Abstract
Purpose
Quantifying the association of chemotherapy relative dose intensity (RDI) with overall survival may enable supportive care interventions that improve chemotherapy RDI to estimate their magnitude of potential clinical benefit.
Methods
This cohort study included 533 patients with stage II-III colon cancer who initiated a planned regimen of 12 cycles of 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX) chemotherapy. The primary exposure was chemotherapy RDI. The primary outcome was overall survival. Restricted cubic splines estimated hazard ratios (HR).
Results
Chemotherapy regimen RDI was associated with overall survival in an L-shaped pattern (linear P=0.006; nonlinear P=0.057); the risk of death was flat above 85% but increased linearly below 85%. For example, a decrease in RDI from 85% to 75% was associated with an increased risk of death [HR: 1.20 (95% CI: 1.08, 1.52)], whereas an increase in RDI from 85% to 95% was not associated with the risk of death [HR: 1.06 (95% CI: 0.82, 1.38)].
Conclusion
If chemotherapy RDI is considered a potential surrogate of overall survival, supportive care interventions that improve chemotherapy RDI might confer a potential clinical benefit in this population.
Keywords: adherence, chemotherapy, surrogate endpoint, toxicity
Introduction
A postoperative chemotherapy regimen including a fluoropyrimidine and oxaliplatin is the standard of care for patients with stage III or high-risk stage II colon cancer [1]. Among clinical trial participants with stage II-III colon cancer randomized to 5-fluorouracil and oxaliplatin (known as the FOLFOX regimen), 46% required ≥1 dose reduction, 70% required ≥1 dose delay, and 26% discontinued chemotherapy prematurely due to treatment toxicities [2]. Such rates of chemotherapy events may be higher outside of the clinical trial setting [3]. Chemotherapy relative dose intensity (RDI) is a single quantitative measure that integrates dose reductions, delays, and discontinuation by dividing the delivered dose intensity by the standard dose intensity [4].
Retrospective studies have analyzed the association of chemotherapy RDI categorically, reporting that an RDI greater than 60% or 70% is associated with improved disease-free and overall survival [5–7]. However, categorizing continuous variables produces discontinuities in outcome risk estimates that reduce statistical power and are problematic to justify biologically and interpret clinically [8]. Furthermore, if chemotherapy RDI is a potential surrogate of overall survival [9], categorizing chemotherapy RDI hinders the ability of supportive care interventions that improve chemotherapy RDI to estimate their magnitude of potential clinical benefit [10,11].
This manuscript reports the results of a statistical analysis that examined chemotherapy RDI as a continuous variable using restricted cubic splines. Cubic splines accommodate nonlinearity and provide statistically efficient and visually intuitive descriptions of associations [12]. The motivation to conduct this detailed analysis was to enhance the potential clinical relevance of several National Cancer Institute (NCI)-sponsored trials designed to improve the primary endpoint of chemotherapy RDI in patients with resected colon cancer (e.g., NCT03291951, NCT05773144).
Methods
The Colorectal, Sarcopenia, Cancer And Near-term Survival (C-SCANS) study used a retrospective cohort design derived from the Kaiser Permanente Northern California (KPNC) cancer registry [13]. The C-SCANS cohort included all patients aged 18 to 80 years diagnosed with stage I to III colon or rectal adenocarcinoma from 2006 to 2011 who underwent surgical resection with curative intent. The current analysis was restricted to patients with colon cancer who initiated FOLFOX chemotherapy after surgical resection. When this cohort study was initiated, the oral fluoropyrimidine, capecitabine, was infrequently used in clinical practice at KPNC [14]. The study investigators obtained a waiver of written informed consent. The KPNC institutional review board approved this study.
Information regarding chemotherapy administration and treatment dates was obtained from the electronic medical record and chemotherapy infusion tables. The National Comprehensive Cancer Network guidelines recommended 12 cycles of postoperative FOLFOX chemotherapy for patients with stage III and high-risk stage II colon cancer when this patient cohort was treated [15]. The first treatment cycle began with the date of chemotherapy initiation and ended with the date of the following treatment cycle administration.
The primary exposure was chemotherapy RDI, calculated by indexing the delivered dose intensity (DDI) to the standard dose intensity (SDI) using the formula of Weycker [4]. DDI was calculated as the ratio of the delivered total dose in mg/m2 (starting with the dose in the first cycle) with imputed zeros for missed cycles over the actual time to complete chemotherapy through the last day of the last cycle, including the number of days in missed cycles. SDI was calculated as the ratio of starting total dose in mg/m2 over time to complete chemotherapy from the first cycle through the last day of the last cycle. The secondary exposures included drug-specific RDI (e.g., 5-fluorouracil and oxaliplatin).
Demographic information, including age at diagnosis and sex, was abstracted from the KPNC electronic medical record. Cancer stage (American Joint Committee on Cancer [AJCC 7th edition]) was obtained from the KPNC cancer registry. Covariate data were 100% complete.
The primary study outcome was overall survival, defined as the time from diagnosis to death from any cause. The secondary study outcome was cancer-specific survival, defined as the time from diagnosis to death attributed to colon or rectal cancer. Deaths were identified from the California state death registry, National Death Index using Social Security Administration data, and KPNC electronic mortality files.
In contrast to prior studies, chemotherapy RDI was modeled as a continuous variable using restricted cubic splines. Restricted cubic splines accommodate associations that may depart from linearity and are statistically efficient, and graphs of splines can provide visually intuitive descriptions of complex associations [16]. Model parsimony and the Akaike Information Criterion (AIC) were used to select the optimal number of knots for chemotherapy RDI. Knot locations were based on default locations using data-derived quantities (e.g., 10%, 50%, and 90% percentiles for three-knot splines) [16]. Multivariable-adjusted Cox proportional hazards models evaluated associations on the relative hazard [Hazard Ratio (HR)] scale between chemotherapy RDI and overall survival. Models were adjusted for age, sex, and cancer stage. The linearity and nonlinearity of associations were inspected visually using spline plots and tested statistically using likelihood ratio tests [16]. Proportional hazards were examined with log-log plots and scaled Schoenfeld residuals on time.
Results
The average (SD) age of the 533 patients was 58.7 (11.3) years, 55.9% were female, and 85.6% had stage III colon cancer. The median [IQR] follow-up time was 6.6 [5.1, 8.1] years. During follow-up, 115 deaths attributed to all causes were observed, of which 76 deaths were attributed to colorectal cancer.
The mean (SD) chemotherapy RDI for the FOLFOX regimen was 85.9% (12.5), and the median [interquartile range] was 88.0% [78, 96]. Correlates of chemotherapy RDI included age [β = −0.19 per one year increase (95% CI: −0.29, −0.10); P<0.001], sex [males: 88.2% vs. females: 84.1%; mean difference: 4.2% (95% CI: 1.9, 6.2); P<0.001], and cancer stage [stage II: 88.3% vs. stage III: 85.5%; mean difference: 2.8% 95% CI: −0.2, 5.8); P=0.072].
Chemotherapy RDI was associated with overall survival in an L-shaped pattern [(linear P=0.006; nonlinear P=0.057), Figure 1]; the risk of death was flat above 85% but increased linearly below 85%. For example, a decrease in RDI from 85% to 75% was associated with an increased risk of death [HR: 1.20 (95% CI: 1.08, 1.52)], whereas an increase in RDI from 85% to 95% was not associated with the risk of death [HR: 1.06 (95% CI: 0.82, 1.38)]. Analyses of individual drug RDI were similar for 5-fluorouracil and oxaliplatin RDI (Figure 2). Conclusions for the secondary endpoint of cancer-specific survival were like those of the primary endpoint of overall survival (results not shown). For illustrative purposes, analysis of RDI by quartile was not statistically significantly associated with overall survival (Supplementary Table 1).
Figure 1.
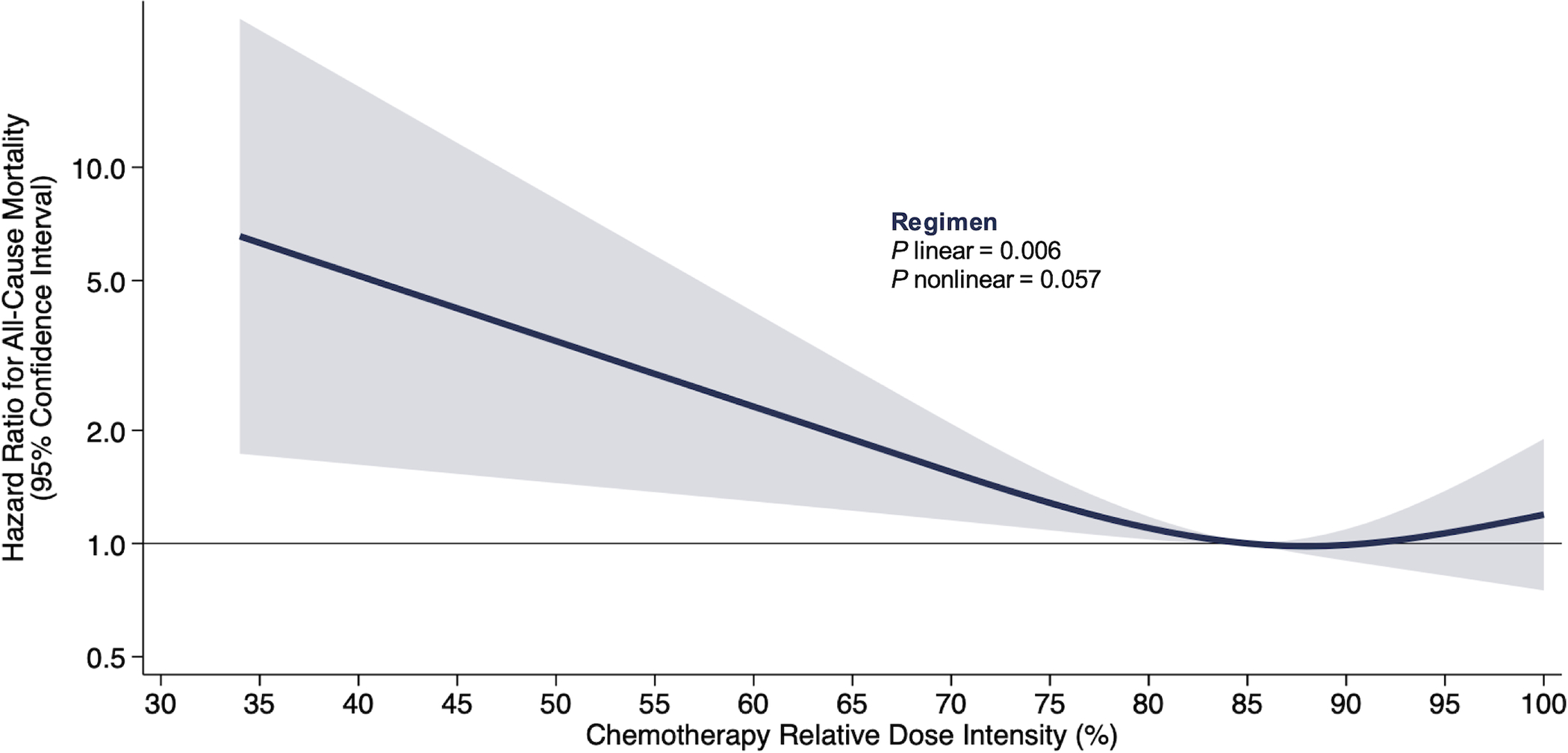
Risk of all-cause mortality by chemotherapy regimen (FOLFOX) relative dose intensity on the relative hazard scale in 533 patients with colon cancer.
Shaded regions indicate 95% confidence bands for mortality risk as a function of chemotherapy relative dose intensity. Estimates are adjusted for age, sex, and cancer stage. Three knots corresponding to the 10%, 50%, and 90% percentiles were placed at 68%, 88%, and 100% chemotherapy regimen RDI. The equation for the presented spline is log (HR) = −0.040 * spline parameter 1 + 0.034 * spline parameter 2 − 0.325 (if sex is female) + 0.282 (if age is ≥75 years) + 0.400 (if cancer stage is 3).
Figure 2.
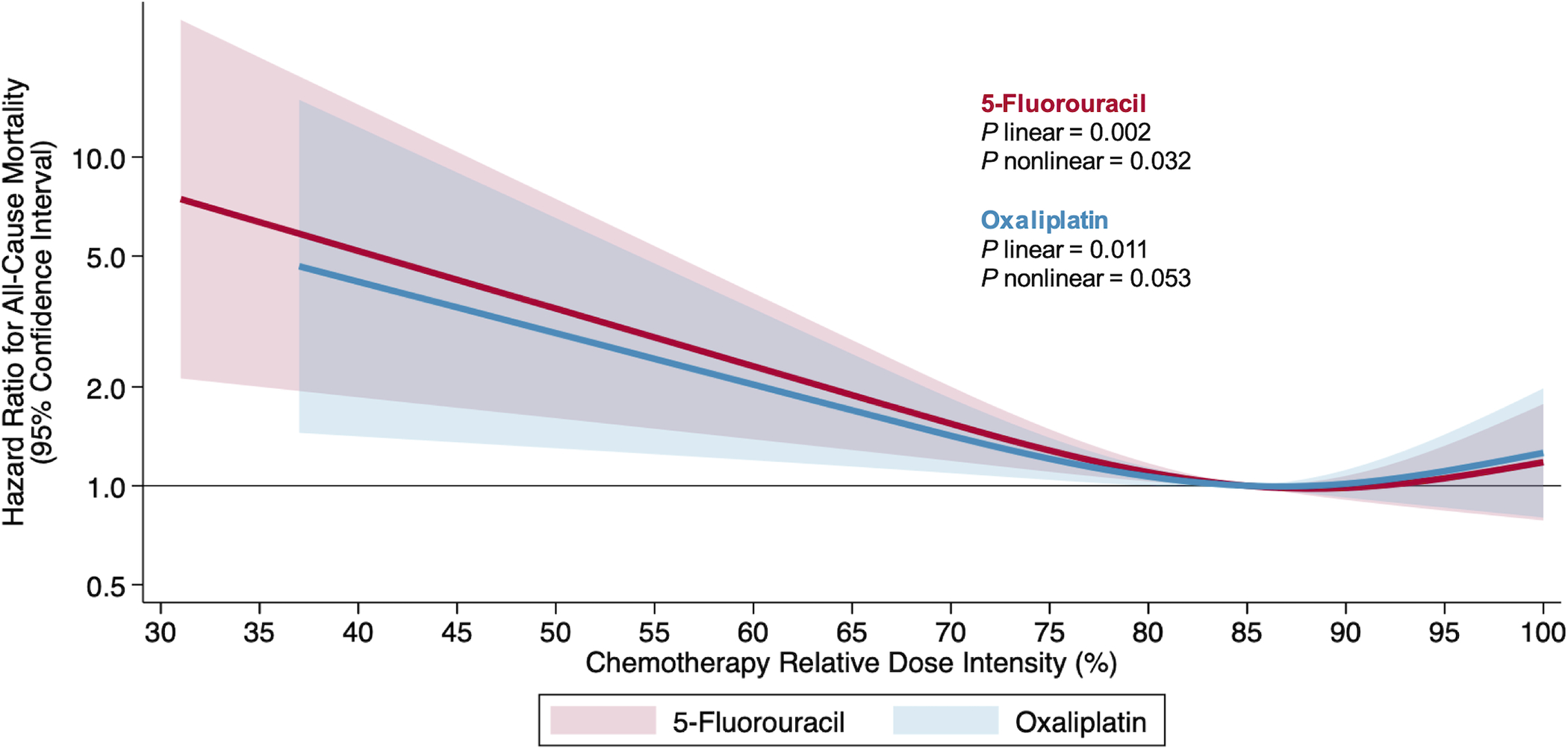
Risk of all-cause mortality by chemotherapy drug relative dose intensity on the relative hazard scale in 533 patients with colon cancer.
Shaded regions indicate 95% confidence bands for mortality risk as a function of 5-fluorouracil (red) and oxaliplatin (blue) chemotherapy relative dose intensity. Estimates are adjusted for age, sex, and cancer stage. Three knots corresponding to the 10%, 50%, and 90% percentiles were placed at 67%, 89%, and 100% for 5-fluorouracil RDI and 66%, 88%, and 100% for oxaliplatin RDI. The equation for the presented spline for 5-fluorouracil is log (HR) = log (HR) = −0.040 * spline parameter 1 + 0.032 * spline parameter 2 − 0.327 (if sex is female) + 0.300 (if age is ≥75 years) + 0.404 (if cancer stage is 3). The equation for the presented spline for oxaliplatin is log (HR) = −0.036 * spline parameter 1 + 0.032 * spline parameter 2 − 0.328 (if sex is female) + 0.279 (if age is ≥75 years) + 0.417 (if cancer stage is 3).
Discussion
In this cohort study of 533 patients with colon cancer, postoperative chemotherapy RDI was associated with overall survival. The use of restricted cubic spline modeling determined that the pattern of the association between chemotherapy RDI and overall survival was L-shaped, such that chemotherapy RDI above 85% was not statistically significantly associated with overall survival (e.g., the hazard was flat); however, as chemotherapy RDI declined below 85%, the risk of death increased.
This analysis is consistent with prior retrospective studies concluding that categorical RDI is associated with disease-free survival and overall survival [5–7]. For example, among 367 patients with stage III colon cancer, compared to receiving less than 70%, receipt of greater than 70% of FOLFOX chemotherapy was associated with improved three-year disease-free survival (66.1% vs. 52.7%; P=0.009) and five-year overall survival (66.3% vs. 50.5%; P<0.001) [6].
Treatment-related toxicities are the principal determinants of chemotherapy dose reductions, delays, and discontinuation in colon cancer. The most common toxicities are gastrointestinal (e.g., nausea, diarrhea, and vomiting) and hematologic (e.g., neutropenia and thrombocytopenia) [17]. Oxaliplatin causes peripheral neuropathy, manifested as numbness, pins and needles, or pain in the hands and feet [17].
Our results indicate that an RDI of ≥85% is associated with improved overall survival. However, dose reductions in response to treatment-related toxicities are expected and potentially undermine the probability of cure when the RDI falls below 85% [2,3]. Interventions that prevent the development of treatment-related toxicities may enable patients to tolerate a higher chemotherapy RDI, potentially improving overall survival. Hypothesis-generating studies have reported that exercise during chemotherapy may reduce toxicity and improve chemotherapy tolerability [10,11]. Prospective randomized trials are ongoing to examine the effects of muscle-strengthening exercise in colon cancer and nutrition and physical activity in breast cancer on the primary endpoint of chemotherapy RDI [18,19].
The American College of Sports Medicine concluded that exercise is safe during chemotherapy, but the evidence that exercise improves chemotherapy RDI continues to represent a significant research gap [20]. Consequently, in 2022, the NCI launched the Exercise and Nutrition Interventions to Improve Cancer Treatment-Related Outcomes (ENICTO) in Cancer Survivors Consortium (https://cancercontrol.cancer.gov/brp/hbrb/enicto-consortium). The ENICTO Consortium trials will provide definitive evidence about lifestyle modification to improve chemotherapy RDI. The information reported in this manuscript between chemotherapy RDI and overall survival will enable the trial of aerobic exercise in colon cancer (NCT05773144) to estimate the magnitude of potential clinical benefit.
The principal limitation of this study is the observational design, which limits our ability to rule out residual confounding. The retrospective design precluded our ability to determine specific medical decisions for chemotherapy deviations, which are often based on subjective elements. The patients in this cohort were treated with FOLFOX chemotherapy; thus, we cannot directly comment on the association between capecitabine and oxaliplatin (CAPEOX) RDI with overall survival. It is possible that the type of fluoropyrimidine does not substantively modify the reported associations with overall survival. It is unknown whether our findings in colon cancer can be generalized to other malignancies. The principal strength of this study is the population-based sample.
Chemotherapy RDI is associated with overall survival in patients with resected colon cancer. If chemotherapy RDI is considered a potential surrogate of overall survival, supportive care interventions that improve chemotherapy RDI might confer a potential clinical benefit.
Supplementary Material
Funding
This work was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers R01-CA175011; R01-CA206196; U01-CA271279, and R25-CA203650. The content is solely the authors’ responsibility and does not necessarily represent the official views of the National Institutes of Health. The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Competing Interests
Dr. Brown reports receiving grants from the National Cancer Institute, Cancer Research UK, and the American Institute for Cancer Research during the study. Dr. Meyerhardt reports receiving grants from the National Cancer Institute during the study and consulting fees from Merck Pharmaceutical during the 36 months before publication (all fees <$5,000). Dr. Caan reports receiving grants from the National Cancer Institute during the study. All other authors report no disclosures.
Data Sharing Statement:
Data described in the manuscript, code book, and analytic code will not be made available because this was a retrospective cohort study using electronic medical records. The institutional review board waived the requirement for informed consent. Consequently, study subjects did not explicitly consent for their data to be shared publicly. Moreover, study subjects were treated within a single health system for an uncommon disease, and our ability to preserve subject anonymity cannot be guaranteed.
REFERENCES
- 1.Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A, Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer I (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350 (23):2343–2351. doi: 10.1056/NEJMoa032709 [DOI] [PubMed] [Google Scholar]
- 2.Webster-Clark M, Keil AP, Sanoff HK, Sturmer T, Westreich D, Lund JL (2021) Introducing longitudinal cumulative dose to describe chemotherapy patterns over time: Case study of a colon cancer trial. Int J Cancer 149 (2):394–402. doi: 10.1002/ijc.33565 [DOI] [PubMed] [Google Scholar]
- 3.Webster-Clark M, Keil AP, Robert N, Frytak JR, Boyd M, Sturmer T, Sanoff H, Westreich D, Lund JL (2022) Comparing Trial and Real-world Adjuvant Oxaliplatin Delivery in Patients With Stage III Colon Cancer Using a Longitudinal Cumulative Dose. JAMA Oncol 8 (12):1821–1824. doi: 10.1001/jamaoncol.2022.4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weycker D, Barron R, Edelsberg J, Kartashov A, Lyman GH (2012) Incidence of reduced chemotherapy relative dose intensity among women with early stage breast cancer in US clinical practice. Breast Cancer Res Treat 133 (1):301–310. doi: 10.1007/s10549-011-1949-5 [DOI] [PubMed] [Google Scholar]
- 5.Zok J, Bienkowski M, Radecka B, Korniluk J, Adamowicz K, Duchnowska R (2021) Impact of relative dose intensity of oxaliplatin in adjuvant therapy among stage III colon cancer patients on early recurrence: a retrospective cohort study. BMC Cancer 21 (1):529. doi: 10.1186/s12885-021-08183-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aspinall SL, Good CB, Zhao X, Cunningham FE, Heron BB, Geraci M, Passero V, Stone RA, Smith KJ, Rogers R, Shields J, Sartore M, Boyle DP, Giberti S, Szymanski J, Smith D, Ha A, Sessions J, Depcinski S, Fishco S, Molina I, Lepir T, Jean C, Cruz-Diaz L, Motta J, Calderon-Vargas R, Maland J, Keefe S, Tague M, Leone A, Glovack B, Kaplan B, Cosgriff S, Kaster L, Tonnu-Mihara I, Nguyen K, Carmichael J, Clifford L, Lu K, Chatta G (2015) Adjuvant chemotherapy for stage III colon cancer: relative dose intensity and survival among veterans. BMC Cancer 15:62. doi: 10.1186/s12885-015-1038-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Eeghen EE, Bakker SD, van Bochove A, Loffeld RJ (2015) High Risk Stage 2 and Stage 3 Colon Cancer, Predictors of Recurrence and Effect of Adjuvant Therapy in a Nonselected Population. Int Sch Res Notices 2015:790186. doi: 10.1155/2015/790186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altman DG, Royston P (2006) The cost of dichotomising continuous variables. BMJ 332 (7549):1080. doi: 10.1136/bmj.332.7549.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burzykowski T, Buyse M, Molenberghs G (2005) The evaluation of surrogate endpoints, vol 427. Springer, [Google Scholar]
- 10.Bland KA, Zadravec K, Landry T, Weller S, Meyers L, Campbell KL (2019) Impact of exercise on chemotherapy completion rate: A systematic review of the evidence and recommendations for future exercise oncology research. Crit Rev Oncol Hematol 136:79–85. doi: 10.1016/j.critrevonc.2019.02.005 [DOI] [PubMed] [Google Scholar]
- 11.Purcell SA, Kok DE, Ketterl T, Garcia MB, Joffe L, Brown JC, Dieli-Conwright CM, Williams GR (2023) Pharmacokinetics of cancer therapeutics and energy balance: the role of diet intake, energy expenditure, and body composition. J Natl Cancer Inst Monogr 2023 (61):3–11. doi: 10.1093/jncimonographs/lgad010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutherford MJ, Crowther MJ, Lambert PC (2013) The use of restricted cubic splines to approximate complex hazard functions in the analysis of time-to-event data: a simulation study. Journal of Statistical Computation and Simulation 85 (4):777–793. doi: 10.1080/00949655.2013.845890 [DOI] [Google Scholar]
- 13.Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, Xiao J, Weltzien E, Feliciano EC, Castillo AL, Quesenberry CP, Kwan ML, Prado CM (2017) Explaining the Obesity Paradox: The Association between Body Composition and Colorectal Cancer Survival (C-SCANS Study). Cancer Epidemiol Biomarkers Prev 26 (7):1008–1015. doi: 10.1158/1055-9965.EPI-17-0200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Twelves C, Wong A, Nowacki MP, Abt M, Burris H 3rd, Carrato A, Cassidy J, Cervantes A, Fagerberg J, Georgoulias V, Husseini F, Jodrell D, Koralewski P, Kroning H, Maroun J, Marschner N, McKendrick J, Pawlicki M, Rosso R, Schuller J, Seitz JF, Stabuc B, Tujakowski J, Van Hazel G, Zaluski J, Scheithauer W (2005) Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 352 (26):2696–2704. doi: 10.1056/NEJMoa043116 [DOI] [PubMed] [Google Scholar]
- 15.Benson AB 3rd, Venook AP, Cederquist L, Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC, Fichera A, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wu CS, Gregory KM, Freedman-Cass D (2017) Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 15 (3):370–398. doi: 10.6004/jnccn.2017.0036 [DOI] [PubMed] [Google Scholar]
- 16.Harrell F (2015) Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. Springer, [Google Scholar]
- 17.Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, Souglakos J, Shi Q, Kerr R, Labianca R, Meyerhardt JA, Vernerey D, Yamanaka T, Boukovinas I, Meyers JP, Renfro LA, Niedzwiecki D, Watanabe T, Torri V, Saunders M, Sargent DJ, Andre T, Iveson T (2018) Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N Engl J Med 378 (13):1177–1188. doi: 10.1056/NEJMoa1713709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caan BJ, Meyerhardt JA, Brown JC, Campbell KL, Cespedes Feliciano EM, Lee C, Ross MC, Quinney S, Quesenberry C, Sternfeld B, Schmitz KH (2021) Recruitment strategies and design considerations in a trial of resistance training to prevent dose-limiting toxicities in colon cancer patients undergoing chemotherapy. Contemp Clin Trials 101:106242. doi: 10.1016/j.cct.2020.106242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanft T, Harrigan M, Cartmel B, Ferrucci LM, Li FY, McGowan C, Zupa M, Nguyen TH, Ligibel J, Neuhouser ML, Hershman DL, Basen-Engquist K, Jones B, Knobf T, Chagpar A, Silber A, Irwin ML (2021) Effect of healthy diet and exercise on chemotherapy completion rate in women with breast cancer: The Lifestyle, Exercise and Nutrition Early after Diagnosis (LEANer) study: Study protocol for a randomized clinical trial. Contemp Clin Trials 109:106508. doi: 10.1016/j.cct.2021.106508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, Zucker DS, Matthews CE, Ligibel JA, Gerber LH, Morris GS, Patel AV, Hue TF, Perna FM, Schmitz KH (2019) Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc 51 (11):2375–2390. doi: 10.1249/MSS.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will not be made available because this was a retrospective cohort study using electronic medical records. The institutional review board waived the requirement for informed consent. Consequently, study subjects did not explicitly consent for their data to be shared publicly. Moreover, study subjects were treated within a single health system for an uncommon disease, and our ability to preserve subject anonymity cannot be guaranteed.


