Abstract
Purpose
To analyze changes in peripapillary retinal nerve fiber layer (RNFL) and ganglion cell complex (GCC) thickness in migraine patients with and without aura compared to healthy controls and to identify factors influencing the occurrence of these anomalies.
Methods
This is a cross-sectional case-control study including migraine patients and control subjects. All patients and controls underwent a complete ophthalmological examination, RNFL and GCC thickness measurements using a spectral domain-OCT device.
The duration of migraine, the frequency and duration of migraine attacks, the migraine disability assessment (MIDAS) and migraine severity scale (MIGSEV) questionnaire scores were recorded.
Results
One hundred and twenty eyes from 60 patients (60 eyes in the migraine without aura (MWoA) group and 60 eyes in the migraine with aura (MWA) group) were included. Control group included 30 age and gender matched healthy participants (60 eyes). OCT revealed that RNFL and GCC thickness were significantly reduced in the migraine without aura (MWoA) and in the migraine with aura (MWA) groups compared to the control group and in the migraine with aura (MWA) group compared to the migraine without aura (MWoA) group. Prolonged disease duration was associated to decreased GCC thickness. RNFL and GCC thickness were correlated to disease severity, attack frequency and duration. In the multivariate study, duration of migraine and attack frequency were the main determinant factors of nasal GCC thickness. Disease severity was the main determinant of RNFL and GCC thickness, with the exception of the nasal sector.
Conclusion
Our study emphasize the significant impact of both types of migraine on retinal structures. OCT would serve as a valuable biomarker in migraine.
Keywords: Retinal nerve fiber layer, Macular ganglion cell complex, Optical coherence tomography, Migraine, Visual aura
Highlights
-
•
Migraine have a significant impact on the optic nerve, which consists in the reduction in RNFL and GCC thickness. Peripapillary retinal nerve fiber layer (RNFL) and the ganglion cell complex (GCC) thickness.
-
•
Optical coherence tomography could serve as a biomarker for monitoring of migraine.
-
•
The duration of migraine and the frequency of attacks are determinants factors of the nasal sector of GCC thickness.
-
•
The severity of the disease is a determinant factor of the mean RNFL and GCC thickness.
1. Introduction
Migraine represents a prevalent chronic neurological condition marked by recurring headache attacks [1]. It is clinically divided into two main subtypes: migraine without aura (MWoA) and migraine with aura (MWA) [2,3].
It is a complex episodic neurovascular disorder characterized by repetitive activation of the trigeminovascular system (STV), leading to cerebral and ocular ischemic complications [4,5]. Although cerebral vasoconstriction is a transient phenomenon, the chronicity of the disease may result in structural and functional alterations of the ocular structures [6]. Cases of ischemic optic neuropathy, retinal vascular occlusion and normal pressure glaucoma (NPG) have been reported in migraine [7]. The advances in optical coherence tomography (OCT) have simplified the evaluation of ocular tissues and the analysis of the optic nerve head (ONH) and the retina [6].
Several studies have focused on studying ocular structural damage and the influence of attack characteristics, disease duration and severity on the appearance of such damage. However, the lack of standardization in the use of these data to date makes the place of OCT in migraine poorly defined.
The purpose of this study was to compare the peripapillary retinal nerve fiber layer (RNFL) and the ganglion cell complex thickness (GCC) of patients with migraine, without and with aura, to those of healthy controls, and to investigate correlation of these measurements with the characteristics of the disease.
2. Methods
This cross-sectional case-control study was conducted at the Ophthalmology and Neurology departments of the Habib Bourguiba University Hospital in Sfax (Tunisia) between April and December 2021. It was approved by the Research Ethics Committee of the Habib Bourguiba hospital Sfax (Tunisia). A detailed written informed consent was obtained from each individual before they were participated in the study.
All patients with migraine with or without visual aura were assessed by a neurologist, according to the revised criteria of the International Classification of Headache Disorders, 3rd Edition (ICHD-3) [3]. The aura in the MWA group was limited to the visual aura. Detailed medical histories were recorded, including years of evolution, the frequency and the duration of migraine attacks. The Migraine severity scale (MIGSEV) was used to assess the severity of individual migraine attack [8]. Moreover, the migraine disability assessment (MIDAS) questionnaire was filled to assess the impact on the patients' quality of life over the preceding 3 months [9]. The control group included healthy volunteers who were age- and gender-matched with the patients.
All the participants underwent ocular examination including slit lamp biomicroscopy, dilated fundus examination, and measurement of refractive error, best-corrected visual acuity and intraocular pressure (IOP). The inclusion criteria were as follows: age between 18 and 50, visual acuity ≥0.6, spherical equivalent between +4 and − 4 diopters, IOP < 18 mmHg and papillary excavation C/D < 0.4. The exclusion criteria were as follows: other types of headache; diabetes; hypertension; pregnancy; smoking; other neurological disorders including infarction, encephalitis, neurodegenerative diseases, demyelinating diseases, and brain trauma; ocular disorders such as optic neuropathy, glaucoma; ocular trauma; ocular surgery or other ocular diseases; intake of vasoactive medicines or background treatment of migraine.
The swept source optic coherence tomography (SS-OCT: DRI OCT, Topcon, Tokyo, Japan). device was used to assess the RNFL and macular GCC thickness. The deep range imaging OCT (DRI OCT) device used in this study produced a swept laser with a wavelength of 1050 nm and a wide-angle (12 × 9 mm) scan centered on the posterior pole. This longer wavelength, with an axial resolution of 8 μm and a speed of 100,000 A-scans per second, enables deeper tissue penetration. The 12 × 9 mm scan consists of 256 B-scans, each containing 512 A-scans. The RNFL thickness and macular GCC thickness were automatically measured using the segmentation software.
For RNFL measurements, a peripapillary circle with a diameter of 3.4 mm is automatically centered on the optic disc. For average or sectoral measurements of macular GCC thickness, a circle with a diameter of 6 mm is automatically centered on the fovea. The measurement of the macular ganglion cell inner plexiform layer (GCIPL) involves the distance from the interface between the nerve fiber layer and ganglion cell layer to the interface between the inner plexiform layer and inner nuclear layer. The GCC comprises the GCIPL and RNFL. The nerve fiber layer represents the thickness between the internal limiting membrane and the interface between the nerve fiber layer and ganglion cell layer.
Statistical analysis was conducted using SPSS version 20.0. The normality of the distribution of quantitative variables was assessed using the Shapiro-Wilk test. The independent t-test was used for group comparisons of normally distributed variables. Linear correlation coefficients were employed to identify correlations between two quantitative variables within a single group, with Pearson's correlation analysis being used for relevance analysis. Regression analyses were performed to evaluate the different factors that may affect GCC and RNFL in patients. Statistical significance was considered for p-values <0.05.
3. Results
Patients with migraine were subdivided into 30 patients (60 eyes) having MWoA and 30 (60 eyes) patients having MWA. Control group included 30 age and gender matched healthy participants (60 eyes)(Table 1). Additionally, there were no statistically significant differences between the migraine patients and the healthy volunteers in terms of intraocular pressure (IOP), visual acuity, or spherical equivalent.
Table 1.
Comparison of demographic features among the study groups.
| Migraine | Control | P | |
|---|---|---|---|
| Age (years) | 37.20 ± 8,8 | 36.60 ± 8,79 | 0.962 |
| Gender male/female | 12/48 | 6/24 | 1 |
Data expressed as mean ± SD, Significant (p < 0.05).
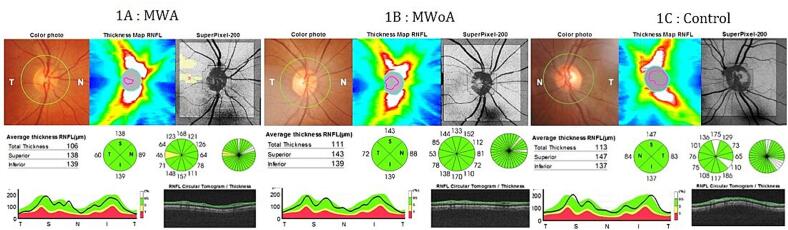
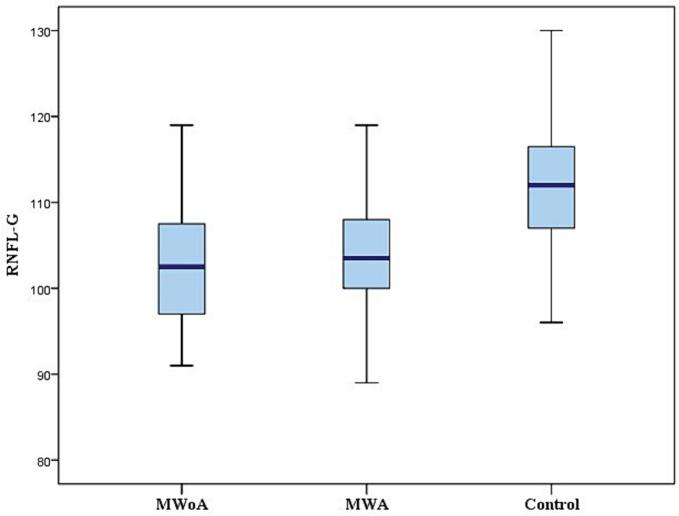
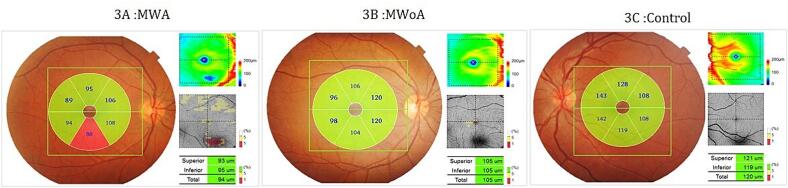
All patients were in the headache-free period and none of them used medication for pain during the study. The RNFL was measured in the four quadrants (superior, inferior, nasal, and temporal) for cases and controls (Fig. 1) and reported in Table 2. The mean RNFL thickness was significantly thinner in MWA and MWoA groups compared to the control group (p < 0.001)(Fig. 2). In patients with MWA, a statistically significant thinning was detected in the nasal, inferior and superior quadrant RNFL thickness measurements compared to the control group (p < 0,001). Patients with MWA had a thinner RNFL in all quadrants compared to the control group (p < 0.001). We noted a significant decrease in RNFL thickness in the temporal quadrant in the MWA group compared to the MWoA group (p = 0,037) and a significant decrease in the nasal quadrant in the MWoA group compared to the MWA group (p = 0,032)(Table 2). The GCC thickness was estimated in the six sectors (superior, superonasal, inferonasal, inferior, inferotemporal, superotemporal) among the patients and controls (Fig. 3) and reported in Table 3. We noted a significant decrease in the mean global GCC thickness in MWoA and MWA groups compared to the control group (p < 0,001) (Fig. 4). When the groups were compared with the control group, the GCC was significantly thinner in all sectors (p < 0.001) except the temporal sectors for the MWoA group. The inferonasal sector of GCC was significantly thinner in MWoA group than in MWA group (p = 0,006). T((he superotemporal sector of GCC was significantly thinner in MWA group than in MWoA group (p = 0,027)(Table 3).
Fig. 1.
A, B and C: Peripapillary retinal nerve fiber layer (RNFL) map mode images of SS − OCT (DRI OCT, Topcon, Tokyo, Japan).
A: RNFL images of a patient with migraine with aura (MWA), B: RNFL images of a patient with migraine without aura (MWoA) and C: RNFL images of a healthy control.
Table 2.
Comparison of the retinal nerve fiber layer (RNFL) thickness (μm) among the migraine patients and control group.
| Thickness (μm) | MWoA (n = 60) | MWA (n = 60) | Control (n = 60) | P1 | P2 | P3 |
|---|---|---|---|---|---|---|
| RNFL-G | 103,02 ± 8,01 | 104,10 ± 7,25 | 111,83 ± 7,47 | <0,001 | <0.001 | NS |
| RNFL-S | 127,80 ± 13,30 | 128,02 ± 20,05 | 140,40 ± 11,69 | <0,001 | <0.001 | NS |
| RNFL-N | 78,90 ± 15,58 | 84,13 ± 10,22 | 89,95 ± 13,33 | <0,001 | 0,008 | 0,032 |
| RNFL-I | 132,45 ± 14,56 | 132,98 ± 14,27 | 142,62 ± 11,97 | <0,001 | <0,001 | NS |
| RNFL-T | 74,22 ± 15,38 | 69,38 ± 8,78 | 74,35 ± 7,92 | NS | 0,001 | 0,037 |
RNFL-G retinal nerve fiber layer-global, RNFL-S retinal nerve fiber layer-superior, RNFL-N retinal nerve fiber layer-nasal, RNFL-I retinal nerve fiber layer-inferior, RNFL-T retinal nerve fiber layer-temporal, MWA, migraine with aura; MWoA, migraine without aura, Data expressed as mean ± SD, Significant (p < 0.05), NS: not significant,P1: MWoA Vs Control, P2: MWA Vs Control, P3: MWoA Vs MWA.
Fig. 2.
Measurements of the RNFL-G thickness among the MWoA, MWA and control groups.
Fig. 3.
A, B and C: Ganglion cell complex (GCC) map mode images of SS − OCT (DRI OCT, Topcon, Tokyo, Japan).
A: GCC images of a patient with migraine with aura (MWA), B: GCC images of a patient with migraine without aura (MWoA), C: GCC images of a healthy control.
Table 3.
Comparison of the ganglion cell complex (GCC) thickness (μm) among the migraine patients and control group.
| Thickness (μm) | MWoA (n = 60) | MWA (n = 60) | Control (n = 60) | P1 | P2 | P3 |
|---|---|---|---|---|---|---|
| GCC-G | 103,78 ± 6,86 | 103,83 ± 6,56 | 109,33 ± 6,21 | <0,001 | <0,001 | NS |
| GCC-S | 108,75 ± 6,45 | 108,90 ± 7,89 | 114,20 ± 7,92 | <0,001 | <0,001 | NS |
| GCC-SN | 117,38 ± 6,96 | 119,87 ± 7,88 | 125,78 ± 8,10 | <0,001 | <0,001 | NS |
| GCC-IN | 117,37 ± 7,85 | 121,45 ± 8,15 | 126,73 ± 7,71 | <0,001 | <0,001 | 0,006 |
| GCC-I | 95,33 ± 9,30 | 96,25 ± 10,25 | 102,22 ± 7,89 | <0,001 | 0,001 | NS |
| GCC-IT | 97,77 ± 8,12 | 96,15 ± 5,21 | 99,88 ± 5,38 | NS | <0,001 | NS |
| GCC-ST | 96,10 ± 7,51 | 93,25 ± 6,35 | 98,33 ± 6,14 | NS | <0,001 | 0,027 |
GCC-G ganglion cell complex-global, GCC-S ganglion cell complex-superior, GCC-SN ganglion cell complex-superonasal, GCC-IN ganglion cell complex-inferonasal, GCC-I ganglion cell complex-inferior, GCC-IT ganglion cell complex-inferotemporal, GCC-ST ganglion cell complex-superotemporal.
Fig. 4.
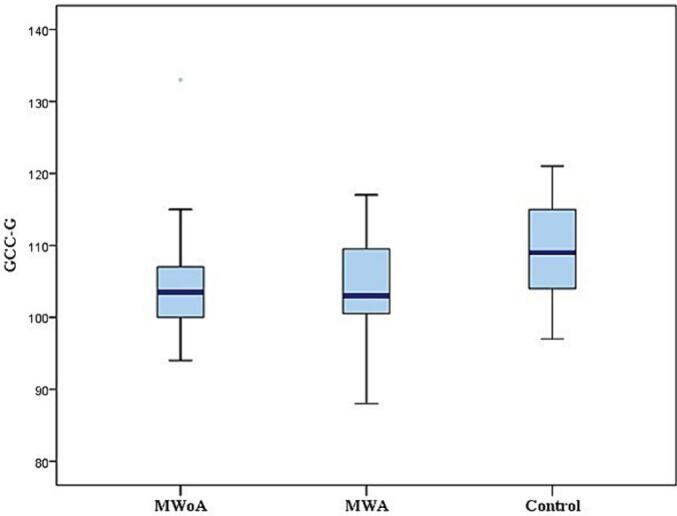
Measurements of the GCC-G thickness among the MWoA, MWA and control groups.
The duration of migraine in the MWoA and MWA groups was 12,07 and 12,47 years, respectively. Most patients experienced one to 4 attacks per month (66.7 % and 56.7 % in the MWoA and MWA groups respectively). The majority of patients had attacks lasting between 4 and 12 h (50 % and 76.7 % in the MWoA and MWA groups respectively). According to the MIGSEV scale, migraine attacks were considered of intermediate severity in 43.3 % of the MWoA group and 50 % of the MWA group. According to the MIDAS score, migraine caused moderate disability (Grade III) in 43.3 % of MWoA group and 26.7 % of MWA group respectively.
The severity of migraine assessed with MIDAS and MIGSEV scale, the frequency and the duration of migraine attacks were significant inversely correlated with the mean RNFL and GCC thickness. The duration of migraine was inversely correlated with the mean GCC thickness and all the sectors except the temporal one (Table 4).
Table 4.
Correlation between duration, characteristics of attacks and severity of migraine and RNFL and GCC thickness in patients with migraine.
| Duration of migraine (years) |
Characteristics of attacks |
Severity |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency of attacks |
Duration of attacks |
MIDAS |
MIGSEV |
|||||||
| R | P | R | P | R | P | R | P | R | P | |
| RNFL | ||||||||||
| RNFL-G | -,148 | ,107 | -,190 | 0,038 | -,191 | 0,037 | -,496 | <0,001 | -,611 | <0,001 |
| RNFL-S | -,023 | ,807 | -,041 | ,657 | -,149 | ,105 | -,238 | 0,009 | -,303 | 0,001 |
| RNFL-N | -,024 | ,794 | -,114 | ,216 | -,127 | ,167 | -,234 | 0,010 | -,373 | <0,001 |
| RNFL-I | -,103 | ,264 | -,289 | 0,001 | ,011 | ,904 | -,322 | <0,001 | -,413 | <0,001 |
| RNFL-T | -,155 | ,091 | ,020 | ,825 | -,219 | 0,016 | -,358 | <0,001 | -,270 | 0,003 |
| GCC | ||||||||||
| GCC-G | -,292 | 0,001 | -,333 | <0,001 | -,304 | 0,001 | -,555 | <0,001 | -,539 | <0,001 |
| GCC-S | -,227 | 0,013 | -,296 | 0,001 | -,235 | 0,010 | -,455 | <0,001 | -,436 | <0,001 |
| GCC-SN | -,260 | 0,004 | -,294 | 0,001 | -,176 | ,055 | -,238 | 0,009 | -,260 | 0,004 |
| GCC-IN | -,328 | <0,001 | -,324 | <0,001 | -,263 | 0,004 | -,322 | <0,001 | -,305 | 0,001 |
| GCC-I | -,257 | 0,005 | -,356 | <0,001 | -,165 | ,072 | -,497 | <0,001 | -,476 | <0,001 |
| GCC-IT | -,136 | ,139 | -,228 | 0,012 | -,250 | 0,006 | -,449 | <0,001 | -,482 | <0,001 |
| GCC-ST | -,119 | ,197 | -,123 | ,182 | -,173 | ,059 | -,419 | <0,001 | -,438 | <0,001 |
RNFL retinal nerve fiber layer, RNFL-G retinal nerve fiber layer-global, RNFL-S retinal nerve fiber layer-superior, RNFL-N retinal nerve fiber layer-nasal, RNFL-I retinal nerve fiber layer-inferior, RNFL-T retinal nerve fiber layer-temporal.
GCC ganglion cell complex, GCC-G ganglion cell complex- global, GCC-S ganglion cell complex-superior, GCC-SN ganglion cell complex-superonasal, GCC-IN ganglion cell complex-inferonasal, GCC-I ganglion cell complex-inferior, GCC-IT ganglion cell complex-inferotemporal, GCC-ST ganglion cell complex-superotemporal.
Data expressed as mean ± SD. Significant (p < 0.05).
Multiregression analysis showed that the duration of the migraine and the frequency of migraine attacks are determinants factors of the nasal sector of GCC thickness while the severity of the disease is a determinant factor of the mean RNFL and GCC thickness, with the exception of the nasal sector (Table 5).
Table 5.
Multiple regression analysis to detect the regression of RNFL, and GCC thickness under the effect of duration, characteristics of attacks and severity of migraine.
| Duration of migraine (years) |
Characteristics of attacks |
Severity |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency of attacks |
Duration of attacks |
MIDAS |
MIGSEV |
|||||||
| Β | p | β | p | Β | p | β | p | β | p | |
| RNFL | ||||||||||
| RNFL-G | -,025 | ,737 | ,037 | ,640 | ,102 | ,213 | -,149 | ,134 | -,611 | <0,001 |
| RNFL-S | ,040 | ,654 | ,079 | ,402 | -,017 | ,862 | -,059 | ,626 | -,303 | 0,001 |
| RNFL-N | ,104 | ,235 | ,025 | ,786 | ,049 | ,607 | ,038 | ,746 | -,373 | <0,001 |
| RNFL-I | -,020 | ,816 | -,160 | ,076 | ,244 | ,009 | -,076 | ,507 | -,464 | <0,001 |
| RNFL-T | -,028 | ,761 | ,197 | ,035 | -,069 | ,480 | -,358 | <0,001 | -,050 | ,672 |
| GCC | ||||||||||
| GCCM | -,120 | ,135 | -,100 | ,223 | -,011 | ,894 | -,351 | 0,001 | -,300 | 0,004 |
| GCCS | -,083 | ,341 | -,110 | ,216 | ,008 | ,935 | -,295 | 0,009 | -,236 | 0,035 |
| GCCSN | -,233 | 0,008 | -,271 | 0,002 | -,060 | ,510 | -,060 | ,553 | -,137 | ,145 |
| GCCIN | -,298 | 0,001 | -,294 | 0,001 | -,139 | ,115 | -,129 | ,184 | -,164 | ,070 |
| GCCI | -,102 | ,228 | -,161 | ,063 | ,131 | ,148 | -,323 | 0,003 | -,256 | 0,018 |
| GCCIT | ,015 | ,861 | -,021 | ,809 | ,001 | ,995 | -,226 | 0,039 | -,328 | 0,003 |
| GCCST | ,024 | ,783 | ,087 | ,334 | ,077 | ,418 | -,285 | 0,012 | -,225 | 0,046 |
RNFL retinal nerve fiber layer, RNFL-G retinal nerve fiber layer-global, RNFL-S retinal nerve fiber layer-superior, RNFL-N retinal nerve fiber layer-nasal, RNFL-I retinal nerve fiber layer-inferior, RNFL-T retinal nerve fiber layer-temporal,
GCC ganglion cell complex, GCC-G ganglion cell complex- global, GCC-S ganglion cell complex-superior, GCC-SN ganglion cell complex-superonasal, GCC-IN ganglion cell complex-inferonasal, GCC-I ganglion cell complex-inferior, GCC-IT ganglion cell complex-inferotemporal, GCC-ST ganglion cell complex-superotemporal,
Data expressed as mean ± SD. Significant (p < 0.05).
4. Discussion
Although the precise pathophysiology of migraines remains uncertain, neurovascular changes linked to trigeminovascular system (STV) activation are implicated in the onset of migraine headaches. It leads to disruptions in both cerebral and retinal circulation [1]. These changes may potentially result in damage to the brain, retina, or even the optic nerve [10]. The present study, which is the first study realised among a north african population to the best of our knowledge, aims to evaluate RNFL and GCC thickness in patients with MWoA and MWA, and compare these measurements to those of healthy controls for early detection of ocular damage in migraine.
In our study, mean RNFL thickness was significantly reduced in migraineurs without and with aura compared with controls. Several studies have found that migraine is associated with decreased RNFL thickness [[11], [12], [13]].
The vascular instability observed during migraines, marked by arteriolar constriction preceding the subsequent dilatation phase may explain this decrease.
To date, it is well-known that trigemino-vascular system (TGVS) activation plays a key role in migraine attack phenomenon. Sensitization of the TGVS during migraine attack involves the network of intra- and extracranial meningeal blood vessels and ocular structures. This process influences vascular tone and the transmission of pain signals [14]. Therefore, fluctuations in perfusion during headaches affect not only the cerebrovascular system but also the ocular structure. This perfusion instability can result in decrease in ocular blood flow, leading to ischemic lesions in the ONH and consequent thinning of the RNFL.
To support this hypothesis, Kara et al. observed decreased blood flow in the central retinal artery and posterior ciliary artery of migraine patients compared to healthy individuals using color Doppler ultrasound during interictal periods, indicating retinal vascular and perfusion changes [15]. Therefore, while vasospasm of the cerebral and retrobulbar vessels is a temporary phenomenon, chronic course of migraines may lead to permanent structural abnormalities in the brain and ONH [12].
ONH ischemia in migraine has also been proven by Arnold et al., who found that migraine was a risk factor in nonarteritic anterior ischemic optic neuropathy in patients younger than 50 years due to vasospasm [16].
Furthermore, neurogenic inflammation contributes to RFLN thickness changes. Indeed, one of the most relevant pathophysiological mechanisms of migraine is the increase in oxidative stress. Inflammation, intensified by oxidative stress, results in the death of cerebral neurons [17]. The decline in neurons within the brain can impact retinal ganglion cells and the RNFL through retrograde trans-synaptic neuronal degeneration triggered by cortical depression during the visual aura [17,18].
Some studies demonstrate a relation between RNFL thinning and the cerebral white matter lesions (WML) detected on brain MRI in migraine patients. These lesions serve as indirect indicators of focal cerebral hypoperfusion resulting from recurrent migraine attacks. Statistical findings indicate that the RNFL in migraine patients with white matter lesions (WML) is thinner compared to those without WML [19]. These findings emphasize the ischemic and retrograde degenerative nature of RNFL thinning in migraine [19,20].
Our results align with papillary OCT-Angiograhy studies, which have shown a decrease in peripapillary vessel density, as well as reduced GCC and RNFL thickness in migraine [21]. These observations may contribute to the increased risk of ocular complications, including ischemic optic neuropathy and normal tension glaucoma, in individuals with migraines [7,21].
Authors speculate that thinning of RNFL thickness may be more affected by migraine with aura [12] attributed to the pathophysiology of visual aura. Occipital hemispheric vasospasm and the subsequent decrease in blood flow in MWA, along with hypoperfusion around the ONH, could contribute to the death of retinal ganglion cells [13].
Shazly et al. found that the RNFL thickness were less in patients with migraine examined during an aura attack than the control group [22]. This thickness increased after the aura, while remaining lower than in controls. Thus, alterations are accentuated during the attack, with partial resolution intercritically. Failure of the RNFL thickness to come back to the normal values after subsidence of the aura may reflect a progressive nature of migraine effect and depletion of the vascular supply pathways of the ONH [22].
RNFL thickness changes were thinner in the side of the headache compared to the contralateral side, indicating potential permanent lateralized cortical changes. The decrease in cerebral perfusion during attacks and the proposed theory of focal retinal blood flow decrease may contribute to the RNFL thinning in migraine [[23], [24], [25]]. The bilateral involvement of RNFL seen reflect diffuse brain and neuronal involvement in migraine [24].
In the present study, we found that all RNFL quadrants thickness were significantly reduced in migraineurs without and with aura compared with controls, except the temporal quadrant for the MWoA group. Ekinci et al. reported a significant thinning of RNFL in all quadrants in migraine patients with aura, but no significant differences in migraine patients without aura [26]. Some studies have found a significant decrease in the nasal quadrant in the MWA group compared with the control group. This is due to the fact that the nasal quadrant is more sensitive to neuronal degeneration because it is thinner [13,18].
Other authors have shown a significant decrease in the upper and lower quadrants. This selective damage has been explained by the differential vulnerability of retinal axons to ischemia [6,12]. On the other hand, some authors have not demonstrated a reduction in all quadrants [23,27].
This disparity may be explained by different measurement protocols and segmentation algorithms depending on the device used. However, the various OCT-TD (Time-Domain) and SD (Spectral Domain) devices demonstrated a decrease in mean RNFL thickness, supporting RNFL impairment in migraine [28].
In our study, the reduction in mean GCC thickness was significant in the 2 migraine subtypes compared with the control group, in line with the results of Gunes et al. [23].
This reduction is due to impaired blood supply to the anterior ONH, resulting in hypoxia and consequent ganglion cell death [23]. Also, the loss of optic fiber axons is likely to induce ganglion cell thinning through the phenomenon of retrograde trans-neuronal degeneration [18,23].
Recent studies indicates that the disease affect not only retinal ganglion cell axons, also ganglion cells bodies and dendrites, most of which are located in the macula. Therefore, SD- OCT scans centred on the macula to measure the GCC thickness provide valuable insights into neural damage in migraine [13]. Ganglion cell involvement can progress to glaucomatous optic neuropathy. In this context, the normal pressure glaucoma (NPG) Society has reported that migraine is a risk factor for the development and progression of NPG [29,30].
Supporting this hypothesis, Drance et al. showed that patients with NPG and migraine have more severe visual field loss than patients with NPG without migraine [29].
In our study, we found a decrease in GCC thickness in the MWoA group compared with the MWA group, in line with the results of Tunc et al. [12]. In the MWA group, the decrease in GCC thickness was significant in all sectors. In the MWoA group, the decrease was significant except in the temporal sectors.
Authors have found a significant decrease in the superior and inferior sectors [13,31]. This sectoral impairment may be linked to the vulnerability of certain sectors to hypoperfusion.
This greater decrease in MWA has been explained in various studies by the severity of hypoxia and the reduction in blood flow during aura [13,26].
However, we showed a significant decrease in GCC thickness of the inferonasal sector in the MWoA group compared with the MWA group, in line with the study of Tunc et al. [12]. More severe impairment in migraineurs without aura may prompt us to look again for other pathophysiological mechanisms responsible for GCC impairment, since hypoperfusion is generally greater in aura.
In our study, the severity of migraine, the frequency and duration of migraine attacks were negatively correlated with RNFL and GCC, while the duration of migraine showed significant negative correlation with GCC, suggesting that the duration and the severity of migraine influence the thinning of GCC and RNFL [12,23,[31], [32], [33], [34], [35]].
The multiregression analysis indicated that the duration of migraine and the frequency of migraine attacks are determinants factors of the nasal sector of GCC while the severity is an important determinant factor of RNFL and GCC thickness with the exception of the nasal sector. In fact, prolonged duration of migraine could lead to prolonged hypoxia, affecting RNFL and GCC thickness [31]. Repeated or prolonged episodes of oligemia during migraine attacks are thought to cause microcirculatory damage to both cerebral and ocular structures [36]. Other factors may be present during severe attacks, such as dehydration (related to vomiting and reduced intake), leading to hyperviscosity and an increased risk of cerebral and ocular intravascular thrombosis. These disorders could be responsible for hypoperfusion affecting all ocular structures [36]. Consequently, the severity of neuronal degeneration tends to increase with longer durations, severity and more frequent occurrences of migraines attacks. Therefore, effective management of migraine attacks, particularly those accompanied by aura, is crucial in order to mitigate these effects [24].
In conclusion, our results suggest that both types of migraine have a significant impact on the optic nerve, which consists in the reduction in RNFL and GCC thickness.
Assessment of RNFL and GCC thickness by OCT could improve our understanding of migraine pathophysiology and could be serve as a biomarker for the diagnosis and monitoring of this pathology.
It is important to emphasize that further studies will be required to consolidate the role of oct as biomarker in migraine.
CRediT authorship contribution statement
Yasmin Walha: Methodology. Mona Rekik: Data curation. Khadija Sonda Moalla: Data curation. Sonda Kammoun: Conceptualization. Chokri Mhiri: Supervision. Mariem Dammak: Supervision. Amira Trigui: Validation, Supervision.
Declaration of competing interest
None.
References
- 1.Aguilar-Shea A.L., Membrilla Md J.A., Diaz-de-Teran J. Migraine review for general practice. Aten. Primaria. 2022 Feb;54(2) doi: 10.1016/j.aprim.2021.102208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stovner L.J., Nichols E., Steiner T.J., Abd-Allah F., Abdelalim A., Al-Raddadi R.M., et al. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954–976. doi: 10.1016/S1474-4422(18)30322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olesen J. Headache classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 4.Brennan K.C., Charles A. An update on the blood vessel in migraine. Curr. Opin. Neurol. 2010;23(3):266–274. doi: 10.1097/WCO.0b013e32833821c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schain A.J., Melo-Carrillo A., Strassman A.M., Burstein R. Cortical spreading depression closes paravascular space and impairs glymphatic flow: Implications for migraine headache. J. Neurosci. 2017;37(11):2904–2915. doi: 10.1523/JNEUROSCI.3390-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin X.G., Yi Z.Q., Zhang X.L., Liu Q.Q., Cai R.Y., Chen C.C., et al. Retinal nerve fiber layer changes in migraine: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2020;99(33) doi: 10.1097/MD.0000000000021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gramer G., Weber B.H.F., Gramer E. Migraine and vasospasm in glaucoma: age-related evaluation of 2027 patients with glaucoma or ocular hypertension. Investig. Ophthalmol. Vis. Sci. 2015;56(13):7999–8007. doi: 10.1167/iovs.15-17274. [DOI] [PubMed] [Google Scholar]
- 8.El Hasnaoui A., Vray M., Richard A., Nachit-Ouinekh F., Boureau F. Assessing the severity of migraine: development of the MIGSEV scale. Headache. 2003;43(6):628–635. doi: 10.1046/j.1526-4610.2003.03105.x. [DOI] [PubMed] [Google Scholar]
- 9.Lipton R.B., Stewart W.F., Sawyer J., Edmeads J.G. Clinical utility of an instrument assessing migraine disability: the migraine disability assessment (MIDAS) questionnaire. Headache. 2001;41(9):854–861. [PubMed] [Google Scholar]
- 10.McKendrick A.M., Nguyen B.N. The eye in migraine: a review of retinal imaging findings in migraine [Internet] Clin. Exp. Optom. 2022;105:186–193. doi: 10.1080/08164622.2021.1971045. [DOI] [PubMed] [Google Scholar]
- 11.Feng Y.F., Guo H., Huang J.H., Yu J.G., Yuan F. Retinal nerve fiber layer thickness changes in migraine: a meta-analysis of case–control studies. Curr. Eye Res. 2016;41(6):814–822. doi: 10.3109/02713683.2015.1056373. [DOI] [PubMed] [Google Scholar]
- 12.Tunç A., Güngen B.D., Evliyaoğlu F., Aras Y.G., Tekeşin A.K. Evaluation of retinal nerve fiber layer, ganglion cell layer and macular changes in patients with migraine. Acta Neurol. Belg. 2017;117(1):121–129. doi: 10.1007/s13760-016-0715-1. [DOI] [PubMed] [Google Scholar]
- 13.Kanar H.S., Toz H.T., Penbe A. Comparison of retinal nerve fiber layer, macular ganglion cell complex and choroidal thickness in patients with migraine with and without aura by using optical coherence tomography. Photodiagn. Photodyn. Ther. 2021;34(May):102–108. doi: 10.1016/j.pdpdt.2021.102323. [DOI] [PubMed] [Google Scholar]
- 14.Russo A., Tessitore A., Tedeschi G. Migraine and trigeminal system - I can feel it coming. Curr. Pain Headache Rep. 2013;17(10):22–28. doi: 10.1007/s11916-013-0367-2. [DOI] [PubMed] [Google Scholar]
- 15.Kara S.A., Erdemoǧlu A.K., Karadeniz M.Y., Altmok D. Color Doppler sonography of orbital and vertebral arteries in migraineurs without aura. J. Clin. Ultrasound. 2003;31(6):308–314. doi: 10.1002/jcu.10181. [DOI] [PubMed] [Google Scholar]
- 16.Arnold A.C., Costa R.M.S., Dumitrascu O.M. The spectrum of optic disc ischemia in patients younger than 50 years. Trans. Am. Ophthalmol. Soc. 2013;111 [PMC free article] [PubMed] [Google Scholar]
- 17.Bulboacă A.C.A.E., Stănescu I.C., Bolboacă S.D., Bulboacă A.C.A.E., Bodizs G.I., Nicula C.A. Retinal nerve fiber layer thickness and oxidative stress parameters in migraine patients without aura: a pilot study. Antioxidants. 2020;9(6):1–13. doi: 10.3390/antiox9060494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ao R., Wang R., Yang M., Wei S., Shi X., Yu S. Altered retinal nerve fiber layer thickness and choroid thickness in patients with migraine. Eur. Neurol. 2019;80(3–4):130–137. doi: 10.1159/000494671. [DOI] [PubMed] [Google Scholar]
- 19.Simsek I.B. Retinal nerve fibre layer thickness of migraine patients with or without white matter lesions. Neuro-Ophthalmology. 2017;41(1):7–11. doi: 10.1080/01658107.2016.1243131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taşli N.G., Ersoy A. Altered macular vasculature in migraine patients without Aura: is it associated with ocular vasculature and white matter hyperintensities? J. Ophthalmol. 2020;2020:1–7. doi: 10.1155/2020/3412490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang M.Y., Phasukkijwatana N., Garrity S., Pineles S.L., Rahimi M., Sarraf D., et al. Foveal and peripapillary vascular decrement in migraine with aura demonstrated by optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 2017;58(12):5477–5484. doi: 10.1167/iovs.17-22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Shazly A.A.E.F., Farweez Y.A., Hamdi M.M., El-Sherbiny N.E. Pattern visual evoked potential, pattern electroretinogram, and retinal nerve fiber layer thickness in patients with migraine during and after Aura. Curr. Eye Res. 2017;42(9):1327–1332. doi: 10.1080/02713683.2017.1319490. [DOI] [PubMed] [Google Scholar]
- 23.Gunes A., Karadag A.S., Yazgan S., Celik H.U., Simsek A. Evaluation of retinal nerve fibre layer, ganglion cell layer and choroidal thickness with optical coherence tomography in migraine patients: a case-control study. Clin. Exp. Optom. 2018;101(1):109–115. doi: 10.1111/cxo.12585. [DOI] [PubMed] [Google Scholar]
- 24.Gunes A., Demirci S., Tok L., Tok O., Demirci S., Kutluhan S. Is retinal nerve fiber layer thickness change related to headache lateralization in migraine? Korean J. Ophthalmol. 2016;30(2):134–139. doi: 10.3341/kjo.2016.30.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demircan S., Ataş M., Arik Yüksel S., Ulusoy M.D., Yuvaci I., Arifoʇlu H.B., et al. The impact of migraine on posterior ocular structures. J. Ophthalmol. 2015;2015:1–8. doi: 10.1155/2015/868967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ekinci M., Ceylan E., Çaǧatay H.H., Keleş S., Hüseyinoǧlu N., Tanyildiz B., et al. Retinal nerve fibre layer, ganglion cell layer and choroid thinning in migraine with aura. BMC Ophthalmol. 2014;14(1):1–6. doi: 10.1186/1471-2415-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yener A.Ü., Korucu O. Quantitative analysis of the retinal nerve fiber layer, ganglion cell layer and optic disc parameters by the swept source optical coherence tomography in patients with migraine and patients with tension-type headache. Acta Neurol. Belg. 2019;119(4):541–548. doi: 10.1007/s13760-018-1041-6. [DOI] [PubMed] [Google Scholar]
- 28.Lin X.G., Yi Z.Q., Zhang X.L., Liu Q.Q., Zhang H., Cai R.Y., et al. Retinal nerve fiber layer changes in migraine: a systematic review and meta-analysis. Neurol. Sci. 2021;42(3):871–881. doi: 10.1007/s10072-020-04992-4. [DOI] [PubMed] [Google Scholar]
- 29.Drance S., Anderson D.R.S.M., Collaborative Normal - Tension Glaucoma Study Group Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am. J. Ophthalmol. 2001;131:699–708. doi: 10.1016/s0002-9394(01)00964-3. [DOI] [PubMed] [Google Scholar]
- 30.Lee J.W.Y., Chan P.P., Zhang X.J., Chen L.J., Jonas J.B. Latest developments in normal-pressure glaucoma: diagnosis, epidemiology, genetics, etiology, causes and mechanisms to management. Asia-Pacific J. Ophthalmol. 2019;8(6):457–468. doi: 10.1097/01.APO.0000605096.48529.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdellatif M.K., Fouad M.M. Effect of duration and severity of migraine on retinal nerve fiber layer, ganglion cell layer, and choroidal thickness. Eur. J. Ophthalmol. 2018;28(6):714–721. doi: 10.1177/1120672117750054. [DOI] [PubMed] [Google Scholar]
- 32.Raga-Martínez I., Povedano-Montero F.J., Hernández-Gallego J., López-Muñoz F. Decrease retinal thickness in patients with chronic migraine evaluated by optical coherence tomography. Diagnostics. 2023;13(1):1–17. doi: 10.3390/diagnostics13010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cankaya C., Tecellioglu M. Foveal thickness alterations in patients with migraine. Med. Arch. (Sarajevo, Bosnia Herzegovina) 2016;70(2):123–126. doi: 10.5455/medarh.2016.70.123-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gürakar Özçift S., Aydın E., Eriş E. Assessment of the choroidal thickness, central macular vascular and optic disk perfusion in migraine patients with optical coherence tomography angiography. Photodiagn. Photodyn. Ther. 2021;35 doi: 10.1016/j.pdpdt.2021.102397. [DOI] [PubMed] [Google Scholar]
- 35.Martinez A., Proupim N., Sanchez M. Retinal nerve fibre layer thickness measurements using optical coherence tomography in migraine patients. Br. J. Ophthalmol. 2008;92(8):1069–1075. doi: 10.1136/bjo.2008.137471. [DOI] [PubMed] [Google Scholar]
- 36.Porter A., Gladstone J.P., Dodick D.W. Migraine and white matter hyperintensities. Curr. Pain Headache Rep. 2005;9(4):289–293. doi: 10.1007/s11916-005-0039-y. [DOI] [PubMed] [Google Scholar]