Abstract
Migraine is a common neurological illness that causes a great burden on individuals and society. Many migraine patients seek relief through complementary and alternative therapies, with Traditional Chinese medicine (TCM) often being their preferred choice. Acupuncture, Chinese herbal medicine, and massage are important components of TCM, and are commonly used in clinical treatment of migraine. This review aims to consolidate the current knowledge regarding the mechanisms of the three TCM interventions for migraine: acupuncture, herbs, and massage, and how they relieve pain. However, the mechanisms underlying the effectiveness of TCM therapies in treating migraine remain unclear. Therefore, we reviewed the research progress on acupuncture, herbal medicine, and massage as TCM approaches for the treatment of migraine. We conducted a comprehensive search of CNKI, PubMed, Web of Science, and Cochrane databases using keywords such as migraine, acupuncture, needle, herbs, herbal, prescription, decoction, massage, Tuina, and TCM, covering the period from 2000 to 2023. The literature included in the review was selected based on specified exclusion criteria. We discussed the mechanism of TCM therapies on migraine from the perspective of modern medicine, focusing on changes in inflammatory factors, neurotransmitters, and other relevant biomarkers. TCM can relieve migraine by decreasing neuropeptide levels, inhibiting inflammation, modulating neuronal sensitization, changing brain function and structure, changing blood brain barrier permeability, regulating hormone levels, and relieving muscle tension. The purpose of this paper is to provide a basis for improving the clinical strategies of TCM for the treatment of migraine.
Keywords: traditional Chinese medicine, migraine, acupuncture, herbs, massage, mechanism
Introduction
Migraine is a recurrent brain disorder influenced by genetic and environmental factors.1 It is characterized by unilateral or bilateral pulsatile headache and is often accompanied by autonomic nervous system dysfunction, such as nausea, vomiting, photophobia, and phonophobia. The latest International Classification of Headache Disorders (ICHD-3) identifies over 250 different types of headaches, categorized into primary headaches, secondary headaches, neuropathies and facial pains, and other headache types. Migraine is considered a major type of primary headache.2 The prevalence of migraine is approximately 1 billion cases worldwide, with 42 million years lived with disability.3 Epidemiological studies have shown that the prevalence of migraine is 14.9% in the United States4 and 8.4–12.7% in Asia.5
According to the Global Burden of Disease study (2019), migraine is considered the second leading cause of disabilities worldwide.6 Although the mechanism of migraine remains unclear, it is known that genetic predisposition, inflammatory processes, and central sensitization have a great influence on migraine.7 Currently, the pathogenesis of migraine mainly involves activation of trigeminovascular pain pathways,8 which is believed to mediate the occurrence of migraine through the release of neuropeptides, such as calcitonin gene-related peptide (CGRP), substance P, and pituitary adenylate cyclase-activating polypeptide.9,10 CGRP has a strong regulatory effect on vasodilation and secondary and tertiary neurons and is widely expressed in peripheral and central neurons.11 The elevation of CGRP in patients with migraine is associated with a reduction in descending inhibitory mechanisms, which may contribute to migraine susceptibility through the sensitization of multiple central nervous circuits.12 Additionally, these released neuropeptides can also improve the activity of some signaling pathways, increase blood vessel permeability, plasma protein exudation, sterile inflammation, and stimulate pain fiber afferent center, triggering migraine.13–15 Conversely, the activation of trigeminovascular pain pathways8 is closely related to inflammatory factors, including interleukin-1β (IL-1β), cyclooxygenase-2 (COX2), and prostaglandin E2 (PGE2).16,17
Conventional therapies for migraine include acute medication, preventive medication, and nonpharmacological therapies. The current medications include paracetamol, non-steroidal anti-inflammatory drugs, beta-blockers, anticonvulsants, tricyclic antidepressants, and calcium channel blockers.18 These drugs are helpful, but because of their suboptimal efficacy, adverse effects, and contraindications, these drugs do not fully meet the needs of patients with migraine.19 Currently, China has formulated relatively mature guidelines for the diagnosis and treatment of migraine, recommending TCM treatment for migraine.20 TCM is a systematic healthcare system developed from clinical experience based on a scientific model of regulation. TCM therapy mainly includes acupuncture, herbs, and massage and has been implemented worldwide and obtained sufficient evidence.21 In clinical trials, acupuncture is more effective and provides longer-lasting relief for migraines compared to sham acupuncture and standard care.22,23 Compared to a placebo, the extract MIG-99 from Chinese herbal feverfew has also been demonstrated to reduce the frequency and severity of migraine attacks in multicenter randomized controlled trials (RCTs).24 Additionally, the massage group experienced a reduction in migraine attack frequency and an improvement in sleep quality compared to the control group.25
With increasing scholarly interest in TCM treatments for migraine, several high-quality studies have emerged exploring their mechanisms. Chen et al demonstrated that acupuncture influences peripheral and central sensitization by modulating neuronal-sensitization-related mediators, potentially altering the abnormal functional activity and connectivity of the descending pain modulatory system.26 The meta-analysis conducted by Sun et al indicates that true acupuncture effectively increased the pain threshold and reduced hyperalgesia in migraine rats.27 Additionally, Chen et al systematically summarized the potential applications of herbal medicines in ameliorating migraine through multiple therapeutic targets and pathways.28 Previous studies on the mechanism of TCM in treating migraine have mostly focused on a single treatment method, with acupuncture and herbs being the main treatment method, and research on massage-related mechanisms is relatively rare. Based on RCTs and animal experiments of acupuncture, herbs, and massage therapy for migraine, this research comprehensively discusses the mechanisms of the three main treatment methods of TCM for migraine, making the research perspective more diversified and making up for the limitation of a single perspective in previous studies. Meanwhile, the methods of research on the mechanism of the three treatment methods can be mutually referenced providing valuable directions for future research.
In the theory of TCM, migraine belongs to the category “head wind”.29 The onset of migraine is often due to wind pathogen invasion, deficiency of Qi and blood, damage to the meridians, and malnutrition of the brain.30 Treatments such as acupuncture, herbs, or massage can help disperse wind and cold, replenish Qi and blood, unblock meridians, and promote the flow of Qi and blood. Previous studies have also demonstrated that TCM can improve pain symptoms and quality of life in patients with migraine.31–33 However, the specific mechanism of acupuncture, herbs, and massage for migraine has not been unified, involving the inhibition of the generation and release of nociceptive neurotransmitters,25,34,35 deceleration of pain induced by inflammatory factors, such as interleukin and COX2,36,37 and alterations in brain function and structure or the permeability of the blood-brain barrier (BBB).38,39 To our knowledge, the exact mechanisms of different types of TCM therapies for migraine have not been clarified. This article reviews mechanisms of the aforementioned three types of TCM treatments for migraine by screening published literature. The aim is to provide valuable insights for future research and support the advancement of TCM in the treatment of migraine.
Methodology
In this paper, the mechanisms of acupuncture, herbs, and massage in the treatment of migraine are discussed by reviewing published Chinese and English literature from CNKI, PUBMED, Web of Science, and COCHRANE (2000–2023). The keywords for searcher were migraine, acupuncture, needle, herbs, herbal, prescription, decoction, massage, tuina, TCM. The articles that met the following criteria were included: a) the studies were written in Chinese or English; b) the studies focused on patients with migraine or involved basic research on migraine models; c) the patients or migraine models received acupuncture, herbs or massage; and d) the studies evaluated the mechanism of acupuncture, herbs or massage on migraine.
Result
Mechanisms of Acupuncture on Migraine
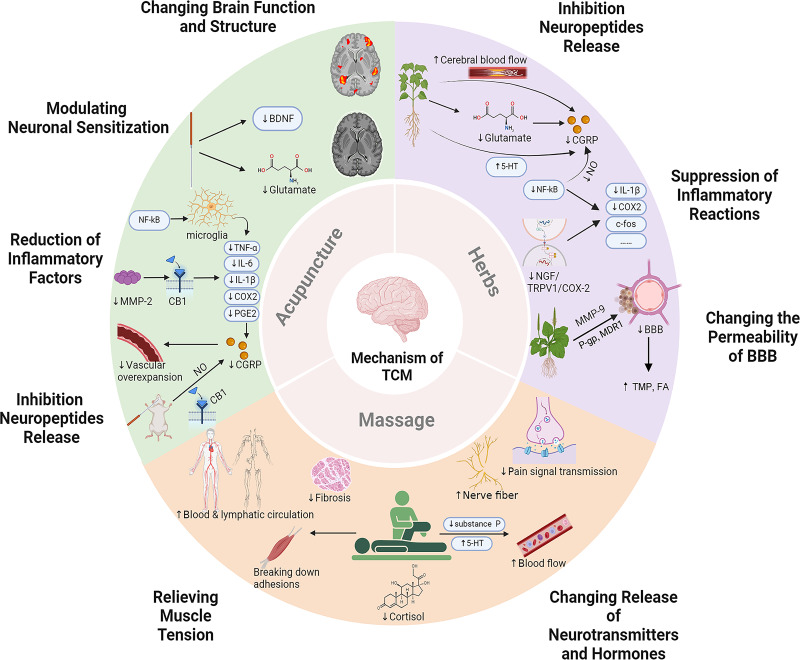
Acupuncture is widely used to manage migraine in China and Western countries.40 Migraine is a good indication of acupuncture, with unique advantages in efficacy, compliance, and safety.41–43 The onset of migraine is related to the production of CGRP and the occurrence of aseptic inflammation caused by pain stimulation. CGRP not only dilates blood vessels but also increases vascular permeability and the secondary inflammatory response. In addition, inflammatory factors can further activate the trigeminovascular system.19,20,31 The mechanisms of acupuncture to improve the pain symptoms and quality of life in patients with migraine involve (Figure 1 and Table 1): 1) inhibiting CGRP neuropeptide release;16,44–46 2) reducing the overexpression of inflammatory factors such as IL-1β, COX2 and PGE2;16,46–48 3) inhibiting central sensitization by modulating neurotrophic factors and neurotransmitters;46,49 4) changing the function and structure of brain.38,50,51
Figure 1.
The mechanisms of acupuncture, herbs, and massage in the treatment of migraine.
(1) Acupuncture inhibits CGRP by reducing NO release, activating CB1 receptors, and lowering levels of inflammatory factors. Acupuncture can reduce BDNF and Glutamate levels, change the interaction between the limbic regions and the autonomic nervous system, and improve the GMV of the cuneus. (2) Herbs can inhibit CGRP by increasing cerebral blood flow, promoting glutamate metabolism, and increasing 5-HT level. Herbs can also inhibit neuroinflammation and inflammatory signaling pathways, thereby reducing the expression of inflammatory factors. Herbs can reduce the permeability of the BBB by regulating MMP-9 and its competitive or non-competitive binding with metabolic enzymes. (3) Massage can inhibit the pain signal transmission of the nerve fiber, reduce substance P, increase 5-HT contents, improve local blood flow, accelerate the metabolism of pain-causing substances, reduce cortisol levels, and relieve muscle tension. Created with BioRender.com.
Abbreviations: BBB, Blood-brain barrier; BDNF, Brain-derived neurotrophic factor; CB1, Type 1 cannabinoid; CGRP, Calcitonin gene-related peptide; COX2, Cyclooxygenase-2; FA, Ferulic acid; IL-1β, Interleukin-1β; MDR1, Multidrug resistance protein 1; MMP-2, Matrix metalloproteinase-2; MMP-9, Matrix metalloproteinase-9; NF-κB, Nuclear factor-kappa B; NGF, Nerve growth factor; NO, Nitric oxide; PGE2, Prostaglandin E2; P-gp, P–glycoprotein; TCM, Traditional Chinese Medicine; TMP, Tetramethylpyrazine; TNF-α, Tumor necrosis factor-α; TRPV1, Transient receptor potential vanilloid 1; 5-HT, 5-hydroxytryptamine.
Table 1.
Summary of Clinical Application of Acupuncture in Treatment of Migraine
| Intervention | Model | Method | Mechanisms | Migraine Types | Model Methods | Intervention | Comparison | Refs. |
|---|---|---|---|---|---|---|---|---|
| Acupuncture | Rats | Animal experiment | 1. Decreased levels of CGRP and inflammatory cytokines 2. Regulated TLR4/NF-κB to inhibit microglial activation 3. Reduced BDNF levels |
NA | Dural cannula implantation and repeated inflammatory decoction injection | EA | Blank control Sham EA |
[36] |
| 1. Decreased PGE2 levels, as well as IL-1β and COX2 protein expression, and neurogenic plasma protein extravasation levels 2. Decreased MMP-2 activity and affected CB1 receptors 3. Reduced the release of CGRP |
NA | Unilateral electrical stimulation of the trigeminal ganglion | EA | Minimal acupuncture | [15] | |||
| 1. Inhibited dural mast cells, macrophages, and serum inflammatory factors 2. Inhibited hyperalgesia by alleviating inflammatory factors |
NA | Dural electrical stimulation | EA | Sham EA | [24] | |||
| 1. Decreased serum PGE2 and CGRP concentrations, IL-1β mRNA and protein, NF-κB p65, NF-κB Ac-p65 and COX2 protein expression | NA | Electrical stimulation of the trigeminal ganglion | EA | EA plus EX527 (a SIRT1 inhibitor) | [38] | |||
| Rats | Systematic review | 1. Alleviated neurogenic inflammation and central nociceptive conduction via the regulation of meningeal vasodilation and inflammatory factors | NA | NTG-induced migraine rats | Manual acupuncture or EA | Blank control | [34] | |
| Human | Before-after study | 1. Reduced the levels of serum NO 2. Reduced the release of CGRP |
Migraine with aura and without aura | NA | Acupuncture | Healthy control | [35] | |
| 1. Decreased levels of glutamate | Migraine without aura | NA | Manual acupuncture | Healthy control | [45] | |||
| 1. Increased FCs between AMYG and MFG, AMYG and INS, IPL and SFG, IPL and MFG, Hipp and SFG, Hipp and MFG, Hipp and INS, and CG and SFG 2. Restored the pain processing function and regulates pain perception |
Migraine without aura | NA | Manual acupuncture | Healthy control | [51] | |||
| 1. Modulated the abnormal function of the frontal gyrus, temporal gyrus, precuneus, and cuneus in patients with migraine 2. Increased the GM volume of left cuneus |
Migraine without aura | NA | Acupuncture | Blank control | [52] | |||
| RCT | 1. Alleviated emotional disorders by modulating the frontal-limbic regions | Menstrual migraine without aura | NA | Manual acupuncture | Sham acupuncture | [53] |
Abbreviations: AMGY, Amygdala; BDNF, Brain-derived neurotrophic factor; CB1, Type 1 cannabinoid; CG, Cingulate gyrus; CGRP, Calcitonin gene-related peptide; COX2, Cyclooxygenase-2; EA, Electroacupuncture; FC, Functional connectivity; GM, Gray matter; Hipp, Hippocampus; IL-1β, Interleukin-1β; INS, Insular gyrus; IPL, Inferior parietal lobule; MFG, Middle frontal gyrus; MMP-2, Matrix metalloproteinase-2; NA, Not applicable; NF-κB, Nuclear factor-kappa B; NO, Nitric oxide; NTG, nitroglycerine; PGE2, Prostaglandin E2; RCT, Randomized controlled trial; SFG, Superior frontal gyrus; SIRT1, Silent mating type information regulation 2 homolog-1; TLR4, Toll-like receptor 4.
Inhibition Neuropeptides Release
Neuroinflammation plays a crucial role in the pathophysiology of migraine and is a potential underlying cause of the condition.53 Neuropeptides involved in trigeminal nerve activation, including CGRP and substance P, are thought to be crucial in migraine development. CGRP is the most abundant neuropeptide in trigeminal sensory nerve stores.9,54 CGRP binds to receptors on endothelial cells and triggers the phosphokinase A-mediated cascade to activate endothelial nitric oxide (NO) synthase and diffuses NO into the smooth muscle of adjacent blood vessels to dilate the blood vessels.52,55 Systematic reviews involving migraine rats have provided evidence that acupuncture can alleviate neuroinflammation by reducing the release of trigeminal nerve-activating neuropeptides.44 A before-and-after clinical trial has shown that acupuncture can decrease the release of NO, which is an intermediate substance in the vasomotor mechanism of CGRP. Consequently, inhibiting CGRP can improve vascular smooth muscle overexpansion and relieve pain symptoms.45 A previous animal experiment has demonstrated that electroacupuncture (EA) is an effective method to inhibit the expression of CGRP. A pre-clinical study shows that EA reduces CGRP levels in the trigeminovascular system ascending pathway, suggesting that EA relieves migraine pain by reducing CGRP levels.46 In addition, Zhang et al conducted a single-blind, randomized controlled animal experiment that revealed EA can inhibit the release of CGRP in the peripheral branches of the trigeminal nerve by activating type 1 cannabinoid (CB1) receptors, thereby preventing the internal flow of pain signals.16
Reduction of Inflammatory Factors
Previous experimental studies in rats have indicated that acupuncture can play a therapeutic role in treating migraine through anti-inflammatory effects.46,47 Several studies involving rats have confirmed that acupuncture can alleviate migraine by decreasing matrix metalloproteinase-2 (MMP-2) activity and affecting the CB1 receptor regulation of inflammatory mediators, including inhibiting PGE2 release, decreasing IL-1β and COX2 protein levels in the trigeminal ganglion, and alleviating neurogenic plasma protein extravasation of migraine.16,47 In addition, the IL-1β/COX2/PGE2 inflammatory pathway may be involved in the release of CGRP during trigeminovascular system activation in migraine,56 and previous studies have shown that EA can inhibit the trigeminal ganglion IL-1β/COX2 inflammatory response signal, reduce the synthesis and release of PGE2, and inhibit the release of CGRP in rat models of migraine.48 An animal study has shown that the activation of microglia leads to the release of pro-inflammatory cytokines, such as IL-1β, tumor necrosis factor-α (TNF-α), and IL-6, in nitroglycerin (NTG)-induced chronic migraine rats model, which are involved in the inflammatory response and contribute to the initiation and maintenance of migraine.57 Zhou et al conducted an animal experiment on EA for the inflammatory soup-induced migraine-like rats demonstrating that EA can reduce microglial activation and improve microglia-mediated inflammatory response by regulating Toll-like receptor 4/nuclear factor-kappa B (NF-κB).46
Modulating Neuronal Sensitization
Central sensitization, which plays an important role in the development and progression of migraine, is defined as the increased reactivity of nociceptive neurons in the central nervous system (CNS) to normal or subliminal afferent inputs. Central sensitization occurs when the function of nociceptive pathway circuits is enhanced and leads to abnormal sensitivity.58 Brain-derived neurotrophic factor (BDNF) is a key molecule essential for neural plasticity and development, and is upregulated by inflammatory activation relevant factors.59,60
Studies have shown that EA can significantly reduce serum BDNF levels in rats with migraine.46 Glutamate is the main excitatory neurotransmitter in the CNS, which is related to central sensitization. High levels of glutamate can lead to excitatory toxicity of the nervous system, which disrupts normal neurotransmission and leads to neuronal damage or death.61 Liu et al conducted a before-after study with 20 participants, revealing that glutamate levels were elevated in blood samples of patients during and between attacks, and decreased after acupuncture treatment.49 Acupuncture may provide additional potential benefits in neuronal sensitization by modulating neurotrophic factors and neurotransmitters; however, the evidence on how acupuncture affects neurotrophic factors and neurotransmitters is not clear.
Changing Brain Function and Structure
Neuroimaging has revealed that migraine is linked to alterations in both the structure and function of the brain. Numerous observational studies have shown that patients with migraine have functional abnormalities in brain regions involving the cingulate cortex,62 caudate nucleus,63 thalamus,64 and cerebellum.65 Especially, The limbic region (the cingulate cortex, amygdala, and parahippocampal gyrus) is a pivotal area associated with brain function abnormalities in patients with migraine, which are also believed to be involved in emotional, cognitive, and autonomic functions.66 The acupuncture might modulate pain through a shift in the interaction of limbic regions with the autonomic nervous system in a before-after neuroimaging study.38 In addition, an RCT involving 44 participants has shown that it can also regulate emotional disorders by modulating the frontal-limbic regions and improving the symptoms of patients with migraine.50 Magnetic resonance imaging (MRI) studies of patients with migraine consistently show a brain structural abnormality characterized by decreased and increased gray matter volume (GMV).66 The current clinical neuroimaging studies support that the occurrence of migraine is related to the frontal and parietal lobes, and the results show that untreated patients with migraine have reduced GMV in the upper brain regions.67,68 Yang et al showed that the reduction in migraine days after acupuncture was associated with the baseline GMV in the parietal and frontal gyri and cuneus in a before-after study involving 41 patients with migraine. In addition, patients with more than 50% reduction in headache days showed a longitudinal increase in GMV in the left cuneus.51
Mechanisms of Chinese Herbs on Migraine
Previous studies have shown that several promising ingredients and herbal formulae have been identified that have central and peripheral analgesic effects and can inhibit the transmission of nociceptive information, thereby playing an anti-migraine role.69,70 A meta-analysis of RCTs of herbal medicine for migraine indicated that herbal medicine significantly reduced migraine frequency, migraine days, migraine duration, migraine intensity and the consumption of analgesics compared with placebo.71 The mechanism of Chinese herbs in treating migraine involves the following aspects (Figure 1 and Table 2): 1) inhibiting the release and expression of CGRP neuropeptide;72–74 2) suppressing inflammatory reactions such as inhibition of inflammatory factors and modulation of NF-κB and nerve growth factor (NGF) signaling pathways;34,75,76 3) changing the permeability of BBB.39,77
Table 2.
Summary of Clinical Application of Herbs in Treatment of Migraine
| Intervention | Model | Method | Mechanisms | Migraine Types | Model Methods | Intervention | Comparison | Refs. |
|---|---|---|---|---|---|---|---|---|
| Herbs | Rats | Animal experiment | 1. Improved the metabolic profile of serum and TCC in migraine rats, mainly involved in amino acid metabolism (glutamate) 2. Increased the expressions of glutamine synthetase |
NA | NTG-induced migraine rats | Dachuanxiong prescription | Blank control | [63] |
| 1. Increased the levels of 5-HT 2. Decreased the levels of CGRP |
NA | Inflammatory soup-induced chronic migraine rats | Wuzhuyu Decoction | Blank control | [64] | |||
| 1. Increased cerebral blood flow 2. Decreased the expression of CGRP, and regulated the release of CGRP 3. Relieved neurogenic inflammation |
NA | NTG-induced migraine rats | Chuanxiong Rhizoma and Cyperi Rhizoma | Blank control | [65] | |||
| 1. Decreased the plasma CGRP and serum NO levels 2. Reduced NF-κB p65 and pro-inflammatory cytokines, including IL-1β and COX-2 in the brainstem |
NA | Glyceryl trinitrate induced migraine rats | Du Liang soft capsule | Blank control | [68] | |||
| 1. Suppressed glutamate 2. Decreased BBB permeability and maintain its integrity through regulating MMP-9 in migraine rats |
NA | NTG-induced migraine rats | Chuanxiong and Gastrodia elata | Blank control | [76] | |||
| 1. Increased the permeation rate across the BBB | NA | Blood-stasis migraine model | TMP and FA | Blank control | [77] | |||
| Network pharmacology and animal experiment | 1. Increased the expression level of 5-HT 2. Reduced the expression of CGRP, NF-κB, c-fos and IL-1β 3. Inhibited NF-κB and phospho-NF-κB levels |
NA | NTG-induced migraine rats | Yufeng Ningxin tablet | Blank control | [69] | ||
| Rats | Animal experiment | 1. Enhanced the 5-HT contents and declined NO contents 2. Inhibited the NGF/TRPV1/COX-2 signaling pathway |
NA | NTG-induced migraine rats | Shaoyao Gancao Decoction | Blank control | [71] |
Abbreviations: BBB, Blood-brain barrier; CGRP, Calcitonin gene-related peptide; COX2, Cyclooxygenase-2; FA, Ferulic acid; IL-1β, Interleukin-1β; MMP-9, Matrix metalloproteinase-9; NA, Not applicable; NF-κB, Nuclear factor-kappa B; NGF, Neurotrophic factor; NO, Nitric oxide; NTG, nitroglycerine; TCC, Trigeminocervical complex; TMP, Tetramethylpyrazine; TRPV1, Transient receptor potential vanillic acid subfamily protein 1 receptor; 5-HT, 5-hydroxytryptamine.
Inhibition Neuropeptides Release
There are large amounts of free amino acids in the CNS.78 These amino acids play a role as neurotransmitters in signal transduction and participate in metabolic pathways.79 Among them, glutamate plays a key role in pain transmission, central sensitization, and cortical spreading depression. A large body of clinical evidence has suggested that glutamate and its receptors are involved in migraine pathophysiology.80 In addition, study also has shown that glutamate is closely related to the release of CGRP.81 An animal study indicated that Dachuanxiong prescription could improve the metabolic profile of trigeminocervical complex (TCC) in NTG-induced migraine rats, which could promote the metabolism of glutamate in TCC, thereby inhibiting the production of CGRP and improving headache.72 Furthermore, the current results revealed that Wuzhuyu decoction significantly prevented hyperalgesia in migraine rats by inhibiting the withdrawal threshold reduction, increasing the level of Serotonin (5-HT), and decreasing the expression of CGRP.73 Wu et al have shown that Chuanxiong and Cyperi can reduce the expression of CGRP and CGRP mRNA by increasing cerebral blood flow in NTG-induced migraine rats.74 These above findings demonstrated that Chinese herbs could exert an analgesic effect on migraine by adjusting the levels of neuropeptides.
Suppression of Inflammatory Reactions
NF-κB and NGF are important signaling pathways involved in promoting migraine-related allodynia, and even maintaining, exacerbating migraine. These findings imply that inhibiting a migraine attack might be efficient by blocking these signaling pathways.82 NF-κB signaling pathway plays a major role in inflammatory reactions, which is triggered by microbial products and pro-inflammatory cytokines such as TNF-α and IL-1. This activation causes the development of inflammation and pain.83 Experiments on rats showed that Chuanxiong and Baizhi down-regulated the expression levels of NF-κB, IL-1β and COX-2. Additionally, these substances were found to lower serum levels of NO and CGRP.75 The network pharmacology and experimental verification research have also proved that one herb Gegen could inhibit neuroinflammation by NF-κB in BV2 cells, and reduce the expression of NF-κB, c-fos and IL-1β.34 NGF binding to tyrosine receptor kinase receptors of sensory nociceptive neurons can evoke potent algogenic effects via multiple signal transduction mechanisms impinging upon the P2X3 and transient receptor potential vanilloid 1 (TRPV1) channels.84 An animal study revealed that Shaoyao Gancao decoction significantly inhibited the NGF/TRPV1/COX-2 signaling pathway, which mediates central hyperalgesia in an NTG-induced migraine rat model.76 In conclusion, there is already a certain amount of research supporting that Chinese herbs can alleviate migraine by regulating inflammatory reactions.
Changing the Permeability of BBB
The BBB plays a crucial role in maintaining normal neuronal function. It is responsible for the stability of the brain’s internal environment and protects nervous tissue from potentially harmful substances by restricting the entry of certain molecules into the CNS.85 The change of BBB permeability associated with neuroinflammation is important when considering therapeutic strategies.86 There is accumulating evidence that migraine can increase BBB permeability in rats by activating MMP-9.86,87 In clinical research, elevation of MMP-9, which is considered a typical marker suggestive of BBB disorders, is observed in patients with migraine.88 Related animal experiment suggest that Chuanxiong and Gastrodia elata may regulate MMP-9 to reduce the permeability and maintain the integrity of BBB in migraine rats.39 Moreover, previous animal experiment has shown that Gastrodin can improve the brain targeting index of Tetramethylpyrazine (TMP) and Ferulic acid (FA) in blood–stasis migraine models, which are bioactive ingredients of Ligusticum chuanqing, indicating that gastroetin can improve the BBB permeability of TMP and FA.77 The specific mechanism may be that the inhibitors of P–glycoprotein and multidrug resistance protein 1 can promote the transmembrane transport of gastrodin.89 Gastrodin competes with TMP and FA for competitive or non–competitive binding of metabolic enzymes, thereby reducing the permeability of the blood-brain barrier, slowing down the metabolism of TMP and FA and increasing their residence time in vivo, allowing TMP and FA to rapidly reach the brain tissue to treat migraine.77 Chinese herbs could be beneficial for the treatment of migraine by changing and regulating the BBB permeability, which has been confirmed by the above experiments.
Mechanisms of Massage on Migraine
Migraine is often treated with medications, but some patients cannot tolerate them because of the drug’s toxicity and adverse effects. Therefore, non-pharmacological management such as acupuncture and massage may be an alternative treatment option. However, some patients fear the sensation of the needles passing through their skin. As a result, massage is increasingly respected by migraine sufferers.33,90 Massage, known as Tuina in China, is widely used to treat migraine and is effective in alleviating the frequency, intensity, and duration of migraine attacks.91,92 The diversity of massage also provides targeted treatment for patients with migraine. Although there is limited literature on massage therapy for migraine, researches have confirmed its effectiveness. An RCT involving 47 migraine participants has highlighted that massage therapy improves the symptoms of migraine by reducing pain-related neurotransmitters and relieving muscle tension.25 Studies in recent years have drawn some conclusions that its underlying mechanisms are associated with (Figure 1 and Table 3): 1) altering the release of 5-HT, substance P, and cortisol;25,93 2) relieving muscle tension.94,95
Table 3.
Summary of Clinical Application of Massage in Treatment of Migraine
| Intervention | Model | Method | Mechanisms | Migraine Types | Model Methods | Intervention | Comparison | Refs. |
|---|---|---|---|---|---|---|---|---|
| Massage | Human | RCT | 1. Inhibited the action of the nociceptive fibers 2. Inhibited the pain pathway through the pain gate mechanism |
Cervicogenic headache | NA | Spinal manipulation therapy or conventional massage therapy | Mulligan mobilization therapy | [86] |
| 1. Reduced substance P and increased 5-HT contents 2. Decreased the level of cortisol |
Migraine | NA | Massage | Blank control | [82] | |||
| 1. Reduced muscle tension by breaking down subcutaneous adhesions, preventing fibrosis, and improving blood and lymphatic circulation | Chronic pain in head | NA | Classic massage | Standard medical care | [91] |
Abbreviations: NA, Not applicable; RCT, Randomized controlled trial; 5-HT, 5-hydroxytryptamine.
Changing Release of Neurotransmitters and Hormones
A study has shown that patients with migraine have abnormal levels of neurotransmitters such as 5-HT and substance P.96 5-HT can combine with receptors to play a role in the regulation of pain, and interact with a variety of neurotransmitters in the CNS to participate in migraine.97 It has been discussed above that substance P is a neuropeptide that dilates blood vessels and causes migraine. The two neurotransmitters are the pathological basis of migraine.13,98 An RCT has shown that massage can stimulate the nerve fiber through manipulation, inhibit pain signal transmission, and thereby achieve analgesia in migraine patients.94 Studies have also found that massage can reduce substance P and increase 5-HT contents, improve local blood flow, accelerate the metabolism of pain-causing substances, which can weaken pain signals or achieve analgesia.25,93 In addition, cortisol is a stress hormone that rises during the onset of a migraine and then decreases when the headache goes away.99 A clinical trial found that massage can reduce cortisol levels in migraine sufferers.25
Relieving Muscle Tension
Migraine attacks have been classically divided into prodromal, aura, headache, and postdromal phases.100 A retrospective study found that the symptom with the highest co-occurrence throughout all migraine phases was neck stiffness.101 Massage is a passive way of exercising muscles and relaxing the mind because it improves muscle endurance and flexibility. Therefore, it is beneficial to relieve muscle tension, anxiety, and depression caused by migraine. Studies have shown that massage can reduce muscle tension that leads to migraine by breaking down subcutaneous adhesions, preventing fibrosis, and improving blood and lymphatic circulation.94,95 Massage is a commonly used manual treatment in TCM. A systematic review of manual therapy for migraine showed that manual therapy may be as effective as modern drugs for the prevention of migraine. In addition, patients who received massage reported a 27–28% improvement in migraine frequency compared to baseline.92
Discussion
Summary
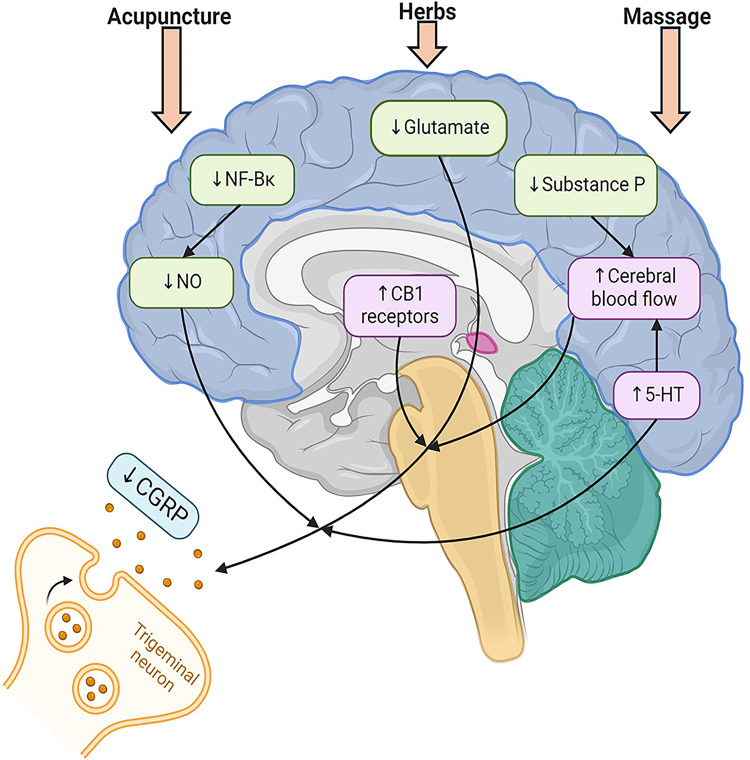
The present study is a comprehensive review of the mechanism of TCM in the treatment of migraine. Acupuncture, herbs, and massage have different mechanisms but all of them are associated with the inhibition of neurotransmitters to relieve migraine (Figure 2). Acupuncture can improve migraine symptoms by reducing the release of NO and activating CB1 receptor, downregulating the expression of CGRP in trigeminal ganglion, inhibiting the microglia and other inflammatory factors, including IL-1β, COX2, and PGE2, reducing BDNF and glutamate levels to regulate central sensitivities, and improving functional connectivity of brain areas and GMV. Herbs can alleviate migraine-related pain by decreasing CGRP expression, inhibiting inflammatory factors and signaling pathways, including NF-κB and NGF, and changing BBB permeability. Massage can achieve analgesia by reducing substance P and cortisol levels, increasing 5-HT content, and relieving muscle tension.
Figure 2.
Mechanism of acupuncture, herbs, and massage to change release of neurotransmitters.
Acupuncture can inhibit the release of CGRP by reducing the release of NO and activating the CB1 receptor. Herbs can reduce CGRP by promoting the metabolism of glutamate in TCC, increasing the level of 5-HT, and increasing cerebral blood flow. Massage can reduce substance P, increase 5-HT contents, improve local blood flow, and accelerate the metabolism of pain-causing neurotransmitters. Created with BioRender.com.
Abbreviations: CB1, Type 1 cannabinoid; CGRP, Calcitonin gene-related peptide; NF-κB, Nuclear factor-kappa B; NO, nitric oxide; 5-HT, 5-hydroxytryptamine.
Future Directions
Experts suggest that preventive treatment is advisable for individuals with migraine.102 However, between 39.2% and 48.2% of patients with migraine stopped preventive medication because of inefficacy, while 34.2–53.2% discontinued it because of side effects.103 Preventive medication can cause dissatisfaction, discontinuation, and occurrence of medication overuse headaches in some patients with migraine,104 which may encourage individuals to seek complementary and alternative treatments for migraine.103,105 TCM, as a major component of complementary and alternative treatments, is commonly used to treat migraine in China and other Western countries. Some patients, including those with contraindications or specific populations, such as pediatric and pregnant patients with chronic migraine, may prefer TCM.106
Many studies have shown that TCM has good therapeutic effects in patients with migraine.92,107,108 However, few studies have investigated the action mechanism of TCM in the treatment of migraine. This study summarizes existing articles on the mechanism of TCM in the treatment of migraine; however, the mechanism remains unclear. Studies analyzing multiple omics data, such as proteomics, metabolomics, genomics, transcriptomics, lipidomics, immunomics, and imaging omics, are needed to clarify the mechanisms of TCM treatment of migraine. With the development of neuroimaging techniques, many studies have found that patients with migraine have abnormalities in brain structure and function, and previous studies have confirmed that TCM can reverse these abnormalities. Therefore, functional MRI, structural MRI, dynamic contrast-enhanced MRI, functional near-infrared spectroscopy, positron emission tomography, electroencephalography, and magnetoencephalography should be performed to assess the effects of TCM on patients with migraine.
Limitation
Despite the substantial advancements in understanding the mechanisms of migraine, several limitations remain. First, Current animal experiments suffer from low quality and significant heterogeneity, primarily focusing on male rats. This neglects sexual dimorphism and limits our understanding of gender-specific factors in migraines, which exhibit strong gender differences. Consequently, this bias restricts the generalizability of the findings. Second, current animal studies often use outdated techniques and lack advanced chemogenetic and optogenetic technologies to explore TCM’s effects on migraine neural circuits. Research mainly focuses on neuroinflammation and neurotransmitters, while the interactions between neurons and glial cells remain underexplored. Third, there is a lack of omics technologies (eg, genomics, proteomics) and advanced imaging techniques to thoroughly examine the mechanisms of TCM for migraine. These approaches could offer deeper insights into the molecular and cellular processes, bridging traditional treatments with modern science. Fourthly, most studies concentrate on migraine without aura, with limited exploration of other subtypes such as migraine with aura, menstrually-related migraine, or chronic migraine. Future research should encompass a broader range of migraine types to enhance the comprehensiveness and representativeness of the findings. Fifthly, many studies focus primarily on the TCC,109–111 with less attention paid to other critical brain regions such as the thalamus, sensory cortex, and limbic system. More research is needed to explore the interactions of neurotransmitters and signaling pathways among these brain regions and their role in the pathogenesis of migraine. Additionally, current research often infers central mechanisms based on peripheral blood samples, which cannot directly reflect changes within the CNS. The absence of effective non-invasive methods to assess CNS pathological changes hinders understanding of migraine’s central mechanisms.
Conclusions
In conclusion, this review aims to provide a scientific and systematic evaluation of TCM in the management of migraine. By integrating existing clinical and preclinical evidence, it offers a comprehensive discussion on the therapeutic efficacy and potential mechanisms of TCM for migraine. Ultimately, it seeks to apply modern Chinese medicine theories and scientific methods to further investigate the pathogenesis of migraine and offer more reliable evidence for its treatment with Chinese medicine.
Acknowledgments
All the authors deserve credit for their contributions to this work. Meanwhile, thanks also to Biorender (www. biorender. com) for the material provided for the picture production.
Funding Statement
This study was funded by China National Natural Science Foundation (82374575, 82074179, 82305389), Beijing Natural Science Foundation (7232270, 7234409), Capital Health Development Scientific Research Project (Capital Development 2024-2-2235), Outstanding Young Talents Program of Capital Medical University (B2207), R&D Program of Beijing Municipal Education Commission (KM202110025005), China Association for Science and Technology Young Talent Lifting Project (2019-2021ZGZJXH-QNRC001), Beijing Hospital Management Centre “Peak” Talent Development Program Team (DFL20241001), Fifth Batch National Excellent TCM Clinical Talents Project, Beijing Hospital of Traditional Chinese Medicine (LY201802), Beijing Institute of Traditional Chinese Medicine (YJS-2022-09), Beijing Hospital Management Centre Key Medical Specialty Project (ZYLX202140), Beijing Administration of Traditional Chinese Medicine “14th Five-Year Plan” Key Specialties Project (BJZKLC0008).
Abbreviations
BBB, Blood-brain barrier; BDNF, Brain-derived neurotrophic factor; CB1, Type 1 cannabinoid; CGRP, Calcitonin gene-related peptide; CNS, central nervous system; COX2, Cyclooxygenase-2; EA, Electroacupuncture; FA, Ferulic acid; GMV, Gray matter volume; IL-1β, Interleukin-1β; MMP-2, Matrix metalloproteinase-2; MRI, Magnetic resonance imaging; NF-κB, Nuclear factor-kappa B; NGF, Nerve growth factor; NO, Nitric oxide; PGE2, Prostaglandin E2; TCC, Trigeminocervical complex; TCM, Traditional Chinese Medicine; TMP, Tetramethylpyrazine; TNF-α, Tumor necrosis factor-α; TRPV1, Transient receptor potential vanilloid 1; 5-HT, 5-hydroxytryptamine.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Loder E, Renthal W. Calcitonin gene-related peptide monoclonal antibody treatments for migraine. JAMA Intern Med. 2019;179(3):421–422. doi: 10.1001/jamainternmed.2018.7536 [DOI] [PubMed] [Google Scholar]
- 2.Olesen J. Headache classification committee of the international headache society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi: 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- 3.Kisa A, Collaborators DI, Oancea B, Kisa S. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/s0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burch RC, Loder S, Loder E, Smitherman TA. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55(1):21–34. doi: 10.1111/head.12482 [DOI] [PubMed] [Google Scholar]
- 5.Wang SJ. Epidemiology of migraine and other types of headache in Asia. Curr Neurol Neurosci Rep. 2003;3(2):104–108. doi: 10.1007/s11910-003-0060-7 [DOI] [PubMed] [Google Scholar]
- 6.Feigin VL, Nichols E, Alam T, et al. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(5):459–480. doi: 10.1016/s1474-4422(18)30499-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreou AP, Edvinsson L. Mechanisms of migraine as a chronic evolutive condition. J Headache Pain. 2019;20(1):117. doi: 10.1186/s10194-019-1066-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashina M, Terwindt GM, Al-Karagholi MAM, et al. Migraine: disease characterisation, biomarkers, and precision medicine. Lancet. 2021;397(10283):1496–1504. doi: 10.1016/S0140-6736(20)32162-0 [DOI] [PubMed] [Google Scholar]
- 9.Iyengar S, Johnson KW, Ossipov MH, Aurora SK. CGRP and the trigeminal system in migraine. Headache. 2019;59(5):659–681. doi: 10.1111/head.13529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zagami AS, Edvinsson L, Goadsby PJ. Pituitary adenylate cyclase activating polypeptide and migraine. Ann Clin Transl Neurol. 2014;1(12):1036–1040. doi: 10.1002/acn3.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol. 2010;6(10):573–582. doi: 10.1038/nrneurol.2010.127 [DOI] [PubMed] [Google Scholar]
- 12.Puledda F, Silva EM, Suwanlaong K, Goadsby PJ. Migraine: from pathophysiology to treatment. J Neurol. 2023;270(7):3654–3666. doi: 10.1007/s00415-023-11706-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haanes KA, Edvinsson L. Pathophysiological mechanisms in migraine and the identification of new therapeutic targets. Cns Drugs. 2019;33(6):525–537. doi: 10.1007/s40263-019-00630-6 [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann J, Baca SM, Akerman S. Neurovascular mechanisms of migraine and cluster headache. J Cereb Blood Flow Metab. 2017;39(4):0271678X1773365. doi: 10.1177/0271678X17733655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borkum JM. Harnessing migraines for neural regeneration. Neural Regen Res. 2018;13(4):609–615. doi: 10.4103/1673-5374.230275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, He S, Hu Y, Zheng H. Antagonism of cannabinoid receptor 1 attenuates the anti-inflammatory effects of electroacupuncture in a rodent model of migraine. Acupunct Med. 2016;34(6):463–470. doi: 10.1136/acupmed-2016-011113 [DOI] [PubMed] [Google Scholar]
- 17.Antonova M, Wienecke T, Olesen J, Ashina M. Prostaglandins in migraine: update. Curr Opin Neurol. 2013;26(3):269–275. doi: 10.1097/WCO.0b013e328360864b [DOI] [PubMed] [Google Scholar]
- 18.Dodick DW. Migraine. Lancet. 2018;391(10127):1315–1330. doi: 10.1016/s0140-6736(18)30478-1 [DOI] [PubMed] [Google Scholar]
- 19.Ashina M, Buse DC, Ashina H, et al. Migraine: integrated approaches to clinical management and emerging treatments. Lancet. 2021;397(10283):1505–1518. doi: 10.1016/S0140-6736(20)32342-4 [DOI] [PubMed] [Google Scholar]
- 20.Neurologists Branch of the Chinese Medical Doctor Association, Headache and Sensory Disorders Professional Committee of China Research Hospital Association, Zhao D, et al. Chinese guidelines for the diagnosis and treatment of migraine 2022 edition). Chin J Pain Med. 2022;28(12):881–898. [Google Scholar]
- 21.Matos LC, Machado JP, Monteiro FJ, Greten HJ. Understanding traditional Chinese medicine therapeutics: an overview of the basics and clinical applications. Healthcare. 2021;9(3):257. doi: 10.3390/healthcare9030257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu SB, Yu LL, Luo X, et al. Manual acupuncture versus sham acupuncture and usual care for prophylaxis of episodic migraine without aura: multicentre, randomised clinical trial. BMJ-Brit Med J. 2020;368:m697. doi: 10.1136/bmj.m697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Chen QY, Zhao LP, et al. Acupuncture plus topiramate placebo versus topiramate plus sham acupuncture for the preventive treatment of chronic migraine: a single-blind, double-dummy, randomized controlled trial. Cephalalgia. 2024;44(6):3331024241261080. doi: 10.1177/03331024241261080 [DOI] [PubMed] [Google Scholar]
- 24.Pfaffenrath V, Diener HC, Fischer M, Friede M, Henneicke-von Zepelin HH. The efficacy and safety of Tanacetum parthenium (feverfew) in migraine prophylaxis--a double-blind, multicentre, randomized placebo-controlled dose-response study. Cephalalgia. 2002;22(7):523–532. doi: 10.1046/j.1468-2982.2002.00396.x [DOI] [PubMed] [Google Scholar]
- 25.Lawler SP, Cameron LD. A randomized, controlled trial of massage therapy as a treatment for migraine. Ann Behav Med. 2006;32(1):50–59. doi: 10.1207/s15324796abm3201_6 [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Liu YH, Song Y, et al. Therapeutic applications and potential mechanisms of acupuncture in migraine: a literature review and perspectives. Front Neurosci. 2022;16:1022455. doi: 10.3389/fnins.2022.1022455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun SQ, Liu L, Zhou MD, Liu Y, Sun MS, Zhao L. The analgesic effect and potential mechanisms of acupuncture for migraine rats: a systematic review and meta-analysis. J Pain Res. 2023;16:2525–2542. doi: 10.2147/Jpr.S422050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Wang S, Wang Y. Role of herbal medicine for prevention and treatment of migraine. Phytother Res. 2021;36(2):730–760. doi: 10.1002/ptr.7339 [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Feng X, Li T, et al. Preliminary study on potential compounds and mechanism of Chuanxiong Chatiao granules in treating migraine. Pharmacol Res Mod Chin Med. 2022;4:100134. doi: 10.1016/j.prmcm.2022.100134 [DOI] [Google Scholar]
- 30.Wang HJ. Peaceful Holy Benevolent Prescriptions. Beijing: People’s Medical Publishing House; 1958. [Google Scholar]
- 31.Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016;2016(6):CD001218. doi: 10.1002/14651858.CD001218.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyu S, Zhang CS, Guo X, et al. Oral Chinese herbal medicine as prophylactic treatment for episodic migraine in adults: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2020;2020:5181587. doi: 10.1155/2020/5181587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Millstine D, Chen CY, Bauer B. Complementary and integrative medicine in the management of headache. BMJ. 2017;357:j1805. doi: 10.1136/bmj.j1805 [DOI] [PubMed] [Google Scholar]
- 34.Yu S, Fan C, Li Y, et al. Network pharmacology and experimental verification to explore the anti-migraine mechanism of Yufeng Ningxin tablet. J Ethnopharmacol. 2023;310:116384. doi: 10.1016/j.jep.2023.116384 [DOI] [PubMed] [Google Scholar]
- 35.Ke HK, Tu SH, Shen YJ, Qu QW. Effect of ZHU Lian’s type II inhibition acupuncture on chronic migraine and serum 5-HT, VEGF, CGRP. Zhongguo Zhen Jiu. 2021;41(10):1079–1083. doi: 10.13703/j.0255-2930.20200925-0001 [DOI] [PubMed] [Google Scholar]
- 36.Jeong HJ, Hong SH, Nam YC, et al. The effect of acupuncture on proinflammatory cytokine production in patients with chronic headache: a preliminary report. Am J Chin Med. 2003;31(6):945–954. doi: 10.1142/s0192415x03001661 [DOI] [PubMed] [Google Scholar]
- 37.Martins LB, Rodrigues A, Rodrigues DF, Dos Santos LC, Teixeira AL, Ferreira AVM. Double-blind placebo-controlled randomized clinical trial of ginger (Zingiber officinale Rosc.) addition in migraine acute treatment. Cephalalgia. 2019;39(1):68–76. doi: 10.1177/0333102418776016 [DOI] [PubMed] [Google Scholar]
- 38.Tian Z, Guo Y, Yin T, et al. Acupuncture modulation effect on pain processing patterns in patients with migraine without aura. Front Neurosci. 2021;15:729218. doi: 10.3389/fnins.2021.729218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Q, Shen L, Ma S, et al. Effects of ligusticum chuanxiong and gastrodia elata on blood-brain barrier permeability in migraine rats. Pharmazie. 2015;70(6):421–426. [PubMed] [Google Scholar]
- 40.Wells RE, Bertisch SM, Buettner C, Phillips RS, McCarthy EP. Complementary and alternative medicine use among adults with migraines/severe headaches. Headache. 2011;51(7):1087–1097. doi: 10.1111/j.1526-4610.2011.01917.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao L, Chen J, Li Y, et al. The long-term effect of acupuncture for migraine prophylaxis: a randomized clinical trial. JAMA Intern Med. 2017;177(4):508–515. doi: 10.1001/jamainternmed.2016.9378 [DOI] [PubMed] [Google Scholar]
- 42.Wang LP, Zhang XZ, Guo J, et al. Efficacy of acupuncture for migraine prophylaxis: a single-blinded, double-dummy, randomized controlled trial. Pain. 2011;152(8):1864–1871. doi: 10.1016/j.pain.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 43.Yang CP, Chang MH, Liu PE, et al. Acupuncture versus topiramate in chronic migraine prophylaxis: a randomized clinical trial. Cephalalgia. 2011;31(15):1510–1521. doi: 10.1177/0333102411420585 [DOI] [PubMed] [Google Scholar]
- 44.Su P, Xie X, Xu Y, Luo X, Niu J, Jin Z. Effectiveness of acupuncture in migraine rats: a systematic review. PLoS One. 2023;18(1):e0280556. doi: 10.1371/journal.pone.0280556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gunduztepe Y, Mit S, Gecioglu E, et al. The impact of acupuncture treatment on nitric oxide (NO) in migraine patients. Acupunct Electrother Res. 2014;39(3–4):275–283. doi: 10.3727/036012914x14109544776178 [DOI] [PubMed] [Google Scholar]
- 46.Zhou M, Pang F, Liao D, He X, Yang Y, Tang C. Electroacupuncture at Fengchi(GB20) and Yanglingquan(GB34) ameliorates paralgesia through microglia-mediated neuroinflammation in a rat model of migraine. Brain Sci. 2023;13(4):541. doi: 10.3390/brainsci13040541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao L, Liu L, Xu X, et al. Electroacupuncture inhibits hyperalgesia by alleviating inflammatory factors in a rat model of migraine. J Pain Res. 2020;13:75–86. doi: 10.2147/JPR.S225431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H, He SD, Zong DD, Zhang XM, Luo J, Zheng JK. Effects of electroacupuncture on miR-34a-5p/SIRT1 signaling in the trigeminal ganglion of rats with migraine. Zhen Ci Yan Jiu. 2020;45(11):868–874. doi: 10.13702/j.1000-0607.200378 [DOI] [PubMed] [Google Scholar]
- 49.Liu L, Li W, Wang L, et al. Proteomic and metabolomic profiling of acupuncture for migraine reveals a correlative link via energy metabolism. Front Neurosci. 2022;16:1013328. doi: 10.3389/fnins.2022.1013328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, Wang Z, Du J, et al. Regulatory effects of acupuncture on emotional disorders in patients with menstrual migraine without aura: a resting-state fMRI study. Front Neurosci. 2021;15:726505. doi: 10.3389/fnins.2021.726505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang XJ, Liu L, Xu ZL, et al. Baseline brain gray matter volume as a predictor of acupuncture outcome in treating migraine. Front Neurol. 2020;11:111. doi: 10.3389/fneur.2020.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Messlinger K, Russo AF. Current understanding of trigeminal ganglion structure and function in headache. Cephalalgia. 2019;39(13):1661–1674. doi: 10.1177/0333102418786261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biscetti L, Cresta E, Cupini LM, Calabresi P, Sarchielli P. The putative role of neuroinflammation in the complex pathophysiology of migraine: from bench to bedside. Neurobiol Dis. 2023;180:106072. doi: 10.1016/j.nbd.2023.106072 [DOI] [PubMed] [Google Scholar]
- 54.Williamson DJ, Hargreaves RJ. Neurogenic inflammation in the context of migraine. Microsc Res Tech. 2001;53(3):167–178. doi: 10.1002/jemt.1081 [DOI] [PubMed] [Google Scholar]
- 55.Karsan N, Gosalia H, Goadsby PJ. Molecular mechanisms of migraine: nitric oxide synthase and neuropeptides. Int J Mol Sci. 2023;24(15):11993. doi: 10.3390/ijms241511993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H, Zhang XM, Zong DD, et al. miR-34a-5p up-regulates the IL-1beta/COX2/PGE2 inflammation pathway and induces the release of CGRP via inhibition of SIRT1 in rat trigeminal ganglion neurons. FEBS Open Bio. 2021;11(1):300–311. doi: 10.1002/2211-5463.13027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun S, Fan Z, Liu X, Wang L, Ge Z. Microglia TREM1-mediated neuroinflammation contributes to central sensitization via the NF-kappaB pathway in a chronic migraine model. J Headache Pain. 2024;25(1):3. doi: 10.1186/s10194-023-01707-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki K, Suzuki S, Shiina T, Kobayashi S, Hirata K. Central sensitization in migraine: a narrative review. J Pain Res. 2022;15:2673–2682. doi: 10.2147/JPR.S329280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohno Y, Kinboshi M, Shimizu S. Inwardly rectifying potassium channel Kir4.1 as a novel modulator of BDNF expression in astrocytes. Int J Mol Sci. 2018;19(11):3313. doi: 10.3390/ijms19113313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dadkhah M, Baziar M, Rezaei N. The regulatory role of BDNF in neuroimmune axis function and neuroinflammation induced by chronic stress: a new therapeutic strategies for neurodegenerative disorders. Cytokine. 2024;174:156477. doi: 10.1016/j.cyto.2023.156477 [DOI] [PubMed] [Google Scholar]
- 61.Martami F, Holton KF. Targeting glutamate neurotoxicity through dietary manipulation: potential treatment for migraine. Nutrients. 2023;15(18):3952. doi: 10.3390/nu15183952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Puledda F, Dipasquale O, Gooddy BJM, et al. Abnormal glutamatergic and serotonergic connectivity in visual snow syndrome and migraine with aura. Ann Neurol. 2023;94(5):873–884. doi: 10.1002/ana.26745 [DOI] [PubMed] [Google Scholar]
- 63.Yuan Z, Wang W, Zhang X, et al. Altered functional connectivity of the right caudate nucleus in chronic migraine: a resting-state fMRI study. J Headache Pain. 2022;23(1):154. doi: 10.1186/s10194-022-01506-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qin ZX, Su JJ, He XW, et al. Altered resting-state functional connectivity between subregions in the thalamus and cortex in migraine without aura. Eur J Neurol. 2020;27(11):2233–2241. doi: 10.1111/ene.14411 [DOI] [PubMed] [Google Scholar]
- 65.Ke J, Yu Y, Zhang X, et al. Functional alterations in the posterior insula and cerebellum in migraine without aura: a resting-state MRI study. Front Behav Neurosci. 2020;14:567588. doi: 10.3389/fnbeh.2020.567588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jia Z, Yu S. Grey matter alterations in migraine: a systematic review and meta-analysis. Neuroimage Clin. 2017;14(C):130–140. doi: 10.1016/j.nicl.2017.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen XY, Chen ZY, Dong Z, Liu MQ, Yu SY. Regional volume changes of the brain in migraine chronification. Neural Regen Res. 2020;15(9):1701–1708. doi: 10.4103/1673-5374.276360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lai KL, Niddam DM, Fuh JL, Chen WT, Wu JC, Wang SJ. Cortical morphological changes in chronic migraine in a Taiwanese cohort: surface- and voxel-based analyses. Cephalalgia. 2020;40(6):575–585. doi: 10.1177/0333102420920005 [DOI] [PubMed] [Google Scholar]
- 69.Luo Y, Wang CZ, Sawadogo R, Tan T, Yuan CS. Effects of herbal medicines on pain management. Am J Chin Med. 2020;48(1):1–16. doi: 10.1142/S0192415X20500019 [DOI] [PubMed] [Google Scholar]
- 70.Zhao Y, Martins-Oliveira M, Akerman S, Goadsby PJ. Comparative effects of traditional Chinese and Western migraine medicines in an animal model of nociceptive trigeminovascular activation. Cephalalgia. 2018;38(7):1215–1224. doi: 10.1177/0333102417728245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi YH, Wang Y, Fu H, Xu Z, Zeng H, Zheng GQ. Chinese herbal medicine for headache: a systematic review and meta-analysis of high-quality randomized controlled trials. Phytomedicine. 2019;57:315–330. doi: 10.1016/j.phymed.2018.12.039 [DOI] [PubMed] [Google Scholar]
- 72.Ni N, Wang Q, Lin X, Hong Y, Feng Y, Shen L. Studies on the mechanism of glutamate metabolism in NTG-induced migraine rats treated with DCXF. Evid Based Complement Alternat Med. 2019;2019:1324797. doi: 10.1155/2019/1324797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nan N, Gong MX, Wang Q, et al. Wuzhuyu decoction relieves hyperalgesia by regulating central and peripheral 5-HT in chronic migraine model rats. Phytomedicine. 2022;96:153905. doi: 10.1016/j.phymed.2021.153905 [DOI] [PubMed] [Google Scholar]
- 74.Wu S, Guo L, Qiu F, Gong M. Anti-migraine effect of the herbal combination of chuanxiong rhizoma and Cyperi rhizoma and UPLC-MS/MS method for the simultaneous quantification of the active constituents in rat serum and cerebral cortex. Molecules. 2019;24(12). doi: 10.3390/molecules24122230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hou M, Tang Q, Xue Q, et al. Pharmacodynamic action and mechanism of Du Liang soft capsule, a traditional Chinese medicine capsule, on treating nitroglycerin-induced migraine. J Ethnopharmacol. 2017;195:231–237. doi: 10.1016/j.jep.2016.11.025 [DOI] [PubMed] [Google Scholar]
- 76.Luo Y, Qiu Y, Zhou R, et al. Shaoyao Gancao decoction alleviates the central hyperalgesia of recurrent NTG-induced migraine in rats by regulating the NGF/TRPV1/COX-2 signal pathway. J Ethnopharmacol. 2023;317:116781. doi: 10.1016/j.jep.2023.116781 [DOI] [PubMed] [Google Scholar]
- 77.Mi Y, Guo S, Cheng H, et al. Pharmacokinetic comparative study of tetramethylpyrazine and ferulic acid and their compatibility with different concentration of gastrodin and gastrodigenin on blood-stasis migraine model by blood-brain microdialysis method. J Pharm Biomed Anal. 2020;177:112885. doi: 10.1016/j.jpba.2019.112885 [DOI] [PubMed] [Google Scholar]
- 78.Niddam DM, Lai KL, Tsai SY, et al. Neurochemical changes in the medial wall of the brain in chronic migraine. Brain. 2018;141(2):377–390. doi: 10.1093/brain/awx331 [DOI] [PubMed] [Google Scholar]
- 79.Tai S. On the detection amino of amino acids neurotransmitter in rat brain by RP-HPLC. J Jilin Agric Sci Technol College. 2009;(4):18–20. [Google Scholar]
- 80.Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97(2):553–622. doi: 10.1152/physrev.00034.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gasparini CF, Griffiths LR. The biology of the glutamatergic system and potential role in migraine. Int J Biomed Sci. 2013;9(1):1–8. doi: 10.59566/IJBS.2013.9001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gong Q, Lin Y, Lu Z, Xiao Z. Microglia-astrocyte cross talk through IL-18/IL-18R signaling modulates migraine-like behavior in experimental models of migraine. Neuroscience. 2020;451:207–215. doi: 10.1016/j.neuroscience.2020.10.019 [DOI] [PubMed] [Google Scholar]
- 83.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang DG, Gao YY, Yin ZQ, et al. Roxadustat alleviates nitroglycerin-induced migraine in mice by regulating HIF-1alpha/NF-kappaB/inflammation pathway. Acta Pharmacol Sin. 2023;44(2):308–320. doi: 10.1038/s41401-022-00941-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.DosSantos MF, Holanda-Afonso RC, Lima RL, DaSilva AF, Moura-Neto V. The role of the blood-brain barrier in the development and treatment of migraine and other pain disorders. Front Cell Neurosci. 2014;8:302. doi: 10.3389/fncel.2014.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamanaka G, Suzuki S, Morishita N, et al. Role of neuroinflammation and blood-brain barrier permutability on migraine. Int J Mol Sci. 2021;22(16):8929. doi: 10.3390/ijms22168929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mi X, Li R, Chen L, Qin G. Recurrent headache increases blood-brain barrier permeability and VEGF expression in rats. Pain Physician. 2018;21(6):E633–E642. [PubMed] [Google Scholar]
- 88.Imamura K, Takeshima T, Fusayasu E, Nakashima K. Increased plasma matrix metalloproteinase-9 levels in migraineurs. Headache. 2008;48(1):135–139. doi: 10.1111/j.1526-4610.2007.00958.x [DOI] [PubMed] [Google Scholar]
- 89.Zheng Q, Liu D, Hu PY, Wang J, Tang Y. Studies on effects and mechanisms of P–gp and MRP1 inhibitor on transportation of gastrodin. Chin Tradit Herb Drugs. 2016;47(21):3840–3847. [Google Scholar]
- 90.Probyn K, Bowers H, Mistry D, et al. Non-pharmacological self-management for people living with migraine or tension-type headache: a systematic review including analysis of intervention components. BMJ Open. 2017;7(8):e016670. doi: 10.1136/bmjopen-2017-016670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wojciech K, Pawel L, Halina RZ. Effects of feet reflexology versus segmental massage in reducing pain and its intensity, frequency and duration of the attacks in females with migraine: a pilot study. J Tradit Chin Med. 2017;37(2):214–219. doi: 10.1016/s0254-6272(17)30047-x [DOI] [PubMed] [Google Scholar]
- 92.Chaibi A, Tuchin PJ, Russell MB. Manual therapies for migraine: a systematic review. J Headache Pain. 2011;12(2):127–133. doi: 10.1007/s10194-011-0296-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Field T, Hernandez-Reif M, Diego M, Schanberg S, Kuhn C. Cortisol decreases and serotonin and dopamine increase following massage therapy. Int J Neurosci. 2005;115(10):1397–1413. doi: 10.1080/00207450590956459 [DOI] [PubMed] [Google Scholar]
- 94.Nambi G, Alghadier M, Ebrahim EE, et al. Comparative effects of Mulligan’s mobilization, spinal manipulation, and conventional massage therapy in cervicogenic headache—a prospective, randomized, controlled trial. Healthcare. 2022;11(1):107. doi: 10.3390/healthcare11010107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walach H, Guthlin C, Konig M. Efficacy of massage therapy in chronic pain: a pragmatic randomized trial. J Altern Complement Med. 2003;9(6):837–846. doi: 10.1089/107555303771952181 [DOI] [PubMed] [Google Scholar]
- 96.Frederiksen SD, Bekker-Nielsen Dunbar M, Snoer AH, Deen M, Edvinsson L. Serotonin and neuropeptides in blood from episodic and chronic migraine and cluster headache patients in case-control and case-crossover settings: a systematic review and meta-analysis. Headache. 2020;60(6):1132–1164. doi: 10.1111/head.13802 [DOI] [PubMed] [Google Scholar]
- 97.Deen M, Hougaard A, Hansen HD, et al. Migraine is associated with high brain 5-HT levels as indexed by 5-HT(4) receptor binding. Cephalalgia. 2019;39(4):526–532. doi: 10.1177/0333102418793642 [DOI] [PubMed] [Google Scholar]
- 98.Villalon CM, VanDenBrink AM. The role of 5-hydroxytryptamine in the pathophysiology of migraine and its relevance to the design of novel treatments. Mini Rev Med Chem. 2017;17(11):928–938. doi: 10.2174/1389557516666160728121050 [DOI] [PubMed] [Google Scholar]
- 99.Kogelman LJA, Falkenberg K, Ottosson F, et al. Multi-omic analyses of triptan-treated migraine attacks gives insight into molecular mechanisms. Sci Rep. 2023;13(1):12395. doi: 10.1038/s41598-023-38904-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dodick DW. A phase-by-phase review of migraine pathophysiology. Headache. 2018;58(Suppl 1):4–16. doi: 10.1111/head.13300 [DOI] [PubMed] [Google Scholar]
- 101.Messina R, Cetta I, Colombo B, Filippi M. Tracking the evolution of non-headache symptoms through the migraine attack. J Headache Pain. 2022;23(1):149. doi: 10.1186/s10194-022-01525-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ailani J, Burch RC, Robbins MS. The American headache society consensus statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021–1039. doi: 10.1111/head.14153 [DOI] [PubMed] [Google Scholar]
- 103.Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II). Headache. 2013;53(4):644–655. doi: 10.1111/head.12055 [DOI] [PubMed] [Google Scholar]
- 104.Hepp Z, Dodick DW, Varon SF, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: a retrospective claims analysis. Cephalalgia. 2017;37(5):470–485. doi: 10.1177/0333102416678382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lipton RB, Buse DC, Serrano D, Holland S, Reed ML. Examination of unmet treatment needs among persons with episodic migraine: results of the American migraine prevalence and prevention (AMPP) study. Headache. 2013;53(8):1300–1311. doi: 10.1111/head.12154 [DOI] [PubMed] [Google Scholar]
- 106.Kim CY, Hwang EH, Heo I, Park SY, Shin BC, Hwang MS. Effectiveness and safety of scalp acupuncture for treating migraine: a systematic review and meta-analysis. Complement Ther Med. 2023;78:102991. doi: 10.1016/j.ctim.2023.102991 [DOI] [PubMed] [Google Scholar]
- 107.Liu Y, Wang Y, Mi C, et al. Efficacy of acupuncture-related therapy for migraine: a systematic review and network meta-analysis. J Pain Res. 2024;17:1107–1132. doi: 10.2147/JPR.S452971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou L, Chen P, Liu L, et al. Systematic review and meta-analysis of traditional Chinese medicine in the treatment of migraines. Am J Chin Med. 2013;41(5):1011–1025. doi: 10.1142/S0192415X13500687 [DOI] [PubMed] [Google Scholar]
- 109.Wen Q, Wang Y, Pan Q, et al. MicroRNA-155-5p promotes neuroinflammation and central sensitization via inhibiting SIRT1 in a nitroglycerin-induced chronic migraine mouse model. J Neuroinflammation. 2021;18(1):287. doi: 10.1186/s12974-021-02342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.He W, Long T, Pan Q, et al. Microglial NLRP3 inflammasome activation mediates IL-1β release and contributes to central sensitization in a recurrent nitroglycerin-induced migraine model. J Neuroinflammation. 2019;16(1):78. doi: 10.1186/s12974-019-1459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jing F, Zhang Y, Long T, et al. P2Y12 receptor mediates microglial activation via RhoA/ROCK pathway in the trigeminal nucleus caudalis in a mouse model of chronic migraine. J Neuroinflammation. 2019;16(1):217. doi: 10.1186/s12974-019-1603-4 [DOI] [PMC free article] [PubMed] [Google Scholar]