Key Points
-
•
In contrast to non-HL, pediatric HL does not involve the CNS parenchyma but extends into the extra-axial CNS space from surrounding tissue.
-
•
Among the patients with HL evaluated in this report, the incidence of lesions involving the extra-axial CNS was 0.9%.
Visual Abstract
Abstract
Hodgkin lymphoma (HL) involving the central nervous system (CNS) is exceedingly rare. Information regarding the presentation, management, treatment, and outcome of patients with CNS HL is limited to case reports or small series. We describe 45 pediatric patients with 55 extra-axial CNS lesions at diagnosis with HL from a cohort of 4995 patients enrolled on Children’s Oncology Group AHOD1331 and the European Network for Pediatric Hodgkin lymphoma C1 and C2 trials, with an overall incidence of 0.9%. Up to 82.2% of patients had a single CNS lesion in the thoracic, lumbar, or sacral spine. In the evaluated cohort, HL did not occur within the CNS parenchyma. Lesions extended into the extra-axial CNS space from adjacent soft tissue or bone and never directly infiltrated through the dura into the brain or spinal cord. Patients with CNS involvement had a twofold greater incidence of extranodal lesions than previously reported cohorts without CNS involvement. After 2 cycles of chemotherapy, 89.1% of CNS lesions demonstrated a complete metabolic response and >75% decrease in volume. Thirteen CNS lesions (23.6%) received irradiation; none were sites of disease relapse. Relapse occurred at the site of 2 lesions involving the CNS, both of which had an adequate interim response to chemotherapy. In summary, we present, to our knowledge, the largest reported cohort of systemic HL involving the CNS at diagnosis, demonstrating that these lesions originate from surrounding tissues, extend into the extra-axial CNS space, and respond similarly to other nodal and extranodal disease. The trials were registered at www.clinicaltrials.gov as #NCT02166463, #NCT00433459, and #NCT02684708.
Introduction
Hodgkin lymphoma (HL) constitutes ∼7% of all childhood cancer, with an annual incidence of 2.6 per 100 000 people of all ages in the United States,1 or 1 in 2300 children aged <18 years enrolled in the German Childhood Cancer Registry.2 It is most common in adolescents and young adults, often presenting with painless adenopathy, elevated inflammatory markers, and symptoms of fever, night sweats, and weight loss. Up to 33% of pediatric patients with HL demonstrate extranodal lesions (E-lesions) at the time of diagnosis, commonly involving bone, lung, or liver.3, 4, 5, 6, 7 Involvement of the central nervous system (CNS) at diagnosis with HL is exceedingly rare, historically reported to occur in <0.5% of patients,8 in contrast to non-HL (NHL) in which CNS involvement has been reported in up to 6% of patients.9,10 NHL has been described to involve the brain parenchyma (hemispheres, basal ganglia, corpus callosum, and posterior fossa), leptomeninges, cerebrospinal fluid, and eyes.11,12 Compression of the spinal cord by epidural or leptomeningeal lesions is an uncommon initial presentation of NHL, however, it can be seen with recurrent or progressive disease.13 Descriptions of CNS involvement in patients with HL are limited to small case reports or series,14, 15, 16, 17, 18, 19, 20 often in patients with advanced disease.21,22 A report from the German Hodgkin Study Group identified only 2 possible patients with CNS involvement among a cohort of 14 868 individuals; 1 in the context of multiply-relapsed HL and the other that was not biopsy proven.23 Our review of previously published cases identified 24 patients with HL involving the CNS at diagnosis (6 patients within the pediatric age range of 8-13 years). This literature review revealed that CNS involvement can present in patients of all ages, with an average length of symptoms before diagnosis of 5.6 months (see supplemental Data). The majority of patients presented with pain and neurologic symptoms consistent with the location of their disease. Many patients demonstrated thoracic-level epidural involvement, potentially related to direct spread from mediastinal lymph nodes.24,25 Among the previously published cases, approximately half of the patients (56%) were reported to undergo an emergency decompressive surgical procedure at the time of presentation. Given the paucity of literature describing this occurrence, risk factors for CNS involvement in HL have not been defined.8
The ability to detect CNS involvement is closely related to the sensitivity of the imaging modality that is used; therefore, identification of this was limited by earlier, less-sensitive techniques. Since the 1980s, computed tomography (CT) scans have been the primary mode of anatomical imaging for patients with HL.26 The Lugano criteria, defined in 2013, supported the use of 18F-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) with CT for the assessment of both metabolic activity and morphology.26 Magnetic resonance imaging (MRI) is increasingly recognized as a viable alternative for morphologic assessment with minimal radiation exposure, which can also be combined with PET.27, 28, 29
Treatment protocols in HL have evolved to include risk- and response-adapted combination chemotherapy, along with radiotherapy in a subset of patients. HL is now a largely curable disease, with novel therapeutics contributing to decreased relapse rates. Brentuximab vedotin (Bv), an antibody-drug conjugate targeting CD30 used in conjunction with standard chemotherapy treatment, was demonstrated to significantly improve event-free survival in high-risk pediatric HL.30,31 Radiotherapy is also an effective local treatment option in HL; however, due to the risk of late effects, the indications for radiotherapy have been reduced to patients at highest risk of relapse.32 The impact of radiotherapy for patients with CNS involvement of HL is unclear. Secondly, it is not known whether the impact of radiotherapy on CNS lesions is comparable with the efficacy of radiotherapy on peripheral lymph nodes and other organs such as bone, lung, or liver.
Therefore, given the rarity of CNS involvement in HL, very little is known regarding the presentation of these lesions, response to treatment, or outcomes with frontline therapy. This report describesa large cohort of pediatric patients with HL with CNS involvement at diagnosis, using combined data from the recent Children’s Oncology Group (COG) and European Network for Pediatric HL (EuroNet-PHL) consortium clinical trials.
Methods
General
The imaging studies evaluated in this report include those from newly diagnosed patients with HL enrolled between 2007 and 2021 on the COG trial AHOD1331 (NCT02166463)30 and EuroNet-PHL trials PHL-C1 (NCT00433459; EudraCT 2006-000995-33)33 and PHL-C2 (NCT02684708; EudraCT 2012-004053-88; see Table 1). These were all multicenter trials with central imaging review. All cases with CNS involvement at the time of diagnosis were identified from the central databases of the 3 clinical trials. E-lesions were defined as per the proposed Staging, Evaluation and Response Criteria Harmonization (SEARCH) for Childhood, Adolescent and Young Adult Hodgkin Lymphoma (CAYAHL) consensus definition34 as contiguous infiltration of a lymph node mass into extralymphatic structures or organs. The COG cases with CNS involvement were identified by the primary study committee radiologist (K.M.) at the time of first protocol-mandated central review, and all COG trial patients were rereviewed to ensure that none were missed. The EuroNet-PHL cases were identified retrospectively because the central trial database was queried for patients with E-lesions or disseminated organ involvement. The nuclear physician (L.K.) and radiologist (D.S.) from the EuroNet-PHL central review board re-evaluated the imaging from these patients for potential CNS involvement. Upon identification, original imaging of all patients (COG and EuroNet-PHL) with confirmed or suspected CNS involvement was then rereviewed by radiologists (K.M., D.S., and H.L.) and nuclear medicine physicians (L.K. and H.L.) with extensive expertise in HL from both COG and EuroNet-PHL. The imaging review was also attended by radiation oncologists (K.D.) and pediatric oncologists (R.P. and C.M.-K.) and occurred over the course of >25 virtual meetings, with the final consensus presented in this report. All cases had morphologic imaging (CT and/or MRI), and 44 of 45 cases had FDG-PET performed before enrollment and the start of therapy. All patients had disease response assessment after 2 cycles of treatment. FDG-PET/CT or FDG-PET/MRI were performed at the end of therapy only if disease uptake was FDG-PET positive after 2 cycles, as per the specific trial protocol.
Table 1.
Comparison of trials
| EuroNet-PHL-C1 | EuroNet-PHL-C2 | AHOD1331 | |
|---|---|---|---|
| Dates of enrollment | 31 January 2007 to 30 January 2013 | 1 October 2015 to 31 December 2020 | 16 March 2015 to 2 August 2019 |
| Median follow-up | 66.5 mo (IQR, 62.7-71.7) | N/A (follow-up ongoing at time of publication) | 42.1 mo (range, 0.1-80.9) |
| Ages enrolled | <18 y | <25 y | 2-21 y |
| Risk groups included in trial | Early (TG-1): IA, IB, and IIA Intermediate (TG-2): IAE, IBE, IIB, IIAE, and IIIA Advanced (TG-3): IIBE, IIIAE, IIIB, IIIBE, all IV |
Early (TL-1): IA, IB, and IIA without risk factors (either bulk >200 mL or ESR >30 mm/h) Intermediate (TL-2): IA, IE, IIA + risk factors or IIB, IIIA Advanced (TL-3): IIBE, IIIAE, IIIB, IIIE, IIIBE, all IV |
High risk (HR): IIB + bulk, IIIB, all IV |
| Time of randomization | After 2 courses of initial chemotherapy and interim FDG-PET | After 2 courses of initial chemotherapy and interim FDG-PET | Before treatment initiation |
| Induction treatment | 2 × OEPA (vincristine, etoposide, prednisone, doxorubicin) | 2 × OEPA (vincristine, etoposide, prednisone, doxorubicin) | 2 × ABVE-PC (adriamycin, bleomycin, vincristine, etoposide, prednisone, cyclophosphamide) or 2 × Bv-AVE-PC (brentuximab vedotin, adriamycin, vincristine, etoposide, prednisone, cyclophosphamide) |
| Chemotherapy treatment after interim FDG-PET | 2 (IR) or 4 (HR) × COPP (cyclophosphamide, vincristine, prednisone, procarbazine) or 2 (IR) or 4 (HR) × COPDAC (cyclophosphamide, vincristine, prednisone, dacarbazine) |
2 (TL-2) or 4 (TL-3) × COPDAC (cyclophosphamide, vincristine, prednisone, dacarbazine) or 2 (TL-2) or 4 (TL-3) × DECOPDAC (doxorubicin, etoposide, cyclophosphamide, vincristine, prednisone, dacarbazine) |
3 additional cycles of chemotherapy based on randomization before treatment start (3 × ABVE-PC or 3 × Bv-AVE-PC) |
| Indication for radiotherapy | All initially involved sites (except for bone marrow lesions) if interim FDG-PET was positive (Deauville score 3, 4, or 5) or volume reduction <50% | COPDAC group: All initially involved sites (except for bone marrow lesions) if interim FDG-PET was positive (Deauville score >3) plus boost on lesions still FDG-PET positive (Deauville score >3) at the end of chemotherapy or DECOPDAC group: 30 Gy on all lesions still FDG-PET positive (Deauville score >3) at the end of chemotherapy |
Large mediastinal adenopathy and all sites which were still positive (Deauville score 4 or 5) in interim FDG-PET |
IQR, interquartile range; HR, high risk; IR, intermediate risk; N/A, not applicable.
The variables evaluated in this report were decided upon a priori by consensus agreement of the multidisciplinary expert panel. The variables included the following: age, sex, Ann Arbor stage, histology, symptoms at the time of presentation (pain and neurologic symptoms), corticosteroid administration before FDG-PET scan, number and location of E-lesions (defined below), number and location of CNS lesions, type of tissue from which the CNS lesion originated, anatomic description of the involvement of the CNS lesion (including whether the neural foramina or spinal cord was affected), whether emergency irradiation or an operative procedure were performed at diagnosis, intensity of FDG tracer uptake at the time of initial staging (characterized by the Deauville score), metabolic and morphologic response of the CNS lesions after 2 cycles of chemotherapy, and whether relapse occurred and in which location.
Definition of CNS involvement
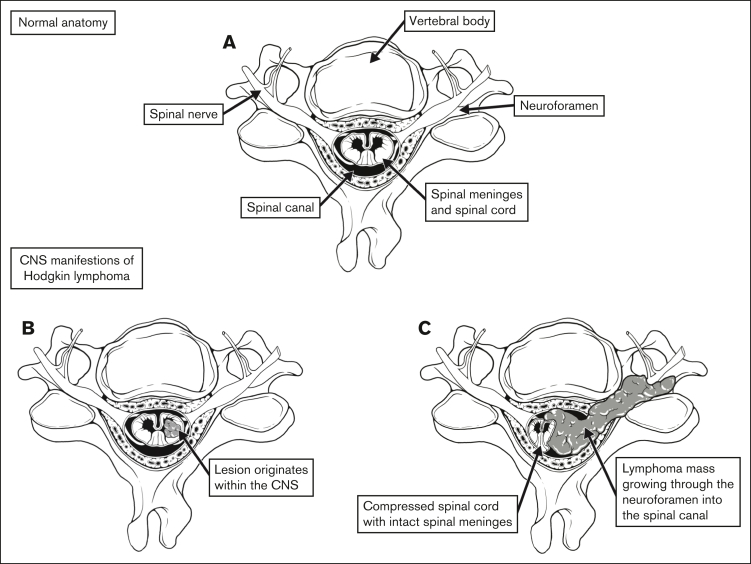
CNS involvement can be defined in 2 distinct ways: (1) lesions originating within the intra-axial CNS space; and (2) lesions extending into the extra-axial CNS space. For example, lesions included in the former group would include those originating within the CNS parenchyma or subdural space. The latter group would include lesions that extend into the intracranial, extra-axial space or the spinal canal, including those which extend from surrounding soft tissue or bone (Figure 1). This is an important distinction, because the biology and response to treatment of intra-axial and extra-axial lesions would likely be unique.
Figure 1.
Defining CNS involvement. (A) Normal anatomy of the spine and surrounding anatomic structures. (B) Tumor originating within the spinal cord. (C) Tumor infiltrating through the neuroforamen, compressing the spinal cord.
Trials included in cohort
COG protocol AHOD133130,35 was a randomized, response-adapted, phase 3 study for patients with newly diagnosed high-risk classic HL (stages IIB with bulk, IIIB, IVA, and IVB) aged 2 to 21 years. The primary aim of this trial was to compare the efficacy of Bv with doxorubicin, vincristine, etoposide, prednisone, and cyclophosphamide (Bv-AVE-PC) with the standard dose-intensive regimen doxorubicin, bleomycin, vincristine, etoposide, prednisone and cyclophosphamide (ABVE-PC). EuroNet-PHL-C17,33 was a titration study with an embedded noninferiority, randomized controlled trial for patients with classic HL aged <18 years with early (treatment group 1 [TG-1]), intermediate (TG-2), or advanced-stage (TG-3) classic HL. The aim of this study was to determine whether radiotherapy could be omitted in patients with an adequate response to vincristine, etoposide, doxorubicin and prednisone (OEPA) and whether modified consolidation therapy (replacing procarbazine with dacarbazine) reduces gonadotoxicity. Patients enrolled on EuroNet-PHL-C1 from France and Austria (n = 574) were not included in this report, because they did not participate in central imaging review. EuroNet-PHL-C2 (NCT02684708; EudraCT 2012-004053-88) was a comprehensive risk-stratified and response-adapted first-line treatment strategy for patients with classic HL aged <25 years. Patients were designated early (treatment level 1 [TL-1]), intermediate (TL-2), or advanced-stage (TL-3) HL. The aim of this study was to determine whether radiotherapy can be reduced without compromising cure rates and to investigate a randomization of chemotherapy intensification. Follow-up for this trial is ongoing. See Table 1 for additional details.
Results
Overview and clinical presentation
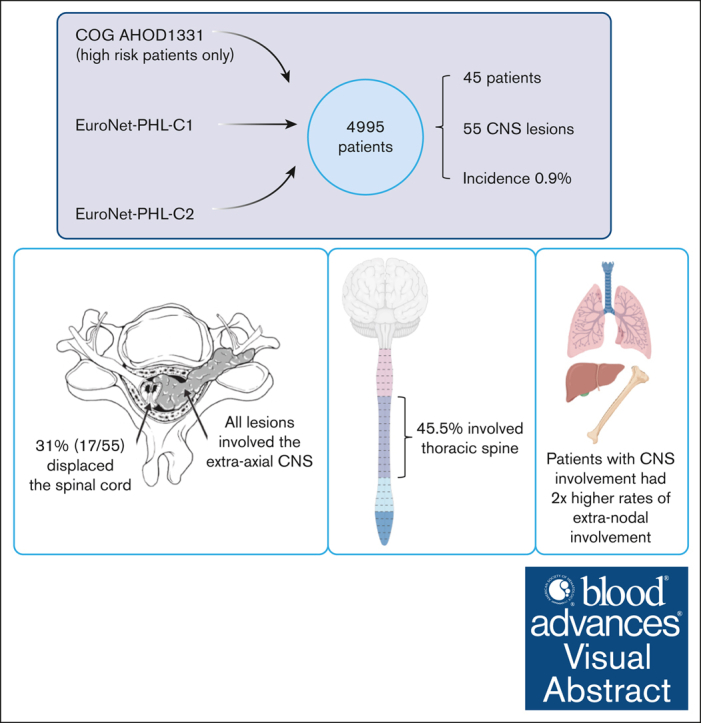
We identified 45 patients (0.9%) with 55 unique CNS lesions at the time of diagnosis from a large international cohort of 4995 clinical trial patients (AHOD1331, n = 587; EuroNet-PHL-C1, n = 1528; EuroNet-PHL-C2, n = 2880). All of the identified lesions involved the extra-axial CNS space. As described in Table 2, 20 patients (3.4%) were found to have CNS involvement from AHOD1331, nine (0.6%) from EuroNet-PHL-C1, and 16 (0.6%) from EuroNet-PHL-C2.
Table 2.
Baseline characteristics
| CNS HL cohort, n (%) | COG AHOD1331, n (%) | EuroNet-PHL-C1 (excluding France and Austria),∗ n (%) | |
|---|---|---|---|
| Sex | |||
| Female | 13 (28.9) | 276 (47) | 716 (47) |
| Male | 32 (71.1) | 311 (53) | 812 (53) |
| Age | <10 y: 9 (20.0) | <12 y: 90 (15.3) | <13 y: 472 (31) |
| 10-18 y: 35 (77.8) | 12-21 y: 497 (84.7) | ≥13 y: 1056 (69) | |
| >18 y: 1 (2.2) | |||
| Ann Arbor stage | |||
| IA | 28 (1.8) | ||
| IB | 5 (0.3) | ||
| IIA | 514 (33.6) | ||
| IIAE | 66 (4.3) | ||
| IIB | 149 (9.8) | ||
| IIBE | 121 (20.6) | 88 (5.6) | |
| IIIA | 2 (4.4) | 130 (8.5) | |
| IIIAE | 17 (1.1) | ||
| IIIB | 1 (2.2) | 111 (7.3) | |
| IIIBE | 2 (4.4) | 113 (19.3) | 35 (2.3) |
| IVA | 12 (26.7) | 128 (8.4) | |
| IVAE | 7 (15.6) | 167 (28.4) | 41 (2.7) |
| IVB | 9 (20.0) | 186 (31.7) | 126 (8.2) |
| IVBE | 12 (26.7) | 90 (5.9) | |
| Histology | |||
| Nodular sclerosing | 31 (68.9) | 449 (76.5) | 1063 (69.6) |
| Mixed cellularity | 5 (11.1) | 33 (5.6) | 305 (20) |
| Lymphocyte rich | 1 (2.2) | 7 (1.2) | 32 (2.1) |
| Lymphocyte depleted | 6 (0.4) | ||
| Lymphocyte predominant | |||
| HL, not otherwise specified | 3 (6.7) | 98 (16.7) | 81 (5.3) |
| Gray zone lymphoma | 5 (0.3) | ||
| Information not available | 5 (11.1) | 36 (2.3) | |
| Trial | |||
| AHOD1331 | 20 (44.4) | ||
| EuroNet-PHL-C1 | 9 (20) | ||
| EuroNet-PHL-C2 | 16 (35.6) | ||
| Number of CNS lesions per patient | |||
| 1 | 37 (82.2) | ||
| 2 | 6 (13.3) | ||
| 3 | 2 (4.4) | ||
| Location | |||
| Skull | 2 (3.6) | ||
| Cervical spine | 3 (5.5) | ||
| Thoracic spine | 25 (45.5) | ||
| Lumbar spine | 12 (21.8) | ||
| Sacrum | 13 (23.6) | ||
| Origin of the CNS mass | |||
| CNS | |||
| Skeletal | 25 (45.5) | ||
| Muscle/soft tissue (including lymph node) | 22 (40.0) | ||
| Origin unclear | 8 (14.5) | ||
| Intensity of FDG uptake of CNS lesions at initial staging (according to Deauville scoring system) | |||
| 3 | 2 (3.6) | ||
| 4 | 5 (9.1) | ||
| 5 | 47 (85.5) | ||
| Information not available | 1 (1.8) |
EuroNet-PHL-C2 primary results have not yet been published; therefore, patient details from that trial are not included here.
Information regarding clinical symptoms relating to the CNS lesion was available for 11 of 45 patients (24.4%) in this cohort. Of these 11 patients, 9 presented with pain (82%), and 4 had neurologic symptoms (36%; see supplemental Materials). Two of the 45 patients had emergency operative procedures at presentation; 1 of these was due to spinal cord compression, and the indication for the second operative procedure was not available within our data set. Four lesions received pretreatment with corticosteroids. None of the patients in our cohort received emergency radiotherapy at presentation. The most common histologic subtype was nodular sclerosis (68.9%), followed by mixed cellularity (11.1%).
Baseline characteristics of the CNS lesions
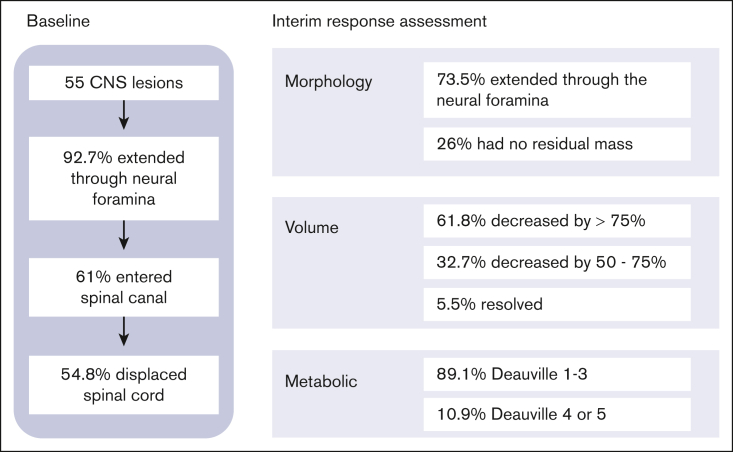
None of the lesions involved the intra-axial CNS space. All lesions extended into the extra-axial CNS space from surrounding tissue. At the time of diagnosis, the majority of patients (82.2%) had a single lesion involving the CNS, whereas 4.4% had up to 3 discrete lesions in the CNS. The lesions extended into the thoracic spine (45.5%), sacrum (23.6%), lumbar spine (21.8%), cervical spine (5.5%), and skull (3.6%; Figure 2). In our cohort, 45% of lesions originated from adjacent bone and 40% from adjacent soft tissue. We were unable to determine the tissue of origin for 15% of lesions (Figure 3).
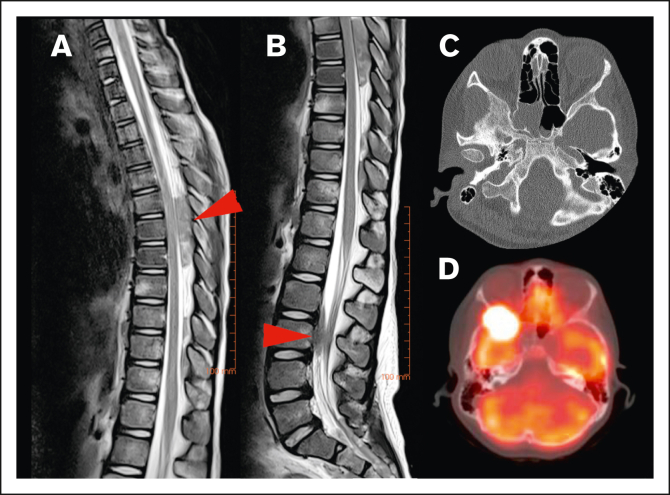
Figure 2.
Locations of HL CNS involvement. (A) Tumor in the dorsal part of the thoracic spinal canal and (B) ventral part of the lumbar spine (T2-weighted MRI sequences of the same patient, red arrow indicating tumor location), (C) tumor originating from the skull (bone window with subtle osteolysis, and (D) PET-fusion demonstrating the same lesion as in panel C extending into orbit and middle cranial fossa.
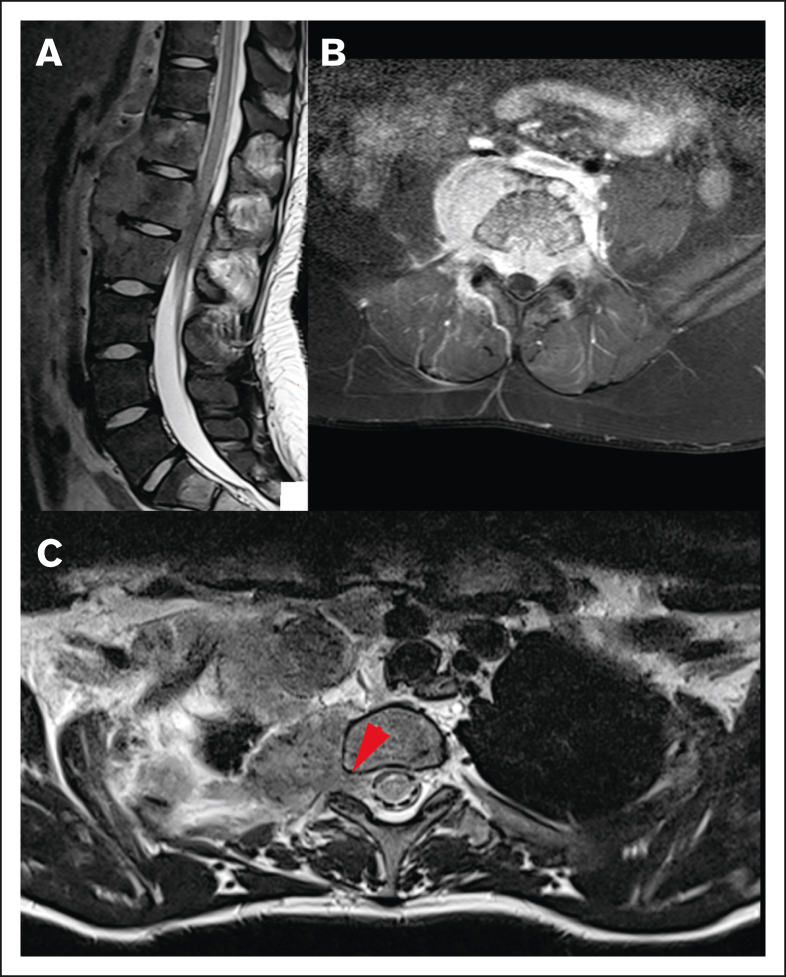
Figure 3.
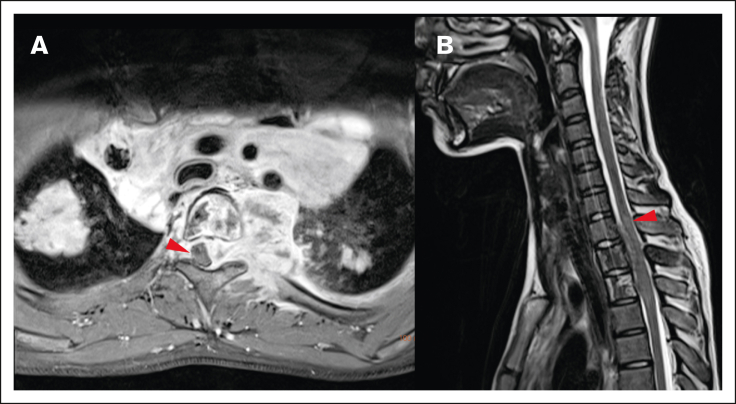
Different origins of CNS HL lesions. (A) Tumor originating from bone (vertebral body of T12, L1, and L2). (B) Unclear tumor origin but very likely bone, with circumferential involvement and an associated soft tissue component. (C) Paravertebral tumor mass of the upper thoracic spine infiltrating through the right neuroforamen, demonstrated by the red arrow, likely originating from soft tissue.
CNS involvement was associated with high-risk HL, with 89% of patients presenting with stage IV disease. Many patients (68.8%) demonstrated evidence of extranodal disease, most frequently in the lung pleura (56%), followed by bone (20%), lung (16%), and pericardium (8%).
Most lesions (92.7%) extended through the neural foramina of either the vertebrae or sacral bone; 2 lesions did not extend through the neural foramina, and 2 lesions could not be evaluated (Figure 4). Thirty-one lesions entered the spinal canal. Of these, 54.8% caused displacement of the spinal cord, 25.8% were adjacent to the spinal cord but did not cause displacement, and 19.4% did not contact the spinal cord (Figure 5). None of the lesions infiltrated into the spinal cord itself. Spinal cord edema was evaluated for patients who had MRI available (17 of 31 lesions). Among these evaluable lesions, only 12.9% had evidence of spinal cord edema.
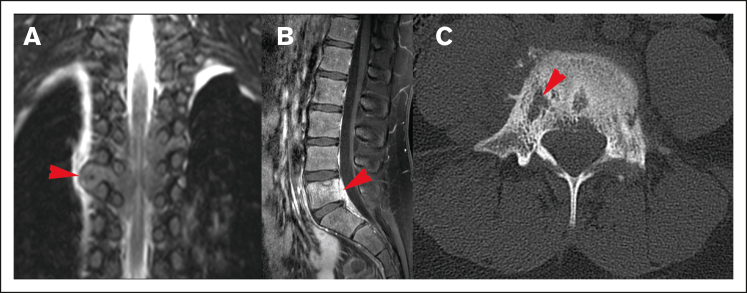
Figure 4.
Spreading pattern of CNS HL. (A) Tumor spreading through the neural foramina into the thoracic spine. (B) Direct spread from lumbar spinal vertebrae, (C) Lytic cortical bony lesions (same patient as shown in panel B).
Figure 5.
Local impact on CNS structures. (A) Displacement and mild compression of spinal cord to the right without contrast enhancement in the transverse T1 fat saturated image. (B) The same patient with edema clearly visible in the spinal cord in a sagittal T2-weighted MRI image.
Metabolic assessment of disease at initial staging was available for 44 of 45 patients. Of these 44 patients, 96.3% of CNS lesions had high FDG uptake, comparable with Deauville 4 or 5. Two lesions were comparable with Deauville 3, one of which had received pretreatment with corticosteroids.
Characteristics of the CNS lesions at IRA
All 45 patients, including those who received emergency surgery or steroid pretreatment, have been included in the following description of response assessment. After 2 cycles of chemotherapy, 67.3% of CNS lesions had decreased in volume by >75% (61.8%) or had resolved completely (5.5%), whereas 32.7% CNS lesions decreased in volume by 50% to 75%.
Thirty-seven of the lesions (74%) involving the neural foramina demonstrated a residual mass after 2 cycles of chemotherapy, whereas no residual mass was detected within the neural foramina for 13 lesions (26%), and 5 lesions could not be adequately assessed on the available imaging studies. Among the lesions that previously entered the spinal canal, 34.5% demonstrated complete resolution at interim response assessment (IRA), 18% continued to demonstrate a residual mass within the spinal canal, and 6.5% were unable to be assessed on the available imaging. Among the lesions that continued to demonstrate a residual mass within the spinal canal, 10% still displaced the cord, 20% closely approximated the cord but did not cause displacement, and 70% had decreased enough in size that they had no proximity to the cord.
Most CNS lesions demonstrated an adequate metabolic response at IRA, with 89.1% PET negative (Deauville score 1-3) and 10.9% remaining PET positive (Deauville score 4 or 5; Figure 6). Only 1 of the CNS lesions that remained PET positive at IRA was irradiated, and none of these lesions were sites of relapse. Two CNS lesions (3.6%) that were PET negative at IRA (Deauville 1 and 3) were associated with relapse, and neither received irradiation. Thirteen CNS lesions (23.6%) in 13 patients received irradiation, and none were sites of disease relapse. These results have been summarized in Figure 7.
Figure 6.
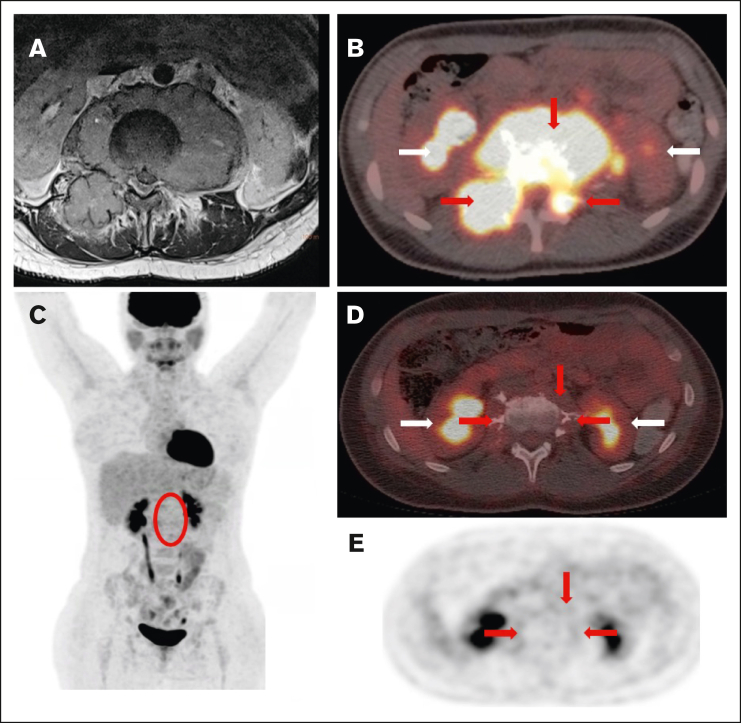
Staging and response assessment. Imaging at staging shows a large paraspinal mass (A, MRI) with intense glucose metabolism (B, 18F-FDG-PET/CT; red arrows = lymphoma; white arrows = normal kidneys). The mass originates from the bone and extends into the neuroforamen and the spinal canal (A-B). After 2 courses of OEPA chemotherapy, 18F-FDG-PET shows a good metabolic response (C, maximum intensity projection; red circle surrounds the slight residual uptake of the former CNS lesion; [D-E] 18F-FDG-PET/CT; same height as panel B; minimal glucose metabolism within the residual mass [red arrows]).
Figure 7.
Summary of baseline and IRA results.
Discussion
Lymphoma involving the CNS has been reported to occur in ∼6% of all patients with NHL,9,10 however, it is very infrequently described in HL.8 Given the rarity of this event, there is little information available about the presentation and response to treatment of patients with HL with CNS involvement at the time of diagnosis. We present, to our knowledge, the largest retrospective study of CNS HL lesions, all of which extended into the extra-axial CNS space, combining data from 3 large cooperative group clinical trials through an international collaborative effort. This report confirms the rarity of CNS involvement with an overall incidence in our evaluated cohort of 0.9%. The incidence of CNS involvement on AHOD1331 was 3.4%, whereas the incidence on the EuroNet-PHL trials was 0.6%; a difference that reflects the different patient risk groups eligible for and enrolled on each study and the methodology for identifying cases within the 2 cooperative groups. As described above, the EuroNet-PHL trials enrolled patients from all risk groups, whereas AHOD1331 enrolled patients defined as high risk.
HL lesions extending into the extra-axial CNS space were highly metabolically active at staging (equivalent to Deauville score 4 or 5). There were 2 CNS lesions that were noted to have a metabolic activity level equivalent to Deauville score 3, one of which received pretreatment with corticosteroids, and the other for which information about corticosteroid pretreatment was unknown. Pretreatment with corticosteroids is known to reduce tracer uptake and may have influenced these results.36 Interestingly, 1 lesion with a Deauville score of 4 and 2 lesions with Deauville score of 5 also received pretreatment with corticosteroids; therefore, pretreatment with corticosteroids did not necessarily correlate with low tracer uptake in our cohort; however, absolute numbers are small, and therefore, it is challenging to draw any clear conclusions from this finding.
CNS involvement in our cohort did not occur in isolation but extended into the extra-axial CNS space from adjacent bone or soft tissue. In our cohort, there were 31 lesions that extended into the spinal canal at the time of initial staging. None of these lesions appeared to infiltrate through the dura into the spinal cord itself; however, some of these lesions caused displacement of the cord through mass effect. This observation, along with the lack of isolated intra-axial CNS lesions, leads us to conclude that it would be extremely unlikely for HL to infiltrate through the dura directly into the CNS parenchyma, in contrast to what is typically observed in NHL.
The majority of CNS lesions demonstrated a rapid response to the first 2 cycles of chemotherapy, with 10.9% of CNS lesions remaining PET positive (Deauville score 4 or 5) at this time point. On AHOD1331, 18.8% and 19.3% of patients remained PET positive after 2 cycles of therapy (standard-care and Bv groups, respectively). The EuroNet trials categorized interim response as adequate or inadequate, in which an adequate response was defined as a partial remission or greater and a negative PET (Deauville score 1 or 2). Of the patients in TG2 or TG3, 60% had an inadequate response after 2 cycles of OEPA, with the vast majority (772/773) remaining PET positive. The different definitions of response (rapid response vs adequate response) and the types of chemotherapy used prevent a direct comparison between the previously published response rates and those of the patients in our cohort.
Two patients demonstrated relapse in the area of the CNS lesions, both which were PET negative at IRA. The patient with a Deauville score of 1 in the CNS lesion had poor IRA overall (Deauville score 5 at other disease sites). The patient with Deauville score of 3 in the CNS lesion had an adequate metabolic response overall (Deauville score 3 at other disease sites). Neither of these CNS lesion received irradiation. Given the small number of patients who demonstrated relapse in the area of a CNS lesion, we are unable to draw conclusions in regard to the clinical significance, but relapse remains a very rare event among this large patient cohort.
Many patients in our cohort (68.8%) had additional E-lesions (pleura, pericardium, skeletal, or lung) as determined by the additional imaging review performed for this study, in contrast to the EuroNet-PHL-C1 trial in which 429 of 1297 intermediate and advanced-stage patients (33%) had evidence of E-lesions.7
Limitations of this study include the retrospective nature of our study, preventing determination of the true incidence of CNS involvement, and the inclusion of patients enrolled on clinical trials, which may have excluded patients if they were clinically unwell and needed interventions emergently. This may have affected our findings in that patients with atypical presentations or intra-axial CNS disease were not likely to be included in this cohort, which could potentially explain why there was no evidence of HL infiltrating through the dura into the CNS parenchyma. In addition, the EuroNet-PHL patients included in this study were identified from those who were noted to have “other” E-lesions or disseminated disease; therefore, there is the potential that some patients with CNS involvement could have been missed if this was not flagged on initial review. Information regarding clinical symptoms at the time of presentation was available for only 11 patients in this cohort, and as a result, we are unable to draw further conclusions about the clinical presentation of HL with extension into the CNS. However, of the patients for whom documentation of clinical symptoms was available, these symptoms clearly correlated with the location that was affected by the CNS lesion (supplemental Materials).
Despite these limitations, our study provides important descriptive information about rare CNS involvement in pediatric classic HL at diagnosis. Among the 3 large cooperative group trials evaluated here, there were no patients who had an intra-axial, parenchymal, CNS lesion at the time of disease presentation. All lesions affecting the CNS were as a result of extension from nearby bone or soft tissue into the extra-axial space. These lesions occurred in patients who had a higher frequency of extranodal lesions, and in general, these lesions responded well to therapy. Our study also highlights that CNS involvement should be considered in patients with HL at the time of diagnosis if suggested clinically by neurologic symptoms or pain.
Conflict-of-interest disclosure: K.M.K., J.F., and S.M.C. served on a scientific advisory board for Seagen. C.M.-K. has received institutional research funding from Merck on a joint clinical trial research project. H.L. is a consultant for Innervate Radiopharmaceuticals. The remaining authors declare no competing financial interests.
Acknowledgments
The authors thank AnnElizabeth White for the medical illustration in Figure 1.
Research reported in this publication was supported by the COG and The National Cancer Institute of the National Institutes of Health under the following awards: St. Baldrick’s Foundation; NCI's National Clinical Trials Network (NCTN) Operations grant U10CA180886; NCTN Statistics and Data Center grants U10CA180899, U10CA98543, and U10CA098413; and the Imaging and Radiation Oncology Core grant U24CA180803. The research funding of the EuroNet-PHL consortium was granted by the Deutsche Krebshilfe e.V., Mitteldeutsche Kinderkrebsforschung-Stiftung für Forschung und Heilung.
The content of this publication is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Authorship
Contribution: C.M.-K., S.M.C., and K.M.K. designed and conducted the trials from which the cases were identified; K.M., H.L., D.S., and L.K. identified cases with CNS involvement and evaluated the images; R.P., J.F., C.M.-K., K.D., and S.K. participated in the evaluation of images; R.P., K.M., J.F., C.M.-K., K.D., D.S., and L.K. determined the data to be collected; L.K. summarized the data; R.P. wrote the manuscript with feedback from all authors; M.P. provided administrative support; and all authors provided input and approval of the final manuscript.
Footnotes
D.S. and L.K. contributed equally to this study.
Original data are available upon reasonable request from the corresponding author, Reena Pabari (reena.pabari@sickkids.ca).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.National Cancer Institute. SEER Cancer Stat Facts: Hodgkin Lymphoma. https://seer.cancer.gov/statfacts/html/hodg.html
- 2.Deutsches Kinderkrebsregister German Childhood Cancer Registry Annual Report 2019. https://www.kinderkrebsregister.de/dkkr/ergebnisse/jahresberichte/jahresbericht-2019.html
- 3.Englund A, Glimelius I, Rostgaard K, et al. Hodgkin lymphoma in children, adolescents and young adults - a comparative study of clinical presentation and treatment outcome. Acta Oncol. 2018;57(2):276–282. doi: 10.1080/0284186X.2017.1355563. [DOI] [PubMed] [Google Scholar]
- 4.Hagleitner MM, Metzger ML, Flerlage JE, et al. Liver involvement in pediatric Hodgkin lymphoma: a systematic review by an international collaboration on Staging Evaluation and Response Criteria Harmonization (SEARCH) for Children, Adolescent, and Young Adult Hodgkin Lymphoma (CAYAHL) Pediatr Blood Cancer. 2020;67(8) doi: 10.1002/pbc.28365. [DOI] [PubMed] [Google Scholar]
- 5.Lewis J, McCarten K, Kurch L, et al. Definition of cortical bone involvement in the staging of newly diagnosed pediatric Hodgkin lymphoma: a report from the International Working Group on Staging Evaluation and Response Criteria Harmonization (SEARCH) Pediatr Blood Cancer. 2020;67(4) doi: 10.1002/pbc.28142. [DOI] [PubMed] [Google Scholar]
- 6.Das J, Ray S, Sen S, Chandy M. Extranodal involvement in lymphoma - a pictorial essay and retrospective analysis of 281 PET/CT studies. Asia Ocean J Nucl Med Biol. 2014;2(1):42–56. [PMC free article] [PubMed] [Google Scholar]
- 7.Mauz-Körholz C, Landman-Parker J, Balwierz W, et al. Response-adapted omission of radiotherapy and comparison of consolidation chemotherapy in children and adolescents with intermediate-stage and advanced-stage classical Hodgkin lymphoma (EuroNet-PHL-C1): a titration study with an open-label, embedded, multinational, non-inferiority, randomised controlled trial. Lancet Oncol. 2022;23(1):125–137. doi: 10.1016/S1470-2045(21)00470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerstner ER, Abrey LE, Schiff D, et al. CNS Hodgkin lymphoma. Blood. 2008;112(5):1658–1661. doi: 10.1182/blood-2008-04-151563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salzburg J, Burkhardt B, Zimmermann M, et al. Prevalence, clinical pattern, and outcome of CNS involvement in childhood and adolescent non-Hodgkin’s lymphoma differ by non-Hodgkin's lymphoma subtype: a Berlin-Frankfurt-Munster Group Report. J Clin Oncol. 2007;25:3915–3922. doi: 10.1200/JCO.2007.11.0700. [DOI] [PubMed] [Google Scholar]
- 10.Akkas BE, Vural GU. The incidence of secondary central nervous system involvement in patients with non-Hodgkin’s lymphoma as detected by 18F-FDG PET/CT. Nucl Med Commun. 2013;34(1):50–56. doi: 10.1097/MNM.0b013e32835aaa48. [DOI] [PubMed] [Google Scholar]
- 11.Scott BJ, Douglas VC, Tihan T, Rubenstein JL, Josephson SA. A systematic approach to the diagnosis of suspected central nervous system lymphoma. JAMA Neurol. 2013;70(3):311–319. doi: 10.1001/jamaneurol.2013.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simonin M, Auperin A, Bertrand Y, et al. In childhood mature B-NHL with CNS disease, patients with blasts in cerebrospinal fluid are at higher risk of failure. Blood Adv. 2020;4(15):3621–3625. doi: 10.1182/bloodadvances.2019001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanneuville B, Janssens A, Lemmerling M, de Vlam K, Mielants H, Veys EM. Non-Hodgkin’s lymphoma presenting with spinal involvement. Ann Rheum Dis. 2000;59(1):12–14. doi: 10.1136/ard.59.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayram N, Yaman Y, Elli M, et al. Unusual clinical presentation of Hodgkin lymphoma in a child: both spinal cord compression and hydronephrosis. J Pediatr Hematol Oncol. 2021;43(6):e900–e902. doi: 10.1097/MPH.0000000000002186. [DOI] [PubMed] [Google Scholar]
- 15.Ghedira K, Matar N, Bouali S, Zehani A, Boubaker A, Jemel H. Hodgkin lymphoma revealed by epidural spinal cord compression. J Spinal Cord Med. 2019;42(3):402–404. doi: 10.1080/10790268.2018.1427519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashi S, Goodwin CR, Ahmed AK, Sciubba DM. Management of extranodal lymphoma of the spine: a study of 30 patients. CNS Oncol. 2018;7(2):CNS11. doi: 10.2217/cns-2017-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salomone G, La Spina M, Belfiore G, et al. Spinal cord compression as tumor onset: an unusual case report of Hodgkin lymphoma in a teenager. BMC Pediatr. 2021;21(1):358. doi: 10.1186/s12887-021-02834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samadian M, Vahidi S, Khormaee F, Ashraf H. Isolated, primary spinal epidural Hodgkin’s disease in a child. Pediatr Neurol. 2009;40(6):480–482. doi: 10.1016/j.pediatrneurol.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Baroni L, Fornaciari S, Predieri B, et al. Paraplegia by spinal cord compression as the initial manifestation of Hodgkin’s disease: a case report. Acta Biomed. 2014;85(2):171–174. [PubMed] [Google Scholar]
- 20.Gupta V, Srivastava A, Bhatia B. Hodgkin disease with spinal cord compression. J Pediatr Hematol Oncol. 2009;31(10):771–773. doi: 10.1097/MPH.0b013e31819c1ff0. [DOI] [PubMed] [Google Scholar]
- 21.Sapozink MD, Kaplan HS. Intracranial Hodgkin’s disease. A report of 12 cases and review of the literature. Cancer. 1983;52(7):1301–1307. doi: 10.1002/1097-0142(19831001)52:7<1301::aid-cncr2820520728>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Higgins SA, Peschel RE. Hodgkin’s disease with spinal cord compression. A case report and a review of the literature. Cancer. 1995;75(1):94–98. doi: 10.1002/1097-0142(19950101)75:1<94::aid-cncr2820750116>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Re D, Fuchs M, Schober T, Engert A, Diehl V. CNS involvement in Hodgkin’s lymphoma. J Clin Oncol. 2007;25(21):3182. doi: 10.1200/JCO.2007.12.5088. [DOI] [PubMed] [Google Scholar]
- 24.Al-Khayat H, Al-Khayat H, Al-Baker O, et al. Cervical radiculopathy secondary to Hodgkin’s lymphoma. Surg Neurol. 2007;67(5):540–543. doi: 10.1016/j.surneu.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 25.Kasper EM, Lam FC, Luedi MM, Zinn PO, Pihan GA. Primary epidural lymphocyte-depleted Hodgkin’s lymphoma of the thoracic spine - presentation of a rare disease variant. BMC Neurol. 2012;12:64. doi: 10.1186/1471-2377-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verhagen MV, Menezes LJ, Neriman D, et al. 18F-FDG PET/MRI for staging and interim response assessment in pediatric and adolescent Hodgkin lymphoma: a prospective study with 18F-FDG PET/CT as the reference standard. J Nucl Med. 2021;62(11):1524–1530. doi: 10.2967/jnumed.120.260059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurch L, Kluge R, Sabri O, et al. Whole-body [18F]-FDG-PET/MRI for staging of pediatric non-Hodgkin lymphoma: first results from a single-center evaluation. EJNMMI Res. 2021;11(1):62. doi: 10.1186/s13550-021-00804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Georgi TW, Stoevesandt D, Kurch L, et al. Optimized whole-body positron emission tomography magnetic resonance imaging sequence workflow in pediatric Hodgkin lymphoma patients. J Nucl Med. 2023;64(1):96–101. doi: 10.2967/jnumed.122.264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castellino SM, Pei Q, Parsons SK, et al. Brentuximab vedotin with chemotherapy in pediatric high-risk Hodgkin’s lymphoma. N Engl J Med. 2022;387(18):1649–1660. doi: 10.1056/NEJMoa2206660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metzger ML, Link MP, Billett AL, et al. Excellent outcome for pediatric patients with high-risk Hodgkin lymphoma treated with brentuximab vedotin and risk-adapted residual node radiation. J Clin Oncol. 2021;39(20):2276–2283. doi: 10.1200/JCO.20.03286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall MD, Terezakis SA, Lucas JT, et al. Radiation therapy across pediatric Hodgkin lymphoma research group protocols: a report from the Staging, Evaluation, and Response Criteria Harmonization (SEARCH) for Childhood, Adolescent, and Young Adult Hodgkin Lymphoma (CAYAHL) Group. Int J Radiat Oncol Biol Phys. 2022;112(2):317–334. doi: 10.1016/j.ijrobp.2021.07.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mauz-Körholz C, Landman-Parker J, Fernández-Teijeiro A, et al. Response-adapted omission of radiotherapy in children and adolescents with early-stage classical Hodgkin lymphoma and an adequate response to vincristine, etoposide, prednisone, and doxorubicin (EuroNet-PHL-C1): a titration study. Lancet Oncol. 2023;24(3):252–261. doi: 10.1016/S1470-2045(23)00019-0. [DOI] [PubMed] [Google Scholar]
- 34.Zijtregtop EAM, Zeal J, Metzger ML, et al. Significance of E-lesions in Hodgkin lymphoma and the creation of a new consensus definition: a report from SEARCH. Blood Adv. 2023;7(20):6303–6319. doi: 10.1182/bloodadvances.2023010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoppe BS, McCarten KM, Pei Q, et al. Importance of central imaging review in a Pediatric Hodgkin Lymphoma trial using positron emission tomography response adapted radiation therapy. Int J Radiat Oncol Biol Phys. 2023;116(5):1025–1030. doi: 10.1016/j.ijrobp.2023.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamane T, Daimaru O, Ito S, et al. Decreased 18F-FDG uptake 1 day after initiation of chemotherapy for malignant lymphomas. J Nucl Med. 2004;45(11):1838–1842. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.