Take Home Message
Optimal follow-up for testicular cancer is not well defined. We prospectively analysed the clinical impact of different imaging modalities for detection of relapse in the large SAGT CCS cohort. We recommend follow-up schedules according to our study findings, with an emphasis on abdominal imaging.
Keywords: Testicular cancer, Follow-up, Germ cell tumours
Abstract
Background and objective
Follow-up for patients with testicular cancer should ensure early detection of relapses. Optimal schedules and minimum requirements for cross-sectional imaging are not clearly defined, and guideline recommendations differ. Our aim was to analyse the clinical impact of different imaging modalities for detection of relapse in a large prospective cohort (Swiss Austrian German Testicular Cancer Cohort Study, SAG TCCS).
Methods
Patients with seminoma or nonseminoma were prospectively enrolled between January 2014 and February 2023 after initial treatment (n = 1175). Follow-up according to the study schedule was individualised for histology and disease stage. Only patients who had received primary treatment were considered. We analysed the total number of imaging modalities and scans identifying relapse and the timing of relapse.
Key findings and limitations
We analysed data for 1006 patients (64% seminoma, 36% nonseminoma); 76% had stage I disease. Active surveillance was the most frequent management strategy (65%). Recurrence occurred in 82 patients, corresponding to a 5-yr relapse-free survival rate of 90.1% (95% confidence interval 87.7–92.1%). Median follow-up for patients without relapse was 38.4 mo (interquartile range 21.6–61.0). Cross-sectional imaging of the abdomen was the most important indicator of relapse 57%, abdominal CT accounting for 46% and MRI for 11%. Marker elevation indicated relapse in 24% of cases. Chest X-ray was the least useful modality, indicating relapse in just 2% of cases.
Conclusions and clinical implications
On the basis of findings from our prospective register, we have adapted a follow-up schedules with an emphasis on abdominal imaging and a reduction in chest X-rays. This schedule might provide additional guidance for clinicians and will be prospectively evaluated as SAG TCCS continues to enrol patients.
Patient summary
We analysed the value of different types of imaging scans for detection of relapse of testicular cancer. We used our findings to propose an optimum follow-up schedule for patients with testicular cancer.
1. Introduction
Testicular cancer is the most common malignancy among men aged 20–40 yr and its incidence has been increasing in recent decades in industrialised countries [1], [2]. The prognosis is excellent, especially in earlier disease stages, with a long-term survival rate of more than 98%. To achieve these outstanding outcomes, optimal follow-up, early detection of relapse, and appropriate treatment according to disease stage and prognosis group are crucial. Follow-up after treatment and on active surveillance usually consists of regular imaging, clinical examination, and analysis of the serum tumour markers α-fetoprotein (AFP; for nonseminoma), human chorionic gonadotropin (β-hCG), and lactate dehydrogenase (LDH).
Cross-sectional imaging with computed tomography (CT) is an essential component of follow-up and is recommended three to four times within the first 2 yr by most guidelines (European Society for Medical Oncology [ESMO], European Association of Urology [EAU], National Comprehensive Cancer Network [NCCN]) [3], [4], [5]. However, repeat CT scans can result in considerable cumulative radiation exposure in this young patient population. Results from a recent randomised trial showed that CT scans of the abdomen can be safely replaced by magnetic resonance imaging (MRI) to reduce radiation exposure in patients with stage I seminoma [6]. X-Ray assessments of the lung are also often part of recommended follow-up, even though their value in detection of pulmonary metastases in testicular cancer is not well defined.
In summary, the optimal follow-up schedule and minimum requirements for cross-sectional imaging for detection of relapses at an early metastatic stage with good prognosis are still an area of uncertainty, and the recommendations available are not based on prospective data.
The Swiss Austrian German Testicular Cancer Cohort Study (SAG TCCS) was set up in 2014 as a prospective register to answer questions on the diagnostic performance and clinical impact of imaging and laboratory tests for detection of relapse and to document late toxicities and secondary malignancies after treatment of testicular cancer.
To date, 1175 patients have been included in this registry. Here we present the first results on the value of different imaging modalities for detection of relapses in testicular cancer.
2. Patients and methods
The SAG TCCS registry was started in January 2014 and prospectively enrols patients with histologically proven seminoma or nonseminoma who completed treatment within 3 mo before enrolment; the protocol is provided in the Supplementary material. Men with malignancies other than testicular cancer within the previous 5 yr were excluded. Patients underwent follow-up according to a predefined study schedule, individualised according to disease stage, histology, risk group, and management for stage I disease; the development of follow-up schedules has been described elsewhere [7] and individual forms are provided in the Supplementary material. The registry protocol recommends following the schedule provided; however, treating physicians were free to amend or add additional imaging in cases with suspicion of relapse. On confirmation of relapse, investigators were asked to report the single most relevant indicator of relapse among the following options: patient history/symptoms, clinical examination, various imaging modalities, and tumour markers.
The registry received approval from institutional review boards and local ethics committees (EKSG13_083; ClinicalTrials.gov NCT02229916). Written informed consent was obtained from each patient before enrolment.
Clinical data on disease characteristics and relapse events were collected, as well as laboratory results, including tumour markers. The imaging modalities used and the results obtained were also documented. The primary objective of the analysis was to determine the diagnostic performance and the clinical impact of conventional radiographs, CT, MRI, abdominal ultrasound, tumour markers, and clinical signs/symptoms for detection of relapse and the time of relapse.
Only patients on follow-up after primary treatment for localised or metastatic disease were considered for this analysis; patients who had already received treatment for relapses were excluded.
2.1. Statistical analysis
Baseline patient characteristics were compared between the seminoma and nonseminoma groups using a χ2 test or nonparametric Wilcoxon rank-sum test, as appropriate.
Frequencies of the single most relevant follow-up indicator were summarised over time using bar plots and were tabulated by stage categories. The total number of examinations performed was summarised by method and by time window, depicted in a stacked bar chart, together with the number of relapses confirmed.
Follow-up time was computed as the time between the primary treatment date and the last visit recorded in the database. Time to relapse was computed as the time between primary treatment and the date of first relapse.
All analyses were conducted using Stata 18 (Stata Corporation, College Station, TX, USA).
3. Results
Between January 2014 and time of data cutoff for the current analysis (February 20, 2023) 1175 patients were enrolled by 21 centres in Switzerland, Austria, and Germany. Of these, 169 patients were excluded because of missing information or follow-up data, so 1006 patients were included in the analysis. Median follow-up for patients without relapse (n = 924) was 38.4 mo (interquartile range 21.6–61.0); information on the primary treatment date was missing for 27 patients.
3.1. Baseline characteristics and survival
Baseline patient characteristics are listed in Table 1. The majority of the patients had seminoma (64%). Men with nonseminoma were significantly younger than those with seminoma (median age 30.6 vs 40.4 yr; p < 0.001). In total, 76% of all patients presented with stage I disease (83% of seminoma and 64% of nonseminoma cases). Of patients initially presenting with metastatic disease, most were classified as having good prognosis (82%; Table 1) according to the International Germ Cell Cancer Collaborative Group (IGCCCG) scheme.
Table 1.
Baseline characteristics of the overall cohort
| Nonseminoma | Seminoma | |
|---|---|---|
| Patients, n (%) | 365 (36) | 641 (64) |
| Median age at entry, yr (IQR) | 30.6 (25.9–37.5) | 40.4 (33.0–50.3) |
| Median follow-up, mo (IQR) a | 39.9 (23.4–61.2) | 37.9 (19.6–60.9) |
| Disease stage at study entry, n (%) | ||
| Stage I | 235 (64) | 531 (83) |
| Stage IIA | 36 (10) | 25 (4) |
| Stage IIB | 22 (6) | 33 (5) |
| Stage IIC | 8 (2) | 24 (4) |
| Stage III | 64 (18) | 28 (4) |
| Extragonadal primary tumour | 10 (3) | 7 (1) |
| IGCCCG prognosis group for patients with metastasis, n (%) b | ||
| Good prognosis | 96 (74) | 101 (92) |
| Intermediate prognosis | 20 (15) | 9 (8) |
| Poor prognosis | 14 (11) | 0 (0) |
| Abnormal AFP at baseline, n (%) | 216 (61) | 15 (2) |
| Data missing | 12 | 22 |
| Median AFP at baseline, per ULN (IQR) | 9.2 (2.8–39.0) | 1.5 (1.3–2.0) |
| Abnormal β-hCG at baseline, n (%) | 205 (58) | 155 (25) |
| Data missing | 14 | 20 |
| Median β-hCG at baseline, per ULN (IQR) | 18.8 (4.7–150.3) | 4.0 (2.0–28.8) |
| Abnormal LDH at baseline, n (%) | 96 (29) | 166 (28) |
| Data missing | 28 | 37 |
| Median LDH at baseline, ULN (median, IQR) | 1.5 (1.2–2.4) | 1.5 (1.2–2.4) |
| Number of markers elevated at baseline, n (%) | ||
| None | 95 (26) | 382 (60) |
| 1 marker | 78 (21) | 186 (29) |
| 2 markers | 137 (38) | 69 (11) |
| 3 markers | 55 (15) | 4 (1) |
| Median tumour size, cm (IQR) | 3.3 (2.2–4.7) | 3.6 (2.5–5.1) |
| Tumour size unknown, n (%) | 13 (4) | 15 (2) |
| Lymphovascular invasion, n (%) | 164 (47) | 135 (22) |
| Data missing | 18 | 30 |
| Rete testis infiltration, n (%) | 103 (31) | 301 (49) |
| Data missing | 29 | 29 |
| Treatment received, n (%) | ||
| Active surveillance | 140 (38) | 358 (56) |
| Radiotherapy (± chemotherapy) | 2 (0.5) | 26 (4) |
| Adjuvant treatment | 1 (0.3) | 3 (0.5) |
| Treatment for metastatic disease | 1 (0.3) | 23 (4) |
| Chemotherapy | 214 (59) | 255 (40) |
| Adjuvant treatment | 92 (25) | 171 (27) |
| Treatment for metastatic disease | 122 (33) | 84 (13) |
| Surgery (retroperitoneal lymph node dissection) | 9 (2) | 2 (0) |
IQR = interquartile range; LDH = lactate dehydrogenase; AFP = α-fetoprotein; hCG = human chorionic gonadotropin; IGCCCG = International Germ Cell Cancer Collaborative Group; ULN = upper limit of normal.
Calculated for patients without relapse.
Percentages provided in relation to the number of patients with metastatic disease; all other percentages relate to the overall population.
Most patients with stage I disease were managed with active surveillance (65%). In the seminoma stage I group, 32% of patients received adjuvant chemotherapy (1 cycle of carboplatin). For nonseminoma stage I, adjuvant treatment (1 cycle of bleomycin, etoposide, and cisplatin in 84%) was the treatment of choice in 39% of patients (Table 1). Other management strategies such as radiotherapy and surgery were used in just 3% and 1% of cases, respectively.
The 5-yr relapse-free and overall survival rates for the overall cohort were 90.1% (95% confidence interval [CI] 87.7–92.1%) and 99.5% (95% CI 98.5–99.8), respectively. In total, four deaths were observed, only one of which was disease-related. One other fatal event was attributed to gastric lymphoma in a patient with stage I seminoma on active surveillance. The two other deaths were attributed to SARS-CoV-2 infection and a cardiac event, respectively. The latter was documented approximately 1 yr after completion of chemotherapy (4 cycles of etoposide and cisplatin) for stage IIB seminoma in a 54-yr-old smoker with a history of cardiovascular disease.
3.2. Relapse characteristics
In total, 82 patients experienced an imaging-proven relapse of their testicular cancer. The majority of patients (88%) were relapses after initial stage I disease; details of the stage I treatment approaches are provided in Supplementary Table 1 and Supplementary Figure 2.
Of patients with information on IGCCCG prognostic group at relapse, 87% presented with good, 7% with intermediate, and 2% with poor prognosis features (Table 2).
Table 2.
Characteristics of relapse
| Nonseminoma | Seminoma | |
|---|---|---|
| Patients, n (%) | 26 (32) | 56 (68) |
| Median time from semicastration to relapse, mo (IQR) a | 4.6 (3.3–12.7) | 12.0 (6.9–18.1) |
| Initial disease stage, n (%) | ||
| Stage I | 20 (77) | 52 (93) |
| Stage IIA | 1 (4) | 0 |
| Stage IIB | 1 (4) | 2 (4) |
| Stage IIC | 0 | 0 |
| Stage III | 4 (15) | 2 (4) |
| Disease stage at relapse, n (%) b | ||
| Stage I S | 0 | 1 (3) |
| Stage IIA | 5 (33) | 13 (34) |
| Stage IIB | 3 (20) | 15 (40) |
| Stage IIC | 0 | 3 (8) |
| Stage III | 7 (47) | 6 (16) |
| Data missing | 0 | 1 |
| IGCCCG prognosis group at relapse, n (%) b | ||
| Good prognosis | 14 (93) | 33 (89) |
| Intermediate prognosis | 0 | 4 (11) |
| Poor prognosis | 1 (7) | 0 |
| Data missing | 0 | 2 |
IQR = interquartile range; IGCCCG = International Germ Cell Cancer Collaborative Group.
Excluding patients who entered the study <2 yr before analysis or lost to follow-up after <2 yr.
Only available for 54 patients who had a second entry in the trial database after treatment of relapse.
Among the whole cohort, five contralateral primary tumours were detected during follow-up, four documented by ultrasound and one found on testis palpation, corresponding to a 5-yr contralateral tumour–free survival rate of 99.1% (95% CI 97.6–99.7%).
3.3. Indicators of relapse
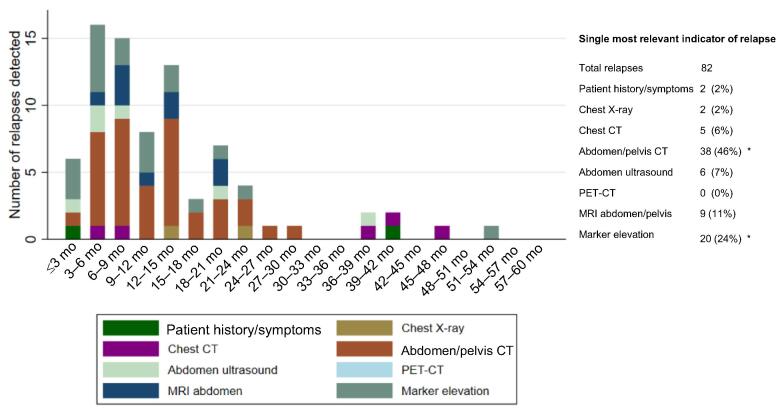
The first indicator of relapse and the corresponding time point were available for 80 patients (96%) with relapse; the results are depicted in Figure 1. Most relapses were detected on CT cross-sectional imaging of the abdomen (46%). MRI scans of the abdomen provided the first indication of relapse in 11% of cases. Patient history or symptoms gave the first indication of relapse in 2% of patients, one early and one late relapse after >3 yr of follow-up. CT scans of the chest revealed evidence of relapse in 6% of patients, which occurred in two patients in the first year of follow-up and in three others as late relapses after year 3 (Supplementary Table 2).
Fig. 1.
Single most relevant indicator of relapse. Investigators had to choose the single most relevant indicator that led to diagnosis of relapse. Indicators and their frequencies are depicted over time as absolute numbers and percentages. CT = computed tomography; MRI = magnetic resonance imaging; PET = positron emission tomography. * One patient with information available on the relapse indicator but no time point was excluded.
Despite being frequently used as part of follow-up, chest X-ray was the least useful diagnostic modality, providing indication of relapse in only 2% of the overall cohort.
Modalities indicating relapse and time points in relation to histology, disease stage, and stage I management are shown in Supplementary Figures 2 and 3.
For men with stage I seminoma, imaging of the chest (X-ray) indicated relapse in only one patient (2%). Apart from seven (13%) additional cases with marker elevation providing the first indication, other relapses in this population were almost exclusively detected by abdominal imaging (n = 43; 83%), most frequently via cross-sectional imaging with CT (n = 30; 58%) or MRI (n = 8; 15%), but also via abdominal ultrasound in five patients (10%).
The number of relapses among patients with stage I nonseminoma at high risk of relapse was limited, most likely because of the widespread use of adjuvant chemotherapy in this population. The first indication of relapse was provided by tumour markers in three cases (60%) and cross-sectional abdominal imaging in two cases (40%).
For patients with stage I nonseminoma at low risk of relapse, marker elevation (six cases; 40%) was the most frequent indicator of relapse, followed by abdominal CT (five cases; 33%).
The number of patients with relapse after initial treatment for metastatic disease was low (4%; Supplementary Table 1). Marker elevation (three cases; 38%) and a CT scan of the chest (three cases; 38%) were the most frequent indicators of relapse for these patients. Four of these events were late relapses detected after more than 2 yr of follow-up (Supplementary Fig. 3).
3.4. Development of new follow-up schedules
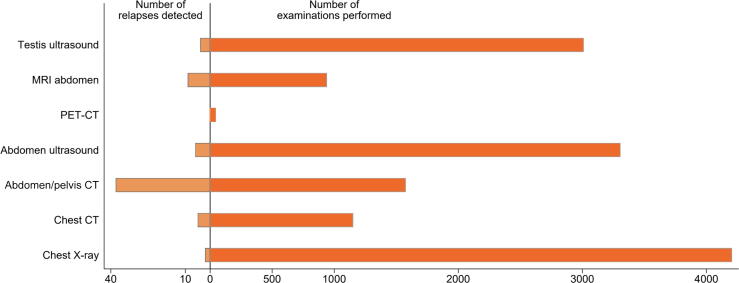
We analysed the total number of examinations performed with each imaging modality for the entire cohort and the follow-up time in relation to the number of relapses detected with each modality (Fig. 2). Chest X-ray, abdominal ultrasound, and ultrasound of the contralateral testis were by far the most frequently performed examinations at 4139, 3237, and 2870, respectively. However, the yield of relapse events detected with these examinations was minimal, with positivity rates (relapses found/total number of scans performed) of 0.05%, 0.19%, and 0.14%, respectively.
Fig. 2.
Total number of scans performed in relation to relapses documented. CT = computed tomography; MRI = magnetic resonance imaging; PET = positron emission tomography.
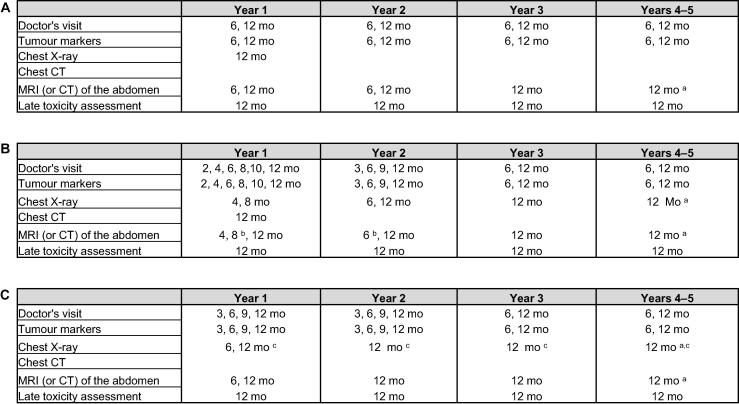
On the basis of the results from our prospective analysis, we have amended the follow-up schedules for future patients enrolled in SAG TCCS, as shown in Figure 3. Examinations not resulting in detection of a relevant number of relapse events (positivity rate <0.5%; Supplementary Fig. 4) were removed from the previous schedules. Modalities that detected the greatest number of relapse events were prioritised and ordered according to the occurrence of relapses over time. Emphasis in the new schedules is now on abdominal imaging with a reduction in chest imaging, especially chest X-rays. On the basis of data from TRISST [6] and other trials on MRI [8], [9], cross-sectional imaging of the abdomen with MRI is now recommended instead of CT. Routine ultrasound of the contralateral testis in the absence of clinical suspicion has been removed.
Fig. 3.
Proposed new follow-up schedules. (A) Patients with stage I seminoma on active surveillance or after adjuvant carboplatin. (B) Patients with low- or high-risk stage I nonseminoma on active surveillance. (C) Patients with stage I nonseminoma after adjuvant chemotherapy and patients with metastatic seminoma/nonseminoma with good prognosis (International Germ Cell Cancer Collaborative Group classification) after treatment completion. Late toxicity checks include weight, blood pressure, kidney function, testosterone levels, lipid profile, and assessment for secondary malignancies. CT = computed tomography; MRI = magnetic resonance imaging. a Only in year 5 (60 mo). b Only in cases with high-risk disease/lymphovascular invasion. c Chest CT instead of X-ray in cases with lung metastases at diagnosis.
4. Discussion
Optimised follow-up for patients with testicular cancer should ensure detection of potential relapses at an early metastatic stage for the highest chance of cure and to potentially minimise the treatment burden. At the same time, unnecessary radiation exposure should be avoided to protect these young patients from potential radiation-induced secondary malignancies later in life.
There are remarkable differences in recommended follow-up schedules among clinical practice guidelines published by the EAU/ESMO, NCCN, and other national and multinational specialist groups for testicular cancer [10] and most are not based on any prospective data. SAG TCCS was explicitly set up to answer questions on the diagnostic performance and clinical impact of different follow-up modalities. Here we provide the first SAG TCCS analysis of the value of different imaging modalities for detection of relapses in testicular cancer.
Cross-sectional imaging of the abdomen was by far the most important indicator of relapse (57% of cases), in line with previous retrospective data [11], [12]. In our cohort, abdominal CT was still much more widely used than MRI. However, as recent data from TRISST and other trials [6], [8], [9] have demonstrated that MRI scans of the abdomen are not inferior to CT scans and do not lead to an increase in the number of relapses at higher stages, this is likely to change in the future.
Imaging of the chest provided an indication of relapse in only a minority of patients in our cohort, with X-ray of the chest being the least useful imaging modality. Only a single seminoma relapse was indicated first by chest imaging. This observation confirms the trend whereby regular imaging with chest X-rays has been removed from most guidelines for follow-up of patients with stage I seminoma. Recent reports have demonstrated that reducing the frequency of chest imaging during active surveillance for stage I disease is safe and does not lead to upstaging at relapse or to more cancer-related deaths [13].
Elevation of tumour markers still accounted for 24% of the first indications of relapse in our cohort, making it the second most important tool after imaging of the abdomen. Measurement of tumour markers therefore still has a role in follow-up, although attention must be paid to the potential for false-positive results [14], [15], [16], [17].
Our initial follow-up schedules also included a recommendation for annual ultrasound of the contralateral testis. However, only five cases of a contralateral tumour were documented in our cohort, four of which were detected via ultrasound. Annual routine contralateral ultrasound was therefore removed from the follow-up schedules for future patients and is now only recommended for cases with clinical suspicion of a contralateral tumour.
Limitations of our study include an imbalance between the two histological groups, with 68% of the relapses occurring in the seminoma group. The data for nonseminoma cases and for patients with relapse after treatment of metastatic disease must therefore be interpreted with appropriate caution because of the limited numbers. However, this distribution reflects both the usual presentation in clinics, where stage I seminoma is the most frequent presentation (53% of our cohort), and the use of effective adjuvant treatment for stage I nonseminoma. Adjuvant treatment was frequently used in our cohort and given to 78% of high-risk/LVI positive NS stage I patients.
We also had very low numbers of patients with intermediate- or poor-prognosis metastatic disease. Therefore, no clear recommendations can be made for this patient group and follow-up needs to be individualised.
Another limitation of our study is that confirmation of relapse was generally based on unequivocal signs on imaging, with additional biopsies left to the discretion of the treating physician. Besides, as our cohort was not randomised, we could not conduct direct comparisons for the different imaging modalities.
A strength of our data is the consistency of follow-up owing to the protocol-recommended schedules. Moreover, SAG TCCS provides prospective and contemporary evaluation of the diagnostic tools used for detection of relapse, whereas data from other large cohorts have mostly been retrospective so far [11], [12], [13].
5. Conclusions
We prospectively assessed the value of different imaging modalities for detection of relapse and the timing of disease recurrence in the large SAG TCCS cohort of >1000 patients. On the basis of our data, we propose updated schedules that can guide follow-up for patients with testicular cancer in addition to the common guideline recommendations.
SAG TCCS will continue to enrol patients and prospectively evaluate relapse patterns and outcome data after implementation of the new follow-up schedules. For future patients, these will also include prospective assessment of miR-371a-3p [18], [19], [20] to further evaluate the role of microRNA as a potentially new and more accurate biomarker for even earlier detection of relapse.
Author contributions: Stefanie Fischer had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: S. Fischer, Gillessen, Cathomas, Rothermundt.
Acquisition of data: S. Fischer, Terbuch, Cathomas, Schmid, Zihler, Müller, Fankhauser, Hirschi-Blickenstorfer, Kluth, Seifert, Templeton, Mingrone, Ufe, N. Fischer, Beyer, Woelky, Omlin, Vogl, Hoppe, Kamradt, Rothermundt.
Analysis and interpretation of data: S. Fischer, Cathomas, Rothermundt.
Drafting of the manuscript: S. Fischer, Rothermundt.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Stalder.
Obtaining funding: Rothermundt.
Administrative, technical, or material support: Rothermundt.
Supervision: Gillessen, Cathomas.
Other: None.
Financial disclosures: Stefanie Fischer certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This study was supported by Ostschweizer Stiftung für klinische Krebsforschung, VB Bank, Alfred und Annelies Sutter-Stöttner Stiftung, Dr. Hans Altschüler Stiftung, Hanne Liebermann-Stiftung, Padella Stiftung, Stiftung zur Krebsbekämpfung and Anna-Lisa Stiftung. The sponsors played no direct role in the study.
Associate Editor: M. Carmen Mir
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2024.08.008.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Nigam M., Aschebrook-Kilfoy B., Shikanov S., Eggener S. Increasing incidence of testicular cancer in the United States and Europe between 1992 and 2009. World J Urol. 2015;33:623–631. doi: 10.1007/s00345-014-1361-y. [DOI] [PubMed] [Google Scholar]
- 2.Gurney J.K., Florio A.A., Znaor A., et al. International trends in the incidence of testicular cancer: lessons from 35 years and 41 countries. Eur Urol. 2019;76:615–623. doi: 10.1016/j.eururo.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oldenburg J., Berney D.M., Bokemeyer C., et al. Testicular seminoma and non-seminoma: ESMO-EURACAN clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:362–375. doi: 10.1016/j.annonc.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Laguna MP, Albers P, Algaba F, et al. EAU guidelines on testicular cancer. Arnhem, The Netherlands: European Association of Urology; 2022.
- 5.National Comprehensive Cancer Network. Treatment by cancer type. https://www.nccn.org/guidelines/category_1.
- 6.Joffe J.K., Cafferty F.H., Murphy L., et al. Imaging modality and frequency in surveillance of stage I seminoma testicular cancer: results from a randomized, phase III, noninferiority trial (TRISST) J Clin Oncol. 2022;40:2468–2478. doi: 10.1200/JCO.21.01199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cathomas R., Helbling D., Stenner F., et al. Interdisciplinary evidence-based recommendations for the follow-up of testicular cancer patients: a joint effort. Swiss Med Wkly. 2010;140:356–369. doi: 10.4414/smw.2010.12993. [DOI] [PubMed] [Google Scholar]
- 8.Larsen S.K.A., Løgager V., Bylov C., et al. Can whole-body MRI replace CT in management of metastatic testicular cancer? A prospective, non-inferiority study. J Cancer Res Clin Oncol. 2023;149:1221–1230. doi: 10.1007/s00432-022-03996-1. [DOI] [PubMed] [Google Scholar]
- 9.Laukka M., Mannisto S., Beule A., Kouri M., Blomqvist C. Comparison between CT and MRI in detection of metastasis of the retroperitoneum in testicular germ cell tumors: a prospective trial. Acta Oncol Stockh Swed. 2020;59:660–665. doi: 10.1080/0284186X.2020.1725243. [DOI] [PubMed] [Google Scholar]
- 10.Kaufmann E., Antonelli L., Albers P., et al. Oncological follow-up strategies for testicular germ cell tumours: a narrative review. Eur Urol Open Sci. 2022;44:142–149. doi: 10.1016/j.euros.2022.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aparicio J., García Del Muro X., Maroto P., et al. Patterns of relapse and treatment outcome after active surveillance or adjuvant carboplatin for stage I seminoma: a retrospective study of the Spanish Germ Cell Cancer Group. Clin Transl Oncol. 2021;23:58–64. doi: 10.1007/s12094-020-02393-9. [DOI] [PubMed] [Google Scholar]
- 12.Kollmannsberger C., Tandstad T., Bedard P.L., et al. Patterns of relapse in patients with clinical stage I testicular cancer managed with active surveillance. J Clin Oncol. 2015;33:51–57. doi: 10.1200/JCO.2014.56.2116. [DOI] [PubMed] [Google Scholar]
- 13.Gariscsak P.J., Anson-Cartwright L., Atenafu E.G., et al. Safety of minimizing intensity of follow-up on active surveillance for clinical stage I testicular germ cell tumors. Eur Urol Open Sci. 2022;40:46–53. doi: 10.1016/j.euros.2022.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer S., Rothermundt C., Stalder O., et al. The value of tumour markers in the detection of relapse—lessons learned from the Swiss Austrian German Testicular Cancer Cohort Study. Eur Urol Open Sci. 2023;50:57–60. doi: 10.1016/j.euros.2023.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris M.J., Bosl G.J. Recognizing abnormal marker results that do not reflect disease in patients with germ cell tumors. J Urol. 2000;163:796–801. [PubMed] [Google Scholar]
- 16.Mohler J.L., Siami P.F., Flanigan R.C. False positive beta-human chorionic gonadotropin in testicular cancer. Urology. 1987;30:252–254. doi: 10.1016/0090-4295(87)90247-0. [DOI] [PubMed] [Google Scholar]
- 17.Albany C., Einhorn L. Pitfalls in management of patients with germ cell tumors and slight elevation of serum α-fetoprotein. J Clin Oncol. 2014;32:2114–2115. doi: 10.1200/JCO.2014.56.0607. [DOI] [PubMed] [Google Scholar]
- 18.Dieckmann K.P., Radtke A., Geczi L., et al. Serum levels of microRNA-371a-3p (M371 test) as a new biomarker of testicular germ cell tumors: results of a prospective multicentric study. J Clin Oncol. 2019;37:1412–1423. doi: 10.1200/JCO.18.01480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leão R., Albersen M., Looijenga L.H.J., et al. Circulating microRNAs, the next-generation serum biomarkers in testicular germ cell tumours: a systematic review. Eur Urol. 2021;80:456–466. doi: 10.1016/j.eururo.2021.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Fankhauser C.D., Christiansen A.J., Rothermundt C., et al. Detection of recurrences using serum miR-371a-3p during active surveillance in men with stage I testicular germ cell tumours. Br J Cancer. 2022;126:1140–1144. doi: 10.1038/s41416-021-01643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.