Abstract
Purpose
Substantial evidence has established a strong association between non-alcoholic fatty liver disease (NAFLD), type 2 diabetes mellitus (T2DM) and insulin resistance (IR). Insulin resistance metabolic score (METS-IR) is a new and more effective comprehensive indicator for measuring IR. Our aim was to investigate the relationship between METS-IR and NAFLD in T2DM population.
Patients and methods
This cross-sectional study included 1097 adult patients with T2DM. Anthropometric measurements and biochemical indicators were collected, and the NAFLD was diagnosed by ultrasound. The METS-IR was calculated. Based on the presence of NAFLD, the population was divided into non-NAFLD and NAFLD groups. The relationship between METS-IR and NAFLD was evaluated.
Results
Compared with the non-NAFLD group, the METS-IR was higher in the NAFLD group (P < 0.001). The incidence rate of NAFLD increased across the quartiles of the METS-IR (P < 0.001). Spearman correlation analysis showed that METS-IR was positively correlated with NAFLD (Correlation Coefficient: 0.441, P < 0.001). The binary logistic regression analysis indicated that METS-IR was independently associated with NAFLD (OR: 1.120, 95% CI 1.080–1.161). Furthermore, the area under the receiver operating characteristic curve of the METS-IR was 0.781 (95% CI 0.746–0.817) and relatively higher than other evaluation variables.
Conclusion
In patients with T2DM, METS-IR is closely associated with NAFLD, and might be a valuable predictor of NAFLD. Further research is needed to verify this association.
Keywords: Type 2 diabetes mellitus, NAFLD, insulin resistance, METS-IR
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide and is a progressive chronic condition.1 The potential disease risk of NAFLD is a significant factor threatening public health.2 Recent evidence suggests that NAFLD is closely associated with various chronic diseases, including tumors, cardiovascular diseases, kidney diseases and sarcopenia, among others.3–7 Moreover, NAFLD is strongly linked to insulin resistance (IR) and type 2 diabetes mellitus (T2DM). Individuals with T2DM are five times more likely to develop NAFLD compared to those without T2DM.8 Therefore, timely identifying and screening of NAFLD in individuals with T2DM is crucial for the prevention and treatment of NAFLD-related complications.
A large body of research indicates that IR is closely linked to the pathogenesis of NAFLD through related molecular-biochemical and immune mechanisms.9 The gold standard for IR assessment is the hyperinsulinemic euglycemic clamp,10 but its high cost and invasiveness limit its clinical utility. Therefore, it is necessary to explore a convenient and practical method for assessing IR. Researchers from Mexico introduced the IR metabolic score (METS-IR) as a novel indicator for evaluating insulin sensitivity.11 It was calculated through simple anthropometric measurements and biochemical parameters including fasting plasma glucose (FPG), triglycerides (TG), body mass index (BMI) and high-density lipoprotein cholesterol (HDL-c). Studies showed that this score was consistent with the hyperinsulinemic-euglycemic clamp technique and was widely used in clinical practice to reflect IR sensitivity.12 In addition to metabolic syndrome, METS-IR could also early predict coronary artery disease, T2DM and hyperuricemia.13,14 Recently, a research report from South Korea showed that METS-IR could detect and prevent NAFLD early in the general population.15 However, the utility of METS-IR in predicting NAFLD in individuals with T2DM remains unclear.
Liver biopsy is regarded as the standard diagnostic method for NAFLD, but its invasive nature restricts its clinical usefulness. Currently, ultrasound is commonly used for diagnosing NAFLD in clinical practice. Thus, this study aims to analyze the correlation between METS-IR and based on ultrasound-diagnosed NAFLD in Chinese T2DM population, and to preliminarily understand the predictive value of METS-IR for NAFLD.
Methods
Study Participants
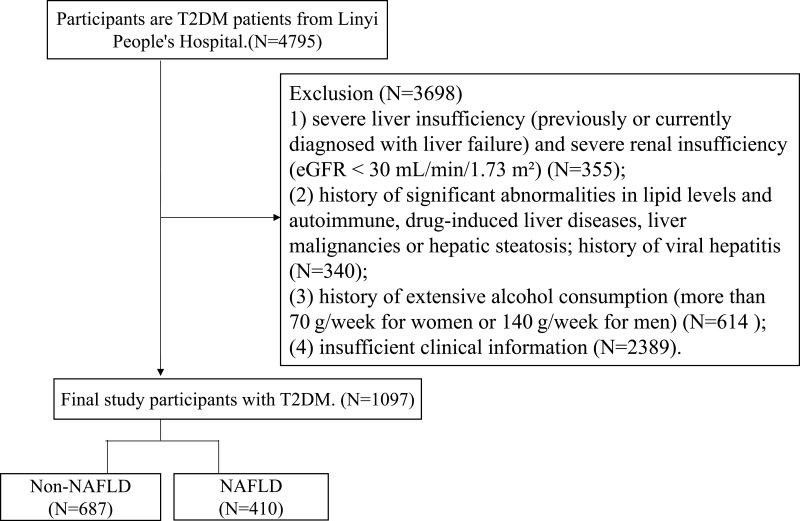
Based on the research objective, the data were collected from inpatients in the Department of Endocrinology of the Linyi People’s Hospital, Shandong Province, China, between January 2020 and March 2023. The inclusion criteria for participants included: individuals with T2DM aged 18 and above. Exclusion criteria for participants included: (1) severe liver insufficiency (previously or currently diagnosed with liver failure) and severe renal insufficiency (eGFR < 30 mL/min/1.73 m²); (2) history of significant abnormalities in lipid levels and autoimmune, drug-induced liver diseases, liver malignancies or hepatic steatosis; history of viral hepatitis; (3) history of extensive alcohol consumption (more than 70 g/week for women or 140 g/week for men); (4) insufficient clinical information. In the end, a total of 1097 T2DM participants aged 18 and above were included in the study (Figure 1).
Figure 1.
The flow chart of study participants selection.
Anthropometric and Biochemical Measurements
The collection of basic medical history and health habits of participants included age, gender, height, weight, diabetes duration, smoking habits and alcohol consumption. In a quiet environment, participants rested in a seated position for at least 5 minutes, then blood pressure was monitored using a standard electronic sphygmomanometer, recording systolic and diastolic blood pressure (S/DBP). Fasting venous blood samples were collected to measure total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), TG, high-density lipoprotein cholesterol (HDL-c), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), FPG, serum creatinine (Scr), uric acid (UA) and glycated hemoglobin (HbA1c, high-performance liquid chromatography), using a clinical chemistry analyzer (Cobas c 702, Roche, Germany). Fasting insulin (FINS, direct chemiluminescence method) was tested by the fully automated sample processing system (Aptio Automation, SIEMENS, USA). This study utilized the Omron DUALSCAN BIA machine (HDS-2000, Omron, Kyoto, Japan) to collect measurements of visceral fat area (VFA) and subcutaneous fat area (SFA), with units in cm².
Definitions
The diagnosis of NAFLD was based on liver ultrasound examination, in conjunction with the participant’s history of alcohol consumption and medication use, to exclude autoimmune, alcohol-induced and drug-induced fatty liver disease before confirming the diagnosis.
Parameter Calculations
Statistical Analysis
Data statistical analysis was performed using SPSS 26.0 (SPSS Inc, Chicago, IL, USA). Mean ± SD was used to describe normally distributed data, while median (interquartile range) was used for skewed data. The independent sample T-test and Mann–Whitney U-test were employed to compare continuous variables between groups with normal and non-normal distributions, respectively. One-way analysis of variance (ANOVA) and Kruskal–Wallis H were used to compare the differences in continuous variables among the four METS-IR groups with normal distribution and abnormal distributions, respectively. Chi square test was used to compare categorical variables. Spearman correlation analyses was used to assess the NAFLD-related correlations. Binary logistic stepwise regression analysis was conducted to identify independent risk factors for NAFLD. Analysis of the area under the receiver operating characteristic (ROC) curve was used to evaluate the ability of METS-IR in predicting NAFLD identification, and further conducted a differential analysis. A two-tailed P value <0.05 was considered statistically significant.
Results
Clinical Characteristics of the Study Subjects
The clinical characteristics of the participants are displayed in Table 1. Compared to the non-NAFLD group (n=687), the BMI, smoking, SBP, DBP, TC, LDL-c, TG, ALT, GGT, UA, FPG, FINS, HOMA-IR, TyG, VFA, SFA, TG/HDL-c ratio and METS-IR were higher, while the HDL-c was significantly lower in the NAFLD group (n=410) (all P < 0.05). There were no statistically significant difference in HbA1c, AST and Scr between the two groups (all P > 0.05).
Table 1.
Comparison of Clinical and Biochemical Characteristics Between Non-NAFLD and NAFLD Groups
| Variables | Non-NAFLD | NAFLD | P value |
|---|---|---|---|
| Number(n) | 687 | 410 | |
| Female (%) | 494 (71.9%) | 278 (67.8%) | < 0.001 |
| Age (years) | 60.60 ± 11.32 | 57.50 ± 11.95 | < 0.001 |
| Diabetes duration(years) | 10 (5.00 ~ 15.00) | 7.00 (3.00 ~ 11.00) | < 0.001 |
| Smoking (%) | 30 (4.4%) | 31 (7.6%) | < 0.001 |
| BMI (kg/m2) | 24.11 ± 3.22 | 27.05 ± 3.42 | < 0.001 |
| SBP (mmHg) | 129.28 ± 19.45 | 132.76 ± 17.82 | 0.002 |
| DBP (mmHg) | 77.96 ± 10.31 | 82.15 ± 11.02 | < 0.001 |
| TC (mmol/L) | 4.64 ± 1.16 | 4.94 ± 1.18 | < 0.001 |
| LDL-c (mmol/L) | 2.90 ± 0.97 | 3.10 ± 0.99 | 0.001 |
| TG (mmol/L) | 1.20 (0.86 ~ 1.71) | 1.73 (1.26 ~ 2.54) | < 0.001 |
| HDL-c (mmol/L) | 1.25 ± 0.31 | 1.12 ± 0.27 | < 0.001 |
| HbA1c (%) | 9.04 ± 2.20 | 9.24 ± 1.88 | 0.152 |
| ALT (U/L) | 15.90 (12.20 ~ 22.60) | 18.95 (14.30 ~ 28.33) | <0.001 |
| AST (U/L) | 17.10 (14.10 ~ 21.00) | 17.35 (14.20 ~ 22.10) | 0.169 |
| GGT (U/L) | 17.00 (13.00 ~ 23.40) | 25.00 (18.00 ~ 35.00) | < 0.001 |
| UA (μmol/L) | 261.17 ± 84.23 | 296.73 ± 84.53 | < 0.001 |
| Scr (μmol/L) | 63.06 ± 19.66 | 61.65 ± 17.06 | 0.239 |
| FPG (mmol/L) | 8.36 ± 3.20 | 9.20 ± 3.21 | < 0.001 |
| FINS (µIU/mL) | 16.04 (7.31 ~ 22.77) | 17.83 (12.65 ~ 23.67) | 0.004 |
| HOMA-IR | 5.29 (2.33 ~ 8.90) | 6.91 (4.14 ~ 9.84) | < 0.001 |
| TyG | 8.95 ± 0.68 | 9.45 ± 0.72 | < 0.001 |
| VFA (cm2) | 77.57 ± 36.88 | 108.54 ± 36.23 | < 0.001 |
| SFA (cm2) | 162.66 ± 59.26 | 215.63 ± 64.63 | < 0.001 |
| TG/HDL-c ratio | 3.02 ± 2.97 | 5.00 ± 4.98 | < 0.001 |
| METS-IR | 37.95 ± 6.85 | 45.13 ± 7.52 | < 0.001 |
Notes: Data were presented as mean ± SD for normally distributed variables, and median (interquartile ranges) for abnormal distributions. Independent-Samples T test and Mann–Whitney U-test were used for comparisons of normally and abnormally distributed continuous variables between Non-NAFLD and NAFLD groups, respectively. Categorical variables were presented as percentage (%), and were compared by chi-square test. Statistical differences were defined by P (two-tailed) less than 0.05.
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; LDL-c, low-density lipoprotein cholesterol; TG, triglyceride; HDL-c, high-density lipoprotein cholesterol; HbA1c, glycated hemoglobin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ- Glutamyl transpeptidase; UA, uric acid; Scr, serum creatinine; FPG, fasting plasma glucose; FINS, fasting insulin; HOMA-IR, homeostatic model assessment for insulin resistance; TyG, triglyceride glucose index; VFA, visceral fat area; SFA, subcutaneous fat area; METS-IR, metabolic score for IR; NAFLD, non-alcoholic fatty liver disease.
The study population was subsequently divided into four groups, including Q1 (22.71–35.41), Q2 (35.41–39.99), Q3 (39.99–45.41) and Q4 (45.41–68.72) based on the quartiles of the METS-IR (Table 2). As the quartiles of METS-IR increased, the smoking, BMI, SBP, DBP, TG, ALT, GGT, UA, FPG, FINS, HOMA-IR, TyG, VFA, SFA and TG/HDL-c ratio showed a gradual increased, while the HDL-c exhibited a gradual decreased (all P < 0.05). There was no statistically significant difference in diabetes duration, TC and AST among the four groups (all P > 0.05).
Table 2.
Comparison of Variables According to the Categories of the METS-IR
| Variables | Q1 (22.71~35.41) | Q2 (35.41~39.99) | Q3 (39.99~45.41) | Q4 (45.41~68.72) | P |
|---|---|---|---|---|---|
| Female | 223 (81.4%) | 196 (71.5%) | 187 (68%) | 166 (60.6%) | < 0.001 |
| Age | 60.43 ± 11.33 | 60.32 ± 10.70 | 59.62 ± 11.08 | 57.40 ± 13.16 | < 0.001 |
| Diabetes duration | 9.50 (4.00 ~ 15.00) | 10.00 (4.00 ~ 15.00) | 10.00 (4.00 ~ 13.00) | 8.00 (4.00 ~ 13.00) | < 0.001 |
| Smoking | 7 (2.6%) | 14 (5.1%) | 15 (5.5%) | 25 (9.1%) | < 0.001 |
| BMI | 21.32 ± 2.12 | 24.45 ± 1.77 | 25.98 ± 1.64 | 27.97 ± 2.91 | < 0.001 |
| SBP | 127.31 ± 20.17 | 129.31 ± 18.07 | 132.47 ± 18.48 | 133.22 ± 18.41 | 0.764 |
| DBP | 77.20 ± 11.13 | 79.05 ± 10.36 | 79.96 ± 9.73 | 81.90 ± 11.31 | 0.002 |
| TC | 4.81 ± 1.17 | 4.82 ± 1.11 | 4.71 ± 1.11 | 4.67 ± 1.30 | 0.312 |
| LDL-c | 2.97 ± 1.00 | 3.09 ± 0.97 | 3.02 ± 0.94 | 2.82 ± 1.02 | 0.672 |
| TG | 0.90 (0.70 ~ 1.26) | 1.26 (0.99 ~1 0.72) | 1.54 (1.18 ~ 2.09) | 2.10 (1.42 ~ 3.08) | < 0.001 |
| HDL-c | 1.46 ± 0.30 | 1.25 ± 0.25 | 1.12 ± 0.21 | 0.97 ± 0.21 | < 0.001 |
| ALT | 15.85 (11.88 ~ 23.43) | 16.50 (12.75 ~ 22.53) | 16.60 (13.10 ~24.10) | 18.10 (14.00 ~ 28.00) | < 0.001 |
| AST | 17.50 (14.40 ~ 22.50) | 17.40 (13.68 ~ 21.00) | 16.70 (13.90 ~ 20.30) | 17.10 (14.55 ~ 21.80) | < 0.001 |
| GGT | 16.00 (12.00 ~ 21.00) | 18.00 (14.00 ~ 25.00) | 21.00 (16.00 ~ 29.00) | 25.00 (17.00 ~ 36.00) | < 0.001 |
| UA | 245.97 ± 84.60 | 269.33 ± 83.37 | 273.28 ± 81.86 | 309.26 ± 82.63 | < 0.001 |
| Scr | 59.60 ± 16.60 | 62.84 ± 19.28 | 61.71 ± 1 7.55 | 65.99 ± 20.78 | < 0.001 |
| FPG | 7.68 ± 3.15 | 7.91 ± 2.81 | 8.99 ± 3 0.00 | 10.12 ± 3.35 | < 0.001 |
| FINS | 15.10 (5.63 ~ 22.87) | 15.09 (7.19 ~2 2.06) | 17.61 (12.11 ~ 24.12) | 18.58 (12.88 ~ 23.95) | < 0.001 |
| HOMA-IR | 4.14 (1.79 ~ 7.86) | 4.95 (2.61 ~ 8.00) | 6.82 (4.43 ~ 9.82) | 7.94 (4.86 ~ 11.70) | < 0.001 |
| TyG | 8.59 ± 0.59 | 8.96 ± 0.56 | 9.27 ± 0.52 | 9.73 ± 0.72 | < 0.001 |
| VFA | 51.00 (36.00 ~ 70.00) | 80.50 (62.00 ~ 101.00) | 95.00 (78.00 ~ 112.00) | 121.00 (97.00 ~ 148.00) | < 0.001 |
| SFA | 120.00 (85.00 ~ 151.25) | 164.50 (138.75 ~ 200.25) | 193.00 (164.00 ~ 221.00) | 234.00 (196.00 ~ 283.25) | < 0.001 |
| TG/HDL-c ratio | 1.74 ± 1.11 | 2.77 ±1.58 | 3.62 ± 1.85 | 6.89 ± 6.40 | < 0.001 |
| NAFLD | 30 (10.9%) | 80 (29.2%) | 112 (40.7%) | 188 (68.6%) | < 0.001 |
Notes: Data with a skewed distribution were shown as median (interquartile range). One-way analysis of variance (ANOVA) and Kruskal–Wallis H were used to compare the differences in continuous variables among the four METS-IR groups with normal distribution and abnormal distributions, respectively. Chi square test was used to compare categorical variables. Spearman correlation analyses was used to assess the NAFLD-related correlations. Statistical differences were defined by P (two-tailed) less than 0.05.
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; LDL-c, low-density lipoprotein cholesterol; TG, triglyceride; HDL-c, high-density lipoprotein cholesterol; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ- Glutamyl transpeptidase; UA, uric acid; Scr, serum creatinine; FPG, fasting plasma glucose; FINS, fasting insulin; HOMA-IR, homeostatic model assessment for insulin resistance; TyG, triglyceride glucose index; VFA, visceral fat area; SFA, subcutaneous fat area.
Univariate Analysis
As indicated by the Spearman correlation analysis in Table 3, NAFLD exhibited positive correlations with smoking, BMI, SBP, DBP, TC, LDL-c, TG, HbA1c, ALT, GGT, UA, FPG, FINS, HOMA-IR, TyG, VFA, SFA, TG/HDL-c ratio and METS-IR, while showing inverse correlations with age, diabetes duration and HDL-c (all P < 0.05). Gender, AST and Scr showed no correlation with NAFLD (all P > 0.05).
Table 3.
The Correlation NAFLD by Univariate Analysis
| Variables | Correlation Coefficient | P |
|---|---|---|
| Age | −0.115 | < 0.001 |
| Diabetes duration | −0.165 | < 0.001 |
| Sex | −0.043 | 0.150 |
| Smoking | 0.064 | 0.035 |
| BMI | 0.394 | < 0.001 |
| SBP | 0.096 | 0.001 |
| DBP | 0.190 | < 0.001 |
| TC | 0.122 | < 0.001 |
| LDL-c | 0.106 | < 0.001 |
| TG | 0.346 | < 0.001 |
| HDL-c | −0.222 | < 0.001 |
| HbA1c | 0.073 | 0.016 |
| AST | 0.040 | 0.188 |
| ALT | 0.184 | < 0.001 |
| GGT | 0.353 | < 0.001 |
| UA | 0.213 | < 0.001 |
| Scr | −0.008 | 0.790 |
| FPG | 0.148 | < 0.001 |
| FINS | 0.115 | 0.004 |
| HOMA-IR | 1.171 | < 0.001 |
| VFA | 0.385 | < 0.001 |
| SFA | 0.383 | < 0.001 |
| TG/HDL-c ratio | 0.354 | < 0.001 |
| METS-IR | 0.441 | < 0.001 |
Notes: Data were presented as mean ± SD for normally distributed variables, and median (interquartile ranges) for abnormal distributions. Independent-Samples T test and Mann–Whitney U-test were used for comparisons of normally and abnormally distributed continuous variables NAFLD groups, respectively. Correlation coefficients NAFLD and different variables were determined by Spearman correlation analysis, respectively.
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; LDL-c, low-density lipoprotein cholesterol; TG, triglyceride; HDL-c, high-density lipoprotein cholesterol; HbA1c, glycated hemoglobin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ- Glutamyl transpeptidase; UA, uric acid; Scr, serum creatinine; FPG, fasting plasma glucose; FINS, fasting insulin; HOMA-IR, homeostatic model assessment for insulin resistance; TyG, triglyceride glucose index; VFA, visceral fat area; SFA, subcutaneous fat area; METS-IR, metabolic score for IR; NAFLD, non-alcoholic fatty liver disease.
Logistic Regression Analysis
NAFLD was defined as the dependent variable, and the above-mentioned relevant indicators (Table 3) as independent variables. Following the adjustment for potential confounders including the BMI, SBP, DBP, LDL-c, TG, HbA1c, FPG, FINS, HOMA-IR, TyG, TG/HDL-c ratio, age and HDL-c, the binary logistic regression analysis (Table 4) revealed that the METS-IR (OR: 1.120, 95% CI 1.080–1.161), TC (OR: 1.343, 95% CI 1.129–1.599), DBP (OR: 1.024, 95% CI 1.004–1.044), VFA (OR: 1.011, 95% CI 1.005–1.018), UA (OR: 1.003, 95% CI 1.001–1.005) and diabetes duration (OR: 0.967, 95% CI 0.937–0.996) were independently associated with NAFLD.
Table 4.
The Relative Risk for NAFLD by Logistic Regression Analysis
| Variables | B | SE | Wals | P | OR | 95.0% CI for OR |
|---|---|---|---|---|---|---|
| METS-IR | 0.113 | 0.018 | 38.255 | < 0.001 | 1.120 | 1.080–1.161 |
| TC | 0.295 | 0.089 | 11.082 | 0.001 | 1.343 | 1.129–1.599 |
| DBP | 0.024 | 0.010 | 5.615 | 0.018 | 1.024 | 1.004–1.044 |
| VFA | 0.011 | 0.003 | 10.817 | 0.001 | 1.011 | 1.005–1.018 |
| UA | 0.003 | 0.001 | 6.078 | 0.014 | 1.003 | 1.001–1.005 |
| Diabetes duration | −0.034 | 0.016 | 4.776 | 0.029 | 0.967 | 0.937–0.996 |
Abbreviations: METS-IR, metabolic score for IR; TC, total cholesterol; VFA, visceral fat area; DBP, diastolic blood pressure; UA, uric acid; NAFLD, non-alcoholic fatty liver disease. SE, standard error; CI, confidence interval; OR, odd ratio.
Areas Under the ROC Curve Analysis
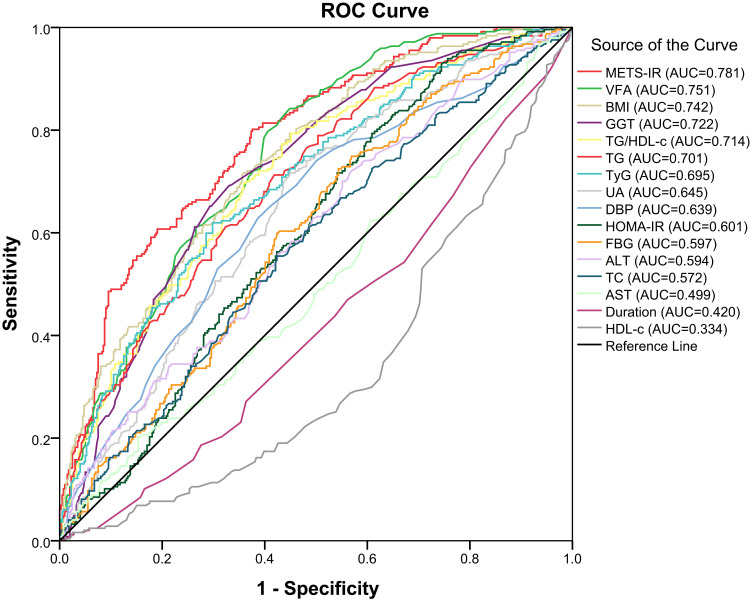
Finally, we compared the area under the ROC curves of METS-IR with those of the variables that constitute METS-IR (BMI, TG, FPG and HDL-c), traditional indicators related to NAFLD (AST, ALT and GGT), and commonly used effective IR-related indicators (TG/HDL-c ratio, TyG and HOMA-IR), and indicators entering the regression model (VFA, DBP, TC, UA and diabetes duration) in Table 5. The findings indicated that the area under the ROC curve for METS-IR was 0.781 (95% CI 0.746–0.817, P < 0.001) (Figure 2). Moerover, differential analysis of ROC showed that METS-IR was higher than BMI, GGT, TG/HDL-c ratio, TG, TyG, UA, DBP, HOMA-IR, FPG, ALT, TC, AST, diabetes duration and HDL-c (all P < 0.05), whereas the difference between METS-IR and VFA was not statistically significant (P = 0.077).
Table 5.
Analysis of Areas Under the ROC Curves for Predicting NAFLD
| Variables | Area | Cut-off value | Sensitivity | Specificity |
|---|---|---|---|---|
| METS-IR | 0.781 | 38.770 | 0.802 | 0.642 |
| VFA | 0.751 | 75.500 | 0.842 | 0.551 |
| BMI | 0.742 | 24.940 | 0.717 | 0.642 |
| GGT | 0.722 | 20.800 | 0.666 | 0.677 |
| TG/HDL-c ratio | 0.714 | 2.360 | 0.794 | 0.549 |
| TG | 0.701 | 116.509 | 0.713 | 0.581 |
| TyG | 0.695 | 9.250 | 0.619 | 0.702 |
| UA | 0.645 | 251.450 | 0.717 | 0.551 |
| DBP | 0.639 | 79.500 | 0.628 | 0.386 |
| HOMA-IR | 0.601 | 3.890 | 0.794 | 0.391 |
| FPG | 0.597 | 129.870 | 0.725 | 0.456 |
| ALT | 0.594 | 14.550 | 0.746 | 0.419 |
| TC | 0.572 | 4.805 | 0.555 | 0.576 |
| AST | 0.499 | 24.250 | 0.198 | 0.868 |
| Diabetes duration | 0.420 | 11.50 | 0.231 | 0.647 |
| HDL-c | 0.334 | 45.090 | 0.300 | 0.391 |
Abbreviations: METS-IR, metabolic score for IR; VFA, visceral fat area; BMI, body mass index; GGT, γ- Glutamyl transpeptidase; TG, triglyceride; HDL-c, high-density lipoprotein cholesterol; TyG, triglyceride glucose index; UA, uric acid; DBP, diastolic blood pressure; HOMA-IR, homeostatic model assessment for insulin resistance; FPG, fasting plasma glucose; ALT, alanine aminotransferase; TC, total cholesterol; AST, aspartate aminotransferase; NAFLD, non-alcoholic fatty liver disease. SE, standard error; CI, confidence interval.
Figure 2.
ROC analyses for NAFLD.
Discussion
Through this cross-sectional study, we found a close association between METS-IR and NAFLD, with the incidence of NAFLD gradually increasing as the quartiles of METS-IR increase. Following adjustment for confounding factors using logistic regression, multivariate analysis revealed a persistent independent correlation between METS-IR and NAFLD. Furthermore, areas of ROC curve analysis indicated that METS-IR was relatively higher than other evaluation variables.
Obesity, hypertriglyceridemia, impaired FPG, diabetes mellitus, IR and other metabolic diseases were the main risk factors for NAFLD.19 METS-IR was considered an independent predictive indicator of IR.20 IR has been confirmed as a key factor in the development of NAFLD in previous studies.21,22 Currently, some studies had investigated the relationship between METS-IR and NAFLD in different populations. Cai et al pointed out that METS-IR was independently associated with NAFLD risk in 10,730 non-obese Chinese adults.2 Li et al’s study confirmed that among 72225 adults aged 60 and above in China, compared to the other six IR substitutes, METS-IR might more reliably identify NAFLD.1 In addition, Lee et al pointed out that in a general population of 10,030 individuals aged between 40 and 69 years in South Korea, METS-IR outperformed other indicators in predicting the occurrence of NAFLD.15 However, there was no evidence to indicate the ability of METS-IR to predict NAFLD in T2DM population. Our research findings showed that METS-IR was an independent risk factor for NAFLD in patients with T2DM, further demonstrating the tight connection between these two variables in different populations.
IR is a key mechanism in the development of NAFLD. HOMA-IR is one of the most commonly used indicators to assess IR. Studies had shown that HOMA-IR was significantly associated with NAFLD.23 The TG/HDL-c ratio and TyG index are newly proposed indicators of IR in recent years, reflecting lipid and glucose metabolism. Research indicated that they are also closely related to NAFLD.24,25 We included above the IR-related indicators. The results showed that they did not enter the regression model, and had significantly smaller areas under the ROC curve compared to METS-IR.
The GGT, ALT and AST levels were considered as the common indicators for evaluating liver cell damage. Research has shown that GGT, ALT and AST were closely associated with NAFLD and could be used as alternative markers for evaluating the condition.26–28 In our study, Spearman correlation analysis showed that both ALT and GGT were associated with NAFLD. However, after adjusting confounding factors, the regression model showed that the ALT and GGT did not enter the model. Furthermore, an analysis of the area under the ROC curve for METS-IR showed that area under the ROC curve was greater than that of ALT, AST and GGT. Therefore, this suggested that single ALT, AST or GGT might not be superior to METS-IR in identifying NAFLD in T2DM.
Limitations
This study has several limitations. Firstly, it is a cross-sectional study, so we can only analyze the relationship between METS-IR and NAFLD, and cannot establish a significant causal relationship. Secondly, in this study, the diagnosis of NAFLD was established through abdominal ultrasound, which may have led to the omission of some NAFLD patients in comparison to the gold standard method of liver biopsy. Additionally, an insulin clamp test should be conducted to compare the specificity of identifying IR between METS-IR and other indicators. Thirdly, our results were based on data analysis from hospitalized patients only, further prospective and multicenter studies are needed to support our findings.
Conclusion
METS-IR is a new index for assessing IR. Our study showed that METS-IR was an independent risk factor for NAFLD in T2DM population, and it might outperform other commonly used variables in identifying NAFLD in Chinese T2DM population. Future research should pay more attention to the value of METS-IR in identifying NAFLD in T2DM patients.
Acknowledgments
Xuan Ma and Baolan Ji are co-first authors for this study. Guanqi Gao and Bo Ban are co-corresponding authors for this study. All of our authors would like to express gratitude to everyone involved in this research.
Funding Statement
This study was funded by grants from the Postdoctoral Program of Affiliated Hospital of Jining Medical University (JYFY322152).
Ethics Approval and Consent to Participate
The Human Ethics Committee of the People’s Hospital of Linyi has reviewed and approved this study. We conducted this research in accordance with the ethical principles regarding human subjects as outlined in the Helsinki Declaration. And informed consent was obtained from the patients.
Disclosure
The authors declare that they have no competing interests in this study.
References
- 1.Li H, Shi Z, Chen X, et al. Relationship between six insulin resistance surrogates and nonalcoholic fatty liver disease among older adults: a cross-sectional study. DMSO. 2023;16:1685–1696. doi: 10.2147/DMSO.S409983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai X, Gao J, Hu J, et al. Dose-response associations of metabolic score for insulin resistance index with nonalcoholic fatty liver disease among a nonobese Chinese population: retrospective evidence from a population-based cohort study. Dis Markers. 2022;2022:1–10. doi: 10.1155/2022/4930355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrne CD, Targher G. NAFLD as a driver of chronic kidney disease. J Hepatol. 2020;72(4):785–801. doi: 10.1016/j.jhep.2020.01.013 [DOI] [PubMed] [Google Scholar]
- 4.Fujiwara N, Qian T, Koneru B, Hoshida Y. Omics-derived hepatocellular carcinoma risk biomarkers for precision care of chronic liver diseases. Hepatol Res. 2020;50(7):817–830. doi: 10.1111/hepr.13506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brouwers MCGJ, Simons N, Stehouwer CDA, Isaacs A. Non-alcoholic fatty liver disease and cardiovascular disease: assessing the evidence for causality. Diabetologia. 2020;63(2):253–260. doi: 10.1007/s00125-019-05024-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koo BK, Allison MA, Criqui MH, Denenberg JO, Wright CM. The association between liver fat and systemic calcified atherosclerosis. J Vasc Surg. 2020;71(1):204–211.e4. doi: 10.1016/j.jvs.2019.03.044 [DOI] [PubMed] [Google Scholar]
- 7.Tarantino G, Sinatti G, Citro V, Santini SJ, Balsano C. Sarcopenia, a condition shared by various diseases: can we alleviate or delay the progression? Intern Emerg Med. 2023;18(7):1887–1895. doi: 10.1007/s11739-023-03339-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujii H, Kawada N, Japan Study Group Of Nafld Jsg-Nafld. The role of insulin resistance and diabetes in nonalcoholic fatty liver disease. Int J Mol Sci. 2020;21(11):3863. doi: 10.3390/ijms21113863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindenmeyer CC, McCullough AJ. The natural history of nonalcoholic fatty liver disease-an evolving view. Clin Liver Dis. 2018;22(1):11–21. doi: 10.1016/j.cld.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–223. doi: 10.1152/ajpendo.1979.237.3.E214 [DOI] [PubMed] [Google Scholar]
- 11.Bello-Chavolla OY, Almeda-Valdes P, Gomez-Velasco D, et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. 2018;178(5):533–544. doi: 10.1530/EJE-17-0883 [DOI] [PubMed] [Google Scholar]
- 12.Liu XZ, Mb JF, Mb SJP. METS‐IR, a novel simple insulin resistance indexes, is associated with hypertension in normal‐weight Chinese adults. The Journal of Clinical Hypertension. 2019;21(8):1075–1081. doi: 10.1111/jch.13591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon J, Jung D, Lee Y, Park B. The Metabolic Score for Insulin Resistance (METS-IR) as a predictor of incident ischemic heart disease: a longitudinal study among Korean without diabetes. J Pers Med. 2021;11(8):742. doi: 10.3390/jpm11080742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai XT, Zhu Q, Liu SS, et al. Associations between the metabolic score for insulin resistance index and the risk of Type 2 Diabetes mellitus among non-obese adults: insights from a population-based cohort study. Int J Gen Med. 2021;14:7729–7740. doi: 10.2147/IJGM.S336990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JH, Park K, Lee HS, Park HK, Han JH, Ahn SB. The usefulness of metabolic score for insulin resistance for the prediction of incident non-alcoholic fatty liver disease in Korean adults. Clin Mol Hepatol. 2022;28(4):814–826. doi: 10.3350/cmh.2022.0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee YC, Lee JW, Kwon YJ. Comparison of the triglyceride glucose (TyG) index, triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio, and metabolic score for insulin resistance (METS-IR) associated with periodontitis in Korean adults. Ther Adv Chronic Dis. 2022;13:20406223221122671. doi: 10.1177/20406223221122671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 18.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304. doi: 10.1089/met.2008.0034 [DOI] [PubMed] [Google Scholar]
- 19.Jarvis H, Craig D, Barker R, et al. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of population-based observational studies. PLoS Med. 2020;17(4):e1003100. doi: 10.1371/journal.pmed.1003100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji L, Cai X, Bai Y, Li T. Application of a novel prediction model for predicting 2-year risk of non-alcoholic fatty liver disease in the non-obese population with normal blood lipid levels: a large prospective cohort study from China. Int J Gen Med. 2021;14:2909–2922. doi: 10.2147/IJGM.S319759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinn DH, Gwak GY, Park HN, et al. Ultrasonographically detected non-alcoholic fatty liver disease is an independent predictor for identifying patients with insulin resistance in non-obese, non-diabetic middle-aged Asian adults. Am J Gastroenterol. 2012;107(4):561–567. doi: 10.1038/ajg.2011.400 [DOI] [PubMed] [Google Scholar]
- 22.Young S, Tariq R, Provenza J, et al. Prevalence and profile of nonalcoholic fatty liver disease in lean adults: systematic review and meta-analysis. Hepatol Commun. 2020;4(7):953–972. doi: 10.1002/hep4.1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutierrez-Buey G, Núñez-Córdoba JM, Llavero-Valero M, Gargallo J, Salvador J, Escalada J. Is HOMA-IR a potential screening test for non-alcoholic fatty liver disease in adults with type 2 diabetes? Eur J Intern Med. 2017;41:74–78. doi: 10.1016/j.ejim.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 24.Potential screening indicators for early diagnosis of NAFLD/MAFLD and liver fibrosis: triglyceride glucose index-related parameters - PubMed. Accessed August 19, 2024. https://pubmed.ncbi.nlm.nih.gov/36120429/. [DOI] [PMC free article] [PubMed]
- 25.Catanzaro R, Selvaggio F, Sciuto M, et al. Triglycerides to high-density lipoprotein cholesterol ratio for diagnosing nonalcoholic fatty liver disease. Minerva Gastroenterol. 2022;68(3):261–268. doi: 10.23736/S2724-5985.21.02818-X [DOI] [PubMed] [Google Scholar]
- 26.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59(9):1265–1269. doi: 10.1136/gut.2010.216077 [DOI] [PubMed] [Google Scholar]
- 27.Martin-Rodriguez JL, Gonzalez-Cantero J, Gonzalez-Cantero A, Arrebola JP, Gonzalez-Calvin JL. Diagnostic accuracy of serum alanine aminotransferase as biomarker for nonalcoholic fatty liver disease and insulin resistance in healthy subjects, using 3T MR spectroscopy. Medicine. 2017;96(17):e6770. doi: 10.1097/MD.0000000000006770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ha Y, Chon YE, Kim MN, Lee JH, Hwang SG. Gamma-glutamyl transpeptidase dynamics as a biomarker for advanced fibrosis in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2022;37(8):1624–1632. doi: 10.1111/jgh.15871 [DOI] [PubMed] [Google Scholar]