Abstract
Vaccine-elicited antibodies specific for the third hypervariable domain of the surface gp120 of human immunodeficiency virus type 1 (HIV-1) (V3 loop) were assessed for their contribution to protection against infection in the simian-human immunodeficiency virus (SHIV)/rhesus monkey model. Peptide vaccine-elicited anti-V3 loop antibody responses were examined for their ability to contain replication of SHIV-89.6, a nonpathogenic SHIV expressing a primary patient isolate HIV-1 envelope, as well as SHIV-89.6P, a pathogenic variant of that virus. Low-titer neutralizing antibodies to SHIV-89.6 that provided partial protection against viremia following SHIV-89.6 infection were generated. A similarly low-titer neutralizing antibody response to SHIV-89.6P that did not contain viremia after infection with SHIV-89.6P was generated, but a trend toward protection against CD4+ T-lymphocyte loss was seen in these infected monkeys. These observations suggest that the V3 loop on some primary patient HIV-1 isolates may be a partially effective target for neutralizing antibodies induced by peptide immunogens.
It has long been known that the strain-specific virus-neutralizing activity in the serum of human immunodeficiency virus type 1 (HIV-1)-infected individuals is mediated at least in part by antibodies that recognize a loop structure formed by a cysteine-cysteine bond in the third hypervariable domain of the surface gp120 (42, 45). This V3 loop is known as the principal neutralizing domain of T-cell-line-adapted (TCLA) strains of HIV-1 (15, 26, 31). The potential importance of the V3 loop as a target in the development of an HIV-1 vaccine was suggested by the finding that chimpanzees infused with a V3-specific neutralizing monoclonal antibody were passively protected from challenge with a TCLA strain of HIV-1 (10). Moreover, when chimpanzees were immunized with the envelope glycoprotein and then received a boosting immunization with the same V3 sequence peptide, they generated antibodies that neutralized HIV-1 in vitro and were protected from intravenous challenge with cell-free HIV-1 (12). The enthusiasm of investigators for pursuing a V3 loop-based strategy in HIV-1 vaccine development was, however, dampened by the observation that the V3 loop on the envelope glycoproteins of primary patient HIV-1 isolates can be relatively inaccessible to antibodies (1, 2, 36, 41). Nevertheless, interest in this antigenic domain of HIV-1 envelope as a target for antibody-mediated neutralization persists because of its importance in virus entry (6, 7, 21, 37–40, 43).
One of the interesting and perhaps useful characteristics of the V3 loop with regard to a vaccine is that it can be mimicked by synthetic peptides. Not only do V3-specific antibodies in the serum of individuals infected with HIV-1 recognize synthetic peptides of the appropriate amino acid sequences (5, 34), but also HIV-1-neutralizing V3-specific antibodies can be elicited by immunizing laboratory animals with synthetic peptides (14, 26, 27). Thus, a native three-dimensional antigen may not be required to elicit a V3 loop-specific antibody with anti-HIV-1 activity.
In spite of the extensive work that has gone into characterizing the role of the V3 loop of the HIV-1 envelope in antibody-mediated viral neutralization, a V3 loop-based immunogen has not been carefully assessed for eliciting protection against a pathogenic viral challenge in a nonhuman primate species. In this regard, the recently developed simian-human immunodeficiency viruses (SHIVs) provide powerful tools for assessing the potential role of V3 loop-specific antibodies in vaccine-elicited protective immunity. SHIVs, viral constructs that express HIV-1 envelopes on a simian immunodeficiency virus (SIV) backbone, have been developed that express primary patient HIV-1 envelopes and induce AIDS in macaques. Such viral constructs can be used as challenge viruses in macaque species to evaluate the protection against infection conferred by vaccine-induced anti-HIV-1 Env antibodies (17, 30).
The present study was done to determine the potential contribution of vaccine-elicited V3 loop-specific antibody protection against infection by SHIVs. Specifically, peptide-elicited anti-V3 loop antibody responses have been assessed for their ability to contain replication of SHIV-89.6, a nonpathogenic SHIV expressing a primary patient isolate HIV-1 envelope, as well as SHIV-89.6P, a pathogenic variant of that virus.
MATERIALS AND METHODS
Animals.
Adult rhesus macaques were maintained in accordance with the guidelines of the Committee on Animals for the Harvard Medical School and the Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services publication 85-23, revised 1985). All monkeys were colony born and seronegative for simian retrovirus and simian T-lymphotropic virus type 1.
Immunization.
For each immunization, 1 mg of peptide was solubilized in 0.5 ml of phosphate-buffered saline and then emulsified with an equal volume of incomplete Freund's adjuvant (Sigma, St. Louis, Mo.). The peptide emulsion (1 ml) was inoculated by the intramuscular route in four sites on the back of each animal.
Virus stocks.
Cell-free stocks of SHIV-89.6, SHIV-89.6P, SHIV-KB9, and SHIV-KU2 were produced in human peripheral blood mononuclear cells (PBMC) (23). Cell-free stocks of SHIV-HXBc2 and HIV-1 strains MN and SF2 were produced in H9 cells (25). Animal challenge stocks of SHIV-89.6 and SHIV-89.6P were prepared in rhesus macaque PBMC. All virus stocks were stored in aliquots at −80°C until use.
Cells.
MT-2 (13) and CEMx174 (33) are human CD4+ lymphoblastoid cell lines permissive to cytopathic infection with the SHIV and HIV-1 variants utilized here. Cell culture growth medium consisted of RPMI 1640 supplemented with 12% heat-inactivated fetal bovine serum and 50 μg of gentamicin/ml. Human PBMC were prepared from buffy coats of healthy, HIV-1-negative individuals obtained through the Laboratory Services of the American Red Cross Carolina Region in Charlotte, N.C. The PBMC were isolated by centrifugation over lymphocyte separation medium (Organon-Teknika/Akzo, Durham, N.C.). Cells at the interface were washed twice in growth medium containing 20% heat-inactivated fetal bovine serum and resuspended at a density of 2.5 × 107 cells/ml and frozen in 1-ml portions in liquid nitrogen with the aid of a Gordonier controlled-rate cryostat. Prior to their use in neutralization assays, and to grow virus stocks, aliquots of PBMC were thawed in a room temperature water bath and incubated for 1 day at 37°C in 5% CO2–95% humidified air in growth medium supplemented with phytohemagglutinin-P (5 μg/ml) and 4% human interleukin-2 (IL-2).
Peptides.
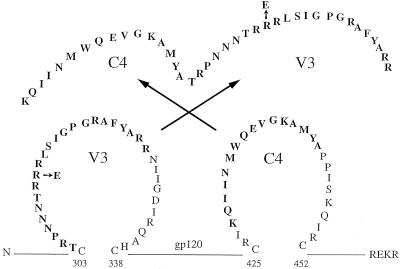
Peptides were synthesized by SynPep Corporation (Dublin, Calif.) and purified by reverse-phase high-pressure liquid chromatography (HPLC). All peptides were >95% purified as determined by HPLC and mass spectrometry. SHIV-89.6 and SHIV-KB9 V3 loop peptides were synthesized C-terminal to a T-helper determinant located in the C4 region of gp120 for enhanced immunogenicity (26). Sequences of the C4/89.6-V3 and C4/KB9-V3 peptides are shown in Fig. 1. A control peptide (C4/scbl-V3) consisted of scrambled amino acid sequences of the SHIV-89.6 V3 loop (RGYFTRRNAPSNTARGRPILRRN) synthesized C-terminal to the C4 helper determinant. Two additional peptides (89.6-V3 and 89.6P-V3) consisted of the V3 loop portions of the C4/89.6-V3 and C4/89.6P-V3 peptides lacking C4.
FIG. 1.
Location and sequence of the C4/89.6-V3 and C4/89.6P-V3 peptides. Peptides 39 amino acids in length contained sequences from the crown and N-terminal portion of the V3 loop synthesized C-terminal to a 16-amino-acid stretch of the C4 helper determinant of gp120. The 89.6 and 89.6P peptides differed by a single arginine (R)-to-glutamic acid (E) substitution in V3 as shown.
Neutralizing antibody assays.
Neutralizing antibodies were assessed in CD4+ cell lines and in human PBMC. Measurements of SHIV neutralization in these human cells have been predictive of antibody efficacy in macaques (17, 19, 20). The cell line assay measured neutralization as a reduction in virus-induced cell killing as described previously (25). Briefly, 50 μl of cell-free virus containing 500 50% tissue culture infectious doses (TCID50) was added to 100 μl of diluted test serum in triplicate in 96-well culture plates for a total of eight dilutions per serum sample. As controls, eight wells contained growth medium alone (cell control) whereas another eight wells contained growth medium plus virus (virus control). Following a 1-h incubation at 37°C, MT-2 or CEMx174 cells (5 × 104 cells in 100 μl) were added to all wells and incubated until 70 to 90% of cells in the virus control wells were involved in syncytium formation (usually 4 to 6 days). Medium was replaced, and cell densities were reduced by 50% on day 3 of incubation. Cell viability was quantified by neutral red uptake as described previously (25). Percent protection from virus-induced cell killing (neutralization) was determined by calculating the difference in absorption (A540) between test wells and virus control wells, dividing this result by the difference in absorption between cell control wells and virus control wells, and multiplying by 100. Neutralization titers are given as the reciprocal serum dilution (dilution in the presence of virus prior to addition of cells) required to protect 50% of cells from virus-induced killing. This 50% protection from cell killing corresponds to an 85 to 90% reduction in viral Gag antigen synthesis in this assay (3). For assays in which peptides were tested for their ability to block neutralizing antibodies, undiluted serum samples were incubated for 1 h at 37°C in the presence and absence of peptide (50 μg/ml). Titers of neutralizing antibodies were then determined in MT-2 cells as described above.
Assays with human PBMC utilized a reduction in SHIV p27 Gag antigen synthesis as a measurement of neutralization (8). Briefly, diluted serum samples were incubated with virus (500 TCID50) in triplicate for 1 h at 37°C in a total volume of 50 μl in 96-well U-bottom culture plates. Six wells containing virus only (no test sample) were included as controls. The mixtures were incubated at 37°C for 1 h, after which time PBMC (4 × 105 cells in 150 μl of IL-2 growth medium) were added to each well. Following an additional 24-h incubation period, the virus inoculum and antibodies were removed by three washes with 200 μl of growth medium. Washed cells were resuspended in 200 μl of IL-2 growth medium and incubated in fresh 96-well U-bottom plates. Culture supernatants (25 μl) were collected on a daily basis thereafter and mixed with 225 μl of 0.5% Triton X-100 for the later quantification of p27 produced by infection. Viral p27 was quantified with an antigen enzyme-linked immunosorbent assay (ELISA) as described by the supplier (Organon-Teknika/Akzo). The 25-μl volume of culture fluid removed each day was replaced with 25 μl of fresh IL-2-containing growth medium. Measurements of p27 for the detection of neutralization were made on a harvest prior to the time when p27 production in virus control wells had reached peak concentrations, which is when optimum sensitivity is achieved in this assay (44). Neutralization was considered positive when p27 synthesis was reduced by ≥80%, which corresponds to a fivefold reduction in infectious virus. This is the minimum cutoff that reliably predicts positive neutralization in this PBMC assay (3) and approximates the cutoff (50% protection from virus-induced cell killing) in the MT-2 and CEMx174 assays described above (3).
ELISA.
Peptide-specific binding antibodies were assessed in Nunc (Roskilde, Denmark) immunoplates (MaxiSorb F96) using alkaline phosphatase-conjugated goat anti-monkey immunoglobulin G as described previously (8). Titers are given as the last serum dilution to yield an absorbance at 405 nm that was twice that of a negative control serum sample (nonimmunized macaque).
Western blotting.
Anti-Gag p27 seroconversion was assessed by HIV-2 Western blotting as described by the supplier of the kit used (Cambridge Biotech, Cambridge, Mass.), with the exception that alkaline phosphatase-conjugated anti-monkey immunoglobulin G (Sigma Biosciences) was substituted for the antihuman reagent supplied in the kit.
SHIV challenge studies.
Vaccinated monkeys were challenged by intravenous inoculation with 20 TCID50 of a cell-free SHIV-89.6 stock (kindly provided by Yichen Lu, Viral Research Institute, Cambridge, Mass.) or cell-free SHIV-89.6P with a 1:500 dilution of a standard viral stock, the smallest inoculation of virus that initiates an infection in 100% of rhesus monkeys. Following SHIV inoculation, viral SIVmac gag RNA levels in plasma samples were determined by branched-DNA (bDNA) assay (Bayer Diagnostics, Emeryville, Calif.) and CD4+ peripheral blood lymphocytes (PBL) were quantitated by routine flow-cytometric analysis and determination of complete blood counts and differentials (35).
bDNA quantitation of SIV RNA.
SIV RNA was quantitated by a bDNA signal amplification assay (P. J. Dailey, M. Zamroud, R. Kelso, J. Kolberg, and M. Urdea, Abstr. 13th Annu. Symp. Nonhuman Primate Models AIDS, abstr. 99, 1995). The target probes were designed to hybridize with the pol region of the SIVmac group of virus strains, including SIVmac 251, SIVmac239, and SIVmne. SIV RNA per 106 CD4+ cells was quantified by comparison with a standard curve produced by purified, quantified, in vitro-transcribed SIVmac239 pol RNA. The lower quantitation limit of this assay was 3,000 SIV RNA equivalents per sample.
Statistical tests.
Statistical significance was assessed by comparing data from the groups of monkeys using Wilcoxon rank sum tests.
RESULTS
Peptide vaccination strategy.
To explore the contribution of vaccine-elicited V3 loop-specific immunity in the protection of rhesus monkeys against infection with a lentivirus expressing a primary patient isolate HIV-1 envelope, monkeys were immunized in two experiments with either an 89.6 V3 loop peptide conjugate or an 89.6P V3 loop peptide conjugate and challenged with either SHIV-89.6 or SHIV-89.6P. These V3 loop peptides were conjugated to the C4 peptide to provide helper epitopes to the immunogens (27). In the first experiment, one group of monkeys received the C4/89.6-V3 peptide, one control group of monkeys received the C4 sequence conjugated to a peptide representing a scrambled sequence of the 89.6 V3 loop amino acids (C4/scbl-V3), and a second control group of monkeys received the C4 sequence conjugated to the 89.6P V3 loop peptide (C4/89.6P-V3). The 89.6P V3 loop differs from the 89.6 V3 loop by a single amino acid (Fig. 1). However, this single amino acid substitution results in a change in the strain-specific neutralizing capacity of sera generated through immunization with that peptide (18). Therefore, monkeys immunized with the C4/89.6P control immunogen would be expected to have antibodies that bind to the 89.6 V3 loop but do not neutralize a virus expressing the 89.6 V3 loop sequence. Monkeys were immunized by the intramuscular route on a schedule of 0, 4, 12, 24, 89, 95, 101, and 111 weeks with 1 mg of peptide adjuvanted with incomplete Freund's adjuvant and were challenged with the nonpathogenic SHIV-89.6 at week 113.
Since SHIV-89.6-infected naive rhesus monkeys do not maintain a level of replicating virus that is high enough to allow detection of plasma viral RNA following primary infection and do not develop clinical manifestations of AIDS, challenges of immunized monkeys with this virus do not allow determination of whether vaccinees have a lower viral set point than control immunized monkeys and whether an immunization strategy alters the pathogenic consequences of a lentivirus infection. To examine these issues, a second experiment was performed. As in the first experiment, a control group of monkeys received the C4 sequence conjugated to a peptide representing a scrambled sequence of the 89.6 V3 loop peptide. The other group of monkeys received the C4/89.6P V3 loop peptide immunogen. These monkeys were immunized by the intramuscular route on a schedule of 0, 6, 12, 18, 24, and 31 weeks with 1 mg of peptide adjuvanted with incomplete Freund's adjuvant and were challenged with the pathogenic SHIV-89.6P at week 33. We chose fewer inoculations in this experiment because in the first experiment the antibody response did not improve after six inoculations.
Antibody responses following peptide immunization.
Serum antibodies were assessed by ELISA for their ability to bind the C4/scbl-V3, C4/89.6-V3, 89.6-V3, and 89.6P-V3 peptides. As shown in Tables 1 and 2, immunization with either C4/89.6-V3 or C4/89.6P-V3 generated antibodies that bound all peptides. The antibodies bound the 89.6-V3 and 89.6P-V3 peptides equally well in most cases, but binding was higher for animals immunized with C4/89.6P-V3 than for animals immunized with C4/89.6-V3. This differential antibody induction could be due to the different vaccination schedules used for these two groups of animals. Alternatively, it might be an indication that the C4/89.6P-V3 peptide was a better immunogen. In most cases antibody titers did not improve after six or eight inoculations compared to three inoculations. Only a minor fraction of the antibodies bound the C4/scbl-V3 peptide, indicating that the majority of vaccine-elicited antibodies were specific for genuine V3 sequences. Of special note, immunization with the C4/89.6-V3 peptide generated 10-fold-higher titers of V3-specific antibodies than infection with SHIV-89.6 (Table 3). As expected, antibodies generated by the C4/scbl-V3 peptide bound C4/scbl-V3 and C4/89.6-V3 but failed to bind the two V3 peptides in which the C4 sequences were absent.
TABLE 1.
ELISA reactivities of sera from animals immunized with either C4/scbl-V3 or C4/89.6-V3 and challenged with SHIV-89.6 in the first experiment
| Immunogena | Animal no. | Inoculationb | ELISA titerc for peptide:
|
|||
|---|---|---|---|---|---|---|
| C4/scbl-V3 | C4/89.6-V3 | 89.6-V3 | 89.6P-V3 | |||
| C4/scbl-V3 | 206-93 | 3rd | 12,150 | 450 | <50 | <50 |
| 8th | nt | nt | <50 | <50 | ||
| 259-94 | 3rd | 1,350 | 450 | <50 | <50 | |
| 8th | nt | nt | <50 | <50 | ||
| 287-94 | 3rd | 12,150 | 4,050 | <50 | <50 | |
| 8th | nt | nt | <50 | <50 | ||
| 305-94 | 3rd | 1,350 | 1,350 | <50 | <50 | |
| 8th | nt | nt | <50 | <50 | ||
| C4/89.6-V3 | 321-94 | 3rd | 50 | 1,350 | 1,350 | 1,350 |
| 8th | nt | nt | 450 | 1,350 | ||
| 358-95 | 3rd | 150 | 1,350 | 1,350 | 1,350 | |
| 8th | nt | nt | 1,350 | 1,350 | ||
| 539-92 | 3rd | 150 | 1,350 | 1,350 | 1,350 | |
| 8th | nt | nt | 450 | 1,350 | ||
| 556-92 | 3rd | 450 | 1,350 | 1,350 | 1,350 | |
| 8th | nt | nt | 1,350 | 1,350 | ||
Animals were inoculated with the designated peptide immunogen.
Serum samples were obtained 2 weeks after the designated peptide inoculations. The day of challenge was 2 weeks after the eighth peptide inoculation.
Titers are the reciprocals of the highest serum dilutions to score positive. nt, not tested.
TABLE 2.
ELISA-reactivities of sera from animals immunized with either C4/scbl-V3 or C4/89.6P-V3 peptide and challenged with either SHIV-89.6 or SHIV-89.6P in the second experiment
| Immunogena | Animal no. | Inoculationb | ELISA titerc for peptide:
|
|||
|---|---|---|---|---|---|---|
| C4/scbl-V3 | C4/89.6-V3 | 89.6-V3 | 89.6P-V3 | |||
| C4/scbl-V3 | 13369 | 3rd | 4,050 | 150 | <50 | <50 |
| 6th | nt | nt | <50 | <50 | ||
| 13398 | 3rd | 4,050 | <50 | <50 | <50 | |
| 6th | nt | nt | <50 | <50 | ||
| 13718 | 3rd | 4,050 | <50 | <50 | <50 | |
| 6th | nt | nt | <50 | <50 | ||
| C4/89.6P-V3 | 13789 | 3rd | 150 | 12,150 | 1,350 | >12,150 |
| 6th | nt | nt | 1,350 | 4,050 | ||
| 15075 | 3rd | 150 | 4,050 | 450 | 4,050 | |
| 6th | nt | nt | 450 | 1,350 | ||
| 15475 | 3rd | 150 | 4,050 | 1,350 | 4,050 | |
| 6th | nt | nt | 4,050 | 4,050 | ||
| 18351 | 3rd | 50 | 4,050 | 450 | 4,050 | |
| 6th | nt | nt | 450 | 450 | ||
| 13947 | 3rd | 4,050 | 109,350 | 12,150 | >12,150 | |
| 6th | nt | nt | 12,150 | 12,150 | ||
| 15032 | 3rd | 450 | 4,050 | 1,350 | 4,050 | |
| 6th | nt | nt | 1,350 | 4,050 | ||
| 17071 | 3rd | 50 | 1,350 | 450 | 1,350 | |
| 6th | nt | nt | 1,350 | 1,350 | ||
Animals were inoculated with the designated peptide immunogen.
Serum samples were obtained 2 weeks after the designated peptide inoculations. The day of challenge was 2 weeks after the sixth peptide inoculation.
Titers are the reciprocals of the highest serum dilutions to score positive. nt, not tested.
TABLE 3.
ELISA reactivities of sera from nonimmunized animals infected with SHIV-89.6
| Animal | Wks infected | ELISA titera for peptide:
|
||
|---|---|---|---|---|
| C4/scbl-V3 | C4/89.6-V3 | 89.6-V3 | ||
| 483-92 | 21 | 50 | 50 | 150 |
| 545-92 | 21 | 50 | <50 | 150 |
| 123-92 | 32 | 50 | 50 | 150 |
| 504-92 | 32 | 50 | <50 | 50 |
Titers are the reciprocals of the highest serum dilutions to score positive.
Neutralizing antibody induction following peptide immunization.
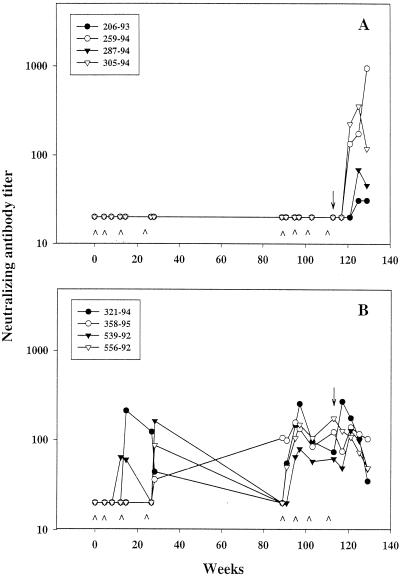
Two to four inoculations with either the C4/89.6-V3 peptide or C4/89.6P-V3 peptide immunogen generated neutralizing antibodies in all animals, detectable in the MT-2 cell killing assay (Fig. 2 and 3). Antibodies generated by the C4/89.6-V3 peptide neutralized SHIV-89.6 (Fig. 2B) but not SHIV-89.6P (data not shown), despite the fact that the antibodies bound the V3 loop peptide of both viruses equally well (Table 1). Titers after the fourth inoculation (week 28) declined in three of four animals by week 89 to undetectable levels in the absence of subsequent boosts. Unexpectedly, neutralizing antibodies in the fourth animal (monkey 358-95) increased between week 28 and week 89. A fifth inoculation with C4/89.6-V3 peptide at week 89 boosted the neutralization titers to detectable levels in three animals and increased the titer in animal 358-95 (Fig. 2 and Table 4). These titers were maintained from week 91 to the day of challenge (week 113), during which time the animals received three additional inoculations of C4/89.6-V3. Peak titers of SHIV-89.6-specific neutralizing antibodies in these immunized monkeys ranged from 1:80 to 1:256 (average, 1:162 ± 1:74).
FIG. 2.
Neutralizing antibody response in peptide-immunized animals before and after challenge with SHIV-89.6. Animals were inoculated with either C4/scbl-V3 (A) or C4/89.6-V3 (B) at weeks 0, 4, 12, 24, 89, 95, 101, and 111 and were challenged with SHIV-89.6 at week 113. Neutralizing antibodies to SHIV-89.6 were measured in MT-2 cells at multiple times before and after challenge. Peptide inoculations were made at the times indicated (∧). Arrow, day of challenge. The lowest serum dilution tested was 1:20 (negative results were given a value of 20 for presentation).
FIG. 3.
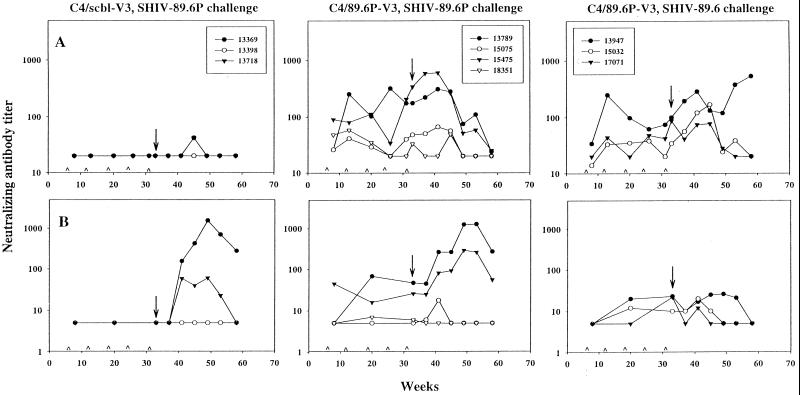
Neutralizing antibody response in peptide-immunized animals before and after challenge with either SHIV-89.6P or SHIV-89.6. Animals were inoculated with either C4/scbl-V3 or C4/89.6P-V3 at weeks 0, 6, 12, 18, 24, and 31 and were challenged with either SHIV-89.6P or SHIV-89.6 at week 33. Neutralizing antibodies to SHIV-89.6 (A) and SHIV-89.6P (B) were measured in MT-2 cells at multiple times throughout the immunization schedule and postchallenge. Peptide inoculations were made at the times indicated (∧). Arrow, day of challenge. The lowest serum dilution tested was 1:20 for SHIV-89.6 and 1:5 for SHIV-89.6P (negative results were given values of 20 and 5, respectively, for presentation).
TABLE 4.
Neutralization of SHIV-89.6 in MT-2 cells and human PBMC by sera from animals immunized with the C4/89.6-V3 peptide
| Animal no. | Inoculationa | Titerb in MT-2 cells | % Reduction in p27 in human PBMCc |
|---|---|---|---|
| 321-94 | 5th | 1:55 | 52 |
| 6th | 1:256 | 77 | |
| 7th | 1:97 | 84 | |
| 8th | 1:74 | 54 | |
| 358-95 | 5th | 1:99 | 48 |
| 6th | 1:134 | 67 | |
| 7th | 1:85 | 68 | |
| 8th | 1:124 | 53 | |
| 539-92 | 5th | 1:20 | 57 |
| 6th | 1:80 | 94 | |
| 7th | 1:58 | 91 | |
| 8th | 1:62 | 81 | |
| 556-92 | 5th | 1:50 | 76 |
| 6th | 1:150 | 93 | |
| 7th | 1:106 | 96 | |
| 8th | 1:177 | 92 |
Animals were inoculated with the C4/89.6-V3 peptide immunogen. Serum was obtained 2 weeks after the indicated inoculation.
Titers are the serum dilutions at which 50% of cells were protected from virus-induced killing.
Serum samples were tested at a 1:5 dilution in triplicate. The percent reduction in p27 synthesis is calculated relative to the amount of p27 produced in the presence of the corresponding prebleed sample for animals 321-94 (1,969 pg/ml), 358-95 (1,292 pg/ml), 539-92 (1,967 pg/ml) and 556-92 (1,383 pg/ml).
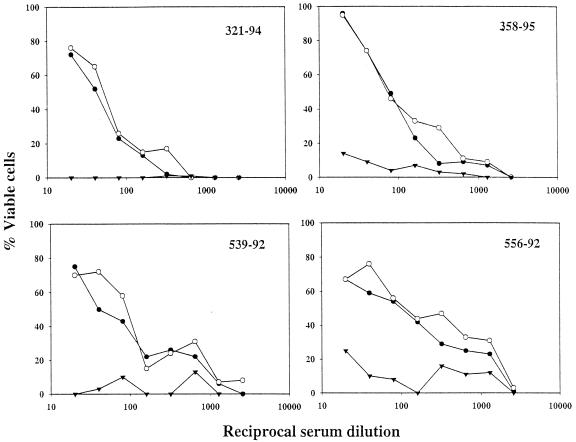
The V3 specificity of the neutralizing antibodies in sera from the C4/89.6-V3-immunized animals was confirmed in peptide competition assays. Serum samples collected on the day of challenge were preincubated with either C4/scbl-V3, C4/89.6-V3, or no peptide prior to assay for neutralization of SHIV-89.6. Incubation of the sera with the C4/89.6-V3 peptide removed all detectable neutralizing activity (≤50% viable cells), whereas incubation with the C4/scbl-V3 peptide had no effect on serum neutralizing antibody titers (Fig. 4). Similar peptide competition assays were performed on prechallenge serum samples from C4/scbl-V3-immunized animals. No neutralizing antibody titers were detected in these sera (data not shown), indicating that the sera of these immunized monkeys had no antiviral activity that might complicate the interpretation of the experiments. We conclude that the neutralizing antibodies in the sera of the immunized monkeys were directed to V3 and not to C4.
FIG. 4.
Neutralizing antibody specificity generated by C4/89.6-V3 peptide immunization. Serum samples were obtained 2 weeks after final boosting with the C4/89.6-V3 peptide. Each serum sample was incubated for 1 h at 37°C with either no peptide (solid circles), C4/scbl-V3 peptide (open circles), or C4/89.6-V3 peptide (solid inverted triangles) and then assessed for neutralizing activity against SHIV-89.6 in MT-2 cells.
Immunization with the C4/89.6P-V3 peptide generated antibodies capable of neutralizing SHIV-89.6 and SHIV-89.6P, although the magnitude of SHIV-89.6 neutralization exceeded that of SHIV-89.6P neutralization (Fig. 3; note the differences in scales on the y axes). Considerable variation was seen in these neutralizing antibody responses, where peak titers were 1:38 to 1:341 for SHIV-89.6 (average, 1:163 ± 1:135) and <1:5 to 1:68 for SHIV-89.6P (average for positive animals was 1:29 ± 1:24).
Serum samples were further assessed for their ability to neutralize SHIV-89.6 and SHIV-89.6P in human PBMC. These two SHIV variants, like their HIV-1 89.6 parent, are approximately fivefold less sensitive to neutralization in PBMC than in MT-2 cells (8, 22, 24). As shown in Table 4, serum from three of four of the C4/89.6-V3-immunized animals neutralized SHIV-89.6 in human PBMC at a 1:5 dilution (≥80% reduction in p27). Serum dilutions of 1:15 or greater failed to neutralize (values ranged from −8 to 55% reductions in p27; data not shown). In addition, serum samples from two of seven of the C4/89.6P-V3-immunized animals were capable of neutralizing SHIV-89.6P in human PBMC (Table 5). Because SHIV-89.6P was less sensitive to neutralization than SHIV-89.6 in human PBMC (Fig. 3), serum samples were tested at a 1:2 starting dilution rather than the 1:5 dilution used for the SHIV-89.6 neutralization assays. No significant neutralization of SHIV-89.6P was seen at a 1:8 serum dilution (values ranged from −8 to 55% reductions in p27; data not shown), indicating that the titers were relatively low. In general, neutralization potencies in the PBMC assay tracked with relative neutralization potencies in the MT-2 assay for both viruses (Tables 4 and 5).
TABLE 5.
Neutralization of SHIV-89.6P in MT-2 cells and human PBMC by sera from animals immunized with the C4/89.6P-V3 peptide
| Challenge group | Animal no.a | Titerb in MT-2 cells | % Reduction in p27 in human PBMCc |
|---|---|---|---|
| SHIV-89.6P | 13789 | 1:47 | 91 |
| 15075 | <1:5 | 51 | |
| 15475 | 1:26 | 76 | |
| 18351 | 1:5 | 45 | |
| SHIV-89.6 | 13947 | 1:23 | 84 |
| 15032 | 1:5 | 67 | |
| 17071 | 1:22 | 57 |
Animals were inoculated with the C4/89.6P-V3 peptide immunogen. Serum samples were obtained on the day of challenge (2 weeks after the sixth inoculation).
Titers are the serum dilutions at which 50% of cells were protected from virus-induced killing.
Serum samples were tested at a 1:2 dilution in triplicate. The percent reduction in p27 synthesis is calculated relative to the amount of p27 produced in the presence of prebleed serum from animal 13718 (3,407 pg/ml).
Serum samples collected after final boosting were assessed for their ability to neutralize SHIV-KB9 (the molecular pathogenic clone of SHIV-89.6P) and four heterologous viruses in the MT-2 assay. As shown in Table 6, SHIV-KB9 was neutralized by sera from animals immunized with C4/89.6P-V3 but not by sera from animals immunized with C4/89.6-V3. There were no major discrepancies between the titers measured with SHIV-KB9 and those measured with SHIV-89.6P (see Table 5 for a comparison), providing evidence that the detection of neutralization was not affected by minor quasispecies complexity in the uncloned stock. Immunization with either peptide generated antibodies that could neutralize HIV-1 MN, but this cross-neutralization was limited in that two additional TCLA strains (SHIV-HXBc2 and HIV-1 SF2) and another primary isolate-like SHIV variant (SHIV-KU2) resisted neutralization by these antibodies. The only exceptions to this finding were one animal in each of the two immunization groups whose sera had weak neutralizing activity against SHIV-HXBc2.
TABLE 6.
Assessment of neutralizing antibody cross-reactivity against heterologous virus variants
| Peptide immunogen | Animal no.a | Titer of neutralizing antibodies to indicated strain ofb:
|
||||
|---|---|---|---|---|---|---|
| SHIV
|
HIV-1
|
|||||
| KB9 | HXBc2 | KU2 | MN | SF2 | ||
| C4/89.6-V3 | 321-94 | <1:20 | <1:20 | <1:20 | 1:62 | <1:20 |
| 358-95 | <1:20 | <1:20 | <1:20 | 1:471 | <1:20 | |
| 539-92 | <1:20 | 1:33 | <1:20 | 1:294 | <1:20 | |
| 556-92 | <1:20 | <1:20 | <1:20 | 1:188 | <1:20 | |
| C4/89.6P-V3 | 13947 | 1:28 | <1:20 | <1:20 | 1:1,089 | <1:20 |
| 15032 | 1:21 | <1:20 | <1:20 | 1:97 | <1:20 | |
| 17071 | <1:20 | <1:20 | <1:20 | 1:253 | <1:20 | |
| 13789 | 1:61 | 1:25 | <1:20 | 1:44 | <1:20 | |
| 15075 | <1:20 | <1:20 | <1:20 | 1:106 | <1:20 | |
| 15475 | 1:25 | <1:20 | <1:20 | 1:892 | <1:20 | |
| 18351 | <1:20 | <1:20 | <1:20 | 1:89 | <1:20 | |
Serum samples were obtained on the day of challenge (2 weeks after the final peptide inoculation).
SHIV-89.6, SHIV-KB9 and SHIV-KU2 were grown in human PBMC. SHIV-HXBc2, HIV-1 MN, and HIV-1 SF2 were grown in H9 cells. All assays were performed in MT-2 cells with the exception of that for HIV-1 SF2, which was assayed in CEMx174 cells. Titers are the serum dilutions at which 50% of cells were protected from virus-induced killing.
CTL responses following peptide immunization.
The V3 loop sequence contained in the peptide immunogens includes a cytotoxic T-lymphocyte (CTL) epitope restricted in humans by HLA-B7 (32). The monkeys in experiment 1 were, therefore, assessed prospectively to determine whether the peptide immunizations had elicited CTLs. CTLs were monitored by assessing the ability of V3 loop peptide-stimulated PBMC from the vaccinated monkeys to lyse autologous B-lymphoblastoid cell lines pulsed with the V3 loop peptide. No evidence of peptide-pulsed target cell lysis by peptide-stimulated PBMC was demonstrated in samples obtained 2, 8, and 10 weeks after the vaccination protocol was initiated, suggesting that CTLs were not generated in this outbred population of monkeys.
SHIV-89.6 challenge of vaccinated monkeys.
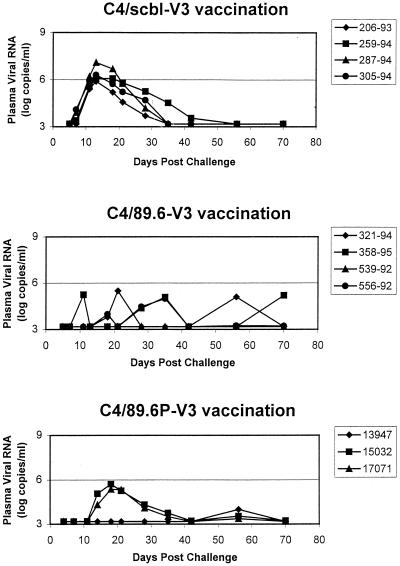
The three groups of monkeys in the first experiment were challenged by the intravenous route with 20 TCID50 of SHIV-89.6 2 weeks following the final immunization. Since this viral construct expresses a primary patient isolate HIV-1 envelope and replicates in rhesus monkeys to relatively high levels during primary infection, this SHIV-89.6 challenge provided a means to determine whether V3 loop-specific antibodies generated through vaccination might contribute to early containment of lentivirus replication. The challenged monkeys were assessed prospectively for changes in circulating CD4+ PBL counts and plasma viral RNA levels following virus inoculation. As expected, since SHIV-89.6 is nonpathogenic in rhesus monkeys, no consistent changes in circulating CD4+ T lymphocytes were observed in the animals after viral challenge (data not shown). However, all animals immunized with either C4/89.6-V3 or C4/89.6P-V3 showed delayed anti-Gag seroconversion relative to control immunized animals (Table 7). Also, consistent differences in the levels of plasma viral RNA were detected during the period of primary infection in these groups of monkeys (Fig. 5). As previously reported for infections of naive rhesus monkeys with this chimeric virus, peak levels of viral RNA in plasma reached between 106 and 107 copies/ml in the C4/scbl-V3-immunized control animals. Two of the three monkeys immunized with the C4/89.6P-V3 peptide had peak virus loads of between 105 and 106 copies/ml detected in their plasma, while the third had no detected plasma viral RNA. This last animal (13947) also had no evidence of anti-Gag seroconversion for up to 12 weeks postchallenge, whereas all control animals had strongly seroconverted by week 6 (Table 7). Importantly, the monkeys immunized with the C4/89.6-V3 peptide had low measurable plasma viral RNA during this period of primary infection. These observations suggested that the V3 loop-specific antibodies elicited by peptide vaccination did provide partial protection against the spread of this virus. Interestingly, two of the four monkeys vaccinated with the 89.6 V3 loop peptide and one of the three monkeys vaccinated with the 89.6P V3 loop peptide had plasma viral RNA detected at least transiently in the postacute phase of infection. While this phenomenon may be vaccine related, insufficient data are available for large numbers of normal rhesus monkeys infected with SHIV-89.6 to predict with confidence the level of viral containment that might be expected in the absence of vaccination.
TABLE 7.
Gag-specific seroconversion postchallenge
| Immunogen | Challenge virus | Animal no. | Anti-Gag reactivitya at indicated wk postchallenge
|
|||
|---|---|---|---|---|---|---|
| 4 | 6 | 8 | 12 | |||
| C4/scbl-V3 | SHIV-89.6 | 206-93 | nt | +++ | +++ | nt |
| 259-94 | nt | +++ | +++ | nt | ||
| 287-94 | nt | +++ | +++ | nt | ||
| 305-94 | nt | +++ | +++ | nt | ||
| C4/89.6-V3 | SHIV-89.6 | 321-94 | nt | − | − | + |
| 358-95 | nt | − | +++ | +++ | ||
| 539-92 | nt | − | + | + | ||
| 556-92 | nt | − | − | − | ||
| C4/scbl-V3 | SHIV-89.6P | 13369 | ++ | nt | +++ | +++ |
| 13398 | + | nt | + | + | ||
| 13718 | + | nt | ++ | + | ||
| C4/89.6P-V3 | SHIV-89.6 | 13947 | − | nt | − | − |
| 15032 | + | nt | + | ++ | ||
| 17071 | − | nt | + | ++ | ||
| C4/89.6P-V3 | SHIV-89.6P | 13789 | + | nt | ++ | +++ |
| 15075 | + | nt | + | + | ||
| 15475 | +++ | nt | +++ | +++ | ||
| 18351 | − | nt | − | − | ||
Anti-Gag reactivity was determined by HIV-2 Western blotting on serum obtained at weeks 6, 8, and 12 postchallenge. Scores represent relative band intensities as determined by visual observation. nt not tested.
FIG. 5.
Sequential plasma viral load measurements in rhesus monkeys immunized with a control peptide (C4/scbl-V3), an experimental homologous peptide (C4/89.6-V3), or a control heterologous peptide (C4/89.6P-V3) after intravenous challenge with SHIV-89.6.
Since lentivirus replication occurs predominantly in secondary lymphatic tissue, viral loads were also assessed at the time of peak viral replication during primary infection in two groups of the challenged monkeys in sampled lymph nodes. In these studies viral RNA was quantitated by a bDNA assay in pellets of lymph node lymphocytes. As shown in Table 8, the viral RNA levels in lymph node lymphocytes were lower in the monkeys that received the experimental vaccine than in those that received the control vaccine (P = 0.028; two-sided Wilcoxon rank sum test).
TABLE 8.
Viral RNA in lymph nodes of SHIV-89.6-challenged monkeys at time of peak viral replicationa
| Immunogen | Animal no. | Viral RNA (copies/5 × 106 cells) |
|---|---|---|
| C4/scbl-V3 | 206-93 | 8,200 |
| 259-94 | 46,500 | |
| 287-94 | 39,780 | |
| 305-94 | 14,190 | |
| C4/89.6-V3 | 321-94 | <3,000 |
| 358-95 | <3,000 | |
| 539-92 | 4,710 | |
| 536-92 | 3,340 |
RNA was quantitated in lymph node lymphocytes by bDNA technology using a pal probe. There was a statistically significant difference between groups (P = 0.028 by Wilcoxon rank sum test).
Neutralizing antibody responses after SHIV-89.6 challenge.
To determine whether the vaccine-elicited V3-specific neutralizing antibodies were boosted following infection, we measured neutralizing antibodies in postchallenge serum samples. Postchallenge neutralization titers increased at 4 weeks postchallenge in the serum of one animal (321-94) and declined steadily thereafter (Fig. 2). The fact that neutralizing antibodies were undetectable at 4 weeks postchallenge in the sera of all the control animals suggests that the V3-specific neutralizing antibodies in C4/89.6-V3-immunized animal 321-94 were boosted transiently by infection. Two of the remaining three C4/89.6-V3-immunized animals showed a decline in neutralization titers at 4 weeks postchallenge; this was followed by a modest increase in titer at week 8 and a steady decline thereafter. This modest increase in titer at week 8 could have been a de novo response to regions outside the V3 loop, since most SHIV-89.6-infected macaques generate little if any V3-specific neutralizing antibodies (8, 11). De novo neutralizing antibody responses in sera of the control-immunized animals (C4/scbl-V3 peptide) were first detected at 8 to 12 weeks postchallenge. A fourth animal in the C4/89.6-V3-immunized group (556-92) showed a steady decline in neutralization titers without any evidence of a boosting effect postchallenge. This animal had the highest titer of neutralizing antibodies on the day of challenge, the lowest levels of plasma viral RNA (Fig. 5), and longest delay in anti-Gag seroconversion (Table 7) in this group.
The inability of SHIV-89.6 infection to boost or even maintain the neutralizing antibodies induced by C4/89.6-V3 peptide immunization in three of four animals most likely reflects the poor immunogenicity of the V3 loop as it exists on gp120 during infection (Table 3). Declining titers of neutralizing antibodies postchallenge in C4/89.6-V3-immunized animals also suggests that the vaccine-induced antibodies suppressed virus replication to levels below the threshold needed to generate a de novo neutralizing antibody response. It is unlikely that these declining titers were due to virus-induced suppression of the B-cell response, since SHIV-89.6 has no detectable immunosuppressive effects early in infection in juvenile and adult macaques.
In the SHIV-89.6-challenged animals immunized with the C4/89.6P-V3 peptide, titers of SHIV-89.6-specific neutralizing antibodies on the day of challenge correlated with plasma RNA levels and anti-Gag seroconversion postchallenge. For example, animal 13947 had the most-potent neutralizing antibodies in this group on the day of challenge in both MT-2 cells and PBMC (Table 5) and also had the most-potent and sustained neutralizing antibody response postchallenge (Fig. 3). This same animal had the lowest levels of plasma viral RNA (Fig. 5) and the longest delay in anti-Gag seroconversion (Table 7) in this group. Postchallenge neutralizing antibodies in the remaining two C4/89.6P-V3-immunized animals exhibited a delayed rise followed by a steady decline during the period of observation. As expected, infection with SHIV-89.6 generated little or no neutralizing antibodies specific for SHIV-89.6P, although it is possible that animal 13947 had a transient mild response to this virus.
SHIV-89.6P challenge of vaccinated monkeys.
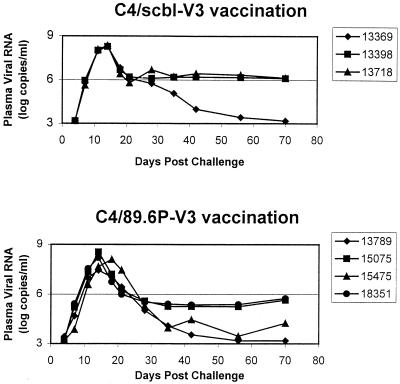
The two groups of vaccinated monkeys in experiment 2 were challenged by the intravenous route with the highly pathogenic SHIV-89.6P 2 weeks following the final immunization. These animals were then assessed prospectively for plasma viral load and CD4+ PBL counts. The peak plasma viral loads during primary infection in the C4/89.6P-V3 and control C4/89.6P-V3 peptide-immunized monkeys were indistinguishable, reaching between 107 and 109 copies/ml (Fig. 6). Moreover, while set point plasma viral RNA levels were higher in two of the three control C4/scbl-V3 peptide-immunized monkeys than in the C489.6P-V3 peptide-immunized monkeys, there was sufficient overlap in values between the groups of monkeys that no statistically significant difference in these values could be documented. Consistent with these findings, no obvious vaccine effect was observed by anti-Gag seroconversion (Table 7). Assessments of seroconversion in SHIV-89.6P-infected animals is, however, complicated by rapid CD4 loss in most nonimmunized animals, which often results in poor seroconversion.
FIG. 6.
Sequential plasma viral load measurements in rhesus monkeys immunized with a control peptide (C4/scbl-V3) or experimental homologous peptide (C4/89.6P) after intravenous challenge with SHIV-89.6P.
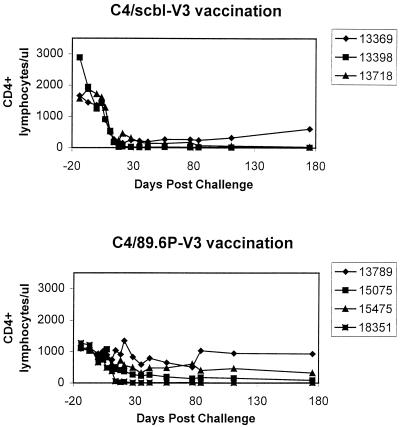
Interestingly, differences in CD4+ PBL loss between these groups of monkeys were demonstrated. By 2 weeks after viral inoculation, the control C4/scbl-V3 peptide-vaccinated monkeys had almost no detectable circulating CD4+ T lymphocytes, and these values remained low thereafter (Fig. 7). CD4+ PBL loss in the infected C4/89.6P-V3 peptide-immunized monkeys was less profound, with only one of four of the monkeys having undetectable CD4+ PBLs in the weeks following infection. This difference in groups did not achieve statistical significance (P = 0.11 for a comparison of CD4+ T-lymphocyte counts at day 84 postchallenge using a one-sided Wilcoxon rank sum test) because of the small number of monkeys in each experimental group. A trend toward protection was, however, evident. Thus, while the degree of protection afforded monkeys by this peptide immunization strategy was less dramatic in experiment 2, in which a highly pathogenic challenge virus was employed, than it was in the study in which a nonpathogenic challenge virus was employed, the outcome of this second experiment was consistent with the results of the first experiment. That is, the peptide vaccine-elicited antibody response did afford some degree of protection against a lentivirus challenge.
FIG. 7.
Sequential peripheral blood CD4+ lymphocyte counts in rhesus monkeys immunized with a control peptide (C4/scbl-V3) or experimental homologous peptide (C4/89.6P) after intravenous challenge with SHIV-89.6P.
Neutralizing antibody responses following SHIV-89.6P challenge.
Animals 13789 and 15475 in this group had the highest titers of SHIV-89.6P-specific neutralizing antibodies as measured in MT-2 cells on the day of challenge (Table 5; Fig. 3). They also maintained their CD4+ PBL counts and controlled the challenge virus at set point to a greater extent than did the other two animals in this group (Fig. 6 and 7). These same two animals exhibited dramatic increases in SHIV-89.6P-specific neutralizing antibodies postchallenge (Fig. 3) and had the greatest potency of SHIV-89.6P-specific neutralization on the day of challenge as measured in human PBMC (91 and 76% neutralization, respectively) (Table 5). Two additional animals in this group (animals 15075 and 18351) had little or no SHIV-89.6P-specific serum neutralizing antibodies before or after challenge. The latter two animals experienced pronounced CD4+ PBL loss and did not control their virus as well as animals 13789 and 15475 after challenge.
We also monitored the production of SHIV-89.6-specific neutralizing antibodies in the above animals. Titers of peptide-induced neutralizing antibodies present on the day of challenge rose slightly for a short time and then declined to low or undetectable levels after SHIV-89.6P challenge (Fig. 3). In particular, SHIV-89.6-specific neutralizing antibodies were not maintained as well as SHIV-89.6P-specific neutralizing antibodies in the two best seroconverters (animals 13789 and 15475). Thus, infection with SHIV-89.6P was not able to sustain the SHIV-89.6-specific neutralizing antibodies generated by peptide immunization. A plausible explanation for this outcome is that the V3 loop of SHIV-89.6P, like that of SHIV-89.6, is poorly immunogenic in its native form in infected animals.
As expected, two animals that were immunized with the control C4/scbl-V3 peptide and challenged with SHIV-89.6P developed a neutralizing antibody response that was specific for the challenge virus and did not neutralize SHIV-89.6 (Fig. 3). A third animal in this group (animal 13398) failed to develop a neutralizing antibody response to either SHIV isolate. This last animal had a very high level of plasma viremia and developed a rapid and profound CD4+ PBL depletion (Fig. 6 and 7). SHIV-89.6P-specific neutralizing antibodies in the two seroconverters were first detected at 8 weeks following challenge. Titers of neutralizing antibodies peaked at 8 to 16 weeks following infection and declined steadily thereafter. Titers in one seroconverter (animal 13718) later declined to undetectable levels, associated with a rapid and profound loss of CD4+ PBLs (Fig. 6 and 7). Notably, animal 13369, which had the most-potent SHIV-89.6P-specific neutralizing antibody response postchallenge, was able to control virus replication better than the other animals in this group.
DISCUSSION
A number of experimental observations have suggested that the V3 loop of HIV-1 Env may prove an important target for vaccine-elicited antibodies. For example, it is well established that the V3 loop is the principal neutralizing determinant of TCLA strains of HIV-1. Moreover, it has been reported that this loop structure plays an important role in the interactions of HIV-1 Env with chemokine receptors as the virus enters a cell (6, 7, 37, 38). However, the extreme sequence variability of this domain of Env among primary patient viral isolates and the demonstration of its poor accessibility to antibodies in these isolates diminished interest in this region of the virus among HIV-1 vaccine investigators. The present study, however, suggests that vaccine-elicited antibodies that bind to the V3 loop can in certain cases confer some degree of protective immunity against primary isolate-like variants of the virus.
The results of the present experiments indicate that neutralizing antibodies directed against the V3 loop of nonpathogenic and pathogenic lentiviruses containing the envelope glycoproteins of primary HIV-1 isolates afforded measurable protection against high virus loads and CD4+ lymphocyte depletion after experimental intravenous challenge with homologous virus. This was seen in spite of the fact that the neutralizing antibodies were strain specific and low in titer. Partial protection against pathogenic SHIV-89.6P challenge in our studies was achieved with a titer of neutralizing antibody that was at the lowest level of detection in the PBMC assay (1:2 serum dilution). It is plausible that a higher titer of these antibodies might provide even greater protection and possibly a complete barrier to infection. This has been seen in this experimental SHIV model (19, 20) with neutralizing antibodies of other specificities.
While this study clearly indicates that a vaccine-elicited anti-V3 loop antibody can provide partial protection against a SHIV challenge, the experiments do not conclusively demonstrate the function of the antibody that is responsible for that protection. Since the peptide vaccine-elicited antibodies exhibited only very weak neutralizing activity, it is certainly possible that the protection against viral challenge was conferred by an antibody-mediated activity other than neutralization of the virus. It is also possible that a barely detectable neutralizing antibody is all that is needed to provide some degree of protection in vivo, where the antibody is undiluted. The latter possibility is more likely than the former since vaccination with a homologous V3 loop peptide, which elicited a neutralizing antibody response to SHIV-89.6, conferred greater protection against SHIV-89.6 challenge than did vaccination with a slightly heterologous V3 loop peptide, which elicited no detectable neutralizing antibody response to SHIV-89.6.
Our observations leave open the possibility that an unidentified region of the V3 loop on primary isolates is a potential target for neutralizing antibodies. For example, some portion of the V3 loop that is presumably exposed to facilitate initial contact with CD4 and the coreceptor during virus attachment and entry (6, 7, 21, 37–40, 43) might be equally exposed for antibody binding and virus neutralization. An important question to ask is whether the structure of the V3 loop on SHIV-89.6 and SHIV-89.6P represents the structure found on other primary isolates. Both viruses were shown to resemble primary isolates by being less sensitive to neutralization than TCLA strains from HIV-1-infected individuals (8). Compared to R5/X4 primary isolates, however, both SHIVs were moderately sensitive to neutralization in MT-2 cells in that study. In addition, although both SHIVs were 14- to 38-fold less sensitive to inhibition by sCD4 than TCLA strains, primary isolates are usually more than 500-fold less sensitive to sCD4 (9). Thus, epitope exposure on SHIV-89.6 and SHIV-89.6P Env appears to be intermediate between TCLA strains and primary isolates.
Another important distinction between these two SHIV variants and primary isolates relates to the immunogenicities of their native V3 loops on the gp 120 produced during infection. HIV-1 gp120 is thought to be present in different forms during infection, including native oligomeric gp120-gp41 heterodimers on the surfaces of the virus and infected cells, uncleaved gp160, and monomeric gp120 that is released from virus particles and infected cells (4). Each form might have a different influence on the immunogenicity of specific epitopes, including epitopes in the V3 loop. Although most primary isolates resist neutralization by the V3-specific antibodies present in sera from infected individuals, the V3 loops of primary isolates are nonetheless sufficiently immunogenic in their native form(s) to generate antibodies that bind V3 peptides (5, 34, 36) and neutralize TCLA strains (1, 36, 41). For SHIV-89.6 and SHIV-89.6P infection in macaques, our results (Table 3) (8) and those of others (11) suggest that their V3 loops are poorly immunogenic. This poor immunogenicity probably explains why V3-specific neutralizing antibodies are rarely detected in the serum from infected animals. Immunization with C4/V3 peptides of appropriate amino acid sequence apparently overcame this poor immunogenicity to generate antibodies that neutralized SHIV-89.6 and SHIV-89.6P and that provided partial protection from challenge. Additional studies are needed to determine whether similar peptides that will target suitable neutralization epitopes on the V3 loop of primary HIV-1 isolates can be designed.
ACKNOWLEDGMENT
This work was supported by National Institutes of Health grants AI35351, AI85343, and RR00163.
REFERENCES
- 1.Beddows S, Louisirirotchanakul S, Cheingsong-Popov R, Eastbrook P J, Simmonds P, Weber J. Neutralization of primary and T-cell line adapted isolates of human immunodeficiency virus type 1: role of V3-specific antibodies. J Gen Virol. 1998;79:77–82. doi: 10.1099/0022-1317-79-1-77. [DOI] [PubMed] [Google Scholar]
- 2.Bou-Habib D C, Roderiquez G, Oravecz T, Berman P W, Lusso P, Norcross M A. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J Virol. 1994;68:6006–6013. doi: 10.1128/jvi.68.9.6006-6013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bures, R., A. Gaitan, T. Zhu, C. Graziosi, K. McGrath, J. Tartaglia, P. Caudrelier, R. E. L. Habib, M. Klein, A. Lazzarin, D. Stablein, M. Deers, L. Corey, M. L. Greenberg, D. H. Schwartz, and D. C. Montefiori. Immunization with recombinant canarypox vectors expressing membrane-anchored gp120 followed by gp160 protein boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses, in press. [DOI] [PubMed]
- 4.Burton D R. A vaccine for HIV type 1: the antibody perspective. Proc Natl Acad Sci USA. 1997;94:10018–10023. doi: 10.1073/pnas.94.19.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheingsong-Popov R, Osmanov S, Pau C-P, Schochetman G, Barin F, Holmes H, Francis G, Ruppach H, Dietrich U, Lister S, Weber J. Serotyping of HIV-1 infections: definition, relationship to viral genetic subtypes, and assay evaluation. AIDS Res Hum Retroviruses. 1998;14:311–318. doi: 10.1089/aid.1998.14.311. [DOI] [PubMed] [Google Scholar]
- 6.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 7.Cocchi F, DeVico A L, Garzino-Demo A, Cara A, Gallo R C, Lusso P. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 8.Crawford J M, Earl P L, Moss B, Reimann K A, Wyand M S, Manson K H, Bilska M, Zhou J T, Pauza C D, Parren P W H I, Burton D R, Sodroski J G, Letvin N L, Montefiori D C. Characterization of primary isolate-like variants of simian-human immunodeficiency virus. J Virol. 1999;73:10199–10207. doi: 10.1128/jvi.73.12.10199-10207.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daar E S, Li X L, Moudgil T, Ho D D. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency virus type 1 isolates. Proc Natl Acad Sci USA. 1990;87:6574–6578. doi: 10.1073/pnas.87.17.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emini E A, Schleif W A, Nunberg J H, Conley A J, Eda Y, Tokiyoshi S, Putney S D, Matsushita S, Cobb K E, Jett C M, Eichberg J W, Murthy K K. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992;355:728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- 11.Etemad-Moghadam B, Karlsson G B, Halloran M, Sun Y, Schenten D, Fernandes M, Letvin N L, Sodroski J. Characterization of simian-human immunodeficiency virus envelope glycoprotein epitopes recognized by neutralizing antibodies from infected macaques. J Virol. 1998;72:8437–8445. doi: 10.1128/jvi.72.10.8437-8445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girard M, Kieny M-P, Pinter A, Barre-Sinoussi F, Nara P, Kolbe H, Kusumi K, Chaput A, Reinhart T, Muchmore E, Ronco J, Kaczorek M, Gomard E, Gluckman J-C, Fultz P N. Immunization of chimpanzees confers protection against challenge with human immunodeficiency virus. Proc Natl Acad Sci USA. 1991;88:542–546. doi: 10.1073/pnas.88.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harada S, Koyanagi Y, Yamamoto N. Infection of HTL V-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985;229:563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- 14.Haynes B F, Torres J V, Langlois A J, Bolognesi D P, Gardner M B, Palker T J, Scearce R M, Jones D M, Moody M A, McDanal C, Matthews T J. Induction of HIVMN neutralizing antibodies in primates using a prime-boost regimen of hybrid synthetic gp120 envelope peptides. J Immunol. 1993;151:1646–1653. [PubMed] [Google Scholar]
- 15.Javaherian K, Langlois A J, McDanal C, Ross K L, Eckler L I, Jellis C L, Profy A T, Rusche J R, Bolognesi D P, Putney S D, Matthews T J. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proc Natl Acad Sci USA. 1989;86:6768–6772. doi: 10.1073/pnas.86.17.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joag S V, Li Z, Foresman L, Stephens E B, Zhao L J, Adany I, Pinson D M, McClure H M, Narayan O. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J Virol. 1996;70:3189–3197. doi: 10.1128/jvi.70.5.3189-3197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Letvin N L, Montefiori D C, Yasutomi Y, Perry H C, Davies M E, Lekutis C, Alroy M, Freed D L, Lord C I, Handt L K, Liu M A, Shiver J W. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc Natl Acad Sci USA. 1997;94:9378–9383. doi: 10.1073/pnas.94.17.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao H, Etemad-Moghadam B, Montefiori D C, Sun Y, Sodroski J, Scearce R M, Doms R W, Thomasch J R, Robinson S, Letvin N L, Haynes B F. Induction of antibodies in guinea pigs and rhesus monkeys against the human immunodeficiency virus type 1 envelope: neutralization of nonpathogenic and pathogenic primary isolate simian/human immunodeficiency virus strains. J Virol. 2000;74:254–263. doi: 10.1128/jvi.74.1.254-263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mascola J R, Lewis M G, Stiegler G, Harris D, VanCott T C, Hayes D, Louder M K, Brown C R, Sapan C V, Frankel S S, Lu Y, Robb M L, Katinger H, Birx D L. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mascola J R, Stiegler G, VanCott T C, Katinger H, Carpenter C B, Hanson C E, Beary H, Hayes D, Frankel S S, Birx D L, Lewis M G. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 21.McDougal J S, Kennedy M S, Orloff S L, Nicholson J K A, Spira T J. Mechanism of human immunodeficiency virus type 1 (HIV-1) neutralization: irreversible inactivation of infectivity by anti-HIV-1 antibody. J Virol. 1996;70:5236–5245. doi: 10.1128/jvi.70.8.5236-5245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montefiori D C, Collman R G, Fouts T R, Zhou J Y, Bilska M, Hoxie J A, Moore J P, Bolognesi D P. Evidence that antibody-mediated neutralization of human immunodeficiency virus type 1 is independent of coreceptor usage. J Virol. 1998;72:1886–1893. doi: 10.1128/jvi.72.3.1886-1893.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montefiori D C, Pantaleo G, Fink L M, Zhou J T, Zhou J Y, Bilska M, Miralles G D, Fauci A S. Neutralizing and infection-enhancing antibody responses to human immunodeficiency virus type 1 in long-term nonprogressors. J Infect Dis. 1996;173:60–67. doi: 10.1093/infdis/173.1.60. [DOI] [PubMed] [Google Scholar]
- 24.Montefiori D C, Reimann K A, Wyand M S, Manson K, Lewis M G, Collman R G, Sodroski J G, Bolognesi D P, Letvin N L. Neutralizing antibodies in sera from macaques infected with chimeric simian-human immunodeficiency virus containing the envelope glycoproteins of either a laboratory-adapted variant or a primary isolate of human immunodeficiency virus type 1. J Virol. 1998;72:3427–3431. doi: 10.1128/jvi.72.4.3427-3431.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montefiori D C, Robinson W E, Jr, Schuffman S S, Mitchell W M. Evaluation of antiviral drugs and neutralizing antibodies to human immunodeficiency virus by a rapid and sensitive microtiter infection assay. J Clin Microbiol. 1988;26:231–235. doi: 10.1128/jcm.26.2.231-235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palker T J, Clark M E, Langlois A J, Matthews T J, Weinhold K J, Randall R R, Bolognesi D P, Haynes B F. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci USA. 1988;85:1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palker T J, Matthews T J, Langlois A, Tanner M E, Martin M E, Scearce R M, Kim J E, Berzofsky J A, Bolognesi D P, Haynes B F. Polyvalent human immunodeficiency virus synthetic immunogen comprised of envelope gp120 T helper cell sites and B cell neutralization epitopes. J Immunol. 1989;142:3612–3619. [PubMed] [Google Scholar]
- 28.Reimann K A, Li J T, Veazey R, Halloran M, Park I-W, Karlsson G B, Sodroski J, Letvin N L. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reimann K A, Li J T, Voss G, Lekutis C, Tenner-Racz K, Racz P, Lin W, Montefiori D C, Lee-Parritz D E, Lu Y, Collman R G, Sodroski J, Letvin N L. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J Virol. 1996;70:3198–3206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson H L, Montefiori D C, Johnson R P, Manson K H, Kalish M L, Lifson J D, Rizvi T A, Lu S, Hu S-L, Mazzara G P, Panicali D L, Herndon J G, Glickman R, Candido M A, Lydy S L, Wyand M S, McClure H M. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat Med. 1999;5:526–534. doi: 10.1038/8406. [DOI] [PubMed] [Google Scholar]
- 31.Rusche J R, Javaherian K, McDanal C, Petro J, Lynn D L, Grimaila R, Langlois A J, Gallo R C, Arthur L O, Fischinger P J, Bolognesi D P, Putney S D, Matthews T J. Antibodies that inhibit fusion of human immunodeficiency virus-infected cells bind a 24-amino acid sequence of the viral envelope gp120. Proc Natl Acad Sci USA. 1988;85:3198–3202. doi: 10.1073/pnas.85.9.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Safrit J T, Lee A Y, Andrews C A, Koup R A. A region of the third variable loop of HIV-1 gp120 is recognized by HLA-B7-restricted CTLs from two acute seropositive patients. J Immunol. 1994;153:3822–3830. [PubMed] [Google Scholar]
- 33.Salter R D, Howell D N, Cresswell P. Gene regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics. 1985;21:235–246. doi: 10.1007/BF00375376. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz G, Wolfs T, Goudsmit J. Characterization of the specificity of the human antibody response to the V3 neutralization domain of HIV-1. AIDS Res Hum Retroviruses. 1992;8:1897–1908. doi: 10.1089/aid.1992.8.1897. [DOI] [PubMed] [Google Scholar]
- 35.Seth A, Ourmanov I, Schmitz J E, Kuroda M J, Lifton M A, Nickerson C E, Wyatt L, Carroll M, Moss B, Venzon D, Letvin N L, Hirsch V M. Immunization with a modified vaccinia virus expressing simian immunodeficiency virus (SIV) Gag-Pol primes for an anamnestic Gag-specific cytotoxic T-lymphocyte response and is associated with reduction of viremia after SIV challenge. J Virol. 2000;74:2505–2509. doi: 10.1128/jvi.74.6.2502-2509.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spenlehauer C, Saragosti S, Fleury H J A, Kim A, Aubertin A-M, Moog C. Study of the V3 loop as a target epitope for antibodies involved in the neutralization of primary isolates versus T-cell-line-adapted strains of human immunodeficiency virus type 1. J Virol. 1998;72:9855–9864. doi: 10.1128/jvi.72.12.9855-9864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strizki J M, Turner J D, Collman R G, Hoxie J, Gonzalez-Scarano F. A monoclonal antibody (12G5) directed against CXCR-4 inhibits infection with dual-tropic human immunodeficiency virus type 1 isolate HIV-189.6 but not the T-tropic isolate HIV-1HxB. J Virol. 1997;71:5678–5683. doi: 10.1128/jvi.71.7.5678-5683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 39.Ugolini S, Mondor I, Parren P W H I, Burton D R, Tilley S A, Klasse P J, Sattentau Q J. Inhibition of virus attachment to CD4+ target cells is a major mechanism of T cell line-adapted HIV-1 neutralization. J Exp Med. 1997;186:1287–1298. doi: 10.1084/jem.186.8.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valenzuela A, Blanco J, Krust B, Franco R, Hovanessian A G. Neutralizing antibodies against the V3 loop of human immunodeficiency virus type 1 gp120 block the CD4-dependent and -independent binding of virus to cells. J Virol. 1997;71:8289–8298. doi: 10.1128/jvi.71.11.8289-8298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vancott T C, Polonis V R, Loomis L D, Michael N L, Nara P L, Birx D L. Differential role of V3-specific antibodies in neutralization assays involving primary and laboratory-adapted isolates of HIV type 1. AIDS Res Hum Retroviruses. 1995;11:1379–1390. doi: 10.1089/aid.1995.11.1379. [DOI] [PubMed] [Google Scholar]
- 42.Vogel T, Kurth R, Norley S. The majority of neutralizing abs in HIV-1-infected patients recognize linear V3 loop sequences. Studies using HIV-1 MN multiple antigenic peptides. J Immunol. 1994;153:1895–1904. [PubMed] [Google Scholar]
- 43.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 44.Zhou J Y, Montefiori D C. Antibody-mediated neutralization of primary isolates of human immunodeficiency virus type 1 in peripheral blood mononuclear cells is not affected by the initial activation state of the cells. J Virol. 1997;71:2512–2517. doi: 10.1128/jvi.71.3.2512-2517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zwart G, Back N K T, Ramautarsing C, Valk M, van der Hoek L, Goudsmit J. Frequent and early HIV-1 MN neutralizing capacity in sera from Dutch HIV-1 seroconverters is related to antibody reactivity to peptides from gp120 V3 domain. AIDS Res Hum Retroviruses. 1994;10:245–251. doi: 10.1089/aid.1994.10.245. [DOI] [PubMed] [Google Scholar]