Abstract
The study investigated the feasibility of enzymatic extraction for guava juice and evaluated the effects of various preservatives on its shelf life. The crushed guava puree was undergone different pectinase enzyme concentrations over three incubation periods. The findings revealed that pectinase concentrations of 0.1 % and 0.2 %, when incubated for 1 and 2 h, were the most effective. Juice yields ranged from 65.24 % to 78.64 %, with Total Soluble Solids (TSS) varying from 9.12°Brix to 11.56°Brix. The physicochemical properties of the guava juice resulted 84.2 % moisture, 2.16 % protein, 0.77 % fat, 3.27 % fiber, 0.65 % acidity, 2.25 % reducing sugars, 8.27 % non-reducing sugars, 79.53 % antioxidant activity, 173.2 mg/100g of ascorbic acid, 10.52 TSS, 109.7 mg/100g of phenolic content, and a pH of 3.2. Eight juice samples were prepared as per formulation with sodium benzoate and potassium metabisulfite (KMS) at concentrations of 150 ppm, 200 ppm, and 250 ppm, in addition to one refrigerated sample and one control. The stability of these guava juice samples was monitored every 15 days over a 90-day period. Results showed that acidity, TSS, pH, reducing sugars, and non-reducing sugars changed over time. Samples with preservatives exhibited slower changes compared to the control. Phenolic compounds diminished more quickly at ambient temperature than in refrigerated or preservative-treated samples. Initially, phenolic content and antioxidant capacity were 44 mg GAE/100g and 44 %, respectively, but declined to 10–15 mg and 15–17 % by the end of the storage period. Color changes were more noticeable in samples stored at room temperature, whereas preservatives effectively reduced color degradation caused by enzymatic browning. Moreover, ascorbic acid retention was better in samples with preservatives and those stored under refrigeration. The ascorbic acid degradation rate was highest at room temperature (0.023 day^-1) and lowest with 250 ppm KMS (0.016 day^-1). Microbiological tests indicated that the juice remained safe for 40 days at room temperature, 90 days under refrigeration, and approximately 85 days with preservatives.
Keywords: Guava juice, Pectinase, Preservatives, Bio-active compound, Storage stability
Graphical abstract
1. Introduction
Guava (Psidium guajava L.) is highly delectable and frequently promoted as a “superfruit” due to its high vitamin A and C content. The seeds include abundant amounts of omega-3 and omega-6 polyunsaturated fatty acids and a notably high concentration of dietary fibre [1]. After guava fruits are harvested, it's critical to minimize postharvest losses and product development is one of the effective ways in this instance [2]. Guava juice is a popular drink renowned for its refreshing flavor and numerous health benefits. Packed with essential nutrients like vitamin C, dietary fiber, and antioxidants, guava juice provides both a pleasing taste and significant nutritional advantages. The fruit's natural sweetness and unique aroma add to its appeal, while its healthful properties bolster immune function, support digestive health, and contribute to overall wellness.
Storage of juice can affect physicochemical and sensory properties as well as reduce its quality, safety, and nutritional value [3]. Preservatives are chemicals added to food to improve its properties like taste, appearance, flavor, and texture, or to prevent it from degrading due to oxidation, enzymatic, and microbiological activity [4,5]. The main goal of preservation is to increasing the shelf life of fruit juice retaining safety, quality, and sensory characteristics [6]. In addition, postharvest practices are also linked with the quality parameters of finished goods that boost their market value [7,8]. The most often used preservatives in food, benzoic acid and its salt, calcium and potassium comply with legislation for non-alcoholic beverages (maximum permissible concentration of 0.05 g/100 mL), sorbic acid and its sodium, potassium, and calcium (maximum permissible concentration of 0.08 g/100 mL), and sulfur dioxide (maximum permissible concentration of 0.004 g/100 mL) are most commonly used [9]. Fruit juice is frequently preserved using potassium metabisulfite (KMS) and sodium benzoate that help preserve the freshness of fruit juice by preventing germs from growing [10].
Sodium benzoate, with the structure C7H5O2Na, has an atomic weight of 144.1 g/mol, has no odor, crystalline powder and can liquefy in ethanol as well as water. Often used as a preservative in some products in the food, cosmetic, and pharmaceutical industries [11]. It is utilized in the food sector for its ability to prevent fungi and bacteria growth in stored foods and drinks, especially in acidic foods. Adding sodium benzoate to fruit juice significantly extends its shelf life by preventing spoilage. It is globally accepted as a food additive, generally recognized by E211 in European food products [12]. The FDA permits a maximum of 0.1 % weight concentration of sodium benzoate in food and drinks [13]. Nonetheless, benzoate could trigger allergic responses in sensitive individuals and increase hyperactivity, particularly when combined with food dyes [14]. One major worry about sodium benzoate is its potential to change into benzene, which is a recognized cancer-causing agent. Benzene can be produced in beverages like soda when they contain both sodium benzoate and vitamin C [15].
Potassium Metabisulphite (KMS) is a recognized derivative of sulfur dioxide and is a proven preservative that has been widely used in the brewing, wine, and food sectors [16]. KMS smells strongly like sulfur dioxide and shows up as a white granular or powdery substance with the chemical formula K2S2O5, and an atomic weight of 222.33 g/mol [17]. It is identified by E244, and its maximum permitted levels of SO2 for EU law in juice and nectar are 10 mg/L [18]. It is used to prevent the oxidation of juice, wine, or beer and to control wild yeasts on fruit. It is sometimes used with potassium sorbate to inhibit additional fermentation. It is a strong antioxidant that guards the food's delicate taste and color [19]. Caution is, for certain people, potassium metabisulfite is limited in use and could cause allergies in some sensitive individuals [20].
This study aims to assess how varying concentrations of pectinase and incubation times affect guava juice extraction. Additionally, to evaluate the influence of sodium benzoate and potassium metabisulfite on the physicochemical properties, antioxidant activity and microbial stability of the juice throughout storage period.
2. Materials and methods
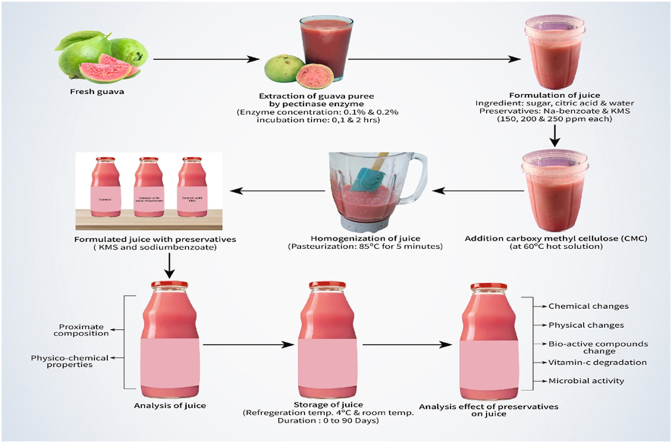
The experiment was conducted in the laboratory of the Department of Food Technology & Rural Industries, Faculty of Agricultural Engineering and Technology, Bangladesh Agricultural University, Mymensingh-2202. Freshly harvested guava of pink mesocarp was collected. Sugar and other materials required for the experiment were received from the laboratory stock.
2.1. Extraction of guava juice
The fruits were washed in running tap water. Then cleaned fruits were cut into small pieces and blended using a blender. After that, crushed guava was strained to remove seeds to obtain guava puree. Enzymatic treatment to optimize juice production was carried out according to the method described by Thongsombat et al. [21] with slight modification. Guava puree of 100 g was placed in 250 ml beakers. Enzyme pectinase was added to 10, 20 and 30 ml in each of the three 100 ml volumetric flasks. Citrate buffer solution (pH 4.0) was immediately added to bring the total volume to 100 ml in each volumetric flask and added to the beakers containing guava puree, stirred well and incubated in a water bath at 45 °C for 1.0, 2.0 and 3.0 h with occasionally stirring for every 30 min intervals. After reaching each incubation period, immediately heat the pectinase-treated sample at 90 °C for 2 min with constant stirring to stop the enzymatic activity. The juice was extracted from the pectinase-treated sample by pressing through a cheesecloth bag. Percent yield (w/w) was calculated by measuring the juice obtained.
2.2. Formulation and preparation of guava juice
The formulation of guava juice is presented in the table-1. Sample without preservatives is considered as the control and kept at room temperature. Room temperature was varied from 36 °C to 40 °C. A sample without preservatives was kept at a refrigerated temperature (4 °C). Six more samples were made by adding KMS and sodium benzoate at the rates of 150, 200 and 250 ppm each. At first sugar and water were mixed to make brix solution. Then carboxy methyl cellulose (CMC) was added to the hot brix solution (60 °C). Subsequently, the mixture of guava puree and citric acid was mixed. Citric acid was added as acidulent. Finally, the mixture was homogenized and filled while hot in glass containers after pasteurization (85 °C for 5 min). Then the bottles were closed with screw caps, cooled and stored at room temperature for further analysis.
Table 1.
Formulation of guava juice.
| Ingredient |
Sample |
|||||||
|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | |
| Guava with pulp (%) | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 |
| KMS (ppm) | 0 | 0 | 150 | 200 | 250 | 0 | 0 | 0 |
| Na-benzoate (ppm) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 150 | 200 | 250 |
| Sugar (%) | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 |
| Citric acid (%) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| CMC (%) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Water (%) | 47 | 47 | 47 | 47 | 47 | 47 | 47 | 47 |
2.3. Physicochemical properties of guava juice
Guava juice extracted using 0.1 % and 0.2 % pectinase were mixed and evaluated for physicochemical properties. The processed guava juice samples were assessed for storage stability for 90 days analyzing physicochemical parameters, bioactive compound and antioxidant activity at 15-day intervals.
Moisture, protein, fiber, fat and ash content were measured as per AOAC [22] method. Total soluble solid (TSS) and pH were measured by direct reading using a portable refractometer and digital pH meter. The reducing sugar and non-reducing sugar content were measured as per the method described by Rangana [23]. Ascorbic acid was determined using the methodology followed by Hasan et al. [24].
The color degradation of juice samples upon storage was analyzed by a colorimeter (Chroma Meter CR400, Konica Minolta, Japan) using L*, a* and b* system, where L* is lightness, a* is redness (+)/greenness (−) and b* indicates yellowness (+)/blueness (−). The difference among L*, a*, and b* is taken into account by the single value known as ΔE* as follows equation (1):
| (1) |
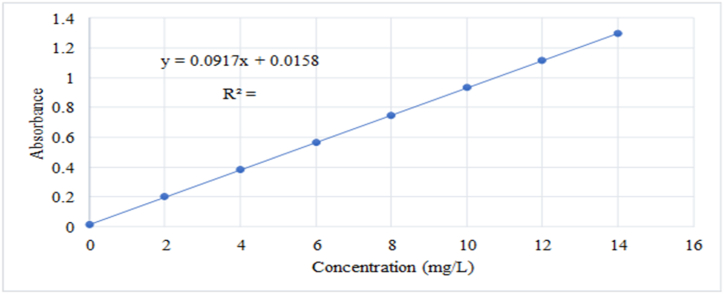
The total phenolic content of juice samples was determined using the Folin-Ciocalteu method as described by Majumder et al. [25]. TPC was presented as mg of gallic acid identical (GAE)/100 g of test employing a standard curve arranged by employing a gallic acid standard solution (0–100 mg/l). The Folin-Ciocaltu reagent (1:10 v/v in distilled water) and 4 ml of 7.5 % Na2CO3 were included in 1.0 ml (1 mg/ml) of each test. The blend was vortexed for 15 s and heated at 40 °C for 30 min to form the color. The absorbance at 765 nm was measured employing a UV/VIS spectrophotometer (Photo-lab 7600, UV–VIS, EU). For the calibration curve, gallic acid was utilized as standard. The concentrations of gallic acid were 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 and 13 mg per ml arrangements of gallic acid in acetone: water (80: 20 v/v). A clear trial comprising water and reagents was moreover utilized as a reference. The results were expressed as milligrams of gallic acid equivalents per ml of juice (mg GAE/g powder) by the gallic acid calibration curve shown in Fig. 1.
Fig. 1.
Gallic acid calibration curve.
Using a method described by Yi et al. [26], the DPPH free radical scavenging activity of juice samples was evaluated. A 200 μl extract sample and 1.0 ml of 0.1 mM DPPH in methanol were combined to conduct the test. After shaking, the mixture was allowed to sit at room temperature for 20 min to react. By comparing the absorbance at 517 nm to methanol blanks, the amount of DPPH free radical left behind was calculated. The percentage scavenging effect was calculated by comparing the decrease in absorbance to the control using the following equation (2):
| (2) |
2.4. Degradation kinetics of Vitamin-C
The reaction's rate is denoted by a rate constant (K) which is represented by the general equation (3) [27,28]:
| (3) |
Where,
= Rate of reaction; K= Reaction rate constant; C = Concentration of reactant at any time t; and m = Order of reaction
The reaction can be of zero order, first order or second order depending on the value of m. Many of the responses that take place in the food system seem to follow a first-order reaction. The following would be a mathematical expression for this behavior by equation (4);
| (4) |
Where,
= Concentration at any time; = Initial concentration; t = time; and K = Reaction rate
Berk [28] explains the link between the reaction rate constant (k) and the temperature (T) by Arrhenius equation (5) as follows:
| (5) |
Where,
k = Reaction rate constant, R = Universal gas constant (8.314 kJ. K−1. Kmol−1), T = Absolute temperature (K), Ea = Activation energy (Kj. Kmol−1).
All the preservative-treated samples along with control were kept at room temperature that varied 36–40 °C. So, the average room temperature was taken as 38 °C and in combination with refrigeration temperature (4 °C) were used for calculating activation energy.
2.5. Microbial analysis
Total plate counts were conducted using the standard plate count method [29]. Twenty-5 mm of juice sample was mixed with 225 ml of sterile buffered peptone water. A 10-fold serial dilution for each sample was made using sterile buffered peptone water up to 12 dilutions. Dilutions were surface spread onto duplicate sterile nutrient agar media and incubated at 37 °C for 24 h. After incubation the incubated plates were selected for counting the bacterial colony based on the number and ease of counting of the colony. The plate containing segregated, overlapping and confusing colonies was avoided. The plates containing 30 to 250 bright, cleared and countable colonies were selected. The total number of colony was determined by equation (6).
| (6) |
2.6. Statistical analysis
All the data collected were analyzed using Microsoft Excel (version 2019). The analysis included various statistical methods such as descriptive statistics, correlation analysis, and Analysis of Variance (ANOVA), all conducted at a 5 % level of significance. To compare means across different groups, the Least Significant Difference (LSD) test was applied. The results were presented as mean ± standard deviation to provide an overview of the data's central tendency and variability. Additionally, Pearson correlation analysis was utilized to evaluate the strength and nature of the relationships between the variables.
3. Result and discussion
3.1. Extraction of guava juice
A progressive increase in Total Soluble Solids (TSS) was observed in the extracted juice as both enzyme concentrations and incubation periods were extended (Table 2). Pectinase facilitated the hydrolysis of pectin, leading to an increase in pulp viscosity and a significant rise in juice yield. These findings are consistent with the research by Chopda and Barrett [30]. The data indicate that higher enzyme concentrations and longer incubation times considerably improved the yield of hazy juice. However, 0.1 % and 0.2 % pectinase concentrations with incubation times of 1 and 2 h were identified as the most cost-effective treatments. Longer incubation periods resulted in juice with increased turbidity. The highest yields of 78.64 % and 78.16 % were obtained with 0.2 % and 0.3 % pectinase, respectively, incubated for 3 min.
Table 2.
Extraction of guava juice.
| Enzyme Concentration (%) | Incubation Time (hr.) | Yield (%) | TSS (°B) |
|---|---|---|---|
| 0 | 0 | 65.24 ± 0.18 | 9.12 ± 0.15 |
| 0.1 | 1 | 68.54 ± 0.22 | 9.47 ± 0.12 |
| 2 | 72.98 ± 0.32 | 9.72 ± 0.24 | |
| 3 | 74.67 ± 0.26 | 9.95 ± 0.12 | |
| 0.2 | 1 | 73.36 ± 0.15 | 10.37 ± 0.25 |
| 2 | 75.44 ± 0.24 | 10.61 ± 0.32 | |
| 3 | 78.16 ± 0.32 | 10.85 ± 0.15 | |
| 0.3 | 1 | 75.47 ± 0.17 | 11.13 ± 0.24 |
| 2 | 77.31 ± 0.26 | 11.42 ± 0.15 | |
| 3 | 78.64 ± 0.34 | 11.56 ± 0.32 |
3.2. Physicochemical properties of guava juice
The physicochemical properties of the extracted guava juice are detailed in Table 3. The juice composition includes 84.2 % moisture, 0.51 % ash, 2.16 % protein, 0.77 % fat, and 3.27 % fiber. Analysis of bioactive compounds revealed ascorbic acid content at 173.2 mg/100g, phenolic compounds at 109.7 mg/100g, and antioxidant activity at 79.53 %. The sugar content was assessed, showing reducing sugars at 2.25 % and non-reducing sugars at 8.27 %, with a Total Soluble Solids (TSS) measurement of 10.52. These characteristics of the fresh guava juice are largely consistent with the findings reported by Yousaf et al. [31].
Table 3.
Physicochemical properties of guava juice.
| Composition/Parameters | Quantity |
|---|---|
| Moisture (%) | 84.2 ± 1.16 |
| Ascorbic acid (mg/100g) | 173.2 ± 3.28 |
| Total Soluble Solids (TSS) | 10.52 ± 0.33 |
| Ash (%) | 0.51 ± 0.07 |
| Protein (%) | 2.16 ± 0.19 |
| Fat (%) | 0.77 ± 0.08 |
| Fiber (%) | 3.27 ± 0.32 |
| Acidity (%) | 0.65 ± 0.15 |
| PH | 3.2 ± 0.21 |
| Reducing Sugar (%) | 2.25 ± 0.31 |
| Non-reducing Sugar (%) | 8.27 ± 0.53 |
| Total Phenolic Content (mg/100g) | 109.7 ± 0.72 |
| Antioxidant Activity (%) | 79.53 ± 2.43 |
3.3. Storage stability analysis
3.3.1. Titratable acidity and pH
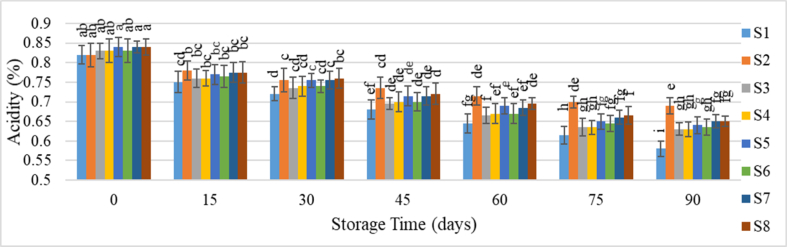
Fig. 2 illustrates the change in titratable acidity of guava juice samples over time. A gradual decline in acidity was observed across different samples, with the reduction being slower for those stored under refrigeration. Titratable acidity, which includes both organic acids naturally present in fruits and any added acids for preservation, initially ranged from 0.82 % to 0.84 % for all samples. By the end of the storage period, acidity levels decreased to between 0.58 % and 0.69 %. Pareek et al. [32] suggest that this reduction in acidity during storage may result from invertase enzymes converting acids into sugars and salts. They also noted that preservatives help minimize acidity loss during storage. Similarly, Falade et al. [33] reported that the titratable acidity in sweetened Julie and Ogbomoso mango juices increased with 18 weeks of storage. Iqbal et al. [34] observed a decrease in acidity in stored Kinnow juice due to oxidation, although the reduction was less pronounced under refrigerated conditions due to enzymatic inactivation.
Fig. 2.
Change of acidity of guava juice during storage.
Samples with the same superscripts don't differ at 5 % level of significance. S1 = Juice (without preservative) at RMT; S2 = Juice (without preservative) at RFT (4 °C); S3 = Juice contained KMS 150 ppm; S4 = Juice contained KMS 200 ppm; S5 = Juice contained KMS 250 ppm; S6 = Juice contained Sodium Benzoate 150 ppm; S7 = Juice contained Sodium Benzoate 200 ppm; S8 = Juice contained Sodium Benzoate 250 ppm.
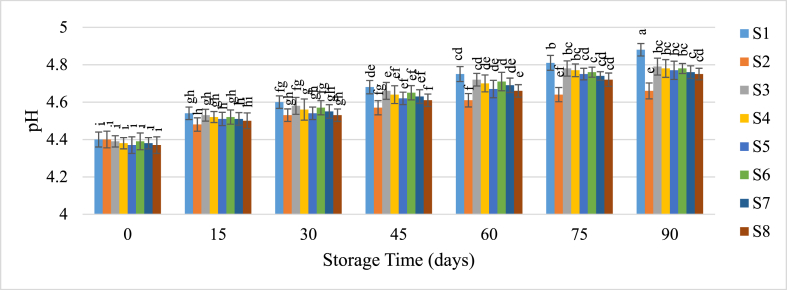
On the other hand, pH, which inversely correlates with acidity, increased over the storage period (Fig. 3). The pH rise was slower in refrigerated samples and those with added preservatives. Initially, pH ranged from 4.37 to 4.4 and increased to between 4.66 and 4.88 by the end of storage. Tiwari et al. [35] also noted an increase in pH in orange juice after 30 days of storage, consistent with the observations of Alaka et al. [36]. Dhaka et al. [37] found that the addition of preservatives helped reduce the loss of juice acidity.
Fig. 3.
Change of pH of guava juice during storage.
Samples with the same superscripts don't differ at 5 % level of significance. S1 = Juice (without preservative) at RMT; S2 = Juice (without preservative) at RFT (4 °C); S3 = Juice contained KMS 150 ppm; S4 = Juice contained KMS 200 ppm; S5 = Juice contained KMS 250 ppm; S6 = Juice contained Sodium Benzoate 150 ppm; S7 = Juice contained Sodium Benzoate 200 ppm; S8 = Juice contained Sodium Benzoate 250 ppm.
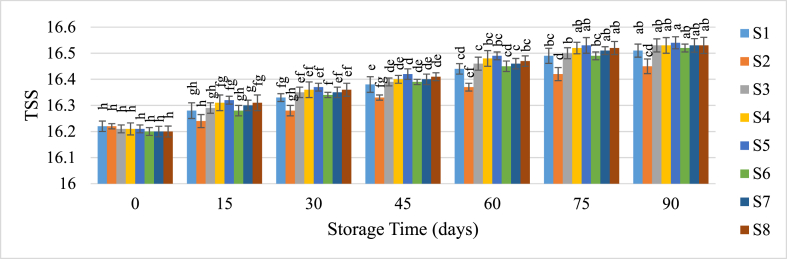
3.3.2. Total soluble solids (TSS)
The storage study revealed a gradual increase in Total Soluble Solids (TSS) of guava juice samples over time (Fig. 4). This increase is likely due to the ongoing hydrolysis of acids and polysaccharides into sugars. Iqbal et al. [34] observed a similar rise in TSS for blended Kinnow juice during storage. Cai et al. [38] also reported this phenomenon in apple juice. Lower temperatures mitigate the rate of hydrolysis, resulting in a slower increase in TSS. According to Le Chatelier's Principle, reduced temperatures slow the hydrolysis of polysaccharides and organic acids [39]. Additionally, Hameed et al. [40] noted the minimal increase in TSS when apple juice was stored under refrigeration. Initially, the TSS of all samples ranged from 16.2 to 16.22, but this increased to between 16.45 and 16.54 as storage progressed.
Fig. 4.
Change of TSS of guava juice during storage.
Samples with the same superscripts don't differ at 5 % level of significance. S1 = Juice (without preservative) at RMT; S2 = Juice (without preservative) at RFT (4 °C); S3 = Juice contained KMS 150 ppm; S4 = Juice contained KMS 200 ppm; S5 = Juice contained KMS 250 ppm; S6 = Juice contained Sodium Benzoate 150 ppm; S7 = Juice contained Sodium Benzoate 200 ppm; S8 = Juice contained Sodium Benzoate 250 ppm.
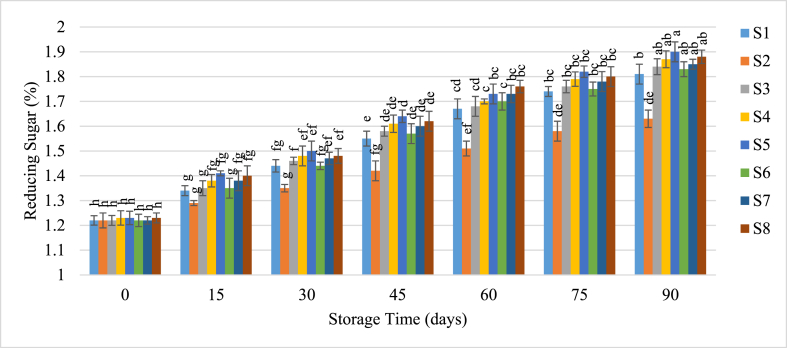
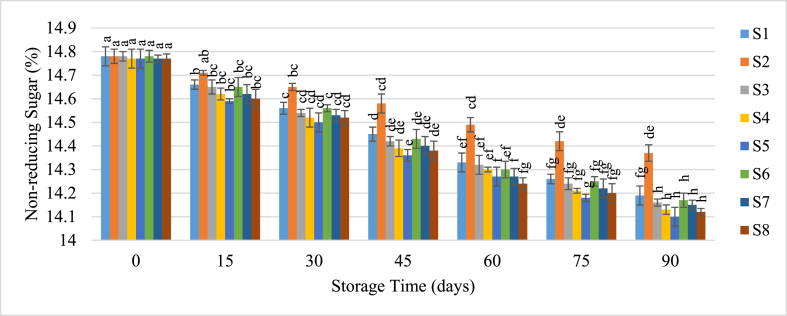
3.3.3. Reducing and non-reducing sugar
Reducing and non-reducing sugars in guava juice samples changed due to various interconversion processes during storage shown in Fig. 5, Fig. 6. Reducing sugars increased with extended storage duration, with the most significant rise occurring in juices with preservatives stored at room temperature. Conversely, refrigeration slowed the conversion process, resulting in a lower increase in reducing sugars. Initially, reducing sugars ranged from 1.22 % to 1.23 %, and by the end of 90 days, they ranged from 1.63 % to 1.90 %. According to Lee and Nagy [41], this increase is likely due to the acid hydrolysis of sucrose into glucose and fructose. Preserved juices with sulfur dioxide or potassium metabisulfite (KMS) typically show higher levels of reducing sugars, as polysaccharides and disaccharides are hydrolyzed into reducing sugars and sulfurous acid [32]. Amin et al. [42] observed a similar effect of chemical preservatives on mango pulp, and Shahnawaz et al. [43] noted increased total and reducing sugars in pear fruits treated with sodium benzoate.
Fig. 5.
Change of Reducing Sugar of guava juice during storage.
Samples with the same superscripts don't differ at 5 % level of significance.
S1 = Juice (without preservative) at RMT; S2 = Juice (without preservative) at RFT (4 °C); S3 = Juice contained KMS 150 ppm; S4 = Juice contained KMS 200 ppm; S5 = Juice contained KMS 250 ppm; S6 = Juice contained Sodium Benzoate 150 ppm; S7 = Juice contained Sodium Benzoate 200 ppm; S8 = Juice contained Sodium Benzoate 250 ppm.
Fig. 6.
Change of Non-reducing Sugar of guava juice during storage.
Samples with the same superscripts don't differ at 5 % level of significance. S1 = Juice (without preservative) at RMT; S2 = Juice (without preservative) at RFT (4 °C); S3 = Juice contained KMS 150 ppm; S4 = Juice contained KMS 200 ppm; S5 = Juice contained KMS 250 ppm; S6 = Juice contained Sodium Benzoate 150 ppm; S7 = Juice contained Sodium Benzoate 200 ppm; S8 = Juice contained Sodium Benzoate 250 ppm.
Iqbal et al. [34] found that higher storage temperatures accelerate the hydrolysis of acids and polysaccharides into simple sugars. Singh and Sharma [39] also reported a significant increase in reducing sugar in Kinnow mandarin juice stored at ambient temperature compared to lower temperatures.
In contrast, non-reducing sugars decreased as reducing sugars increased, likely due to their conversion. Non-reducing sugars initially ranged from 14.77 % to 14.78 % and fell to 14.1 %–14.37 % by the end of the storage period. The decline was slower under refrigeration, while preservatives enhanced sugar interconversion at room temperature. Kishore et al. [44] reported a similar reduction in non-reducing sugars during the storage of purple passion fruit.
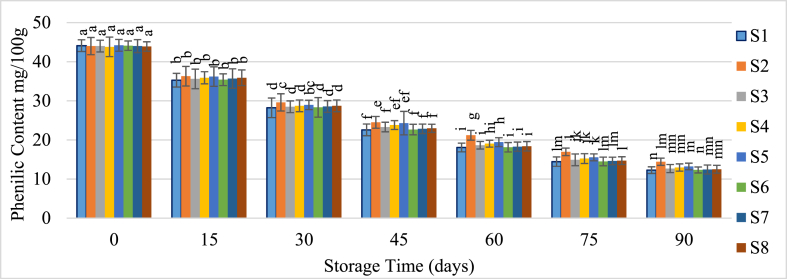
3.3.4. Bioactive compounds
The concentration of phenolic compounds in guava juice gradually diminishes over storage time (Fig. 7). Initially, the phenolic content was approximately 44 mg GAE/100g, but it fell to between 10 and 15 mg after 90 days of storage. The most significant reduction in phenolic concentration was observed in samples stored at ambient temperature. Conversely, samples kept under refrigeration experienced less phenolic degradation. The addition of preservatives had a minimal effect on preserving phenolic content, although juice samples treated with potassium metabisulfite (KMS) and sodium benzoate exhibited a slight reduction in phenolic breakdown. Singh and Pal [45] reported a decrease in phenolic content from 224.26 mg/100g to 190.56 mg/100g in guava juice. Similarly, Freda et al. [46] observed a reduction in phenolic compounds from 128.33 mg to 94.98 mg in hot-canned guava juice stored at ambient temperature for 50–250 days.
Fig. 7.
Change of phenolic content of guava juice during storage.
Samples with the same superscripts don't differ at 5 % level of significance. S1 = Juice (without preservative) at RMT; S2 = Juice (without preservative) at RFT (4 °C); S3 = Juice contained KMS 150 ppm; S4 = Juice contained KMS 200 ppm; S5 = Juice contained KMS 250 ppm; S6 = Juice contained Sodium Benzoate 150 ppm; S7 = Juice contained Sodium Benzoate 200 ppm; S8 = Juice contained Sodium Benzoate 250 ppm.
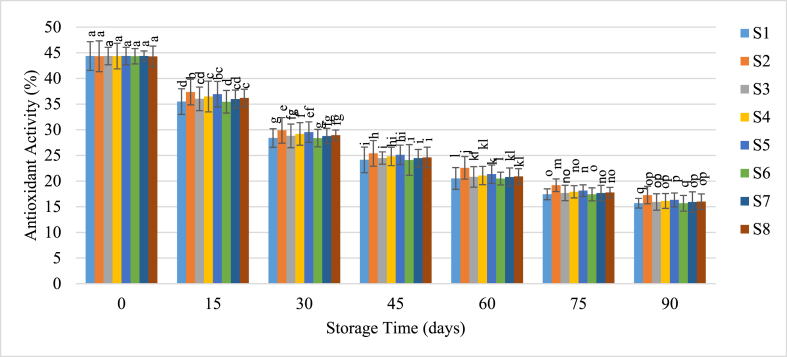
Antioxidant capacity in guava juice also declined progressively with extended storage (Fig. 8). Initially at around 44 %, this capacity fell to between 15 % and 17 % by the end of the storage period. There was no significant correlation between antioxidant capacity and either refrigeration temperature or the presence of preservatives. This finding is consistent with the research by Aishah et al. [47], who noted that antioxidant activity in guava juice decreased with lower storage temperatures. Pasupuleti and Kulkarni [48] also predicted a faster loss of antioxidant activity at higher storage temperatures.
Fig. 8.
Change of antioxidant activity of guava juice during storage.
Samples with the same superscripts don't differ at 5 % level of significance. S1 = Juice (without preservative) at RMT; S2 = Juice (without preservative) at RFT (4 °C); S3 = Juice contained KMS 150 ppm; S4 = Juice contained KMS 200 ppm; S5 = Juice contained KMS 250 ppm; S6 = Juice contained Sodium Benzoate 150 ppm; S7 = Juice contained Sodium Benzoate 200 ppm; S8 = Juice contained Sodium Benzoate 250 ppm.
3.3.5. Color
Significant color changes were observed in guava juice samples as storage time progressed (Table 4). Juices stored at room temperature darkened more noticeably over time compared to that kept under refrigeration, which retained their color better. Additionally, juices containing sodium benzoate and potassium metabisulfite (KMS) were more effective at preventing color degradation.
Table 4.
Color degradation (ΔE) of guava juice.
| Sample |
Storage (days) |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | 75 | 90 | |
| S1 | 0j | 4.63 ± 0.08g | 5.79 ± 0.12de | 8.21 ± 0.03de | 9.82 ± 0.14cd | 12.33 ± 0.04b | 14.45 ± 0.18a |
| S2 | 0j | 2.61 ± 0.07hi | 3.76 ± 0.10gh | 4.57 ± 0.04gh | 5.19 ± 0.09fg | 6.11 ± 0.05ef | 7.06 ± 0.12ef |
| S3 | 0j | 3.11 ± 0.33hi | 5.04 ± 0.07fg | 6.07 ± 0.12f | 7.25 ± 0.02de | 8.87 ± 0.15d | 10.13 ± 0.04cd |
| S4 | 0j | 2.06 ± 0.26i | 3.10 ± 0.10hi | 3.79 ± 0.08gh | 5.41 ± 0.18fg | 6.42 ± 0.06ef | 7.15 ± 0.17ef |
| S5 | 0j | 1.02 ± 0.10i | 1.33 ± 0.13i | 1.88 ± 0.05i | 2.33 ± 0.18hi | 3.28 ± 0.12hi | 3.61 ± 0.25gh |
| S6 | 0j | 3.63 ± 0.18gh | 4.81 ± 0.09fg | 5.72 ± 0.10fg | 7.51 ± 0.04e | 9.19 ± 0.02cd | 10.20 ± 0.12c |
| S7 | 0j | 2.18 ± 0.15hi | 3.34 ± 0.05h | 3.92 ± 0.16gh | 5.16 ± 0.09fg | 6.20 ± 0.13fg | 7.08 ± 0.18ef |
| S8 | 0j | 1.34 ± 0.10i | 1.52 ± 0.02i | 1.94 ± 0.12i | 2.47 ± 0.06hi | 3.15 ± 0.19hi | 3.51 ± 0.22gh |
Samples with the same superscripts don't differ at 5 % level of significance. S1 = Juice (without preservative) at RMT; S2 = Juice (without preservative) at RFT (4 °C); S3 = Juice contained KMS 150 ppm; S4 = Juice contained KMS 200 ppm; S5 = Juice contained KMS 250 ppm; S6 = Juice contained Sodium Benzoate 150 ppm; S7 = Juice contained Sodium Benzoate 200 ppm; S8 = Juice contained Sodium Benzoate 250 ppm.
Color changes in fruit juice during storage are primarily due to the Maillard reaction between amino acids and reducing sugars, which produces hydroxy-methyl-furfural (HMF) and results in sugar caramelization [49]. The formation of HMF is accelerated by both storage time and temperature [50]. Saini et al. [51] found that KMS application significantly reduced browning in mango pulp. Similarly, Falade et al. [33] reported a linear increase in non-enzymatic browning in sweetened Julie and Ogbomoso mango juices over 12 weeks of storage.
3.3.6. Degradation of ascorbic acid
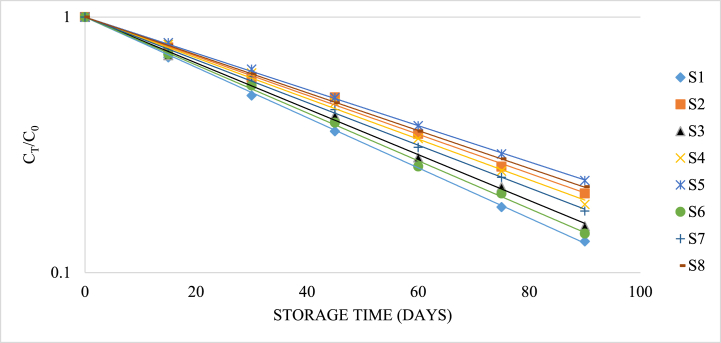
The initial ascorbic acid content among different samples was approximately 93.72 mg/100 g which significantly decreased throughout the storage period. Comparatively more retention of ascorbic acid at refrigeration temperature was observed. However, results showed that using preservatives is relatively effective in increasing the stability of vitamin C during the storage period. Vitamin C concentration ratio (Ct/Co) versus storage time (days) was plotted on a semi-log coordinate (Figure-9) and the following regression equations were developed:
Fig. 9.
Degradation of ascorbic acid of guava juice during storage.
Samples with the same superscripts don't differ at 5 % level of significance. S1 = Juice (without preservative) at RMT; S2 = Juice (without preservative) at RFT (4 °C); S3 = Juice contained KMS 150 ppm; S4 = Juice contained KMS 200 ppm; S5 = Juice contained KMS 250 ppm; S6 = Juice contained Sodium Benzoate 150 ppm; S7 = Juice contained Sodium Benzoate 200 ppm; S8 = Juice contained Sodium Benzoate 250 ppm.
From the figure-9 and Table 5, it may be concluded that the ratio of vitamin C concentration (Ct/Co) decreases exponentially with storage time. It is also observed that the reaction rate constant (k) is found to be the highest (0.023 day−1) in the case of sample kept at room temperature, while the lowest (0.016 day−1) in the case of sample preserved with 250 ppm of KMS. KMS was found comparatively more effective than sodium benzoate in the retention of ascorbic acid in guava juice.
Table 5.
Regression equations for ascorbic acid degradation of guava juice.
| Sample | Regression Equation | R2 | Activation Energy(kj/mol) |
|---|---|---|---|
| S1 | Ct/C0 = e−0.023t | 0.996 | 5163.62 |
| S2 | Ct/C0 = e−0.018t | 0.997 | |
| S3 | Ct/C0 = e−0.021t | 0.992 | |
| S4 | Ct/C0 = e−0.019t | 0.995 | |
| S5 | Ct/C0 = e−0.016t | 0.993 | |
| S6 | Ct/C0 = e−0.022t | 0.998 | |
| S7 | Ct/C0 = e−0.019t | 0.997 | |
| S8 | Ct/C0 = e−0.017t | 0.995 |
S1 = Juice (without preservative) at RMT; S2 = Juice (without preservative) at RFT (4 °C); S3 = Juice contained KMS 150 ppm; S4 = Juice contained KMS 200 ppm; S5 = Juice contained KMS 250 ppm; S6 = Juice contained Sodium Benzoate 150 ppm; S7 = Juice contained Sodium Benzoate 200 ppm; S8 = Juice contained Sodium Benzoate 250 ppm.
In liquid systems with high water activity and oxygen presence, ascorbic acid primarily degrades through oxidation, converting to dehydroascorbic acid, which further breaks down into 2,3-diketogulonic acid [52,53]. This degradation leads to the immediate loss of vitamin efficacy upon the hydrolysis of dehydroascorbic acid. Increased water activity or moisture content accelerates ascorbic acid degradation [54]. Additionally, ascorbic acid can degrade via an anaerobic pathway, where the keto forms of L-ascorbic acid hydrolyze to produce 2,3-diketogulonic acid. This pathway involves complex reactions that ultimately generate reductones, furfural, and furan carboxylic acid as end products. Key factors affecting Vitamin C stability include pH, light exposure, temperature, and the presence of trace metal ions, oxygen, and degradative enzymes [55].
Carvalho et al. [56] observed a reduction in vitamin C concentration in cashew apple juice combined with coconut water during storage. Kabasakalis et al. [57] reported a 29–41 % loss of ascorbic acid in fruit juices stored at room temperature for 4 months. Burdulu et al. [58] noted a 27.3%–45.3 % decrease in ascorbic acid in orange juice stored at 28 °C for two months. Majumdar et al. [59] found a 74 % loss of ascorbic acid after 6 months of storing a blended juice of cucumber, litchi, and lemon. Preservatives can enhance ascorbic acid retention during fruit juice storage. Mathooko and Kiniiya [60] demonstrated that sodium metabisulfite significantly stabilized ascorbic acid, with its concentration directly affecting ascorbic acid retention.
3.3.7. Microbial analysis
Table 6 presents the total viable bacterial counts in various samples. After 90 days of storage, the sample kept at room temperature showed the highest bacterial count, reaching 53.67 × 109. In contrast, samples treated with preservatives exhibited lower levels of contamination. Those stored under refrigeration demonstrated the most favorable microbial safety results. According to FDA guidelines [61], the maximum allowable total microbial load in ready-to-serve (RTS) juice is between 5 × 104 and 105 CFU/g. Based on these guidelines, the room-temperature sample is considered safe for up to 40 days, whereas refrigerated samples remain safe for over 90 days. Additionally, KMS-treated samples were deemed safe for up to 65, 70, and 85 days at concentrations of 150 ppm, 200 ppm, and 250 ppm, respectively. Samples with sodium benzoate were considered safe for 60, 65, and 70 days at the same concentrations.
Table 6.
Total microbial count of guava juice during storage.
| Sample |
Storage (Days) |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | 75 | 90 | |
| S1 | 4.33 ± 0.47 | 90.33 ± 9.98 | (24.33 ± 7.04) × 10 | (47.33 ± 7.41) × 104 | (76.33 ± 7.36) × 107 | (30.33 ± 4.99) × 109 | (53.67 ± 7.76) × 109 |
| S2 | 4.67 ± 1.70 | 7.00 ± 1.63 | 14.33 ± 3.40 | 15.33 ± 5.31 | 20.67 ± 4.50 | 34.00 ± 6.98 | 51.33 ± 6.94 |
| S3 | 6.33 ± 1.25 | 26.67 ± 7.59 | (13.33 ± 5.79) × 10 | (57.00 ± 7.35) × 10 | (23.00 ± 4.90) × 103 | (12.33 ± 2.87) × 105 | (22.00 ± 4.55) × 106 |
| S4 | 4.33 ± 1.25 | 16.67 ± 3.30 | 53.67 ± 12.23 | (21.67 ± 4.92) × 10 | (97.33 ± 8.22) × 102 | (17.33 ± 4.92) × 104 | (12.33 ± 3.30) × 105 |
| S5 | 2.67 ± 1.25 | 8.33 ± 2.87 | 15.33 ± 4.92 | 36.00 ± 6.16 | (92.00 ± 6.16) × 10 | (18.33 ± 5.31) × 103 | (20.67 ± 3.68) × 104 |
| S6 | 3.67 ± 1.25 | 60.67 ± 9.81 | (22.67 ± 6.55) × 10 | (16.33 ± 6.55) × 102 | (58.00 ± 7.79) × 102 | (32.00 ± 7.79) × 105 | (11.67 ± 3.40) × 107 |
| S7 | 4.00 ± 0.82 | 28.00 ± 6.16 | 71.33 ± 7.59 | (35.67 ± 6.94) × 10 | (17.33 ± 3.68) × 102 | (47.00 ± 6.53) × 104 | (79.00 ± 4.08) × 105 |
| S8 | 3.67 ± 1.70 | 11.67 ± 3.40 | 33.67 ± 7.04 | (21.33 ± 7.41) × 10 | (65.00 ± 9.09) × 102 | (76.33 ± 5.31) × 103 | (78.33 ± 6.13) × 104 |
S1 = Juice (without preservative) at RMT; S2 = Juice (without preservative) at RFT (4 °C); S3 = Juice contained KMS 150 ppm; S4 = Juice contained KMS 200 ppm; S5 = Juice contained KMS 250 ppm; S6 = Juice contained Sodium Benzoate 150 ppm; S7 = Juice contained Sodium Benzoate 200 ppm; S8 = Juice contained Sodium Benzoate 250 ppm.
Evelyn and Chairul [62] found that sodium benzoate is effective in inactivating spores, thereby enhancing food safety and extending the shelf life of guava juice. Chauhan et al. [63] observed that sugarcane juice stored at ambient temperature had a higher microbial count compared to juice stored under refrigeration. Similarly, Ogiehor et al. [64] noted reduced microbial growth in KMS-treated samples, and Sama et al. [65] reported lower microbial loads in sodium benzoate-treated cassava fufu.
3.4. Correlation analysis
Table 7 presents the correlation coefficients among various physicochemical properties of guava juice samples. The analysis showed that pH strongly correlated with Total Soluble Solids (TSS), Reducing Sugars (RS), and ΔE (color difference), indicating that as pH changed, there were significant associations with variations in TSS, RS, and color intensity. However, pH had weak correlations with Non-Reducing Sugars (NRS), Antioxidant Activity (AA), and Vitamin C (Vit-C), suggesting that pH had minimal influence on these properties.
Table 7.
Relationship among different parameters of guava juice treated with various concentrations of preservatives during storage.
| pH | TA | TSS | RS | NRS | TPC | AA | Vit-C | ΔE | CFU | |
|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | |||||||||
| TA | −0.405 | 1 | ||||||||
| TSS | 0.960 | −0.454 | 1 | |||||||
| RS | 0.956 | −0.464 | 0.994 | 1 | ||||||
| NRS | −0.956 | 0.466 | 0.994 | −1.000 | 1 | |||||
| TPC | −0.969 | 0.435 | −0.971 | −0.964 | 0.963 | 1 | ||||
| AA | −0.964 | 0.423 | −0.965 | −0.955 | 0.955 | 0.998 | 1 | |||
| Vit-C | −0.972 | 0.415 | −0.949 | −0.939 | 0.939 | 0.994 | 0.994 | 1 | ||
| ΔE | 0.835 | −0.269 | 0.677 | 0.664 | −0.664 | −0.760 | −0.757 | −0.811 | 1 | |
| CFU | 0.339 | −0.078 | 0.202 | 0.204 | −0.205 | −0.221 | −0.210 | −0.236 | 0.520 | 1 |
Here, TSS = Total Soluble Solids, TA = Titratable Acidity, RS = Reducing Sugar. NRS = Non-Reducing Sugar, TPC = Total Phenolic Content, AA = Antioxidant Activity, CFU = Colony Forming Unit, ΔE = Color difference with Respect to L* a* b*.
Titratable Acidity (TA) exhibited strong positive correlations with NRS, Total Phenolic Content (TPC), AA, and Vit-C, indicating that higher acidity was associated with increased levels of these components. On the other hand, TA showed weak correlations with TSS and RS, suggesting that changes in acidity were not strongly linked to soluble solids and reducing sugars.
TSS showed strong positive correlations with RS, NRS, and ΔE, meaning that increases in TSS were associated with higher levels of RS, NRS, and color difference. TSS had weak correlations with TPC, AA, and Vit-C, implying that TSS did not significantly affect phenolic content, antioxidant activity, and vitamin C levels.
RS was strongly correlated with ΔE, suggesting a close relationship between reducing sugars and color intensity. However, RS had weak correlations with NRS, TPC, AA, and Vit-C. NRS showed strong correlations with TPC, AA, and Vit-C but weak correlations with ΔE. TPC had strong positive correlations with AA and Vit-C, reflecting that higher phenolic content was associated with greater antioxidant activity and vitamin C content. TPC showed weak correlations with ΔE, indicating minimal impact on color changes. AA and Vit-C were strongly correlated with each other but had weak correlations with ΔE, reflecting limited impact on color difference.
4. Conclusion
Enzymatic hydrolysis of guava juice proved effective with up to 0.2 % pectinase concentration and incubation periods of 2–3 h. Beyond these conditions, either extended incubation or higher enzyme concentrations led to a decline in juice quality. During the storage study, a gradual decrease in acidity and an increase in pH were observed over time. Additionally, total soluble solids (TSS), as well as reducing and non-reducing sugars, rose with prolonged storage. Conversely, phenolic content, ascorbic acid levels, and DPPH scavenging activity diminished over time. Significant color changes were also noted among the different juice samples. Furthermore, the total microbial count increased in all samples as storage time lengthened. Notably, juices stored under refrigeration exhibited significantly smaller changes in pH, acidity, TSS, sugar content, and bioactive compounds compared to those kept at room temperature, whether or not preservatives were used.
Ethics declarations
Review and/or approval by an ethics committee was not needed for this study because we don't work with humans or animals.
Data availability statement
The data supporting the findings of this study are available upon request from the corresponding author. The authors are committed to ensuring the transparency and reproducibility of the study's results and will promptly respond to any reasonable requests for data sharing.
CRediT authorship contribution statement
Imroze Zahan: Writing – original draft, Investigation, Formal analysis, Data curation. Md Momin Khan: Writing – review & editing, Visualization, Software, Methodology, Investigation, Formal analysis, Data curation. Md Suman Rana: Writing – review & editing, Visualization, Software, Formal analysis. Md Sahabuddin: Writing – review & editing, Software. Md Rezwan Rasik: Writing – review & editing. M. Burhan Uddin: Visualization, Validation, Supervision, Project administration, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors wish to acknowledge the teachers, students and staff of the Department of Food Technology and Rural Industries, Bangladesh Agricultural University, Mymensimgh-2200.
References
- 1.Kadam D.M., Kaushik P., Kumar R. Evaluation of guava products quality. Int. J. Food Sci. Nutr. Eng. 2012;2(1):7–11. doi: 10.5923/j.food.20120201.02. [DOI] [Google Scholar]
- 2.Rawan S., Bibi F., Khan N., Khattak A.M., Shah Z., Iqbal A., A M., Haq S. Ul, Kamal A., Shah F.A., Naeem A., Ali W. Postharvest life of guava (PsidiumguajavaL.) varieties as affected by storage intervals at room temperature. Pakistan J. Agric. Res. 2017;30(2):155–161. doi: 10.17582/journal.pjar/2017/30.2.155.161. [DOI] [Google Scholar]
- 3.Bensid A., El Abed N., Houicher A., Regenstein J.M., Özogul F. Antioxidant and antimicrobial preservatives: properties, mechanism of action and applications in food – a review. Cri. Revi. Food Sci. Nutr. 2017;62(11):2985–3001. doi: 10.1080/10408398.2020.1862046. [DOI] [PubMed] [Google Scholar]
- 4.Peng Z. Progress in the application of food preservatives in food. H. Sci. Eng. and Tech. 2023;74:1570–1575. doi: 10.54097/05gj7k40. [DOI] [Google Scholar]
- 5.Seetaramaiah K., Smith A.A., Murali R., Manavalan R. Preservatives in food products – review. Int. J. of Phar. & Bio. Arch. 2011;2(2):583–599. [Google Scholar]
- 6.Panigrahi C., Shaikh A.E.Y., Bag B.B., Mishra H.N., De S. A technological review on processing of sugarcane juice: spoilage, preservation, storage, and packaging aspects. J. Food Process. Eng. 2021;44(6) doi: 10.1111/jfpe.13706. [DOI] [Google Scholar]
- 7.Zhong Q., Xia W.S., Jiang Y. Effects of 1-methylcyclopropene treatments on ripening and Quality of harvested sapodilla fruit. Food Tech. biotech. 2006;44(4):535–539. [Google Scholar]
- 8.Chien P.J., Sheu F., Yang F.H. Effects of edible Chitosan coating on quality and shelf life of sliced mango fruit. J. Food Eng. 2007;78(1):225–229. doi: 10.1016/j.jfoodeng.2005.09.022. [DOI] [Google Scholar]
- 9.Natalia K.V. da S., Luiz B. de S.S., Luciana S. de O., Lucicléia B. de V.T., de Paulo M. Effect of food additives on the antioxidant properties and microbiological quality of red guava juice. Rev. Cienc. Agron. 2016;47(1):77–85. doi: 10.5935/1806-6690.20160009. [DOI] [Google Scholar]
- 10.Chen H., Brashears M.M., Zhong Q. Sodium benzoate and sodium bisulfate as preservatives in apple juice and alternative sanitizers for washing cherry tomatoes. Int. J. Food Microbiol. 2022;372 doi: 10.1016/j.ijfoodmicro.2022.109697. [DOI] [PubMed] [Google Scholar]
- 11.Lennerz B.S., Vafai S.B., Delaney N.F., Clish C.B., Deik A.A., Pierce K.A., Ludwig D.S., Mootha V.K. Effects of sodium benzoate, a widely used food preservative, on glucose homeostasis and metabolic profiles in humans. Mol. Genet. Metabol. 2015;114(1):73–79. doi: 10.1016/j.ymgme.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nettis E., Colanardi M.C., Ferrannini A., Tursi A. Sodium benzoate-induced repeated episodes of acute urticaria/angio-oedema: randomized controlled trial. Br. J. Dermatol. 2004;151(4):898–902. doi: 10.1111/j.1365-2133.2004.06095.x. [DOI] [PubMed] [Google Scholar]
- 13.Olmo A.D., Calzada J., Nuñez M. Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: uses, exposure, and controversy. Crit. Rev. Food Sci. Nutr. 2017;57(14):3084–3103. doi: 10.1080/10408398.2015.1087964. [DOI] [PubMed] [Google Scholar]
- 14.Skypala I.J., Williams M., Reeves L., Meyer R., Venter C. Sensitivity to food additives, vaso-active amines and salicylates: a review of the evidence. Clin. Transl. Allergy. 2015;5(34) doi: 10.1186/s13601-015-0078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos V.P. S.d., Salgado A.M., Torres A.G., Pereira K.S. Benzene as a chemical hazard in processed foods. Int. J. Food Sci. 2015;2015 doi: 10.1155/2015/545640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santos M.C., Nunes C., Saraiva J.A., Coimbra M.A. Chemical and physical methodologies for the replacement/reduction of sulfur dioxide use during winemaking: review of their potentialities and limitations. Eur. Food Res. Technol. 2012;234:1–12. doi: 10.1007/s00217-011-1614-6. [DOI] [Google Scholar]
- 17.EFSA Panel on Food additives and Nutrient Sources added to Food (ANS), Scientific Opinion on the re-evaluation of sulfur dioxide (E 220), sodium sulfite (E 221), sodium bisulfite (E 222), sodium metabisulfite (E 223), potassium metabisulfite (E 224), calcium sulfite (E 226), calcium bisulfite (E 227) and potassium bisulfite (E 228) as food additives. Eur. Food Saf. J. 14(4) 92016) 4438, 10.2903/j.efsa.2016.4438. . [DOI] [PMC free article] [PubMed]
- 18.European Commission. (n.d.). Food Additives Database: Potassium metabisulfite (E224). Retrieved July 13, 2024, from https://ec.europa.eu/food/food-feed-portal/screen/food-additives/search/details/POL-FAD-IMPORT-3044. .
- 19.Varo M., Martin-Gomez J., Serratosa M.P., Merida J. Effect of potassium metabisulphite and potassium bicarbonate on color, phenolic compounds, vitamin C and antioxidant activity of blueberry wine. LWT--Food Sci. Technol. 2022;163 doi: 10.1016/j.lwt.2022.113585. [DOI] [Google Scholar]
- 20.Taylor S.L., Bush R.K., Nordlee J.A., Metcalfe Sulfites D.D., Sampson H.A., Simon R.A. Blackwell Publishing; UK: 2008. Food Allergy: Adverse Reaction to Foods and Food Additives, Fourth Edi; pp. 353–368.https://www.wiley.com/enus/Food+Allergy%3A+Adverse+Reaction+to+Foods+and+Food+Additives%2C+5th+Edition-p-9781118744147 [Google Scholar]
- 21.Thongsomba W., Sirichote A., Chanthachum S. The production of guava juice fortified with dietary fiber. Songklanakarin. J. Sci. Technol. 2007;29(1):187–196. [Google Scholar]
- 22.AOAC . nineteen ed. Association of Official Analytical Chemist; Washington DC: 2012. Official Methods of Analysis.https://www.scirp.org/reference/ReferencesPapers?ReferenceID=1819676 [Google Scholar]
- 23.Ranganna S. second ed. Tata McGraw Hill Publications Company Limited; New Delhi: 1997. Handbook of Analysis of Quality Control for Fruit and Vegetable Products; pp. 11–12.https://www.scirp.org/reference/referencespapers?referenceid=3248147 [Google Scholar]
- 24.Hasan M.M., Khan M.M., Jony M.E., Akter F., Alim M.A. Studies on the preparation and shelf life of aloe vera juice. 2023;22(9) doi: 10.9734/AFSJ/2023/v22i9658. 64-73. [DOI] [Google Scholar]
- 25.Majumder S., Das P.C., Sami R., Ismail K., Almushhin A., Iqbal A., Ranganathan T.V., Mazumder M.A.R. Effect of spent green tea leaf extracts, butylated hydroxytoluene and repeated freezing-thawing on physicochemical and oxidative properties of Chevon. J. Biobased Mater. Bioenergy. 2022;22:56–67. doi: 10.1166/jbmb.2022.2113. [DOI] [Google Scholar]
- 26.Yi Z., Yu Y., Liang Y., Zeng B. In vitro antioxidant and antimicrobial activities of the extract of pericarpium citri reticulatae of a new citrus cultivar and its main flavonoids. LWT-Food Sci. Technol. 2008;41(4):597–603. doi: 10.1016/j.lwt.2007.04.008. [DOI] [Google Scholar]
- 27.Heldman D.R., Singh R.P. The AVI Pub. second ed. Co. reprint edition. West port; USA: 1981. Food process engineering; pp. 1–24.https://link.springer.com/book/10.1007/978-94-010-9337-8 [Google Scholar]
- 28.Berk Z. third ed. Academic press, Elsevier; Amsterdam: 2018. Food Process Engineering and Technology; pp. 115–124.https://shop.elsevier.com/books/food-process-engineering-and-technology/berk/978-0-12-812018-7 [Google Scholar]
- 29.Morton D. In: Compendium of Methods for the Microbiological Examination of Foods. fourth ed. Downes F.P., Ito K., editors. American Public Health Association; Washington, D.C.: 2001. Aerobic plate count; pp. 63–67.https://lib.ugent.be/en/catalog/rug01:000842770 [Google Scholar]
- 30.Chopda C.A., Barrett D.M. Optimization of guava juice and powder production. J. Food Process. 2001;25:411–417. doi: 10.1111/j.1745-4549.2001.tb00470.x. [DOI] [Google Scholar]
- 31.Yousaf A.A., Abbasi K.S., Ahmad A., Hasan I., Shohail A., Qayyum A., Akram M.A. Physico-chemical and nutraceutical characterization of selected indigenous guava (psidium guajava L.) cultivars. J. Food Sci. Technol. 2021;41(1):47–58. doi: 10.1590/fst.35319. [DOI] [Google Scholar]
- 32.Pareek S., Paliwal R., Mukherjee S. Effect of juice extraction methods, potassium meta-bi-sulphite concentration and storage temperature on the extent of degradation and reactivity of chemical constituents in Mandarin (Citrus reticulata Blanco) juice. J. Food Agric. Environ. 2015;13:39–44. doi: 10.1234/4.2015.3924. [DOI] [Google Scholar]
- 33.Falade K.O., Babalola S.O., Akinyemi S.O.S., Ogunlade A.A. Degradation of quality attributes of sweetened Julie and ogbomoso mango juices during storage. Eur. Food Res. Technol. 2004;218:456–459. doi: 10.1007/s00217-004-0878-5. [DOI] [Google Scholar]
- 34.Iqbal A., Nadeem M., Aineea A., Qureshib T.M., Khalidc W., Malika F., Ur Rehmand S., Rehmane A., Khalidf M.Z., Ahmadg N., Nawazh A., Ahmedi I.A.M. Quality evaluation of ozone-processed Kinnow (Citrus reticulata Blanco) juice at ambient temperature. Int. J. Food Prop. 2023;26:2420–2432. doi: 10.1080/10942912.2023.2249266. [DOI] [Google Scholar]
- 35.Tiwari B.K., O’ Donnell C.P., Muthukumarappan K., Cullen P.J. Effect of sonication on orange juice quality parameters during storage. Int. J. Food Sci. Technol. 2009;44(3):586–595. doi: 10.1111/j.1365-2621.2008.01858.x. [DOI] [Google Scholar]
- 36.Alaka O.O., Aina J.O., Falade K.O. Effect of storage conditions on the chemical attributes of ogbomoso mango juice. J. European Food Res. Technol. 2003;37:213–217. doi: 10.1007/s00217-003-0800-6. [DOI] [Google Scholar]
- 37.Dhaka A., Sharma M., Singh S.K. Use of additives to reduce browning, MicrobialLoad and quality loss of Kinnow juice under ambient storage. Indian J. Sci. Technol. 2016;9(14) doi: 10.17485/ijst/2016/v9i14/80907. [DOI] [Google Scholar]
- 38.Cai M., Xie C., Lv Y., Yang K., Sun P. Changes in physicochemical profiles and quality of apple juice treated by ultrafiltration and during its storage. J. Food Sci. Nutri. 2020;8(6):2913–2919. doi: 10.1002/fsn3.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh S.K., Sharma M. Review on biochemical changes associated with storage of fruit juice. Int. J. Curr. Microbiol. App. Sci. 2017;6(8):236–245. doi: 10.20546/ijcmas.2017.608.032. [DOI] [Google Scholar]
- 40.Hameed F., Kumar A., Hamid N. Effect of thermal treatment and storage on the quality of apple juice. J. Pharmacogn. Phytochem. 2019;8(1):1976–1979. https://www.phytojournal.com/archives/2019.v8.i1.7058/effect-of-thermal-treatment-and-storage-on-the-quality-of-apple-juice [Google Scholar]
- 41.Lee H.S., Nagy S. Quality changes and nonenzymic browning intermediates in grapefruit Juice during storage. J. Food Sci. 1988;53(1):168–172. doi: 10.1111/j.1365-2621.1988.tb10201.x. [DOI] [Google Scholar]
- 42.Amin M., Aman U.M., Mazhar M.S., Islam U.D., Khalid M.S., Saeed A. Mango fruit desapping in relation to time of harvesting. Pakistan J. Bot. 2008;40(4):1587–1593. https://www.yumpu.com/en/document/view/11498222/mango-fruit-desapping-in-relation-to-time-of-harvesting [Google Scholar]
- 43.Kaur A., Gill P.P.S., Jawandha S.K. Effect of sodium benzoate application on quality and enzymatic changes of pear fruits during low temperature. J. Food Sci. Technol. 2019;56(7):3391–3398. doi: 10.1007/s13197-019-03823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kishore K., Pathak K.A., Shukla R., Bharali R. Effect of storage temperature on physico-chemical and sensory attributes of purple passion fruit (Passiflora edulis Sims) J. Food Sci. Technol. 2011;48(4):484–488. doi: 10.1007/s13197-010-0189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh S.P., Pal R.K. Controlled atmosphere storage of guava (Psidiumguajava L.) fruit. Postharvest Biol. Technol. 2008;47:296–306. doi: 10.1016/j.postharvbio.2007.08.009. [DOI] [Google Scholar]
- 46.Freda S.A., Krumreich F.D., Rutz J.k., Hartwig N., Zambiazi R.C. Bioactive compounds during processing and storage of sweet guava (conventional and light) Int. Food Res. J. 2018;25(3):1181–1188. 10.10.26656/fr.2017.5(3).554. [Google Scholar]
- 47.Aishah B., Hannah K., Zati O.A. Stability of selected quality attributes of Guava juice during storage at elevated temperatures. Int. Food Res. J. 2016;23(5):1918–1925. https://www.proquest.com/scholarly-journals/stability-selected-quality-attributes-pink-guava/docview/1833108259/se-2 [Google Scholar]
- 48.Pasupuleti V., Kulkarni S.G. Lycopene fortification on the quality characteristics of beverage formulations developed from flesh Guava. J. Food Sci. Technol. 2013;51(12):4126–4131. doi: 10.1007/s13197-013-0932-z. 10.1007%2Fs13197-013-0932-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arribas-Lorenzo G., Morales F.J. Estimation of dietary intake of 5-hydroxymethylfurfural and related substances from coffee to Spanish population. Food Chem. Toxicol. 2010;48(2):644–649. doi: 10.1016/j.fct.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 50.Serra-Cayuela A., Jourdes M., Riu Aumatell M., Buxaderas S., Teissedre P.L., López-Tamames E. Kinetics of browning, phenolics and 5- hydroxymethylfurfural in commercial sparkling wines. J. Agric. Food Chem. 2014;62(5):1159–1166. doi: 10.1021/jf403281y. [DOI] [PubMed] [Google Scholar]
- 51.Saini D. S. Sogi, Bawa A.S. Shelf-life studies on chemically preserved sand pear (Pyrus pyrifolia cv. patharnakh) pulp. J. Food Sci. Technol. 2000;40(2):230–232. [Google Scholar]
- 52.Al Fata N., Georgé S., Dlalah N., Renard b C.M.G.C. Influence of partial pressure of oxygen on ascorbic acid degradation at canning temperature. Innov. Food Sci. Emerg. 2018;49:215–221. doi: 10.1016/j.ifset.2017.11.007. [DOI] [Google Scholar]
- 53.Klu M.W., Addy B.S., Oppong E.E., Sakyi E.S. Effect of storage conditions on the stability of ascorbic acid in some formulations. Int. J. Appl. Pharm. 2016;8(4):26–31. doi: 10.22159/ijap.2016v8i4.14131. [DOI] [Google Scholar]
- 54.Lee S.H., Labuza T.P. Destruction of ascorbic acid as a function of water activity. J. Food Sci. 1975;40:370–373. doi: 10.1111/j.1365-2621.1975.tb02204.x. [DOI] [Google Scholar]
- 55.Gregory J.F. In: Food Chemistry. third ed. Fennema O.R., editor. Marcel Dekker; New York, USA: 1996. Vitamins; pp. 531–616. [Google Scholar]
- 56.Carvalho J.M.D., Maia G.A., Figueiredo R.W., Brito E.S.D., Rodrigues S. Storage stability of a stimulant coconut water-cashew apple juice beverage. J. Food Process. Preserv. 2007;31(2):178–189. doi: 10.1111/j.1745-4549.2007.00121.x. [DOI] [Google Scholar]
- 57.Kabasakalis V., Siopidou D., Moshatou E. Ascorbic acid content of commercial fruit juices and its rate of loss upon storage. J. Food Chem. 2000;70:325–328. doi: 10.1016/S0308-8146(00)00093-5. [DOI] [Google Scholar]
- 58.Burdulu H.S., Koca N., Karadeniz F. Degradation of vitamin C in citrus juices concentrates during storage. J. Food Eng. 2006;74(2):211–216. doi: 10.12691/jfnr-3-8-10. [DOI] [Google Scholar]
- 59.Majumdar T.K., Vasudish C.R., Premavalli K.S., Bawa A.S. Development and storage stability of cucumber-litchi-lemon Juice. J. Food Sci. Technol. 2009;46:269–270. [Google Scholar]
- 60.Mathooko F.M., Kiniiya E.N. Ascorbic acid retention in canned lime juice preserved with sulfur acid dioxide and benzoic. Afr. J. Food Nutr. Sci. 2002;2(1):33–37. doi: 10.4314/ajfand.v2i1.19120. [DOI] [Google Scholar]
- 61.Food and Drug Administration Revised guidelines for the assessment of microbiological quality of processed foods. 2013. https://WW2.FDA.GOV.PH/attachments/article/17218/FC2013-010 Retrieved from Food and Drug Administration Philippines website:
- 62.Evelyn Chairul. Bacteria and mold spore heat resistance in guava juice and its control by pH and sodium benzoate. International Journal of Food Science. 2021;2021:1–7. doi: 10.1155/2021/5594362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chauhan O.P., Singh D., Tyagi S.M., Balyan D.K. Studies on preservation of sugarcane juice. Int. J. Food Prop. 2002;5(1):217–229. doi: 10.1081/JFP-120015603. [DOI] [Google Scholar]
- 64.Ogiehor I.S., Ikenebomehclearly M.J. Antimicrobial effects of sodium benzoate on the growth, survival and aflatoxin production potential of some species of Aspergillus in Garri during storage. Pakistan J. Nutr. 2004;3:300–303. doi: 10.3923/pjn.2004.300.303. [DOI] [Google Scholar]
- 65.Sama V., Molua E.L., Nkongho R.N., Ngosong C. Potential of sodium benzoate additive to control food-borne pathogens and spoilage microbes on cassava (Manihot esculenta Crantz) fufu and shelf-life extension. J. of Agri. Food Res. 2023;11 doi: 10.1016/j.jafr.2023.100521. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available upon request from the corresponding author. The authors are committed to ensuring the transparency and reproducibility of the study's results and will promptly respond to any reasonable requests for data sharing.