Summary
Background
Cardiometabolic multimorbidity (CMM) and depression are often co-occurring in older adults and associated with neurodegenerative outcomes. The present study aimed to estimate the independent and joint associations of CMM and depression on cognitive function in multi-regional cohorts, and to validate the generalizability of the findings in additional settings, including clinical.
Methods
Data harmonization was performed across 14 longitudinal cohort studies within the Cohort Studies of Memory in an International Consortium (COSMIC) group, spanning North America, South America, Europe, Africa, Asia, and Australia. Three external validation studies with distinct settings were employed for generalization. Participants were eligible for inclusion if they had data for CMM and were free of dementia at baseline. Baseline CMM was defined as: 1) CMM 5, ≥2 among hypertension, hyperlipidemia, diabetes, stroke, and heart disease and 2) CMM 3 (aligned with previous studies), ≥2 among diabetes, stroke, and heart disease. Baseline depression was primarily characterized by binary classification of depressive symptom measurements, employing the Geriatric Depression Scale and the Center for Epidemiological Studies-Depression scale. Global cognition was standardized as z-scores through harmonizing multiple cognitive measures. Longitudinal cognition was calculated as changes in global cognitive z-scores. A pooled individual participant data (IPD) analysis was utilized to estimate the independent and joint associations of CMM and depression on cognitive outcomes in COSMIC studies, both cross-sectionally and longitudinally. Repeated analyses were performed in three external validation studies.
Findings
Of the 32,931 older adults in the 14 COSMIC cohorts, we included 30,382 participants with complete data on baseline CMM, depression, and cognitive assessments for cross-sectional analyses. Among them, 22,599 who had at least 1 follow-up cognitive assessment were included in the longitudinal analyses. The three external studies for validation had 1964 participants from 3 multi-ethnic Asian older adult cohorts in different settings (community-based, memory clinic, and post-stroke study). In COSMIC studies, each of CMM and depression was independently associated with cross-sectional and longitudinal cognitive function, without significant interactions between them (Ps > 0.05). Participants with both CMM and depression had lower cross-sectional cognitive performance (e.g. β = −0.207, 95% CI = (−0.255, −0.159) for CMM5 (+)/depression (+)) and a faster rate of cognitive decline (e.g. β = −0.040, 95% CI = (−0.047, −0.034) for CMM5 (+)/depression (+)), compared with those without either condition. These associations remained consistent after additional adjustment for APOE genotype and were robust in two-step random-effects IPD analyses. The findings regarding the joint association of CMM and depression on cognitive function were reproduced in the three external validation studies.
Interpretation
Our findings highlighted the importance of investigating age-related co-morbidities in a multi-dimensional perspective. Targeting both cardiometabolic and psychological conditions to prevent cognitive decline could enhance effectiveness.
Funding
Natural Science Foundation of China and National Institute on Aging/National Institutes of Health.
Keywords: Cardiometabolic multimorbidity, Depression, Cognitive decline, Multi-regional study
Research in context.
Evidence before this study
We searched PubMed, Web of Science, and Google Scholar for studies in English published from database inception until May 1, 2024, using the following combination of search terms: (“cognitive impairment” OR “cognitive decline” OR “mild cognitive impairment” OR “dementia”) AND (“cardiometabolic multimorbidity” OR “cardiometabolic disease”) AND (“depression”) AND (“co-occurrence” OR “synergistic effect” OR “multimorbidity”). To date, evidence has demonstrated that both cardiometabolic multimorbidity and depression are individual risk factors for cognitive decline and dementia. Furthermore, it has been established that cardiometabolic multimorbidity and depression are interrelated. Recent pathological evidence suggests that the co-existence of cardiometabolic multimorbidity and mental disorders may be associated with brain dysfunction. However, it remains unknown how the co-occurrence of cardiometabolic multimorbidity and depression is associated with cognitive function among older adults. Additionally, considering the disparities in the prevalence of individual cardiometabolic diseases and depression across different countries and ethnic backgrounds, population-level evidence needs to be derived from data from various ethno-regional groups.
Added value of this study
To the best of our knowledge, no study has investigated the independent and joint associations of cardiometabolic multimorbidity and depression on cognitive function across cohorts from various regions and ethnicities. Using the individual participant data analysis in multi-regional community-based cohorts, we found that baseline cardiometabolic multimorbidity and depression were independently associated with cross-sectional and longitudinal cognitive function among older adults. The joint association of cardiometabolic multimorbidity and depression was associated with lower cognitive performance and faster cognitive decline. In our external validation analysis, the joint association of cardiometabolic multimorbidity and depression on cognition was cross-sectionally confirmed in one community-based and two clinic-based studies, and longitudinally confirmed in two clinic-based studies. Furthermore, the associations between the comorbidity of cardiometabolic multimorbidity and depression and longitudinal cognitive function may vary across different sex and region groups.
Implications of all the available evidence
Our findings highlighted the importance of investigating age-related co-morbidities in a multi-dimensional perspective, including physical and mental conditions, to explore their independent and joint effects on cognitive function. Targeting both cardiometabolic and psychological conditions to prevent cognitive decline could enhance effectiveness.
Introduction
Dementia is a major global health challenge. Given the absence of a widely accessible, effective, and safe treatment,1 reducing the risk of developing dementia has become increasingly important.2 Individuals with a heightened susceptibility to dementia frequently exhibit a confluence of multiple risk factors, chronic conditions, and medical predispositions prior to the manifestation of dementia.3, 4, 5 Individual cardiometabolic diseases (CMDs) are well-established risk factors for cognitive impairment and dementia.6, 7, 8, 9, 10, 11, 12 “Multimorbidity”, the occurrence of two or more chronic conditions within an individual,5 has been associated with heightened risks of incident mild cognitive impairment and dementia compared to those with a single chronic condition.13,14
CMDs are the leading form of morbidity,15 and include a group of interrelated conditions which range between cardiovascular diseases (such as stroke and heart disease) and metabolic diseases (hypertension, hyperlipidemia, obesity and diabetes).16,17 Cardiometabolic multimorbidity (CMM),18 the concurrent presence of two or more CMDs, has been growing in prevalence among older adults across the world.19, 20, 21, 22 Recent investigations have shown that CMM conveys an increased rate of global cognitive decline and a 2–5 fold heightened risk of dementia, compared with healthy controls.23, 24, 25, 26, 27 However, CMDs included in previous literature were primarily diabetes, heart disease, and stroke. In addition to these, hypertension and hyperlipidemia are prevalent among older adults and are individually associated with an increased risk of cognitive impairment.12,28 Therefore, incorporating these two common CMDs is likely to provide more comprehensive insights into the impact of CMM on cognitive decline.29,30
As a well-known risk factor for cognitive decline,7 depression often co-occurs with CMM in older populations,31 possibly due to pathophysiological processes, such as dysregulation of the hypothalamic-pituitary-adrenal axis and platelet activation, neuroinflammation, as well as shared lifestyle risk factors (e.g. physical inactivity and heavy alcohol consumption).32 Recent studies have shown that not only were individuals with more severe depressive symptoms at greater risk of developing CMM,33,34 but older adults with CMM were also more likely to develop depression in the long term.35 While CMM and depression are both associated with neurodegenerative outcomes,23, 24, 25,36 mixed results have been observed. In a prior cross-sectional study among male late-life depression patients, no significant difference in MMSE scores was observed between individuals with and without any CMDs.21 However, in a one-year longitudinal study, patients with late-life depression exhibited elevated risks of cognitive decline if also having CMDs.37 As there are disparities in the prevalence of individual CMDs and depression across different countries and ethnic backgrounds,23,38 it is crucial to investigate the association between CMM, depression, and cognitive decline in various ethno-regional groups. Moreover, recent pathological evidence suggested that the co-existence of CMM and mental disorder may be associated with brain dysfunction.39 Therefore, such in-depth comprehension of these physical-mental multimorbidity cannot only facilitate the elucidation of pathophysiological mechanisms underpinning dementia development,40 but also pave the way towards developing effective prevention and management strategies.2,7
Our aim in the present study was to assess both independent and joint associations of CMM and depression on cognitive function at a population-level. We performed a pooled individual participant data (IPD) analysis on a harmonized data set of 14 community-based longitudinal studies across diverse ethnicities and regions. To enhance generalizability, we externally validated and extended our findings using three multi-ethnic Asian older adults studies of varied settings, including community and clinic. Finally, we conducted interaction analyses to explore the association between the co-occurrence of CMM and depression with cognitive function stratified by different age, sex, education, APOE genotype, and study region groups.
Methods
Contributing studies and participants
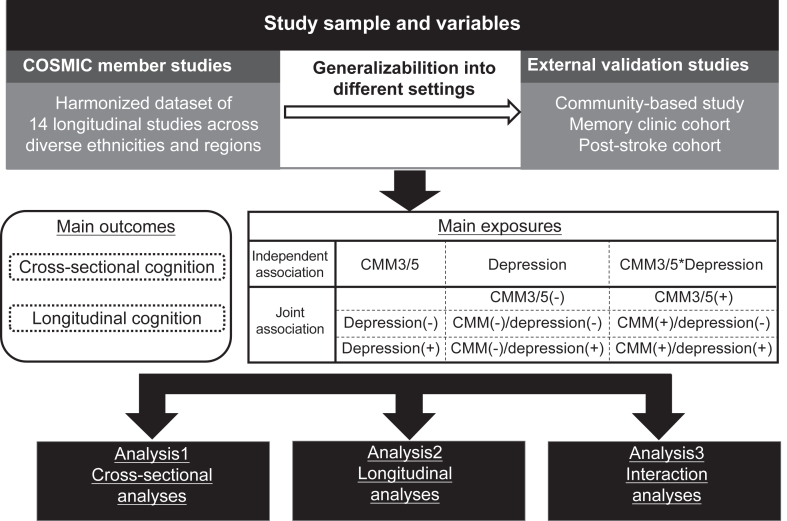
For the IPD analysis we used data from 14 community-based longitudinal studies of aging, all of whom are members of The Cohort Studies of Memory in an International Consortium (COSMIC).41,42 The COSMIC member studies included were those who had collected sufficient data on CMDs and cognitive function (Table 1). These cohorts were situated across various regions, including Africa (2), Asia (1), Europe (6), North America (3), South and Latin America (1), and comprised multi-country studies (1). They exhibited diverse assessment schedules spanning from 2 to 19 waves and follow-up durations ranging from 2 to 19 years (Appendices Figure S1). To determine the generalizability of our results, we used data from three multi-ethnic Asian older adults studies with different study settings (Table 1). The inclusion of the post-stroke cohort was due to the heightened vulnerability of post-stroke patients to both CMM and cognitive impairment.56 Specifically, the community-based study only provided a baseline assessment and therefore did not contribute to the external validation of longitudinal findings. An overview of study description, recruitment, procedures, inclusion and exclusion criteria, and data collection strategy for all contributing studies is provided in Appendices Table S2. A total of 32,931 individuals participated in the COSMIC studies, while 2057 individuals participated in the external validation studies. Within each study, we included participants who had complete baseline CMDs, depression, and cognition data. We excluded participants with baseline dementia. COSMIC and external validation studies used different ways to classify baseline dementia, including: Diagnostic and Statistical Manual of Mental Disease 4th edition criteria; International Classification of Diseases 10th; Revision criteria, a clinical dementia rating (CDR) scale score ≥1, or others (Appendices Table S3). For longitudinal analyses, we additionally excluded participants without any follow-up cognitive assessment. This project was approved by the University of New South Wales Human Research Ethics committee (HC220222) and by the Medical Ethics Committee of the Zhejiang University School of Public Health (ZGL202101-1). All the contributing studies had prior ethics approvals. Written informed consent was obtained from all participants or their legal representatives in their preferred language prior to their recruitment into these studies (Appendices Table S4). The study is reported as per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline (Appendices Table S1), and the prospective analysis plan is given in Fig. 1.
Table 1.
Contributing studies.
| Study | Abbreviation | Location | Main race/ethnicity | Study setting | Start date | Maximum waves, No. | Length of follow-up, median, y (IQR) | N1 | N2 |
|---|---|---|---|---|---|---|---|---|---|
| COSMIC studies | Primary analyses using the individual participant data analysis | ||||||||
| Bambui Cohort Study of Ageing43 | Bambui | Brazil | Brazilian | Community-based | 1997 | 16 | 11 (8) | 1387 | 1323 |
| Einstein Aging Study44 | EAS | USA | White, Black | Community-based | 1993 | 19 | 4 (5) | 2021 | 1325 |
| Etude Sante' Psychologique Pre'valence Risques et Traitement45 | ESPRIT | France | White | Community-based | 1999 | 5 | 10 (11) | 1920 | 1707 |
| The International Mobility In Aging Study46 | IMIAS | Albania, Canada, Brazil, and Colombia | No data | Community-based | 2012 | 2 | 4 (0) | 1907 | 1661 |
| Invecchiamento Cerebrale in Abbiategrasso47 | Invece.Ab | Italy | White | Community-based | 2010 | 4 | 8 (4) | 1126 | 998 |
| Monongahela Youghiogheny Healthy Aging Team48 | MYHAT | USA | White, Black | Community-based | 2006 | 15 | 6 (8) | 1585 | 948 |
| Sacramento Area Latino Study on Aging49 | SALSA | USA | White | Community-based | 1998 | 7 | 7.5 (4) | 1642 | 1401 |
| Singapore Longitudinal Ageing Studies (I)50 | SLASI | Singapore | Chinese | Community-based | 2003 | 3 | 3 (2) | 2702 | 2003 |
| Vallecas Project51 | Vallecas | Spain | White | Community-based | 2011 | 10 | 5.5 (4) | 1169 | 987 |
| Hellenic Longitudinal Investigation of Aging and Diet52 | HELIAD | Greece | White | Community-based | 2010 | 2 | 3 (1) | 1445 | 695 |
| Zaragoza Dementia Depression Project | ZARADEMP | Zaragoza, Spain | White | Community-based | 1994 | 4 | 5 (9) | 4399 | 2100 |
| Leipzig Longitudinal Study of the Aged | LEILA75+ | Leipzig, Germany | White | Community-based | 1997 | 7 | 5 (4) | 1038 | 811 |
| Ibadan Study of Ageing | Ibadan | Ibadan, Nigeria | Black, African | Community-based | 2003 | 7 | 5 (5.5) | 4193 | 3633 |
| The Indianapolis Ibadan Dementia Project | IIDPIN | Ibadan, Nigeria and Indianapolis, USA | Black, African | Community-based | 1992 | 7 | 5 (5) | 3848 | 3013 |
| External validation studies | Generalization to different healthcare settings | ||||||||
| The Singapore Epidemiology of Eye Diseases study53 | Community-based | Singapore | Chinese, Malay, Indian |
Community-based | 2010 | NA | NA | 911 | NA |
| Singapore memory clinic study54 | Memory clinic | Singapore | Chinese, Malay, Indian |
Clinic-based | 2010 | 5 | 4 (3) | 422 | 356 |
| The COgnition After STroke (COAST) cohort55 | Post-stroke | Singapore | Chinese, Malay, Indian |
Clinic-based | 2010 | 4 | 5 (5) | 318 | 221 |
Note. N1: Sample size for cross-sectional analyses. N2: Sample size for longitudinal and stratification analyses.
Fig. 1.
Study schematic. Schematic diagram showing the study design. CMM: cardiometabolic multimorbidity; CMM(+)/depression (+): both CMM and depression; CMM(+)/depression (−): CMM but no depression; CMM(−)/depression (+): depression but no CMM; and CMM(−)/depression (−): neither depression nor CMM; IPD: individual participant data; COSMIC: Cohort Studies of Memory in an International Consortium. In summary, we first conducted IPD meta analyses in each step (from cross-sectional to interaction analyses), and then replicated the cross-sectional and longitudinal analyses via same analytical procedure using 3 Asian studies for external validation.
Procedures and measures
COSMIC members are independent studies with different ranges and depths of data and different collection methods (Appendices Table S2). Consequently, we needed to harmonize their data on CMDs, depression, cognitive function, and other covariates. Standardized survey questionnaires were conducted by certified neurologists, trained nurses or physicians, research staff, or trained interviewers in all COSMIC studies to collect for demographics, medical history, medications, and lifestyle factors. Five studies sourced medical records of diseases and medications from local hospitals or healthcare centers' databases. Eleven studies had between 1 and 3 direct measures of blood pressure, obtained with participants seated, while 6 studies collected blood samples for biochemical testing of hyperlipidemia and diabetes. Three studies lacked available data on hyperlipidemia. The three external validation studies adhered to similar data collection procedures, as detailed in Appendices Table S2.
We included 5 baseline CMDs: hypertension, hyperlipidemia, diabetes, stroke and heart disease. Specifically, hypertension was defined by blood pressure measurement (systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg), self-reported hypertension, medical history, and/or any antihypertensive medication use. Hyperlipidemia was defined by total cholesterol (≥240 mg/dL or >6.2 mmol/L), triglycerides (≥200 mg/dL or >2.3 mmol/L), self-reported hyperlipidemia, medical history, and/or any lipid-lowering treatment. Diabetes was defined by fasting blood glucose (≥126 mg/dL or >7 mmol/L), self-reported diabetes, medical history, and/or any glucose-lowering medication use. Stroke was defined by medical history of stroke and/or transient ischemic attack (TIA). Heart disease was classified as medical history of having any of the following: angina, arrhythmia, atrial fibrillation, heart failure, myocardial infarction, or any related treatment. Similar definitions were used in the external validation studies. Detailed measures and harmonization protocols for CMDs are described in the in Appendices Table S5.
Baseline depression was operationalized through clinical interviews/diagnosis (2 studies) or self-reported diagnosis (1 study). In studies where clinical interviews were lacking, we defined depression as scoring above clinically validated cutoffs on self-report questionnaires for depressive symptoms, using Geriatric Depression Scale-15 (GDS ≥5)57 or Center for Epidemiological Studies Depression (CES-D ≥ 16).58 Depression status was assessed by a GDS score ≥5 in three external validation studies. Harmonization protocols of depression are given in Appendices Table S6.
Two definitions of CMM were used in this study. In our main analyses, CMM5 was defined as having two or more from five CMDs including hypertension, hyperlipidemia, diabetes, stroke, and heart disease. Consistent with prior studies,24,25,36 we used CMM3 an alternate definition of CMM, defined as having two or more of diabetes, stroke, and heart disease. Baseline co-occurrence of CMM and depression was categorized into one of four groups according to participants’ CMM and depression status: 1) both CMM and depression (CMM(+)/depression (+)); 2) CMM but no depression (CMM(+)/depression (−)); 3) depression but no CMM (CMM(−)/depression (+)); and 4) neither depression nor CMM (CMM(−)/depression (−)).
For the IPD analysis, covariates included in the models were harmonized in accordance with previous research by COSMIC.59 Age, sex, education years, race, study region, and current smoking and drinking status were controlled for in the main models using data from all 14 cohorts. Race was mostly self-reported in the individual studies and categorized as Asian, Black, White, and other (encompassing a range of different groups that did not fit within the other categories, eg, American Indian or First Nations Australian). Study region categorized as high income countries (HICs) and lower middle income countries (LMICs) according to the World Bank classification.60 The COSMIC studies are from 10 HICs (EAS, ESPRIT, Invece. Ab, MYHAT, SALSA, SLASI, Vallecas, HELIAD, ZARADEMP, LEILA75+) and 4 LMICs (Bambui, IMIAS, Ibadan, and IIDPIN). Current smoking status was categorized as either current smoking or non-current smoking, while current drinking status was defined by current alcohol consumption, harmonized across various questions in each study (Appendices Table S7). Factors such as physical inactivity and obesity were not included due to limited availability of data from a small number of studies. We additionally ran models controlling for APOE genotype using 11 studies with available gene data. APOE genotype was categorized as having either at least one ε4 allele or none. Covariates in the three external validation studies mirrored those in the main models, with the exception of adjustment for race, study region, and APOE genotype.
Cognitive outcomes
To create comparable cognitive measures across COSMIC studies and provide stable estimates in the pooled analysis, we applied a statistical harmonization on cognitive data to compute a global cognitive z-score.61 We assessed global cognitive function within the COSMIC studies as standardized scores on brief cognitive screening tests, including the Mini-Mental State Examination (MMSE), Leganés cognitive test (LCT), and Community Screening Interview for Dementia (CSI-D) (Appendices Table S8). The steps to obtain the standardized cognitive scores were firstly, within each study, the transformation of raw cognitive scores, pooled across all waves, to have a Gaussian (or normal) distribution, calculated so that the transformed value has the same percentile value as the value in the original distribution. Transformed score outliers were then Winsorized to values plus or minus 3 standard deviations (SDs) from the mean scores. The scores were then standardized by converting to z-scores within each study, using estimated means and SDs of baseline scores within each study at common values of age, sex, and education. The common values were the average values at baseline from data pooled across all studies (common values: age = 71.8 years, education = 8.7 years, and sex = 0.40, indicating 40% males). SDs used for the calculation of the z-scores were the estimated SDs of the residuals (i.e., the standard errors [SEs] of the estimates) obtained from the regression models for each study after adjustment for age, sex, and education. The standardization process for global cognition has been previously reported elsewhere.41,42 In this study, primary outcomes included cognitive performances at baseline and longitudinal changes in global cognitive z-scores.
All participants in the three external validation studies completed formal neuropsychological assessments at baseline and all subsequent follow-ups. Details of the administrated neuropsychological tests and assessed cognitive domains are shown in Appendices Table S12. Raw scores of the comprehensive neuropsychological tests were standardized into z-scores for all individual tests using the means and SDs, setting the baseline measurement as the reference group, as used previously.62 Global cognition scores (global cognitive z-scores) were obtained by averaging domain z-scores and standardizing using the mean and SD of the reference group.
Statistical analysis
Demographic characteristics are presented as mean ± SD or number (%). The proportion and reasons for loss to follow-up for each study were examined. The baseline characteristics of participants with follow-up assessments were compared with those who had only a baseline assessment using χ2 tests or ANOVA as appropriate.
A pooled IPD analysis using mixed models with study as a random effect assessed the independent and joint associations of CMM and depression with cognitive function, both cross-sectionally and longitudinally. This approach was warranted given that we included small studies with low rates for the co-occurrence of CMM and depression, where interrogation of interaction effects may reduce power in two-step IPD approaches.63 We examined the overall linear effect of time (years) (β = −0.048, 95% CI = [−0.050, −0.046]) in study on cognitive function and the quadratic effect of time in study (β = −0.012, 95% CI = [−0.015 to 0.008]). As the quadratic time-in-study effect was not significant, we used a linear effect in all analyses. To test for any synergistic effects, CMM, depression, and their interaction terms were entered into the models. Specifically, a two-way interaction of CMM∗depression was tested in cross-sectional analyses while a three-way interaction of CMM∗depression∗time in study was tested in longitudinal analyses. The joint association of CMM and depression on cognitive function was assessed using participants without CMM or depression (CMM(−)/depression (−)) as the reference groups. The main models included age, sex, education years, race, study region, and current smoking and drinking status as covariates. Covariates were defined consistently across studies. APOE genotype were additionally controlled in separate models according to data availability. In longitudinal models, a significant interaction between a predictor and time indicated an association with the annual rate of cognitive change (change in global cognitive z-score per year). To examine potential moderating factors of baseline age (≤65 years: middle-life vs. >65 years: late-life), sex (female vs. male), education level (<8 years vs. ≥8 years), APOE ε4 status (non-carriers vs. carriers), and study region (HICs vs. LMICs) on the relationship between the co-occurrence of CMM5 and depression and cognitive change over time, we implemented interaction analyses with these variables in separate models. We conducted stratified analyses when the interaction term was significant.
To test the robustness of the pooled IPD analysis results, we additionally used a two-step IPD random-effects meta-analysis to pool the cohort-wise linear mixed model results to obtain pooled estimates of effect sizes for the CMM(+)/depression (+) group, in contrast to the CMM(−)/depression (−) group, both cross-sectionally and longitudinally. The heterogeneity and potential bias was tested by the I2 statistic and funnel plots.64,65
We performed both cross-sectional and longitudinal analyses in our validation using data from three Asian older adults studies. This included simple linear regressions within each study, with CMM, depression, or their co-occurrence as primary predictors. For the longitudinal analyses we used linear mixed models in each study.
Differences in baseline characteristics between the included and excluded participants in the longitudinal analyses were assessed. Sensitivity analyses were conducted using multiple imputation with the Markov Chain Monte Carlo method to impute missing data for baseline demographics and covariates,66 incorporating information from auxiliary variables and generating 20 imputed datasets within the pooled dataset. Estimates derived from each imputed dataset were subsequently combined using Rubin's rules.67 Additional sensitivity analyses were performed to compare the major findings when only complete cases (i.e., 0% missing baseline data) were included. Furthermore, we conducted a third sensitivity analysis by repeating our main analyses after excluding participants with potential undiagnosed dementia, defined as those who were diagnosed with dementia at the first follow-up after the baseline assessment. R Studio software version 4.1.2 was used, with a statistical significance level set at < 0.05.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Results
Summary statistics
The study flowchart is shown in Appendices Figure S2. Participants who had been diagnosed with dementia at baseline were excluded. Further details regarding the exclusions of each study are provided in Appendices Table S9. Of the 32,931 participants in the COSMIC studies, we included 30,382 participants with complete data on CMM, depression, and cognitive assessment for cross-sectional analyses, and with 22,599 of these having at least one follow-up cognitive assessment included in our longitudinal analyses. The median follow-up period was 4.9 years (IQR = 5.0 y). For the external validation studies, we included 1651 participants for cross-sectional analyses, and 577 participants (from memory clinic and post-stroke studies) for longitudinal analyses. The median follow-up period for each study is shown in Table 1. In the COSMIC studies, the prevalence of CMM tripled when hypertension and hyperlipidemia were included (CMM5 = 24.6%) in addition to diabetes mellitus, stroke, and heart disease (CMM3 = 7.9%). Individuals with CMM5 tended to be younger and cognitively healthier than those with CMM3 (data not shown). We identified that 2056 individuals (6.8%) had both CMM5 and depression (CMM5 (+)/depression (+)), and 673 individuals (2.2%) had both CMM3 and depression (CMM3 (+)/depression (+)) in the COSMIC studies. In three external validation studies, a lower prevalence of both CMM5 (+)/depression (+) and CMM3 (+)/depression (+) was observed in the community-based study, while a higher prevalence was observed in the memory clinic and post-stroke studies. Baseline characteristics are presented in Table 2. Study profiles for each COSMIC study, including numbers of missing data, are provided in Appendices Table S10.
Table 2.
Baseline characteristics.
| COSMICa studies |
External validation studies |
||||
|---|---|---|---|---|---|
| No. of contributing studies | Overall (N = 30,382) | Community-based (N = 911) | Memory clinic (N = 422) | Post-stroke (N = 318) | |
| Age | 14 | 72.8 (7.3) | 69.8 (6.3) | 71.6 (7.9) | 59.4 (11.1) |
| Sex, female | 14 | 18,584 (61.2%) | 459 (50.4%) | 227 (53.8%) | 94 (29.6%) |
| Race | 13 | ||||
| White (COSMIC)/Chinese (external) | 15,662 (51.6%) | 293 (32.2%) | 364 (86.3%) | 228 (71.7%) | |
| Black (COSMIC)/Malay (external) | 8683 (28.6%) | 296 (32.5%) | 25 (5.9%) | 61 (19.2%) | |
| Asian (COSMIC)/Indian (external) | 2710 (8.9%) | 322 (35.3%) | 28 (6.6%) | 26 (8.2%) | |
| Others (COSMIC)/Others (external) | 1416 (4.7%) | 0 | 5 (1.2%) | 3 (0.9%) | |
| Education, years | 14 | 7.5 (5.5) | 7.5 (4.8) | 8.1 (5.1) | 7.8 (3.1) |
| Smoking, current | 14 | 6957 (22.9%) | 257 (28.2%) | 34 (8.1%) | 85 (26.7%) |
| Drinking, current | 14 | 9625 (31.7%) | 15 (6.4%) | 44 (10.4%) | 120 (37.7%) |
| Individual CMDb s | |||||
| Hypertension | 14 | 16,591 (54.6%) | 731 (80.2%) | 267 (63.3%) | 224 (70.4%) |
| Hyperlipidemia | 11 | 6137 (20.2%) | 692 (76.0%) | 295 (69.9%) | 243 (76.4%) |
| Diabetes | 14 | 4895 (16.1%) | 335 (36.8%) | 115 (27.3%) | 125 (39.3%) |
| Stroke | 14 | 1776 (5.8%) | 43 (4.7%) | 117 (27.7%) | 318 (100.0%) |
| Heart disease | 14 | 6489 (21.4%) | 94 (10.3%) | 82 (19.4%) | 96 (30.2%) |
| Depression | 14 | 6177 (20.3%) | 76 (8.3%) | 56 (13.3%) | 103 (32.4%) |
| CMM5c | 11 | 7462 (24.6%) | 258 (28.3%) | 272 (64.5%) | 294 (92.5%) |
| CMM3d | 14 | 2390 (7.9%) | 63 (6.9%) | 75 (17.8%) | 186 (58.5%) |
| Co-occurrence of CMM5 and depression | 11 | ||||
| CMM5 (−)/depression (−) | 9374 (30.9%) | 595 (65.3%) | 129 (30.6%) | 16 (5.0%) | |
| CMM5 (+)/depression (−) | 5406 (17.8%) | 240 (26.3%) | 233 (55.2%) | 199 (62.6%) | |
| CMM5 (−)/depression (+) | 2298 (7.6%) | 58 (6.4%) | 16 (3.8%) | 8 (2.5%) | |
| CMM5 (+)/depression (+) | 2056 (6.8%) | 18 (2.0%) | 38 (9.0%) | 95 (29.9%) | |
| Co-occurrence of CMM3 and depression | 14 | ||||
| CMM3 (−)/depression (−) | 22,488 (74.0%) | 780 (85.6%) | 308 (73.0%) | 96 (30.2%) | |
| CMM3 (+)/depression (−) | 1717 (5.7%) | 55 (6.0%) | 56 (13.3%) | 119 (37.4%) | |
| CMM3 (−)/depression (+) | 5504 (18.1%) | 68 (7.5%) | 37 (8.8%) | 36 (11.3%) | |
| CMM3 (+)/depression (+) | 673 (2.2%) | 8 (0.9%) | 19 (4.5%) | 67 (21.1%) | |
| APOE ε4 carriere, yes | 11 | 3728 (12.3%) | NA | NA | NA |
| Global cognitive z-score | 14 | 0.1 (1.0) | −2.6 (2.2) | −1.4 (1.7) | 0.1 (1.0) |
COSMIC: Cohort Studies of Memory in an International Consortium.
CMD: Cardiometabolic disease.
CMM5: Cardiometabolic multimorbidity, defined as having two or more CMDs including hypertension, hyperlipidemia, diabetes, stroke, and heart disease.
CMM3: An alternative definition of CMM, defined as having two or more CMDs including diabetes, stroke, and heart disease.
APOE: apolipoprotein E genotype.
Independent associations of CMM and depression on cognitive outcomes
In cross-sectional analyses, CMM (CMM5: β, −0.044, 95% CI = [−0.074, −0.014]; CMM3: β, −0.086, 95% CI = [−0.129, −0.043]; Table 3) and depression (adjusted for CMM5: β, −0.166, 95% CI = [−0.200, −0.133]; adjusted for CMM3: β, −0.073, 95% CI = [−0.103, −0.044]; Table 3) were each independently associated with lower cross-sectional global cognitive z-scores. In longitudinal analyses, CMM (CMM5: β, −0.028, 95% CI = [−0.032, −0.024]; CMM3: β, −0.018, 95% CI = [−0.025, −0.010]; Table 3) and depression (adjusted for CMM5: β, −0.013, 95% CI = [−0.018, −0.008]; adjusted for CMM3: β, −0.008, 95% CI = [−0.012, −0.003]; Table 3) were each independently associated with a faster rate of global cognitive z-score decline. We observed no significant interactions of CMM with depression (CMM∗depression and CMM∗depression∗time for cross-sectional and longitudinal analyses, respectively) on both cross-sectional and longitudinal cognitive outcomes (Ps > 0.05, Table 3). These associations remained consistent after additionally adjusting for APOE genotype (Appendices Table S11). In the external validation studies, cross-sectionally, CMM was independently associated with cognitive performance in community-based and memory clinic studies, but not in the post-stroke study. Depression was independently associated with lower cognitive performance in all three studies. However, regarding the longitudinal analyses, significant association was were only observed for CMM3 in the post-stroke cohort (Table 3).
Table 3.
Independent and joint associations of CMM and depression, and p for interaction on cognitive function in cross-sectional and longitudinal analyses in COSMIC and external validation studies.
| COSMIC studies |
External validation studies |
|||||||
|---|---|---|---|---|---|---|---|---|
| Community-based |
Memory clinic |
Post-stroke |
||||||
| CMM5a |
CMM3b |
CMM5 |
CMM3 |
CMM5 |
CMM3 |
CMM5 |
CMM3 |
|
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Cross-sectional analyses | N = 21,303 | N = 30,382 | N = 911 | N = 422 | N = 318 | |||
| Independent association | ||||||||
| CMM (reference = free of CMM) | −0.044 (−0.074, −0.014)∗ | −0.086 (−0.129, −0.043)∗ | −0.363 (−0.599, −0.127)∗ | −0.398 (−0.611, −0.186)∗ | −0.36 (−0.621, −0.099)∗ | −0.458 (−0.777, −0.139)∗ | −0.037 (−0.409, 0.335) | −0.150 (−0.354, 0.054) |
| Depression (reference = free of depression) | −0.166 (−0.200, −0.133)∗ | −0.073 (−0.103, −0.044)∗ | −0.393 (−0.775, −0.010)∗ | −0.388 (−0.77, −0.007)∗ | −0.48 (−0.726, −0.234)∗ | −0.457 (−0.702, −0.212)∗ | −0.264 (−0.472, −0.055)∗ | −0.247 (−0.456, −0.039)∗ |
| Interaction between CMM and depression | 0.010 (−0.055, 0.076) | −0.021 (−0.117, 0.075) | 0.216 (−0.676, 1.108) | 0.086 (−0.676, 0.847) | −0.384 (−0.906, 0.139) | 0.265 (−0.375, 0.906) | −0.208 (−0.966, 0.551) | −0.191 (−0.606, 0.223) |
| Joint association | ||||||||
| CMM(−)/depression (−) | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| CMM(−)/depression (+) | −0.171 (−0.215, −0.126)∗ | −0.071 (−0.102, −0.040)∗ | −0.530 (−1.910, 0.850) | −0.430 (−0.960, 0.101) | −0.225 (−0.651, 0.201) | −0.505 (−0.776, −0.234) | 0.058 (−0.452, 0.568) | −0.128 (−0.460, 0.203) |
| CMM(+)/depression (−) | −0.047 (−0.081, −0.013)∗ | −0.080 (−0.112, −0.048)∗ | −0.557 (−1.335, 0.221) | −0.406 (−0.628, −0.184)∗ | −0.205 (−0.504, 0.131) | −0.606 (−1.087, −0.126) | −0.073 (−0.801, 0.655) | −0.077 (−0.336, 0.181) |
| CMM(+)/depression (+) | −0.207 (−0.255, −0.159)∗ | −0.172 (−0.251, −0.094)∗ | −0.720 (−1.189, −0.250)∗ | −0.750 (−1.292, −0.207)∗ | −0.813 (−1.156, −0.470)∗ | −0.846 (−1.253, −0.439)∗ | −0.222 (−0.735, 0.290) | −0.397 (−0.673, −0.121)∗ |
| Longitudinal analyses | N = 15,142 | N = 22,599 | N = 356 | N = 221 | ||||
| Independent association | ||||||||
| CMM (reference = free of CMM) | −0.028 (−0.032, −0.024)∗ | −0.018 (−0.025, −0.010)∗ | NA | NA | −0.024 (−0.067, 0.018) | −0.038 (−0.093, 0.018) | −0.008 (−0.074, 0.058) | −0.028 (−0.054, −0.002)∗ |
| Depression (reference = free of depression) | −0.013 (−0.018, −0.008)∗ | −0.008 (−0.012, −0.003)∗ | NA | NA | −0.030 (−0.072, 0.012) | −0.027 (−0.070, 0.016) | −0.025 (−0.051, 0.001) | −0.022 (−0.048, 0.004) |
| Interaction between CMM and depression | 0.007 (−0.003, 0.016) | 0.011 (−0.006, 0.028) | NA | NA | −0.107 (−0.195, 0.019) | −0.022 (−0.133, 0.089) | 0.034 (−0.100, 0.168) | 0.004 (−0.051, 0.058) |
| Joint association | ||||||||
| CMM(−)/depression (−) | Reference | Reference | Reference | Reference | Reference | Reference | ||
| CMM(−)/depression (+) | −0.013 (−0.019, −0.006)∗ | −0.008 (−0.012, −0.003)∗ | NA | NA | 0.038 (−0.033, 0.108) | −0.023 (−0.070, 0.025) | −0.057 (−0.189, 0.074) | −0.024 (−0.068, 0.020) |
| CMM(+)/depression (−) | −0.028 (−0.032, −0.023)∗ | −0.018 (−0.026, −0.009)∗ | NA | NA | 0.015 (−0.038, 0.069) | −0.025 (−0.108, 0.058) | −0.022 (−0.108, 0.063) | −0.030 (−0.062, 0.003) |
| CMM(+)/depression (+) | −0.040 (−0.047, −0.034)∗ | −0.026 (−0.040, −0.012)∗ | NA | NA | −0.058 (−0.110, −0.001)∗ | −0.070 (−0.139, −0.001)∗ | −0.045 (−0.132, 0.041) | −0.050 (−0.085, −0.015)∗ |
∗p < 0.05.
CMM5: Cardiometabolic multimorbidity, defined as having two or more CMDs including hypertension, hyperlipidemia, diabetes, stroke, and heart disease.
CMM3: An alternative definition of CMM, defined as having two or more CMDs including diabetes, stroke, and heart disease. Each model was adjusted for age, sex, education years, race, study region, and current smoking and drinking status. In the longitudinal models, effect size describes the change in cognitive function (global z-scores) per year.
Joint associations of CMM and depression on cognitive outcomes
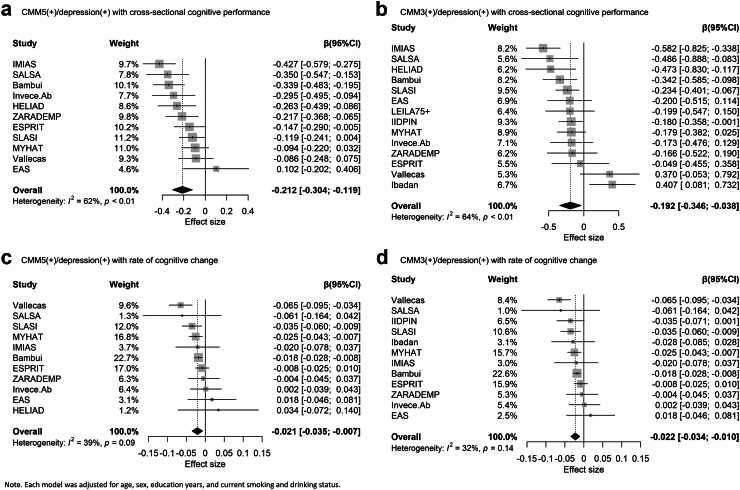
In cross-sectional analyses, participants with both CMM and depression had lower global cognitive z-scores than participants without depression or CMM (CMM5 (+)/depression (+): β, −0.207, 95% CI = [−0.255, −0.159]; CMM3 (+)/depression (+): β, −0.172, 95% CI = [−0.251, −0.094]; Table 3). Our two-step IPD random-effects meta-analysis replicated these findings (Fig. 2), showing an effect size of −0.212 (95% CI = [−0.304, −0.119]) for CMM5 (+)/depression (+), and −0.192 (95% CI = [−0.346, −0.038]) for CMM3 (+)/depression (+). Heterogeneity was 0.62 for CMM5 (+)/depression (+) and 0.64 for CMM3 (+)/depression (+). Funnel plots for the cross-sectional analysis are provided in Appendices Figure S3. In longitudinal analyses, participants in the CMM(+)/depression (+) group also exhibited a faster rate of global cognitive z-score decline than participants without depression or CMM (CMM5 (+)/depression (+): β, −0.040, 95% CI = [−0.047, −0.034]; CMM3 (+)/depression (+): β, −0.026, 95% CI = [−0.040, −0.012]; Table 3). The two-step IPD random-effects meta-analysis demonstrated consistent findings (CMM5 (+)/depression (+): β, −0.021, 95% CI = [−0.035, −0.007]; CMM3 (+)/depression (+): β, −0.022, 95% CI = [−0.034, −0.010]; Fig. 2). Heterogeneity estimates across studies were 0.39 for CMM5 (+)/depression (+) and 0.32 for CMM3 (+)/depression (+). Funnel plots for the longitudinal analysis are in Appendices Figure S3. These associations remained stable after additionally adjusting for APOE genotype (Appendices Table S11). In the external validation studies, the co-occurrence of CMM and depression was associated with lower cognitive performance cross-sectionally, except for cases of CMM5 (+)/depression (+) in the post-stroke study. In longitudinal analyses, we observed consistent associations between the co-occurrence of CMM and depression and a faster rate of cognitive decline, except for CMM5 (+)/depression (+) in the post-stroke cohort (Table 3).
Fig. 2.
Forest plots from the 2-step IPD meta-analysis of the joint associations of CMM and depression on cognitive function in COSMIC studies. Note. Each model was adjusted for age, sex, education years, and current smoking and drinking status. Reference group:CMM(−)/depression (−).
Interaction and sensitivity analysis
Interaction analyses found significant moderation by baseline sex and study region on the association between CMM5 (+)/depression (+) and the rate of global cognitive z-score change. Females, but not males, who were CMM5 (+)/depression (+) at baseline, had faster decline in global cognitive z-scores (β, −0.038, 95% CI [−0.045, −0.031] for female; β, −0.011,95% CI [−0.022, 0.001] for male). Compared with LIMCs, participants in HICs had faster decline in global cognitive z-scores (β, −0.037, 95% CI [−0.046, −0.27] for HICs; β, −0.021, 95% CI [−0.031, 0.011] for LMICs). Results of the interaction analyses are shown in Table 4.
Table 4.
Subgroup differences in the association of the joint associations of CMM5a and depression on longitudinal cognitive function.
| Interaction term | β (95% CI) | p-value | Stratified analyses when the interaction term is significant, β (95% CI) |
|---|---|---|---|
| Age interaction: age is classified as middle-life (≤65, N = 3158) and late-life (>65, N = 19,441) | |||
| CMM5 (−)/depression (+)∗Age∗Time | −0.005 (−0.020, 0.011) | 0.496 | |
| CMM5 (+)/depression (−)∗Age∗Time | 0.004 (−0.007, 0.014) | 0.554 | |
| CMM5 (+)/depression (+)∗Age∗Time | 0.003 (−0.012, 0.018) | 0.671 | |
| Education interaction: education (years) is classified as low education (<8, N = 10,930) and high education ( ≥ 8, N = 11,616) | |||
| CMM5 (−)/depression (+)∗Education∗Time | 0.001 (0.0001, 0.003) | 0.029 | −0.006 (−0.016, 0.003) for high −0.025 (−0.032, −0.018) for low |
| CMM5 (+)/depression (−)∗Education∗Time | 0.001 (−0.001, 0.002) | 0.077 | |
| CMM5 (+)/depression (+)∗Education∗Time | −0.001 (−0.002, 0.001) | 0.277 | |
| Sex interaction: sex is classified as male (N = 8608) and female (N = 13,991) | |||
| CMM5 (−)/depression (+)∗Sex∗Time | 0.009 (−0.006, 0.024) | 0.246 | |
| CMM5 (+)/depression (−)∗Sex∗Time | 0.014 (0.005, 0.024) | 0.002 | −0.030 (−0.036, −0.024) for female −0.017 (−0.024, −0.010) for male |
| CMM5 (+)/depression (+)∗Sex∗Time | 0.029 (0.014, 0.040) | <0.001 | −0.038 (−0.045, −0.031) for female −0.011 (−0.022, 0.001) for male |
| APOE genotype interaction: APOE genotype is classified as ε4 non-carriers (N = 12,579) and ε4 carriers (N = 3151) | |||
| CMM5 (−)/depression (+)∗APOE genotype∗Time | −0.003 (−0.024, 0.017) | 0.747 | |
| CMM5 (+)/depression (−)∗APOE genotype∗Time | −0.013 (−0.026, −0.001) | 0.040 | −0.030 (−0.036, −0.024) for ε4 non−carriers −0.041 (−0.053, −0.030) for ε4 carriers |
| CMM5 (+)/depression (+)∗APOE genotype∗Time | −0.008 (−0.027, 0.010) | 0.390 | |
| Study region interaction: study region is classified as HICsb (N = 12,969) and LMICsc (N = 9630) | |||
| CMM5 (−)/depression (+)∗Study region∗Time | −0.012 (−0.026, 0.002) | 0.332 | |
| CMM5 (+)/depression (−)∗Study region∗Time | 0.005 (−0.005, 0.015) | 0.101 | |
| CMM5 (+)/depression (+)∗Study region∗Time | 0.017 (0.003, 0.031) | 0.015 | −0.037 (−0.046, −0.270) for HICs −0.021 (−0.031, −0.011) for LMICs |
Reference group was CMM5(−)/depression(−). Each model was adjusted for age, sex, education years, race, study region, and current smoking and drinking status (though not when the stratification variable, e.g. sex was not adjusted in the sex stratification).
CMM5: Cardiometabolic multimorbidity, defined as having two or more CMDs including hypertension, hyperlipidemia, diabetes, stroke, and heart disease.
HICs: high income countries.
LMICs:lower middle income countries. Effects were estimated using 11 COSMIC studies and 13,570 participants.
The proportions of loss to follow-up ranged from 4.7% to 27.8% across the COSMIC and external validation studies. Participants included in the longitudinal analyses were younger, more educated, and more likely to be female. Details of the comparisons of the baseline characteristics between participants included or excluded in the longitudinal analyses are shown in Appendices Table S12. Findings from our main analyses were replicated in our sensitivity analyses using multiple imputation with the Markov Chain Monte Carlo method and complete cases (Appendices Table S13 & Table S14). We additionally repeated the main analyses by excluding 568 participants (in COSMIC studies) who were diagnosed with dementia at the first follow-up after the baseline assessment, and similar results were found (Appendices Table S15).
Discussion
The present study examined the independent and joint associations of CMM and depression on both cross-sectional and longitudinal cognitive function at a population level. In the pooled IPD analysis, we found that both CMM and depression were independently associated with lower cross-sectional cognitive performance and longitudinal cognitive decline. The co-occurrence of CMM and depression was associated with cognitive deterioration in comparison to those without both conditions. In our external validation analysis, the joint association of CMM and depression on cognition was cross-sectionally confirmed in one community-based and two clinic-based studies, and longitudinally confirmed in two clinic-based studies. Finally, we identified sex and region differences in the associations of the comorbidity and CMM and depression with longitudinal cognitive function.
A recent study using UK Biobank data found a hazard ratio of 5.39 (95% CI: 3.30–8.82) for incident dementia in individuals with CMM.24 Likewise, a study from the Swedish Twin Registry, which included 17,913 dementia-free individuals, found a hazard ratio of 2.10 (95% CI: 1.73–2.57) for incident dementia in individuals with CMM compared to those without.24 Regarding the longitudinal cognitive trajectory, our findings were consistent with two recent studies that demonstrated an association between CMM and a faster rate of global cognitive decline.26,27 However, previous studies did not include middle/late-life hyperlipidemia and hypertension, which have been reported as consistently associated with an increased risk of cognitive impairment.12,28 The slightly discrepant results between CMM3 and CMM5 may be due to the differential operational definitions. The additionally included hypertension and hyperlipidemia in CMM5 could be considered risk factors for stroke and heart disease. Additionally, midlife hypertension and hyperlipidemia has been associated with an increased risk of dementia in later life. However, associations between these 2 CMDs and dementia, when assessed in late life, may have been mixed and sometimes even reversed as compared to midlife assessments. In COSMIC studies, we found that the participants with CMM5 tended to be younger and cognitively healthier compared with those with CMM3. The more extensive definition of CMM (CMM5) demonstrated a persistent relationship between CMM and cognition, highlighting the importance of incorporating more prevalent and early stage chronic conditions such as hypertension and hyperlipidemia into the strategic planning for the prevention and health management of cognitive decline and dementia in the community. However, due to the small sample size of the co-occurrence of CMM3 and depression, we did not conduct further exploration on the difference of the joint association of CMM and depression defined by CMM3 and CMM5 (e.g. interaction analyses). Further studies should explore the joint effect between various constellations of CMDs and psychological conditions on cognition.
Our IPD meta-analysis, using a large and diverse ethno-regional sample, provided population-based evidence that CMM and depression, independent of each other, were associated with both cross-sectional and longitudinal cognitive function. The absence of a significant interaction between CMM and depression implied that no synergistic effect existed between these variables. In our external validation analysis, these independent associations were not consistently observed in two clinic-based cohorts. This discrepancy may be due to the high prevalence of individuals at higher risk of different subtypes of dementia, which may have underestimated the individual effects of CMM or depression on adverse cognitive outcomes. Therefore, validation of the independent associations of CMM and depression on cognition in clinical settings remains inconclusive; future studies with larger sample size, longer follow-up, and more in-depth etiological investigations are warranted.
Our findings highlight the importance of considering multi-dimensional age-related co-morbidities ie., both physical and psychological conditions. Limited evidence exists on the joint associations of CMM and depression on adverse cognitive outcomes. We found that the participants with both CMM and depression have both lower cross-sectional cognitive performance and a faster rate of cognitive decline, compared with those without both conditions. We observed consistent relationships between the co-occurrence of CMM and depression in the two clinic-based studies, in which both cross-sectional and longitudinal association were confirmed. This suggests that the joint associations of CMM and depression on cognition persists in populations potentially with varying pathological statuses: neurodegeneration (typically associated with ageing)68 in a memory clinic, and vascular pathology69 in a post-stroke cohort. However, we didn't include time-variant exposures in this study. Given that CMM and depression might fluctuate or progress over time, it is worthwhile to explore long-term variation in CMM and depression status and cognition in future research.
The comorbidity of CMM and depression may be linked with cognitive decline via complex mechanisms involving both cerebrovascular damage and neurodegeneration.5,70 CMM has been associated with lower total hippocampal and gray matter volumes, as well as higher white matter hyperintensity volumes. Similarly, the pathogenesis from depression to cognitive impairment involves impaired white matter integrity and reduced brain volume. Recent research has revealed a cross-sectional correlation between the simultaneous presence of CMM, severe mental disorders, including depression, and pronounced structural brain abnormalities, including diminished gray matter volume and increased white matter hyperintensities.71 Another shared pathological explanation is neuroinflammation. Disruptions in cerebral blood flow, oxygen supply, and insulin signaling caused by CMM are associated with several hallmarks of brain aging, including oxidative stress and the accumulation of advanced glycation end products. Simultaneously, depression may activate pro-inflammatory mediators, which may lead to cerebral small vessels impairment, with a consequent reduction in cerebral blood flow, causing cognitive deficits. In the current study, we did not incorporate any brain imaging or blood-based inflammatory markers. The sole potential pathological predictor we examined was the APOE genotype, which did not influence the correlation between the co-occurrence of CMM and depression and cognition. Future research is warranted to offer a more comprehensive exploration of the pathophysiological mechanisms that underlie the co-occurrence of CMM and depression, as well as their impact on cognition.
The prevalence of overall multimorbidity burden has experienced a rapid escalation, ranging from 30% to 82% across the transition from middle-aged to old-aged populations.72 Identifying diverse patterns of multimorbidity in terms of different combinations of physical and psychological disorders could provide useful implications for the future management of cognitive impairment. Moreover, our study adds to the growing body of evidence that preventing cognitive decline and dementia may require multiple interventions addressing related risk factors rather than focusing solely on individual factors.7 While this has been demonstrated for CMM comprising hypertension and diabetes,73 our results suggest that additionally targeting depression may enhance the effectiveness of interventions.
Our study has several strengths. We harmonized data from 14 longitudinal cohorts representing different ethno-racial groups and countries. Our analyses were well powered for assessing how the co-occurrence of CMM and depression is associated with cognitive outcomes. Using an IPD meta-analysis, we controlled for the same covariates across studies. We conducted both one-step and two-step IPD meta-analyses and consistent results were found between these two methods. We validated our results in three Asian studies with different study settings, thus demonstrating that our results may have generalizability to other populations.
However, there were limitations to our study. Harmonizing data from multiple studies entailed a loss of granularity and posed the risk of misclassification. Self-reported disease diagnoses could underestimate the measurement of disease prevalence. Simultaneously, multimorbidity might be influenced by detection biases (i.e., one disease is detected, prompting testing for others). Definitions of hypertension, hyperlipidemia, diabetes, stroke, and heart disease evolve over time and vary across locations, potentially resulting in differences in diagnosis. Similarly, the cohort studies we included varied in the cognitive instruments and criteria for dementia. Notably, some studies adhered to the diagnostic criteria of DSM-IV which although still widely applied, could be outdated. In terms of depression definition, our analyses primarily relied on self-reported questionnaires for depressive symptoms, potentially leading to over-estimation of depressive patients. Additionally, the inconsistent prevalence may also be attributed to the heterogeneous definition of depression. Moreover, this study did not include adjustment for additional confounding factors, including various socioeconomic and lifestyle factors, due to data availability constraints, despite our adjustments for numerous known potential confounders. More covariates will be needed in our future investigations. Additionally, the reverse causation between the co-occurrence of CMM and depression and cognitive decline remains possible, although we performed sensitivity analysis by excluding potential undiagnosed dementia participants to minimize the risk. The drop-out rate after baseline and the varying number of participants at each visit may introduce bias through attrition and data loss. However, we performed sensitivity analyses addressed missing values by multiple imputation and completed cases, and that results were consistent with our main findings. In this study, we did not account for death as a competing risk for cognitive decline. This may have led to an underestimation of the coefficients we identified, as older adults with more severe CMM and depression may be at a higher risk of death. Furthermore, it may explain the sex and region differences in the associations between the comorbidity of CMM and depression and cognitive decline, given the longer disability-adjusted life years due to CMDs and depression among females and older adults in HICs.74,75 Therefore, future studies should consider including death as a competing risk.
In conclusion, our study demonstrated that baseline CMM and depression were independently associated with cross-sectional and longitudinal cognitive function among older adults. The joint association of CMM and depression was associated with lower cognitive performance and faster cognitive decline. Our findings highlighted the importance of investigating age-related co-morbidities in a multi-dimensional perspective. Targeting both cardiometabolic and psychological conditions to prevent cognitive decline could enhance effectiveness.
Contributors
XinX and XiaoX conceptualized the study. XHZ performed the data analysis and wrote the first draft. CC and XX designed the study, developed the protocol and obtained the ethics of this study and conceptualized this study. DML, JC, PSS, YCZ, CC, XLX and XX, reviewed the biostatistical results and revised the manuscript, conceived the study and methodology, and interpreted the data. DML, JC, PSS, CC, XLX and XX, were responsible for funding acquisition, data curation, and investigation. All authors revised the manuscript and provided critical revisions to the manuscript. All authors were responsible for reviewing and editing the manuscript.
Data sharing statement
For COSMIC: Data were provided by the contributing studies on the understanding and proviso that the relevant study leaders be contacted for further use of their data and additional formal data sharing agreements be made. Researchers can apply to use COSMIC data by completing a COSMIC Research Proposal Form available from https://cheba.unsw.edu.au/consortia/cosmic/research-proposals.
For external validations studies: The data that support the findings of this study are available from the Memory, Ageing, and Cognition Centre (MACC), Department of Pharmacology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore. All identified raw data of each participant in the EDIS, Harmonization and Coast are accessible on reasonable request to Christopher Chen (christopher_chen@nuhs.edu.sg) and Xin Xu (xuxinsummer@zju.edu.cn) following appropriate research ethical approvals and with permission of the authors.
Declaration of interests
CC declares funding to their institution from the National Medical Research Council of Singapore. RBL declares receiving payments for royalties or licenses from Wolff's Headache (7th and 8th Editions), Oxford University Press (2009), Wiley, and Inform. RBL has received consulting fees, honoraria for lectures, support for attending meetings and/or travel, or served on data safety monitoring boards or advisory boards for AbbVie (Allergan), the American Academy of Neurology, the American Headache Society, Amgen, Avanir, Axon, Axsome, Biohaven, Biovision, Boston Scientific, Dr. Reddy's (Promius), Electrocore, Eli Lilly, eNeura Therapeutics, Equinox, GlaxoSmithKline, Grifols, Lundbeck (Alder), Manistee, Merck, Pernix, Pfizer, Satsuma, Supernus, Teva, Trigemina, Vector, and Vedanta. RBL's institution has received funding from the FDA, the National Institutes of Health (NIH), and the National Institute on Aging (NIA). RBL holds stock in Axon, Biohaven Holdings, CoolTech, and Manistee. MJK declares funding to their institution from the NIH (grant numbers NIA P01 AG03949). NS declares participation as a member of the data safety monitoring board (uncompensated) for the public-private funded Phase II study PRimus AD in Germany and reports that their institution has received funding from Novo Nordisk, unrelated to the submitted work. Both HCH and SG declare funding to their institution from the NIH (grant numbers R01AG009956, P30AG072976, and K07AG076659). MG declares funding to their institution from the NIA (grant number R37AG023651). C–CHC declares that their institution received funding from the NIH. AL, CDC and EL declare that their institution received funding from the Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness, Madrid, Spain (grant numbers 94/1562, 97/1321E, 98/0103, 01/0255, 03/0815, 06/0617, G03/128, 12/02254, 16/00896, 19/01874), as well as from the European Regional Development Fund (FEDER) of the European Union and the Government of Aragón (grant number B15_17R). CDC also declares receiving support for attending scientific meetings from Almirall, Lilly, Pfizer, Esteve, AstraZeneca, Novartis, Lundbeck, Casen Recordati, Janssen, and Rovi. PSS declares receiving payments for advisory board meetings for Biogen Australia and Roche Australia, honoraria for lectures from Alkem Labs; and funding to their institution from the National Health and Medical Research Council of Australia (grant number APP1169489). Both PSS and DL declare funding to their institution from the NIH (grant number 1RF1AG057531–01). Both PSS and JDC declare funding to their institution from the NIH (grant number 2R01AG057531-02A1). DL holds editor roles in the Frontiers journals. PSS holds leadership roles in the VASCOG Society (Executive Committee; unpaid) and the World Psychiatric Association (Planning Committee; unpaid). All other authors declare no competing interests.
Acknowledgements
For COSMIC: The head of COSMIC is PSS, and the study coordinator is DML. The research scientific committee leads the scientific agenda of COSMIC and provides ongoing support and governance; it is comprised of member study leaders. The COSMIC research scientific committee and additional principal investigators are listed at https://cheba.unsw.edu.au/consortia/cosmic/scientific-committee. We thank the participants and their informants for their time and generosity in contributing to this research. We also thank the research teams for the contributing cohort studies. Funding for COSMIC comes from the NIA–NIH (award numbers 1RF1AG057531–01 and 2R01AG057531-02A1). The Bambuí Cohort Study of Ageing was funded by Financiadora de Estudos eProjetos, Conselho Nacional de Desenvolvimento Científico e Tecnológicos, and Fundação de Amparo Pesquisa do Estado de Minas Gerais. The EAS cohort was funded by NIA P01 AG03949. The ESPRIT project is financed by the regional government of Languedoc-Roussillon, the Agence Nationale de la Recherche Project 07 LVIE 004, and an unconditional grant from Novartis. The IMAS study was supported by the Canadian Institutes of Health Research, Mobility in Aging Initiative (funding reference number AAM108751). The Invece. Ab study was funded by own funds and supported in part by "Federazione Alzheimer Italia”, Milan, Italy (AG); The MYHAT study was funded by a grant made by NIA to the University of Pittsburgh (grant number R37AG023651). The SALSA study was supported by National Institute on Aging grants R01 AG12975 (M Haan PI), R01 AG10220 (D Mungas PI), and P30 AG043097 (L Hinton PI). The SLASI was supported by a research grant (number 03/1/21/17/214) from the Biomedical Research Council, Agency for Science, Technology and Research, Singapore. The Vallecas Project is carried out in the Alzheimer Center Reina Sofía Foundation, funded by CIEN Foundation and Reina Sofía Foundation. The HELIAD cohort was funded by the Alzheimer's Association, European Social Fund, and Greek Ministry of Health. The ZARADEMP project was Supported by grants from the Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness, Madrid, Spain (grants 94/1562, 97/1321E, 98/0103, 01/0255, 03/0815, 06/0617, G03/128, 12/02254, 16/00896, 19/01874), and the Fondo Europeo de Desarrollo Regional of the European Union and Gobierno de Aragón, (grant B15_17R). The LEILA75+ study was funded by the Interdisciplinary Centre for Clinical Research at the University of Leipzig (grant 01KS9504). The Ibadan&IIDPIN were funded by NIH grant R01 AG09956 and WellCome Trust.
For external validations studies: This work was funded by Center Grant from the National Medical Research Council Singapore (NMRC/CG/NUHS/2010 and NMRC/CG/013/2013, grant number R-184-006-184-511).
Research reported in this publication was supported by the Natural Science Foundation of China (grant number NSFC/72274170 and NSFC/72474197) and NIA-NIH (grant number R01AG057531). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2024.101198.
Appendix A. Supplementary data
References
- 1.van Dyck C.H., Swanson C.J., Aisen P., et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388:9–21. doi: 10.1056/NEJMoa2212948. [DOI] [PubMed] [Google Scholar]
- 2.Amoretti M., Amsler C., Bonomi G., et al. Production and detection of cold antihydrogen atoms. Nature. 2002;419:456–459. doi: 10.1038/nature01096. [DOI] [PubMed] [Google Scholar]
- 3.Peters R., Booth A., Rockwood K., Peters J., D'Este C., Anstey K.J. Combining modifiable risk factors and risk of dementia: a systematic review and meta-analysis. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-022846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khondoker M., Macgregor A., Bachmann M.O., Hornberger M., Fox C., Shepstone L. Multimorbidity pattern and risk of dementia in later life: an 11-year follow-up study using a large community cohort and linked electronic health records. J Epidemiol Community Health. 2023;77:285–292. doi: 10.1136/jech-2022-220034. [DOI] [PubMed] [Google Scholar]
- 5.Grande G., Marengoni A., Vetrano D.L., et al. Multimorbidity burden and dementia risk in older adults: the role of inflammation and genetics. Alzheimer's Dementia. 2021;17:768–776. doi: 10.1002/alz.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albanese E., Launer L.J., Egger M., et al. Body mass index in midlife and dementia: systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimer's Dementia. 2017;8:165–178. doi: 10.1016/j.dadm.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livingston G., Huntley J., Sommerlad A., et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbiellini Amidei C., Fayosse A., Dumurgier J., et al. Association between age at diabetes onset and subsequent risk of dementia. JAMA. 2021;325:1640–1649. doi: 10.1001/jama.2021.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Justin B.N., Turek M., Hakim A.M. Heart disease as a risk factor for dementia. Clin Epidemiol. 2013;5:135–145. doi: 10.2147/CLEP.S30621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuźma E., Lourida I., Moore S.F., Levine D.A., Ukoumunne O.C., Llewellyn D.J. Stroke and dementia risk: a systematic review and meta-analysis. Alzheimers Dement. 2018;14:1416–1426. doi: 10.1016/j.jalz.2018.06.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma L., Wang J., Li Y. Insulin resistance and cognitive dysfunction. Clin Chim Acta. 2015;444:18–23. doi: 10.1016/j.cca.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Ou Y.-N., Tan C.-C., Shen X.-N., et al. Blood pressure and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 209 prospective studies. Hypertension. 2020;76:217–225. doi: 10.1161/HYPERTENSIONAHA.120.14993. [DOI] [PubMed] [Google Scholar]
- 13.Vassilaki M., Aakre J.A., Cha R.H., et al. Multimorbidity and risk of mild cognitive impairment. J Am Geriatr Soc. 2015;63:1783–1790. doi: 10.1111/jgs.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben Hassen C., Fayosse A., Landré B., et al. Association between age at onset of multimorbidity and incidence of dementia: 30 year follow-up in Whitehall II prospective cohort study. BMJ. 2022 doi: 10.1136/bmj-2021-068005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steffens D.C. Vascular depression: is an old research Construct finally ready for clinical prime time? Biol Psychiatry. 2019;85:441–442. doi: 10.1016/j.biopsych.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Sinclair A.J., Abdelhafiz A.H. Cardiometabolic disease in the older person: prediction and prevention for the generalist physician. Cardiovasc Endocr Me. 2020;9:90–95. doi: 10.1097/XCE.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agbonlahor O., DeJarnett N., Hart J.L., Bhatnagar A., McLeish A.C., Walker K.L. Racial/ethnic discrimination and cardiometabolic diseases: a systematic review. J Racial Ethn Health. 2023 doi: 10.1007/s40615-023-01561-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Angelantonio E., Kaptoge S., Wormser D., et al. Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314:52. doi: 10.1001/jama.2015.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerdts E., Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med. 2019;25:1657–1666. doi: 10.1038/s41591-019-0643-8. [DOI] [PubMed] [Google Scholar]
- 20.Ling J., Koye D., Buizen L., Khunti K., Montvida O., Paul S.K. Temporal trends in co-morbidities and cardiometabolic risk factors at the time of type 2 diabetes diagnosis in the UK. Diabetes Obes Metabol. 2021;23:1150–1161. doi: 10.1111/dom.14323. [DOI] [PubMed] [Google Scholar]
- 21.Chang Y.-H., Liu M.-E., Huang C.-C., et al. Cognitive performance in older elderly men with late-life depression and cardiovascular comorbidities: symptomatological correlation. Ann Gen Psychiatr. 2013;12:36. doi: 10.1186/1744-859X-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang D., Tang X., Shen P., et al. Multimorbidity of cardiometabolic diseases: prevalence and risk for mortality from one million Chinese adults in a longitudinal cohort study. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-024476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kontari P., Fife-Schaw C., Smith K. Clustering of cardiometabolic risk factors and dementia incidence in older adults: a cross-country comparison in england, the United States, and China. J Gerontol. 2023;78:1035–1044. doi: 10.1093/gerona/glac240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tai X.Y., Veldsman M., Lyall D.M., et al. Cardiometabolic multimorbidity, genetic risk, and dementia: a prospective cohort study. Lancet Healthy Longev. 2022;3:e428–e436. doi: 10.1016/S2666-7568(22)00117-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dove A., Guo J., Marseglia A., et al. Cardiometabolic multimorbidity and incident dementia: the Swedish twin registry. Eur Heart J. 2023;44:573–582. doi: 10.1093/eurheartj/ehac744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dove A., Guo J., Wang J., et al. Cardiometabolic disease, cognitive decline, and brain structure in middle and older age. Alzheimers Dement. 2024;16 doi: 10.1002/dad2.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin Y., Liang J., Hong C., Liang R., Luo Y. Cardiometabolic multimorbidity, lifestyle behaviours, and cognitive function: a multicohort study. Lancet Healthy Longev. 2023;4:e265–e273. doi: 10.1016/S2666-7568(23)00054-5. [DOI] [PubMed] [Google Scholar]
- 28.Neergaard J.S., Dragsbæk K., Christiansen C., et al. Metabolic syndrome, insulin resistance, and cognitive dysfunction: does your metabolic profile affect your brain? Diabetes. 2017;66 doi: 10.2337/db16-1444. [DOI] [PubMed] [Google Scholar]
- 29.Kontari P. Association of cardiometabolic and genetic risk with incidence of dementia. Lancet Healthy Longev. 2022;3:e374–e375. doi: 10.1016/S2666-7568(22)00127-1. [DOI] [PubMed] [Google Scholar]
- 30.Fadini G.P., Morieri M.L. Deciphering dementia in the cardiometabolic continuum. Eur Heart J. 2023;44:583–585. doi: 10.1093/eurheartj/ehac691. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y., Chen Y., Ma L. Depression and cardiovascular disease in elderly: current understanding. J Clin Neurosci. 2018;47:1–5. doi: 10.1016/j.jocn.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 32.Howren M.B., Lamkin D.M., Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 33.Harshfield E.L., Pennells L., Schwartz J.E., et al. Association between depressive symptoms and incident cardiovascular diseases. JAMA. 2020;324:2396. doi: 10.1001/jama.2020.23068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiao Y., Ding Y., Li G., Lu Y., Li S., Ke C. Role of depression in the development of cardiometabolic multimorbidity: findings from the UK Biobank study. J Affect Disord. 2022;319:260–266. doi: 10.1016/j.jad.2022.09.084. [DOI] [PubMed] [Google Scholar]
- 35.Huang Z.-T., Luo Y., Han L., et al. Patterns of cardiometabolic multimorbidity and the risk of depressive symptoms in a longitudinal cohort of middle-aged and older Chinese. J Affect Disord. 2022;301:1–7. doi: 10.1016/j.jad.2022.01.030. [DOI] [PubMed] [Google Scholar]
- 36.Dove A., Marseglia A., Shang Y., et al. Cardiometabolic multimorbidity accelerates cognitive decline and dementia progression. Alzheimer's Dementia. 2023;19:821–830. doi: 10.1002/alz.12708. [DOI] [PubMed] [Google Scholar]
- 37.Zhong X., Wu Z., Ouyang C., et al. Cardiovascular diseases and related risk factors accelerated cognitive deterioration in patients with late-life depression: a one-year prospective study. Int Psychogeriatr. 2019;31:1483–1489. doi: 10.1017/S1041610218002041. [DOI] [PubMed] [Google Scholar]
- 38.Hu P., Lee J., Beaumaster S., et al. Cognitive function and cardiometabolic-inflammatory risk factors among older Indians and Americans. J Am Geriatr Soc. 2020;68:S36–S44. doi: 10.1111/jgs.16734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carney R.M., Freedland K.E., Miller G.E., Jaffe A.S. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res. 2002;53:897–902. doi: 10.1016/s0022-3999(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 40.Vassilaki M., Aakre J.A., Mielke M.M., et al. Multimorbidity and neuroimaging biomarkers among cognitively normal persons. Neurology. 2016;86:2077–2084. doi: 10.1212/WNL.0000000000002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lipnicki D.M., Makkar S.R., Crawford J.D., et al. Determinants of cognitive performance and decline in 20 diverse ethno-regional groups: a COSMIC collaboration cohort study. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samtani S., Mahalingam G., Lam B.C.P., et al. Associations between social connections and cognition: a global collaborative individual participant data meta-analysis. Lancet Healthy Longev. 2022;3:e740–e753. doi: 10.1016/S2666-7568(22)00199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lima-Costa M.F., Firmo J.O., Uchoa E. Cohort profile: the Bambui (Brazil) cohort study of ageing. Int J Epidemiol. 2011;40:862–867. doi: 10.1093/ije/dyq143. [DOI] [PubMed] [Google Scholar]
- 44.Katz M.J., Lipton R.B., Hall C.B., et al. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and alzheimer dementia in blacks and whites: a report from the einstein aging study. Alzheimer Dis Assoc Disord. 2012;26:335–343. doi: 10.1097/WAD.0b013e31823dbcfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ritchie K., Carriere I., Ritchie C.W., Berr C., Artero S., Ancelin M.-L. Designing prevention programmes to reduce incidence of dementia: prospective cohort study of modifiable risk factors. BMJ. 2010;341:c3885. doi: 10.1136/bmj.c3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caldas V.V.D.A., Zunzunegui M.V., Freire A.D.N.F., Guerra R.O. Translation, cultural adaptation and psychometric evaluation of the Leganés cognitive test in a low educated elderly Brazilian population. Arq Neuro-psiquiat. 2012;70:22–27. doi: 10.1590/s0004-282x2012000100006. [DOI] [PubMed] [Google Scholar]
- 47.Guaita A., Colombo M., Vaccaro R., et al. Brain aging and dementia during the transition from late adulthood to old age: design and methodology of the “Invece.Ab” population-based study. BMC Geriatr. 2013;13:98. doi: 10.1186/1471-2318-13-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ganguli M., Snitz B., Bilt J.V., Chang C.-C.H. How much do depressive symptoms affect cognition at the population level? The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) study. Int J Geriatr Psychiatr. 2009;24:1277–1284. doi: 10.1002/gps.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haan M.N., Mungas D.M., Gonzalez H.M., Ortiz T.A., Acharya A., Jagust W.J. Prevalence of dementia in older latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003;51:169–177. doi: 10.1046/j.1532-5415.2003.51054.x. [DOI] [PubMed] [Google Scholar]
- 50.Niti M., Yap K.-B., Kua E.-H., Tan C.-H., Ng T.-P. Physical, social and productive leisure activities, cognitive decline and interaction with APOE-ε4 genotype in Chinese older adults. Int Psychogeriatr. 2008;20 doi: 10.1017/S1041610207006655. [DOI] [PubMed] [Google Scholar]
- 51.Olazarán J., Valentí M., Frades B., et al. The Vallecas project: a cohort to identify early markers and mechanisms of Alzheimer's disease. Front Aging Neurosci. 2015;7 doi: 10.3389/fnagi.2015.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dardiotis E., Kosmidis M.H., Yannakoulia M., Hadjigeorgiou G.M., Scarmeas N. The Hellenic longitudinal investigation of aging and diet (HELIAD): rationale, study design, and cohort description. Neuroepidemiology. 2014;43:9–14. doi: 10.1159/000362723. [DOI] [PubMed] [Google Scholar]
- 53.Majithia S., Tham Y.-C., Chee M.-L., et al. Cohort profile: the Singapore Epidemiology of eye diseases study (SEED) Int J Epidemiol. 2021;50:41–52. doi: 10.1093/ije/dyaa238. [DOI] [PubMed] [Google Scholar]
- 54.Shaik M.A., Venketasubramanian N., Cheng C.-Y., et al. Ankle brachial index, MRI markers and cognition: the epidemiology of dementia in Singapore study. Atherosclerosis. 2017;263:272–277. doi: 10.1016/j.atherosclerosis.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Zhao X., Chong E.J.Y., Qi W., Pang T., Xu X., Chen C. Domain-Specific cognitive trajectories among patients with minor stroke or transient ischemic attack in a 6-year prospective Asian cohort: serial patterns and indicators. J Atten Disord. 2021;83:557–568. doi: 10.3233/JAD-210619. [DOI] [PubMed] [Google Scholar]
- 56.Mijajlović M.D., Pavlović A., Brainin M., et al. Post-stroke dementia – a comprehensive review. BMC Med. 2017;15:11. doi: 10.1186/s12916-017-0779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitchell A.J., Bird V., Rizzo M., Meader N. Diagnostic validity and added value of the geriatric depression scale for depression in primary care: a meta-analysis of GDS30 and GDS15. J Affect Disord. 2010;125:10–17. doi: 10.1016/j.jad.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 58.Vilagut G., Forero C.G., Barbaglia G., Alonso J. Screening for depression in the general population with the center for epidemiologic studies depression (CES-D): a systematic review with meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lipnicki D.M., Crawford J.D., Dutta R., et al. Age-related cognitive decline and associations with sex, education and apolipoprotein E genotype across ethnocultural groups and geographic regions: a collaborative cohort study. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wdi–the world by income and region. https://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html
- 61.Griffith L., van den Heuvel E., Fortier I., et al. Agency for Healthcare Research and Quality (US); Rockville (MD): 2013. Harmonization of cognitive measures in individual participant data and aggregate data meta-analysis.http://www.ncbi.nlm.nih.gov/books/NBK132553/ [PubMed] [Google Scholar]
- 62.Xu X., Chan Q.L., Hilal S., et al. The diagnostic utility of the NINDS-CSN neuropsychological battery in memory clinics. Dement Geriatr Cogn Dis Extra. 2016;6:276–282. doi: 10.1159/000445050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burke D.L., Ensor J., Riley R.D. Meta-analysis using individual participant data: one-stage and two-stage approaches, and why they may differ. Stat Med. 2017;36:855–875. doi: 10.1002/sim.7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jackson D., White I.R., Riley R.D. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med. 2012;31:3805–3820. doi: 10.1002/sim.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Little R.J., Rubin D.B. John Wiley & Sons; 2019. Statistical analysis with missing data. [Google Scholar]
- 67.Schafer J.L., Graham J.W. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- 68.Hou Y., Dan X., Babbar M., et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 69.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han L.K.M., Dinga R., Hahn T., et al. Brain aging in major depressive disorder: results from the ENIGMA major depressive disorder working group. Mol Psychiatry. 2021;26:5124–5139. doi: 10.1038/s41380-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ryan M.C., Hong L.E., Hatch K.S., et al. The additive impact of cardio-metabolic disorders and psychiatric illnesses on accelerated brain aging. Hum Brain Mapp. 2022;43:1997–2010. doi: 10.1002/hbm.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skou S.T., Mair F.S., Fortin M., et al. Multimorbidity. Nat Rev Dis Prim. 2022;8:48. doi: 10.1038/s41572-022-00376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ngandu T., Lehtisalo J., Solomon A., et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 74.Timmis A., Vardas P., Townsend N., et al. European Society of Cardiology: cardiovascular disease statistics 2021. Eur Heart J. 2022;43(8):799. doi: 10.1093/eurheartj/ehab892. [DOI] [PubMed] [Google Scholar]
- 75.GBD 2019 Mental Disorders Collaborators Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatr. 2022;9(2):137–150. doi: 10.1016/S2215-0366(21)00395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.