TyG-BMI and the likelihood of developing UI is shown in Table 2. Weighted multivariate logistic regression was used, adjusting models in a tiered approach: unadjusted (Model 1), moderately adjusted (Model 2), and fully adjusted (Model 3) for a precise assessment of the relationship. This research revealed that the three forms of UI had substantially greater likelihoods linked with increasing TyG-BMI values.
Table 2.
Associations between TyG-BMI and urinary incontinence
| SUI | OR (95% CI), P-value | ||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Continuous |
1.00 (1.00,1.01), < 0.0001 |
1.01 (1.00,1.03), < 0.0001 |
1.01 (1.00,1.01), < 0.0001 |
| Categories | |||
| Q1 | Reference | Reference | Reference |
| Q2 |
1.21 (1.08,1.33), 0.0005 |
1.36 (1.17,1.59), < 0.0001 |
1.38 (1.17,1.62), < 0.0001 |
| Q3 |
1.34 (1.21,1.49), < 0.0001 |
1.53 (1.33,1.77), < 0.0001 |
1.49 (1.25,1.71), < 0.0001 |
| Q4 |
1.86 (1.63,2.11), < 0.0001 |
2.46 (2.14,2.84), < 0.0001 |
2.36 (2.03,2.78), < 0.0001 |
| P for trend | < 0.001 | < 0.001 | < 0.001 |
| UUI | OR (95% CI), P-value | ||
| Model 1 | Model 2 | Model 3 | |
| Continuous |
1.00 (1.00,1.01), < 0.0001 |
1.01 (1.00,1.02), < 0.0001 |
1.01 (1.00,1.01), < 0.0001 |
| Categories | |||
| Q1 | Reference | Reference | Reference |
| Q2 |
1.26 (1.11,1.43), 0.0011 |
1.13 (0.97,1.31), 0.1076 |
1.19 (1.06,1.334), 0.0042 |
| Q3 |
1.35 (1.17,1.55), < 0.0001 |
1.23 (1.05,1.43), < 0.0101 |
1.28 (1.14,1.44), < 0.0001 |
| Q4 |
2.01 (1.76,2.28), < 0.0001 |
1.82 (1.58,2.08), < 0.0001 |
1.86 (1.65,2.09), < 0.0001 |
| P for trend | < 0.001 | < 0.001 | < 0.001 |
| MUI | OR (95% CI), P-value | ||
| Model 1 | Model 2 | Model 3 | |
| Continuous |
1.01 (1.00,1.01), < 0.0001 |
1.00 (1.00,1.01), < 0.0001 |
1.01 (1.00,1.02), < 0.0001 |
| Categories | |||
| Q1 | Reference | Reference | Reference |
| Q2 |
1.32 (1.11,1.59), 0.0034 |
1.38 (1.13,1.69), 0.0017 |
1.34 (1.09,1.65), 0.0004 |
| Q3 |
1.47 (1.22,1.78), < 0.0001 |
1.62 (1.32,1.94), < 0.0001 |
1.41 (1.12,1.76), < 0.0001 |
| Q4 |
2.38 (2.01,2.84), < 0.0001 |
2.46 (2.03,2.98), < 0.0001 |
2.07 (1.71,2.51), < 0.0001 |
| P for trend | < 0.001 | < 0.001 | < 0.001 |
Model 1: unadjusted
Model 2: adjusted for age, gender and race/ethnicity
Model 3: adjusted for variables, including age, gender, race/ethnicity, education level, marital status, the family poverty ratio, smoking status, alcohol use, vigorous activity, moderate activity, diabetes, hypertension, high cholesterol, and health insurance coverage
Abbreviation: TyG-BMI Triglyceride glucose body mass index, SUI Stress Urinary incontinence, UUI Urge urinary incontinence, MUI Mixed urinary incontinence, OR odds ratio, 95%CI 95% confidence interval
The likelihood of UI and TyG-BMI all exhibited statistically significant positive connections in all three different adjusted models, with statistical significance confirmed (P < 0.0001). For additional validation, the TyG-BMI variable was analyzed as a continuous variable in categorical form, dividing the cohort into quartiles (Q1–Q4). Individuals in the top quartile (Q4) had a much higher probability of experiencing any UI compared to those in the bottom quartile (Q1). This association remained significant in all three different adjusted models: unadjusted (SUI: OR = 1.86; 95% CI = 1.63–2.11, UUI: OR = 2.01; 95% CI = 1.76–2.28, MUI: OR = 2.38; 95% CI = 2.01–2.84); moderately adjusted (SUI: OR = 2.46; 95% CI = 2.14–2.84, UUI: OR = 1.82; 95% CI = 1.58–2.08, MUI: OR = 2.46; 95% CI = 2.03–2.98); and fully adjusted (SUI: OR = 2.36; 95% CI = 2.03–2.78, UUI: OR = 1.86; 95% CI = 1.65–2.09, MUI: OR = 2.07; 95% CI = 1.71–2.51).
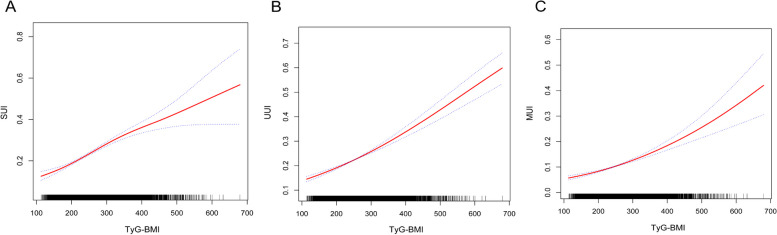
Further investigation of the relationship between UI likelihood and TyG-BMI, employing smoothed curve fitting techniques, verified a statistically significant, consistent positive relationship across all three models (Fig. 2).
Fig. 2.
Smooth curve fitting for the relationship between TyG-BMI and three UI types. A, B, and C represent the linear associations between TyG-BMI and SUI, UUI, and MUI, respectively. The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval from the fit. The Y-axis represents the probability of occurrence of each of the three UI types
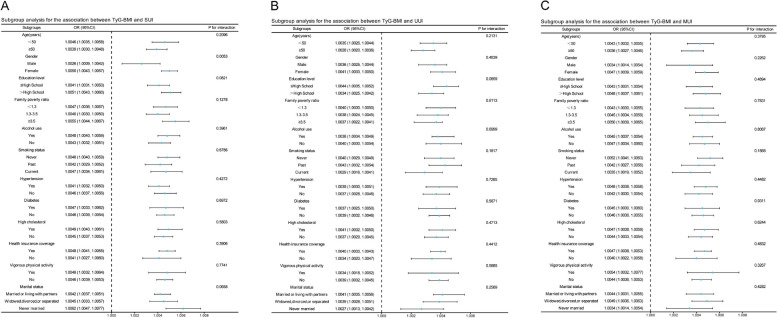
Subgroup analysis
In this study, subgroup analyses were conducted, and interactions within diverse demographic segments were tested to assess the robustness of the UI and TyG-BMI association. Notably, gender was found to modulate the link between SUI and TyG-BMI, with a more pronounced association observed in females than in males, as evidenced by a significant interaction term (P < 0.05) (Fig. 3A). Conversely, gender-based analyses for UUI and MUI failed to reveal significant differences, with all interaction P-values exceeding 0.05 (Fig. 3B and C). Furthermore, subgroup evaluations across various classifications did not significantly alter the positive connection between UI and TyG-BMI, affirming the consistency across all examined cohorts.
Fig. 3.
Subgroup analysis of the association between TyG-BMI and three UI types. A, B and C represent the subgroup analysis results for the relationships between TyG-BMI and SUI, UUI, and MUI in different stratifications
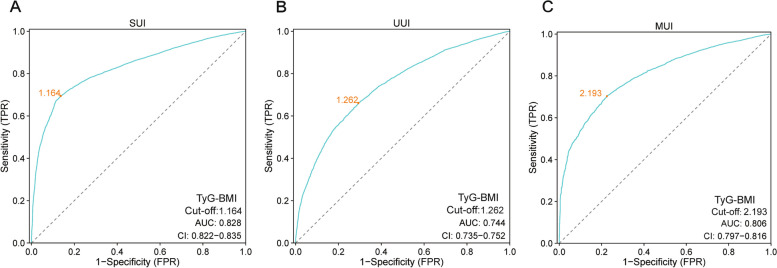
The ROC curve of TyG-BMI for the UI
The relationship between TyG-BMI and the risk of UI (SUI, MUI, UUI) was assessed using the ROC curve. Figure 4 shows that the association of TyG-BMI with the risk of UI is indicated by AUC values for SUI (AUC = 0.828, 95% CI 0.822–0.835), MUI (AUC = 0.806, 95% CI 0.797–0.816), and UUI (AUC = 0.744, 95% CI 0.735–0.752). Table 3 provides detailed information on the performance of TyG-BMI in predicting the three types of UI, including cutoff values, sensitivity, and specificity. For SUI, the optimal cutoff value was 1.164, yielding a sensitivity of 69.4% and a specificity of 86.3%. In the case of MUI, the cutoff value was 2.193, with corresponding sensitivity and specificity values of 70.3% and 77.5%, respectively. For UUI, the optimal cutoff value was 1.262, with a sensitivity of 66.1% and a specificity of 70.6%. These cutoff points represent the optimal thresholds between the TyG-BMI index and UI risk.
Fig. 4.
Receiver operating characteristic (ROC) curve analysis for assessing the association with UI. A TyG-BMI was assessed to identify SUI. B TyG-BMI was assessed to identify MUI. (C) TyG-BMI was assessed to identify UUI
Table 3.
The TyG-BMI for assessing the association with three types of UI
| Test | AUC | 95%CI low | 95%CI upp | Cut-off value | Specificity | Sensitivity |
|---|---|---|---|---|---|---|
| SUI | ||||||
| TyG-BMI | 0.828 | 0.822 | 0.835 | 1.164 | 0.863 | 0.694 |
| UUI | ||||||
| TyG-BMI | 0.744 | 0.735 | 0.752 | 1.262 | 0.706 | 0.661 |
| MUI | ||||||
| TyG-BMI | 0.806 | 0.797 | 0.816 | 2.193 | 0.775 | 0.703 |
AUC area under curve, 95% CI 95% confidence interval
Discussion
This investigation elucidates the relationship between UI and the TyG-BMI in a diverse cohort of US citizens, analyzing NHANES data from 2001 to 2018. Our findings show a robust correlation between higher TyG-BMI values and a higher chance of developing several types of UI, such as SUI, UUI, and MUI. Converting TyG-BMI from a numerical to a grouped variable by dividing it into quartiles confirmed the strong connection between increases in TyG-BMI and susceptibility to UI. Subgroup analyses further revealed a pronounced predisposition for SUI in females over males, underscoring the potential gender-specific impacts of TyG-BMI on UI. The possible reason for the differences in subgroup analyses is that pregnancy, childbirth, and the decline in estrogen in menopausal women can cause damage to the pelvic support structures and atrophy of the tissues around the vesico-urethral area in women [1, 2], all of which increase the risk of SUI in women. Secondly, UUI is often associated with overactive bladder (OAB), and the primary cause of OAB is abnormal contractions of the detrusor muscle. This process is regulated by the autonomic nervous system, which functions similarly in both males and females. Hence, gender has a lesser impact on UUI [21, 22].
We found that an elevated TyG-BMI is associated with a higher incidence of UI, consistent with previous studies linking IR with UI. For example, Uzun et al. demonstrated a correlation between IR and OAB in women, indicating that IR increases the incidence of urgency [8]. Furthermore, Nazzal et al. found that the significantly higher incidence of UI in women with diabetes is related to IR [23]. Our study further validates this association by using TyG-BMI as a surrogate marker for IR in evaluating UI. Compared to TyG, the TyG-BMI index, by incorporating weight factors, provides a more comprehensive assessment of metabolic status.
The underlying pathophysiology is hypothesized to involve IR, a pivotal factor in metabolic syndrome, contributing to UI onset via mechanisms related to inflammation, neuronal disruption, oxidative stress, and alterations in muscle mass and functionality [24].
Identifying straightforward and clinically valuable indicators of IR has garnered significant interest due to the costly and cumbersome nature of the HIEC procedures for diagnosing IR [17]. The HOMA-IR is another often utilized technique for measuring IR. It is a non-invasive method for studying IR, but it is insulin-dependent and has limited utility in patients on insulin therapy or without functional β cells [25]. Therefore, there are few application situations for either approach, mostly due to the rarity of measuring fasting insulin levels in typical clinical settings and extensive epidemiological research. Within this context, Prof. Er first introduced the TyG-BMI in 2016 [10]—a composite variable comprising fasting plasma glucose (FPG), triglycerides (TG), and body mass index (BMI). TyG-BMI offers a more straightforward and economical way to evaluate IR. It may be used for any patient, regardless of whether they are receiving insulin therapy, and does not require the measurement of insulin.
According to recent research, the TyG-BMI is a more accurate indicator of IR, surpassing traditional measures such as the Triglyceride-Glucose (TyG) index, BMI, visceral adiposity index, lipid accumulation products, adipokines, and lipid ratios [10]. Findings showed that the TyG-BMI performed better in IR prediction than the TyG index [26]. Moreover, the application of TyG-BMI extends across diverse clinical contexts, highlighting its versatility. For instance, Yang X et al. [12] demonstrated that TyG-BMI and the severity of coronary artery disease (CAD) in females have a direct linear connection, while Du Z et al. [27] highlighted its substantial correlation with ischemic stroke and suggested its utility in refining risk stratification for these conditions. In a population-based study, HOMA-IR and the TyG index were less effective than the TyG-BMI [28]. Additionally, the TyG-BMI has been independently associated with osteoporotic fractures and non-small cell lung cancer (NSCLC), underscoring its potential to predict a broad spectrum of diseases beyond metabolic disorders [29, 30]. In summary, the TyG-BMI represents a practical and potent tool for identifying and assessing IR and its associated risks across diverse diseases.
Additionally, TyG-BMI is also closely associated with pre-hypertension, diabetes, all-cause mortality in patients suffering from atrial fibrillation, and non-alcoholic fatty liver disease [13, 31, 32]. Nevertheless, studies investigating the correlation between UI and TyG-BMI are scarce. The problem of UI may cause great suffering among a wide range of people, especially women and the elderly. Nonetheless, few markers exist that can reliably predict the onset of UI. The clinical application of TyG-BMI could, to some extent, reduce the incidence of UI by facilitating early intervention and health counselling for high-risk populations.
One fundamental process associated with IR is oxidative stress. Mitochondrial dysfunction, characterized by reduced mitochondrial size and DNA content that elevates oxidative stress, represents a primary pathogenic feature of IR [33]. Additionally, insulin regulates oxidative capacity and mitochondrial metabolism through PI3K/Akt signalling [34, 35]. The induction of oxidative stress in the bladder suggests that IR could likewise provoke oxidative damage within the urethral sphincter, potentially altering the functionality of the lower urinary tract [36]. Blaha I et al. showed that IR correlates with higher levels of cellular injury caused by oxidative stress. Furthermore, elevated oxidative stress and diminished blood flow to the bladder could lead to neurological dysfunction of the bladder and injury to the urethral muscle cells, culminating in urinary urgency [37]. These findings offer additional support for the distinct correlation between IR and UI. Moreover, mitochondrial damage leads to significant production of reactive oxygen species (ROS), which subsequently leads to higher levels of inflammatory cytokines, hindering the function of pancreatic β-cells, worsening IR, and starting a harmful cycle of IR, mitochondrial damage, and inflammation [38, 39].
To our knowledge, prior research has established a correlation between inflammation and IR, and numerous studies have demonstrated that IR is positively correlated with high-sensitivity C-reactive protein [40–43]. Research involving ischemic stroke patients without diabetes also corroborated this association [44]. Moreover, IR not only diminishes enzyme activity in adipocytes but also activates the NLRP3 inflammasome, thereby leading to increased levels of inflammatory molecules like TNF-α and IL-6, which initiate the inflammatory process [45, 46]. Furthermore, although inflammation is both a cause and a result of insulin resistance, known to exacerbate each other cyclically [47], their synergistic role in driving UI still requires further investigation. And, their synergistic contribution to the pathophysiology of UI may involve complex multifactorial pathways. The study revealed that the inflammatory cytokine TNF-α impeded myogenic differentiation in human urethral transverse sphincter cells. This indicates that TNF-α could potentially elevate the likelihood of SUI among older individuals [48]. Moreover, overactive bladder syndrome (OAB) and UI, conditions exacerbated by inflammation, were studied. According to research by Chung SD et al. [22], individuals with OAB exhibited significantly elevated levels of CRP compared to those without OAB.
IR is strongly associated with the start of diabetes and is a significant contributor to the risk of the disease's progression [49, 50]. On the other hand, Nazzal Z et al. have indicated that diabetes significantly enhances the likelihood of UI. Among the 400 diabetic females in the study, 44%, aged 30–83, reported UI [23]. Furthermore, compared to females without Type 2 Diabetes Mellitus (T2DM), the prevalence of UI is considerably greater in females with T2DM, according to many research findings [51, 52].
The TyG-BMI, an adapted index derived from both BMI and TyG, is designed to assess the association with the incidence of UI more accurately. The potential benefits of TyG-BMI in relation to UI lie in its simple calculation method, which requires only routine physical examination data. Additionally, as an important assessment tool for IR, TyG-BMI can indirectly reflect the risk of UI. Our study presents multiple advantages. In order to increase the study's representativeness, we considered sample weights when analyzing NHANES data. Furthermore, adjustments were made for several confounding variables to bolster the data's reliability. Given the cross-sectional nature of our study, establishing causation was beyond our reach. Secondly, Self-reporting of UI is indeed the main means of obtaining disease diagnosis information, so the application and promotion of TyG-BMI does have limitations to a certain extent. Furthermore, despite accounting for numerous factors, the potential influence of additional confounders could only partially be excluded. Additionally, due to limitations inherent in the NHANES database's UI questionnaire design, symptoms and medical information for all three UI types were self-reported through interview responses, potentially reducing the count of verifiable UI cases. Given the reliance on NHANES database data, further high-quality cohort studies are necessary to support this connection.
Conclusions
The results of this investigation showed a correlation between elevated TyG-BMI levels and an increased risk of UI, a relationship that persisted even after adjusting for multiple covariates. We speculated that TyG-BMI could be used as a valuable clinical indicator. To confirm this relationship, additional prospective studies will be essential in the coming years.
Acknowledgements
We are grateful to the National Health and Nutrition Examination Survey for the data provided and to all participants for their selfless dedication.
Abbreviations
- NHANES
National Health and Nutrition Examination Survey
- TyG-BMI
Triglyceride glucose body mass index
- UI
Urinary incontinence
- SUI
Stress Urinary incontinence
- UUI
Urge urinary incontinence
- MUI
Mixed urinary incontinence
- OR
Odds ratio
- CI
Confidential interval
- IR
Insulin resistance
- METS
Metabolic syndrome
- PIR
Income-to-Poverty Ratio
- OAB
Overactive bladder
Authors’ contributions
JHL and RJX designed the research. JHL, DW, and MMS collected, analyzed the data and drafted the manuscript. HT, WBG, and JKY revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported in part by grants from the Natural Science Foundation of Guangdong Province (2020A1515010541).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The portions of this study involving human participants, human materials, or human data were conducted in accordance with the Declaration of Helsinki and were approved by the NCHS Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
JianKun Yang, Email: 592053507@qq.com.
WenBin Guo, Email: gwb200350514@163.com.
References
- 1.Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, Monga A, Petri E, Rizk DE, Sand PK, Schaer GN. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21:5–26. [DOI] [PubMed] [Google Scholar]
- 2.Diokno AC, Newman DK, Low LK, Griebling TL, Maddens ME, Goode PS, Raghunathan TE, Subak LL, Sampselle CM, Boura JA, et al. Effect of group-administered behavioral treatment on urinary incontinence in older women: a randomized clinical trial. JAMA Intern Med. 2018;178:1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durigon Keller K, La Rosa VL, Cerentini TM, Machado de Souza C, Langlois Costa F, Viana da Rosa P, da Silva Klahr P, de Almeida Pereira E, Telles da Rosa LH. Quality of life and urinary incontinence symptoms in women undergoing bariatric surgery: a combined case-cohort study. Female Pelvic Med Reconstr Surg. 2020;26:e62–7. [DOI] [PubMed] [Google Scholar]
- 4.Anger JT, Saigal CS, Stothers L, Thom DH, Rodríguez LV, Litwin MS. The prevalence of urinary incontinence among community dwelling men: results from the national health and nutrition examination survey. J Urol. 2006;176:2103–8 discussion 2108. [DOI] [PubMed] [Google Scholar]
- 5.Fwu CW, Schulman IH, Lawrence JM, Kimmel PL, Eggers P, Norton J, Chan K, Mendley SR, Barthold JS. Association of obesity, metabolic syndrome, and diabetes with urinary incontinence and chronic kidney disease: analysis of the national health and nutrition examination survey, 2003–2020. J Urol. 2024;211:124–33. [DOI] [PubMed] [Google Scholar]
- 6.Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45–62. [DOI] [PubMed] [Google Scholar]
- 7.Alwahsh SM, Dwyer BJ, Forbes S, Thiel DH, Lewis PJ, Ramadori G. Insulin production and resistance in different models of diet-induced obesity and metabolic syndrome. Int J Mol Sci. 2017;18:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uzun H, Yilmaz A, Kemik A, Zorba OU, Kalkan M. Association of insulin resistance with overactive bladder in female patients. Int Neurourol J. 2012;16:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med. 2002;19:527–34. [DOI] [PubMed] [Google Scholar]
- 10.Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, Ko YL. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS ONE. 2016;11: e0149731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huo RR, Zhai L, Liao Q, You XM. Changes in the triglyceride glucose-body mass index estimate the risk of stroke in middle-aged and older Chinese adults: a nationwide prospective cohort study. Cardiovasc Diabetol. 2023;22:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Li K, Wen J, Yang C, Li Y, Xu G, Ma Y. Association of the triglyceride glucose-body mass index with the extent of coronary artery disease in patients with acute coronary syndromes. Cardiovasc Diabetol. 2024;23:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang M, Chang M, Shen P, Wei W, Li H, Shen G. Application value of triglyceride-glucose index and triglyceride-glucose body mass index in evaluating the degree of hepatic steatosis in non-alcoholic fatty liver disease. Lipids Health Dis. 2023;22:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhan C, Peng Y, Ye H, Diao X, Yi C, Guo Q, Chen W, Yang X. Triglyceride glucose-body mass index and cardiovascular mortality in patients undergoing peritoneal dialysis: a retrospective cohort study. Lipids Health Dis. 2023;22:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang R, Chen C, Xu G, Jin Z. Association of triglyceride glucose-body mass index and hemoglobin glycation index with heart failure prevalence in hypertensive populations: a study across different glucose metabolism status. Lipids Health Dis. 2024;23:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang H, Kuang M, Qiu J, He S, Yu C, Sheng G, Zou Y. Relative importance of triglyceride glucose index combined with body mass index in predicting recovery from prediabetic state to normal fasting glucose: a cohort analysis based on a Chinese physical examination population. Lipids Health Dis. 2024;23:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang C, Yang R, Kuang M, Yu M, Zhong M, Zou Y. Triglyceride glucose-body mass index in identifying high-risk groups of pre-diabetes. Lipids Health Dis. 2021;20:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y, Chao J, Bao M, Zhang N, Wang L. Construction of predictive model for osteoporosis related factors among postmenopausal women on the basis of logistic regression and Bayesian network. Prev Med Rep. 2023;35: 102378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- 20.Abdalla SM, Yu S, Galea S. Trends in cardiovascular disease prevalence by income level in the United States. JAMA Netw Open. 2020;3: e2018150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersson KE. LUTS treatment: future treatment options. Neurourol Urodyn. 2007;26:934–47. [DOI] [PubMed] [Google Scholar]
- 22.Chung SD, Liu HT, Lin H, Kuo HC. Elevation of serum c-reactive protein in patients with OAB and IC/BPS implies chronic inflammation in the urinary bladder. Neurourol Urodyn. 2011;30:417–20. [DOI] [PubMed] [Google Scholar]
- 23.Nazzal Z, Khatib B, Al-Quqa B, Abu-Taha L, Jaradat A. The prevalence and risk factors of urinary incontinence among women with type 2 diabetes in the north West Bank: a cross-sectional study. Lancet. 2021;398(Suppl 1):S42. [DOI] [PubMed] [Google Scholar]
- 24.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–23. [DOI] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 26.Lim J, Kim J, Koo SH, Kwon GC. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: an analysis of the 2007–2010 Korean national health and nutrition examination survey. PLoS ONE. 2019;14: e0212963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du Z, Xing L, Lin M, Sun Y. Estimate of prevalent ischemic stroke from triglyceride glucose-body mass index in the general population. BMC Cardiovasc Disord. 2020;20:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu S, Wu Y, Fang L, Zhao J, Cai Y, Xia W. A negative association between triglyceride glucose-body mass index and testosterone in adult males: a cross-sectional study. Front Endocrinol (Lausanne). 2023;14:1187212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang F, He T, Wang G, Han T, Yao Z. Association of triglyceride glucose-body mass index with non-small cell lung cancer risk: a case-control study on Chinese adults. Front Nutr. 2022;9: 1004179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen Z, Li Y, Xu L, Yue C, Wang Q, Chen R, Ding N, Qu X, Ou Y, Yang Y, et al. Triglyceride glucose-body mass index is a reliable indicator of bone mineral density and risk of osteoporotic fracture in middle-aged and elderly nondiabetic Chinese individuals. J Clin Med. 2022,;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Y, Zhao Y, Zhang J, Li C. The association between triglyceride glucose-body mass index and all-cause mortality in critically ill patients with atrial fibrillation: a retrospective study from MIMIC-IV database. Cardiovasc Diabetol. 2024;23:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao Y, Hu H, Cao C, Han Y, Wu C. Elevated triglyceride-glucose-body mass index associated with lower probability of future regression to normoglycemia in Chinese adults with prediabetes: a 5-year cohort study. Front Endocrinol (Lausanne). 2024;15:1278239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng Z, Tseng Y, White MF. Insulin signaling meets mitochondria in metabolism. Trends Endocrinol Metab. 2010;21:589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stiles BL. PI-3-K and AKT: onto the mitochondria. Adv Drug Deliv Rev. 2009;61:1276–82. [DOI] [PubMed] [Google Scholar]
- 35.Kim B, Feldman EL. Insulin resistance in the nervous system. Trends Endocrinol Metab. 2012;23:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nocchi L, Daly DM, Chapple C, Grundy D. Induction of oxidative stress causes functional alterations in mouse urothelium via a TRPM8-mediated mechanism: implications for aging. Aging Cell. 2014;13:540–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blaha I, López-Oliva ME, Martínez MP, Recio P, Agis-Torres Á, Martínez AC, Benedito S, García-Sacristán A, Prieto D, Fernandes VS, Hernández M. Bladder dysfunction in an obese zucker rat: the role of TRPA1 channels, oxidative stress, and hydrogen sulfide. Oxid Med Cell Longev. 2019;2019:5641645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaribeygi H, Farrokhi FR, Butler AE, Sahebkar A. Insulin resistance: review of the underlying molecular mechanisms. J Cell Physiol. 2019;234:8152–61. [DOI] [PubMed] [Google Scholar]
- 39.Montgomery MK, Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect. 2015;4:R1-r15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan MA, Khan ZA, Shoeb F, Fatima G, Khan RH, Khan MM. Role of de novo lipogenesis in inflammation and insulin resistance in Alzheimer’s disease. Int J Biol Macromol. 2023;242:124859. [DOI] [PubMed] [Google Scholar]
- 41.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–80. [DOI] [PubMed] [Google Scholar]
- 42.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. [DOI] [PubMed] [Google Scholar]
- 43.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin A, Wang S, Li J, Wang M, Lin J, Li H, Meng X, Wang Y, Pan Y. Mediation of systemic inflammation on insulin resistance and prognosis of nondiabetic patients with ischemic stroke. Stroke. 2023;54:759–69. [DOI] [PubMed] [Google Scholar]
- 45.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bai B, Yang Y, Wang Q, Li M, Tian C, Liu Y, Aung LHH, Li PF, Yu T, Chu XM. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020;11:776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Püschel GP, Klauder J, Henkel J. Macrophages, low-grade inflammation, insulin resistance and hyperinsulinemia: a mutual ambiguous relationship in the development of metabolic diseases. J Clin Med. 2022;11:4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shinohara M, Sumino Y, Sato F, Kiyono T, Hashimoto N, Mimata H. Tumor necrosis factor-alpha inhibits differentiation of myogenic cells in human urethral rhabdosphincter. Int J Urol. 2017;24:461–7. [DOI] [PubMed] [Google Scholar]
- 49.Lin JY, Cheng J, Du YQ, Pan W, Zhang Z, Wang J, An J, Yang F, Xu YF, Lin H, et al. In vitro expansion of pancreatic islet clusters facilitated by hormones and chemicals. Cell Discov. 2020;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Urakaze M, Kobashi C, Satou Y, Shigeta K, Toshima M, Takagi M, Takahashi J, Nishida H. The beneficial effects of astaxanthin on glucose metabolism and modified low-density lipoprotein in healthy volunteers and subjects with prediabetes. Nutrients. 2021;13:4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lifford KL, Curhan GC, Hu FB, Barbieri RL, Grodstein F. Type 2 diabetes mellitus and risk of developing urinary incontinence. J Am Geriatr Soc. 2005;53:1851–7. [DOI] [PubMed] [Google Scholar]
- 52.Jackson SL, Scholes D, Boyko EJ, Abraham L, Fihn SD. Urinary incontinence and diabetes in postmenopausal women. Diabetes Care. 2005;28:1730–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.