Abstract
Background
Urinary tract infections (UTIs) are the second most common infection, affecting 150 million people each year worldwide. Enterobacteriaceae species expressing extended-spectrum β-lactamases (ESBLs) are on the rise across the globe and are becoming a severe problem in the therapeutic management of clinical cases of urinary tract infection. Knowledge of the prevalence and antibiogram profile of such isolates is essential to develop an appropriate treatment methodology. This study aimed to investigate the prevalence of Enterobacteriaceae isolates exhibiting ESBL and their selective oral antibiogram profile at the district general hospital, Polonnaruwa.
Results
A total of 4386 urine specimens received to the Microbiology Laboratory during the study period. Among them, 1081 (24.6%) showed positive results for urine culture while 200/1081 specimens showed ESBL isolates. Out of the selected 200 specimen’s majority (67.5%) of samples received from the In-Patient Department. There were 200 patients and reported that 115 (57.5%) were females and 85 (42.5%) were males. The majority (51%) of the patients belong to the age group of 55–74 years. Among the ESBLs positive specimens, the majority 74.5% (n = 149) identified organisms were E. coli followed by Klebsiella spp.17.5% (n = 35), Enterobacteriaceae 7% (n = 14) and only1% (n = 2) isolate of Proteus spp. Mecillinam (87.92%) and Nitrofurantoin (83.2%) showed higher effectiveness against E. coli. Nitrofurantoin showed the highest effectiveness against Klebsiella spp. (40%), other Enterobacteriaceae spp. (100%). Proteus spp. showed 100% effectiveness and resistance respectively against Ciprofloxacin, Cotrimoxazole and Nitrofurantoin.
Conclusion
The most predominant ESBLs producing uro-pathogen was the E. coli in the study setting and E. coli had higher sensitivity rate against Mecillinam. Among currently used oral antibiotics Nitrofurantoin was the best choice for UTIs caused by ESBL producers.
Keywords: Escherichia coli, Proteus spp., Klebsiella spp., Enterobacteriaceae, Extended spectrum β-lactamase, Urinary tract infection
Background
Urinary Tract Infection (UTI) is the most common bacterial infection prevalent in both females and males. The incidence is more frequent in women than men due to shortness of urethra, easy contamination with fecal flora, and pregnancy [1, 2]. Mostly, neonates, girls, young women, infants, young children, and older men are mainly vulnerable to UTIs [3]. Escherichia coli (E. coli) is the most frequent uropathogen accounting for 70–80% of cases of acute uncomplicated lower UTIs [4]. However, Klebsiella spp, Proteus spp, and Pseudomonas spp. are also reported as uropathogen which cause uncomplicated UTIs [5, 6].
Extended-spectrum beta-lactamases (ESBLs) are plasmid-mediated enzymes [7, 8] produced by the Enterobacteriaceae family, Klebsiella pneumoniae, and E. coli. Other Enterobacteriaceae, non-fermenting bacteria, Acinetobacter spp., and Pseudomonas spp. also produce ESBLs but the prevalence rate is low [5]. ESBLs are hydrolyzed and inactivated beta-lactam antibiotics, rendering them ineffective in treating infections caused by ESBLs-producing bacteria [9]. It’s responsible for the increasing antibiotic resistance globally to UTIs. Major risk factors of UTIs produced by ESBLs positive uropathogens are patients with numerous comorbidities, diabetes, long stay in nursing homes, frequent use of antibiotics, recurrent UTIs, older age, sex (male), and intravenous treatments or urinary tract abnormalities [9].
According to a WHO report published in 2021, ESBLs-producing Enterobacteriaceae are part of the group posing the highest risk to public health [10]. The increasing isolation of ESBLs-producing bacteria causing UTIs worldwide due to the failure of empirical therapy which may result in serious clinical complications such as sepsis, renal scarring, and prolonged hospitalization, compared to infection with non-ESBL strains [11].
Hence, ESBL producers have become a major multidrug-resistant pathogen, and several significant changes in ESBL-producing isolates have been witnessed worldwide in the last two decades [12]. Therefore ESBL production is needed to guide appropriate antimicrobial therapy [13] and minimize the risk of developing resistance to certain drugs soon [14]. Identification of drug-resistant microorganisms and available oral treatment options are essential for choosing an appropriate antibiotic that would enable to avoid the waste of time, and high medical costs and reduce the potential complications or rate of mortality. Further, that may reduce the development of multidrug-resistant bacteria [15].
Methodology
Patients suggestive of Urinary Tract Infection (UTI) including the clinical symptoms of dysuria, haematuria, pain in the lower abdominal area, fever, and chills, were considered in the study. Among them patients with significant bacteriuria with extended spectrum beta lactamase producers were included in the study [1, 2]. Patient recruitment process was done with the help of Consultant Microbiologist.
A total of 200 patients with positive urine cultures for ESBL-producing bacteria and suggestive of having UTI were enrolled in the study. Only ESBL producing organisms were included in this study from those UTI causing organisms, the recruitment of patients was done by the Consultant Microbiologist at District General Hospital Polonnaruwa, depending on the clinical symptoms and medical history of the patients.
Urine specimens were processed according to the standard guidelines [7]. On the first day of the samples receiving macroscopic appearance, culture on CLED (Cystine Lactose Electrolyte Deficient Agar) media and wet film examination were conducted. On the following day, culture plates were observed and gram stained and biochemical tests were conducted accordingly to identify the bacterial species. The uropathogen were grown on Hi-Crome UTI agar was presumptively identified based on the different contrasted colony colors produced by reactions of genus or species-specific enzymes and chromogenic substrates according to the manufacturer’s instructions. Antibiotic susceptibility testing was done according to the Clinical Laboratory Standard Institute guidelines M100 2021. Thus, ESBL production was identified according to the CLSI disc diffusion method, measuring the zone diameters of each antibiotic. In the Sri Lankan Clinical Laboratories ESBL screening was done by placing the Cefotaxime/Ceftriaxone or Ceftazidime disc and amoxycillin-clavulanic acid/ ticarcillin-clavulanic acid disc with their centres 20 mm apart. Presence of ESBL is indicated by an enhanced clear inhibitory zone resembling a “Keyhole” or an elliptical area between the discs (Fig. 1).
Fig. 1.
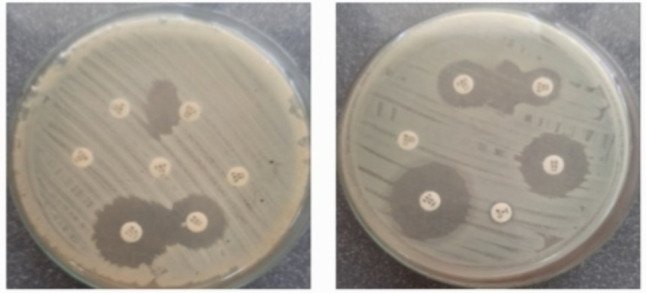
ESBL screening test A- elliptical area B-keyhole appearance. AMC - Amoxycillin-clavulanic acid CTX- Cefotaxime
Further, ESBL screening positive organisms confirmed by placing Cefpodoxime (10 µg), Ceftazidime (30 µg), Aztreonam (30 µg), Cefotaxime (30 µg), or Ceftriaxone (30 µg) antibiotics discs. Noting specific zone diameters that indicate a high level of suspicion for ESBL production. (Cefpodoxime 10 µg ≤ 17 mm, Ceftazidime 30 µg ≤ 22 mm, Cefotaxime 30 µg ≤.
27 mm or Ceftriaxone 30 µg ≤ 25 mm or aztreonam 30 µg ≤ 27 mm) (Fig. 2.).
Fig. 2.
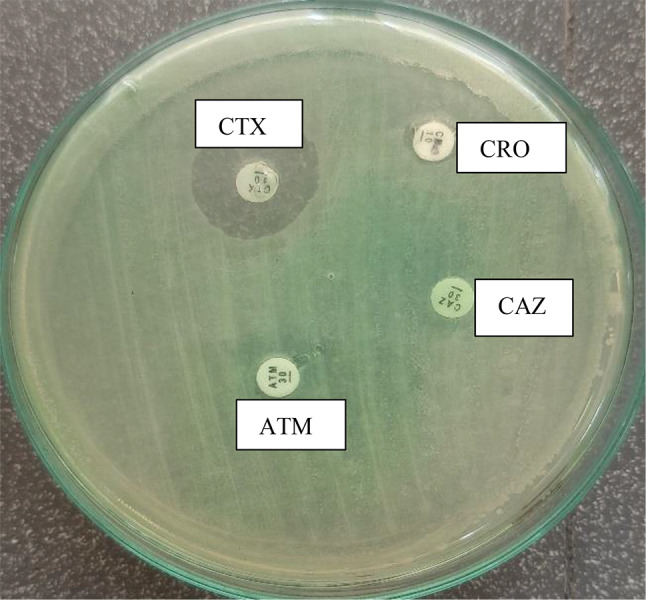
ESBL confirmation test. CRO- Ceftriaxone CAZ- Ceftazidime ATM- Aztreonam
For the identified ESBL producing bacteria, the antimicrobial susceptibility testing (AST) was performed using Ciprofloxacin 10 µg, Nitrofurantoin 300 µg, Mecillinam 10 µg, Cotrimoxazole and interpretation was done according to the CLSI guidelines, 2021. Statistical software SPSS version 21.0 was used for statistical analysis and calculations of the data set. The p values less than 0.05 were considered as statistical significance.
Results
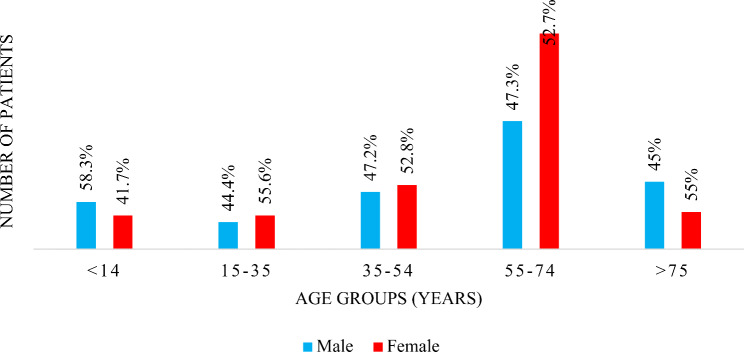
A total of 4386 urine specimens were received at the Microbiology Laboratory during the sample collection period of 1st April to 31st July 2023. Out of the 4386 specimens, 1081(24.6%) specimens showed culture-positive results with more than 105 CFU/ml. Among these culture-positive urine specimens, 200 (18.5%) specimens showed ESBLs producing uropathogens. the majority 135 (67.5%) specimens received from the inward departments (IWD) while 65 (32.5%) specimens received from the Out-Patient Departments (OPD). There were 200 patients and reported 115 (57.5%) were females and 85 (42.5%) were males. Frequency of UTI infection according to gender and age are shown in Fig. 1. The highest number of patients reported ESBL producers in between the age category of 55–74 years while the majority were females (Fig. 3).
Fig. 3.
Frequency of UTI infection according to gender and age
Among the 200 ESBLs positive specimens, majority 74.5% (149/200) were E. coli followed by Klebsiella spp.17.5% (35/200), Enterobacteriaceae 7% (14/200) and only1% (2/200) isolate of Proteus spp. Number of cases according to the organism type and the age category (Child < 14 years and Adults > 14 years) [37] is tabulated in Table 1.
Table 1.
Frequency of the isolated uropathogens according to the age
| Uro-pathogen | UTI patient with ESBLS producer | Statistic | ||
|---|---|---|---|---|
| Total | Adult | Children | ||
| E. coli | 149 | 127 (85.2%) | 22 (14.8%) |
χ2 = 2.17 p = 0.537 |
| Klebsiella spp. | 35 | 33 (94.3%) | 2 (5.7%) | |
| Proteus spp. | 2 | 2 (100%) | 0 (0.0%) | |
| Enterobacteriaceae | 14 | 14 (100%) | 0 (0.0%) | |
| Total | 200 | 178 | 22 | |
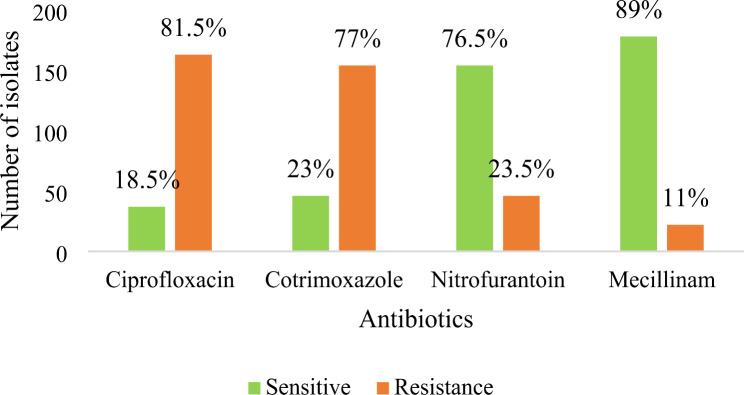
ESBL producers showed highest effectiveness against Mecillinam (89%) and least effectiveness against Ciprofloxacin (Fig. 4.).
Fig. 4.
Distribution of drug sensitivity and resistance of antibiotics among ESBL producers
Antibiotic susceptibility is shown in Table 2. The E. coli showed the highest sensitivity toward Nitrofurantoin and Mecillinam (83.2%) and (87.9%) respectively. Klebsiella spp. showed resistance to all three antibiotics including Ciprofloxacin (88.5%), Cotrimoxazole (88.5%), and Nitrofurantoin (60.0%). Further, Proteus spp. showed (100%) sensitivity to both Ciprofloxacin and Cotrimoxazole and 100% resistance to Nitrofurantoin. Other Gram-Negative isolates showed 100%sensitivity to Nitrofurantoin and 100% resistance to Ciprofloxacin and Cotrimoxazole.
Table 2.
Antibiotic susceptibility according to the organism type
| Antibiotics | Susceptibilit-y | Organisms | |||
| E. coli | Klebsiella spp. | Proteus spp. | Other coliforms | ||
| Ciprofloxa–cin | Sensitive | 31 (20.8%) | 4 (11.4%) | 2 (100%) | 0 |
| Resistance | 118 (79.1%) | 31 (88.6%) | 0 | 14 (100%) | |
| Nitrofurant-oin | Sensitive | 124 (83.2%0 | 14 (40%) | 0 | 14 (100%) |
| Resistance | 25 (16.8%) | 21 (60%) | 2(100%) | 0 | |
| Cotrimoxa–zole | Sensitive | 40 (26.8%) | 4 (11.4%) | 2 (100%) | 0 |
| Resistance | 109 (73.2%) | 31 (88.6%) | 0 | 14 (100%) | |
| Mecillinam | Sensitive | 131 (87.9%) | - | - | - |
| Resistance | 18 (12.1%) | - | - | - | |
Discussion
The present study revealed that most of the UTI patients reported from inward departments of the hospital while only a limited number of cases were reported from outpatient departments, suggestive of hospital-acquired infection of ESBLs producing Enterobacteriaceae. The urine-positive culture-positive rate was reported as 24.6%. Similar results have been reported by in Nepal of 20.41% positive urine cultures out of 2029 non-repetitive mid-stream urine specimens [16]. Yadav and Prakash, in 2017 reported a 62.9% rate of urine culture positivity in Nepal [1]. In contrast, a very low percentage of urine culture positivity was reported at 6.52% in Iran [17]. These differences may be due to geographical area, time, and the diagnostic technique used [18].
Increasing coliform production of ESBLs is a global healthcare concern because of increasing antibiotic resistance and restricted treatment options [19–21]. In the present study, ESBL production was found in 18.5% (200/1081) in Polonnaruwa District. Another study done in different districts of Sri Lanka reported 28% and 33% in Colombo (2012), and 40% in Galle (2016) [22, 23]. Comparatively in Sri Lanka, the rate of ESBLs producing isolation has changed with time and location.
In 2019 the Centre for Disease Control and Prevention (CDC) reported that the global prevalence rate of ESBL production by coliforms has increased by 50% between 2012 and 2017 in the United States [24]. A similar study for Monitoring Antimicrobial Resistance Trends (SMART) observed the prevalence of urinary tract infections caused by ESBLs producing E. coli to increase from 7.8 to 18.3% between 2010 and 2014 in the US [25]. The ESBL-producing E.coli isolation rates increased at a faster rate in healthcare-associated settings than in the community between 2014 and 2020 in the USA [26].
Females are more prone to have UTI infections than males depending on several factors such as the anatomical structure of the urethra, hormonal imbalances, and behavioral changes. The present study also reported higher incidences of UTI in females compared to males [27]. The present study reported that many of the UTI patients belong to the age category of 55–74 years. Many studies have reported that immune-compromised patients, elderly patients, diabetes mellitus patients, and those who are taking chemotherapy and prolonged antibiotic treatments are more prone to develop UTIs than the other populations [28]. Similarly, In 2021 Iran reseacher reported that the age groups of 60–75 (20.11%) and 45–60 (18%) years were frequently infected with uropathogens. The least number of patients were found in the age group 15–34 years (9%) and children (12%) [17].
The E. coli found to be the most predominant ESBLs producing uropathogens in this study. These results are in line with the previous studies conducted in India 2019,Nepal 2013 and in 2022 China who reported E. coli isolation rates as 90% ,83.9% and 73.3% respectively [7, 29, 30]. The members of the Enterobacteriaceae family, E. coli and Klebsiella spp. are identified as common causative agents of UTIs [31–33]. As they possess several factors including adhesion, pili, fimbriae, and P1 blood group genotype receptor, which contribute to the attachment of bacteria to the urothelium [34].
The development of novel antimicrobials is not keeping up with developing resistance, from this time it is necessary to re-evaluate and revive overlooked and older antimicrobials. Fosfomycin, Colistin, Rifampicin, and Polymyxin B are some of the older revived antimicrobials that are used effectively these days for the treatment of superbugs. Mecillinam is an Amido penicillin with selective and good activity against Gram-negative isolates. Many European and Scandinavian countries have recently included Mecillinam in their empirical treatment guidelines, especially for the treatment of community-based urinary tract Infections (UTIs). Many studies from Belgium, the United Kingdom, France, and Norway have reported good in vitro as well as In vivo activity of Mecillinam against extended-spectrum beta-lactamase-producing Gram-negative isolates. The important prospective benefits of Mecillinam are that it is available as an oral prodrug, it has a suitable twice-daily dosage regimen, it replaces carbapenems and its use is less associated with a high risk of Clostridium difficile associated diarrhea [6, 35].
In ourstudy, Mecillinam identified as the most effective antibiotic against E. coli. Similarly, Raja et al., 2019 [30]. and Kresken et al., 2022 [36] reported Mecillinam sensitivity toward E. coli was 96% and 86.3% respectively.
Conclusion
In the present study, E. coli was the most predominant ESBL-producing uropathogen with the highest sensitivity towards Mecillinam. Among currently used oral antibiotics Nitrofurantoin is the best choice for UTIs caused by ESBL producers.
Acknowledgements
Acknowledge the academic and non-academic staff members attached to the Department of Medical Laboratory Sciences, Faculty of Health Sciences, The Open University of Sri Lanka Nawala, Nugegoda, Sri Lanka, and Teaching Hospital, Polonnaruwa, Sri Lanka.
Abbreviations
- E. coli
Escherichia coli
- UTI
Urinary Tract Infection
- ESBL
Extended Spectrum of Beta-Lactamase
- CDC
Disease Control and Prevention
- IWD
Inward Department OPD: Out-Patients Department
Author contributions
G.G.Y.H. Weerasinghe, N.S. Weerakkody, and U. Priyadarshana served as supervisors for the research project. A.M.W.G.K. Aththanayaka and S.H.G.G. Samarasinghe developed the proposal and collected the data. All authors contributed to data analysis, statistical analysis, manuscript writing and manuscript reviewing.
Funding
There was no funding bodies associated with this research.
Data availability
All information on patient’s clinical and demographic data are not publicly available due to ethical policies. Data base was password protected and access were given only to the investigators of the project. Data are available upon request from the authors. kpasun93@gmail.com.
Declarations
Ethical approval and consent to participate
The study was approved by the Ethical Review Committee of the Teaching Hospital, Polonnaruwa (DGHP/ERC/2023/01). A letter of authorization was obtained from Teaching hospital, Polonnaruwa before data collection. All participants involved in the study have voluntarily agreed to participate and understand the nature, purpose, and potential risks of the research.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yadav K, Prakash S. Screening of ESBL Producing Multidrug Resistant E. Coli from urinary tract infection suspected cases in Southern Terai of Nepal. J Infect Dis Diagnosis. 2017;02(02). 10.4172/2576-389x.1000116.
- 2.Tula A, Mikru A, Alemayehu T, Dobo B. Bacterial Profile and Antibiotic susceptibility pattern of urinary tract infection among pregnant women attending Antenatal Care at a Tertiary Care Hospital in Southern Ethiopia. Can J Infect Dis Med Microbiol. 2020;2020. 10.1155/2020/5321276. [DOI] [PMC free article] [PubMed]
- 3.Yadav K, Prakash S, Serayi R, Shilpkar T, Shrestha S. Antimicrobial susceptibility test of pathogens isolated from urinary tract infection suspected cases. Janaki Med Coll J Med Sci. 2014;2(1):28–34. 10.3126/jmcjms.v2i1.11393. [Google Scholar]
- 4.Care P. Resistance to Mecillinam and Nine other antibiotics for oral use in Escherichia coli isolated from urine specimens of, 2022. [DOI] [PMC free article] [PubMed]
- 5.Priyadharshana U, Piyasiri LB, Wijesinghe C. Prevalence, antibiotic sensitivity pattern and genetic analysis of extended-spectrum beta-lactamase producing Escherichia coli and Klebsiella spp among patients with community acquired urinary tract infection in Galle district, Sri Lanka. Ceylon Med J. Dec. 2019;64(4):140. 10.4038/cmj.v64i4.8990. [DOI] [PubMed]
- 6.FME, Wagenlehner U, Hoyme M, Kaase R, Fünfstück KG, Naber, Schmiemann G. Unkomplizierte Harnwegsinfektionen. Dtsch Arztebl. 2011;108(24):415–23. 10.3238/arztebl.2011.0415. [DOI] [PMC free article] [PubMed]
- 7.Liu H, Qiu S, Chen M, Lyu J, Yu G, Xue L. A clinical prediction tool for extended-spectrum β-lactamase-producing Enterobacteriaceae urinary tract infection. BMC Infect Dis. 2022;22(1):1–13. 10.1186/s12879-022-07040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwaber MJ, Navon-Venezia S, Schwartz D, Carmeli Y. High levels of antimicrobial coresistance among extended-spectrum-β- lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2005;49(5):2137–9. 10.1128/AAC.49.5.2137-2139.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aboumarzouk OM. Extended spectrum beta – lactamase urinary tract infections, 6, 2, pp. 2012–3, 2014. [PMC free article] [PubMed]
- 10.Naushad VA et al. Epidemiology of urinary tract infection in adults caused by extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae – a case–control study from Qatar, IJID Reg., vol. 3, no. May, pp. 278–286, 2022, 10.1016/j.ijregi.2022.05.001 [DOI] [PMC free article] [PubMed]
- 11.Mandracchia VJ, Hayes DW, Yoho RM, Hayes MF. Diagnosis, Differential and Treatment options. Nat Rev Microbiol. 2016;13:34. 10.1038/nrmicro3432.Urinary. [Google Scholar]
- 12.Doi Y, Iovleva A, Bonomo RA. The ecology of extended-spectrum b-lactamases (ESBLs) in the developed world. J Travel Med. 2017;24:44–51. 10.1093/jtm/taw102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aggarwal A, et al. Clinical & immunological erythematosus patients characteristics in systemic lupus Maryam. J Dent Educ. 2012;76(11):1532–9. 10.4103/ijmr.IJMR.23144490 [Google Scholar]
- 14.Li B, Webster TJ. Bacteria antibiotic resistance: new challenges and opportunities for implant-associated orthopedic infections. J Orthop Res. 2018;36(1):22–32. 10.1002/jor.23656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryce A, Hay AD, Lane IF, Thornton HV, Wootton M, Costelloe C. Global prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: Systematic review and meta-analysis, BMJ (Online), vol. 352. BMJ Publishing Group, Mar. 15, 2016. 10.1136/bmj.i939 [DOI] [PMC free article] [PubMed]
- 16.Shakya P, Shrestha D, Maharjan E, Sharma VK, Paudyal R. ESBL Production among E. Coli and Klebsiella spp. Causing urinary tract infection: a Hospital based study. Open Microbiol J. 2017;11(1):23–30. 10.2174/1874285801711010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gharavi MJ, Zarei J, Roshani-Asl P, Yazdanyar Z, Sharif M, Rashidi N. Comprehensive study of antimicrobial susceptibility pattern and extended spectrum beta-lactamase (ESBL) prevalence in bacteria isolated from urine samples. Sci Rep. 2021;11(1):1–11. 10.1038/s41598-020-79791-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nanoty VV, Agrawal GN, Tankhiwale SS. Evaluation of Antibiotic Resistance and β- lactamase production in clinical isolates from a Tertiary Care Hospital in Central India. J Clin Basic Res. 2018;2(1):1–5. 10.29252/jcbr.2.1.1. [Google Scholar]
- 19.Falodun OI, Morakinyo YM, Fagade OE. Determination of water quality and detection of Extended Spectrum Beta-Lactamase producing Gram-negative bacteria in selected rivers located in Ibadan, Nigeria. Jordan J Biol Sci. 2018;11(1):107–12. [Google Scholar]
- 20.Habeeb MA, Sarwar Y, Ali A, Salman M, Haque A. Rapid emergence of ESBL producers in E. Coli causing urinary and wound infections in Pakistan. Pakistan J Med Sci. 2013;29(2):540–4. 10.12669/pjms.292.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukherjee M, Basu S, MuKherjee SKM, Majumder M. Multidrug-resistance and extended spectrum beta-lactamase production in uropathogenic E. Coli which were isolated from hospitalized patients in Kolkata, India. J Clin Diagn Res. 2013;7(3):449–53. 10.7860/JCDR/2013/4990.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dissanayake D, Fernando S, Chandrasiri N. The distribution and characteristics of extended-spectrum β-Lactamase producing Escherichia coli and Klebsiella species among urinary isolates in a tertiary care hospital. Sri Lankan J Infect Dis. Aug. 2012;2(2):30. 10.4038/sljid.v2i2.4235.
- 23.Tillekeratne LG, et al. Extended-spectrum β-lactamase-producing Enterobacteriaceae as a common cause of urinary tract infections in Sri Lanka. Infect Chemother. 2016;48(3):160–5. 10.3947/ic.2016.48.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CDC. Antibiotic Resistance Threats in The United States 2019, Cdc, vol. 10, no. 1, 2019, 10.1186/s13756-020-00872-w
- 25.Ontario P. Trends in the rates of extended-Spectrum- b -Lactamase-producing enterobacterales isolated from urine cultures during the COVID-19, 2021. [DOI] [PMC free article] [PubMed]
- 26.Raphael E, Glymour MM, Chambers HF. Trends in prevalence of extended-spectrum beta-lactamase-producing Escherichia coli isolated from patients with community- and healthcare-associated bacteriuria: results from 2014 to 2020 in an urban safety-net healthcare system. Antimicrob Resist Infect Control. 2021;10(1):1–13. 10.1186/s13756-021-00983-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wijesooriya W, Herath YB, Sugandhi RAI. Antibiotic sensitivity pattern for non-beta lactam antibiotics and carbapenems in extended-spectrum beta-lactamase (ESBL) producing uropathogens versus non- ESBL producing uropathogens, no. October, pp. 92–99, 2017.
- 28.Nandagopal B, et al. Frequency of extended spectrum β-lactamase producing urinary isolates of Gram-negative bacilli among patients seen in a multispecialty hospital in Vellore district, India. Indian J Med Microbiol. 2015;33(2):282–5. 10.4103/0255-0857.153563. [DOI] [PubMed] [Google Scholar]
- 29.Chander A, Shrestha CD. Prevalence of extended spectrum beta lactamase producing Escherichia coli and Klebsiella pneumoniae urinary isolates in a tertiary care hospital in Kathmandu, Nepal. BMC Res Notes. 2013;6(1). 10.1186/1756-0500-6-487. [DOI] [PMC free article] [PubMed]
- 30.Raja NS. Oral treatment options for patients with urinary tract infections caused by extended spectrum βeta-lactamase (ESBL) producing Enterobacteriaceae. J Infect Public Health. 2019;12(6):843–6. 10.1016/j.jiph.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Akhtar N, Rahman R, Sultana S, Rahman MR. Antimicrobial Sensitivity Pattern of Bacterial Pathogens Associated with urinary tract infection. Delta Med Coll J. 2017;5(2):57–62. 10.3329/dmcj.v5i2.33342. [Google Scholar]
- 32.HAKKOYMAZ H. The assessment of etiology and risk factors of urinary tract infections in geriatric patients admitted to emergency department. J Surg Med. 2022;6(5):1–1. 10.28982/josam.726628. [Google Scholar]
- 33.Brumfitt W. Efficacy and safety profile of long-term nitrofurantoin in urinary infections: 18 years’ experience, pp. 363–71, 1998. [DOI] [PubMed]
- 34.Shilpakar A, Ansari M, Rai KR, Rai G, Rai SK. Prevalence of multidrug-resistant and extended-spectrum beta-lactamase producing Gram-negative isolates from clinical samples in a tertiary care hospital of Nepal. Trop Med Health. 2021;49(1). 10.1186/s41182-021-00313-3. [DOI] [PMC free article] [PubMed]
- 35.36Kaleem. Reviving the role of mecillinam against extended spectrum beta-lactamase producing enterobacterales. Iran J Microbiol vol. 2022;14(5):662–8. 10.18502/ijm.v14i5.10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kresken M, Pfeifer Y, Wagenlehner F, Werner G, Wohlfarth E. Jun., Resistance to Mecillinam and Nine other antibiotics for oral use in Escherichia coli isolated from urine specimens of primary care patients in Germany, 2019/20, Antibiotics, 11, 6, 2022, 10.3390/antibiotics11060751 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All information on patient’s clinical and demographic data are not publicly available due to ethical policies. Data base was password protected and access were given only to the investigators of the project. Data are available upon request from the authors. kpasun93@gmail.com.