Highlights
-
•
There is limited research on the cosmetic outcome of different PBI techniques.
-
•
Several techniques are available for PBI, including IOERT and EB-APBI.
-
•
5-year cosmetic outcome was evaluated by patients, physicians and software.
-
•
IOERT and EB-APBI yield comparable, satisfactory cosmetic outcomes.
Keywords: Breast cancer, Accelerated partial breast irradiation, External beam radiotherapy, Intraoperative electron radiotherapy, Cosmetic outcome
Abstract
Purpose
The aim of this study is to evaluate the cosmetic outcome among early stage breast cancer patients who underwent accelerated partial breast irradiation with either intraoperative electron radiotherapy (IOERT) or photon external beam radiotherapy (EB-APBI).
Materials and methods
This prospective multicenter cohort study enrolled women aged 60 years and older who underwent breast-conserving therapy. Following breast-conserving surgery, patients were treated with either IOERT or EB-APBI. Cosmetic outcome was evaluated over a 5 year follow-up period using both subjective scoring by patients and physicians, as well as objective scoring using BCCT.core software. Differences between treatments over time were described with mixed model analyses.
Results
A total of 241 patients treated with IOERT and 164 patients treated with EB-APBI were eligible for cosmetic analysis. In both groups, the majority of patients reported a satisfactory cosmetic outcome, with no significant differences between treatments over time (p = 0.538). This was also observed by physicians, with satisfactory outcomes ranging from 94 % (170/181) to 91 % (69/76) over time in the IOERT group and from 93 % (124/133) to 95 % (54/57) in the EB-APBI group (p = 0.579). BCCT.core analysis returned satisfactory cosmetic outcomes in 75 % (54/72) of the IOERT patients at 3 years and in 77 % (20/26) at 5 years. These numbers were 86 % (72/84) and 90 % (36/40) for the EB-APBI patients, with no significant differences between treatment over time (p = 0.834).
Conclusion
Regarding the cosmetic results, IOERT and EB-APBI yield comparable and satisfactory outcomes over 5 years follow-up in the treatment of early stage breast cancer.
Introduction
Achieving an optimal cosmetic outcome following treatment for breast cancer is widely recognized as an important aspect of care. Breast-conserving therapy (BCT) was introduced as a feasible alternative to mastectomy, with the objective of achieving equivalent survival and local control rates while preserving an acceptable aesthetic outcome [1]. Adjuvant radiotherapy forms an integral part of BCT [2], but is also known to have potential impact on the cosmetic result. Large treatment volumes have been associated with increased treatment burden, high toxicity rates and unfavorable cosmetic outcome [3], [4]. Therefore, accelerated partial breast irradiation (APBI) has been thoroughly investigated as an alternative to whole breast irradiation (WBI), as it targets only the tumor bed area. Numerous studies have shown that APBI achieves comparable local control rates to WBI in low-risk patients, while reducing treatment burden and improving cosmetic outcome [5], [6], [7].
Various techniques are available for the administration of APBI. External-beam APBI (EB-APBI) is a non-invasive method that is widely available. However, due to the external application, the skin is subject to radiation exposure, potentially resulting in skin toxicity. In contrast, intraoperative electron radiotherapy (IOERT) involves the delivery of a single dose of radiation directly to the lumpectomy cavity immediately after surgery. As a result, healthy skin and subcutaneous tissue are spared [8]. Additionally, IOERT offers a significant advantage in terms of reduced treatment time for patients. On the downside, its application requires a larger incision due to the placement of a protection disk in the surgical cavity, that may negatively impact postoperative toxicity and cosmetic outcome.
The evaluation of the benefits and drawbacks of different APBI modalities offers valuable insights for both patients and clinicians, enabling them to make more informed decisions regarding the optimal treatment strategy. In this context, a prospective cohort study was initiated to evaluate outcomes after IOERT and EB-APBI. The previous publications have highlighted limited toxicity and excellent quality of life for both treatment groups [9], [10]. The oncological outcomes, however, showed unexpectedly high rates of local recurrences in the IOERT group, and acceptable rates in the EB-APBI group [11]. In the current analysis, the cosmetic results of these treatment modalities are reported.
Materials and methods
Study design and participants
Detailed information on the study design, patient selection and treatment has been reported previously [10]. This prospective cohort study was conducted at three centers in the Netherlands, investigating the clinical outcomes following IOERT and EB-APBI. Patients received treatment based on the center to which they were referred: those at Haaglanden Medical Center received IOERT, while patients at Haga Teaching Hospital and Isala Clinics received EB-APBI. Patients who, although intended, did not undergo IOERT but were still eligible for EB-APBI, were treated with EB-APBI in the Haaglanden Medical Center. As Isala Clinics did not participate in the cosmetic sub study, these patients were excluded from the current analysis. Time of inclusion was between January 2011 and November 2016.
Patient eligibility was determined based on classification as low to intermediate risk according to the 2010 GEC-ESTRO recommendations [6]. Inclusion criteria were female patients aged ≥ 60 years with invasive or in situ breast tumors measuring ≤ 30 mm (classified as cT1 with any receptor status or cT2 with ER/PR-positive and HER2-negative status), clinical N0 status, and eligibility for breast-conserving therapy with a sentinel node procedure. Exclusion criteria were multicentric or multifocal tumors, extensive intraductal or lymphovascular invasion, positive surgical margins, > pN1a after sentinel node procedure (or a positive sentinel node perioperatively in the case of IOERT), neoadjuvant chemotherapy, previous malignancy in the past 5 years, and previous radiation on the ipsilateral breast.
Additionally, patients were included for current analysis, if data regarding the cosmetic result were available from at least one of the three cosmetic assessments. Patients with previous or synchronous treatment of the contralateral breast, either for a malignancy or a benign lesion, were excluded. Patients initially included in the analysis were subsequently excluded from ongoing assessments in case of an ipsilateral breast tumor recurrence (IBTR), a new contralateral malignancy or death within the 5 years of follow-up.
The study was approved by the local medical ethical committee (10-042 METC ZuidwestHolland; NTR2931). All patients gave written informed consent.
Treatment
Breast-conserving surgery at center of inclusion was performed according to at least level 1 oncoplastic surgery principles [12]. IOERT was administered directly after surgery (Mobetron, INTRAOP, USA). A protection disk was placed below the surgical cavity in front of the pectoralis muscle to protect the organs at risk [13]. A total dose of 23.3 Gy (21 Gy prescribed at the 90 % isodose) was delivered using high-energy electron (6–12 MeV) beam radiation therapy. EB-APBI was administered with photons (4–10 MV) within six weeks after wide local excision in 10 daily fractions of 3.85 Gy, 5 days a week. Either Intensity Modulated Radiotherapy or 3D-Conformal Radiotherapy was used. Patients received systemic therapy when indicated, based on national Dutch guidelines.
Outcomes
The current study was intended to evaluate the cosmetic outcome after either IOERT or EB-APBI, by assessing the cosmetic result as scored by patients, physicians and digital software over a 5 year follow-up period.
Cosmesis
Patients received questionnaires in which they could rate the cosmetic result of the treated breast in comparison to the contralateral breast on a numerical scale of 0 to 10 (0 indicating a poor result, 10 indicating an excellent result). Additionally, patients could assess the extent of nipple deformation, breast asymmetry, skin discoloration or retraction and breast firmness on the same scale, where 0 signified no alteration and 10 a severe alteration. Questionnaires were send to patients 3, 6, 12, 24, 36 and 60 months after completion of radiotherapy. Cosmetic evaluation at baseline was not performed. As the last fraction of EB-APBI was given approximately 7 weeks after surgery, the time points differ between treatment groups. Only questionnaires completed within specific time ranges were included for analysis: 1.5–4.5 months for 3 months, 4.5–9 for 6, 9–16 for 12, 16–30 for 24, 30–42 for 36 and 54–66 for 60 months.
In the same time period, radiation oncologists rated the cosmetic outcome during follow-up visits by comparing the treated breast with the contralateral breast on a categorical 5 point-scale (excellent, good, fair, poor, intervention needed). For analysis, “poor” and “intervention needed” were combined into the score “poor”, resulting in a 4-point categorical scale.
Objective analysis of the cosmetic result was performed using digital software BCCT.core [14]. Digital photographs were obtained in anteroposterior view at 3 and 5 years follow-up. Photographs were evaluated by the software, which returned a cosmetic score on a categorical 4-point scale (excellent, good, fair, poor) based on symmetry, skin discoloration and scar appearance.
Toxicity
Up till 3 months after surgery, acute toxicity was scored by radiation oncologists on a categorical 5-point scale. However, as this deviates from the CTCAE v3.0, it was decided to retrospectively score toxicity according to the CTCAE v3.0 [15]. Detailed information has been reported previously [10]. Adverse events included wound infection, seroma, wound dehiscence, hematoma and hemorrhage with surgery.
During the 5 year follow-up period, the area of fibrosis was measured with a ruler by physical examination and scored on a categorical 5 point-scale (varying from none to the whole breast affected).
Statistical analysis
Descriptive and explorative analyses were performed using statistical software R version 4.3.3. Clinical and pathological characteristics were described and compared using either the Mann-Whitney U test or chi-square test, as deemed appropriate. Postoperative toxicity was compared using the Fisher’s exact test. The patient reported cosmetic outcome was presented as the mean score along with the 95 % confidence interval. Differences in cosmetic outcome between treatments over time were assessed using a linear mixed model, with patients included as random effects and time, treatment, and the interaction between time and treatment as fixed effects. Time was included in the model as a numerical variable in months. For mixed model analysis of the cosmetic outcome as evaluated by physicians and BCCT.core software, results were dichotomized into “good and excellent” or “fair and poor”. To assess these results over time, generalized linear mixed models were employed using the same methods as in the linear mixed models. The threshold for statistical significance was set at p < 0.05.
Results
Patient characteristics
A total of 444 patients were enrolled from two participating centers, with 405 patients meeting the criteria for cosmetic analysis. Among these, 241 were assigned to the IOERT group, and 164 to the EB-APBI group (Supplementary Fig. 1). Ten patients initially intended for IOERT but unable to receive it due to logistical constraints were treated with EB-APBI. The baseline characteristics are presented in Table 1. The median age was 69 years in the IOERT group and 68 years in the EB-APBI group. In both groups, the majority of patients had a luminal tumor subtype, and most tumors were classified as pT1 and pN0. A significantly higher number of patients in the EB-APBI group had a high body mass index (p = 0.002).
Table 1.
Clinical and pathological baseline characteristics. Data are shown as median (interquartile range) or absolute numbers (percentage). * Smoking defined as actively smoking or stopped smoking in the last 12 months. ** Tumor size as measured on mammography or MRI. *** Excision volume based on pathologic measurements.
| IOERT, n = 241 |
EB-APBI, n = 164 |
p-value | |||
|---|---|---|---|---|---|
| n; median | %; IQR | n; median | %; IQR | ||
| Age (years) | 69 | 65–73 | 68 | 64–73 | 0.846 |
|
Body mass index (kg/m2) < 25 ≥ 25 Unknown |
97 144 1 |
40 60 0 |
38 126 1 |
23 77 1 |
0.002 |
|
Diabetes mellitus Yes No |
28 213 |
12 88 |
18 146 |
11 89 |
0.968 |
|
Smoking* Yes No |
83 158 |
34 66 |
67 97 |
41 59 |
0.227 |
| Tumor size** (mm) | 12 | 9–16 | 11 | 8–17 | 0.862 |
| Excision volume (mL)*** | 104 | 63–154 | 98 | 63–138 | 0.592 |
|
Tumor location Medial Central/lateral |
59 182 |
25 75 |
43 121 |
26 74 |
0.780 |
|
pT stage Tis T1 T2 |
13 204 24 |
5 85 10 |
22 118 24 |
13 72 15 |
0.004 |
|
pN stage pN0 ≥ pN1mi Unknown |
213 18 10 |
88 8 4 |
138 5 21 |
84 3 13 |
0.001 |
|
Grade 1 2 3 Unknown |
75 98 49 19 |
31 41 20 8 |
49 67 23 25 |
30 41 14 15 |
0.069 |
|
Tumor subtype Luminal HER2+ Triple-negative Unknown |
200 12 13 16 |
83 5 5 7 |
120 9 6 29 |
73 6 4 18 |
0.006 |
During the follow-up period, 46 patients in the IOERT group were excluded from cosmetic analysis onwards because of an IBTR (26), death (14) or new contralateral malignancy (6). In the EB-APBI group, these numbers were 7, 9 and 2, respectively.
Cosmetic outcome by patients
The response rates for the patient questionnaires in the IOERT group were 96 % at 3 months, 94 % at 6 months, 92 % at 1 year, 87 % at 2, 82 % at 3 and 70 % at 5 years. The corresponding response rates for the EB-APBI group were 94 %, 92 %, 92 %, 84 %, 81 % and 71 %, respectively. In both groups, 6 patients returned their questionnaires outside the predefined time windows, consequently their results were excluded from analysis.
The mean cosmetic score at 3 months after radiotherapy was 7.24 [95 %CI 7.00–7.48] in the IOERT group and 7.29 [95 %CI 7.01–7.58] in the EB-APBI group. At 5 years, the scores were 7.67 [95 %CI 7.37–7.96] and 7.57 [95 %CI 7.25–7.89], respectively. Fig. 1 displays the mean scores of the patients at different time points. Mixed model analysis showed no statistically significant differences between treatment groups in cosmetic outcome throughout the 5 year follow-up period (p = 0.538).
Fig. 1.
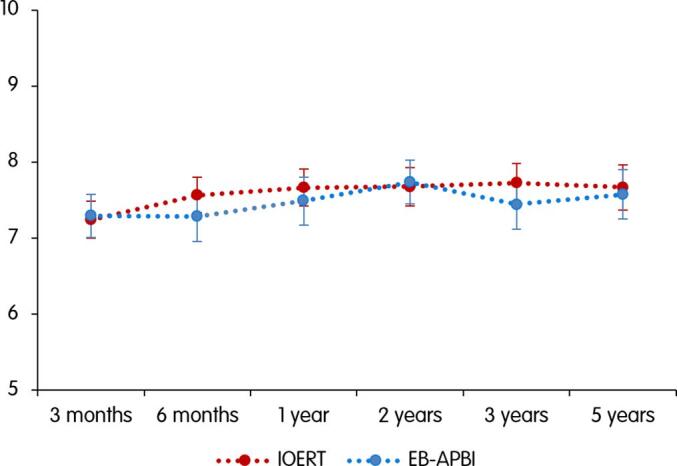
Cosmetic result rated by the patients at different time points. Patients could score the result on a numerical scale from 0 to 10. A score near 0 indicates a poor result and a score near 10 an excellent result. The scores are displayed as means along with the 95% confidence intervals.
In the subcategories on the questionnaires, patients rated similar degrees of asymmetry, skin discoloration and breast firmness. In the EB-APBI group, patients reported a slightly worse degree of skin discoloration and retraction (Supplementary Fig. 2).
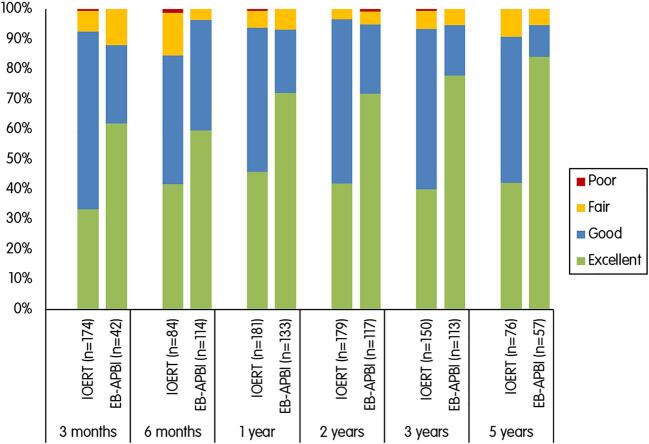
Cosmetic outcome by physicians
Physicians filled out the cosmetic forms for the IOERT group in 72 % of the patients at 3 months, in 35 % at 6 months, 75 % at 1 year, 74 % at 2, 62 % at 3 and 32 % at 5 years. For EB-APBI, these numbers were 26 %, 70 %, 81 %, 71 %, 69 % and 35 %, respectively.
In the IOERT group, a good to excellent outcome as rated by the physicians ranged from 94 % (170/181) at 1 year to 91 % (69/76) at 5 years. The EB-APBI group had similar findings, with a good to excellent outcome in 93 % (124/133) at 1 year and 95 % (54/57) at 5 years.
Overall, the generalized linear mixed model showed no significant differences between the IOERT and EB-APBI groups regarding a good to excellent cosmetic result over the follow-up period (p = 0.579).
Among the patients with a good to excellent cosmesis, the EB-APBI group showed higher rates of excellent cosmetic results over time compared to the IOERT group (Fig. 2).
Fig. 2.
Cosmetic result rated by the physicians at different time points.
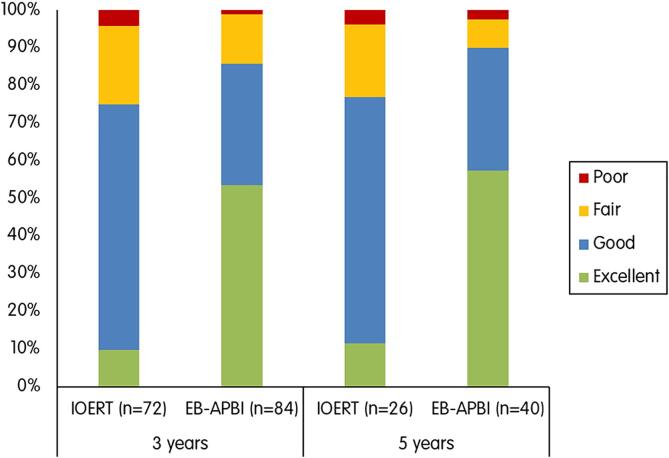
Cosmetic outcome by BCCT.core
Digital photographs were available in 72 (30 %) IOERT and 84 (51 %) EB-APBI patients at 3 years, and in 26 (11 %) and 40 (24 %) at 5 years. In the IOERT group, the software returned a good to excellent cosmesis in 75 % (54/72) of the patients at 3 years and 77 % (20/26) at 5 years. In the EB-APBI group, 86 % (72/84) of the patients at 3 years and 90 % (36/40) at 5 years had a good to excellent cosmesis.
Over the 3 to 5 year follow-up period, no significant differences were observed between the IOERT and EB-APBI groups (p = 0.834).
Fig. 3 shows an overview of the scoring by BCCT.core software. Even though achieving a good to excellent cosmesis was similar for both treatment groups, the EB-APBI group showed higher rates of excellent outcomes over time.
Fig. 3.
Cosmetic result rated by BCCT.core software.
Toxicity
A total of 31 patients experienced postoperative toxicity grade 2 or higher, of whom 25 in the IOERT and 6 in the EB-APBI group. Seven patients experienced multiple symptoms. Toxicity rates per symptom are displayed in Table 2. Postoperative wound infection was significantly more frequent in the IOERT group compared to the EB-APBI group (7 % vs. 1 %, p = 0.012).
Table 2.
Acute toxicity as scored by physicians up till 3 months after radiotherapy, defined as toxicity grade 2 or higher according to the CTCAEv3.0 classification.
| Toxicity | IOERT, n = 241 |
EB-APBI, n = 164 |
p-value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Bleeding | 3 | 1 | 1 | 1 | 0.393 |
| Hematoma | 2 | 1 | 2 | 1 | 0.443 |
| Seroma | 5 | 2 | 1 | 1 | 0.236 |
| Dehiscence | 6 | 3 | 4 | 2 | 0.479 |
| Infection | 17 | 7 | 2 | 1 | 0.012 |
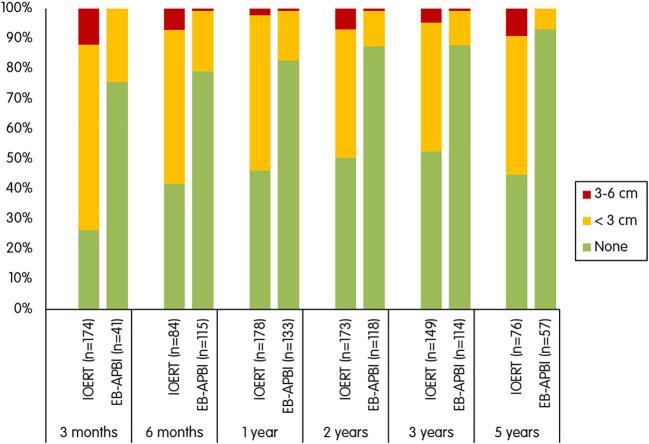
As shown in Fig. 4, higher rates of fibrosis were observed in patients treated with IOERT. In this group, the rates of any degree of fibrosis ranged from 74 % (128/174) at 3 months to 55 % (42/76) at 5 years. In the EB-APBI group, these numbers were 24 % (10/41) to 7 % (4/57), respectively.
Fig. 4.
Fibrosis rated by physicians at different time points.
Discussion
In this prospective cohort study, we aimed to evaluate the cosmetic outcomes of two distinct techniques for APBI, namely IOERT and EB-APBI. Our findings did not indicate statistically significant differences in good to excellent cosmetic outcome between patients treated with either IOERT or EB-APBI. These findings were based on evaluations made by both patients and physicians, as well as an objective assessment conducted by digital software.
Moreover, we observed a higher incidence of fibrosis in the IOERT group compared to the EB-APBI group. Radiation-induced fibrosis can lead to structural changes in the breast, such as retraction and fixation, which often translates to unfavorable cosmetic outcomes. Despite the increased occurrence of fibrosis in the IOERT patients, cosmetic outcomes remained comparable between the two treatment groups. A study by Veronesi et al. assessed fibrosis in breast cancer patients treated with a similar IOERT technique [16]. They evaluated 1246 patients treated with ELIOT (1 x 21 Gy prescribed at the 90 % isodose), reporting that 4 % displayed some degree of fibrosis during follow-up. This is notably lower compared to the findings in our cohort. They stated that fibrosis cases did not seriously impact the cosmetic results, however, their assessment methodology was not explicitly detailed. Additionally, differences in scoring systems and subjectivity in fibrosis evaluation should be considered when comparing these findings.
Previous studies have identified several risk factors for adverse cosmetic outcomes following breast cancer treatment, including tumor size [17], [18], excision volume [17], [18], tumor location [17], [18], [19], postoperative complications [19], diabetes mellitus [20], body mass index [21], [22], and smoking [19]. The impact of these factors can vary depending on outcome measures, follow-up duration and treatment techniques. In our cohorts, most factors were balanced between the two treatment groups, except for body mass index and postoperative infection. While the EB-APBI group had a higher body mass index, patient-reported outcomes were comparable between groups, with the EB-APBI group even receiving higher excellent scores from both physicians and BCCT.core software, suggesting minimal bias. Postoperative infection rates were higher in the IOERT group, which may have adversely affected the results, but the small number of cases made a correlation analysis unfeasible.
Previous studies predominantly compared cosmetic outcomes between different APBI techniques and WBI. In the Florence trial involving 520 breast cancer patients treated with either EB-APBI or WBI, results demonstrated significantly better cosmetic outcomes in the APBI group (p < 0.001) [23]. The IMPORT-LOW trial including 2018 breast cancer patients did not specifically address cosmetic outcome in their results, however, they did report significantly lower rates of patient-reported changes in breast appearance and texture in the EB-APBI cohort compared to the WBI cohort [24]. Conversely, both the IRMA and RAPID trial reported a less favorable cosmetic outcome in the EB-APBI group compared to WBI [19], [25]. However, this could be attributed to the twice-daily administration of EB-APBI in both trials, which may not allow sufficient time for normal tissue repair between treatment fractions [26]. In a sub study of the TARGIT-A trial, cosmetic outcome was evaluated among 126 breast cancer patients receiving either TARGIT-IORT or WBI [27]. At 5 year follow-up, TARGIT-IORT showed higher rates of favorable cosmetic outcomes compared to WBI.
As stated above, there is variability in the reported cosmetic outcomes following APBI. This variability can be attributed to several factors, including differences in treatment techniques and dosage, varied treatment schedules, and varying timings of outcome assessments. Therefore, careful consideration of the nuances of each APBI approach is necessary to accurately interpret and apply study findings.
Several trials support the idea that specific APBI schedules have the potential to improve the cosmetic results following treatment [23], [27], [28]. However, there is a lack of research comparing the cosmetic outcomes of different APBI techniques, which prompted our study. To the best of our knowledge, this is the first study to evaluate the cosmetic results of both IOERT and EB-APBI. In addition, the current cosmetic results, along with the previously published data on acute toxicity, quality of life and oncological outcomes for both treatment groups, offer a comprehensive and unique overview of all critical aspects of these APBI techniques [9], [10], [11]. This provides valuable insights for clinicians and patients, enabling them to make more informed decisions about the most appropriate treatment option.
The study was designed as a two-armed prospective cohort study with identical inclusion criteria for both treatment groups. However, since the study was not randomized, there is a risk of selection and treatment bias. Additionally, the assessment of cosmetic outcome by physicians within their own hospital may have introduced variability in the evaluation standards. Moreover, the response of physicians filling out cosmetic forms during the follow-up visits was moderate and the number of digital photographs obtained from patients during follow-up was low. Nonetheless, the compliance of patients in returning the cosmetic questionnaires was excellent, and the results from both the patients, physicians and software were consistent with each other. Further limitations include the lack of cosmetic measurement at baseline and the collection of measurements relative to radiotherapy rather than baseline, potentially introducing time lag bias due to varying surgical recovery times. Nevertheless, given the comprehensive 5 year follow-up period, we believe that any potential impact on the current findings was not substantial.
The previously reported assessments on health-related quality of life and acute toxicity in the current cohort did not show clinically substantial differences between patients treated with IOERT or EB-APBI [9], [10]. However, the locoregional recurrence rate at 5 years was significantly higher in the IOERT group compared to the EB-APBI group (10.6 % vs. 3.6 %, respectively) [11]. In the current study, we demonstrated similar cosmetic results in both treatment groups.
While IOERT offers unique advantages such as one-day treatment, the above findings highlight the need for careful consideration of its clinical application. Moreover, recent studies on hypofractionation have resulted in the adoption of regimens consisting of only five fractions for EB-APBI patients, significantly reducing treatment burden for these patients [29], [30]. In light of these advancements and our previous observations of higher recurrence rates among IOERT patients, our collective results favor EB-APBI as the preferred treatment modality. Nonetheless, IOERT may still be suitable for carefully selected patients with minimal recurrence risk who prefer one-day treatment, provided they receive adequate counselling.
CRediT authorship contribution statement
Jetske L.B. Gunster: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Visualization, Project administration, Writing – original draft, Writing – review & editing. Daphne H.M. Jacobs: Conceptualization, Data curation, Writing – review & editing. Mirjam E. Mast: Conceptualization, Resources, Supervision, Writing – review & editing. Antoinette Verbeek-de Kanter: Conceptualization, Resources, Supervision, Writing – review & editing. Ursula J. Fisscher: Data curation, Resources, Writing – review & editing. Anna L. Petoukhova: Conceptualization, Resources, Writing – review & editing. Gabrielle Speijer: Conceptualization, Resources, Writing – review & editing. Marieke Straver: Conceptualization, Resources, Writing – review & editing. Jos Merkus: Conceptualization, Resources, Writing – review & editing. Corrie A.M. Marijnen: Conceptualization, Methodology, Supervision, Writing – review & editing. Astrid N. Scholten: Conceptualization, Methodology, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank all participating patients. Physicians and IT-personnel contributed to patient inclusion, treatment and follow-up of study patients. K. Ritzen, A. van Hemert and D. Verweij-Steeneveld helped obtaining digital photographs at the Haga Hospital. R. Kessels advised on and reviewed the statistical analysis.
Funding
We did not receive any grants from funding agencies in the public, commercial, or not-for-profit sectors.
Data sharing statement
Research data are not available at this time.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2024.100844.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Veronesi U., Cascinelli N., Mariani L., Greco M., Saccozzi R., Luini A., et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative G., Darby S., McGale P., Correa C., Taylor C., Arriagada R., et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olivotto I.A., Whelan T.J., Parpia S., Kim D.H., Berrang T., Truong P.T., et al. Interim cosmetic and toxicity results from RAPID: a randomized trial of accelerated partial breast irradiation using three-dimensional conformal external beam radiation therapy. J Clin Oncol. 2013;31(32):4038–4045. doi: 10.1200/JCO.2013.50.5511. [DOI] [PubMed] [Google Scholar]
- 4.Thomsen M.S., Alsner J., Nielsen H.M., Jakobsen E.H., Nielsen M.H., Moller M., et al. Volume matters: breast induration is associated with irradiated breast volume in the Danish Breast Cancer Group phase III randomized Partial Breast Irradiation trial. Radiother Oncol. 2022;177:231–235. doi: 10.1016/j.radonc.2022.09.024. [DOI] [PubMed] [Google Scholar]
- 5.Correa C., Harris E.E., Leonardi M.C., Smith B.D., Taghian A.G., Thompson A.M., et al. Accelerated partial breast irradiation: executive summary for the update of an ASTRO evidence-based consensus statement. Pract Radiat Oncol. 2017;7(2):73–79. doi: 10.1016/j.prro.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Polgar C., Van Limbergen E., Potter R., Kovacs G., Polo A., Lyczek J., et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Europeen de Curietherapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009) Radiother Oncol. 2010;94(3):264–273. doi: 10.1016/j.radonc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Strnad V., Ott O.J., Hildebrandt G., Kauer-Dorner D., Knauerhase H., Major T., et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a randomised, phase 3, non-inferiority trial. Lancet. 2016;387(10015):229–238. doi: 10.1016/S0140-6736(15)00471-7. [DOI] [PubMed] [Google Scholar]
- 8.Njeh C.F., Saunders M.W., Langton C.M. Accelerated Partial Breast Irradiation (APBI): a review of available techniques. Radiat Oncol. 2010;5:90. doi: 10.1186/1748-717X-5-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs D.H.M., Horeweg N., Straver M., Roeloffzen E.M.A., Speijer G., Merkus J., et al. Health-related quality of life of breast cancer patients after accelerated partial breast irradiation using intraoperative or external beam radiotherapy technique. Breast. 2019;46:32–39. doi: 10.1016/j.breast.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs D.H.M., Speijer G., Petoukhova A.L., Roeloffzen E.M.A., Straver M., Marinelli A., et al. Acute toxicity of intraoperative radiotherapy and external beam-accelerated partial breast irradiation in elderly breast cancer patients. Breast Cancer Res Treat. 2018;169(3):549–559. doi: 10.1007/s10549-018-4712-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs D.H.M., Mast M.E., Horeweg N., Speijer G., Petoukhova A.L., Straver M., et al. Accelerated partial breast irradiation using external beam or intraoperative electron radiation therapy: 5-year oncological outcomes of a prospective cohort study. Int J Radiat Oncol Biol Phys. 2022;113(3):570–581. doi: 10.1016/j.ijrobp.2022.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Bertozzi N., Pesce M., Santi P.L., Raposio E. Oncoplastic breast surgery: comprehensive review. Eur Rev Med Pharmacol Sci. 2017;21(11):2572–2585. [PubMed] [Google Scholar]
- 13.Petoukhova A., Russel I., Nijst-Brouwers J., van Wingerden K., van Egmond J., Jacobs D., et al. In vivo dosimetry with MOSFETs and GAFCHROMIC films during electron IORT for Accelerated Partial Breast Irradiation. Phys Med. 2017;44:26–33. doi: 10.1016/j.ejmp.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Cardoso M.J., Cardoso J., Amaral N., Azevedo I., Barreau L., Bernardo M., et al. Turning subjective into objective: the BCCT.core software for evaluation of cosmetic results in breast cancer conservative treatment. Breast. 2007;16(5):456–461. doi: 10.1016/j.breast.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Cancer Therapy Evaluation Program (2003) Common terminology criteria for adverse events version 3.0.
- 16.Veronesi U., Orecchia R., Luini A., Galimberti V., Gatti G., Intra M., et al. Full-dose intra-operative radiotherapy with electrons (ELIOT) during breast-conserving surgery: experience with 1246 cases. Ecancermedicalscience. 2008;2:65. doi: 10.3332/eCMS.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vrieling C., Collette L., Fourquet A., Hoogenraad W.J., Horiot J.H., Jager J.J., et al. The influence of patient, tumor and treatment factors on the cosmetic results after breast-conserving therapy in the EORTC 'boost vs. no boost' trial. EORTC Radiotherapy and Breast Cancer Cooperative Groups. Radiother Oncol. 2000;55(3):219–232. doi: 10.1016/s0167-8140(00)00210-3. [DOI] [PubMed] [Google Scholar]
- 18.Immink J.M., Putter H., Bartelink H., Cardoso J.S., Cardoso M.J., van der Hulst-Vijgen M.H.V., et al. Long-term cosmetic changes after breast-conserving treatment of patients with stage I-II breast cancer and included in the EORTC 'boost versus no boost' trial. Ann Oncol. 2012;23(10):2591–2598. doi: 10.1093/annonc/mds066. [DOI] [PubMed] [Google Scholar]
- 19.Peterson D., Truong P.T., Parpia S., Olivotto I.A., Berrang T., Kim D.H., et al. Predictors of adverse cosmetic outcome in the RAPID trial: an exploratory analysis. Int J Radiat Oncol Biol Phys. 2015;91(5):968–976. doi: 10.1016/j.ijrobp.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 20.Ciammella P., Podgornii A., Galeandro M., Micera R., Ramundo D., Palmieri T., et al. Toxicity and cosmetic outcome of hypofractionated whole-breast radiotherapy: predictive clinical and dosimetric factors. Radiat Oncol. 2014;9:97. doi: 10.1186/1748-717X-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim M.K., Kim T., Moon H.G., Jin U.S., Kim K., Kim J., et al. Effect of cosmetic outcome on quality of life after breast cancer surgery. Eur J Surg Oncol. 2015;41(3):426–432. doi: 10.1016/j.ejso.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Weng J.K., Lei X., Schlembach P., Bloom E.S., Shaitelman S.F., Arzu I.Y., et al. Five-year longitudinal analysis of patient-reported outcomes and cosmesis in a randomized trial of conventionally fractionated versus hypofractionated whole-breast irradiation. Int J Radiat Oncol Biol Phys. 2021;111(2):360–370. doi: 10.1016/j.ijrobp.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meattini I., Marrazzo L., Saieva C., Desideri I., Scotti V., Simontacchi G., et al. Accelerated partial-breast irradiation compared with whole-breast irradiation for early breast cancer: long-term results of the randomized phase III APBI-IMRT-florence trial. J Clin Oncol. 2020;38(35):4175–4183. doi: 10.1200/JCO.20.00650. [DOI] [PubMed] [Google Scholar]
- 24.Coles C.E., Griffin C.L., Kirby A.M., Titley J., Agrawal R.K., Alhasso A., et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet. 2017;390(10099):1048–1060. doi: 10.1016/S0140-6736(17)31145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meduri B., Baldissera A., Iotti C., Scheijmans L., Stam M.R., Parisi S., et al. Cosmetic results and side effects of accelerated partial-breast irradiation versus whole-breast irradiation for low-risk invasive carcinoma of the breast: the randomized phase III IRMA trial. J Clin Oncol. 2023;41(12):2201–2210. doi: 10.1200/JCO.22.01485. [DOI] [PubMed] [Google Scholar]
- 26.Boutrus R.R., El Sherif S., Abdelazim Y., Bayomy M., Gaber A.S., Farahat A., et al. Once daily versus twice daily external beam accelerated partial breast irradiation: a randomized prospective study. Int J Radiat Oncol Biol Phys. 2021;109(5):1296–1300. doi: 10.1016/j.ijrobp.2020.11.044. [DOI] [PubMed] [Google Scholar]
- 27.Corica T., Nowak A.K., Saunders C.M., Bulsara M., Taylor M., Vaidya J.S., et al. Cosmesis and breast-related quality of life outcomes after intraoperative radiation therapy for early breast cancer: a substudy of the TARGIT-A trial. Int J Radiat Oncol Biol Phys. 2016;96(1):55–64. doi: 10.1016/j.ijrobp.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 28.Kumar R., Krupa K., Yehia Z.A., Kumar S., Potdevin L., Eladoumikdachi F., et al. Long-term clinical and cosmetic outcomes of once-daily accelerated partial breast irradiation in early breast cancer. Adv Radiat Oncol. 2024;9(1) doi: 10.1016/j.adro.2023.101324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunt A.M., Haviland J.S., Wheatley D.A., Sydenham M.A., Bloomfield D.J., Chan C., et al. One versus three weeks hypofractionated whole breast radiotherapy for early breast cancer treatment: the FAST-Forward phase III RCT. Health Technol Assess. 2023;27(25):1–176. doi: 10.3310/WWBF1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meattini I., Becherini C., Boersma L., Kaidar-Person O., Marta G.N., Montero A., et al. European Society for Radiotherapy and Oncology Advisory Committee in Radiation Oncology Practice consensus recommendations on patient selection and dose and fractionation for external beam radiotherapy in early breast cancer. Lancet Oncol. 2022;23(1):e21–e31. doi: 10.1016/S1470-2045(21)00539-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.