Abstract
Objectives:
Human papillomavirus (HPV) positivity is a favorable prognostic factor in the general population of head and neck squamous cell carcinoma (HNSCC) patients. However, its impact on the survival of metastatic HNSCC of pharynx (mHNSC-P) patients is unclear. This study aims to investigate the associations between HPV status and survival in mHNSC-P patients.
Methods:
735 mHNSC-P patients diagnosed at first presentation from 2010 to 2016 were retrieved from the Surveillance, Epidemiology and End Result database (SEER). Chi-Squared test, univariate and multivariate cox proportional hazards model, Kaplan–Meier analysis, and log-rank test were applied to compare HPV-positive and -negative mHNSC-P patients.
Result:
Using univariate cox proportional hazards analysis, HPV status, primary site, T stage, treatment and distant metastatic site correlate with the overall survival (OS) and disease-specific survival (DSS) in mHNSC-P patients. Multivariate cox regression analysis shows that HPV-positive mHNSC-P patients experienced significantly better OS (HR: 0.62 CI: 0.51–0.76, p < 0.001) and DSS (HR: 0.73 CI: 0.58–0.91, p < 0.01) as compared to HPV-negative mHNSC-P patients. Subgroup analysis indicates that HPV-associated OS and DSS benefits exist in patients with metastatic HNSCC of oropharynx (mHNSC-OP) but not in patients with metastatic HNSCC of non-oropharynx (mHNSC-non-OP). Among mHNSC-OP patients, HPV positivity confers disease-specific survival benefit in patients with oligometastatic rather than polymetastatic patients. Furthermore, HPV associated OS and DSS advantages in mHNSC-OP with lung metastasis was observed.
Conclusion:
HPV-positive mHNSC-OP patients with lung metastasis show better survival than HPV-negative mHNSC-OP patients, providing key information to guide patient treatment approaches.
Keywords: Human papillomavirus associated head and neck squamous cell carcinoma; The Surveillance, Epidemiology and End Result database; Overall survival; Disease-specific survival, metastatic head and neck squamous cell carcinoma of pharynx; Oropharyngeal squamous cell carcinoma; Non-oropharyngeal pharyngeal squamous cell carcinoma; Lung metastasis; Bone metastasis
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a heterogeneous group of malignancies that arise in the mucosal epithelium of the oral cavity, oropharynx, hypopharynx, and larynx [1]. Approximately 890,000 new cases are diagnosed worldwide, and 430,000 deaths occur annually [2]. The prevalence of HNSCC is geographically variable; carcinogen-driven HNSCC is common among patients from Asia and Australia, whereas HPV infection now accounts for a significant percentage of HNSCC cases in the USA and western Europe with a continued rise in prevalence [3–5].
HPV-positive HNSCC differs from HPV-negative HNSCC in epidemiological, etiological, biological, and clinical presentations [6]. HPV infection causes around 70% of oropharyngeal squamous cell carcinoma (OPSCC) and a small percentage of cancers from non-oropharyngeal anatomical sites, including 25% of larynx and hypopharynx and 3% of the oral cavity [7]. The tumor cell of HPV-positive HNSCC tends to spread to the cervical lymph node at a relatively early T stage [8]. Notably, HPV-positive OPSCC patients with cervical lymph node metastases have better survival outcomes than HPV-negative OPSCC patients [9]. In addition to cervical lymph node involvement, around 15% of HPV-positive OPSCC patients can also develop distant metastasis [10,11], even though numerous studies consistently show that HPV positivity is a well-known favorable prognosis indicator for a general HNSCC patient [1,12]. There are very few studies examining the relationship between HPV positivity and survival of patients with metastatic HNSCC. The importance of knowing the association of HPV positivity with prognosis in late stage HNSCC patients with distant metastases can be of tremendous benefit in patient consulting as well as clinical trial design since new trials would more likely enroll late stage HNSCC patients with metastases rather than early stage HNSCC patients.
Currently, there are very few studies that value HPV positivity in relation to survival of metastatic HNSCC of oropharynx (mHNSC-OP) patients [13–16]. No statistical survival difference between the recurrent/metastatic HPV-positive and HPV-negative HNSCC patients [17], however, this study is underpowered due to its very small sample size. The role of HPV positivity in metastatic HNSCC patients, especially in metastatic HNSCC of non-oropharynx (mHNSC-non-OP) patients require more confirmatory investigations. In this study, a cohort of HNSCC patients retrieved from the Surveillance, Epidemiology and End Result database (SEER), which collects U.S. nationwide cancer cases, was used for analysis to avoid small sample size, single institute limitations, and provide sufficient cases for further sub-grouped analysis. Patient cohort used for analysis was narrowed down to metastatic oropharyngeal cancer and non-oropharyngeal cancer cases. Our results show a survival advantage in overall survival (OS) and disease-specific survival (DSS) in HPV-positive mHNSC-OP compared to HPV-negative mHNSC-OP and no evidence to confirm the survival benefits from HPV positivity in mHNSC-non-OP patients, providing physicians clues in patients consultant and therapy discussions.
Patients and methods
Study population
The National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) database (http://seer.cancer.gov) recording the morbidity, mortality, and disease status of millions of cancer patients in the United States, was used for in this study. We retrieved 40,866 cases from SEER*Stat Database: Incidence - SEER 18 Regs Custom Data Head and Neck (select schemas with HPV recode and additional treatment fields), Nov 2018 Sub (2010–2016) by selecting Site and Morphology. Site recode ICD-O-3/WHO 2008 = ‘Oral Cavity and Pharynx’, ‘Tongue’, ‘Gum and Other Mouth’, ‘Nasopharynx’, ‘Tonsil’, ‘Oropharynx’, ‘Hypopharynx’, or ‘Other Oral Cavity and Pharynx’. We narrowed down the HSNCC cases to a 735-patient cohort that was used in this study after fulfilling the following criteria: 1) with distant metastases (M1 for patients diagnosed with the 7th derived AJCC M stage); 2) confirmed HPV status are available. The detailed ICD-O-3 site codes were summarized in supplementary Table 1. Only pharyngeal cancers were included after above selections. Metastatic site was identified under variables of “SEER Combined Mets at DX-bone (2010+)”, “SEER Combined Mets at DX-liver (2010+)”, “SEER Combined Mets at DX-lung (2010+)”, “CS mets at dx (2004–2015)”, “Mets at DX-Distant LN (2016+)” and “Mets at DX-Other (2016+)”.
Patient characteristics
All patients were divided into two groups: HPV-negative and HPV-positive. The clinical characteristics obtained from the database were: age of diagnosis (age was divided into ≤62 years old and >62 years old.), sex, race, primary site, tumor grade, T stage, N classification, insurance, marital status, treatment, and distant metastatic sites which included bone, brain, liver, and lung. We recorded the number of metastatic sites and classified it as single or multiple organ metastasis. The median OS follow-up was 19 months (range: 17–23 months) versus 10 months (range: 9–12 months) in the HPV-positive group and HPV-negative group, respectively. The median DSS follow-up was 23 months (range: 18–32 months) versus 15 months (range: 12–17 months) in the HPV-positive group and HPV-negative group, respectively.
Statistical analysis
The Chi-squared test or Fisher’s exact test was used to compare the difference between HPV-positive and HPV-negative groups. The one-year, two-year, and three-year OS and DSS of HNSCC patients were compared using Kaplan–Meier analysis and log-rank test. The univariate and multivariate Cox proportional hazard regression models were performed to calculate the hazard ratio (HR) of death and were used to determine independent factors that may affect prognosis. A two-sided p-value < 0.05 was considered statistically significant. All analyses were performed using R software (Version 4.0.3, https://www.rproject.org/).
Results
Demographics of the cohort
The patient cohort used for analysis consisted of 735 metastatic HNSCC of pharynx (mHNSC-P) patients with 392 HPV-negative (53.3%) and 343 HPV-positive (46.7%) cases. The different clinical features among patient cohort are summarized in Table 1. Majority of patients (552 of 735) have oropharynx carcinoma. Median age is 62 years old. Briefly, there are more male, white, advanced N-stage, and married status patients in HPV-positive group than HPV-negative group.
Table 1.
Demographics and clinical characteristics for mHNSC-P patients.
| Characteristic | HPV Negative N = 3921 | HPV Positive N = 3431 | p-value2 |
|---|---|---|---|
|
| |||
| Age | > 0.9 | ||
| <=62 | 201 (51%) | 177 (52%) | |
| >62 | 191 (49%) | 166 (47%) | |
| Sex | 0.011 | ||
| Female | 91 (23%) | 54 (16%) | |
| Male | 301 (77%) | 289 (84%) | |
| Race | 0.008 | ||
| Black | 56 (14%) | 38 (11%) | |
| White | 276 (70%) | 290 (85%) | |
| Others | 60 (15%) | 15 (4.4%) | |
| Primary site | <0.001 | ||
| Non-Oropharynx Hypopharynx | 64 (16%) | 18 (5.2%) | |
| Nasopharynx | 78 (20%) | 23 (6.7%) | |
| Oropharynx | 250 (64%) | 302 (88%) | |
| Grade | 0.008 | ||
| I, II | 123 (31%) | 73 (21%) | |
| III, IV | 153 (39%) | 152 (44%) | |
| Unknown | 116 (30%) | 118 (34%) | |
| T stage | 0.8 | ||
| T1, T2 | 103 (26%) | 96 (28%) | |
| T3, T4 | 154 (39%) | 127 (37%) | |
| Unknown | 135 (34%) | 120 (35%) | |
| N stage | <0.001 | ||
| N0, N1 | 105 (27%) | 52 (15%) | |
| N2, N3 | 198 (51%) | 203 (59%) | |
| Unknown | 89 (23%) | 88 (26%) | |
| Insurance | 0.3 | ||
| Insured | 262 (67%) | 251 (73%) | |
| Medicaid | 98 (25%) | 70 (20%) | |
| Uninsured | 23 (5.9%) | 16 (4.7%) | |
| Unknown | 9 (2.3%) | 6 (1.7%) | |
| Marriage | 0.003 | ||
| Married | 158 (40%) | 182 (53%) | |
| Separated | 106 (27%) | 62 (18%) | |
| Unmarried | 105 (27%) | 80 (23%) | |
| Unknown | 23 (5.9%) | 19 (6%) | |
| Treatment | 0.045 | ||
| Comprehensive Treatment a | 210 (54%) | 209 (61%) | |
| Single treatment | 116 (30%) | 96 (28%) | |
| No/Unknown | 66 (17%) | 38 (11%) | |
| Bone | 0.5 | ||
| Yes | 120 (31%) | 98 (29%) | |
| No/Unknown | 272 (69%) | 245 (71%) | |
| Lung | 0.06 | ||
| Yes | 197 (50.2%) | 149 (43%) | |
| No/Unknown | 195 (49.8%) | 194 (57%) | |
| Liver | 0.9 | ||
| Yes | 73 (19%) | 61 (18%) | |
| No/Unknown | 319 (81%) | 273 (82%) | |
| Brain | 0.02 | ||
| Yes | 23 (5.9%) | 8 (2.3%) | |
| No/Unknown | 369 (94.1%) | 335 (97.7%) | |
| Number of metastatic sites | >0.5 | ||
| 1 | 163 (42%) | 151 (44163%) | |
| >=2 | 229 (58%) | 192 (56%) | |
n (%);
Pearson’s Chi-squared test;
two or more than two treatments were given to patients such as chemotherapy plus radiotherapy.
HPV positivity correlates with better survival in metastatic HNSCC-P patients
The univariate cox proportional hazards analyses of potential predictors for the OS and DSS in metastatic HNSCC patients are shown in Table 2. Primary site, HPV status, T stage, treatment, bone metastasis, lung metastasis and brain metastasis were significantly associated with both OS and DSS (p < 0.05). Meanwhile, grade I-II and separated marital status were associated with worse OS (p < 0.05), while N2-N3 and uninsured status were associated with worse DSS (p < 0.05).
Table 2.
Hazard ratio for overall and disease specific death in HNSCC-DM patients by univariate Cox-proportional hazard model analysis.
| Characteristic | Overall death |
Disease specific death |
||
|---|---|---|---|---|
| HR1 (95 %CI2) | p-value | HR1 (95 %CI2) | p-value | |
|
| ||||
| Age | ||||
| <=62 | Reference | Reference | ||
| >62 | 1.07 (0.893–1.29) | 0.448 | 0.909 (0.733–1.13) | 0.382 |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 1.07 (0.854–1.35) | 0.546 | 0.937 (0.711–1.23) | 0.644 |
| Race | ||||
| White | Reference | Reference | ||
| Black | 1.06 (0.804–1.39) | 0.691 | 1.01 (0.731–1.4) | 0.938 |
| Others | 0.764 (0.548–1.06) | 0.111 | 0.945 (0.664–1.35) | 0.756 |
| Primary site | ||||
| Hypopharynx | Reference | Reference | ||
| Nasopharynx | 0.471 (0.324–0.684) | < 0.001 | 0.604 (0.393–0.93) | 0.022 |
| Oropharynx | 0.622 (0.474–0.816) | < 0.001 | 0.705 (0.507–0.982) | 0.038 |
| Grade | ||||
| I, II | Reference | Reference | ||
| III, IV | 0.737 (0.589–0.923) | < 0.001 | 0.782 (0.602–1.02) | 0.065 |
| Unknown | 0.819 (0.644–1.04) | 0.104 | 0.85 (0.642–1.12) | 0.255 |
| HPV status | ||||
| HPV Negative | Reference | Reference | ||
| HPV Positive | 0.594 (0.492–0.717) | < 0.001 | 0.698 (0.563–0.866) | 0.001 |
| T stage | ||||
| T1, T2 | Reference | Reference | ||
| T3, T4 | 1.37 (1.11–1.71) | 0.004 | 1.36 (1.05–1.75) | 0.019 |
| Unknown | 1.2 (0.927–1.56) | 0.166 | 1.27 (0.944–1.71) | 0.114 |
| N stage | ||||
| N1, N2 | Reference | Reference | ||
| N2, N3 | 1.13 (0.905–1.4) | 0.285 | 1.42 (1.09–1.85) | 0.0105 |
| Unknown | 1.02 (0.719–1.43) | 0.931 | 1.09 (0.716–1.66) | 0.69 |
| Insurance | ||||
| Insured | Reference | Reference | ||
| Medicaid | 1.18 (0.946–1.46) | 0.144 | 1.2 (0.936–1.55) | 0.148 |
| Uninsured | 1.39 (0.947–2.03) | 0.0931 | 1.57 (1.03–2.39) | 0.036 |
| Unknown | 0.89 (0.474–1.67) | 0.717 | 1.1 (0.564–2.14) | 0.783 |
| Marriage | ||||
| Married | Reference | Reference | ||
| Separated | 1.63 (1.3–2.05) | < 0.001 | 1.32 (1 –1.73) | 0.048 |
| Unmarried | 1.41 (1.12–1.78) | 0.004 | 1.45 (1.12–1.88) | 0.004 |
| Unknown | 1.14 (0.764–1.71) | 0.513 | 1.04 (0.644–1.68) | 0.875 |
| Treatment | ||||
| Single treatment | Reference | Reference | ||
| Comprehensive Therapya | 0.593 (0.48–0.733) | < 0.001 | 0.642 (0.501–0.823) | < 0.001 |
| No/Unknown | 3.35 (2.56–4.4) | < 0.001 | 3.58 (2.61–4.89) | < 0.001 |
| Bone | ||||
| Yes | Reference | Reference | ||
| No | 0.642 (0.527–0.782) | < 0.001 | 0.552 (0.442–0.69) | < 0.001 |
| Lung | ||||
| Yes | Reference | Reference | ||
| No | 0.761 (0.632–0.917) | 0.004 | 0.702 (0.566–0.871) | 0.001 |
| Liver | ||||
| Yes | Reference | Reference | ||
| No | 0.796 (0.626–1.01) | 0.062 | 0.702 (0.538–0.918) | 0.01 |
| Brain | ||||
| Yes | Reference | Reference | ||
| No | 0.559 (0.37–0.845) | 0.006 | 0.494 (0.314–0.777) | 0.002 |
| Number of metastatic sites | ||||
| 1 | Reference | Reference | ||
| >=2 | 0.91 (0.685–1.21) | 0.517 | 0.862 (0.63–1.18) | 0.355 |
HR = Hazard Ratio;
CI = Confidence Interval
two or more than two treatments were given to patients such as chemotherapy plus radiotherapy
After adjusting for age, race, gender, grade, T stage, N stage and treatment by multivariate cox proportional hazard model, HPV-positive mHNSC-P patients still had better OS and DSS compared to HPV-negative mHNSC-P patients (p < 0.05). As shown in Fig. 1, mHNSC-P patients with HPV-positive status are more likely to have better OS and DSS than patients with HPV-negative status (OS: aHR: 0.62, 95 %CI: 0.51–0.76, p < 0.001; DSS: aHR: 0.73, 95 %CI:0.58–0.91, p < 0.01). The 1-year, 2-year and 3-year OS rates were 63.6%, 41.0% and 30.1% versus 42.9%, 24.5% and 19.2% in the HPV-positive patients and HPV-negative patients, respectively. In addition, the 1-year, 2-year and 3-year DSS rates of HPV-positive patients and HPV-negative patients were 68.3%, 49.2% and 38.1% versus 54.8%, 38.6% and 31.5%.
Fig. 1.
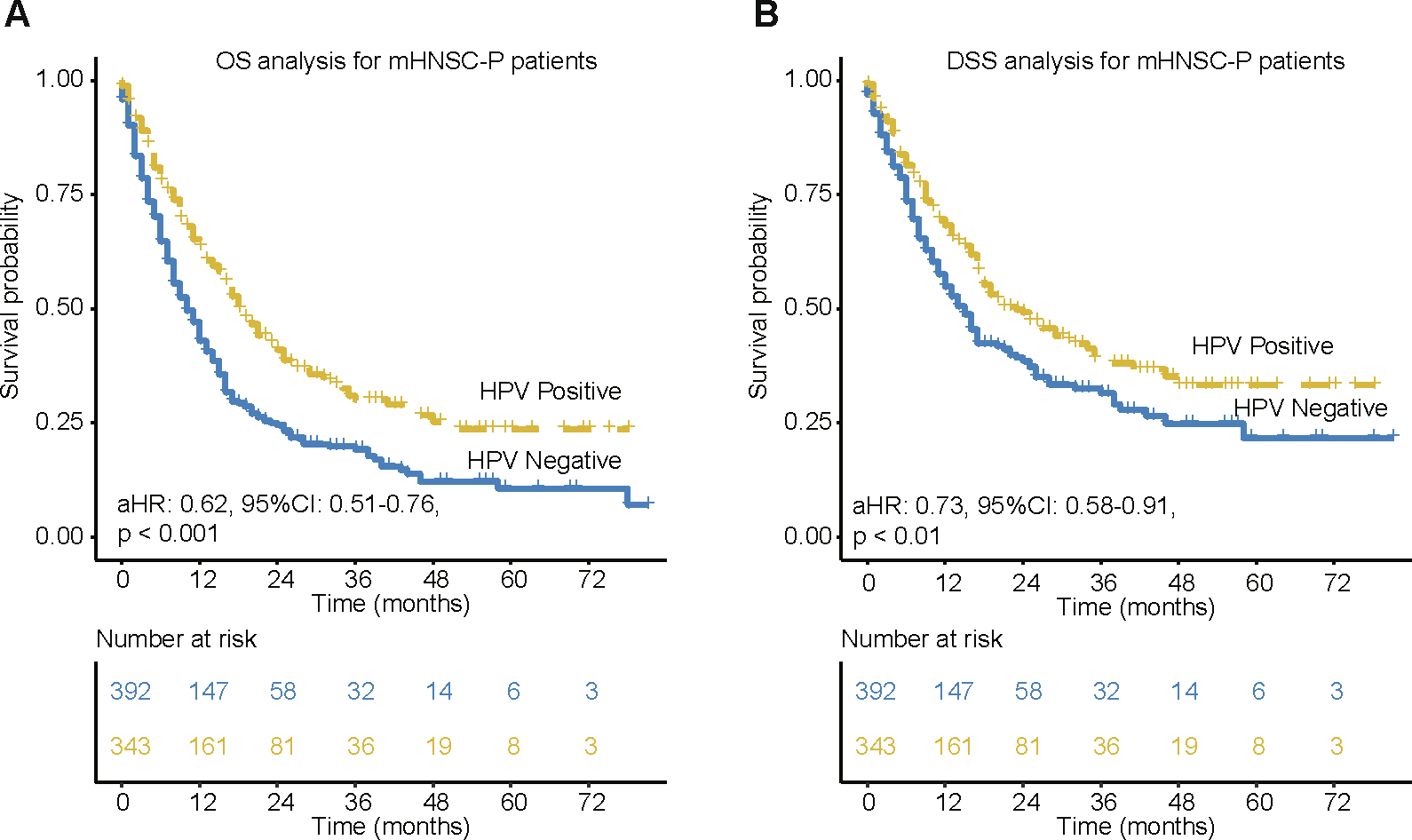
Kaplan-Meier of OS and DSS for metastatic head and neck cancer of pharynx patients. (A) OS based on HPV status in mHNSC-P patients. (B) DSS based on HPV status in mHNSC-P patients. aHR means Hazard ratio of HPV-positive vs. HPV-negative patients by adjusting for age, race, gender, grade, T stage, N stage and treatment. 95 %CI means the range of estimates with 95% confidence intervals.
The HPV-positive associated survival benefit occurs in metastatic oropharyngeal cancer rather than metastatic non-oropharyngeal cancer
Due to the skewed numbers of oropharyngeal cancer vs non-oropharyngeal cancer in our patient cohort, we applied subgroup analysis by splitting the cohort into mHNSC-OP and mHNSC-non-OP. As shown in Fig. 2A and 2B, HPV-positive mHNSC-OP patients had significantly better OS (p < 0.0001) and DSS (p < 0.01) than HPV-negative mHNSC-OP patients. The DSS rate after 1-year, 2-year and 3-year in HPV-positive patients were 69.6%, 51.3% and 39.1% compared with 49.7%, 37.7%, and 31.6% for HPV-negative patients respectively. However, there was no significant survival difference observed between HPV-positive and HPV-negative mHNSC-non-OP patients, including nasopharyngeal and hypopharyngeal cancers (Fig. 2C–F).
Fig. 2.
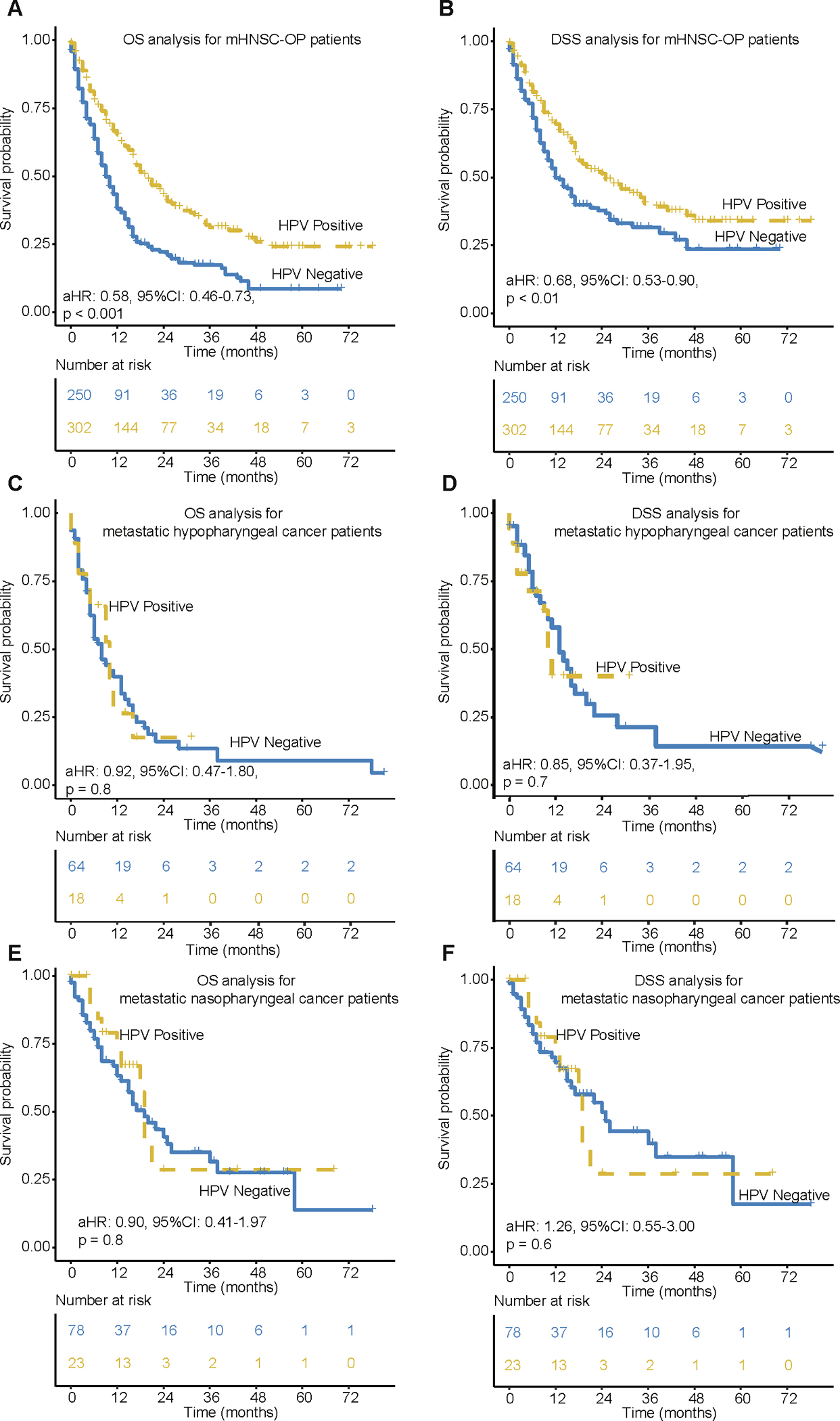
Kaplan-Meier of OS and DSS for mHNSC-OP and mHNSC-non-OP patients. (A-B) OS and DSS for metastatic oropharyngeal cancer patients by HPV status. (C-D) OS and DSS for metastatic nasopharyngeal cancer patients by HPV status. (E-F) OS and DSS for metastatic hypopharyngeal cancer patients by HPV status. aHR means Hazard ratio of HPV-positive vs. HPV-negative patients by adjusting for age, race, gender, grade, T stage, N stage and treatment. 95 %CI means the range of estimates with 95% confidence intervals.
The HPV positivity associated survival benefit differs between OPSCC patients with oligo and ploymetastatic sites
HPV-positive mHNSC-OP patients had better OS and DSS than HPV-negative counterparts when only the oligometastatic organ was involved (p < 0.05, Fig. 3A–B). As for the polymetastatic OPSCC patients, HPV-positive patients seem to have better OS and DSS rates than HPV-negative counterparts (Fig. 3C–D, log-rank p < 0.05). However, after adjusting for T, N, age, gender, race and treatment, the adjusted HR for DSS is 0.84 with 95 %CI (0.6–1.15) (p = 0.3), indicating no survival benefit in HPV positivity in polymetastatic OPSCC patients.
Fig. 3.
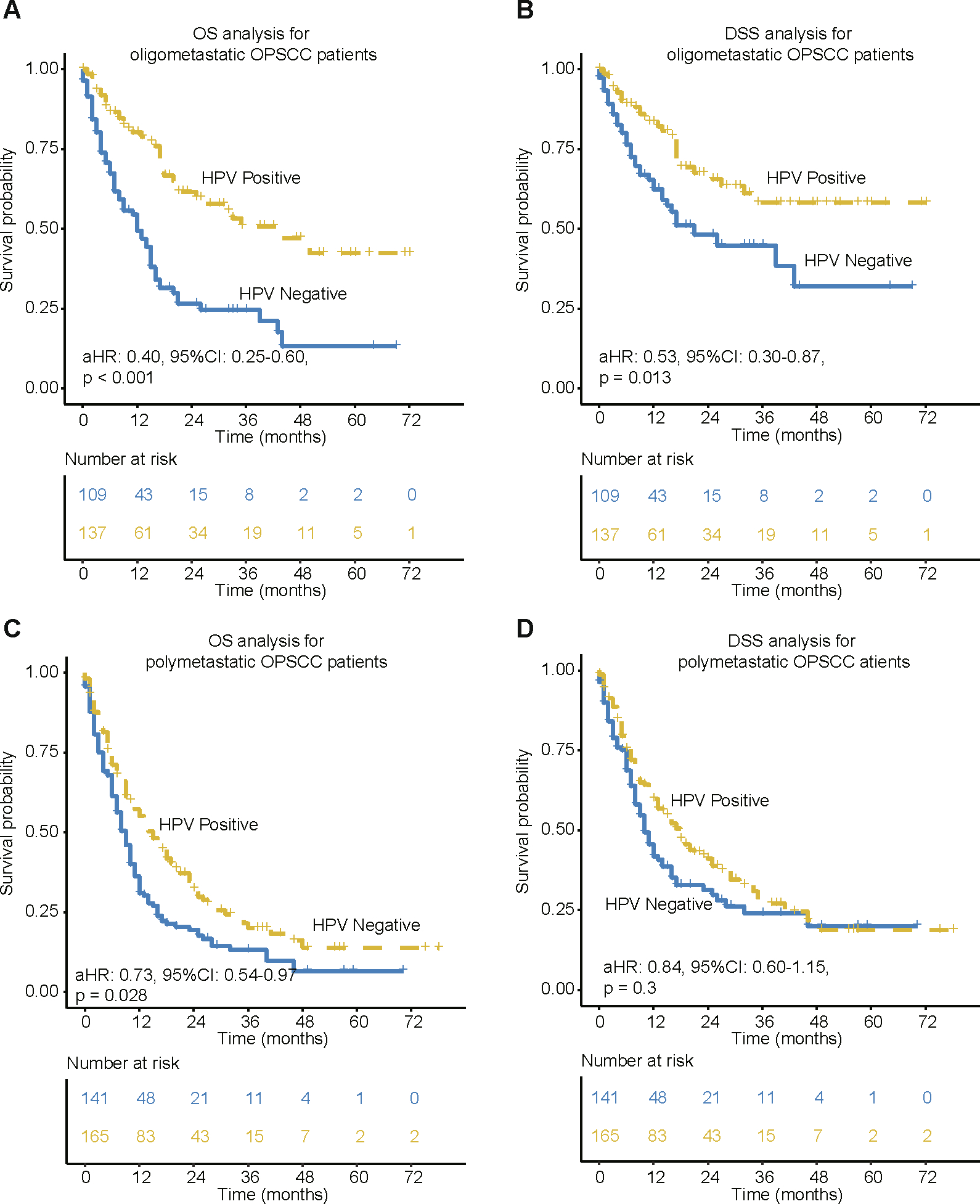
Kaplan-Meier of OS and DSS for mHNSC-OP patients with different numbers of distant metastatic sites. (A-B) OS and DSS for oligometastatic (only metastases in 1 site) mHNSC-OP patients by HPV status. (C-D) OS and DSS for ploymetastatic (>=2 sites) mHNSC-OP patients by HPV status. aHR means Hazard ratio of HPV-positive vs. HPV-negative patients by adjusting for age, race, gender, grade, T stage, N stage and treatment. 95 %CI means the range of estimates with 95% confidence intervals.
To investigate the association of survival with the specific metastatic site, patients were grouped accordingly based on presence of lung and bone metastases (patients with liver and brain metastases were not analyzed due to small sample size). The HPV-positive mHNSC-OP patients with lung metastasis had significantly better DSS (aHR:0.04, 95 % CI 0.002–0.55, p = 0.017; Fig. 4A) and OS (aHR:0.02, 95 %CI: 0.002–0.33, p = 0.006; Supplementary Fig. 1) compared to HPV-negative patients. In contrast, there was no observed significant difference in DSS between HPV-positive and -negative mHNSC-OP patients with bone metastasis (aHR: 0.04, 95 %CI:0.0007–2.4, p =0.12, Fig. 4B).
Fig. 4.
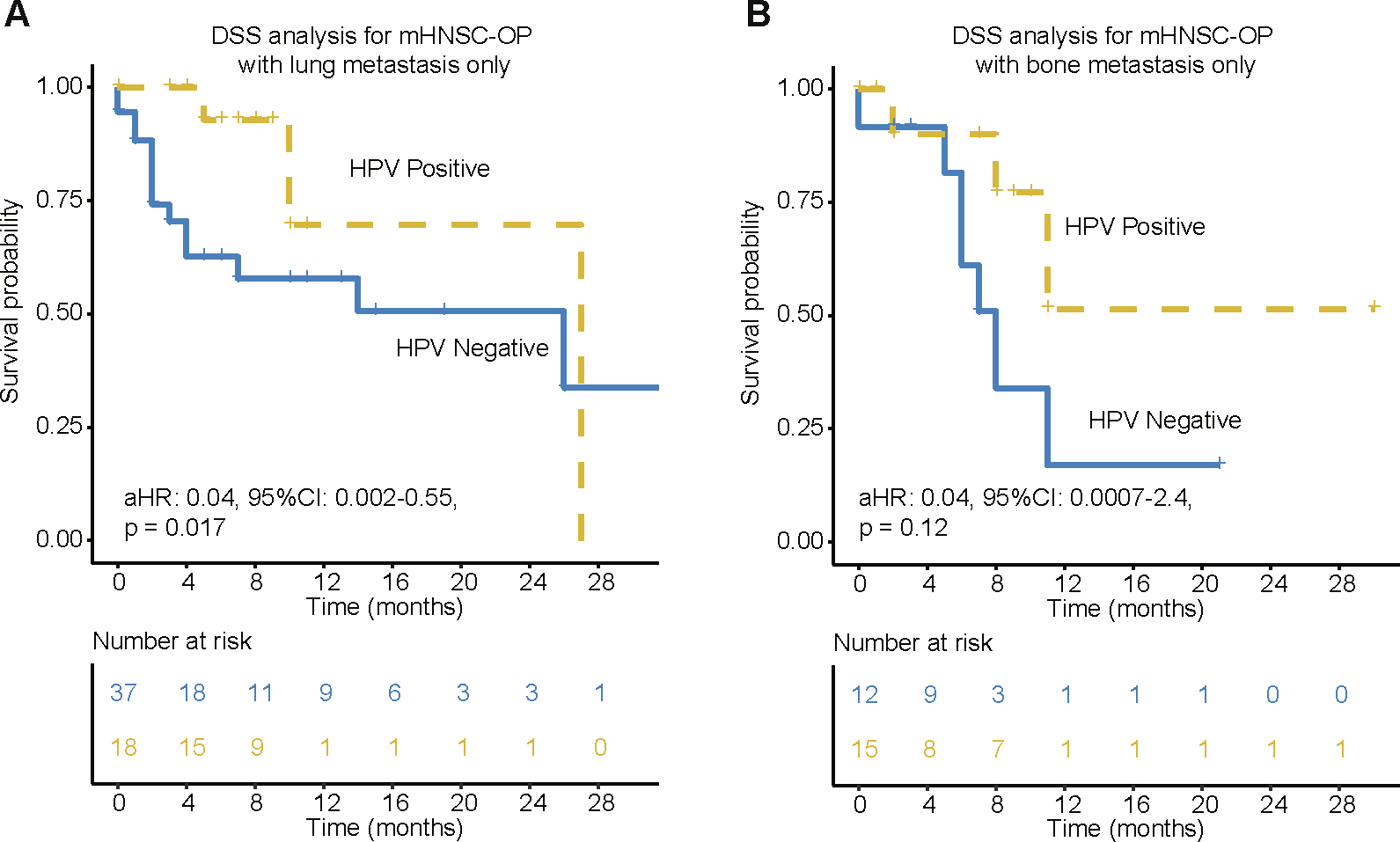
Kaplan-Meier of DSS for mHNSC-OP patients with lung or bone metastasis only. (A) DSS for HPV associated oligometastatic (only metastases in 1 site) mHNSC-OP patients with lung metastasis only. (B) DSS for HPV associated oligometastatic (only metastases in 1 site) mHNSC-OP patients with bone metastasis only. aHR means Hazard ratio of HPV-positive vs. HPV-negative patients by adjusting for age, race, gender, grade, T stage, N stage and treatment. 95 %CI means the range of estimates with 95% confidence intervals.
Discussion
The association of HPV positivity with the survival of head and neck cancer patients has been investigated for more than a decade and numerous studies have shown that HPV-positive HNSCCs are distinct from HPV-negative HNSCC in terms of survival and biology. However, majority of these studies consisted of a large proportion of locoregionally advanced HNSCC therefore do not provide sufficient information to address potential correlation between HPV positivity and survival benefit in metastatic HNSCC. Here, we show that HPV-association is an independently favorable indicator of survival in 735 metastatic HNSCC patients. These findings contrast with results shown by Morris et al., which analyzed 35 cases of metastatic and 18 local/regional cases of HNSCC, where HPV-positive HNSCC did not have improved survival benefit compared to HPV-negative HNSCC [17]. The small sample size (only 53 patients in total) and the inclusion of local regional cases in this study may limit the survival difference to reach statistical significance. Because clinical trial recruitment usually involves metastatic cancer patients, the effect of HPV positivity on metastatic HNSCC patients’ survival should be considered during patient consultant and clinical trial.
In this study, we observed that HPV positivity resulted in survival benefit for metastatic HNSCC patients specifically with oropharyngeal cancer. This finding is consistent with previous studies showing that HPV-positive metastatic oropharyngeal cancer is likely to survive longer than their HPV-negative counterparts [13,14]. Our study here makes the conclusion from the previous studies more robust by a relative bigger number of patients. Additionally, this is the first investigation based on the U.S. nationwide dataset to describe that there is no observed survival benefit between HPV-positive and -negative mHNSC-non-OP patients, which consists with a study from The Danish Head and Neck Cancer Group displaying no different outcomes between HPV-positive and -negative non-OPSCC patients [18]. Although Chung et al. found the p16-positive non-oropharyngeal cancer patients have a significantly better outcome than the p16-negative non-oropharyngeal cancer patients [19], we should be aware that majority of their cases are locally advanced stage, which cannot be directly compared to metastatic patients in our study.
Here, we show that the outcome benefit for metastatic HPV associated HNSCCs is limited to the oropharyngeal site. This may indicate that the rejection of virus-related cancer cell requires a unique tumor immune environment. Single-cell RNA sequencing analysis on HPV associated HNSCC show that HPV-positive oropharyngeal cancer displays a divergent spectrum of helper CD4 + T cells and B cells [20]. Non-oropharyngeal mucosa may not have enough infiltrating immune cells to kill tumor cells loaded with viral antigens. Furthermore, the oncogenesis activated by HPV virus may accelerate tumor growth and invasion since not enough ejections of immune killings in non-oropharyngeal mucosa. Regardless of HPV status, the survival outcomes of different sub-anatomical HNSCCs show huge differences [21], which is consistent with our notion of tumor subsite’s effect in HNSCCs. The sub-anatomic site to some extent determines the outcomes of host immune-virus interactions coordinating with findings shown in preclinical mouse model where HPV-positive tumors are significantly sensitive to anti-PD1 treatment when implanted in base of tongue compared to the flank [22]. Taken together, HPV status and primary tumor site should be considered as key factors in patient consultant and trial design for metastatic HNSCCs.
The metastatic OPSCCs were classified into oligometastatic OPSCC (single metastatic site) and polymetastatic OPSCC (multiple metastatic sites) [23]. A recent study by Saiyed et al. indicate a survival advantage in oligometastatic OPSCC compared to polymetastatic OPSCC [24], however, this is not a HPV associated study. Here, we show that there is significantly better prognosis for HPV-positive oligometastatic OPSCC than their HPV-negative counterparts, but there was no survival benefit if patients developed polymetastasis. In agreement with previous studies [13,14,25], we show that the OPSCC metastases are located preferentially in lung (52% and 47% for HPV-positive and HPV-negative), followed by bone (26% and 25% for HPV-positive and HPV-negative) and liver (14% and 13% for HPV-positive and HPV-negative). Furthermore, when comparing different oligometastatic sites, lung metastasis is the only site reaching statistical significance in DSS when comparing HPV-positive oligometastatic OPSCC with their HPV-negative counterparts. The mechanism underlying this finding is still poorly understood, but maybe involved with host immunity. An immune analysis on metastatic organs showed that the samples from metastatic cancer in lung have a higher immunogenic cell infiltrates than the samples from other metastatic sites including liver and bone [26]. And emerging studies indicate that cancer metastasize to liver could result in the systemic suppression of antitumor immunity by the coordinated activation of regulatory T cells and modulation of intratumoral CD11b + monocytes [27,28]. Bone marrow is a primary hematopoietic organ and a common site of cancer metastasis in breast, prostate, lung and head and neck cancers [29]. Specific cellular and molecular niches in the bone marrow with metastatic cancer may include high levels of Treg cells and myeloid-derived suppressor cells (MDSCs) [30], which could lead to systemic immunosuppression.
There are some limitations of this study that should be acknowledged. Despite being retrospective, our study provides a comprehensive and large cohort size that limited by the nature of SEER data and may contain inherent bias. Furthermore, samples in SEER dataset lack HPV information on metastatic oral cavity and laryngeal cancers. The small number of HPV-positive metastatic non-oropharyngeal cancer patients (nasopharyngeal cancer, n = 101; hypopharyngeal cancer, n = 82) analyzed in this study may not be enough to make a definitive conclusion. Currently, we do not have enough evidence to exclude a possible minimal outcome improvement in HPV-positive metastatic non-oropharyngeal cancer patients due to small sample size. Future studies using larger cohorts must be done to address this issue. Even though the survival analysis was adjusted using clinical parameters including T, N, and treatments, we lack patient information on tobacco and alcohol consumption, which can also influence the analysis. Thus, we cannot exclude the potential biases from the heterogeneity of HPV testing methods, treatment order, smoking and alcohol status. Overall, HPV-positive mHNSC-OP patients had a more favorable outcome, particularly in patients with lung metastasis indicating that anatomy of primary and metastatic site are important factors to consider prior to treatment and clinical trial recruitment.
Supplementary Material
Acknowledgements
Dr. Robert L. Ferris supervised the study. Drs. Xin Zhang and Gangcai Zhu designed the study. We thank Drs. Patricia Santos Ph.D. and Housaiyin Li Ph.D. Candidate (University of Pittsburgh, Pittsburgh, PA, USA) for polishing the manuscript. All authors have read and approved the final submitted manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations:
- HNSCC
head and neck squamous cell carcinoma
- HPV
human papillomavirus
- SEER
the Surveillance, Epidemiology and End Result database
- mHNSC-P
metastatic HNSCC of pharynx
- mHNSC-OP
metastatic HNSCC of oropharynx
- mHNSC-non-OP
metastatic HNSCC of non-oropharynx
- OPSCC
oropharyngeal squamous cell carcinoma
- OS
overall survival
- DSS
disease-specific survival
- HR
hazard ratio
- CI
confidence interval
- MDSCs
myeloid-derived suppressor cells
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.oraloncology.2021.105675.
References
- [1].Cramer JD, Burtness B, Le QT, Ferris RL. The changing therapeutic landscape of head and neck cancer. Nat Rev Clin Oncol 2019;16(11):669–83. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- [3].Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado MP, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst 2007;99(10):777–89. [DOI] [PubMed] [Google Scholar]
- [4].Mehanna H, Beech T, Nicholson T, El-Hariry I, McConkey C, Paleri V, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer–systematic review and meta-analysis of trends by time and region. Head Neck 2013;35(5):747–55. [DOI] [PubMed] [Google Scholar]
- [5].Jiang H, Livingston M, Room R, Gan Y, English D, Chenhall R. Can public health policies on alcohol and tobacco reduce a cancer epidemic? Australia’s Exp BMC Med 2019;17:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 2010;11(8):781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kobayashi K, Hisamatsu K, Suzui N, Hara A, Tomita H, Miyazaki T. A Review of HPV-Related Head and Neck Cancer. J Clin Med 2018;7(9):241. 10.3390/jcm7090241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Humanˆ papillomavirus and survival of patients with oropharyngeal cancer. New England J Med 2010;363(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].O’Sullivan B, Huang SH, Su J, Garden AS, Sturgis EM, Dahlstrom K, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol 2016;17(4):440–51. [DOI] [PubMed] [Google Scholar]
- [10].Leeman JE, Li J-G, Pei X, Venigalla P, Zumsteg ZS, Katsoulakis E, et al. Patterns of Treatment Failure and Postrecurrence Outcomes Among Patients With Locally Advanced Head and Neck Squamous Cell Carcinoma After Chemoradiotherapy Using Modern Radiation Techniques. JAMA Oncol 2017;3(11):1487. 10.1001/jamaoncol.2017.0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tiwana MS, Wu J, Hay J, Wong F, Cheung W, Olson RA. 25 year survival outcomes for squamous cell carcinomas of the head and neck: population-based outcomes from a Canadian province. Oral Oncol 2014;50(7):651–6. [DOI] [PubMed] [Google Scholar]
- [12].Maxwell JH, Grandis JR, Ferris RL. HPV-Associated Head and Neck Cancer: Unique Features of Epidemiology and Clinical Management. Annu Rev Med 2016;67(1):91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fakhry C, Zhang Q, Nguyen-Tan PF, Rosenthal D, El-Naggar A, Garden AS, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol 2014;32(30):3365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Huang SH, Perez-Ordonez B, Weinreb I, Hope A, Massey C, Waldron JN, et al. Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral Oncol 2013;49(1):79–85. [DOI] [PubMed] [Google Scholar]
- [15].Trosman SJ, Koyfman SA, Ward MC, Al-Khudari S, Nwizu T, Greskovich JF, et al. Effect of human papillomavirus on patterns of distant metastatic failure in oropharyngeal squamous cell carcinoma treated with chemoradiotherapy. JAMA Otolaryngol – Head Neck Surg 2015;141(5):457. 10.1001/jamaoto.2015.136. [DOI] [PubMed] [Google Scholar]
- [16].Modesto A, Siegfried A, Lusque A, Vergez S, Sarini J, Brouchet L, et al. Distinct Outcomes of Oropharyngeal Squamous Cell Carcinoma Patients after Distant Failure According to p16 Status: Implication in Therapeutic Options. Current Oncol (Toronto, Ont) 2021;28(3):1673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Morris LGT, Chandramohan R, West L, Zehir A, Chakravarty D, Pfister DG, et al. The Molecular Landscape of Recurrent and Metastatic Head and Neck Cancers: Insights From a Precision Oncology Sequencing Platform. JAMA Oncol 2017;3(2):244. 10.1001/jamaoncol.2016.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lassen P, Primdahl H, Johansen J, Kristensen CA, Andersen E, Andersen LJ, et al. Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiotherapy Oncol: J Europ Soc Therap Radiol Oncol 2014;113(3):310–6. [DOI] [PubMed] [Google Scholar]
- [19].Chung CH, Zhang Q, Kong CS, Harris J, Fertig EJ, Harari PM, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol: Off J Am Soc Clin Oncol 2014;32(35):3930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cillo AR, Kürten CHL, Tabib T, Qi Z, Onkar S, Wang T, et al. Immune Landscape of Viral- and Carcinogen-Driven Head and Neck Cancer. Immunity 2020;52(1):183–199.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu J, Zhu W, Li Z, Cai G, Wang J, Tang Q, et al. Proteomic analysis of hypopharyngeal and laryngeal squamous cell carcinoma sheds light on differences in survival. Sci Rep 2020;10(1). 10.1038/s41598-020-76626-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dorta-Estremera S, Hegde VL, Slay RB, Sun R, Yanamandra AV, Nicholas C, et al. Targeting interferon signaling and CTLA-4 enhance the therapeutic efficacy of anti-PD-1 immunotherapy in preclinical model of HPV(+) oral cancer. J ImmunoTher Cancer 2019;7(1). 10.1186/s40425-019-0728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Albergotti WG, Abberbock S, Mathews F, Ferris RL, Johnson JT, Duvvuri U, et al. Oligometastatic status as predictor of survival in metastatic human papillomavirus-positive oropharyngeal carcinoma. Head Neck 2018;40:1685–90. [DOI] [PubMed] [Google Scholar]
- [24].Saiyed FK, Guo T, Johnson F, Myers JN. Characterizing distant metastases and survival in oropharyngeal squamous cell carcinoma. Head Neck 2021;43(7):2101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ruzevick J, Olivi A, Westra WH. Metastatic squamous cell carcinoma to the brain: an unrecognized pattern of distant spread in patients with HPV-related head and neck cancer. J Neurooncol 2013;112(3):449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].García-Mulero S, Alonso MH, Pardo J, Santos C, Sanjuan X, Salazar R, et al. Lung metastases share common immune features regardless of primary tumor origin. J ImmunoTher Cancer 2020;8(1):e000491. 10.1136/jitc-2019-000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lee JC, Mehdizadeh S, Smith J, Young A, Mufazalov IA, Mowery CT, et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol 2020;5(52). 10.1126/sciimmunol.aba0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med 2021;27(1):152–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Landi L, D’Incà F, Gelibter A, Chiari R, Grossi F, Delmonte A, et al. Bone metastases and immunotherapy in patients with advanced non-small-cell lung cancer. J ImmunoTher Cancer 2019;7(1). 10.1186/s40425-019-0793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhao E, Xu H, Wang L, Kryczek I, Wu Ke, Hu Yu, et al. Bone marrow and the control of immunity. Cell Mol Immunol 2012;9(1):11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


