Abstract
Purpose
To evaluate the efficacy of a cloud-based surgical planning platform with regards to refractive target accuracy.
Methods
This was a retrospective chart review of consecutive cases from January 2022 through December 2023. Surgical planning was performed using the SMARTCataract platform, eyes were implanted with Clareon monofocal IOLs, and power calculations were done using the Barrett Universal II formula. Data were collected for the percentage of eyes within ±0.5 D of target refraction, mean absolute error (MAE), and postoperative visual acuity.
Results
A total of 148 eyes were identified that met the inclusion/exclusion criteria. The percentage of eyes within ±0.5 D of the planned target was 94%. The MAE was 0.25 ± 0.17 D. In addition, 57%, 93%, 98%, and 100% of eyes had MAE ≤ 0.25 D, ≤ 0.5 D, ≤ 0.75 D, and ≤ 1.0 D, respectively.
Conclusion
The results of this study suggest high refractive accuracy when using the SMARTCataract planning platform with the Barrett Universal II formula and excellent distance visual acuity.
Keywords: SMARTCataract, biometry, accuracy, intraocular lens
Plain Language Summary
When the natural lens inside the eye develops a cataract (becomes opaque) it can be replaced with a clear artificial intraocular lens (IOL). Good visual outcomes after surgery are heavily reliant on implanting the optimal lens power. A new cloud-based surgical planning tool (SMARTCataract) aims to automatically use patient data, surgeon preferences, favored IOL power calculation formulas, and desired IOL type to guide surgical planning. However, to date there are no data on the refractive outcomes when using the SMARTCataract platform. The purpose of this study was to evaluate the efficacy of the SMARTCataract platform with regards to refractive target accuracy. The results of this study suggest high refractive accuracy when using the SMARTCataract platform with the Barrett Universal II formula and excellent distance visual acuity.
Introduction
Achieving the postoperative target refraction with cataract surgery and intraocular lens (IOL) implantation is critical for good patient outcomes. Technology continues to advance, and surgeons have many tools at their disposal to enable successful outcomes. For instance, assessments with corneal topographers or tomographers,1 optical biometers,2–6 and aberrometers7,8 provide crucial information for surgical planning. These biometry data can then be fed into the latest IOL power calculation formulas to determine the ideal IOL power for the intended refractive outcome.
Despite these innovations, recent estimates are that 73% of eyes are within 0.5 D of target postoperatively.9 One source of inaccuracy may be data entry errors,10 such as transcribing preoperative measurements into surgical planning software or online IOL power calculators. Eyes with previous refractive surgery11,12 or long and short axial lengths13–15 can also be challenging cases to achieve successful refractive outcomes.
SMARTCataract (Alcon Vision, LLC) is a cloud-based platform. It integrates ocular history (such as previous refractive surgery), preoperative measurements, IOL selection with options to vary the model, power, and formula, astigmatism planning with arcuate incisions and toric IOLs, and exports data for intraoperative use.16 With these integrations, patient data can be automatically transferred between devices, which can decrease workflow time,16 and may reduce transcriptions errors. In addition, SMARTCataract aims to automatically use patient data, surgeon preferences, favored IOL power calculation formulas, and desired IOL type to guide surgical planning. While the platform is not meant to replace a surgeon’s judgement, it may augment decision making. However, to date there are no data on the refractive outcomes when using SMARTCataract.
The purpose of this study was to evaluate the efficacy of the SMARTCataract planning platform with the Barrett Universal II formula with regards to refractive target accuracy.
Methods
This was a retrospective chart review from a single site. The study was conducted in private practice, thus an independent institutional review board (Salus IRB) reviewed and approved the study (approval EJ-23-01). The IRB granted a waiver of informed consent as this was a retrospective review of anonymized data. This was a retrospective chart review, thus there was no requirement to register the study in a clinical trials database. All data were maintained with confidentiality. Good clinical practice, International Harmonization (ICH) guidelines, and the tenets of the Declaration of Helsinki were adhered to. Data are not available for sharing.
Consecutive charts were reviewed from January 2022 through December 2023. Data were included from cases that were planned with the SMARTCataract platform using the Barrett Universal II IOL power formula. The Barrett Universal II formula is commonly used and was selected to ensure consistency. Charts were excluded if there was astigmatism requiring correction with a T3 and higher, no preoperative biometry captured, or visually significant comorbidities (age-related macular degeneration, primary open angle glaucoma, irregular astigmatism, prior corneal refractive surgery). All cataract surgeries were performed by one experienced surgeon (EJ) using their preferred method. All eyes were implanted with a non-toric Clareon monofocal IOL (Alcon Vision, LLC).
Data were collected from preoperative and 1 month or greater postoperative visits. The primary outcome measure was the percentage of eyes within 0.50 D of the planned target. Other outcome measures included the percentage of eyes within 0.25 D, 0.75 D, and 1.0 D of the planned target, mean absolute error (MAE), corrected distance visual acuity (CDVA), and uncorrected distance visual acuity (UDVA). The MAE was calculated as the absolute difference between postoperative spherical equivalent and the predicted spherical equivalent using the actual IOL implanted.
The study was intended to be descriptive, thus there is no relevant sample size justification. All cases that met the inclusion/exclusion criteria were included in the final dataset. Statistical analysis was performed using R (version 4.3.1, The R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 148 eyes of 96 patients (62% female) were identified that fit the inclusion and exclusion criteria above. All eyes were targeted for −0.25 D. Preoperative and patient demographics are summarized in Table 1.
Table 1.
Preoperative and Patient Demographics
| Parameter | Mean ± SD (Range) |
|---|---|
| Age (Years) | 68.7 ± 6.9 (52 to 84) |
| Cylinder (D) | −0.75 ± 0.80 (−3.50 to 3.75) |
| MRSE (D) | −0.74 ± 3.07 (−12.75 to 4.25) |
| Axial Length (mm) | 23.9 ± 1.2 (21.5 to 29.0) |
| CDVA (logMAR) | 0.20 ± 0.27 (−0.12 to 2.00) |
Abbreviations: CDVA, corrected distance visual acuity; D, diopters; MRSE, manifest refraction spherical equivalent; SD, standard deviation.
Postoperative refractive outcomes are summarized in Table 2. The percentage of eyes within 0.5 D of target was high, at 94%. Additionally, MAE was low with 93% of eyes within 0.5 D or less. Postoperative MRSE and cylinder were also within 0.5 D of emmetropia in 95% and 88% of eyes respectively.
Table 2.
Postoperative Refractive Outcomes
| Outcome | Mean ± SD (Range) | ≤0.25 D (%) | ≤0.50 D (%) | ≤0.75 D (%) | ≤1.00 D (%) |
|---|---|---|---|---|---|
| SE – Target (D) | −0.17 ± 0.24 (−0.75 to 0.75) | 86 | 94 | 100 | 100 |
| MAE (D) | 0.25 ± 0.17 (0.00 to 0.89) | 57 | 93 | 98 | 100 |
| Cylinder (D) | −0.17 ± 0.30 (−1.00 to 0.00) | 78 | 88 | 96 | 100 |
| MRSE (D) | −0.08 ± 0.24 (−1.00 to 0.50) | 83 | 95 | 98 | 100 |
Abbreviations: MAE, mean absolute error; MRSE, manifest refraction spherical equivalent; SE, spherical equivalent.
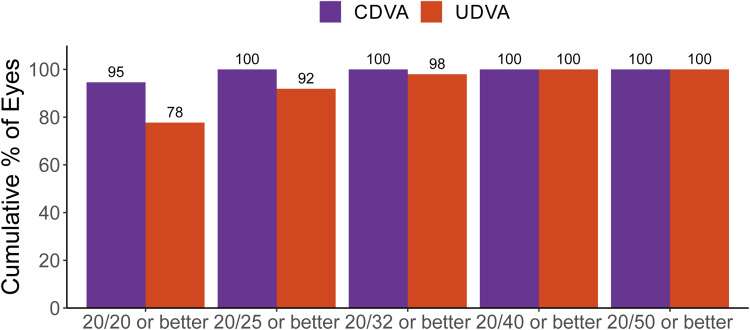
Postoperative visual outcomes are summarized in Figure 1. Mean postoperative UDVA and CDVA were and 0.02 ± 0.07 logMAR and 0.00 ± 0.03 logMAR, respectively.
Figure 1.
Cumulative postoperative monocular visual acuity.
Discussion
Cataract surgery is a refractive procedure and hitting the target refraction is critical for good postoperative outcomes. In a large retrospective study, Lundstrom et al9 observed that 75% of eyes were within 0.5 D of target refraction (0.0 D) using a variety of IOL power calculation formulas. In our study, we investigated the refractive outcomes with a new cloud-based surgical planning software. To the best of our knowledge, this is the first report of clinical outcomes with the SMARTCataract platform.
Using the BUII formula, 94% of eyes were within 0.5 D of target refraction. There was no direct comparator in our study, and we are not aware of other studies that have reported refractive outcomes with the SMARTCataract platform. However, for context, we can compare to other reports of monofocal implants using the BUII formula. Spekreijs et al17 reported that 77% of eyes within 0.5 D target after implantation of the Clareon monofocal IOL using BUII. Roberts et al18 reported that about 80% of eyes implanted with a monofocal using BUII were within 0.5 D of target. Raufi et al19 observed that 84% were within 0.5 D of target after IOL implantation (64% of IOLs were monofocal non-toric) using BUII. In addition, Ma et al20 reported that 81% of eyes implanted with a variety of IOL types were within 0.5 D of target. Given previous reports17–20 of spherical equivalent accuracy using the BUII formula, the results of our study suggests good refractive outcomes using the SMARTCataract platform.
The SMARTCataract platform is not the only cloud-based surgical planning software available. Veracity Surgical (Carl Zeiss Meditec AG) is a web-based surgical planning software. Gujral and Hovanesian21 reported on the refractive outcomes between the Veracity software and using traditional paper of electronic scans, and observed no differences in prediction accuracy (68% of eyes were within 0.5 D for both traditional and online planning groups). Eyetelligence was announced by Bausch + Lomb October 2023, but we are not aware of any studies reporting on the refractive outcomes with this platform. Cassini Connect (Cassini Technologies BV) is another recently launched platform, but we also are not aware of any studies reporting on refractive outcomes. It would appear that the SMARTCataract platform is currently the only online surgical planning software with evidence of excellent refractive and visual outcomes.
A limitation of this study was the single arm nature. A comparator group would enable us to draw conclusions about the effect of using the SMARTCataract platform versus not using it. At best we can conclude that high refractive accuracy and excellent distance visual acuity were achieved when using the SMARTCataract planning platform with the Barrett Universal II formula. Another limitation was that the results are from a single site, which could limit the applicability to other surgeons. Other limitations include the retrospective design and relatively short follow up period.
In conclusion, the results of this study suggest high refractive accuracy when using the SMARTCataract planning platform with the Barrett Universal II formula and excellent distance visual acuity.
Acknowledgment
This paper was presented at the 2024 American Society of Cataract and Refractive Surgery (ASCRS) Annual Meeting as a conference eposter.
Funding Statement
This study was supported with an investigator-initiated study grant (89429475) from Alcon Vision, LLC, Fort Worth, TX, USA.
Disclosure
Dr Eric Jennings reports consulting fees from Alcon, outside the submitted work. The authors report no other conflicts of interest for this work.
References
- 1.Goto S, Maeda N. Corneal topography for intraocular lens selection in refractive cataract surgery. Ophthalmology. 2021;128(11):e142–e152. doi: 10.1016/j.ophtha.2020.11.016 [DOI] [PubMed] [Google Scholar]
- 2.Vogel A, Dick BH, Krummenauer F. Reproducibility of optical biometry using partial coherence interferometry: intraobserver and interobserver reliability. J Cataract Refract Surg. 2001;27(12):1961–1968. doi: 10.1016/S0886-3350(01)01214-7 [DOI] [PubMed] [Google Scholar]
- 3.Hoffer KJ, Shammas HJ, Savini G, Huang J. Multicenter study of optical low-coherence interferometry and partial-coherence interferometry optical biometers with patients from the United States and China. J Cataract Refract Surg. 2016;42(1):62–67. doi: 10.1016/j.jcrs.2015.07.041 [DOI] [PubMed] [Google Scholar]
- 4.Hoffer KJ, Shammas HJ, Savini G. Comparison of 2 laser instruments for measuring axial length. J Cataract Refract Surg. 2010;36(4):644–648. doi: 10.1016/j.jcrs.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 5.Montes-Mico R, Pastor-Pascual F, Ruiz-Mesa R, Tana-Rivero P. Ocular biometry with swept-source optical coherence tomography. J Cataract Refract Surg. 2021;47(6):802–814. doi: 10.1097/j.jcrs.0000000000000551 [DOI] [PubMed] [Google Scholar]
- 6.Yang CM, Lim DH, Kim HJ, Chung TY, Grulkowski I. Comparison of two swept-source optical coherence tomography biometers and a partial coherence interferometer. PLoS One. 2019;14(10):e0223114. doi: 10.1371/journal.pone.0223114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatch KM, Woodcock EC, Talamo JH. Intraocular lens power selection and positioning with and without intraoperative aberrometry. J Refract Surg. 2015;31(4):237–242. doi: 10.3928/1081597X-20150319-03 [DOI] [PubMed] [Google Scholar]
- 8.Ashena Z, Gallagher S, Naveed H, Spalton DJ, Nanavaty MA. Comparison of anterior corneal aberrometry, keratometry and pupil size with scheimpflug tomography and ray tracing aberrometer. Vision. 2022;6(1):6. doi: 10.3390/vision6010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundstrom M, Dickman M, Henry Y, et al. Risk factors for refractive error after cataract surgery: analysis of 282 811 cataract extractions reported to the European registry of quality outcomes for cataract and refractive surgery. J Cataract Refract Surg. 2018;44(4):447–452. doi: 10.1016/j.jcrs.2018.01.031 [DOI] [PubMed] [Google Scholar]
- 10.Hakansson I, Lundstrom M, Stenevi U, Ehinger B. Data reliability and structure in the Swedish national cataract register. Acta Ophthalmol Scand. 2001;79(5):518–523. doi: 10.1034/j.1600-0420.2001.790519.x [DOI] [PubMed] [Google Scholar]
- 11.Chow SSW, Chan TCY, Alk N, Kwok AKH. Outcomes of presbyopia-correcting intraocular lenses after laser in situ keratomileusis. Int Ophthalmol. 2019;39(5):1199–1204. doi: 10.1007/s10792-018-0908-0 [DOI] [PubMed] [Google Scholar]
- 12.McCarthy M, Gavanski GM, Paton KE, Holland SP. Intraocular lens power calculations after myopic laser refractive surgery: a comparison of methods in 173 eyes. Ophthalmology. 2011;118(5):940–944. doi: 10.1016/j.ophtha.2010.08.048 [DOI] [PubMed] [Google Scholar]
- 13.Zaldivar R, Shultz MC, Davidorf JM, Holladay JT. Intraocular lens power calculations in patients with extreme myopia. J Cataract Refract Surg. 2000;26(5):668–674. doi: 10.1016/S0886-3350(00)00367-9 [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Shirayama M, Ma XJ, Kohnen T, Koch DD. Optimizing intraocular lens power calculations in eyes with axial lengths above 25.0 mm. J Cataract Refract Surg. 2011;37(11):2018–2027. doi: 10.1016/j.jcrs.2011.05.042 [DOI] [PubMed] [Google Scholar]
- 15.Hoffer KJ, Savini G. IOL power calculation in short and long eyes. Asia Pac J Ophthalmol. 2017;6:330–331. [DOI] [PubMed] [Google Scholar]
- 16.Zavodni Z, Pan LC, Mok K, Cheng H, O’Boyle D. End-to-end impact of a cloud-based surgical planning system on efficiency in cataract surgery: a time-and-motion study. Clin Ophthalmol. 2023;17:1885–1896. doi: 10.2147/OPTH.S392669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spekreijse LS, Bauer NJC, van den Biggelaar F, et al. Predictive accuracy of an intraoperative aberrometry device for a new monofocal intraocular lens. J Cataract Refract Surg. 2022;48(5):542–548. doi: 10.1097/j.jcrs.0000000000000791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts TV, Hodge C, Sutton G, Lawless M. contributors to the vision eye institute IOLor. comparison of hill-radial basis function, Barrett universal and current third generation formulas for the calculation of intraocular lens power during cataract surgery. Clin Exp Ophthalmol. 2018;46(3):240–246. doi: 10.1111/ceo.13034 [DOI] [PubMed] [Google Scholar]
- 19.Raufi N, James C, Kuo A, Vann R. Intraoperative aberrometry vs modern preoperative formulas in predicting intraocular lens power. J Cataract Refract Surg. 2020;46(6):857–861. doi: 10.1097/j.jcrs.0000000000000173 [DOI] [PubMed] [Google Scholar]
- 20.Ma J, El-Defrawy S, Lloyd J, Rai A. Prediction accuracy of intraoperative aberrometry compared with preoperative biometry formulae for intraocular lens power selection. Can J Ophthalmol. 2023;58(1):2–10. doi: 10.1016/j.jcjo.2021.06.024 [DOI] [PubMed] [Google Scholar]
- 21.Gujral T, Hovanesian J. Cataract surgical planning using online software vs traditional methods: a time/motion and quality of care study. Clin Ophthalmol. 2021;15:3197–3203. doi: 10.2147/OPTH.S318935 [DOI] [PMC free article] [PubMed] [Google Scholar]