Abstract
Introduction
Japan will be transitioning from the free-of-charge COVID-19 vaccination program to annual periodic vaccination under a national immunization program for old adults and high-risk patients from 2024 fall/winter season. The policy transition including out-of-pocket payment requirement may discourage vaccination, leading to a lower vaccination rate. This study aimed to estimate the impact of varying vaccination rates with BNT162b2 COVID-19 mRNA vaccine on economics and public health in an illustrative prefecture which administers and promotes the periodic vaccination program, using budget impact analysis.
Methods
A combined cohort Markov decision tree model estimated the public health outcomes of COVID-19-related symptomatic cases, hospitalizations and deaths; and the economic outcomes including vaccine-related cost, non-vaccine-related medical cost, and productivity loss from the societal perspective. The base case examined the impact on the outcomes when vaccination coverage changed from the reference value of 50% to upper and lower values, respectively. Scenario analyses were performed based on multiple scenarios.
Results
Increase in the vaccination rate demonstrated improvement in all public health outcomes. At 50% vaccination, the vaccine-related cost for 3 years in a prefecture was estimated at JPY 7.58 billion (USD 57.67 million), the non-vaccine-related medical cost at JPY 79.22 billion (USD 602.48 million), the productivity loss at JPY 253.11 billion (USD 1.92 billion), and the total cost at JPY 339.92 billion (USD 2.59 billion). When the vaccination rate increased to 90%, the total cost decreased by JPY 4.88 billion (USD 37.11 million) (1.4%). When the vaccination rate decreased to 10%, the total cost increased by JPY 5.73 billion (USD 43.58 million) (1.7%). Results were consistent across almost all scenario analyses.
Conclusions
Maintaining a high vaccination rate with BNT162b2 is important from both public health and economic perspectives in Japan. The findings highlight to local governments the importance of continued effort to promote vaccination.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-024-01032-y.
Keywords: Booster, BNT162b2, Budget impact analysis, COVID-19, Japan, National immunization program, Periodic vaccination, Pfizer-BioNTech Vaccine, SARS-CoV-2
Key Summary Points
| Why carry out the study? |
| Starting from the fall of 2024, Japan will be transitioning from the free-of-charge COVID-19 vaccination program to annual periodic vaccination under National Immunization Program (NIP) to be implemented by local governments for those aged 65 years and above, and those aged 60-64 years at high risk. |
| Because the periodic COVID-19 vaccination program requires out-of-pocket payment, the vaccination rate is feared to become lower than before, leading to concerns that the full benefits of the vaccine may not be realized. |
| This study evaluated the impact of changes in vaccination rates on the economy of local governments and the health of their citizens. |
| What was learned from the study? |
| Increase in the vaccination rate led to reduction in COVID-19-related symptomatic cases, hospitalizations and deaths. |
| Although increase in the vaccination rate led to increase in vaccine-related costs, non-vaccine-related medical costs and productivity losses decreased even more, leading to a net reduction in total costs. |
| It is hoped that, with consideration given to the public health benefits, the impact on healthcare finance and the entire local society, local governments in Japan will investigate and implement policies of promoting COVID-19 vaccination. |
Introduction
COVID-19 is a highly infectious respiratory illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that has become endemic throughout the world since the outbreak in December 2019 [1, 2]. In particular, senior citizens and those with underlying chronic illnesses affecting heart, liver, kidney, or respiratory organs, are at greater risk of aggravation of COVID-19 or mortality [3–5]. In responding to the massive public health crisis with COVID-19 causing dramatic disruptions in social and economic activities [6, 7], BNT162b2 and other COVID-19 vaccines have reduced symptomatic infections, hospitalization, mortality rates and long-term sequelae (referred to as post-acute sequelae SARS-CoV-2 infection (PASC), or more commonly as “long COVID”) due to COVID-19 [8–10], and they serve as a major tool in humanity’s fight against COVID-19. A growing body of evidence also supports the ongoing need for periodic vaccinations in order to maintain immunity levels and lower risk of symptomatic infections, hospitalization and long COVID [11–13]. Moreover, COVID-19 vaccination can have a significant positive economic impact by reducing treatment costs as well as avoiding or shortening infection-related absences from work [14, 15].
In Japan, vaccination has also led to significant public health benefits by reducing negative public health outcomes of COVID-19 [16–19]. With major peak waves of the pandemic receding in Japan, the special temporary COVID-19 vaccination sponsored by the Ministry of Health, Labour, and Welfare (MHLW) of the Japanese government that allowed vaccination free of charge was ended in March 2024, and annual periodic COVID-19 vaccination under a national immunization program for people aged 65 and above and for people aged 60–64 at high risk has been approved to start in the fall of 2024 [20]. From the fiscal year 2024 onward, the main purpose of COVID-19 vaccination in Japan will be regarded by the national government as the reduction of the number of severe COVID-19 cases by preventing individuals from becoming seriously ill. The annual periodic COVID-19 vaccination program requires a partial out-of-pocket payment from vaccination recipients. This periodic vaccination program is administered by local municipalities in Japan which are also responsible for promotion of vaccination based on public health and economic perspectives. It implies that vaccine policies in each municipality can have a direct impact on the individual residents, as well as the public health and local economy of those communities.
A recent study in Japan has shown that booster vaccination with BNT162b2 is cost-effective versus no vaccination [21]. This result supports Japan’s COVID-19 vaccination policy under the NIP from the fall of 2024, including the eligible population and frequency. However, the implementation of the NIP, which includes the out-of-pocket payment requirement for the vaccination, may discourage vaccination potentially leading to a lower vaccination rate than before. For local governments, maintaining a high vaccination rate is an important policy issue because COVID-19 infection can impose large societal costs on local communities through decreased productivity of patients and increased burden on caregivers [22, 23]. Thus, this study used the budget impact analysis (BIA) approach to evaluate the impact of varying vaccination rates on the number of COVID-19 cases, hospitalizations and deaths, as well as societal costs at the level of local governments.
Methods
Model Structure and Settings
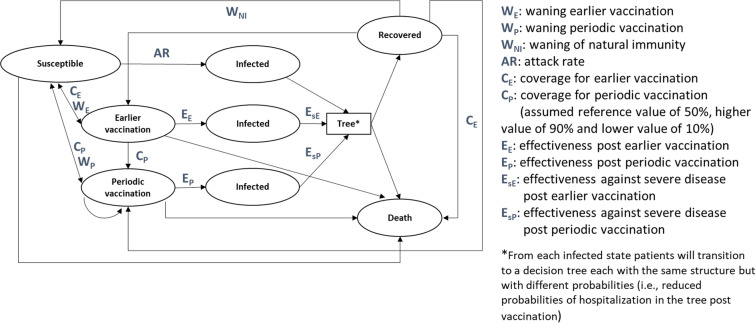
A BIA was conducted comparing the annual periodic vaccination with BNT162b2 at various assumptions of vaccination rates for the vaccine eligible population. Since it is not the central government but the local governments that are responsible for administration of the periodic vaccination program and promotion of immunization, an illustrative example of a prefecture in Japan (hereinafter referred to as Prefecture X) was adopted to conduct the analysis. The BIA model used for the present study was adapted from prior studies [21, 24–26]. The analysis model consisted of two parts: the Markov model that represents the transition of subjects among the susceptible, infected, recovered, and vaccinated states (Fig. 1), and the decision tree model that represents the transition of the condition of COVID-19 patients in the acute phase [24]. The analysis model was implemented as a static model programmed in Microsoft Excel. If available, model inputs were obtained for the period of the Omicron dominance in early 2022. The main model inputs are presented in Table 1.
Fig. 1.
Markov cohort model structure. WE waning earlier vaccination; WP waning periodic vaccination; WNI waning of natural immunity; AR attack rate; CE coverage for earlier vaccination; CP coverage for periodic vaccination (assumed reference value of 50%, higher value of 90% and lower value of 10%); EE effectiveness post earlier vaccination; EP effectiveness post periodic vaccination; EsE effectiveness against severe disease post earlier vaccination; EsP effectiveness against severe disease post periodic vaccination
Table 1.
Main model inputs for the base case
| Category | Input description | 5–11 years | 12–17 years | 18–29 years | 30–59 years | 60–64 years | 65–74 years | ≥ 75 years | References |
|---|---|---|---|---|---|---|---|---|---|
| Effectiveness inputs | Vaccine effectiveness (infection/symptomatic/hospitalization) (%) | 50/60/70 | [27–31] | ||||||
| Vaccine-induced duration of protection against infection | 6 months | [21] | |||||||
| Clinical inputs | Annual attack rate (%) | 32.7 | 30.5 | 27.2 | 21.1 | 11.1 | 9.1 | 9.1 | [32] |
| Duration of protection from infection-induced immunity | 3 months | [21] | |||||||
| Hospitalization rate among symptomatic patients (%) | 0.8 | 0.6 | 1.1 | 1.3 | 2.9 | 6.0 | 22.8 | [33] | |
| Among hospitalized patients: | |||||||||
| General ward with IMV (%) | 0.0 | 0.0 | 0.4 | 4.2 | 4.2 | 6.8 | 6.8 | [34] | |
| ICU without IMV (%) | 0.10 | 0.10 | 1.30 | 7.2 | 7.2 | 10.9 | 10.9 | [34] | |
| ICU with IMV (%) | 0.0 | 0.0 | 0.4 | 4.2 | 4.2 | 6.8 | 6.8 | [34] | |
| Probability of COVID-19-related death among infected individuals: | |||||||||
| General ward without IMV (%) | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.7 | 0.7 | [34] | |
| General ward with IMV (%) | 0.0 | 0.0 | 6.7 | 9.1 | 9.1 | 34.8 | 34.8 | [34] | |
| ICU without IMV (%) | 0.0 | 0.0 | 6.7 | 9.1 | 9.1 | 34.8 | 34.8 | [34] | |
| ICU with IMV (%) | 0.0 | 0.0 | 6.7 | 9.1 | 9.1 | 34.8 | 34.8 | [34] | |
| Outpatient care setting (%) | 0.00 | 0.00 | 0.00 | 0.01 | 0.08 | 0.20 | 1.13 | [35] | |
| Probability of long COVID among infected individuals: | |||||||||
| Patients having received outpatient care (%) | 15.5 | 25.3 | 34.5 | 42.8 | 33.1 | 32.2 | 29.9 | [36] | |
| Patients having received inpatient care (%) | 35.6 | 35.6 | 35.6 | 46.9 | 66.7 | 66.7 | 66.7 | [37] | |
| Share of individuals at high risk within age groups | 0.1 | 0.1 | 0.1 | 0.4 | 0.6 | 0.9 | 2.3 | [38, 39] | |
| Hospitalization risk ratio for individuals at high risk | 1.83 | [40] | |||||||
| Mortality risk ratio for individuals at high risk | 1.78 | [40] | |||||||
| Direct medical cost inputs | Vaccine acquisition cost per dose (JPY) | 11,600 | [41] | ||||||
| Vaccine administration cost per dose (JPY) | 3740 | [41] | |||||||
| COVID-19 test (JPY) | 8500 | [42] | |||||||
| Number of tests/case (asymptomatic/outpatient/inpatient/ICU) | 0/1/3/3 | Assumption | |||||||
| GP visit unit cost (JPY) | 28,054 | [42] | |||||||
| Inpatient treatment (JPY): | |||||||||
| General ward without IMV | 562,650 | 561,632 | 845,761 | 937,469 | 965,039 | 1,239,964 | 1,446,865 | [43] | |
| General ward with IMV | 1,449,227 | 1,449,227 | 1,449,227 | 1,449,227 | 205,148 | 854,644 | 1,986,158 | [43] | |
| ICU without IMV | 3,534,277 | 3,534,277 | 3,534,277 | 1,857,751 | 5,259,124 | 4,079,808 | 3,788,960 | [43] | |
| ICU with IMV | 2,263,806 | 2,263,806 | 3,870,947 | 3,870,947 | 7,413,719 | 6,364,921 | 3,768,221 | [43] | |
| Long COVID treatment cost (JPY) | 107,725 | [25, 42] | |||||||
| Productivity loss inputs | Workforce participation rate (%) | 100.0 | Assumption | ||||||
| Labour cost per week (JPY) | 85,025 | [44] | |||||||
| Working time lost (days): | |||||||||
| Outpatient care | 5.0 | [43, 45] | |||||||
| General ward without IMV | 21.0 | [43, 45] | |||||||
| General ward with IMV | 40.0 | [43, 45] | |||||||
| ICU without IMV | 40.0 | [43, 45] | |||||||
| ICU with IMV | 40.0 | [43, 45] | |||||||
| Patients with long COVID | 60.0 | [43, 45] | |||||||
Refer to the supplemental material for additional model inputs not listed in Table 1
COVID-19 Coronavirus disease 2019; GP general physician; ICU intensive care unit; IMV invasive mechanical ventilation; JPY Japanese Yen; QALY quality-adjusted life year
Population
In the base case, the information in the National Census 2020 Basic Tabulation of Population was used to establish the population for each age group in Prefecture X (Table S1 in the supplementary material) [46]. The analysis population was defined to be the entire population of Prefecture X, excluding those who did not complete the two injections of the primary dose of the vaccine; and the vaccine eligible population was defined to be people aged 65 and above and for people aged 60–64 at high risk within the analysis population. This is consistent with the criteria for the population eligible for the new annual periodic COVID-19 vaccination program in the fall of 2024 [20], analogous to the vaccination program intended for the infectious diseases categorized as Class B by the MHLW, such as seasonal influenza [47]. Individuals at high risk were defined as those who have impaired heart, kidney, or respiratory functions that severely limit their personal lives, or those who have impaired immune function due to the human immunodeficiency virus (HIV) that makes daily living almost impossible [20]; these individuals were deemed equivalent to Grade 1 Disability for the physically disabled designated by the MHLW, and the proportion of individuals at high risk in the population was estimated based on patient surveys conducted by the MHLW [48]. The percentages of susceptible/recovered (infection-induced immunity)/vaccinated states at the start of the analysis were established based on a survey of actual antibody retention rates and several assumptions (Table S2 in the supplementary material) [21, 49]. For the subgroup analysis 1, 3 municipalities in Prefecture X (hereinafter referred to as City A, City B, and City C, where people aged 65 and above make up 19.2%, 26.9%, and 36.1% of the city populations, respectively) with different rates of population aging were considered as the analysis population (Table S1 in the supplementary material) [46]. For the subgroup analysis 2, the analysis population was restricted to those who are 65 years and above within the population of Prefecture X, to focus on the individual-level impact of changes in vaccination rates for the main target of the vaccination program.
Vaccine Effectiveness
COVID-19 vaccines have undergone changes in response to evolving pandemics through modification of the vaccine to be active against the viral strain prevalent at the time. Therefore, vaccine effectiveness is evaluated for each type of vaccine designed to be responsive to a specific viral strain. BNT162b2 vaccine was reported to be effective against COVID-19 infection rates, symptom rates, and hospitalization rates [50–52], and for the current analysis, in order to utilize the most recent and relevant data, we chose to use the effectiveness data of vaccine in use against the Omicron XBB1.5 viral strain at the time of the analysis. Due to the lack of appropriate published data in Japan for vaccine effectiveness against the XBB1.5 viral strain at the time of the current study, effectiveness for infection [27, 28], symptom [27–29], and hospitalization [29–31] rates were set at 50%, 60% and 70% respectively, based on data from multiple foreign studies; these estimates also did not show large deviation from the effectiveness versus XBB1.5 viral strain reported in a non-peer-reviewed study on real-world effectiveness in Japan [53]. The duration of each effectiveness parameter was set at 6 months, based on the cost-effectiveness analysis (CEA) for booster vaccination in Japan [21].
Adverse Events
A large real-world study of subjects 65 years and above showed that vaccination with a vaccine effective against the XBB1.5 viral strain did not increase the incidence of major serious adverse events (AE) compared to non-vaccination [54]. Local or systemic reactions have been reported with the XBB 1.5-adapted vaccine, consistent with previous insights [55]. However, most of them are mild or moderate in severity [55] and thus have a negligible impact on the local economy. For these reasons, this analysis did not include treatment costs attributable to AE.
Clinical Inputs
Since the MHLW had ceased the total count of all COVID-19 cases as a government policy in May 2023 and since then has transitioned to a new policy to collect information on COVID-19 infections from a limited number of designated medical institutions, no accurate data on the latest COVID-19 infection rates were available in Japan. Therefore, the total number of COVID-19 cases for 1 year backward from May 2023 was tabulated to estimate the annual COVID-19 infection rate [32]. In regard to COVID-19 symptomatic infection rates, hospitalization rates, and mortality rates in outpatient settings [33, 35, 56], because of the lack of relevant evidence by age group at the national level, prefectural-level survey reports were used. The percentages of general ward with ventilation, intensive care unit (ICU), and ICU with ventilation among hospitalizations by COVID-19 were based on a large observational study in Japan [34]. The observational study focused on the first three epidemic waves in Japan through May 2021, which took place before the Omicron dominant period. Mortality rates during hospitalization were also derived from the Japanese observational study mentioned above [34]. Long COVID incidence rates in outpatient settings were derived based on a survey report from Yamanashi prefecture [36]. Long COVID incidence rates in inpatient settings were derived from Sugiyama et al. 2022 [37], which was conducted between August 2020 and March 2021. The duration of immunity from COVID-19 infection was assumed to be 3 months as in the previous CEA study in Japan [21]. The risk ratios of hospitalization and death for patients at high risk in comparison to patients without high risk were derived from a study conducted during the pre-Omicron dominant period in the state of New York in the USA [40], as there were no appropriate data in Japan.
Direct Medical Cost
Because the price of BNT162b2 is not published information, we used the average price of COVID-19 vaccines in Japan, as estimated in a survey by the MHLW and disclosed in a consultation meeting with local municipal authorities preparing for transition of the vaccination program [41]. The study did not consider a potential decline in the vaccine price in the future, which was assumed to remain constant throughout the time horizon. For the administration cost of vaccination, the estimate from the MHLW in the aforementioned source was used [41]. The cost per hospitalization was estimated using the MDV DPC hospital claims database [43]. Hospitalizations between January 2022 and December of the same year with COVID-19 as the primary disease were included in the estimation. To exclude the influence due to outliers, the median rather than the mean of hospitalization costs was used as the estimated input for the parameter. Outpatient costs, diagnostic testing costs, and long COVID treatment costs were calculated by identifying relevant medical procedures based on the medical fee schedule published by the MHLW [42]. For cost conversion from JPY to USD, the conversion rate of USD 1 = JPY 131.498 published by OECD for the year 2022 was used [57].
Productivity Loss
The COVID-19 vaccine not only affects healthcare costs through the prevention of COVID-19 infections, hospitalizations, and deaths, but also prevents absence from work due to infection [14, 58]. To assess the broad impact of the COVID-19 vaccine, we adopted a societal perspective and considered direct medical costs and productivity losses. Since it can be assumed that even when people who are not working, such as the elderly, are affected by COVID-19, their family members or caregivers may be forced to take time off from work in order to take care of the affected members [59], the labor participation rate was assumed to be 100% in this analysis in the same manner as the previously published CEA studies on vaccination in Japan [21, 59]. Wages per week were calculated based on Japanese wage statistics published by the Japanese government [44]. The number of days of absence for non-hospitalized patients was obtained from the MHLW guidelines [45], and the number of days of absence for hospitalized patients was estimated from the MDV DPC hospital database [43].
Outcomes
The current study considered both public health and economic outcomes. Public health outcomes considered during the analysis were the number of COVID-19-related symptomatic infections, hospitalizations, and deaths. Economic outcomes considered were vaccine-related costs, non-vaccine-related medical costs, productivity loss, and the total cost summing up those costs. Vaccine-related costs consisted of the administration cost of the vaccine in addition to the price of the vaccine itself. Non-vaccine-related medical costs included diagnostic testing costs, hospitalization costs, outpatient costs, and long COVID treatment costs.
Model Analysis
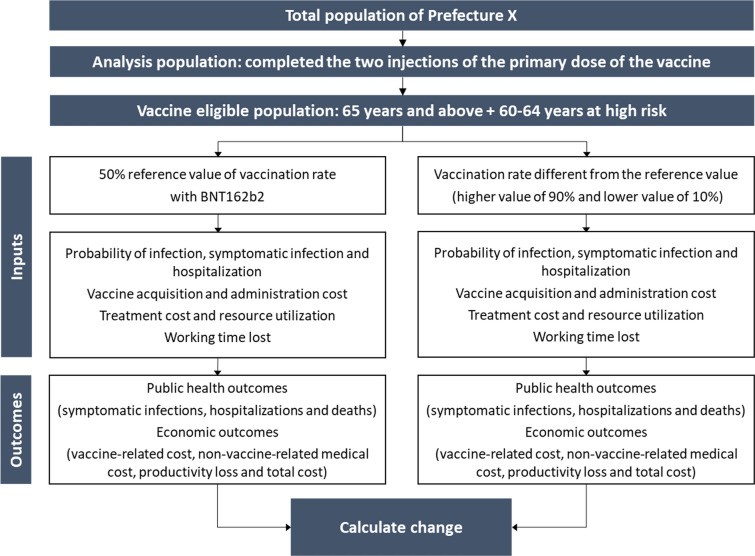
Changes in public health and economic outcomes were evaluated when the vaccination rate was varied from a reference value to higher and lower values in the vaccine eligible population (Fig. 2). Because influenza is the same Class B disease as COVID-19 and the target population for vaccination is the same, the reference value of the vaccination rate was set at 50% based on the vaccination rate estimate of the periodic influenza vaccination program in Japan [60]. The higher value of the vaccination rate was set at 90%, in light of the vaccination rate reported for the first booster dose (third dose) of the COVID-19 vaccines [61]. The lower value of the vaccination rate, 10%, was obtained by subtracting the absolute value of the difference between the reference value and the higher value from the reference value.
Fig. 2.
Budget impact schematic for the base case. BNT162b2, Pfizer–BioNTech COVID-19 vaccine
As the analysis for the base case, we estimated each outcome assuming a 50% reference value for the vaccination rate for the vaccine eligible population in Prefecture X, and then calculated the difference versus the outcomes assuming 90% and 10% vaccination rates. As the subgroup analysis 1, in order to assess the impact of changes in vaccination rates on municipalities that are expected to be primarily responsible for implementation of vaccination in their jurisdictions, 3 illustrative examples of municipalities in Prefecture X with different rates of population aging (City A, City B, and City C in the order of increasing rates of population aging) were chosen as the target population in the analysis. The analysis population and the vaccine eligible population for each municipality were defined similarly to the base case. Since the population size differs by municipality, the rate of change (%) in the results was of primary interest. As the subgroup analysis 2, we calculated public health and economic outcomes by narrowing the analysis population to those 65 years of age and above who were the primary vaccination target population. To assess the impact of vaccinating one individual resident, individual-level economic outcomes were calculated by estimating outcomes when the vaccination rate changed from 0 to 100%, and then dividing each cost at the population level by the size of the corresponding population 65 years of age and above within the analysis population.
Scenario analyses were also conducted based on ISPOR's BIA guideline [62], using multiple plausible scenarios to assess the uncertainty of the analysis. The key parameters for the scenario analyses were chosen for their potential importance in interpreting public health and economic outcomes in light of prior studies [21, 24]. The plausible scenarios incorporate varying assumptions about infection rates, vaccine effectiveness, clinical inputs, and productivity losses. The list of scenarios considered during the scenario analyses is presented in Supplementary Table S5.
For all analyses in the study, the time horizon was set to 3 years which was deemed sufficiently long enough to account for the impact of immunization through the periodic vaccination. Since ISPOR’s BIA guidelines [62] recommended that the BIA should present undiscounted costs, the 0% discount rate was adopted for the analysis.
Ethics Approval
This simulation study did not require human subject participation and used publicly available or anonymized data. Thus, ethics approval was not required, and informed consent was not applicable.
Results
Base Case Analysis
The base case results are presented in Table 2. At the reference vaccination rate of 50%, the total number of COVID-19-related symptomatic cases, hospitalizations and deaths over 3 years were estimated to be 554,435, 15,214 and 1057 respectively, and vaccine-related cost and non-vaccine-related medical cost over 3 years were estimated at JPY 7.58 billion (USD 57.67 million) and JPY 79.22 billion (USD 602.48 million) respectively. When the vaccination rate increased to 90%, the total number of COVID-19-related symptomatic cases, hospitalizations and deaths decreased by 7219 (1.3%), 1188 (7.8%) and 115 (10.8%), respectively, in comparison to the 50% vaccination rate. Moreover, at the 90% vaccination rate, vaccine-related cost increased by JPY 6.11 billion (USD 46.46 million) (80.6%) and non-vaccine-related medical cost decreased by JPY 2.72 billion (USD 20.70 million) (3.4%) in comparison to the 50% vaccination rate. When the vaccination rate decreased to 10%, the total number of COVID-19-related symptomatic cases, hospitalizations and deaths increased by 7759 (1.4%), 1275 (8.4%) and 123 (11.6%), respectively, in comparison to the 50% vaccination rate. Moreover, at the 10% vaccination rate, vaccine-related cost decreased by JPY 6.07 billion (USD 46.17 million) (80.1%) and non-vaccine-related medical cost increased by JPY 2.92 billion (USD 22.22 million) (3.7%) in comparison to the 50% vaccination rate. The breakdown of the changes in non-vaccine-related medical cost in Table 2 shows that changes in vaccination rates have a greater proportional impact on inpatient treatment cost than other components of the non-vaccine-related medical cost.
Table 2.
Base case results of cumulative public health and economic impact of COVID-19 vaccination in Prefecture X under different vaccination rates across 3 years
| Outcomes | 50% vaccination | 90% vaccination | Difference (90% vs. 50%) | 10% vaccination | Difference (10% vs. 50%) |
|---|---|---|---|---|---|
| (A) | (B) | (B–A) (%) | (C) | (C–A) (%) | |
| Number of symptomatic cases (n) | 554,435 | 547,216 | − 7219 (− 1.3%) | 562,194 | 7759 (1.4%) |
| Number of hospitalizations (n) | 15,214 | 14,026 | − 1188 (− 7.8%) | 16,489 | 1275 (8.4%) |
| Number of COVID-19-related deaths (n) | 1057 | 943 | − 115 (− 10.8%) | 1180 | 123 (11.6%) |
| Vaccine-related cost (JPY) | 7,583,852,237 | 13,692,677,882 | 6,108,825,645 (80.6%) | 1,512,122,941 | − 6,071,729,296 (− 80.1%) |
| Non-vaccine-related medical cost (JPY) | 79,224,370,230 | 76,502,517,184 | − 2,721,853,046 (− 3.4%) | 82,146,527,944 | 2,922,157,715 (3.7%) |
| Testing cost (JPY) | 4,971,340,711 | 4,889,784,168 | − 81,556,543 (− 1.6%) | 5,058,965,735 | 87,625,024 (1.8%) |
| Inpatient treatment cost (JPY) | 21,642,778,946 | 19,629,490,245 | − 2,013,288,701 (− 9.3%) | 23,803,362,294 | 2,160,583,348 (10.0%) |
| Outpatient treatment cost (JPY) | 30,254,716,902 | 29,916,300,983 | − 338,415,919 (− 1.1%) | 30,618,547,994 | 363,831,091 (1.2%) |
| Long COVID treatment cost (JPY) | 22,355,533,670 | 22,066,941,787 | − 288,591,882 (− 1.3%) | 22,665,651,921 | 310,118,251 (1.4%) |
| Productivity loss (JPY) | 253,114,483,829 | 244,847,143,806 | − 8,267,340,023 (− 3.3%) | 261,994,530,546 | 8,880,046,717 (3.5%) |
| Total cost (JPY) | 339,922,706,296 | 335,042,338,872 | − 4,880,367,424 (− 1.4%) | 345,653,181,431 | 5,730,475,135 (1.7%) |
The number of symptomatic cases include those cases involving hospitalizations as well. The target population for vaccination is individuals ≥ 65 years old and individuals at high risk at the age of 60 to 64 years old in Prefecture X. The time horizon is over 3 years
COVID-19 Coronavirus disease 2019; JPY Japanese Yen
Once productivity losses were taken into account, the total cost over 3 years at the 50% vaccination rate was estimated to be JPY 339.92 billion (USD 2.59 billion). When the vaccination rate increased to 90%, the total cost decreased by JPY 4.88 billion (USD 37.11 million) (1.4%) in comparison to the 50% vaccination rate. When the vaccination rate decreased to 10%, the total cost increased by JPY 5.73 billion (USD 43.58 million) (1.7%) in comparison to the 50% vaccination rate. These results imply that increase in the vaccination rate would lead to overall decrease in total cost from the societal perspective, because of the reduction in non-vaccine-related medical cost and productivity losses caused by COVID-19 that outweigh the increased cost of vaccination.
Subgroup Analyses
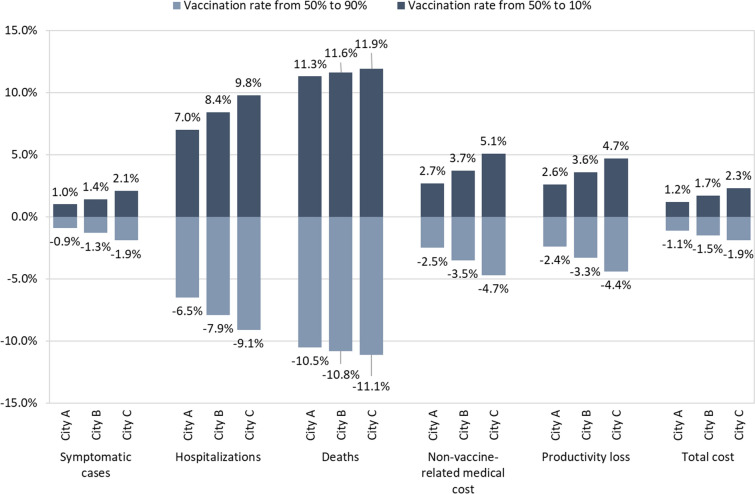
The results of subgroup analyses are presented in Fig. 3, Table 3 as well as Tables S3, S4 in the supplementary material. In the subgroup analysis 1 (Fig. 3), the impact of vaccination rates on public health and economic outcomes was evaluated in 3 illustrative municipalities in Prefecture X. For all key public health and economic outcomes, increasing trend in the rate of population aging among 3 municipalities corresponds to monotonic increase in the absolute value of the impact of changes in vaccination rates, suggesting that changes in vaccination rates can have a greater impact in municipalities with a high rate of population aging. For example, increasing the vaccination rate from 50 to 90% in Cities A, B, and C in the order of increasing rates of population aging, lead to reductions in COVID-19-related death of 10.5%, 10.8%, and 11.1%, respectively, and to reductions in total cost of 1.1%, 1.5%, 1.9%, respectively. Full public health and economic outcomes for the subgroup analysis 1 are presented in Table S3 in the supplementary material.
Fig. 3.
Differences in cumulative public health and economic outcomes of COVID-19 vaccination in 3 municipalities in Prefecture X versus the reference vaccination rate of 50% across 3 years. The percentage changes in vaccine-related cost were the same in each of the three cities, and they were 80.6% (vaccination rate from 50 to 90%) and − 80.1% (vaccination rate from 50 to 10%) respectively
Table 3.
Subgroup analysis 2: results of cumulative economic impact of COVID-19 vaccination for all individuals ≥ 65 years old in Prefecture X at 0% and 100% vaccination rates at the individual level across 3 years
| Outcomes | 0% vaccination | 100% vaccination | Difference (100% vs. 0%) |
|---|---|---|---|
| (A) | (B) | (B–A) (%) | |
| Vaccine-related cost (JPY) | 0 | 42,893 | 42,893 (NA) |
| Non-vaccine-related medical cost (JPY) | 74,905 | 54,920 | − 19,985 (− 26.7%) |
| Testing cost (JPY) | 2442 | 1844 | − 599 (− 24.5%) |
| Inpatient treatment cost (JPY) | 52,820 | 38,036 | − 14,783 (− 28.0%) |
| Outpatient treatment cost (JPY) | 10,876 | 8392 | − 2484 (− 22.8%) |
| Long COVID treatment cost (JPY) | 8766 | 6648 | − 2118 (− 24.2%) |
| Productivity loss (JPY) | 239,507 | 178,782 | − 60,726 (− 25.4%) |
| Total cost (JPY) | 314,412 | 276,594 | − 37,817 (− 12.0%) |
The target population for vaccination is individuals ≥ 65 years old in Prefecture X. The time horizon is over 3 years
COVID-19 Coronavirus disease 2019; JPY Japanese Yen
In the subgroup analysis 2 (Table 3), the analysis population was those aged 65 years and above in Prefecture X in order to estimate the economic impact for the main target population of the annual vaccination program at the individual level. Vaccine-related cost for 3 years was JPY 42,893 (USD 326.19) per person if an individual in the vaccine eligible population was vaccinated. On the other hand, non-vaccine-related medical cost for 3 years was JPY 74,905 (USD 569.63) per person without vaccination and JPY 54,920 (USD 417.65) per person with vaccination, leading to a reduction of JPY 19,985 (USD 151.98) (26.7%). When productivity losses were taken into account, the total cost across 3 years for an unvaccinated individual was estimated to be JPY 314,412 (USD 2,391.00) per person. For a vaccinated individual, the total cost decreased by JPY 37,817 (USD 287.59) per person (12.0%).
At the population level for the subgroup analysis 2 (Table S4), vaccination rates were varied similarly to the base case (the reference value of 50%, the higher value of 90% and the lower value of 10%). The trends observed for the subgroup analysis 2 were similar to the base case, but with a greater relative magnitude of impact than the base case. Full public health and economic outcomes for the subgroup analysis 2 are available in Table S4 in the supplementary material.
Scenario Analyses
The results of the scenario analyses based on plausible modifications of the input parameters of the base case are presented in terms of total cost at key vaccination rates in the vaccine eligible population as Table 4. A total of 14 scenarios were implemented, and reduction in total cost was achieved similarly to the base case by increasing the vaccination rate from 50 to 90% for all scenarios except one. The exception was in regard to the workforce participation rate that displayed a trend different from the base case analysis. The scenario 14 in Table 4 considered the productivity losses from only patients by imputing the approximate labor participation rates of the Japanese population. The result showed that when the productivity impact on caregivers was ignored, increase in the vaccination rate among the vaccine eligible population led to an increase in total cost by JPY 1.91 billion (USD 14.53 million) (0.8%) across 3 years. However, the results of all the other 13 scenario analyses supported the robustness of the main study results by showing that for most reasonable ranges of key input parameters, results were directionally consistent with the findings of the base case analysis.
Table 4.
Results of scenario analyses for cumulative total economic outcomes across 3 years
| Outcomes | 50% vaccination | 90% vaccination | Difference (90% vs. 50%) | 10% vaccination | Difference (10% vs. 50%) |
|---|---|---|---|---|---|
| (A) | (B) | (B–A) (%) | (C) | (C–A) (%) | |
| Base scenario A (reference) | 339,922,706,296 | 335,042,338,872 | − 4,880,367,424 (− 1.4%) | 345,653,181,431 | 5,730,475,135 (1.7%) |
| Vaccine effectiveness (infection/symptomatic/hospitalization) | |||||
|
40%/50%/50% (Scenario 1) |
345,171,720,030 | 343,065,850,289 | − 2,105,869,741 (− 0.6%) | 347,927,036,377 | 2,755,316,347 (0.8%) |
|
60%/70%/80% (Scenario 2) Data from Nagano et al. 2024 [21] |
336,108,056,310 | 329,379,594,701 | − 6,728,461,609 (− 2.0%) | 343,824,609,163 | 7,716,552,854 (2.3%) |
| Vaccine-induced duration of protection against infection | |||||
| 3 months (Scenario 3) | 350,321,101,430 | 350,019,141,277 | − 301,960,153 (− 0.1%) | 350,680,775,282 | 359,673,852 (0.1%) |
| 9 months (Scenario 4) | 331,894,002,698 | 324,619,594,353 | − 7,274,408,345 (− 2.2%) | 341,280,970,842 | 9,386,968,145 (2.8%) |
| Annual attack rate | |||||
| 30% for all age groups (Scenario 5) | 681,047,725,908 | 651,550,749,037 | − 29,496,976,871 (− 4.3%) | 712,804,538,492 | 31,756,812,584 (4.7%) |
| − 25% (75% of the base case) (Scenario 6) | 253,586,893,599 | 251,445,137,801 | − 2,141,755,798 (− 0.8%) | 256,386,400,919 | 2,799,507,320 (1.1%) |
| + 25% (125% of the base case) (Scenario 7) | 428,891,222,860 | 421,281,363,241 | − 7,609,859,618 (− 1.8%) | 437,536,197,034 | 8,644,974,174 (2.0%) |
| Hospitalization rate among symptomatic patients | |||||
| − 25% (75% of the base case) (Scenario 8) | 323,939,709,049 | 320,573,021,300 | − 3,366,687,750 (− 1.0%) | 328,045,267,126 | 4,105,558,077 (1.3%) |
| + 25% (125% of the base case) (Scenario 9) | 355,899,390,369 | 349,506,687,031 | − 6,392,703,338 (− 1.8%) | 363,253,224,117 | 7,353,833,748 (2.1%) |
| Long COVID incidence rate (outpatient) | |||||
| 10% for all age groups (Scenario 10) | 218,904,656,790 | 215,098,002,622 | − 3,806,654,168 (− 1.7%) | 223,480,688,726 | 4,576,031,937 (2.1%) |
| 30% for all age groups (Scenario 11) | 309,117,455,616 | 304,301,720,870 | − 4,815,734,746 (− 1.6%) | 314,778,350,490 | 5,660,894,875 (1.8%) |
| Long COVID incidence rate (inpatient) | |||||
| 50% for all age groups (Scenario 12) | 338,814,169,268 | 334,099,734,231 | − 4,714,435,037 (− 1.4%) | 344,366,559,671 | 5,552,390,403 (1.6%) |
| 70% for all age groups (Scenario 13) | 341,359,540,446 | 336,446,383,987 | − 4,913,156,458 (− 1.4%) | 347,125,205,977 | 5,765,665,531 (1.7%) |
| Workforce participation rate | |||||
|
Consideration of productivity loss from patients only (Scenario 14) Data from e-Stat (Portal Site of Official Statistics of Japan) [65] |
229,806,254,585 | 231,717,116,320 | 1,910,861,735 (0.8%) | 228,243,337,668 | − 1,562,916,917 (− 0.7%) |
The number of symptomatic cases include those cases involving hospitalizations as well. The target population for vaccination is individuals ≥ 65 years old and individuals at high risk at the age of 60 to 64 years old in Prefecture X. The time horizon is over 3 years
COVID-19 Coronavirus disease 2019; JPY Japanese Yen
Discussion
The base case analysis showed that increase in the vaccination rate led to sizable reduction in all public health outcomes of symptomatic infections, hospitalizations and deaths due to COVID-19. Moreover, the rates of reduction in hospitalizations and deaths due to COVID-19 were greater than that for symptomatic infections, implying that the result of the analysis is consistent with the objective of the Japanese government to reduce the number of severe COVID-19 cases through vaccination. Regarding economic outcomes, in the base case, on one hand, increase in the vaccination rate led to increase in vaccine-related cost. On the other hand, vaccination-induced decrease in non-vaccine-related medical cost and productivity loss exceeded the increase in vaccine-related cost, and as a result, vaccination led to overall decrease in total cost.
A close look at the result shows that the relative impact of productivity loss on total cost is large. Many studies on COVID-19 infection have reported a number of significant negative consequences on daily activities, including labor productivity [22, 58, 66, 67], and vaccination has been effective in mitigating such impact [23, 68, 69]. The scenario analyses have demonstrated robustness of the results, except the labor participation rate. However, it has been reported that COVID-19 infection has considerable negative impact on family members and caregivers of patients including productivity loss due to caregiving [23, 70–72]. Therefore, for proper interpretation of the results of the scenario analyses, one needs to be mindful of the possibility that ignoring impact on family members and caregivers may lead to substantial underestimation of the societal value of COVID-19 vaccination.
The subgroup analysis 1 which analyzed the case of 3 municipalities with different degrees of population aging, found that all public health outcomes and total economic outcomes improved in all municipalities when the vaccination rate increased. Moreover, the higher the degree of population aging in a municipality was, the greater the percentages of improvement in all outcomes were when the vaccination rate increased. The results of this study are compelling in light of the reported finding that the older the patient, the higher the rate of severe diseases caused by COVID-19 and the greater the benefit from the vaccine [73]. In this study, municipalities in Prefecture X with relatively large differences in population aging rates were selected for analysis, in order to examine the financial impact of vaccination in regional communities with large differences in population aging rates. Population aging rates in Japan’s approximately 1,700 municipalities vary widely, and the results of this study may be helpful in considering optimal vaccination policies in each municipality.
The subgroup analysis 2 showed that from the societal perspective, vaccinating one person over the age of 65 in a local community would deliver total cost savings at the individual level. Under the NIP, the maximum out-of-pocket payment for one injection of the COVID-19 vaccines is JPY 7,000 (USD 53.23) in 2024 [41]. The out-of-pocket payment after 2025 has not been determined, but the 3-year total out-of-pocket payment is likely to be JPY 21,000 (USD 159.70) or even more. However, since vaccinating one person can achieve savings of the non-vaccine-related medical cost of JPY 19,985 (USD 151.98) per person and reduction of productivity loss of JPY 60,726 (USD 461.80) per person over 3 years, vaccination can result in a positive economic impact on the local economy as well as a positive health impact on individuals.
Some limitations exist in this study and thus the results should be interpreted within that context. The static simulation model in the study may not entirely describe the clinical reality, such as potential seasonal waves of COVID-19 infections [74]. Due to the static nature of the BIA model, it does not capture any herd immunity or population effects, and only considers the direct effects of vaccinations [75]. The BIA model also does not fully cover the broad economic impact of the COVID-19 vaccine, such as the reduction in the productivity loss caused by COVID-19-related premature deaths [76], and the direct impact to mitigate the symptoms of long COVID [77]. Those might lead to an underestimation of the public health and economic impact of the COVID-19 vaccine, implying that the results in this study may be still conservative. Data from the pre-Omicron dominant period and from foreign countries were used for some parameters including clinical inputs, as appropriate data were not available. In addition, due to the lack of accurate data on the current COVID-19 infection rates in Japan, we used the previous year’s COVID-19 incidence data to calculate the annual infection rate, which did not necessarily represent the latest COVID-19 infection trend. To address the uncertainty in these parameters, scenario analyses were conducted to confirm the robustness of the results. Finally, because of the constantly evolving nature of COVID-19 viral strains, the results of this study should be re-assessed in future situations that more appropriately reflect the epidemiological characteristics of future outbreaks.
Conclusion
The results of this study showed that maintaining a high COVID-19 vaccination rate among the vaccine eligible population aged 65 and above and aged 60–64 at high risk would lead to significant public health benefits and societal cost savings at the municipal level in Japan. These benefits would be more pronounced in municipalities with higher rates of population aging. The evidence from this study supports the consideration of public policy at the municipal level to promote annual periodic vaccination as a preventive social investment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing, Editorial, and Other Assistance
Data analysis and medical writing support for the present study were provided by INTAGE Healthcare Inc., and funded by Pfizer Japan Inc. The authors thank Michael LoPresti of INTAGE Healthcare Inc. and Jingyan Yang of Pfizer Inc. for advice throughout this study, Jennifer Nguyen of Pfizer Inc. and Sheetal Raina of Pfizer UK Ltd. for advice regarding clinical input, and Yaoki Sonohara of INTAGE Healthcare Inc. for administrative support
Author Contributions
All the authors (M Nagano, K Tanabe, K Kamei, S Lim, H Nakamura, S Ito) (1) made substantial contribution to the study concept or the data analysis or interpretation, (2) drafted the manuscript or revised it critically for important intellectual content, (3) approved the final version of the manuscript to be published, and (4) agreed to be accountable for all aspects of the work.
Funding
This work, and the journal’s Rapid Service Fee, was supported by Pfizer Japan Inc.
Data Availability
All data generated or analyzed during this study are included in this published article or as supplementary information files.
Declarations
Conflict of interest
The authors declare the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: M Nagano, K Tanabe, K Kamei, and S Ito are full-time employees of Pfizer Japan Inc., and hold stock of Pfizer Inc. S Lim and H Nakamura are full-time employees of INTAGE Healthcare Inc.
Ethical Approval
This simulation study did not require human subject participation and used publicly available or anonymized data. Thus, ethics approval was not required, and informed consent was not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mitsuhiro Nagano and Kosuke Tanabe are co-first authors of the article.
References
- 1.Rahman S, Montero MTV, Rowe K, Kirton R, Kunik F Jr. Epidemiology, pathogenesis, clinical presentations, diagnosis and treatment of COVID-19: a review of current evidence. Expert Rev Clin Pharmacol. 2021;14(5):601–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 cases | WHO COVID-19 dashboard [Internet]. [cited 2024 Mar 18]. Available from: https://data.who.int/dashboards/covid19/cases.
- 3.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;22(369): m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takada R, Takazawa T, Takahashi Y, Fujizuka K, Akieda K, Saito S. Risk factors for mechanical ventilation and ECMO in COVID-19 patients admitted to the ICU: a multicenter retrospective observational study. PLoS ONE. 2022;17(11): e0277641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan G, Casalino S, Chowdhary S, Frangione E, Fung CYJ, Haller S, et al. Characterizing risk factors for hospitalization and clinical characteristics in a cohort of COVID-19 patients enrolled in the GENCOV study. Viruses. 2023;15(8):1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cutler DM, Summers LH. The COVID-19 pandemic and the $16 trillion virus. JAMA. 2020;324(15):1495–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karako K, Song P, Chen Y, Karako T. An average of nearly 200,000 new infections per day over a six-week period: what is the impact of such a severe COVID-19 pandemic on the healthcare system in Japan? Biosci Trends. 2022;16(5):371–3. [DOI] [PubMed] [Google Scholar]
- 8.Altmann DM, Boyton RJ. COVID-19 vaccination: the road ahead. Science. 2022;375(6585):1127–32. [DOI] [PubMed] [Google Scholar]
- 9.Lee SW, Ma D, Davoodian A, Ayutyanont N, Werner B. COVID-19 vaccination decreased COVID-19 hospital length of stay, in-hospital death, and increased home discharge. Prev Med Rep. 2023;1(32): 102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceban F, Kulzhabayeva D, Rodrigues NB, Di Vincenzo JD, Gill H, Subramaniapillai M, et al. COVID-19 vaccination for the prevention and treatment of long COVID: a systematic review and meta-analysis. Brain Behav Immun. 2023;1(111):211–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Y, Liu S, Zhang D. Effectiveness of COVID-19 vaccine booster shot compared with non-booster: a meta-analysis. Vaccines. 2022;10(9):1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyaw MH, Spinardi J, Zhang L, Oh HML, Srivastava A. Evidence synthesis and pooled analysis of vaccine effectiveness for COVID-19 mRNA vaccine BNT162b2 as a heterologous booster after inactivated SARS-CoV-2 virus vaccines. Hum Vaccines Immunother. 2023;19(1):2165856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arbel R, Peretz A, Sergienko R, Friger M, Beckenstein T, Duskin-Bitan H, et al. Effectiveness of a bivalent mRNA vaccine booster dose to prevent severe COVID-19 outcomes: a retrospective cohort study. Lancet Infect Dis. 2023;23(8):914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirson N, Swallow E, Lu J, Mesa-Frias M, Bookhart B, Maynard J, et al. The societal economic value of COVID-19 vaccines in the United States. J Med Econ. 2022;25(1):119–28. [DOI] [PubMed] [Google Scholar]
- 15.Li R, Liu H, Fairley CK, Zou Z, Xie L, Li X, et al. Cost-effectiveness analysis of BNT162b2 COVID-19 booster vaccination in the United States. Int J Infect Dis. 2022;1(119):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arashiro T, Arima Y, Muraoka H, Sato A, Oba K, Uehara Y, et al. Coronavirus disease 19 (COVID-19) vaccine effectiveness against Symptomatic Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection during delta-dominant and omicron-dominant periods in Japan: a multicenter prospective case-control study (Factors associated with SARS-CoV-2 infection and the effectiveness of COVID-19 vaccines study). Clin Infect Dis. 2022;76(3):e108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinugasa Y, Llamas-Covarrubias MA, Ozaki K, Fujimura Y, Ohashi T, Fukuda K, et al. Post-Coronavirus disease 2019 syndrome in Japan: an observational study using a medical database. JMA J. 2023;6(4):416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomioka K, Uno K, Yamada M. Association between vaccination status and severe health consequences among community-dwelling COVID-19 patients during Omicron BA.1/BA.2 and BA.5-predominant periods in Japan. Environ Health Prev Med. 2023;28:35–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kayano T, Ko Y, Otani K, Kobayashi T, Suzuki M, Nishiura H. Evaluating the COVID-19 vaccination program in Japan, 2021 using the counterfactual reproduction number. Sci Rep. 2023;13(1):17762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ministry of Health, Labour, and Welfare. COVID-19 vaccination Q&A [Internet]. [cited 2024 Mar 19]. Available from: https://www.cov19-vaccine.mhlw.go.jp/qa/0180.html.
- 21.Nagano M, Kamei K, Matsuda H, Takahashi C, Yang J, Wada K, et al. Cost-effectiveness analysis of COVID-19 booster vaccination with BNT162b2 in Japan. Expert Rev Vaccines. 2024;23(1):349–61. [DOI] [PubMed] [Google Scholar]
- 22.Miyaji C, Kobayashi T, Habu H, Hagiyama A, Horie Y, Takao S. Effect of COVID-19 infection on presenteeism: cohort study using large health insurance-based data in Japan. J Occup Environ Med. 2023. 10.1097/JOM.0000000000003128. [DOI] [PubMed] [Google Scholar]
- 23.Kwon J, Milne R, Rayner C, Rocha Lawrence R, Mullard J, Mir G, et al. Impact of long COVID on productivity and informal caregiving. Eur J Health Econ. 2023. 10.1007/s10198-023-01653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Fusco M, Marczell K, Thoburn E, Wiemken TL, Yang J, Yarnoff B. Public health impact and economic value of booster vaccination with Pfizer-BioNTech COVID-19 vaccine, bivalent (Original and Omicron BA.4/BA.5) in the United States. J Med Econ. 2023;26(1):509–24. [DOI] [PubMed] [Google Scholar]
- 25.Di Fusco M, Marczell K, Deger KA, Moran MM, Wiemken TL, Cane A, et al. Public health impact of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) in the first year of rollout in the United States. J Med Econ. 2022;25(1):605–17. [DOI] [PubMed] [Google Scholar]
- 26.Mendes D, Chapman R, Aruffo E, Gal P, Nguyen JL, Hamson L, et al. Public health impact of UK COVID-19 booster vaccination programs during Omicron predominance. Expert Rev Vaccines. 2023;22(1):90–103. [DOI] [PubMed] [Google Scholar]
- 27.Huiberts AJ, Hoeve CE, de Gier B, Cremer J, van der Veer B, de Melker HE, et al. Effectiveness of Omicron XBB.1.5 vaccine against infection with SARS-CoV-2 Omicron XBB and JN.1 variants, prospective cohort study, the Netherlands, October 2023 to January 2024. Eurosurveillance. 2024;29(10):2400109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skowronski DM, Zhan Y, Kaweski SE, Sabaiduc S, Khalid A, Olsha R, et al. 2023/24 mid-season influenza and Omicron XBB.1.5 vaccine effectiveness estimates from the Canadian Sentinel Practitioner Surveillance Network (SPSN). Eurosurveillance. 2024;29(7):2400076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tartof SY, Slezak JM, Frankland TB, Puzniak L, Hong V, Ackerson BK, et al. BNT162b2 XBB.1.5-adapted vaccine and COVID-19 hospital admissions and ambulatory visits in US adults. medRxiv. 2023. 10.1101/2023.12.24.23300512v1.38014151 [Google Scholar]
- 30.Hansen CH, Moustsen-Helms IR, Rasmussen M, Søborg B, Ullum H, Valentiner-Branth P. Short-term effectiveness of the XBB.1.5 updated COVID-19 vaccine against hospitalisation in Denmark: a national cohort study. Lancet Infect Dis. 2024;24(2):e73–4. [DOI] [PubMed] [Google Scholar]
- 31.van Werkhoven CH, Valk AW, Smagge B, de Melker HE, Knol MJ, Hahné SJ, et al. Early COVID-19 vaccine effectiveness of XBB.1.5 vaccine against hospitalisation and admission to intensive care, the Netherlands, 9 October to 5 December 2023. Eurosurveillance. 2024;29(1):2300703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ministry of Health, Labour, and Welfare. Visualizing the data: information on COVID-19 infections [Internet]. [cited 2024 Mar 20]. Available from: https://covid19.mhlw.go.jp/en/.
- 33.Hiroshima Prefecture Health and Welfare Bureau. Hiroshima prefecture COVID-19 J-SPEED (J-SPEED: surveillance in post extreme emergencies and disasters, Japan version) data analysis from the 7th wave [Internet]. [cited 2024 Mar 19]. Available from: https://www.mhlw.go.jp/content/10900000/000975398.pdf.
- 34.Matsunaga N, Hayakawa K, Asai Y, Tsuzuki S, Terada M, Suzuki S, et al. Clinical characteristics of the first three waves of hospitalised patients with COVID-19 in Japan prior to the widespread use of vaccination: a nationwide observational study. Lancet Reg Health–West Pac. 2022. 10.1016/j.lanwpc.2022.100421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osaka Prefectural Department of Health and Medical Care. The current situation of infection and medical treatment (COVID-19). [Internet]. [cited 2024 Mar 19]. Available from: https://www.mhlw.go.jp/content/10900000/001039367.pdf.
- 36.Yamanashi Prefecture Infectious Disease Control Center, Welfare, and Health Department. Results of the COVID-19 sequelae (summary) [Internet]. [cited 2024 Mar 19]. Available from: https://www.pref.yamanashi.jp/kansensho/documents/coronavirus_kouisho2.pdf.
- 37.Sugiyama A, Miwata K, Kitahara Y, Okimoto M, Abe K, et al. Long COVID occurrence in COVID-19 survivors. Sci Rep. 2022;12(1):6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ministry of Health, Labour and Welfare of Japan. Social welfare and War victims’ Relief Bureau, Department of Health and Welfare for Persons with Disabilities. 2016 survey results on difficulties on living-national survey of people living with disability at home. Tokyo, Japan: Ministry of Health, Labour and Welfare of Japan [Internet]. [cited 2024 Mar 27]. Available from: https://www.mhlw.go.jp/toukei/list/dl/seikatsu_chousa_c_h28.pdf.
- 39.e-Stat (Portal Site of Official Statistics of Japan). Population Estimates Annual Report Yearly 2020|File|Browse Statistics [Internet]. [cited 2024 Apr 11]. Available from: https://www.e-stat.go.jp/en/stat-search/files?page=1&layout=datalist&toukei=00200524&tstat=000000090001&cycle=7&year=20200&month=0&tclass1=000001011679&result_back=1&tclass2val=0.
- 40.Tesoriero JM, Swain CAE, Pierce JL, Zamboni L, Wu M, Holtgrave DR, et al. COVID-19 outcomes among persons living with or without diagnosed HIV infection in New York State. JAMA Netw Open. 2021;4(2): e2037069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ministry of Health, Labour and Welfare. Briefing session for local governments on ensuring the vaccination system for the COVID-19 vaccine (34th meeting held on Mar 15, 2024) [Internet]. Available from: https://www.mhlw.go.jp/content/10906000/001226290.pdf.
- 42.Ministry of Health, Labour, and Welfare. Various information of medical fee [Internet]. [cited 2024 Mar 20]. Available from: https://shinryohoshu.mhlw.go.jp/shinryohoshu/.
- 43.Medical Data Vision. MDV database [Internet]. [cited 2024 Mar 20]. Available from: https://en.mdv.co.jp/.
- 44.e-Stat (Portal Site of Official Statistics of Japan). Basic statistical survey of wage structure [Internet]. [cited 2024 Mar 20]. Available from: https://www.e-stat.go.jp/stat-search/files?page=1&toukei=00450091&tstat=000001011429.
- 45.Ministry of Health, Labour, and Welfare. Measures to be taken after the transition of the COVID-19 to class 5 infectious diseases [Internet]. [cited 2024 Mar 20]. Available from: https://www.mhlw.go.jp/stf/corona5rui.html.
- 46.e-Stat (Portal Site of Official Statistics of Japan). National Census 2020 Basic Tabulation of Population, etc. (Main contents: sex, age, marital status, composition of households, residential status, single-mother and single-father households, nationality, etc.) October 2020 [Internet]. General Contact for Government Statistics. [cited 2024 Apr 9]. Available from: https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00200521&tstat=000001136464&cycle=0&year=20200&month=24101210&tclass1=000001136466&tclass2val=0.
- 47.Ministry of Health, Labour, and Welfare. Vaccination information Q&A [Internet]. [cited 2024 Mar 19]. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/kekkaku-kansenshou/kihonteki_keikaku/index_00001.html.
- 48.Ministry of Health, Labour and Welfare. 2020 patient survey (confirmed numbers) [Internet]. [cited 2024 Mar 19]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/kanja/20/index.html.
- 49.Ministry of Health, Labour, and Welfare. [Document 5] Survey of Antibody Possession Rates of New Coronavirus Using Residual Blood Samples for Testing at the Second Blood Donation [Internet]. [cited 2024 May 15]. Available from: https://www.mhlw.go.jp/content/10906000/001070846.pdf.
- 50.Arbel R, Hammerman A, Sergienko R, Friger M, Peretz A, Netzer D, et al. BNT162b2 vaccine booster and mortality due to covid-19. N Engl J Med. 2021;385(26):2413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moreira ED, Kitchin N, Xu X, Dychter SS, Lockhart S, Gurtman A, et al. Safety and efficacy of a third dose of BNT162b2 Covid-19 vaccine. N Engl J Med. 2022;386(20):1910–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu X, Xu K, Zhan P, Liu H, Zhang F, Song Y, et al. Comparative efficacy and safety of COVID-19 vaccines in phase III trials: a network meta-analysis. BMC Infect Dis. 2024;24(1):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tropical Medicine Research Institute at Nagasaki University. Vaccine Effectiveness Real-Time Surveillance for SARS-CoV-2 (VERSUS) Study [Internet]. [cited 2024 May 10]. Available from: https://www.tm.nagasaki-u.ac.jp/versus/results/20240228.pdf.
- 54.Andersson NW, Thiesson EM, Hviid A. Adverse events after XBB.1.5-containing COVID-19 mRNA vaccines. JAMA. 2024;331(12):1057–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gayed J, Diya O, Lowry FS, Xu X, Bangad V, Mensa F, et al. Safety and immunogenicity of the monovalent omicron XBB.1.5-adapted BNT162b2 COVID-19 vaccine in individuals ≥ 12 years old: a phase 2/3 trial. Vaccines. 2024;12(2):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishikawa Infectious Disease Information Center. The status of SARS-CoV-2 infection in Ishikawa Prefecture [Internet]. [cited 2024 Mar 19]. Available from: https://www.pref.ishikawa.lg.jp/kansen/documents/202207kansenzyokyo.pdf.
- 57.Organisation for Economic Co-operation and Development. Exchange rates - OECD Data [Internet]. theOECD. [cited 2024 Mar 20]. Available from: http://data.oecd.org/conversion/exchange-rates.htm.
- 58.Sah P, Vilches TN, Moghadas SM, Pandey A, Gondi S, Schneider EC, et al. Return on investment of the COVID-19 vaccination campaign in New York City. JAMA Netw Open. 2022;5(11): e2243127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shinjoh M, Togo K, Hayamizu T, Yonemoto N, Morii J, Perdrizet J, et al. Cost-effectiveness analysis of 20-valent pneumococcal conjugate vaccine for routine pediatric vaccination programs in Japan. Expert Rev Vaccines. Expert Rev Vaccines. 2024. 10.1080/14760584.2024.2345670. [DOI] [PubMed] [Google Scholar]
- 60.Ministry of Health, Labour and Welfare. Number of people receiving periodic immunizations [Internet]. [cited 2024 Apr 9]. Available from: https://www.mhlw.go.jp/topics/bcg/other/5.html.
- 61.Prime Minister’s Office in Japan. Regarding COVID-19 vaccine [Internet]. [cited 2024 Apr 9]. Available from: https://www.kantei.go.jp/jp/headline/kansensho/vaccine.html.
- 62.Sullivan SD, Mauskopf JA, Augustovski F, Jaime Caro J, Lee KM, Minchin M, et al. Budget impact analysis—principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health. 2014;17(1):5–14. [DOI] [PubMed] [Google Scholar]
- 63.DeCuir J, Payne A, Self W, Rowley E, Dascomb K, DeSilva M. Interim Effectiveness of Updated 2023–2024 (Monovalent XBB.1.5) COVID-19 Vaccines Against COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalization Among Immunocompetent Adults Aged ≥ 18 Years—VISION and IVY Networks, September 2023–January 2024. MMWR Morb Mortal Wkly Rep [Internet]. 2024 [cited 2024 May 15];73. Available from: https://www.cdc.gov/mmwr/volumes/73/wr/mm7308a5.htm. [DOI] [PMC free article] [PubMed]
- 64.Link-Gelles R. Vaccine effectiveness of updated (2023–2024) COVID-19 vaccines [Internet]. Centers for Disease Control and Prevention; 2024 [cited 2024 May 15]. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2024-02-28-29/04-COVID-Link-Gelles-508.pdf.
- 65.e-Stat (Portal Site of Official Statistics of Japan). Labour Force Survey Basic Tabulation All Prefectures Summary of Results Year 2023 [Internet]. [cited 2024 May 15]. Available from: https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00200531&tstat=000000110001&cycle=7&year=20230&month=0&tclass1=000001040276&tclass2=000001040277&stat_infid=000040168839&result_back=1&tclass3val=0.
- 66.Luong N, Barnett I, Aledavood T. The impact of the COVID-19 pandemic on daily rhythms. J Am Med Inform Assoc. 2023;30(12):1943–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsuzuki S, Beutels P. The estimated disease burden of COVID-19 in Japan from 2020 to 2021. J Infect Public Health. 2023;16(8):1236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Català M, Mercadé-Besora N, Kolde R, Trinh NTH, Roel E, Burn E, et al. The effectiveness of COVID-19 vaccines to prevent long COVID symptoms: staggered cohort study of data from the UK, Spain, and Estonia. Lancet Respir Med. 2024;12(3):225–36. [DOI] [PubMed] [Google Scholar]
- 69.Trinh NT, Jödicke AM, Català M, Mercadé-Besora N, Hayati S, Lupattelli A, et al. Effectiveness of COVID-19 vaccines to prevent long COVID: data from Norway. Lancet Respir Med. 2024;12(5):e33–4. [DOI] [PubMed] [Google Scholar]
- 70.Anderson S, Parmar J, L’Heureux T, Dobbs B, Charles L, Tian PGJ. Family caregiving during the COVID-19 pandemic in canada: a mediation analysis. Int J Environ Res Public Health. 2022;19(14):8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakamoto I, Murayama H, Takase M, Muto Y, Saito T, Tabuchi T. Association between increased caregiver burden and severe psychological distress for informal caregivers during the COVID-19 pandemic in Japan: a cross-sectional study. Arch Gerontol Geriatr. 2022;1(102): 104756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ohbe H, Goto T, Okada A, Yasunaga H. Association between COVID-19 pandemic and mental disorders in spouses of intensive care unit patients. Intensive Care Med. 2023;49(1):112–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsampasian V, Elghazaly H, Chattopadhyay R, Debski M, Naing TKP, Garg P, et al. Risk factors associated with post−COVID-19 condition: a systematic review and meta-analysis. JAMA Intern Med. 2023;183(6):566–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Majeed B, David JF, Bragazzi NL, McCarthy Z, Grunnill MD, Heffernan J, et al. Mitigating co-circulation of seasonal influenza and COVID-19 pandemic in the presence of vaccination: a mathematical modeling approach. Front Public Health. 2023. 10.3389/fpubh.2022.1086849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma S, Lavelle TA, Ollendorf DA, Lin PJ. Herd immunity effects in cost-effectiveness analyses among low- and middle-income countries. Appl Health Econ Health Policy. 2022;20(3):395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanly P, Ahern M, Sharp L, Ursul D, Loughnane G. The cost of lost productivity due to premature mortality associated with COVID-19: a Pan-European study. Eur J Health Econ. 2022;23(2):249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Watanabe A, Iwagami M, Yasuhara J, Takagi H, Kuno T. Protective effect of COVID-19 vaccination against long COVID syndrome: a systematic review and meta-analysis. Vaccine. 2023;41(11):1783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article or as supplementary information files.