Abstract
Background
Adequate sleep plays a crucial role in maintaining physical, mental, and emotional health. On average, adults require 7–9 h of sleep per night. However, less than two-thirds of women meet this recommendation. During the coronavirus disease 2019 (COVID-19) pandemic, poor sleep quality and moderate-to-severe stress were highly prevalent among healthcare workers (HCWs), especially women. While some interventions have been proposed to address stress/burnout in HCWs, few have focused specifically on women in healthcare. Therefore, this is a protocol for a study that aims to determine the efficacy of a mind–body intervention (MBI) to improve sleep duration among women HCWs aged 40–60 years using the personalized (N-of-1) trial design.
Methods
A personalized (N-of-1) trials model will be employed to evaluate the efficacy of an MBI to improve sleep duration (primary endpoint) and explore its effects on sleep quality, physiological factors, and their relationships with participants’ perceived stress, anxiety, and depression. The series of personalized trials (n = 60) will be conducted over 16 weeks. The MBI will include mindfulness, yoga, and guided walking, delivered in two 2-week block sequences for 12 weeks, with two 2-week periods for baseline and follow-up. Participants will watch 30-min videos three times weekly and wear an activity tracker to monitor sleep and activity. They will receive daily text messages with questions about sleep quality and bi-weekly questionnaires about their stress, anxiety and depression scores, fatigue, concentration, confidence, mood, and pain levels.
Conclusion
Results from this study will inform the development of N-of-1 methodology for addressing the health and wellness needs of middle-aged women.
Keywords: Sleep duration, Personalized trials, N-of-1, Mind-body intervention, Healthcare workers, Women
Highlights
-
•
Poor sleep is common in female healthcare workers, especially during the pandemic.
-
•
A personalized mind–body intervention (MBI) could improve sleep in this cohort.
-
•
MBIs could also be effective in improving other health factors and emotions.
Abbreviations:
- COVID-19
coronavirus disease 2019
- HCWs
healthcare workers
- RCTs
randomized controlled trials
- MBI
mind–body intervention
- IRB
Institutional Review Board
- PSS
Perceived Stress Scale
- PHQ-4
Patient Health Questionnaire-4
- EMA
ecological momentary assessment
- SE
Sleep efficiency
- TST
total sleep time
- TIB
time in bed
- GEE
generalized estimating equation
- ITT
intention-to-treat
- MICE
multivariate imputation by chained equations
Ethics and dissemination:
This trial was approved by the Northwell Health Institutional Review Board (IRB). All participants will be required to complete the written informed consent prior to enrollment. Important protocol modifications will be shared with participants, as per the IRB's discretion. The trial results will be published in a peer-reviewed journal. All publications resulting from this series of personalized trials will follow the CONSORT reporting guidelines. De-identified participant-level data and the study protocol will be uploaded to Open Science Framework (https://osf.io/dtv46/).
Registration details:
This trial is registered on www.ClinicalTrials.gov (NCT05789212).
Protocol version:
10/26/2023, 22-0770-MRB;
1. Introduction
Sleep is an important and complex physiological process for maintaining optimal health, with the National Sleep Foundation recommending ≥7 h of sleep per night for adults aged 18–60 years [1]. Poor sleep duration or quality has been associated with adverse emotional (e.g., stress, depression), cognitive (e.g., memory, cognitive performance), and physiological (e.g., pain) symptoms [2], and recent studies have demonstrated that poor sleep quality affects 19.2 % of United States workers [3]. During the first two waves of the COVID-19 pandemic, findings indicated a high prevalence of both poor sleep quality and moderate-severe stress in healthcare workers (HCWs) [4,5], especially amongst women [6]. Studies conducted in a cohort of New York City HCWs (80 % women, >50 % nurses, median age of 36 years) revealed a 20 % increase in insomnia symptoms (e.g., short duration, poor quality sleep) compared to 2018 [7], establishing an association between poor sleep quality and a higher prevalence of psychological distress [8]. Thus, evidence suggests that sleep interventions could be effective in reducing psychological distress among HCWs. However, while mitigation interventions (e.g., reducing workload) have been proposed to address stress/burnout in HCWs [9], few have focused on women in healthcare and specifically targeted sleep as a symptom of stress.
One possible reason for limited research in this area is the high heterogeneity of treatment effects (HTE) in stress reduction and sleep interventions, which traditional parallel-group trials cannot fully capture. Personalized (N-of-1) trials offer a solution by testing interventions in single patients through randomized crossover sequences, identifying the most effective treatment for each individual [10,11]. In contrast to randomized controlled trials (RCTs), which provide inferences on a population level, N-of-1 trials provide a method to determine what works best for the individual directly [10,11]. The three techniques included in the personalized intervention (meditation, yoga, and walking) for this study have been shown to be safe and effective in addressing sleep problems and reducing stress in middle-aged women [12]. Prior studies examining the effects of mediation [13], yoga [14], and physical activity [15] for stress reduction have shown that all three interventions were associated with significant reductions in self-reported and/or physiological measures of stress. Results from these systematic reviews and meta-analyses for each intervention are supported by reviews of Mind-Body Interventions (MBIs) to improve sleep in the scientific literature [[16], [17], [18], [19]]. The promising behavioral interventions proposed for this study are theorized to improve sleep quantity and quality through targeting multiple cognitive and emotional processes.
The primary goal of this study is to determine the efficacy of a Mind-Body Intervention using a personalized (N-of-1) trial design to produce a meaningful increase in sleep duration among women aged 40–60 years working at Northwell Health, a major healthcare system in New York. This proposed trial follows the National Institutes of Health (NIH) Stage Model for behavioral intervention development [20] and is a Stage II efficacy trial. The MBI will include a personalized intervention comprised of three components (mindfulness, yoga, and guided walking) assigned in two 2-week block sequences for a total period of 12 weeks. Participants will be prompted to complete three 30-min intervention sessions weekly, where the delivery/collection of all components and data will be conducted virtually. Sleep components (duration, sleep latency, sleep efficiency) and physiological factors (daily resting heart rate, daily step count) will be monitored continuously using a Fitbit® device and self-reported questionnaires. The primary outcome is the mean within-subject difference in daily sleep duration between baseline and each treatment period. Exploratory outcomes include mean within-subject differences in daily sleep latency, daily sleep efficiency, daily resting heart rate, daily number of steps, perceived stress score, and anxiety and depression scores. We hypothesize that an MBI will improve sleep duration, positively influencing perceived stress, anxiety and depression, and sleep quality in female HCWs.
2. Materials and methods
2.1. Study design
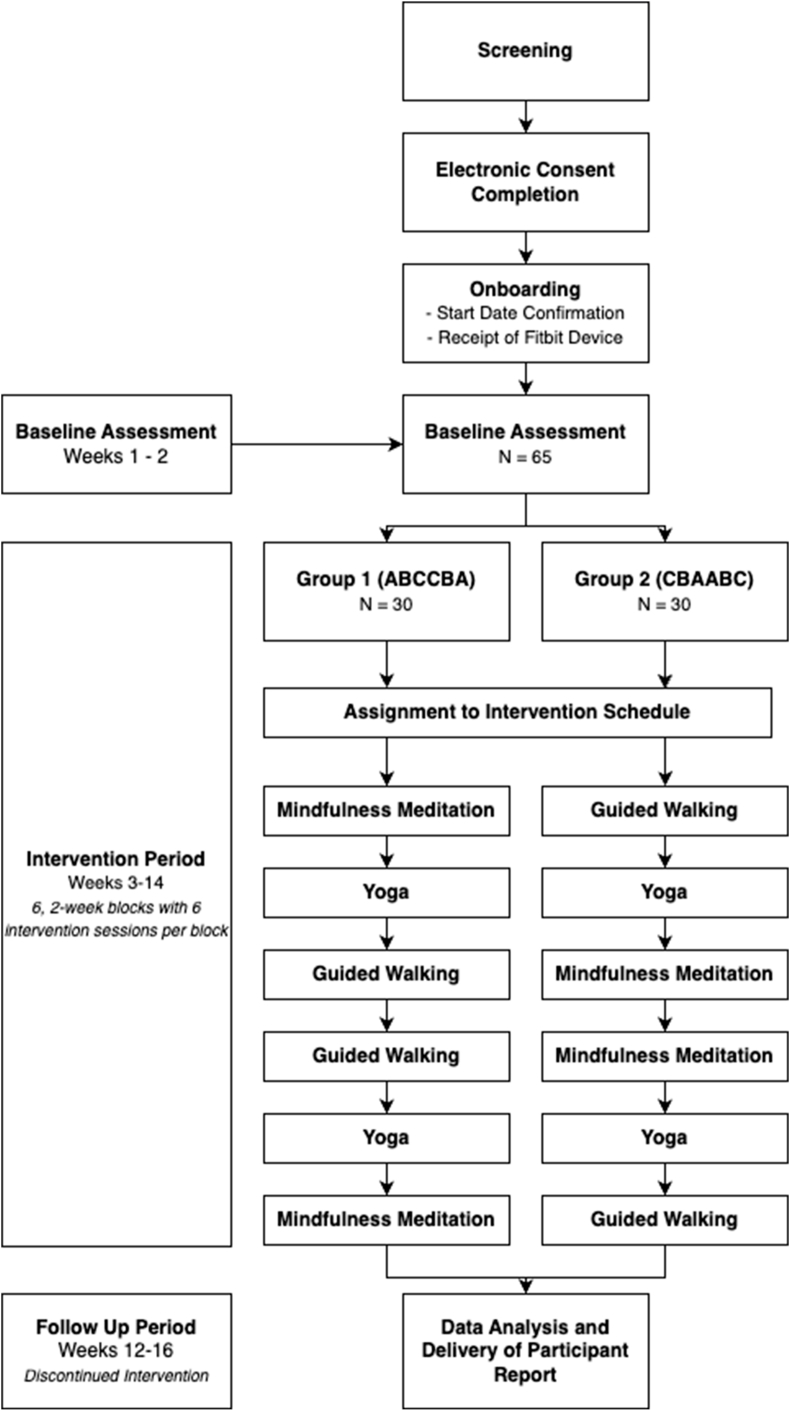
This study will employ a series of 60 randomized, personalized N-of-1 trials examining the effects of an MBI on sleep duration. The interventions will be delivered virtually over a 16-week trial comprising a 2-week baseline period (run-in), a 12-week intervention period, and a 2-week follow-up period (Fig. 1).
Fig. 1.
Participant timeline.
Participants will be provided a Fitbit® device for continuous sleep and activity monitoring and will receive online surveys assessing sleep and other health factors via text messages. Study enrollment will continue until 60 participants have been randomized following baseline and complete research procedures. This study was approved by the Northwell Health Institutional Review Board (IRB).
2.2. Study population
Female (assigned sex at birth) HCWs aged 40–60 years working in Northwell Health, the largest health system in New York, or its affiliates will be recruited. Those who meet a minimum stress threshold (Perceived Stress Scale [PSS-10] raw score ≥18) and with self-reported short sleep duration (<7 h per 24-h period for the last 3 months) will be eligible. The specific inclusion and exclusion criteria are listed in Table 1.
Table 1.
Inclusion and exclusion criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
Participants must meet the following criteria to begin the study:
|
Individuals who meet any of the following criteria will be excluded from participation:
|
2.3. Recruitment
Participants will be recruited from the over 85,000 employees of Northwell Health via e-mail blasts, newsletter advertisements, posts in Northwell's social platforms, and physical flyers at various Northwell Health facilities. Interested individuals will be directed to a HIPAA-compliant web application for data capture and management (REDCap) [21] for further details about the trial and completion of screening surveys regarding the inclusion and exclusion criteria. Ineligible participants will be notified immediately upon screening completion. Personal identifiable data of potential and enrolled participants will be maintained in a REDCap project, accessible only to IRB-approved study team members, and will only be shared as described in the study consent form. Study recruitment began in April 2023 and is expected to finish in May 2024.
2.4. Consent
Electronic informed consent will be obtained using an IRB-approved REDCap form. Eligible participants will be directed to a video outlining key points of the consent form and to a view-only version of the study consent form. Potential participants will be given the option for a 30-min informational phone call with an IRB-approved study team member to further review the consent form, or to proceed to signing the electronic consent form with a four-question comprehension check. Those choosing the phone call will be scheduled for an appointment with a study coordinator before signing. All participants will receive an electronic copy of the signed consent forms. These forms will be stored securely on a HIPAA compliant Northwell Health-approved shared drive, accessible only to IRB-approved study team members. After completing the consent form, participants will complete an onboarding survey on their lifestyle and schedule for personalization of the interventions (e.g., possible start dates, preferred time for survey delivery). Upon completion of the survey, participants will receive a Fitbit® device with instructions for its setup.
2.5. Baseline period
The first 2 weeks of the study will be the baseline (run-in) period. Participants will be asked to wear their Fitbit® device 24/7 and receive daily modified Consensus Sleep Diary [22] surveys on their perceived sleep duration and quality. At the end of the baseline period, participants will receive a health survey, which includes the PSS-10 [23], the Patient Health Questionnaire-4 (PHQ-4) [24], and additional ecological momentary assessment (EMA)-adapted questions from the Numeric Pain Rating Scale (momentary pain, fatigue, stress, mood, confidence, and concentration levels) [25,26]. All study surveys will be administered via text-message links to secure REDCap pages.
By the end of the baseline period, a study coordinator will review the participant's average sleep duration and study procedure adherence (one completed survey translates to one instance of adherence). Participants with ≥12 h of wear time and 180 min of sleep per 24-h period will be considered adherent to Fitbit wear. Participants with an objective, Fitbit-derived average sleep duration <7 h per 24-h period, and who achieved ≥80 % adherence to Fitbit wear and survey completion during the baseline period will be randomized to one of the intervention arms. In contrast, those who fail to meet these criteria during the baseline period will be removed before randomization and instructed to deregister their Fitbit device.
2.6. Randomization
Participants will be randomized 1:1 into one of two 12-week intervention arms, each comprising six 2-week blocks of guided (A) mindfulness meditation, (B) yoga, (C) or walking. Among the 60 eligible participants, 30 will be randomized to receive interventions in the ABCCBA order (Arm 1), while the other 30 will receive interventions in the CBAABC order (Arm 2)(Fig. 1). The counterbalanced treatment sequences were chosen to protect against temporally linear confounders [27].
The randomization schedule will be created by the principial investigator and provided to the study coordinators. The randomization list will be generated with a set seed of the random number generator for reproducibility and auditability. The order in which an individual becomes eligible for one of these randomizations will determine the placement given (e.g., the first eligible participant will be randomized to the intervention order given to participant 1 and will continue sequentially).
2.7. Intervention period
The MBI consists of 30-min guided audiovisual recordings on mindfulness meditation, yoga, and walking created by Zeel (https://www.zeel.com/), a commercial wellness technology platform that connects individuals to in-home or in-office services (such as massage and yoga). Following the baseline period, participants will proceed to the 12-week intervention period, where they will be asked to view and complete the assigned MBI sessions up to three times per week. Participants will access the videos through Vimeo (https://vimeo.com/), a video hosting platform, using password-protected “showcases” (folders) unique to their randomization schedule. They will receive a reminder containing a link and password three times per week, according to their preferences from their onboarding survey.
For monitoring, participants will be asked to continue wearing the Fitbit device 24/7 and submit daily and biweekly surveys during the intervention period. Study coordinators will monitor and review adherence to Fitbit wear, and completion of surveys and interventions weekly. In cases where adherence falls below the 80 % threshold for Fitbit wear or survey completion in a given week, participants will be re-educated on the study protocol via weekly regret lottery email notifications. Additionally, in cases of insufficient data (Fitbit data <5 days, survey data <50 % of 7 days, intervention data not provided for 7 days), the study team will contact participants by phone or email to address potential technological difficulties. Participants will receive the team's contact information via regular text messages and surveys should they have questions or concerns about side effects. The study team will be trained to identify and report any potential adverse events to the principal investigator, who is primarily responsible for monitoring participant safety. The IRB and/or sponsor will be notified of adverse events, as outlined in the data and safety monitoring plan and in accordance with local policy. Participants will also be reminded that they may withdraw from the trial completely at any point, including if they experience any concerning side effects.
2.8. Follow-up period
Following the intervention period, participants will proceed to the 2-week follow-up period to continue collecting data for additional exploratory analyses that may inform future trial designs. During this period, participants will continue to receive study surveys and will be asked to wear their Fitbit device, but they will not receive any interventions. Upon completion of the intervention period, a summary report of each participant's trial results will be sent. All feedback will be presented in narrative and in numerical form. After receiving the summary report, participants will also receive a satisfaction survey and may be asked to participate in a virtual follow-up interview to provide opinions on their experience as research participants and with personalized trials. Data collected from the satisfaction survey and the virtual follow-up will be used to inform future trials.
2.9. Compensation
At the end of the study, participants will be allowed to keep their commercially available Fitbit device (valued up to $160.00), and those who completed all study activities will receive a $50 ClinCard as compensation. Additionally, participants who are 80 % complaint with Fitbit wear and survey responses are eligible for a weekly lottery (maximum win: $400 after eight sessions) during the 14-week intervention and follow-up periods.
3. Outcomes
3.1. Primary outcome
The study's primary outcome is the mean within-subject difference in daily sleep duration between baseline and each treatment period, objectively measured using the Fitbit device and subjectively assessed through self-reported questionnaires. Participants' sleep activity will be aggregated by day.
3.2. Exploratory outcomes
Exploratory outcomes include mean within-subject differences in daily sleep latency, daily sleep efficiency, daily resting heart rate, and daily number of steps between baseline and each treatment period.
Sleep latency, defined as the time from when the lights are turned off (lights out) to sleep onset, will be aggregated daily using the Fitbit and assessed via self-reported questionnaires. Sleep efficiency (SE), an indicator of sleep quality, is defined as the ratio of total sleep time (TST) to time in bed (TIB), with values ≥85 % indicating good sleep quality [28]. SE will be recorded and aggregated daily using the Fitbit device. Resting heart rate and number of steps will also be measured using the Fitbit. Specifically, the device will measure the heart rate when sleep is detected and throughout the day while an individual is inactive (no steps detected).
Other exploratory outcomes include mean within-subject differences in perceived stress scores and anxiety and depression scores between baseline and each treatment period. Perceived stress scores will be measured using the total score of the PSS-10 questionnaire on a scale of 0–4 for 10 items. Scores will be categorized as low (0–13), moderate (14–26), or high stress (27–40). Anxiety and depressive disorder scores will be assessed using the total score of the PHQ-4 questionnaire on a scale of 0–3 for four items. Scores will be rated as normal (0–2), mild (3–5), moderate (6–8), or severe (9–12). Scores ≥3 for the first two and for the last two questions suggest anxiety and depression, respectively. Both PSS-10 and PHQ-4 will be evaluated pre-intervention (run-in), bi-weekly during the intervention, and post intervention.
3.3. Sample size calculation
The primary aim of this study is to evaluate the effect of a personalized MBI on increasing the average daily sleep duration in female healthcare workers at Northwell Health. A sample size of 30 participants per arm achieves 80 % power to detect a minimum effect size of 0.7 expected by one of the three interventions. Calculations were based on generalized estimating equation (GEE) tests for repeated measures considering a total of 28 daily measurements (4 weeks) per intervention at a significance level of 0.05. The within subject standard deviation is anticipated to be maximum 2h, with an auto-regressive AR(1) base correlation of 0.95 taken at two successive timepoints. The auto-regressive correlation structure is one of the most frequently used in personalized trials [29]. Calculations were performed in PASS (Power Analysis and Sample Size Software 2023) using the function GEE Tests for Repeated Measures (Continuous Outcomes) [30]. Estimated effect size and correlation values were derived from a previous fatigue study conducted at Northwell Health that employed a similar intervention/design and collected sleep duration as an exploratory endpoint [31]. The study will enroll up to 65 participants to account for special cases when randomized participants drop out (due to various reasons) before the start of the intervention.
3.4. Data management
Sleep and activity data from Fitbit devices will be collected from the participant study accounts via the Fitabase API and will be stored in Fitabase. All survey responses will be collected via text-message surveys and will be stored in REDCap. Only IRB-approved study team members will have access to the Fitabase account and REDCap project.
3.5. Primary analysis
The effect of the personalized MBI intervention on the participant's sleep duration will be assessed in each arm separately. All analyses will be performed using statistical software R version 4.4.1. The effects of each treatment on sleep duration will be assessed using GEE models with the auto-regressive AR(1) variance-covariance matrix for measures on the same day to that night's sleep (R package gee) [32,33]. This model accounts for possible autocorrelation and linear trends between sleep duration across time. To explore HTE on sleep duration, we will also model the data using linear mixed models (LMM) with random treatment effects (R package lmer4) [34] and evaluate the HTE index based on the LMM fits [35]. Analysis for the primary outcome will be based on intention-to-treat (ITT) principle that includes all randomized participants, regardless of their level of treatment received or protocol adherence. In instances of missing data, we will use multivariate imputation by chained equations (MICE) [36] and report the results alongside with ITT analysis. For descriptive purposes, individual changes in the average daily sleep durations between the run-in and each treatment period will be reported using the mean (95 % confidence intervals CIs). Bland-Altman plots will be constructed to visualize the agreement between the Fitbit-reported and self-reported sleep duration (R package BlandAltmanLeh).
3.6. Exploratory analysis
The effect of the personalized MBI intervention on the participant's sleep quality and physiological factors will be assessed in each arm separately. Individual changes in average daily sleep quality (sleep latency and efficiency), daily heart rate, and daily average number of steps between the run-in and each intervention period will be reported using means (95 % CIs). GEE models will be further employed to test the effect of the interventions on sleep quality and physiological factors. To explore the role of anxiety and depression as a potential mediator (M) between perceived stress (X) and sleep quality (Y), we will employ three regression models to estimate the direct, indirect, and the total effect of perceived stress on sleep quality.
4. Conclusions
This study protocol describes the aims of the proposed Stage II randomized clinical trial to evaluate the heterogeneity of effects of a differing MBI on increasing sleep duration among middle-aged female HCWs, and to determine which intervention component proves to be most efficacious for each individual participant. Thus, this study will provide an opportunity to examine the effectiveness of a scalable and efficacious method for supporting sleep health in women who are health care workers, and will provide data to support larger NIH stage model clinical trials designed to assess MBI efficacy (stage IA) and community effectiveness (stage V). Results from this study data may contribute to the incorporation of N-of-1 trials into the clinical practice of sleep quality management. Additionally, collected data will inform the development of future personalized trials, helping researchers and clinicians discover which wellness options are optimal for certain individuals, such as healthcare workers and women.
This protocol follows the NIH Stage Model for behavioral intervention development. The NIH Stage model was formulated to reduce obstacles encountered in the translation and implementation of basic science demonstrating the efficacy of behavioral interventions. These obstacles include loss of treatment fidelity when the intervention is implemented in the community. This is a proposed Stage II randomized trial. The trial will test behavioral interventions (yoga, mindfulness, and guided walking) for their efficacy to improve sleep duration in participants, and the trial is conducted in a research setting. Each of these promising behavioral interventions are theorized to improve sleep quantity and quality through targeting multiple cognitive and emotional processes that will be tested repeatedly in this Stage II proposed trial. If successful at identifying the right behavioral intervention for improving sleep duration, the anticipated pathway would be to next conduct a Stage IA activity to prepare the training and fidelity materials, and then conduct a Stage V effectiveness trial testing strategies of implementation and adoption for all women healthcare professionals implemented in community settings, using the N-of-1 trial design. As this is a digital (virtual) intervention, the maintenance of fidelity to the treatment delivery is expected to be identical to that found in a research setting. If this Stage II trial is not successful, then a Stage IA test of other behavioral interventions adapted and refined to increase sleep duration would be the next N-of-1 trial in the anticipated pathway.
One potential limitation of this protocol is the lack of generalizability of the study findings due to the sole inclusion of middle-aged female HCWs. While this is the target population to determine the effectiveness of an MBI on sleep duration to improve stress in female HCWs, the results may not be applicable to other populations. However, should the MBI intervention demonstrate efficacy in enhancing sleep duration within this specific demographic and exhibit consistent effects across participants, the subsequent phase would involve a large randomized controlled trial (RCT) to assess implementation and adoption strategies for all female healthcare professionals.
Funding
This work is supported by the National Institute on Aging (NIA) P30AG063786-01. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. NIA information centers can be contacted: https://www.nia.nih.gov/contact.
CRediT authorship contribution statement
Ashley M. Goodwin: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Data curation. Codruta Chiuzan: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Ciaran P. Friel: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Data curation. Danielle Miller: Writing – review & editing, Writing – original draft, Visualization, Project administration, Investigation, Data curation. Jordyn Rodillas: Writing – review & editing, Writing – original draft, Visualization, Project administration, Investigation, Data curation. Joan Duer-Hefele: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Investigation, Data curation. Ying Kuen Cheung: Writing – review & editing, Validation, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Karina W. Davidson: Writing – review & editing, Validation, Supervision, Methodology, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2024.101364.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Hirshkowitz M., Whiton K., Albert S.M., Alessi C., Bruni O., DonCarlos L., Hazen N., Herman J., Katz E.S., Kheirandish-Gozal L., Neubauer D.N., O'Donnell A.E., Ohayon M., Peever J., Rawding R., Sachdeva R.C., Setters B., Vitiello M.V., Ware J.C., Adams Hillard P.J. National Sleep Foundation's sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1:40–43. doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Medic G., Wille M., Hemels M.E. Short- and long-term health consequences of sleep disruption. Nat. Sci. Sleep. 2017;9:151–161. doi: 10.2147/NSS.S134864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yong L.C., Li J., Calvert G.M. Sleep-related problems in the US working population: prevalence and association with shiftwork status. Occup. Environ. Med. 2017;74:93–104. doi: 10.1136/oemed-2016-103638. [DOI] [PubMed] [Google Scholar]
- 4.Jáuregui Renaud K., Cooper-Bribiesca D., Martínez-Pichardo E., Miguel Puga J.A., Rascón-Martínez D.M., Sánchez Hurtado L.A., Colin Martínez T., Espinosa-Poblano E., Anda-Garay J.C., González Diaz J.I., Cardeña E., Avelar Garnica F. Acute stress in health workers during two consecutive epidemic waves of COVID-19. Int. J. Environ. Res. Public Health. 2021;19:206. doi: 10.3390/ijerph19010206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miguel‐Puga J.A., Cooper‐Bribiesca D., Avelar‐Garnica F.J., Sanchez‐Hurtado L.A., Colin‐Martínez T., Espinosa‐Poblano E., Anda-Garay J.C., González-Díaz J.I., Segura-Santos O.B., Vital-Arriaga L.C., Jáuregui-Renaud K. Burnout, depersonalization, and anxiety contribute to post‐traumatic stress in frontline health workers at COVID‐19 patient care, a follow‐up study. Brain Behav. 2021;11 doi: 10.1002/brb3.2007. https://onlinelibrary.wiley.com/doi/10.1002/brb3.2007 (Cited 2022 February 14) e02007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sriharan A., Ratnapalan S., Tricco A.C., Lupea D., Ayala A.P., Pang H., Lee D.D. Occupational stress, burnout, and depression in women in healthcare during COVID-19 pandemic: rapid scoping review. Front. Glob. Womens Health. 2020;1 doi: 10.3389/fgwh.2020.596690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdalla M., Chiuzan C., Shang Y., Ko G., Diaz F., Shaw K., McMurry C.L., Cannone D.E., Sullivan A.M., Lee S.A.J., Venner H.K., Shechter A. Factors associated with insomnia symptoms in a longitudinal study among New York City healthcare workers during the COVID-19 pandemic. Int. J. Environ. Res. Public Health. 2021;18:8970. doi: 10.3390/ijerph18178970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz F., Cornelius T., Bramley S., Venner H., Shaw K., Dong M., Pham P., McMurry C.L., Cannone D.E., Sullivan A.M., Lee S.A.J., Schwartz J.E., Shechter A., Abdalla M. The association between sleep and psychological distress among New York City healthcare workers during the COVID-19 pandemic. J. Affect. Disord. 2022;298:618–624. doi: 10.1016/j.jad.2021.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Correia I., Almeida A.E. Organizational justice, professional identification, empathy, and meaningful work during COVID-19 pandemic: are they burnout protectors in physicians and nurses? Front. Psychol. 2020;11 doi: 10.3389/fpsyg.2020.566139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guyatt G., Sackett D., Taylor D.W., Chong J., Roberts R., Pugsley S. Determining optimal therapy — randomized trials in individual patients. N. Engl. J. Med. 1986;314:889–892. doi: 10.1056/NEJM198604033141406. [DOI] [PubMed] [Google Scholar]
- 11.Lillie E.O., Patay B., Diamant J., Issell B., Topol E.J., Schork N.J. The n-of-1 clinical trial: the ultimate strategy for individualizing medicine?, Pers. Med. 2011;8:161–173. doi: 10.2217/pme.11.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W.L., Chen K.H., Pan Y.C., Yang S.N., Chan Y.Y. The effect of yoga on sleep quality and insomnia in women with sleep problems: a systematic review and meta-analysis. BMC Psychiatr. 2020;20:195. doi: 10.1186/s12888-020-02566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goyal M., Singh S., Sibinga E.M.S., Gould N.F., Rowland-Seymour A., Sharma R., Berger Z., Sleicher D., Maron D.D., Shihab H.M., Ranasinghe P.D., Linn S., Saha S., Bass E.B., Haythornthwaite J.A. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern. Med. 2014;174:357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pascoe M.C., Thompson D.R., Ski C.F. Yoga, mindfulness-based stress reduction and stress-related physiological measures: a meta-analysis. Psychoneuroendocrinology. 2017;86:152–168. doi: 10.1016/j.psyneuen.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Strehli I., Burns R.D., Bai Y., Ziegenfuss D.H., Block M.E., Brusseau T.A. Mind–body physical activity interventions and stress-related physiological markers in educational settings: a systematic review and meta-analysis. Int. J. Environ. Res. Public Health. 2020;18:224. doi: 10.3390/ijerph18010224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y., Wang F., Zheng W., Zhang L., Ng C., Ungvari G., Xiang Y. Mindfulness-based interventions for insomnia: a meta-analysis of randomized controlled trials. Behav. Sleep Med. 2018;18:1–9. doi: 10.1080/15402002.2018.1518228. [DOI] [PubMed] [Google Scholar]
- 17.Tseng A.A. Effectiveness of meditation-based interventions on health problems caused by COVID-19 pandemic: narrative review. Int. J. Yoga. 2023;16(2):72–78. doi: 10.4103/ijoy.ijoy_112_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duarte D.F.B., Libório J.R., Cavalcante G.M.E., Aquino T.L.D., Bezerra L.D.C., Martin A.L.D.A.R.…Paula J.D.A.D. The effects of mindfulness-based interventions in COVID-19 times: a systematic review. Journal of Human Growth and Development. 2022;32(2):315–326. [Google Scholar]
- 19.Garland S.N., Zhou E.S., Gonzalez B.D., Rodriguez N. The quest for mindful sleep: a critical synthesis of the impact of mindfulness-based interventions for insomnia. Current sleep medicine reports. 2016;2:142–151. doi: 10.1007/s40675-016-0050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onken L.S., Carroll K.M., Shoham V., Cuthbert B.N., Riddle M. Reenvisioning clinical science: unifying the discipline to improve the public health. Clin. Psychol. Sci. 2014;2(1):22–34. doi: 10.1177/21677026134979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O'Neal L., McLeod L., Delacqua G., Delacqua F., Kirby J., Duda S.N. REDCap Consortium, the REDCap consortium: building an international community of software platform partners. J. Biomed. Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carney C.E., Buysse D.J., Ancoli-Israel S., Edinger J.D., Krystal A.D., Lichstein K.L., Morin C.M. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35:287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- 24.Kroenke K., Spitzer R.L., Williams J.B.W., Löwe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50:613–621. doi: 10.1176/appi.psy.50.6.613. [DOI] [PubMed] [Google Scholar]
- 25.Jensen M.P., Turner J.A., Romano J.M., Fisher L.D. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83:157–162. doi: 10.1016/s0304-3959(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 26.Goodwin A.M., Miller D., D'Angelo S., Perrin A., Wiener R., Greene B., Romain A.N., Arader L., Chandereng T., Kuen Cheung Y., Davidson K.W., Butler M. Protocol for randomized personalized trial for stress management compared to standard of care. Front. Psychol. 2023;14 doi: 10.3389/fpsyg.2023.1233884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kravitz R.L., Duan N., Vohra S., Li J., The DEcIDE Methods Center N-of-1 Guidance Panel . In: Design and Implementation of N-Of-1 Trials: A User's Guide. Kravitz R.L., Duan N., editors. AHRQ; 2014. Chapter 1. Introduction to N-of-1 trials: indications and barriers; pp. 1–11. AHRQ publication no. 13(14)-EHC122-EF. [Google Scholar]
- 28.Ohayon M., Wickwire E.M., Hirshkowitz M., Albert S.M., Avidan A., Daly F.J., Dauvilliers Y., Ferri R., Fung C., Gozal D., Hazen N., Krystal A., Lichstein K., Mallampalli M., Plazzi G., Rawding R., Scheer F.A., Somers V., Vitiello M.V. National Sleep Foundation's sleep quality recommendations: first report: first report. Sleep Health. 2017;3:6–19. doi: 10.1016/j.sleh.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Chen X., Chen P. A comparison of four methods for the analysis of N-of-1 trials. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0087752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahn C., Heo M., Zhang S. first ed. Chapman and Hall/CRC; 2014. Sample Size Calculations for Clustered and Longitudinal Outcomes in Clinical Research. [DOI] [Google Scholar]
- 31.Butler M., D'Angelo S., Lewis C., Miller D., Perrin A., Suls J., Chandereng T., Cheung Y.K., Davidson K.W. Series of virtual light therapy interventions for fatigue: a feasibility pilot study protocol for a series of personalised (N-of-1) trials. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-055518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang K.Y., Zeger S.L. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 33.Fitzmaurice G.M., Laird Nan M., Ware J.H. Vol. 998. John Wiley & Sons; 2012. (Applied Longitudinal Analysis). [Google Scholar]
- 34.Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using Lme4. J. Stat. Soft. 2015;67:1–48. [Google Scholar]
- 35.Cheung K., Mitsumoto H. Evaluating personalized (N-of-1) trials in rare diseases: how much experimentation is enough? Harvard data science review. 2022. (Special Issue 3) [DOI] [PMC free article] [PubMed]
- 36.Azur M.J., Stuart E.A., Frangakis C., Leaf P.J. Multiple imputation by chained equations: what is it and how does it work? Int. J. Methods Psychiatr. Res. 2011;20:40–49. doi: 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.