Abstract
The 5-hydroxytryptamine 7 receptor (5-HT7) is necessary for 5-HT to cause a concentration-dependent vascular relaxation and hypotension. 5-HT7 is recognized as having biased signaling, transduced through either Gs or β -arrestin. It is unknown whether 5-HT7 signals in a biased manner to cause vasorelaxation/hypotension. We used the recently described β-arrestin selective 5-HT7 receptor agonist serodolin to test the hypothesis that 5-HT7 activation does not cause vascular relaxation or hypotension via the β -arrestin pathway. Isolated abdominal aorta (no functional 5-HT7) and vena cava (functional 5-HT7) from male Sprague Dawley rats were used in isometric contractility studies. Serodolin (1 nM – 10 μM) did not change baseline tone of isolated tissues and did not relax the endothelin-1 (ET-1)-contracted vena cava or aorta. In the aorta, serodolin acted as a 5-HT2A receptor antagonist, evidenced by a rightward shift in 5-HT-induced concentration response curve [pEC50 5-HT [M]: Veh = 5.2α0.15; Ser (100 nM) = 4.49α0.08; p<0.05]. In the vena cava, serodolin acted as a 5-HT7 receptor antagonist, shifting the concentration response curve to 5-HT left and upward (%10 μM NE contraction; Veh = 3.2α1.7; Ser (10 nM) = 58α11; p< 0.05) and blocking relaxation of pre-contracted tissue to the 5-HT1A/7 agonist 5-carboxamidotryptamine. In anesthetized rats, 5-HT or serodolin was infused at 5, 25 and 75 μg/kg/min, iv. Though 5-HT caused concentration-dependent depressor responses, serodolin caused an insignificant small depressor responses at all three infusion rates. With the final dose of serodolin on board, 5-HT was unable to reduce blood pressure. Collectively the data indicate that serodolin functions as a 5-HT7 antagonist with additional 5-HT2A blocking properties. 5-HT7 activation does not cause vascular relaxation or hypotension via the β -arrestin pathway.
Keywords: 5-HT, 5-HT7 receptor, hypotension, biased agonist, cardiovascular disease
Graphical Abstract
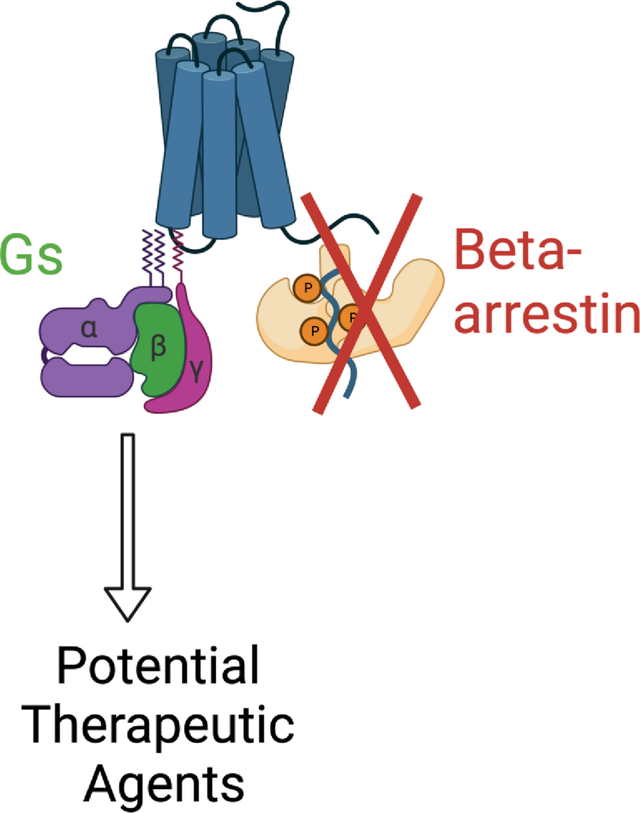
1.0. INTRODUCTION
Serotonin (5-hydroxytryptamine or 5-HT) was discovered as a vasoconstrictor, a substance that could change blood pressure in animal and human (Page and McCubbin 1953). It is without question that 5-HT is a vasoconstrictor primarily through activation of 5-HT2A receptor. However, with the discovery of other classes of 5-HT receptors, the (cardio)vascular actions of 5-HT expanded (Watts et al, 2012). Of specific interest in this study is the 5-HT7 receptor, cloned in 1993 (Ruat et al, 1993; Shen et al, 1993).
The 5-HT7 receptor is best known to play a role in circadian rhythm and photic response (Gardani and Biello, 2008; Sprouse et al, 2005); sleep and wakefulness (Monti and Jantos, 2014); temperature regulation (Hedlund et al, 2003); learning and memory (Roberts and Hedlund, 2012) and inflammation (Guseva et al, 2014). Relative to its function in the cardiovascular system, the 5-HT7 receptor mRNA was first localized to the blood vessels, both arteries and veins, by Ullmer et al (1995).
The functions of this receptor in control of vascular tone and blood pressure are of interest for several reasons. 5-HT possesses low nanomolar affinity for the 5-HT7 receptor such that free circulating 5-HT has the potential to activate this receptor (Watts et al, 2012). The 5-HT7 receptor also possesses interesting properties. It can be allosterically modified (Alberts et al, 2001); has constitutive activity (Andressen et al, 2018; Gellynck et al 2013; Hobson et al, 2003, Krobert et al, 2002; Kvachnina et al, 2009; Mahe et al, 2004; Purohit et al, 2005; Romero et al, 2006); has natural variants that change receptor pharmacology (Bruss et al, 2005; Kiel et al, 2003); can heterodimerize (Renner et al, 2012); and, discussed below, can be biased in its signal transduction.
Independent groups have consistently shown that infusion of 5-HT or its pathway limited precursor 5-hydroxytryptophan can lower blood pressure in animals, primarily the rat, that have either normal or elevated blood pressure (Balasubramaniam 1993, 1995; Baron et al, 1991; Cade and Fregly, 1992; Centurion et al, 2004; Dalton et al, 1986; DeVries et al, 1999; Ding et al, 1989; Echizen and Freed, 1981; Fregly et al, 1987; Itskovitz et al, 1989). Our group has focused on the mechanisms of long term hypotension caused by infusion of 5-HT over the course of a week (Diaz et al 2008). 5-HT, given as such, reduces total peripheral resistance (TPR), invoking involvement of the vasculature (Davis et al, 2012). We now know that 5-HT can relax skeletal muscle arterioles and reduce hindquarter vascular resistance (Jackson et al, 2023; Seitz et al, 2021), and relax veins in vitro and in vivo (Seitz et al, 2016; 2017; Watts et al, 2015). These events are predominantly mediated by activation of the 5-HT7 receptor, validated by a loss of many of these effects in the 5-HT7 KO rat we created (Demireva et al, 2019; Seitz et al, 2019) or antagonism by SB269970 (Hagan et al, 2000). Our findings follow important work by Terron et al whom first suggested the 5-HT7 receptor could be involved in a hypotensive response to 5-HT (Terron 1997; Terron et al, 2007). As such, the 5-HT7 receptor is an attractive target for developing new therapeutics for combating diseases of elevated total peripheral resistance, such as hypertension.
Only recently has the 5-HT7 receptor, a G protein coupled receptor, been observed to show biased agonism, with activation of Gs and β-arrestin pathways those towards which agonist may be differently biased (El Khamlichi et al, 2022). Our goal is to determine the ideal way of activating the 5-HT7 receptor to effect a fall in TPR/blood pressure with minimal side effects. Thus, we test here the hypothesis that the 5-HT7 receptor is not β-arrestin biased in its actions on blood pressure/vascular function. We use a vascular model – the isolated abdominal aorta and vena cava – that we’ve well established as being a vessel with a non-functional (aorta) and functional 5-HT7 receptor (vena cava) (Gonzalez-Pons et al, 2021; Seitz et al, 2019; Watts et al, 2015). We take advantage of the newly created drug serodolin as a β-arrestin biased agonist at the 5-HT7 receptor (El Khamlichi et al, 2022). Using a combination of in vitro and in vivo methods, our findings strongly support that activation of the 5-HT7 receptor unlikely utilizes β-arrestin pathways to effect the fall in vascular resistance/blood pressure.
2.0. MATERIALS AND METHODS
2.1. Animal approval, use and dissection
Male Sprague Dawley rats were purchased from Charles River Laboratories (Matawan, MI, USA). Previous studies indicate no difference in the cardiovascular functioning of the 5-HT7 receptor between male and female (Seitz et al, 2019). Thus, in the interest of reducing animal use, only males were used. Animals were on a normal diet [Teklad 22/5 Rodent diet (Madison WI, USA)]. Food and drinking water were available ad libitum. Procedures using animals complied with National Institutes of Health Guide for the Care and Use of Laboratory Animals (2011). Procedures used in this study were approved by the MSU Institutional Animal Care and Use Committee (PROTO202000009). Finally, this study was conducted with Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (essential 10 and recommended) in mind.
For in vitro work and before tissue removal, rats were given pentobarbital as a deep anesthetic (80 mg kg−1, ip). A bilateral pneumothorax was created prior to vessel dissection. The abdominal vessels [vena cava (AbIVC) and aorta (AbA)] were removed in toto and separated from one another under a stereomicroscope and in a Silastic®-coated dish filled with physiological salt solution [PSS in mM: NaCl 130; KCl 4.7; KH2PO4 1.18; MgSO4 • 7H2O 1.17; NaHCO3 14.8; dextrose 5.5; CaNa2EDTA 0.03, CaCl2 1.6 (pH 7.2).].
2.2. Isometric Contractility
Rings of tissues (3–5 mm wide) were placed onto two L-shaped stainless-steel rings. Rings were mounted in warmed (37°C) and aerated (95% O2, 5% CO2) tissue baths (10 ml or 30 ml volume) on Grass isometric transducers (FT03; Grass instruments, Quincy, MA, USA) connected to an 8 channel PowerLab C through an Octet Bridge (ADInstruments, Colorado Springs, CO, USA) or a 4 channel PowerLab connected to a Quad Bridge. Sample type (aortic or vena cava ring) and exposure to vehicle or inhibitor were randomized daily into different tissue baths. Tissues were placed under optimum resting tension (determined in previous experiments: AbA 4 grams; AbIVC: 1 gram) and allowed to equilibrate for one hour with frequent exchange of buffer. At this time, tissues were challenged with a maximum concentration of norepinephrine (10 μM). Tissues were washed to baseline, and one of the following protocols commenced. Tissues were used in only one of the following protocols.
Test of vehicle or serodolin from baseline: Vehicle (increasing % of DMSO to 0.1% or serodolin) were added in a cumulative fashion (1 nM - 10 μM), waiting at least three minutes before adding the next concentration. If an effect was observed, a plateau was allowed to be achieved before addition of next concentration.
Test of serodolin as a relaxant (vena cava only): AbIVC were contracted with a half maximal concentration of the thromboxane A2 mimetic U46619 (1 μM) or endothelin-1 (ET-1; 1 nM). Once contraction plateaued, either vehicle or serodolin (1 μM) were added to observe whether relaxation ensued.
Test of serodolin vs 5-carboxamidotryptamine (5-CT) (vena cava only): AbIVC were incubated with vehicle (0.01% DMSO) or serodolin (100 nM) for 45 minutes. Tissues were then contracted to a half-maximal concentration of U46619, and 5-CT (1 μM) added to stimulate relaxation.
Test of serodolin vs 5-HT: AbA and AbIVC were incubated with either vehicle (up to 0.1% DMSO) or one concentration of serodolin (10 nM, 100 nM, 1 μM) for one hour without washing. A cumulative concentration response curve to 5-HT (10−9 – 3×10−4 M) was then constructed.
2.4. In vivo administration of 5-HT, serodolin
The catheter of a telemeter probe (Data Sciences International, Minneapolis, MN, USA) was inserted into a femoral artery to measure blood pressure throughout the experiment. An open catheter was inserted via a femoral vein for drug infusion. A 30-minute baseline period was followed by a 20-minute infusion of 5-HT or serodolin at progressive rates of 5, 25 and 75 μg/min by means of an infusion pump. A 30-minute recovery period was allowed between 5-HT and serodolin infusion.
2.5. Data/Statistical analyses and presentation.
All quantitative data are reported as means α SEM for number of animals in parentheses. N represents the number of biological replicates (e.g. individual animals). For isometric contractile studies, contraction is reported as milligrams (tracing) or as a percentage of initial contraction to a maximum concentration of NE (10 μM). Relaxation is reported as a percentage of a half-maximal contraction to the thromboxane A2 mimetic U46619 or endothelin −1 (ET-1). Agonist potencies were calculated using a non-linear regression (curve fit) within GraphPad Prism 9.0 (La Jolla, CA, USA) and are reported as –log EC50 values [M]. Maximums are reported as the maximal effect achieved. Where a maximal response was not achieved, the actual potency (EC50 value) was considered equal or greater than the reported value. The pKB value or the apparent antagonist dissociation constant for an serodolin at the 5-HT2A receptors was calculated using the equation:
where DR is the EC50 value of agonist in the presence of antagonist/EC50 value in the absence of antagonist; [B] is the molar concentration of the antagonist.
Repeated measures two-way ANOVA followed by the Bonferroni post hoc test was used to compare concentration-response curves. In all cases, p < 0.05 was considered significant.
2.6. Materials
5-HT hydrochloride, norepinephrine hydrochloride and dimethylsulfoxide were obtained from Sigma Chemical Company (St. Louis, MO USA). 5-CT and SB269970 were purchased from Tocris (R & D systems, Minneapolis, MN, USA). ET-1 was purchased from Echelon Biosciences (Salt Lake City, UT, USA). U46619 was purchased from Cayman Chemical Co (Ann Arbor, MI USA). Serodolin was provided through a Materials Transfer Agreement with Dr. Morisset-Lopez, the CNRS and Orléans University.
3.0. Results
3.1. Serodolin did not change baseline tone of isolated aorta or vena cava
These two tissues – the AbA and AbIVC – were tested for their ability to respond to serodolin given in a cumulative fashion. When compared to a vehicle that carried an equivalent percentage of DMSO, serodolin neither increased nor decreased the baseline tone of either vessel (figure 1). Tissues were alive given their contraction to NE (in figure legend 1).
Figure 1.
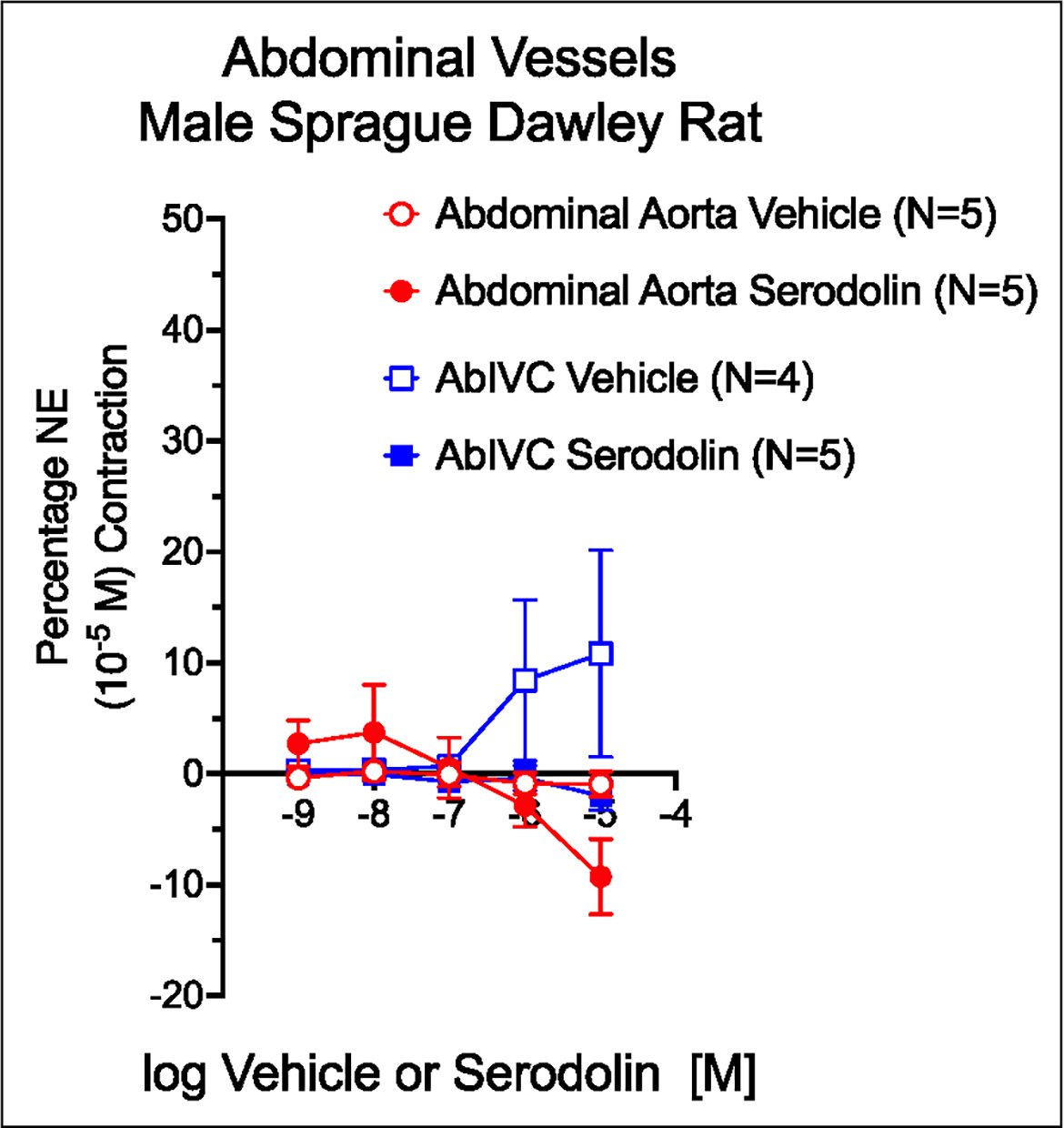
Effect of vehicle (graded DMSO) and serodolin in the abdominal aorta (red) and Vena Cava of the male Sprague Dawley rat. Points are meansαSEM for number of animals stated in parentheses. Contraction to NE (10−5 M; in milligrams): Aorta Vehicle = 2249α196; Aorta Serodolin = 1739α115; AbIVC Vehicle = 383α111; AbIVC Serodolin = 461α88.
3.2. Serodolin did not cause relaxation of contracted vena cava
The lack of response from baseline is not surprising given that this would not be expected unless tone had been established. However, when the AbIVC was contracted with half-maximal ET-1 or U46619, serodolin (1 μM) did not cause relaxation (figure 2A). Collectively, these data support serodolin is likely not an agonist that causes relaxation.
Figure 2.
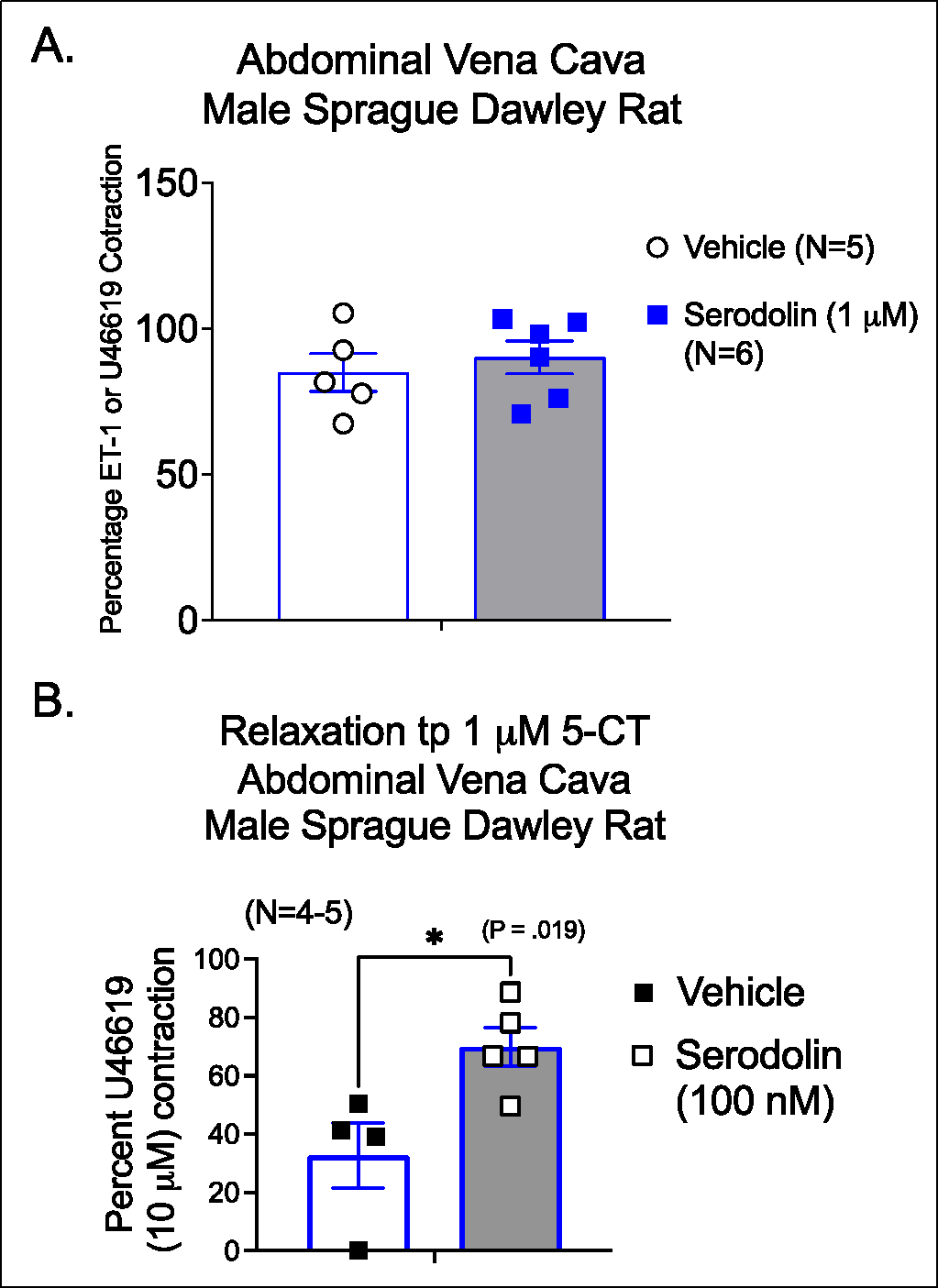
A. Effect of vehicle or serodolin on the contracted Vena Cava of the male Sprague Dawley rat. ET-1 contraction in milligrams: Vehicle = 447α163; Serodolin = 658α125. B. Ability of serodolin (100 nM) to reduce the relaxation caused by 5-CT (1 μM) in the contracted vena cava. ET-1/U46619 contraction in milligrams Vehicle = 294α78; Serodolin = 518α88. *signifies statistically significant differences as determined by an unpaired Students t test. Bars are means+SEM for number of animals stated in parentheses.
3.3. Serodolin antagonized 5-CT-induced relaxation in contracted vena cava.
In the next experiment, serodolin (100 nM) was tested for its ability to antagonize a response recognized as being 5-HT7 receptor dependent, 5-CT-induced relaxation. 5-CT caused a relaxation to 30% of U46619-induced contraction (figure 2B; open bar) that was reduced significantly in the presence of serodolin (figure 2B; shaded bar). This finding supports serodolin acting as a 5-HT7 receptor antagonist in this model.
3.4. Serodolin acted as a 5-HT2A and 5-HT7 receptor antagonist
In final in vitro experiments, increasing concentrations of serodolin (10 nM, 100 nM or 1 μM) were tested for the ability to modify 5-HT-indcued contraction. Figure 3 depicts effects in the AbA (figure 3A) and AbIVC (figure 3B), as well as pharmacological parameters calculated for Table 1. In the aorta, Serodolin caused a concentration dependent rightward shift of 5-HT induced contraction. The antagonist dissociation constant (pKB) calculated for 5-HT in the Ab Awa was 7.57. Contraction in the AbA is largely mediated by the 5-HT2A receptor; serodolin is most likely antagonizing the 5-HT2A receptor.
Figure 3.
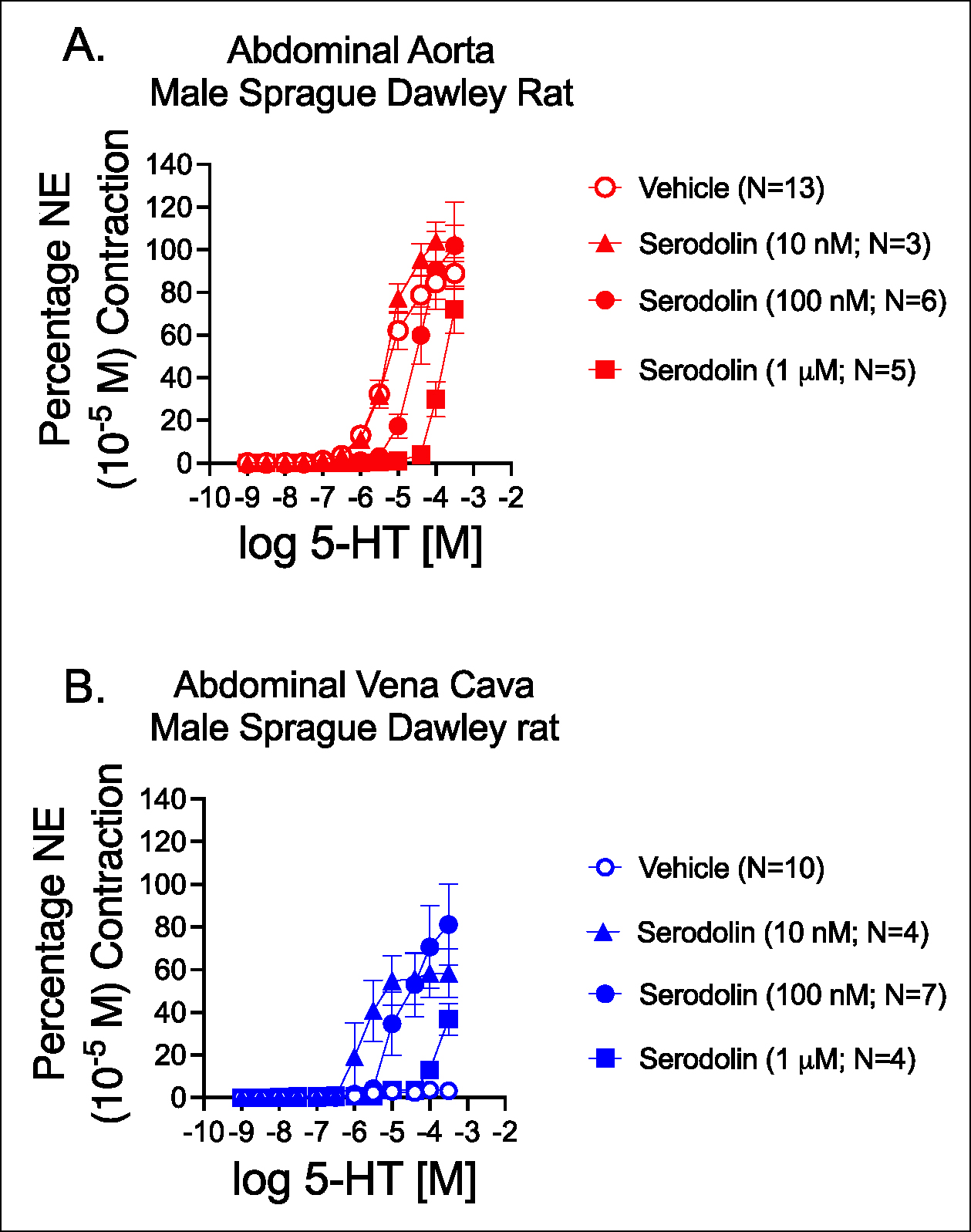
Effect of vehicle (graded DMSO) or increasing concentrations of serodolin in modifying 5-HT-induced contraction in the Abdominal Aorta (A) and Vena Cava (B) of the male Sprague Dawley rat. Points are meansαSEM for number of animals stated in parentheses. (NE maxes in milligrams for aorta were ( Vehicle = 2261α235; 10 nM Ser = 1867α220; 100 nM Ser = 2065α163; 1 μM Ser = 1849α85; p>0.05 by one way ANOVA. NE maxes for vena cava were: Vehicle = 393α96; 10 nM Ser = 606α117; 100 nM Ser = 509α106; 1 μM Ser = 260α109; p0.05 by one way ANOVA).
Table 1.
Potencies and Maximal contraction of 5-HT in vessels incubated with vehicle or serodolin. Potency is reported as −log EC50 [M] and maximums as a percentage of initial NE (10 μM) induced contraction. Reported as means±SEM.
| Tissue | Vehicle | 10 nM Ser | 100 nM Ser | 1 μM Ser |
|---|---|---|---|---|
| Abdominal Aorta (−log EC50) |
5.17±0.15 0/13 unstable |
5.27±0.04 (0/3 unstable) |
4.49±0.07* (1/6 unstable) |
3.86±0.06* (0/5 unstable) |
| Abdominal Vena Cava (−log EC50) |
5.36+0.48 (7/10 unstable) |
5.73+0.17 (0/4 unstable) |
4.93+0.13 (1/7 unstable) |
3.99 (2/4 unstable) |
| Abdominal Aorta Maximum (%) |
89.0±7.2 | 103.1±8.5 | 101.8±20.4 | 71.8±11.1 |
| Abdominal Vena Cava Maximum (%0 |
3.2±1.7 | 58.3±11.4* | 81.2±19.2* | 36.6±7.7 |
signifies statistically significant differences (p<0.05) from vehicle as determined by a one way ANOVA followed by Tukey’s multiple comparisons.
Unstable means Graphpad Prism could not calculate a value. The frequency of this occurrence is reported in the table with the denominator equivalent to the number of tissues tested.
The actions of serodolin were different in the AbIVC. Consistent with past results, 5-HT did not cause a concentration-dependent contraction in the AbIVC. Whereas serodolin alone did not modify contraction in the AbIVC (see Figure 2), it potentiated the effect of 5-HT in stimulating contraction at all tested concentrations. The potency of 5-HT to induce contraction was greatest in the presence of 10 nM serodolin, and progressively decreased as increasing concentrations were used. Table 1 shares the pharmacological parameters of these curves in figure 3. In the AbIVC, the increased potency of 5-HT can be explained by serodolin acting as a 5-HT7 receptor antagonist at a low concentration. With increasing serodolin concentration, 5-HT2A receptor antagonism was observed.
3.5. Serodolin alone did not decrease blood pressure and antagonized 5-HT-induced hypotension
Figures 4 and 5 share data on the effects of serodolin when given in vivo to anesthetized male Sprague Dawley rats. Serodolin, infused independently, did not modify blood pressure. By contrast, 5-HT caused the expected hypotension, best observed at the 25 μg/kg dose (figure 4). If this same dose response curve is constructed after the final addition of serodolin (75 μg/kg on board), 5-HT no longer caused a reduction in blood pressure (figure 5). Here, the ability of serodolin to antagonize a 5-HT7 receptor mediated event in vivo is consistent with 5-HT7 receptor antagonism exerted in vitro.
Figure 4.
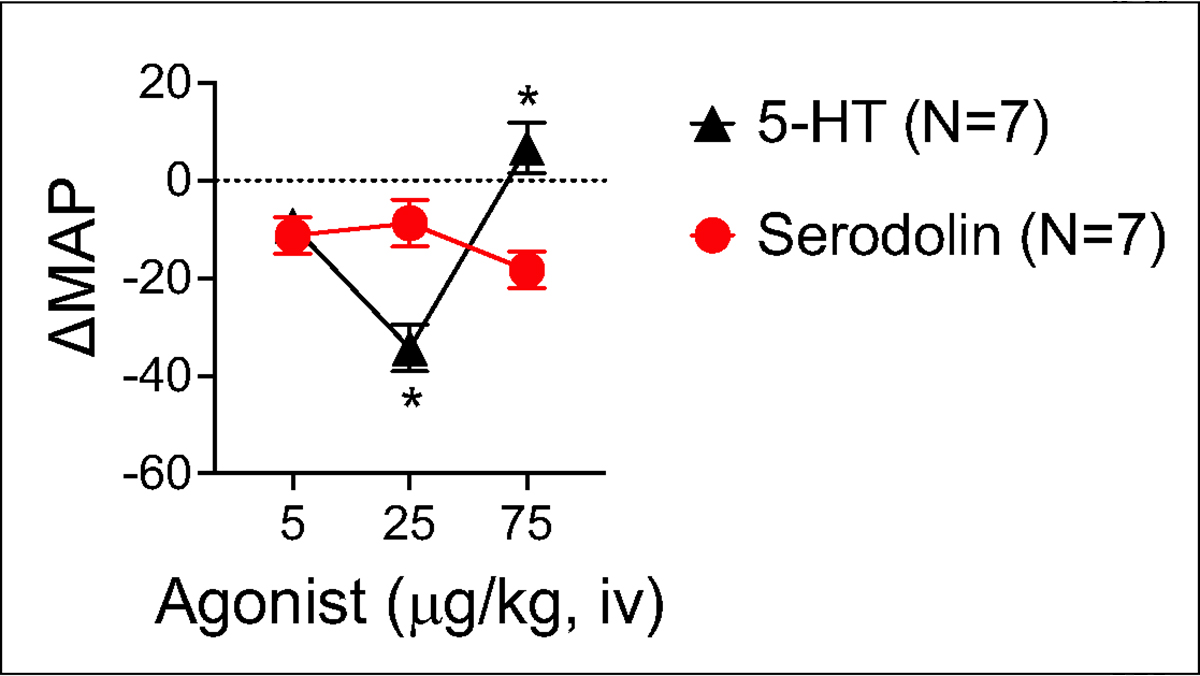
Comparison of change in mean arterial blood pressure (MAP) caused by infusion of 5-HT and serodolin when given iv in a dose-dependent fashion to anesthetized Sprague Dawley male rat. Points represent meansαSEM for number of animals in parentheses. * signifies statistical differences calculated through two way ANOVA.
Figure 5.
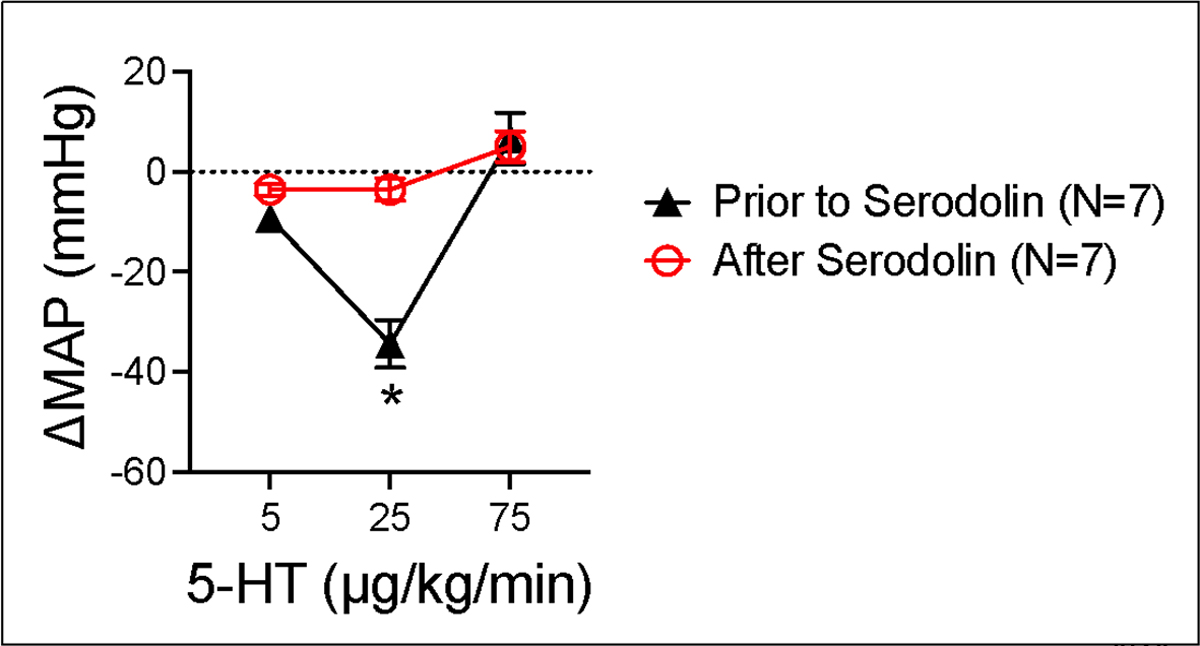
Antagonism of 5-HT-induced hypotension by serodolin (75 μg/kg) when 5-HT was administered iv in a dose-dependent fashion to anesthetized Sprague Dawley male rat. Points represent meansαSEM for number of animals in parentheses. * signifies statistical differences calculated through two way ANOVA.
4.0. Discussion
Blood pressure control is regressing in the US and groups of resistant (3 meds do not decrease blood pressure) and refractory (5 meds do not decrease blood pressure) hypertension exist (Acelajado et al, 2019; Egan et al, 2021; Muntner et al, 2022). These daunting facts raise the idea that we do not fully understand how both normal and elevated blood pressures are regulated. As such, we and others have invested in the idea that agonism at the 5-HT7 receptor could provide a new therapeutic. The present study supports that 5-HT7 receptor agonists that are not β-arrestin biased are likely the drugs with the best potential of therapeutic efficacy.
4.1. Serodolin shows no agonism but acts as an antagonist
Whether serodolin was tested from baseline or in a contracted tissue, serodolin did not stimulate either direct contraction or relaxation in either the isolated abdominal vena cava or aorta. Importantly, this is not because tissues were not viable. All data are reported as a percentage of an initial contraction (NE) or half-maximal contraction (ET-1/U46619). All tissues contracted to an acceptable magnitude to these agonists such that response allowed their continuance in the protocol. We conclude that serodolin does not have the ability to activate a receptor that would directly influence vascular contraction. This was validated by in vivo studies which support little to no direct effect of serodolin on mean arterial pressure.
Rather, our data support that serodolin, in these blood vessels, functions as a 5-HT7 receptor antagonist at low nM concentrations and at higher concentrations as a 5-HT2A receptor antagonist. The ability to antagonize the 5-HT7 receptor was particularly evident in the profound enhancement of 5-HT-induced contraction in the AbIVC. This finding is consistent with previous work which discovered that the 5-HT7 receptor restrained the contractile function of the 5-HT2A receptor in the vena cava (Gonzalez-Pons et al, 2021; Seitz et al, 2019). In these two studies either pharmacological blockade by the antagonist SB269970 (Gonzales-Pons et al, 2021; Hagan et al, 2000) or genetic removal (Seitz et al, 2019) of the 5-HT7 receptor unveiled a 5-HT contraction conducted through the 5-HT2A receptor in the rat abdominal vena cava. The mechanism of how this occurs, such as a physical association of the two 5-HT receptors that is modified with antagonism/loss, is not known. The 5-HT7 receptor can heterodimerize with the 5-HT1A receptor (Renner et al, 2012); we were unable to find reports of the 5-HT7 receptor heterodimerizing with the 5-HT2A receptor.
The ability of serodolin to act as a 5-HT7 receptor antagonist is consistent with its known pharmacology (El Khamlichi et al, 2022). Occupancy of the orthosteric site of the 5-HT7 receptor with no measurable efficacy would permit antagonism of 5-HT-stimulated events.
4.2. 5-HT7 biased ligands
Serodolin is among the first described β -arrestin biased ligands. Kim et al published a series of tetrahydroazepine derivatives with β-arrestin bias (Kim et al, 2018). In parallel with El Khamlichi et al, Onyameh published on β -arrestin biased 5-HT7 receptor agonists (2022).
The intent of production of these molecules was largely focused on effects in the central nervous system, including endpoints such as sleep time, grooming and pain. Here, we use (one of) them in a different way: to examine whether the 5-HT7 receptor, through the β-arrestin pathway, was sufficient to cause vascular relaxation or hypotension. Serodolin caused neither of these events, leading to the conclusion that activation of the β -arrestin pathway through the 5-HT7 receptor is not necessary for producing hypotension. As such, it is most likely activation of Gs through the 5-HT7 receptor that leads to vascular relaxation and hypotension.
There is a small amount of information about the Gs/β-arrestin bias of agonists used to interrogate the 5-HT7 receptor. While important, such information comes with the caveat of bias measures being done in artificial, constructed cells, typically human embryonic kidney or HEK cells, that allow for measure of cAMP (Gs) or β-arrestin trafficking. This makes it impossible to be fully confidentthat the observations made in these artificial systems could be translated to, in our case, the venous smooth muscle cell. In such systems, 5-CT activates cAMP production through activation of the Gs pathway through the 5-HT7 receptor, but may also minimally activate β-arrestin through protein kinase A (El Khamlichi et al 2022). 5-CT is significantly more potent than 5-HT in reducing blood pressure in the rat, in part because it lacks the affinity for the 5-HT2A receptor possessed by 5-HT. Lee et al (2021) calculated the bias factor for 5-HT and the 5-HT7 receptor agonist E-55888, finding that 5-HT was equivalently biased towards Gs and β-arrestin (bias factor 1:1), while E-55888 was more biased towards β-arrestin (bias of 0.34:1). Onyameh et al confirmed that 5-HT is equally biased to Gs and β-arrestin (2022). If these findings translate to the naïve 5-HT7 receptor, then the 5-HT-induced vascular relaxation and hypotension is likely mediated predominantly by Gs stimulation. It will be interesting to compare a 5-HT7 receptor agonist that has pure Gs bias to serodolin.
4.4. Limitations
We did not carry out assays that would demonstrate the ability of serodolin to recruit β-arrestin in any of the cell types of the blood vessel. This was not considered necessary given the significant proof of principle done by El-Khamlichi et al (2022). We have also not compared it to other β-arrestin biased ligands. Again, the present studies are proof of principle for us to now understand the β-arrestin arm of 5-HT7 receptor activation is not necessary for the hypotensive actions of 5-HT. Finally, our conclusions can only be made relative to the rat given that this is the only species tested.
5.0. Conclusions
We conclude that activation of the β-arrestin pathway through the 5-HT7 receptor is not necessary for the relaxant/hypotensive effects of 5-HT (Graphical Abstract). This knowledge allows focus on 5-HT7 receptor ligands that are biased towards Gs for development of cardiovascular therapeutics.
Highlights.
5-HT7 receptor stimulation mediates the hypotension when 5-HT is infused, but the ability of this receptor to function in a biased manner calls to question how 5-HT effects a hypotension: through Gs or β-arrestin?
Serodolin as a β -arrestin biased agonist at the 5-HT7 receptor was unable to relax isolated vessels nor did it cause a dose-dependent hypotension.
These findings point to the actions that proceed from 5-HT stimulation of the 5-HT7 receptor to be dependent on Gs signaling.
6.0. Acknowledgements, Conflicts, Funding and Author Contributions
Graphical abstract created using Biorender.com.
No AI tools were used in the writing of this manuscript.
Funding Sources
This work was funded by the National Heart Lung and Blood Institute through HL151413 and the Region Centre Val de Loire (project APR-IR TheraSEP).
Abbreviations
- 5-HT
5-hydroxytryptamine, serotonin
- 5-CT
5-carboxamidotryptamine
- ET-1
endothelin-1
- SB269970
(2R)-1-[(3-Hydroxyphenyl)sulfonyl]-2-[2-(4-methyl-1-piperidinyl)ethyl]pyrrolidine
Footnotes
Conflict of Interest
The authors have conflicts as noted below.
Greg D Fink: MSU Faculty; Funded by HL151413
Hannah Garver: MSU Employee
Severine Morisset-Lopez: CNRS
Franck Suzenet: Orléans University
Stephanie W Watts: MSU Faculty; funded by NIH HL151413; Keystone Scientific Advisory Board.
Author Contributions (CreDiT roles)
Greg D Fink : Conceptualization; Data Curation; Formal Analysis; Investigation; Methodology; Writing-review & Editing; Funding acquisition
Hannah Garver: Data Curation; Formal Analysis; Investigation; Methodology; Writing-review & Editing
Severine Morisset-Lopez: Conceptualization; Investigation; Resources; Funding acquisition Writing-review & Editing
Franck Suzenet: Supply of Serodolin; Funding acquisition
Stephanie W Watts: Conceptualization; Data Curation; Formal Analysis; Investigation; Methodology; Writing: Original Draft; Writing-review & Editing; Funding acquisition; Visualization.
Data Availability
All data generated during this study are included in this article.
7.0 References
- Acelajado MC, Hughes ZH, Oparil S, Calhoun DA. Treatment of resistant and refractory hypertension. CIRC RES 2019. 124:1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts GL, Chio CL, Im WB (2001) Allosteric modulation of the human 5-HT(7A) receptor by lipid amphipathic compounds. Mol Pharmacol 60:1349–1355. [DOI] [PubMed] [Google Scholar]
- Andressen KW, Ulsund AH, Krobert KA, Lohse MJ, Bunemann M, Levy FO (2018) Related GPCRs couple differently to Gs: preassociation between G protein and 5-HT7 serotonin receptor reveals movement of Gαs upon receptor activation. FASEB J 32:1059–1069. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam G, Lee HS, Mah SC (1993) Antihypertensive effect of chronic iv administration of 5-carboxamidotryptamine in spontaneously hypertensive rats. Eur J Pharmacol 237:207–213. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam G, Lee HS, Mah SC (1995) 5-carboxamidotryptamine attenuates the development of deoxycorticosterone acetate-salt in rats. Eur J Pharmacol 276:183–190. [DOI] [PubMed] [Google Scholar]
- Baron A, Riesselmann A, Fregly MJ (1991) Reduction in the elevated blood pressure of Dahl Salt sensitive rats treated chronically with L-5-hydroxytryptophan. Pharmacology 42:15–22. [DOI] [PubMed] [Google Scholar]
- Bruss M, Kiel S, Bonisch H, Kostanian A, Gothert M (2005) Decreased agonist, but not antagonist, binding to the naturally occurring Thr92Lys variant of the h5-HT7(a) receptor. Neurochem Int 47:196–203. [DOI] [PubMed] [Google Scholar]
- Cade JR, Fregly MJ, Privette M (1992) Effect of tryptophan and 5-hydroxytryptophan on the blood pressure of patients with mild to moderate hypertension. Amino Acids 2:133–140. [DOI] [PubMed] [Google Scholar]
- Centurion D, Glusa E, Sanchez-Lopez A, Valdivia LF, Saxena PR, Villalon CM (2004) 5-HT7, but not 5-HT2B, receptors mediate hypotension in vagosympathetcomized rats. Eur J Pharmacol 19:239–242. [DOI] [PubMed] [Google Scholar]
- Dalton DW, Feniuk W, Humphrey PP (1986) An investigation into the mechanisms of the cardiovascular effects of 5-hydroytryptamine in the conscious normotensive and DOCA-salt hypertensive rats. J Auton Pharmacol 6:219–228. [DOI] [PubMed] [Google Scholar]
- Davis RP, Pattison J, Thompson JM, Tiniakov R, Scrogin KE, Watts SW (2012) 5-hydroxytryptamine (5-HT) reduces total peripheral resistance during chronic infusion: direct arterial mesenteric relaxation is not involved. BMC Pharmacol 12:4 doi: 10.11186/1471-2210-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries P, De Visser PA, Heiligers JPC, Villalón CM, Saxena PR (1999) Changes in systemic and regional haemodynamics during 5-HT7 receptor mediated depressor responses in rats. Naunyn-Schmied Arch Pharmacol 359:331–338. [DOI] [PubMed] [Google Scholar]
- Demireva EY, Xie H, Flood ED, Thompson JM, Seitz BM, Watts SW (2019) Creation of the 5-hydroxytryptamine receptor knockout (5-HT7 KO) rat as a tool for cardiovascular research, Physiological Genomics, 51:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J, Ni W, King A, Fink GD and Watts SW (2008) 5-hydroxytryptamine lowers blood pressure in normotensive and hypertensive rats. J Pharmacol Exp Ther 325:1031–1038. [DOI] [PubMed] [Google Scholar]
- Ding XR, Stier CT, Itskovitz HD (1989) Serotonin and 5-hydroxytryptophan on blood pressure and renal blood flow in anesthetized rats. Am J Med Sci 297:290–293, 1989. [DOI] [PubMed] [Google Scholar]
- Echizen H, Freed CR (1981) Long-term infusion of L-5-hydroxytryptophan increases brain serotonin turnover and decreases blood pressure in normotensive rats. J Pharmacol Exp Ther 220:579–584. [PubMed] [Google Scholar]
- Egan BM, Li J, Sutherland SE, Rakotz MK and Wozniak GD (2021) Hypertension control in the United States 2009 to 2018: Factors underlying falling control rates during 2015 to 2018 across Age- and Race-ethnicity groups. Hypertension 78:578–587. [DOI] [PubMed] [Google Scholar]
- El Khamlichi C, Reverchon F, Hervouet-Coste N, Robin E, Chopin N, Deau E, Madouri F, Guimpied C, Colas C, Menuet A, Inoue A, Bojarski AJ, Guillaumet G, Suzenet F, Reiter E and Morisset-Lopez S (2022) Serodolin, a β-arrestin biased ligand of 5-HT7 receptor, attenuates pain related behaviors. Proc Natl Acad Sci 119(21)e2118847119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregly MJ, Lockley OE, and Sumners C (1987) Chronic treatment with L-5-hydroxytryptophan prevents the development of DOCA-salt induced hypertension in rats. J Hypertens 5:621–628. [DOI] [PubMed] [Google Scholar]
- Gardani M and Biello SM (2008) The effects of photic and nonphotic stimuli in the 5-HT7 receptor knockout mouse. Neurosci 152:245–253. [DOI] [PubMed] [Google Scholar]
- Gellynck E, Heyninck K, Andressen KW, Haegeman G, Levy FO, Vanhoenacker P, Van Craenenbroeck K (2013) The serotonin 5-HT7 receptors: two decades of research. Exp Brain Res 230:555–568. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Pons R, McRae K, Thompson JM and Watts SW: The 5-HT7 receptor restrains 5-HT-induced 5-HT2A mediated contraction in the isolated abdominal vena cava. J Cardiovasc Pharmacol, 78(2):319–327, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guseva D, Wirth A, Ponimaskin E (2014) Cellular mechanisms of the 5-HT7 receptor mediated signaling. Front Behav Neurosci 8:306. Doi: 10.3389/fnbeh.2014.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, Piper D, Smith MI, Upton N, Medhurst AD, Middlemiss DN, Riley GJ, Lovell PJ, Bromidge SM, Thomas DR (2000) Characterization of SB-269970-A, a selective 5-HT7 receptor antagonist. Br J Pharmacol 130:539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Danielson PE, Thomas EA, Slanina K, Carson MJ, Sutcliffe JG (2003) No hypothermic response to serotonin in 5-HT7 receptor knockout mouse. Proc Natl Acad Sci USA 100:1375–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson RJ, Geng J, Gray AD, Komuniecki RW (2003) SER-7b, a constitutively active Galpha S coupled 5-HT7 like receptor expressed in the Caenorhabditis elegans M4 pharyngeal motorneuron. J Neurochem 87:22–29. [DOI] [PubMed] [Google Scholar]
- Itskovitz HD, Werber JL, Sheridan AM, Brewer TF, Stier CT (1989) 5-hydroxytryptophan and carbidopa in spontaneously hypertensive rats. J Hypertens 7:311–315. [PubMed] [Google Scholar]
- Jackson WF, Daci A, Thompson JM, Fink GD, Watts SW: 5-HT7 receptors mediate dilation of rat cremaster muscle arterioles in vivo, Microcirculation e12808. doi: 10.1111/micc.12808, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel S, Bonisch H, Bruss M, Bothert M (2003) Impairment of signal transduction in response to stimulation of the naturally occurring Pro279Leu Variant of the h5-HT7(a) receptor. Pharmacogenetics 13:119–126. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim H, Lee J, Lee JK, Min S-J, Seong J, Rhim H, Tae J, Lee HJ, Choo H (2018) Discovery of β-arrestin biased ligands of 5-HT7R. J Med Chem 61(16):7218–7233. [DOI] [PubMed] [Google Scholar]
- Krobert KA, Levy FO (2002) The human 5-HT7 receptor splice variants and constitutive activity and inverse agonist effects. Br J Pharmacol 135:1563–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvachnina E, Dumuis A, Wlodarczyk J, Renner U, Cochet M, Richter DW, Ponimaskin E (2009) Constitutive Gs-mediated but not G12-mediated, activity of the 5-Hydroxytryptamine 7 receptor is modulated by the palmitoylation of its C-terminal domain. Biochim Biophy Acta 1793:1646–1655. [DOI] [PubMed] [Google Scholar]
- Lee J, Kwag R, Lee S, Kim D, Woo J, Cho J, Kim HF, Kim J, Jeon B, Choo H (2021) Discovery of G protein-biased ligands against 5-HT7R. J Med Chem 64(11):7453–7467. [DOI] [PubMed] [Google Scholar]
- Mahe C, Loetscher E, Feuerbach D, Muller W, Seiler MP, Schoeffter P (2004) Differential inverse agonist efficacies of SB-258719, SB-258741 and SB-269970 as human recombinant serotonin 5-HT7 receptors. Eur J Pharmacol 495:97–102. [DOI] [PubMed] [Google Scholar]
- Monti JM, Jantos H (2014) The role of serotonin 5-HT7 receptor in regulating sleep and wakefulness. Rev Neurosci 25:429–437. [DOI] [PubMed] [Google Scholar]
- Muntner P, Miles MA, Jaeger BC, Hannon L, Hardy ST, Ostchega Y, Wozniak G and Schwartz JE. (2022) Blood pressure control among US adults, 2009 to 2012 through 2017 to 2020. Hypertension 79:1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyameh EK, Ofori E, Bricker BA, Gonela UM, Eyunni SVK, Kang HF, Voshavar C, Ablordeppey SY (2022) Design and discovery of a high affinity, selective and β-arrestin biased 5-HT7 receptor agonist. Med Chem Res 31:274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page IH, McCubbin JW (1953) The variable arterial pressure response to serotonin in laboratory animals and man. Circ Res 1:354–362. [DOI] [PubMed] [Google Scholar]
- Purohit A, Smith C, Herrick-Davis K, Teitler M (2005) Stable expression of constitutively activated mutant 5-HT6 and h5HT7 serotonin receptors: Inverse agonist activity of antipsychotic drugs. Psychopharmacol 179:461–469. [DOI] [PubMed] [Google Scholar]
- Renner U, Zeug A, Woehler A, Niebert M, Dityatev A, Dityateva G, Gorinski N, Guseva D, Abdel-Galil D, Frohlich M, Doring F, Wischmeyer D, Richter DW, Neher E, Ponimaskin EG (2012) Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signaling and trafficking. J Cell Sci 125:2486–2499. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Hedlund PB (2012) The 5-HT7 receptor in learning and memory. Hippocampus 22:762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero G, Pujol M, Pauwels PJ (2006) Reanlaysis of constitutively active rat and human 5-HT7(a) receptors in HEK-293F cells demonstrates lack of silent preoperties for reported netural antagonists. Naunyn Schmiedebergs Arch Pharmacol 374:31–39. [DOI] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Aarrano JM, Schwartz JC (1993) Molecular cloning, characterization and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc Natl Acad Sci USA 90:8547–8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz BM, Demireva EY, Xie H, Fink GD, Krieger-Burke T, Burke WM, Watts SW (2019) 5-HT does not lower blood pressure in the 5-HT7 knockout rat, Physiological Genomics 51: 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz BM, Fink GD, Watts SW. (2021) Reduction in hindquarter vascular resistance supports 5-HT7 receptor mediated hypotension, Front Physiol. Integr Physiol doi: 10.3389/fphys.2021.679809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz BM, Krieger-Burke T, Fink GD, Watts SW (2016) Serial measurements of splanchnic vein diameters in rats using high frequency ultrasound, Frontiers in Pharmacology; experimental Pharmacology and Drug Discovery, 10.3389/fphar.2016.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz BM, Orer H, Krieger-Burke T, Darios E, Thompson J, Fink GD, Watts SW (2017) 5-HT causes splanchnic venodilation, Am J Physiol Heart Circ Physiol 313:H676–H686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Monsma FJ, Metcalf MA, Jose PA, Hambilin MW, Sibley DR (1993) Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J Biol Chem 268:18200–182004. [PubMed] [Google Scholar]
- Sprouse J, Li X, Stock J, McNeish J, Reynolds L (2005) Circadian rhythm phenotype of 5-HT7 receptor knockout mice: 5-HT and 8-OH-DPAT-induced phase advances of SCN neuronal firing. J Biol Rhythms 20:122–131. [DOI] [PubMed] [Google Scholar]
- Terrón JA (1997) Role of 5-ht7 receptors in the long-lasting hypotensive response induced by 5-hydroxytryptamine in the rat. Br J Pharmacol 121: 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terron JA, Martinez-Garcia E (2007) 5-HT7 receptor-mediated dilatation in the middle meningeal artery of anesthetized rats. Eur J Pharmacol 560:56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmer C, Schmuck K, Kalkman HO, Lubbert H (1995) Expression of serotonin receptor mRNAs in blood vessels. FEBS Lett 370:215–221. [DOI] [PubMed] [Google Scholar]
- Watts SW, Darios ES, Seitz BM, Thompson JM (2015) 5-HT is a potent relaxant in rat superior mesenteric veins. Pharmacol Res Perspect 3:e00103. Doi: 10.1002/prp2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts SW, Morrison SF, Davis RP, Barman SM (2012) Serotonin and blood pressure regulation. Pharmacol Rev 64:359–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated during this study are included in this article.


