Abstract
There is growing interest in developing artificial lighting that stimulates intrinsically photosensitive retinal ganglion cells (ipRGCs) to entrain circadian rhythms to improve mood, sleep, and health. Efforts have focused on stimulating the intrinsic photopigment, melanopsin; however, specialized color vision circuits have been elucidated in the primate retina that transmit blue-yellow cone-opponent signals to ipRGCs. We designed a light that stimulates color-opponent inputs to ipRGCs by temporally alternating short- and long-wavelength components that strongly modulate short-wavelength sensitive (S) cones. Two-hour exposure to this S-cone modulating light produced an average circadian phase advance of 1 h and 20 min in 6 subjects (mean age = 30 years) compared to no phase advance for the subjects after exposure to a 500 lux white light equated for melanopsin effectiveness. These results are promising for developing artificial lighting that is highly effective in controlling circadian rhythms by invisibly modulating cone-opponent circuits.
Keywords: circadian phase shift, social jet lag, circadian misalignment, photic entrainment, human circadian entrainment, DLMO
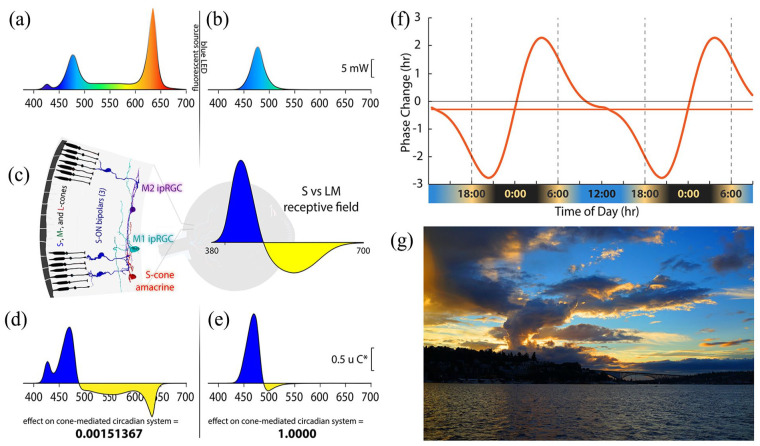
Circadian rhythms are entrained by 24-h environmental cycles. The light-dark cycle emerging from the solar day acts as the most important entraining cycle in virtually all species including humans. Light can reset the phase of the central circadian clock, with light pulses leading to phase delays or advances depending on the phase at which light is applied. We measured the effectiveness of lights with different spectral and temporal properties in generating circadian phase advances. Lights were calibrated to produce the same time-averaged effect on melanopsin, but to have large differences in their effect on the recently discovered color vision circuits that drive M1 and M2 intrinsically photosensitive retinal ganglion cells (ipRGCs; Patterson et al., 2020a, 2020b) underlying color-opponent receptive fields first discovered by Dacey et al. (2005), as illustrated in Figure 1. One light had the same International Commission on Illumination (CIE) 1931 chromaticity diagram coordinates as an equal energy white (x = y = 0.33) and produced an illuminance of 500 lux at the pupil plane (Figure 1a). While a 500 lux exposure for even 30 min can significantly suppress nocturnal melatonin levels, white lights only advance the circadian phase when light intensities and durations are much greater (Hashimoto et al., 1996; Khalsa et al., 2003; Kozaki et al., 2011) and as modeled by St Hilaire et al. (2022). The second light was blue, derived from an LED (476 nm peak; Figure 1b). These 2 lights are predicted to have very different effects on the color vision circuits carrying chromatically opponent signals from short (S-), middle (M-), and long (L-) wavelength-sensitive cones that drive ipRGCs in primates (Patterson et al., 2020a, 2020b; Figure 1c, left). Primate ipRGCs display an S versus (L + M) color-opponent light response (Figure 1c, right). The white light drives both excitatory and inhibitory sides of the color-opponent response, which cancel each other, thus producing little net drive to the ipRGCs from cones. In contrast, the blue light strongly stimulates one side of the color-opponent system. The white light is predicted to produce a null response (Figure 1d), and the blue light is predicted to strongly differentially excite one side of the color-opponent ipRGC (Figure 1e), making blue about 660 times more effective at driving the color-opponent cone pathways upstream of the ipRGCs compared to static white illumination. Finally, we tested a third light that temporally modulated S versus (L + M) cones by rapidly alternating short and long wavelengths. Unlike the sustained melanopsin activation within ipRGCs, the cone inputs have transient responses. The S versus (L + M) inputs to ipRGCs have high temporal sensitivity, while the temporal sensitivity of S-cone inputs to our conscious color vision is very poor. Thus, the conscious perception of alternating short and long wavelengths can fuse to a relatively steady-appearing white light, while ipRGCs are strongly activated via S versus (L + M) circuitry. If further developed, this strategy may work as a practical lighting solution in which modest-intensity white lights can reset the phase delays associated with social jet lag and other circadian disorders.
Figure 1.
Spectral distributions of experimental light stimuli, their predicted effects on the color-opponent inputs to ipRGCs, and a theoretical phase response curve. (a) Spectrum of the experimental white light with chromaticity coordinates 0.333, 0.333. (b) Spectrum of the LED-derived experimental “blue” light with a spectral peak at 476 nm. (c) (Left) Illustration of the color vision circuitry for S-ON and S-OFF types of primate ipRGCs. (Right) Illustration of the spectrally opponent response of an S-ON ipRGC with S - (L + M) cone inputs. (d) The product of wavelength-by-wavelength multiplication of the spectral distribution of the white light (a) times the spectrally opponent response of an ipRGC (c). Integration of the curve in (d) across wavelength yields the predicted very small relative response (0.0015) of the ipRGC to the white light. (e) The product of multiplication of the spectral distribution of the blue light (b) times the spectrally opponent response of an ipRGC (c). Integration across wavelengths yields the predicted large relative response of the ipRGC to the blue light, which we have normalized to 1. The blue light is expected to be about 667 times more effective at activating the S-cone color vision circuit than the white light. (f) Phase response curve based on Khalsa et al. (2003) that is aligned with earth time so that the beginning of the internal biological night occurs at sunset and the end of the internal biological night occurs before wake time just after sunrise as indicated below the x-axis of the curve. (g) Image of sunset in Seattle, Washington, illustrating how contrasting short and long wavelength light near the horizon produces a stimulus capable of driving spectrally opponent inputs to ipRGCs, making them act as sunrise/sunset detectors.
People who spend most of their time under artificial light often suffer from a phase-delayed circadian rhythm (Wright et al., 2013; Stothard et al., 2017; Casiraghi et al., 2020). This is partly because indoor lighting is ineffective in inducing phase advances. This, combined with social schedules that demand early wake-up times, causes “social jet lag” (Wittmann et al., 2006; Roenneberg et al., 2019), which is associated with disturbed sleep, daytime fatigue, reduced cognitive function, and a general feeling of unwellness. Compared to melanopsin, cone-opponent circuits in primates activate ipRGCs at much lower thresholds (Dacey et al., 2005). Thus, at standard indoor low illumination levels, lights optimized to stimulate the color-opponent circuits should be more effective in producing circadian phase advances than typical white artificial lighting, which balances excitatory and inhibitory cone components and has little net effect (Figure 1d). Our goal is to evaluate the most effective lighting approach for circadian photoentrainment at the comparatively low general indoor lighting lux to phase-advance the central circadian clock and, in turn, combat social jet lag and other circadian problems such as seasonal affective disorder.
When humans are exposed only to natural light, the internal circadian clock synchronizes to solar time such that the internal biological night begins at sunset and ends before wake time just after sunrise (Wright et al., 2013; Figure 1f). We used dim light salivary melatonin onset (DLMO) as a measure of circadian phase. Figure 2a shows the rise in evening melatonin levels assayed from saliva samples for the 6 subjects who participated in this study (each subject is represented by a different color). Compared to being synchronized to solar time (shown by the dashed gray curve; Figure 2a), we found that, on average, subjects were phase delayed by 2.8 h. Results for each subject were comparable across multiple days, indicating that while all subjects were phase delayed, they were still entrained to the 24-h environmental cycle. These results are like those observed earlier for young academics (Wright et al., 2013), and the considerable variability in the phase delays among the 6 subjects is consistent with the fact that later chronotypes are associated with longer phase delays (Wright et al., 2013).
Figure 2.
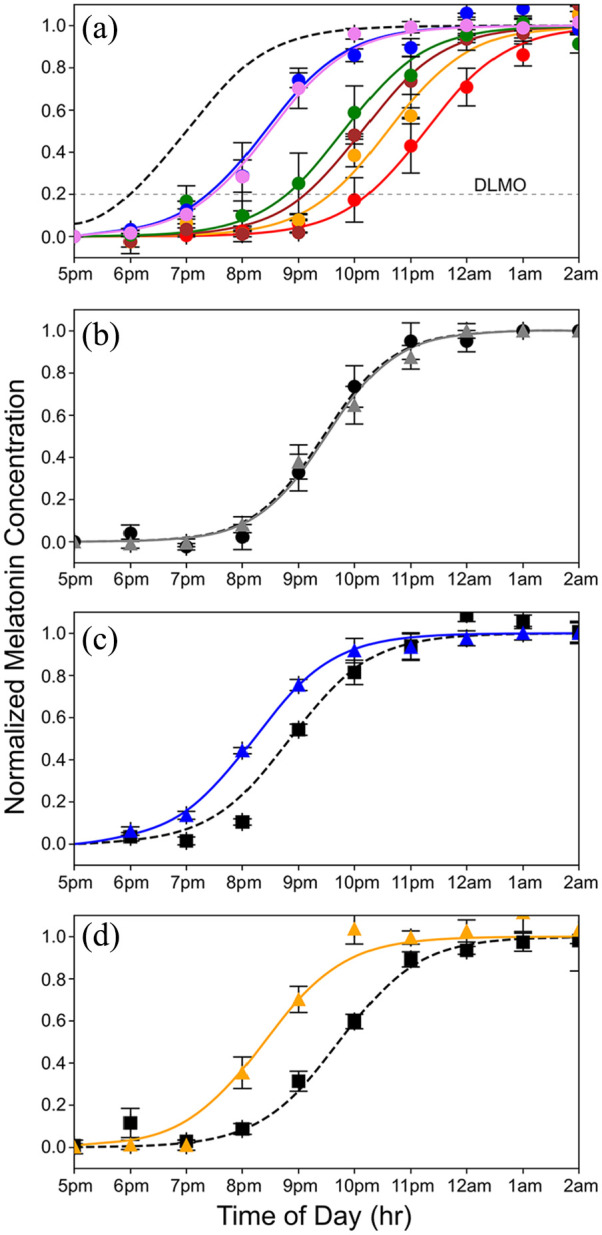
Curves showing the nighttime dim rise in salivary melatonin levels under various conditions equated for melanopsin effectiveness. (a) Rise in evening melatonin levels for the 6 subjects who participated in this study (each is shown in a different color; error bars are standard errors of the mean). The dashed gray curve shows the predicted rise if the subjects were aligned to earth time, where the beginning of internal biological night occurs at sunset, which is 1800 h based on a 12-12 dark light cycle. On average, subjects were phase delayed 2.8 h. (b) Average rise in evening melatonin after 2-h exposure to the static white light (gray curve) of Figure 1a compared to a baseline (dashed curve) measured on day 1 of the 3-day protocol. A slight, nonsignificant phase delay was associated with the white light exposure (n = 6 subjects). (c) Average rise in evening melatonin (blue curve) after a 2-h exposure to the 476 nm blue light of Figure 1b compared to baseline (dashed curve) (n = 6 subjects). The 476 nm light produced a phase advance of 40 min (t = 3.4, p = 0.019). (d) The rise in evening melatonin (orange curve) after 2-h exposure to 19 Hz S-cone modulated light compared to baseline (dashed curve) (n = 6 subjects). This light produced a phase advance of 1 h and 20 min (t = 7.1, p = 0.0009).
The subjects were involved in (or familiar with) studies related to circadian rhythms. As such, they were motivated to adhere to the grueling demands of the protocol. We believe that having motivated, compliant participants was key to obtaining precise and reliable results. However, this means that the highly compliant participants were not blind to the conditions or hypotheses. Salivary melatonin measurements are objective, so the fact that participants were not naïve is unlikely to bias the results. In addition, we took extensive measures to ensure the subjects’ knowledge could not bias the results; for more details, see the supplemental materials.
To evaluate the ability of lights with different spectral and temporal characteristics to advance circadian phase, we followed the same 3-day protocol for each light condition. On the evening of the first day, subjects collected saliva samples every hour from the early evening until 0200 h. The following day, the samples were analyzed to measure the rise in melatonin the evening before, and the time of DLMO was determined for each subject, defined as the time the melatonin levels reached 20% of the maximum (Benloucif et al., 2005). On the morning of the third day of the protocol, each subject viewed a test light for 2 h, centered 10.5 h after their individual DLMO. This corresponds to the time of day expected to produce maximum light-induced phase advances (Figure 1f; Khalsa et al., 2003). On the evening of the same day, subjects again collected saliva samples that were used to evaluate whether the light exposure produced a phase advance.
Figure 2b shows the results for the static white light. After exposure to the static white light, the average rise in evening salivary melatonin levels was phase delayed by 2.8 minutes, a value that did not differ significantly from the baseline measured before exposure (p = 0.75; NS paired t-test). In contrast, the 476 nm blue light that was equated in melanopsin effectiveness to the static white light produced a phase advance of 40 min (and a total difference from the white light condition of 42.8 min). This phase advance was significantly larger than the baseline measured before exposure (t = 3.423, p = 0.019; paired t test; Figure 2c).
Blue lights are particularly effective at suppressing nocturnal melatonin (Brainard et al., 2001; Thapan et al., 2001), and it is often assumed this is mediated by melanopsin. However, the blue and white lights were equated for melanopsin effectiveness; thus, the large effect of blue compared to white cannot be attributed to the activation of melanopsin. Since the white condition nulls the color-opponent response (Figure 1d), we assumed that it effectively isolates the melanopsin drive to the ipRGCs. We conclude that under the relatively low light and short duration conditions used here, melanopsin activation is insufficient to produce any significant circadian phase advance. Moreover, it follows that the substantial phase advance produced by the blue light equated in melanopsin effectiveness to the white light is the result of activation of the color-opponent circuitry, not melanopsin, as commonly assumed.
Our goal is to develop lighting that can replace standard indoor white lighting and give people control of their circadian phase. As an alternative to the static blue light (Figure 2c), which is not a practical indoor lighting solution, we tested a temporally modulated light because, unlike the sustained melanopsin activation within ipRGCs, the cone inputs have transient responses (Weng et al., 2013; Brown et al., 2021; St Hilaire et al., 2022).
Thus, theoretically, a light that alternates between short- and long-wavelength components would stimulate color-opponent cells optimally by the simultaneous offset of one spectral component and the onset of the opposing component. Moreover, it should be possible to produce 2 light phases that, when temporally alternated, appear white but strongly modulate S-cones. The S-cone inputs to ipRGCs are tuned to respond to higher temporal frequencies than those serving hue perception, making it possible to modulate the S-cone input to ipRGCs strongly but minimize (and ideally eliminate) the percept of flicker.
The S-cone modulating light tested here consisted of a 19 Hz alternating pulse train designed to modulate the quantal catch of S-cones with a differential of 100× between the 2 phases. The intensities of LEDs peaking at 427 nm versus 544 nm were alternated, and the addition of light from a 637 nm LED made the S-cone modulated pulse train appear nominally white. The intensity of this light was adjusted to produce a time-averaged quantal catch in melanopsin matched to the 500 lux static white light of Figure 1a. As shown in Figure 2d, the cone-modulated “white light” elicited a striking 1 hr 20 min phase advance compared to the nonsignificant change in circadian phase produced by the steady white light. The difference between the steady white and the 19 Hz S-cone modulated conditions was highly significant (p < 0.01; t = 4.4 Bonferroni Multiple Comparisons Test).
Previously, 1 h of bright white (~8,000 lux) light produced a 27-min advance in circadian phase (St Hilaire et al., 2012). When white lights are sufficiently bright or prolonged, they can produce a phase advance by activating the much less sensitive melanopsin expressed in human ipRGCs compared to the 500 lux static white light that was ineffective here (Figure 2b). However, our light condition that strongly modulates the S-cones for 2 h (500 lux × 2 h vs ~8,000 lux × 1 h) amounts to 8× fewer lux-hours but produced a circadian phase advance that was twice as great. Thus, the S-cone modulating light is as effective as very bright white at 1/20th the intensity.
Our results showing that a light that temporally modulates S cones versus (L + M) cones is highly effective for advancing circadian phase are consistent with those of St Hilaire et al. (2022), who demonstrated that melatonin suppression is driven strongly by S and L + M cones in the first quarter of light exposure and with Brown et al. (2021) who found evidence for an initial S-cone contribution to melatonin suppression that rapidly decays under extended light exposure. Our S-cone modulated light alternated between 2 wavelengths designed to stimulate one side of the cone-opponent response and then the other alternately to produce a strong cone-opponent response. In contrast, Spitschan and colleagues (Spitschan et al., 2019) measured melatonin suppression by comparing 2 light stimuli, which differed exclusively in the amount of S-cone excitation. As predicted, the light with stronger S-cone excitation did not differentially suppress melatonin because to equate the 2 lights for L and M cone effectiveness, the S+ light had to include about equal amounts of long- and short-wavelength light, nulling the color-opponent response.
As a different alternative to static illumination, Zeitzer et al. administered 60 2-msec pulses of 473 lux broad spectral band light over an hour and produced a phase change nearly half that of 1 h 10,000 lux static white light (Zeitzer et al., 2011). We assume that the increased effectiveness is due to the involvement of cone circuits, as in the experiments reported here, since transient white flashes drive spectrally opponent cone inputs to ipRGCs by virtue of differences in the temporal properties of their components. The S-cone modulating light is 4 times more effective than the millisecond flashes of light that Zeitzer et al. administered, and the exchange between long- and short-wavelength components can appear nearly static, whereas bright flashes every minute are not a practical solution for standard indoor illumination. We did not test a condition where the 2 lights in our S-cone modulated condition were modulated in phase rather than out of phase, making an on-off flicker that would temporally modulate S, M, and L cones and may, thus, produce even larger phase advances but the intense flickering sensation such a light would produce would preclude it as a lighting solution.
The color of the sky at sunrise and sunset (Figure 1g) is the ideal cue for synchronizing one’s internal body clock to solar time. The intensity of light overhead is an unreliable indicator of the time of day (Woelders et al., 2018), but the orange color of the sky at the horizon always indicates that it is sunrise or sunset, and it has been shown that mammalian blue-yellow color discrimination provides a more reliable method of tracking twilight progression than simply measuring irradiance (Walmsley et al., 2015). The color-opponent inputs to ipRGCs confer the ability to act as sunrise/sunset detectors. The orange color of the horizon that characterizes the rising and setting sun produces a color contrast with the blue sky (Figure 1g). The blue and orange parts of the image on the retina produced by the sunset moving across the receptive field of a primate ipRGC activate the transient color-opponent response very strongly. As shown in Figure 1f, when our internal clock is aligned with solar time, sunrise occurs after the peak of the phase advance portion of the phase response curve, and sunset occurs before the peak of the phase delay portion. When the ipRGCs are strongly stimulated at both dawn and dusk, the human phase response curve is perfectly tuned to keep the phase of our internal pacemaker precisely aligned with solar time.
Color-opponent mechanisms are associated with sensory systems that regulate circadian activity throughout the animal kingdom, including fish and reptiles (Su et al., 2006; Pauers et al., 2012). Ancient single-celled organisms exhibit color sensitivity that they use to drive their circadian activity (Spudich and Spudich, 2008). It appears that the capacity to sense colors originally evolved to serve circadian rhythms, not for hue perception (Neitz and Neitz, 2011). The fact that primates have evolved multiple independent circuits that provide color-opponent inputs to ipRGCs is a testament to the importance of these sunrise and sunset detectors to our evolutionary survival (Patterson et al., 2021). Thus, it makes perfect sense to develop lighting to use these color vision circuits to take control of our circadian well-being. A light that modulates S-cones almost imperceptibly, with daily exposure, for example, from a bedroom light fixture to get exposure when first waking, or one in the bathroom or kitchen providing exposure in the context of a person’s normal lighting routine, may be capable of offsetting the average 2.8 h delay, therefore eliminating social jet lag. Thus, rather than focusing on melanopsin, further experiments stimulating ipRGCs by modulating S-cones promise to provide insight into how to design healthy indoor lighting to help control circadian rhythms to improve mood, sleep, and health.
Supplemental Material
Supplemental material, sj-docx-1-jbr-10.1177_07487304241262918 for Toward an Indoor Lighting Solution for Social Jet Lag by Alexandra Neitz, Alicia Rice, Leandro Casiraghi, Ivana L. Bussi, Ethan D. Buhr, Maureen Neitz, Jay Neitz, Horacio O. de la Iglesia and James A. Kuchenbecker in Journal of Biological Rhythms
Acknowledgments
This work was supported by NIH grants R01 EY027859, P30EY001730, and T32EY007031 and Research to Prevent Blindness.
Supplementary material is available for this article online.
Footnotes
Author contributions: A.N., J.N., and J.A.K. had full access to the data and take responsibility for the data integrity and accuracy of the analysis. A.N., J.N., M.N., and J.A.K. conceptualized the study. A.N., M.N., A.R., L.C., I.L.B., E.D.B., and J.A.K. did the experiments. A.N., J.A.K., and J.N. wrote the first draft of the manuscript. All authors contributed to the final draft of the manuscript.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The University of Washington has filed U.S. Patent Application, entitled “LIGHTING DEVICES, SYSTEMS, METHODS FOR STIMULATING CIRCADIAN RHYTHMS” serial number 17/612,061 for which authors A.N., M.N., J.N., and J.A.K. receive licensing fees.
ORCID iDs: Ivana L. Bussi  https://orcid.org/0000-0003-4721-763X
https://orcid.org/0000-0003-4721-763X
Horacio O. de la Iglesia  https://orcid.org/0000-0003-0855-6807
https://orcid.org/0000-0003-0855-6807
References
- Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L’hermite-Balériaux M, Zee PC. (2005) Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms 20:178-188. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. (2001) Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci 21:6405-6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM, Thapan K, Arendt J, Revell VL, Skene DJ. (2021) S-cone contribution to the acute melatonin suppression response in humans. J Pineal Res 71:e12719. [DOI] [PubMed] [Google Scholar]
- Casiraghi LP, Plano SA, Fernández-Duque E, Valeggia C, Golombek DA, de la Iglesia HO. (2020) Access to electric light is associated with delays of the dim-light melatonin onset in a traditionally hunter-gatherer Toba/Qom community. J Pineal Res 69:e12689. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Liao H-W, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau K-W, Gamlin PD. (2005) Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 433:749-754. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Nakamura K, Honma S, Tokura H, Honma K. (1996) Melatonin rhythm is not shifted by lights that suppress nocturnal melatonin in humans under entrainment. Am J Physiol 270:R1073-R1077. [DOI] [PubMed] [Google Scholar]
- Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. (2003) A phase response curve to single bright light pulses in human subjects. J Physiol 549:945-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaki T, Toda N, Noguchi H, Yasukouchi A. (2011) Effects of different light intensities in the morning on dim light melatonin onset. J Physiol Anthropol 30:97-102. [DOI] [PubMed] [Google Scholar]
- Neitz J, Neitz M. (2011) The genetics of normal and defective color vision. Vision Res 51:633-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SS, Kuchenbecker JA, Anderson JR, Neitz M, Neitz J. (2020. a) A color vision circuit for non-image -forming vision in the primate retina. Curr Biol 30:1269-1274.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SS, Mazzaferri MA, Bordt AS, Chang J, Neitz M, Neitz J. (2020. b) Another Blue-ON ganglion cell in the primate retina. Curr Biol 30:R1409-R1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SS, Neitz M, Neitz J. (2021) S-cone circuits in the primate retina for non-image-forming vision. Semin Cell Dev Biol 126:66-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauers M, Kuchenbecker J, Neitz M, Neitz J. (2012) Changes in the colour of light cue circadian activity. Anim Behav 83:1143-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Pilz LK, Zerbini G, Winnebeck EC. (2019) Chronotype and social jetlag: a (self-) critical review. Biology 8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitschan M, Lazar R, Yetik E, Cajochen C. (2019) No evidence for an S cone contribution to acute neuroendocrine and alerting responses to light. Curr Biol 29:R1297-R1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J, Spudich E. (2008) The simplest eyes: rhodopsin-mediated phototaxis reception in microorganisms. In: Tsonis P, editor. Animal Models in Eye Research. Elsevier. p. 6-14. [Google Scholar]
- St Hilaire MA, Ámundadóttir ML, Rahman SA, Rajaratnam SMW, Rüger M, Brainard GC, Czeisler CA, Andersen M, Gooley JJ, Lockley SW. (2022) The spectral sensitivity of human circadian phase resetting and melatonin suppression to light changes dynamically with light duration. Proc Natl Acad Sci U S A 119:e2205301119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Hilaire MA, Gooley JJ, Khalsa SBS, Kronauer RE, Czeisler CA, Lockley SW. (2012) Human phase response curve to a 1 h pulse of bright white light. J Physiol 590:3035-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stothard ER, McHill AW, Depner CM, Birks BR, Moehlman TM, Ritchie HK, Guzzetti JR, Chinoy ED, LeBourgeois MK, Axelsson J, et al. (2017) Circadian entrainment to the natural light-dark cycle across seasons and the weekend. Curr Biol 27:508-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C, Luo D, Terakita A, Shichida Y, Liao H, Kazmi M, Sakmar T, Yau KW. (2006) Parietal-eye phototransduction components and their potential evolutionary implications. Science 311:1617-1627. [DOI] [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ. (2001) An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol 535:261-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley L, Hanna L, Mouland J, Martial F, West A, Smedley AR, Bechtold DA, Webb AR, Lucas RJ, Brown TM. (2015) Colour as a signal for entraining the mammalian circadian clock. PLoS Biol 13:e1002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng S, Estevez ME, Berson DM. (2013) Mouse ganglion-cell photoreceptors are driven by the most sensitive rod pathway and by both types of cones. PLoS One 8:e66480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. (2006) Social jetlag: misalignment of biological and social time. Chronobiol Int 23:497-509. [DOI] [PubMed] [Google Scholar]
- Woelders T, Wams E, Gordijn M, Beersma D, Hut R. (2018) Integration of color and intensity increases time signal stability for the human circadian system when sunlight is obscured by clouds. Sci Rep 8:15214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Jr, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. (2013) Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol 23:1554-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer JM, Ruby NF, Fisicaro RA, Heller HC. (2011) Response of the human circadian system to millisecond flashes of light. PLoS One 6:e22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jbr-10.1177_07487304241262918 for Toward an Indoor Lighting Solution for Social Jet Lag by Alexandra Neitz, Alicia Rice, Leandro Casiraghi, Ivana L. Bussi, Ethan D. Buhr, Maureen Neitz, Jay Neitz, Horacio O. de la Iglesia and James A. Kuchenbecker in Journal of Biological Rhythms