Abstract
Background
In recent years, the indication for cementless short stem total hip arthroplasty (THA) has been widened to elderly patients as they might profit by the advantages of the short-curved implant design as well. Therefore, this study was conducted to evaluate the clinical and radiological outcome of a cementless short stem in elderly patients (≥ 75 years) compared to a young control group (≤ 60 years).
Methods
A retrospective cohort of 316 THAs performed between 2014 and 2017 was prospectively examined. In all patients a cementless, curved short stem and press-fit cup (Fitmore® stem; Allofit®/-S cup; both ZimmerBiomet, Warsaw, IN, USA) were implanted via a minimally-invasive anterolateral approach. Clinical and radiological outcome as well as rate of complications and revision were assessed.
Results
In total, 292 patients have been included for analysis of complications and revisions (Øfollow-up: 4.5 years) and 208 patients for clinical and radiological outcome (Øfollow-up: 4.4 years). Complication rate was significantly increased in elderly patients (13.7% vs. 5.8%, p = 0.023), while the revision rate was increased without statistical significance (5.2% vs. 2.2%, p = 0.169). Periprosthetic fractures occurred significantly higher in the elderly patients (5.2% vs. 0.7%; p = 0.026). Both groups showed a comparable clinical outcome in the Harris Hip Score (93.7 vs. 91.9; p = 0.224), Oxford Hip Score (44.5 vs. 43.7; p = 0.350), Forgotten Joint Score (81.7 vs. 81.5; p = 0.952) and WOMAC (7.4 vs. 9.3; p = 0.334).
Conclusion
Cementless short stem total hip arthroplasty shows a comparable clinical and radiological outcome in patients over 75 years of age compared to younger patients under 60 years of age. However, cementless shorts stem THA shows an increased rate of overall complications and periprosthetic fractures in elderly patients over 75 years of age. Cemented fixation of the femoral component should be considered in patients over 75 years of age.
Level of evidence
III Case-controlled study.
Trial registration
Observational study without need for trial registration due to ICMJE criteria.
Keywords: Total hip arthroplasty, THA, Short stem, Cementless, Anterolateral approach, Minimally-invasive
Introduction
Cementless short stems have proven to show excellent survival rates ranging from 95 to 100% after 3–11 years [1–5]. Short stems have been increasingly used in total hip arthroplasty (THA) in parallel with minimally invasive (MIS) approaches as they facilitate soft tissue sparing implantation [6, 7]. Additionally, they preserve more of the proximal femoral bone stock and can restore the proximal femoral anatomy more accurately [7–9]. Short stems can even reduce the rate of periprosthetic fractures in MIS approaches compared to conventional cementless straight stems [10, 11].
Because of its bone preserving quality, the use of short stems in THA has initially been recommended for young and active patients with adequate bone quality [3]. In recent years, the indication for cementless short stem THA has been widened also in geriatric patients, as this patient collective might also profit by the theoretical advantages of short stem THA [12–14]. In a multicenter case study with 400 patients, Gkagkalis et al. [12] report a comparable clinical outcome and complication rate in patients with an advanced age over 75 years, when using a neck-sparing cementless short stem. However, they advocate against the use of this type of stem in patients with a Dorr type C of the proximal femur due the significantly increased risk of periprosthetic fractures [12].
The use of cementless fixation and cementless short stems in elderly patients contradicts registry data [15–17]. Periprosthetic femoral fractures and the safest implant fixation remain the most important aspects in THA in patients older than 75 years, in order to reduce the revision risk and increase the long-term survival of primary THAs [15–17].
As data on the performance of cementless short stems in elderly patients is still rare, this study was conducted to compare the survival, complication and revision rate as well as clinical and radiological outcome of a neck-resecting cementless short stem in elderly patients over 75 years compared to a younger control group with patients under 60 years of age.
Methods
Study design
A retrospective cohort of 316 short stem THAs performed between 2014 and 2017 was prospectively examined in a comparative study. The study was approved by the institutional review board (1239/2019) and conducted according to the Helsinki Declaration of 2008. Informed consent was obtained in every case for participation in the study.
Cohort
A cohort of 316 short stem THAs in 300 patients was analyzed for inclusion. Two age groups were included for the analysis of clinical and radiological follow-up, as well as of complications and rate of revision. One age group with patients under 60 years of age at index surgery and one group of patients older than 75 years of age at index surgery were included in the study. The younger patients were included as the control group and the elderly patients were included as the treatment group. In the younger control group 149 THAs in 140 patients and in the elderly treatment group 167 THAs in 160 patients were performed between 2014 and 2017. Indication for surgery were primary osteoarthritis, avascular necrosis of the femoral head, congenital dysplasia of the hip (Crowe grade I), secondary osteoarthritis such as posttraumatic conditions as well as rheumatoid arthritis. Patients with bilateral THA or prior hip surgery have also been included.
All surgeries were performed at a single tertiary university hospital. Patients were followed at the recommended follow-up examinations. A minimum follow-up of 2 years was required for inclusion. The routine interval for a follow-up examination at the study center are at 3 months, 1, 3, 5, 7 and 10 years postoperatively. If a patient missed a follow-up examination, the patient was invited for a clinical and radiological follow-up examination. If the patient refused to participate personally, the patient was asked for any complication, revision surgery or reoperation within the last follow-up examination given the informed consent. If a patient was deceased, any complication, revision surgery or reoperation was excluded using information from relatives, general practitioners and clinical records. Therefore, the both study groups were reviewed in one collective for complications and revisions as well as one collective for clinical and radiological outcome.
Implants & surgery
A cementless, curved short stem (Fitmore® stem, ZimmerBiomet, Warsaw, IN, USA) and a cementless titanium press-fit cup with or without screws (Allofit®/-S, ZimmerBiomet, Warsaw, IN, USA) was implanted in every case. Fitmore® hip stem is a titanium alloy stem (Ti Al6V4) that has a porolock Ti-VPS coating in the proximal part to enhance bone ingrowth and is available in four different neck angle options (127°, 129°, 137°, 140°). A highly cross-linked polyethylene liner (Alpha Durasul®, ZimmerBiomet, Warsaw, IN, USA) and a ceramic femoral head (BIOLOX forte, CeramTec GmbH, DE; Sulox, ZimmerBiomet, Warsaw, IN, USA) were used. Digital templating was conducted in every case using mediCAD® version 5.1 (Hectec GmbH, Altdorf, Germany).
In total, 13 surgeons performed the surgeries. The surgeries were performed by 6 consultants and 4 residents. 3 surgeons performed the surgeries as residents and after finishing residency also as consultants. Consultants performed at least 50 arthroplasties per year at the institution. Surgeries performed by residents were always conducted under supervision of a consultant.
All surgeries were performed under laminar air flow. Extremity preparation was performed with threefold antiseptic scrub with alcohol disinfectant in all cases. The standardized peri- and postoperative protocol was identical in all cases, including single-shot antibiotics [cefuroxime 1.5 g intravenous (i.v.), directly preoperatively], full weight-bearing as tolerated from the first postoperative day on, indomethacin 75 mg twice daily for the prevention of heterotopic ossification on days 1–4 postoperatively, and 40 mg low-molecular-weight heparin or rivaroxaban 10 mg for 28 days postoperatively as venous thromboembolic event prophylaxis. Suturing was done either by intracutaneous suturing. Fluoroscopy was not routinely used.
A minimally invasive anterolateral approach was performed in supine positioning [18]. A skin incision was centered over the greater trochanter. An incision at the border between the Tensor fasciae latae and the Tractus iliotibilias was performed. Then, the Watson-Jones interval between Tensor fasciae latae and Gluteus medius was bluntly dissected. A capsulectomy was performed in each case. The average operation time from skin incision to closure was 78.3 min (min.: 48,1; max.: 200 min).
Assessment of complications and revisions
All complications and revisions have been analyzed through the follow-up appointments, retrospectively via the electronic institutional database as well as prospectively via consulting all patients. The type of complication was recorded. Complications were defined as a periprosthetic fracture (PFF), periprosthetic joint infection (PJI), nerve lesion (confirmed by electroneurography), wound healing disorder, and hematoma or seroma that lead to an intervention, a prolonged hospital stay, an unplanned follow-up visit or readmission. Implant loosening was defined by the criteria of Engh et al. [19]. PFFs were analyzed depending on the intra- or postoperative occurrence, the classification according to Dorr et al. [20], the fracture type according to the Vancouver Classification [21, 22] and the time of occurrence. Revision was defined as a change of modular parts or removal of the components. Reoperation was defined as an operation without the change of components.
Clinical and radiographic assessment
Clinical follow-up was conducted through postoperative measurements of functional outcome, patient report outcome measurements (PROMs) and assessment of quality of life. At the follow-up every patient was assessed with the Harris hip score (HHS) [23], the Oxford hip score (OHS) [24], the Forgotten Joint Score (FJS) [25] and the Western Ontario and McMaster Universities Arthritis Index (WOMAC) [26]. The sports activity was measured by using the University of California Los Angeles (UCLA) activity score [27]. Quality of life was assessed by using the European Quality of Life 5 Dimension 3 Level (EQ-5D-3L) [28] and the Veterans RAND 12-Item Health Survey (VR-12) [29]. Pain at rest was measured with the visual analog scale (VAS) with a scale of 0–10 [30].
Radiological follow-up was conducted through standardized digital, calibrated low centered anterior–posterior radiograph of the pelvis and a radiograph of the lateral hip [31]. Radiological outcome was assessed for heterotopic ossifications, cortical hypertrophies, radiolucent lines, bone resorptions and osteolysis. Heterotopic ossifications were assessed according to the Brooker-classification [32]. Cortical hypertrophies and radiolucent lines were assessed as previously described [1, 5]. Bone resorptions were evaluated according to the Singh-Index [33]. A Singh-Index of 1–3 was defined as a bone resorption [12, 33]. Cortical hypertrophies, radiolucent lines, bone resorptions and osteolysis were evaluated using the zones described by Gruen et al. [34]. Implant loosening as defined by the criteria of Engh et al. [19].
Statistical analysis
Descriptive analyses were performed for patient demographics. A Shapiro–Wilk test for normality was performed to determine whether continuous data were normally distributed. As the variables were normally distributed, Pearson’s chi square tests were performed for categorial variables and student’s t-tests were performed for continuous variables. Values are given as mean values with standard deviation. The endpoint of stem survival was measured for revision for any reason and for stem revision by using a Kaplan–Meier survival analysis with a 95% confidence interval (CI). A p-value of < 0.05 was considered to be significant. Data was analyzed using SPSS version 28 (IBM SPSS statistics, Chicago, IL) and R version 4.3.3 (R Development Core Team).
Results
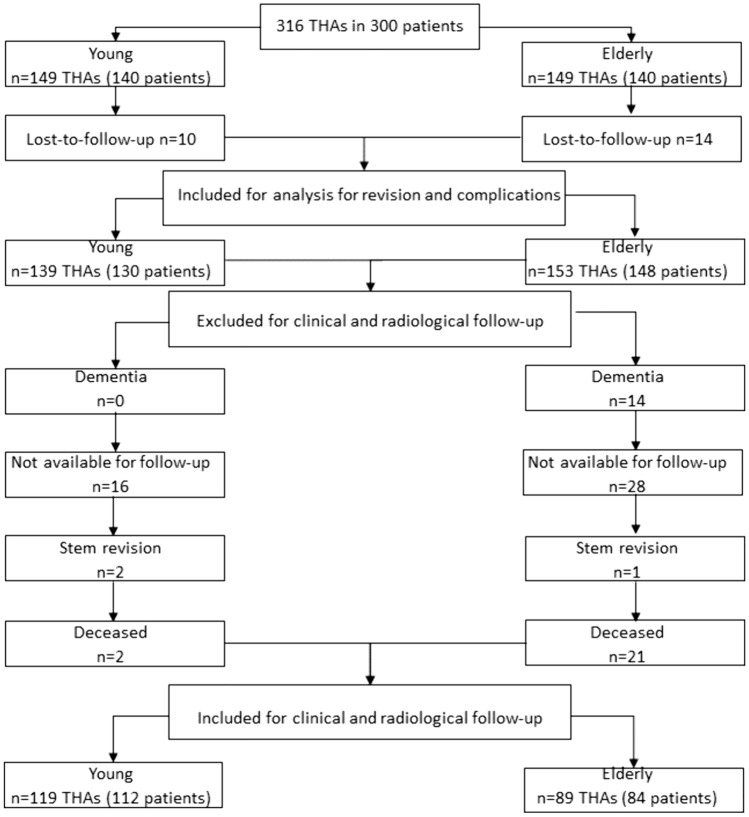
In total, 292 patients (92.4%) were included for analysis of complications and revisions with a conclusive follow-up. For clinical and radiological outcome 208 patients (65.8%) were included in the study (Fig. 1). In the younger group 10 patients (3.2%) and in the elderly group 14 patients (4.4%) were lost-to-follow-up. In the elderly group 14 patients (4.4%) were not available for assessment of the questionnaires due to dementia and 28 patients (8.9%) refused to attend the clinical or radiological follow-up examination due to their advanced age, personal reasons or the COVID-restrictions at that time. One patient (0.3%) was excluded due to stem exchange to a straight stem and therefore not included for the final analysis of clinical and radiological outcome. Furthermore, 21 patients (6.6%) deceased without any revision surgery until death. In the younger group, 2 patients (0.6%) deceased without any revision surgery and 2 patients (0.6%) were excluded for clinical and radiological follow-up due to stem revision. Furthermore, 16 patients (5.1%) refused to attend the clinical or radiological follow-up examination due to personal reasons or the COVID-restrictions at that time.
Fig. 1.
Consort diagram for inclusion and exclusion
Analysis for complications and revisions
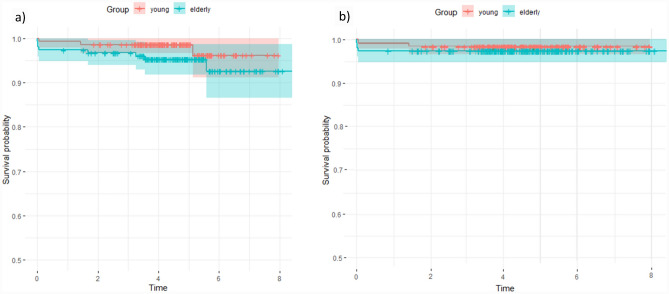
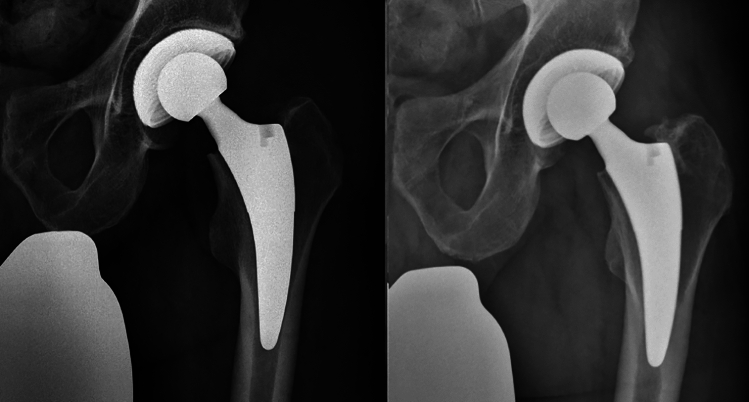
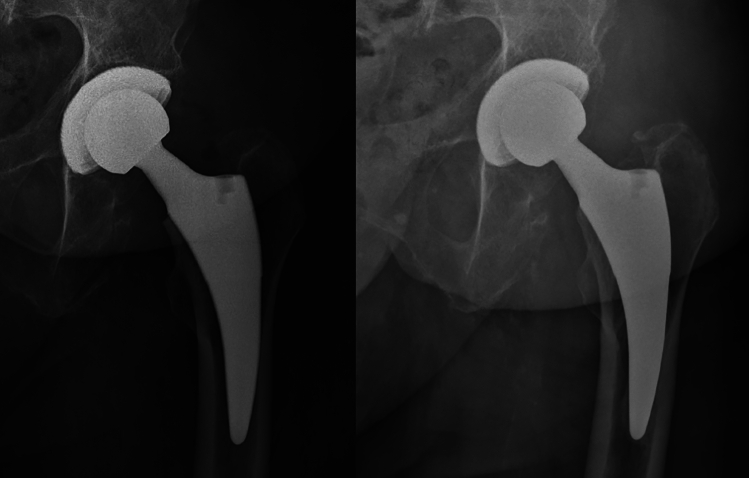
The patient demographics for both groups for the analysis of complications and revisions are given in Table 1. Both groups differed significantly in age at surgery (p < 0.001), gender (p = 0.032), the indication for surgery (p < 0.001), ASA score (< 0.001) and the Dorr type (p = 0.014). The rates of complications and revisions are given in detail in Table 2. Overall complication rate was significantly higher in the elderly patients (13.7% vs. 5.8%, p = 0.023), (Table 2). Periprosthetic fractures also occurred significantly higher in the elderly patients (5.2% vs. 0.7%; p = 0.026). Analysis of the occurrence of PFFs depending on specific factors did not show any statistically significant difference, (Table 2). All other complications were also without any significant difference between both groups (Table 2). Rates for revision for any reason, as well as stem revision were higher in the elderly group, but without statistically significant difference (p = 0.169; p = 0.479) (Tab. 2). Figure 2 shows the Kaplan Meier survival analysis after 8.6 years. Figure 2a shows the survival analysis for the endpoint “revision for any reason” with 97.8% (CI: 91.1–100%) for young patients and 94.8% (CI: 86.5–98.7%) for elderly patients. Figure 2b shows the survival analysis for the endpoint “stem revision” with 98.6% (CI: 96.6–100%) for young patients and 97.4% (CI: 94.9–99.9%) for elderly patients. Radiographic images of a standard case in the younger age group are given in Fig. 3 and in the elderly age group in Fig. 4. Examples of cases with PFFs are shown in Figs. 5 and 6.
Table 1.
Patient demographics for analysis of complications and revisions
| Young < 60 years of age | Elderly > 75 years of age | P Value | |
|---|---|---|---|
| Total number of hips | 139 (130 patients) | 153 (148 patients) | |
| Gender | 0.032 | ||
| Woman | 68 (48.9%) | 94 (61.4%) | |
| Men | 71 (51.1%) | 59 (38.6%) | |
| Age at Surgery (in years) | 52.4 ± 6.2 | 79.8 ± 3.9 | < 0.001 |
| Indication for surgery | < 0.001 | ||
| Primary osteoarthritis | 109 (78.4%) | 129 (84.3%) | |
| Avascular necrosis of the femoral head | 12 (8.6%) | 22 (14.4%) | |
| Congenital dysplasia of the hip | 17 (12.2%) | 2 (1.3%) | |
| Secondary osteoarthritis | 1 (0.7%) | 0 (0.0%) | |
| Side | 0.750 | ||
| Left | 68 (48.9%) | 72 (47.1%) | |
| Right | 71 (51.1%) | 81 (52.9%) | |
| ASA Score | < 0.001 | ||
| 1 | 58 (41.7%) | 9 (5.9%) | |
| 2 | 70 (50.4%) | 79 (51.6%) | |
| 3 | 11 (7.9%) | 65 (42.5%) | |
| 4 | 0 (0.0%) | 0 (0.0%) | |
| BMI | 27.6 ± 5 | 27.2 ± 4 | 0.448 |
| Surgeon’s experience | 0.314 | ||
| Consultant | 103 (74.1%) | 121 (79.1%) | |
| Resident | 36 (25.9%) | 32 (20.9%) | |
| Dorr Classification | 0.014 | ||
| A | 42 (30.2%) | 27 (17.6%) | |
| B | 81 (58.3%) | 95 (62.1%) | |
| C | 16 (11.5%) | 31 (20.3%) |
Bold letters indicate significant values
ASA Score American Society of Anesthesiologists Score; BMI Body Mass Index, kg/m2
Table 2.
Complications and revisions
| Young < 60 years of age |
Elderly > 75 years of age |
P Value | |
|---|---|---|---|
| Average Follow-up (in years) | 4.5 | 4.6 | 0.571 |
| Min.-Max. (in years) | 0.05–8 | 0–8.6 | |
| Complication | 0.023 | ||
| Yes (%) | 8 (5.8%) | 21 (13.7%) | |
| No (%) | 131 (94.2%) | 132 (86.3%) | |
| Periprosthetic Fracture | 0.026 | ||
| Yes (%) | 1 (0.7%) | 8 (5.2%) | |
| No (%) | 138 (99.3%) | 146 (95.4%) | |
| Intraoperative | 0 (0.0%) | 3 (2.0%) | 0.097 |
| Vancouver A | 0 (0.0%) | 2 (1.3%) | 0.176 |
| Vancouver B | 0 (0.0%) | 1 (0.7%) | 0.340 |
| Vancouver C | 0 (0.0%) | 0 (0.0%) | - |
| Postoperative | 1 (0.7%) | 5 (3.3%) | 0.125 |
| Vancouver A | 0 (0.0%) | 2 (1.3%) | 0.176 |
| Vancouver B | 1 (0.7%) | 3 (2.0%) | 0.362 |
| Vancouver C | 0 (0.0%) | 0 (0.0%) | - |
| Time of occurrence (months) | 0 ± 0.0 | 21.3 ± 27.5 | 0.496 |
| < 12 months | 1 (0.7%) | 5 (3.3%) | 0.125 |
| > 12 months | 0 (0.0%) | 3 (2.0%) | 0.097 |
| Dorr Classification | |||
| A | 0 (0.0%) | 3 (2.0%) | 0.097 |
| B | 1 (0.7%) | 3 (2.0%) | 0.362 |
| C | 0 (0.0%) | 2 (1.3%) | 0.176 |
| Sex | - | ||
| Female | 1 (0.7%) | 4 (4.3%) | 0.811 |
| Male | 0 (0.0%) | 3 (5.1%) | |
| Periprosthetic Joint Infection | 0 (0.0%) | 3 (2.0%) | 0.097 |
| Aseptic loosening | 1 (0.7%) | 0 (0.0%) | 0.293 |
| Dislocation | 1 (0.7%) | 1 (0.7%) | 0.946 |
| Femoral Nerve lesion | 0 (0.0%) | 1 (0.7%) | 0.340 |
| Wound healing disorder | 2 (1.4%) | 5 (3.3%) | 0.307 |
| Hematoma/seroma | 1 (0.7%) | 3 (2.0%) | 0.362 |
| Revision | |||
| Revision for any reason | 3 (2.2%) | 8 (5.2%) | 0.169 |
| Stem Revision | 2 (1.4%) | 4 (2.6%) | 0.479 |
| Reoperation | 1 (0.7%) | 0 (0.0%) | 0.293 |
Bold letters indicate significant values
Fig. 2.
a, b: Kaplan Meier survival after 8.6 years for the endpoint: A “revision for any reason” and B “stem revision” for young and elderly patients (n = 292)
Fig. 3.
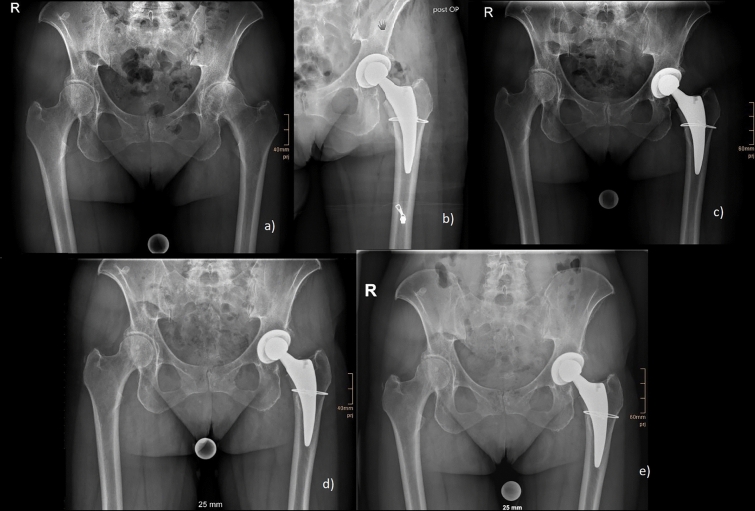
49-year-old-male patient; left: fourth postoperative day; right: at 4.3 years of follow-up
Fig. 4.
79-year-old female; left: fourth postoperative day; right: at 5.2 years of follow-up
Fig. 5.
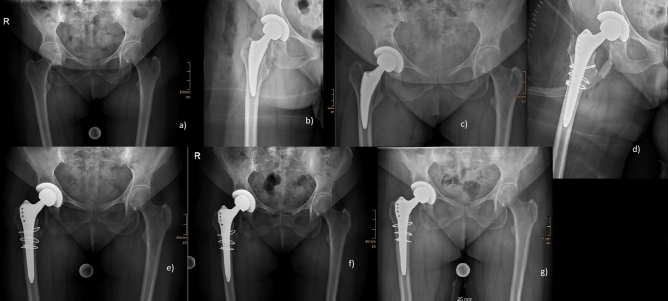
Occult fracture of the medial cortex detected on the fourth postoperative day; a preoperative; b postoperative; c fourth postoperative day; d postoperatively after revision (Alloclassic SL (ZimmerBiomet®) with three cerclage wires); e 6 weeks after revision; f 3 months after revision; g 1 year after revision
Fig. 6.
Intraoperative fracture of the calcar treated with one cerclage wire: a preoperative; b postoperative; c 6 weeks postoperative; d 3 months postoperative; e 1 year postoperative
Clinical and radiological outcome
For clinical and radiological outcome 89 THAs have been included in the elderly group and 119 THAs in the younger group with an average follow-up of 4.4 years in both groups (p = 0.933). The patient demographics for both groups are given in Table 3. Both groups differed significantly in age at surgery (p < 0.001), the indication for surgery (p = 0.013), ASA score (< 0.001) and the Dorr type (p = 0.049). Expectedly, the number of congenital dysplasia of the hip was higher in the younger group (12.6% vs. 1.1%), as well as the number of patients with ASA score 1 (41.2% vs. 7.9%). The number of patients with ASA score 3 was higher in the elderly group (36% vs. 7.6%), as well as patients with a Dorr type C (19.1% vs. 12.6%).
Table 3.
Patient demographics for analysis of clinical and radiological outcome
| Young < 60 years of age | Elderly > 75 years of age | P Value | |
|---|---|---|---|
| Total number of hips | 119 (112 patients) | 89 (84 patients) | |
| Gender | 0.112 | ||
| Woman | 58 | 53 | |
| Men | 61 | 36 | |
| Age at Surgery (in years) | 51.8 ± 6.4 | 79.1 ± 3.3 | < 0.001 |
| Indication for surgery | 0.013 | ||
| Primary osteoarthritis | 93 (78.2%) | 77 (86.5%) | |
| Avascular necrosis of the femoral head | 10 (8.4%) | 11 (12.4%) | |
| Congenital dysplasia of the hip | 15 (12.6%) | 1 (1.1%) | |
| Secondary osteoarthritis | 1 (0.8%) | 0 (0.0%) | |
| Side | 0.643 | ||
| Left | 56 (47.1%) | 39 (43.8%) | |
| Right | 63 (52.9%) | 50 (56.2%) | |
| ASA Score | < 0.001 | ||
| 1 | 49 (41.2%) | 7 (7.9%) | |
| 2 | 61 (51.3%) | 50 (56.2%) | |
| 3 | 9 (7.6%) | 32 (36%) | |
| 4 | 0 (0.0%) | 0 (0.0%) | |
| BMI | 27.5 ± 5.2 | 27 ± 3.5 | 0.360 |
| Surgeon’s experience | 0.101 | ||
| Consultant | 86 (72.3%) | 73 (82%) | |
| Resident | 33 (27.7%) | 16 (18%) | |
| Dorr Classification | 0.049 | ||
| A | 37 (31.1%) | 15 (16.9%) | |
| B | 67 (56.3%) | 57 (64%) | |
| C | 15 (12.6%) | 17 (19.1%) |
Bold letters indicate significant values
ASA Score American Society of Anesthesiologists Score; BMI Body Mass Index, kg/m2
The detailed results for the clinical outcome are given in Table 4. Clinical outcome did not differ between both groups for the HHS (p = 0.224), OHS (p = 0.350), FJS (p = 0.952) and WOMAC (p = 0.334) (Tab. 4). The average score of the VAS at rest was also without any statistically significant difference (p = 0.102) (Table 4). Both groups differed significantly in the UCLA with a higher activity level in the younger age group (6.9 vs. 4.6, p < 0.001). The quality of life was significantly higher for younger patients in the EQ-5D-3L (p = 0.003) (Table 4). The physical component summary (PCS) of the VR-12 questionnaire was also significantly higher for younger patients (p < 0.001), whereas the mental component summary (MCS) of the VR-12 was without any significant difference between both age groups (p = 0.238) (Table 4).
Table 4.
Clinical outcome; Bold letters indicate significant values
| Young < 60 years of age |
Elderly > 75 years of age |
P Value | |
|---|---|---|---|
| Average Follow-up (in years) | 4.4 ± 1 | 4.4 ± 1 | 0.933 |
| Min.-Max. (in years) | 2.1–7.1 | 2–7.1 | |
| Pain | 0.146 | ||
| Yes (%) | 42 (35.3%) | 23 (25.8%) | |
| No (%) | 77 (64.7%) | 76 (74.2%) | |
| VAS (± SD) | .85 ± 1.4 | .56 ± 1.1 | 0.102 |
| Harris Hip Score (± SD) | 93.7 ± 11.2 | 91.9 ± 8.8 | 0.224 |
| Oxford Hip Score (± SD) | 44.5 ± 7.2 | 43.7 ± 5.3 | 0.350 |
| Forgotten Joint Score 12 (± SD) | 81.7 ± 26.3 | 81.5 ± 22 | 0.952 |
| WOMAC Score (± SD) | 7.4 ± 14.8 | 9.3 ± 12.3 | 0.334 |
| UCLA Score (± SD) | 6.9 ± 2.3 | 4.6 ± 2.3 | < 0.001 |
| EQ-5D-3L (%) | 82.3 ± 19 | 75.9 ± 11.9 | 0.003 |
| EQ-5D-3L Q1 (median) | 2.4 (1) | 1.3 (1) | 0.160 |
| EQ-5D-3L Q2 (median) | 1.1 (1) | 1.2 (1) | < 0.001 |
| EQ-5D-3L Q3 (median) | 1.1 (1) | 1.4 (1) | < 0.001 |
| EQ-5D-3L Q4 (median) | 1.4 (1) | 1.6 (2) | 0.002 |
| EQ-5D-3L Q5 (median) | 1.1 (1) | 1.2 (1) | 0.075 |
| VR-12 PCS (± SD) | 54.7 ± 10.9 | 47.9 ± 10.2 | < 0.001 |
| VR-12 MCS (± SD) | 43.4 ± 4.3 | 44.3 ± 5.8 | 0.238 |
The detailed results for the radiological outcome are given in Table 5. Heterotopic ossifications were slightly higher in the younger age group (16% vs. 10.1%, p = 0.221) without any statistical significance, also when assessed according to the Brooker-classification (p = 0.088) (Table 5). Cortical hypertrophies were significantly higher in the younger age group (54.6% vs. 31.5%, p < 0.001) (Table 5). Cortical hypertrophies were detected in the Gruen zones 3 and 5 with statistically significantly higher proportion in the younger age group (P < 0.001; p = 0.002) (Table 5). Radiolucent lines were significantly higher in younger patients (13.4% vs. 2.2%, p = 0.004), especially in Gruen zone 1 (p = 0.008) (Table 5). Bone resorptions have been detected without any statistical significance between groups (p = 0.658) (Table 5). Osteolysis was not detected in any age group.
Table 5.
Radiological outcome
| Young < 60 years of age |
Elderly > 75 years of age |
P Value | |
|---|---|---|---|
| Average Follow-up (in years) | 4.4 ± 1 | 4.4 ± 1 | 0.933 |
| Min.-Max. (in years) | 2.1–7.1 | 2–7.1 | |
| Heterotopic ossifications | 0.221 | ||
| Yes (%) | 19 (16%) | 9 (10.1%) | |
| No (%) | 100 (84%) | 80 (89.9%) | |
| Brooker-Classification | 0.088 | ||
| 0 | 100 (84%) | 80 (89.9%) | |
| 1 | 16 (13.5%) | 7 (7.9%) | |
| 2 | 3 (2.5%) | 0 (0.0%) | |
| 3 | 0 (0.0%) | 2 (2.2%) | |
| Cortical hypertrophy | < 0.001 | ||
| Yes (%) | 65 (54.6%) | 28 (31.5%) | |
| No (%) | 54 (45.4%) | 61 (68.5%) | |
| Gruen zone 1 | 0 (0.0%) | 0 (0.0%) | – |
| Gruen zone 2 | 0 (0.0%) | 0 (0.0%) | – |
| Gruen zone 3 | 64 (53.8%) | 27 (30.3%) | < 0.001 |
| Gruen zone 4 | 0 (0.0%) | 0 (0.0%) | – |
| Gruen zone 5 | 37 (31.1%) | 11 (12.4%) | 0.002 |
| Gruen zone 6 | 0 (0.0%) | 0 (0.0%) | – |
| Gruen zone 7 | 0 (0.0%) | 0 (0.0%) | – |
| Radiolucent Line (< 2mm) | 0.004 | ||
| Yes (%) | 16 (13.4%) | 2 (2.2%) | |
| No (%) | 103 (86.6%) | 87 (97.8%) | |
| Gruen zone 1 | 9 (7.6%) | 0 (0.0%) | 0.008 |
| Gruen zone 2 | 1 (0.8%) | 0 (0.0%) | 0.386 |
| Gruen zone 3 | 3 (2.5%) | 0 (0.0%) | 0.131 |
| Gruen zone 4 | 3 (2.5%) | 2 (2.2%) | 0.898 |
| Gruen zone 5 | 2 (1.7%) | 0 (0.0%) | 0.219 |
| Gruen zone 6 | 0 (0.0%) | 0 (0.0%) | – |
| Gruen zone 7 | 2 (1.7%) | 0 (0.0%) | 0.219 |
| Bone resorption | 0.658 | ||
| Yes (%) | 7 (5.9%) | 4 (4.5%) | |
| No (%) | 112 (94.1%) | 85 (95.5%) | |
| Gruen zone 1 | 0 (0.0%) | 0 (0.0%) | – |
| Gruen zone 2 | 0 (0.0%) | 0 (0.0%) | – |
| Gruen zone 3 | 0 (0.0%) | 0 (0.0%) | – |
| Gruen zone 4 | 0 (0.0%) | 0 (0.0%) | – |
| Gruen zone 5 | 0 (0.0%) | 0 (0.0%) | – |
| Gruen zone 6 | 0 (0.0%) | 0 (0.0%) | – |
| Gruen zone 7 | 7 (5.9%) | 4 (4.5%) | 0.658 |
| Osteolysis | – | ||
| Yes (%) | 0.0 (0%) | 0.0 (0%) | |
| No (%) | 100 (100%) | 100 (100%) |
Bold letters indicate significant values
Discussion
This study reports the clinical and radiological outcome, as well as the rate of complications and revisions in cementless short stem total hip arthroplasty in elderly patients over 75 years of age compared to a young control group under 60 years of age. While clinical outcome and patient satisfaction is comparable in both outcome groups, elderly patients show a higher complication and revision rate.
The clinical and radiological outcome in the study is comparable to other studies of the Fitmore® hip stem. The clinical outcome is comparable for both age groups without any significant difference in the HHS, OHS, FJS-12 as well as WOMAC Score. Our findings are comparable to other studies evaluated the clinical outcome and PROMs after short stem THA [1, 12, 35, 36]. As to be expected, our results show a higher activity level of younger patients with a significantly higher UCLA score as well as PCS-12 of the VR-12 questionnaire. Additionally, the quality of life is significantly lower for elderly patients in the EQ-5D-3L, while the mental health is comparable without any significant difference in the MCS-12 of the VR-12 questionnaire.
The Fitmore® stem shows good to excellent early outcomes [6, 37] with reliable fixation in a radiostereometric analysis 2 years after surgery [37], as well as in Ein-Bild-Roentgen-Analysis Femoral-Component-Analysis (EBRA-FCA) 5 years after surgery [38]. Innmann et al. [5] report a low revision rate of 93.7% for all stem revisions and 99.6% for revision due to aseptic loosening after a follow-up of 8.6 years. Our study finds a comparable revision rates for young and elderly patients with the same stem design. The higher revision rate for revisions for any reason might also be associated with the generally higher age and might not be associated with the stem design and related complications.
In numerous studies cementless fixation in elderly patients is not advocated due to the increased risk for periprosthetic femoral fractures [15–17, 39]. In recent studies, cementless short stems show a low number of PFFs and they are associated with a reduced risk compared to standard cementless straight stems [10, 11, 40]. Gkagkalis et al.[12] do not report an overall increased fracture rate for the Optimys® short stem (Mathys, Bettlach, CH) with 1.5% in patients under 60 years and 1.4% in patients over 75 years of age at index surgery. However, they advocate against the use of this type of short stem in patients with a Dorr type C, as PFFs occurred in 22.2% of all patients with a Dorr type C femur [12]. The rate of PFFs was significantly increased in the presented study for elderly patients with 5.2% compared to 0.7% in young patients (p = 0.026). However, our results do not show a statistical significance for intra- or postoperative occurrence, early or late occurrence, as well as the Dorr type. In a big propensity-score-matched analysis, increased age was found to be a risk factor for the occurrence of a PFFs within the first year for the Fitmore® stem [11]. The results in the presented study supports this finding with a significantly increased overall fracture rate in elderly patients without any significant difference in the subgroup analysis. The higher fracture rate might be associated with the cementless fixation itself, as cementless short stems show a generally low number of PFFs [10–12, 40].
The Fitmore® hip stem is associated with a high number of cortical hypertrophies [1, 5, 41]. CHs are reported up to 56% for this stem design and was not associated with inferior clinical results after 3.3 years and up to 8.6 years after index surgery [1, 5, 37]. The radiological outcome in the presented study shows a significantly increased rate of CHs within the younger age group of 54.6% compared to 31.5% in elderly patients (p < 0.001), primarily determined in the Gruen zones 3 and 5 comparable to other studies [1, 5, 37]. Our results show a higher activity level in the patients under 60 years of age with a statistically higher UCLA score as well as the PCS-12 of the VR-12 questionnaire. The higher activity level and therefore higher pressure on the cortical bone might lead to a higher number of CHs. Other comparable stem designs show high a rate of aseptic loosening in young and active patients under 60 years of age after a follow-up of five years [42], which could not be seen in the presented study, as we report only one case of aseptic loosening within an average clinical follow-up of 4.4 years.
Limitations of the study are primarily the retrospective study cohort. Therefore, we cannot present preoperative clinical scores and PROMs. Furthermore, there has not been any randomized controlled study concept. A low number of patients lost-to-follow-up can be presented in the study in respect of the follow-up for revision. However, a significant number of patients did not participate in the clinical and radiological follow-up due to several reasons, especially the COVID-restrictions at the time of the follow-up examinations.
Conclusion
Cementless short stem total hip arthroplasty shows a comparable clinical and radiological outcome in patients over 75 years of age compared to younger patients under 60 years of age. However, cementless shorts stem THA shows an increased rate of overall complications and periprosthetic fractures in elderly patients over 75 years of age. Cemented fixation of the femoral component should be considered in patients over 75 years of age.
Acknowledgements
Not Applicable
Author contributions
M. Luger: Drafting of the article, Statistical analysis Collection and assembly of data. M. Holzbuaer: Statistical analysis, Final approval of the article. M.C. Klotz: Conceptualization of the study design, Critical revision of the article for important intellectual content, Final approval of the article. F. Fellner: Critical revision of the article for important intellectual content, Final approval of the article. T. Gotterbarm: Conceptualization of the study design, Critical revision of the article for important intellectual content, Final approval of the article.
Funding
Open access funding provided by Johannes Kepler University Linz. The study was conducted without any funding or benefits from a commercial party.
Availability of data and materials
Data and materials are available on request.
Declarations
Conflict of interest
We report personal fees paid to one co-author (T.G.) during the conduct of the study from Zimmer Biomet, Europe and from Depuy Synthes Orthopädie Gmbh, Peter Brehm GmbH, ImplanTec GmbH outside the submitted work. We report research grants paid to our institution during the conduct of the study from Zimmer Biomet, Europe, Mathys AG Switzerland, Anika Therapeutics outside the submitted work. All other co-authors declare no financial support related or non-related to the study.
Ethical approval
The study was approved by the institutional review board (1239/2019) and conducted according to the Helsinki Declaration of 2008.
Consent to participate
Informed consent was obtained in every case for participation in the study.
Consent to publish
Consent for publication was given by from every participant by giving their informed consent for participation. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained by all participating patients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maier MW, Streit MR, Innmann MM, Kruger M, Nadorf J, Kretzer JP, Ewerbeck V, Gotterbarm T (2015) Cortical hypertrophy with a short, curved uncemented hip stem does not have any clinical impact during early follow-up. BMC Musculoskelet Disord 16:371. 10.1186/s12891-015-0830-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutt J, Harb Z, Gill I, Kashif F, Miller J, Dodd M (2014) Ten year results of the collum femoris preserving total hip replacement: a prospective cohort study of seventy five patients. Int Orthop 38(5):917–922. 10.1007/s00264-013-2212-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giardina F, Castagnini F, Stea S, Bordini B, Montalti M, Toni A (2018) Short stems versus conventional stems in cementless total hip arthroplasty: a long-term registry study. J Arthroplasty 33(6):1794–1799. 10.1016/j.arth.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 4.Kendoff DO, Citak M, Egidy CC, O’Loughlin PF, Gehrke T (2013) Eleven-year results of the anatomic coated CFP stem in primary total hip arthroplasty. J Arthroplasty 28(6):1047–1051. 10.1016/j.arth.2012.10.013 [DOI] [PubMed] [Google Scholar]
- 5.Innmann MM, Weishorn J, Bruckner T, Streit MR, Walker T, Gotterbarm T, Merle C, Maier MW (2019) Fifty-six percent of proximal femoral cortical hypertrophies 6 to 10 years after total hip arthroplasty with a short cementless curved hip stem - a cause for concern? BMC Musculoskelet Disord 20(1):261. 10.1186/s12891-019-2645-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gustke K (2012) Short stems for total hip arthroplasty: initial experience with the Fitmore stem. J Bone Joint Surg Br 94(11 Suppl A):47–51. 10.1302/0301-620X.94B11.30677 [DOI] [PubMed] [Google Scholar]
- 7.Khanuja HS, Banerjee S, Jain D, Pivec R, Mont MA (2014) Short bone-conserving stems in cementless hip arthroplasty. J Bone Joint Surg Am 96(20):1742–1752. 10.2106/JBJS.M.00780 [DOI] [PubMed] [Google Scholar]
- 8.Cinotti G, Della Rocca A, Sessa P, Ripani FR, Giannicola G (2013) Thigh pain, subsidence and survival using a short cementless femoral stem with pure metaphyseal fixation at minimum 9-year follow-up. Orthop Traumatol Surg Res 99(1):30–36. 10.1016/j.otsr.2012.09.016 [DOI] [PubMed] [Google Scholar]
- 9.Lombardi AV Jr, Berend KR, Ng VY (2011) Stubby stems: good things come in small packages. Orthopedics 34(9):e464-466. 10.3928/01477447-20110714-26 [DOI] [PubMed] [Google Scholar]
- 10.Dietrich M, Kabelitz M, Dora C, Zingg PO (2018) Perioperative fractures in cementless total hip arthroplasty using the direct anterior minimally invasive approach: reduced risk with short stems. J Arthroplasty 33(2):548–554. 10.1016/j.arth.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 11.Luger M, Feldler S, Pisecky L, Klasan A, Gotterbarm T, Schopper C (2023) Periprosthetic femoral fractures in cementless short versus straight stem total hip arthroplasty: a propensity score matched analysis. J Arthroplasty 38(4):751–756. 10.1016/j.arth.2022.10.027 [DOI] [PubMed] [Google Scholar]
- 12.Gkagkalis G, Goetti P, Mai S, Meinecke I, Helmy N, Bosson D, Kutzner KP (2019) Cementless short-stem total hip arthroplasty in the elderly patient - is it a safe option?: a prospective multicentre observational study. BMC Geriatr 19(1):112. 10.1186/s12877-019-1123-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boller S, Jahnke A, Augustin L, Ahmed G, Rickert M, Ishaque BA (2019) Age-related osseointegration of a short hip stem: a clinical and radiological 24 months follow-up. Arch Orthop Trauma Surg 139(3):405–410. 10.1007/s00402-018-3082-y [DOI] [PubMed] [Google Scholar]
- 14.Kim YH, Park JW, Kim JS (2018) Clinical Performance of ultra-short anatomic cementless versus fourth-generation cemented femoral stems for hip replacement in octogenarians. Orthopedics 41(4):e470–e478. 10.3928/01477447-20180424-01 [DOI] [PubMed] [Google Scholar]
- 15.Carli AV, Negus JJ, Haddad FS (2017) Periprosthetic femoral fractures and trying to avoid them: what is the contribution of femoral component design to the increased risk of periprosthetic femoral fracture? Bone Joint J. 99-B(1 Supple A):50–59. 10.1302/0301-620X.99B1.BJJ-2016-0220.R1 [DOI] [PubMed] [Google Scholar]
- 16.Troelsen A, Malchau E, Sillesen N, Malchau H (2013) A review of current fixation use and registry outcomes in total hip arthroplasty: the uncemented paradox. Clin Orthop Relat Res 471(7):2052–2059. 10.1007/s11999-013-2941-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bunyoz KI, Malchau E, Malchau H, Troelsen A (2020) Has the use of fixation techniques in THA changed in this decade? The uncemented paradox revisited. Clin Orthop Relat Res 478(4):697–704. 10.1097/CORR.0000000000001117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen D, Berger RA (2013) Outpatient minimally invasive total hip arthroplasty via a modified Watson-Jones approach: technique and results. Instr Course Lect 62:229–236 [PubMed] [Google Scholar]
- 19.Engh CA, Bobyn JD, Glassman AH (1987) Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br 69(1):45–55. 10.1302/0301-620X.69B1.3818732 [DOI] [PubMed] [Google Scholar]
- 20.Dorr LD, Faugere MC, Mackel AM, Gruen TA, Bognar B, Malluche HH (1993) Structural and cellular assessment of bone quality of proximal femur. Bone 14(3):231–242. 10.1016/8756-3282(93)90146-2 [DOI] [PubMed] [Google Scholar]
- 21.Davidson D, Pike J, Garbuz D, Duncan CP, Masri BA (2008) Intraoperative periprosthetic fractures during total hip arthroplasty. Evaluation and management. J Bone Joint Surg Am 90(9):2000–2012. 10.2106/JBJS.H.00331 [DOI] [PubMed] [Google Scholar]
- 22.Pike J, Davidson D, Garbuz D, Duncan CP, O’Brien PJ, Masri BA (2009) Principles of treatment for periprosthetic femoral shaft fractures around well-fixed total hip arthroplasty. J Am Acad Orthop Surg 17(11):677–688. 10.5435/00124635-200911000-00002 [DOI] [PubMed] [Google Scholar]
- 23.Harris WH (1969) Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 51(4):737–755 [PubMed] [Google Scholar]
- 24.Dawson J, Fitzpatrick R, Carr A, Murray D (1996) Questionnaire on the perceptions of patients about total hip replacement. J Bone Joint Surg Br 78(2):185–190 [PubMed] [Google Scholar]
- 25.Behrend H, Giesinger K, Giesinger JM, Kuster MS (2012) The “forgotten joint” as the ultimate goal in joint arthroplasty: validation of a new patient-reported outcome measure. J Arthroplasty 27(3):430-436 e431. 10.1016/j.arth.2011.06.035 [DOI] [PubMed] [Google Scholar]
- 26.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15(12):1833–1840 [PubMed] [Google Scholar]
- 27.Zahiri CA, Schmalzried TP, Szuszczewicz ES, Amstutz HC (1998) Assessing activity in joint replacement patients. J Arthroplasty 13(8):890–895. 10.1016/s0883-5403(98)90195-4 [DOI] [PubMed] [Google Scholar]
- 28.EuroQol G (1990) EuroQol–a new facility for the measurement of health-related quality of life. Health Policy 16(3):199–208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 29.Selim AJ, Rogers W, Fleishman JA, Qian SX, Fincke BG, Rothendler JA, Kazis LE (2009) Updated U.S. population standard for the veterans RAND 12-item health survey (VR-12). Qual Life Res 18(1):43–52. 10.1007/s11136-008-9418-2 [DOI] [PubMed] [Google Scholar]
- 30.Boonstra AM, Schiphorst Preuper HR, Reneman MF, Posthumus JB, Stewart RE (2008) Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Int J Rehabil Res 31(2):165–169. 10.1097/MRR.0b013e3282fc0f93 [DOI] [PubMed] [Google Scholar]
- 31.Dastane M, Dorr LD, Tarwala R, Wan Z (2011) Hip offset in total hip arthroplasty: quantitative measurement with navigation. Clin Orthop Relat Res 469(2):429–436. 10.1007/s11999-010-1554-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brooker AF, Bowerman JW, Robinson RA, Riley LH Jr (1973) Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg Am 55(8):1629–1632 [PubMed] [Google Scholar]
- 33.Singh M, Nagrath AR, Maini PS (1970) Changes in trabecular pattern of the upper end of the femur as an index of osteoporosis. J Bone Joint Surg Am 52(3):457–467 [PubMed] [Google Scholar]
- 34.Gruen TA, McNeice GM, Amstutz HC (1979) “Modes of failure” of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res 141:17–27 [PubMed] [Google Scholar]
- 35.Innmann MM, Streit MR, Kolb J, Heiland J, Parsch D, Aldinger PR, Konigshausen M, Gotterbarm T, Merle C (2015) Influence of surgical approach on component positioning in primary total hip arthroplasty. BMC Musculoskelet Disord 16:180. 10.1186/s12891-015-0623-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kutzner KP, Kovacevic MP, Roeder C, Rehbein P, Pfeil J (2015) Reconstruction of femoro-acetabular offsets using a short-stem. Int Orthop 39(7):1269–1275. 10.1007/s00264-014-2632-3 [DOI] [PubMed] [Google Scholar]
- 37.Acklin YP, Jenni R, Bereiter H, Thalmann C, Stoffel K (2016) Prospective clinical and radiostereometric analysis of the fitmore short-stem total hip arthroplasty. Arch Orthop Trauma Surg 136(2):277–284. 10.1007/s00402-015-2401-9 [DOI] [PubMed] [Google Scholar]
- 38.Freitag T, Fuchs M, Woelfle-Roos JV, Reichel H, Bieger R (2019) Mid-term migration analysis of a femoral short-stem prosthesis: a five-year EBRA-FCA-study. Hip Int 29(2):128–133. 10.1177/1120700018772277 [DOI] [PubMed] [Google Scholar]
- 39.Gromov K, Bersang A, Nielsen CS, Kallemose T, Husted H, Troelsen A (2017) Risk factors for post-operative periprosthetic fractures following primary total hip arthroplasty with a proximally coated double-tapered cementless femoral component. Bone Joint J 99-B(4):451–457. 10.1302/0301-620X.99B4.BJJ-2016-0266.R2 [DOI] [PubMed] [Google Scholar]
- 40.Luger M, Hipmair G, Schopper C, Schauer B, Hochgatterer R, Allerstorfer J, Gotterbarm T, Klasan A (2021) Low rate of early periprosthetic fractures in cementless short-stem total hip arthroplasty using a minimally invasive anterolateral approach. J Orthop Traumatol 22(1):19. 10.1186/s10195-021-00583-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thalmann C, Kempter P, Stoffel K, Ziswiler T, Frigg A (2019) Prospective 5-year study with 96 short curved fitmore hip stems shows a high incidence of cortical hypertrophy with no clinical relevance. J Orthop Surg Res 14(1):156. 10.1186/s13018-019-1174-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garavaglia G, Gonzalez A, Barea C, Peter R, Hoffmeyer P, Lubbeke A, Hannouche D (2021) Short stem total hip arthroplasty with the direct anterior approach demonstrates suboptimal fixation. Int Orthop 45(3):575–583. 10.1007/s00264-020-04910-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials are available on request.