Abstract
Cashew trees (Anacardium occidentale L.) are planted for primarily their nuts, but they also generate apples which are mostly thrown away due to their astringent taste. The current study aimed to explore the possible utilization of cashew apple by-products (CABP) in West Africa as an alternative feedstuff for small ruminants’ nutrition. To achieve this aim, five parts of cashew apple by-products (whole, up, down and middle part, and pomace) of two cashew varieties (red and yellow) were collected in two different agroecological zones (Sudanian Zone, SZ and Sudano-Guinea Zone, SZ) to be characterized for the chemical composition, including polyphenols and sugars, and the in vitro fermentation pattern. In general, the results showed that CABP characteristics depend more on sampling area than on variety. The dry matter (DM) in SZ and SGZ varied from 12.76 to 26.10 % and 7.41–22.9 %, respectively. The pomace showed the highest crude protein, lipids, and neutral detergent fiber (NDF) content (SZ: 9.48, 3.94 and 31.66 % DM; SGZ: 14.03, 4.94 and 34.12 % DM, respectively) but the lowest nonstructural carbohydrate (NSC) and sugar for both zones. Regarding the in vitro fermentation, the organic matter degradability (dOM) was higher in the middle part (73.73 %) and whole apple (61.62 %) of SZ and SGZ, respectively. In contrast, the pomace from both zones showed the lowest in vitro fermentation parameters. The total polyphenols were more concentrated in the CABP from SZ (whole: 2736 μg/g DW; pomace: 3813 μg/g DW) compared to those from SGZ (whole: 1755 μg/g DW; pomace: 1374 μg/g DW). Results suggest that CABP should be collected in each cultivation zone regardless of variety, separating pomace from other by-products and may be used as alternative feedstuff for small ruminants during the dry season in the West Africa region.
Keywords: Waste management, Polyphenols, Volatile fatty acids, Ruminant feedstuffs, Sheep
Highlights
-
•
Cashew apple by-products are useful as alternative feedstuff for small ruminants in West Africa.
-
•
Cashew apple pomace must be separated to other cashew by-products independently of the variety in each cultivation zone.
-
•
The astringent taste of the cashew apple is related to its naringin content.
1. Introduction
According to the objective #12 of The 2030 Agenda for Sustainable Development proposed by The United Nations (https://sdgs.un.org/goals), it is necessary to minimize the negative effects on human health and the environment pollution by promoting sustainable management and the efficient use of natural resources, reducing food losses along supply chains and managing all waste throughout its life cycle at a global level. The use of agro-industrial residues in animal nutrition is an eco-sustainable feeding strategy of making use of them, especially in countries where the climate conditions do not always guarantee the necessary feed resources to improve livestock product yields and quality.
The cashew (Anacardium occidentale L.) belongs to the Anacardiaceae Family which includes about 60 genera and 400 species is a tropical and sub-tropical plant capable of growing on various soil types, even dry ones with poor nutrients [1]. It is native to tropical America, but was successfully introduced in several countries of Asia, Africa, and Central America as an important cash crop [2]. Cashew trees are planted principally for nut production (called true fruit) which is outside and linked to the apple (called peduncle or pseudo fruit). The colors of the apples (yellow to red) are the only physical differences observed in cashew plantation give the name to the varieties (red variety vs yellow variety) while the nuts do not present any differences. The farmers often cultivate different varieties in the same field because only the nuts are of interest. In 2020, the cashew nut production in the world was estimated at 4,180,990 tons [3] with West African region (Ivory Coast and Benin) in second position. At the same time, a great number of cashew apples are thrown away annually (ranging from 4 to 62 million tons), considering that 10–15 tons of apples are obtained for every ton of nuts harvested [4]. Cashew apples are low processed (10 %) to juice, vinegar, jam, chutney, or soft drinks compared to other processed fruits generating the pomace as waste [5], while a large quantity of them (90 %) is thrown away [2,6]. These quantities are expected to increase because the cashew trees inventory has revealed that the plantations are young and will live at least 35 years [7]. However, the perishability of the apples, their rapid fermentation and the presence of phenolic compounds, such as tannins with an astringent taste, limit their total transformation by the food industries even if cashew apple juice is richer in vitamins, sugar, minerals, amino acid, and dietary fiber than the most consumed fruits, such as oranges and pineapples [[8], [9], [10]]. Clarification, heat treatment, microfiltration, fermentation or ethanol vapors and CO2 methods could mitigate the presence of tannin and the astringent taste in cashew apples and their juice [11,12]. However, these methods are not available in all cashew cultivation areas.
In West African countries, the technique used to reduce the astringent taste in cashew juice consists of cutting the extremities (up and down part of apples), pressing the middle part, and applying a heat treatment [13]. Therefore, many different types of cashew waste are produced consisting of whole apples left in the fields during the nut harvesting and the extremities and pomace generated during the processing of the apples. The unused cashew waste represents an environmental problem that the decomposition will contribute to global warming and environmental air pollution [14,15]. However, this wastes can be utilized for different purposes, such as bioethanol, hydrolytic enzymes, lactic acid, as biosurfactants and as feed for animals [9,16]. The latter would seem to be the most useful in West Africa due to the cost of animal feed and the scarcity of natural pasture for ruminants during the dry season [17]. But, some studies in Latin America, focusing on chemical composition, intake, digestibility, and growth parameters, advise replacing cashew apple by-products with other ingredients in ruminants’ diets [5,18,19]. In addition, recent studies carried out in West Africa revealed that red pulp by-products are richer in sodium, potassium and non-structural carbohydrates than yellow pulp by-products which are richer in calcium, magnesium, phosphorus, and lignin [20].
Furthermore, tannin, saponin, phytate, oxalate and flavonoids which might have a negative effect on rumen fermentation were reported to be present in cashew apple and its by-products [21,22]. Unfortunately, the literature on cashew apple by-products lacks data on the comparative chemical composition, polyphenols and the rumen fermentation between the by-products of different cashew varieties (red vs yellow) and their origin (Sudanian vs Sudano-Guinea) in West Africa region. Therefore, there is a need to understand the nutritional composition of the different cashew apple by-products and to consider the influence of variety and environmental conditions in the areas where they are grown. This information would be helpful for farmers when planning animal feed including these by-products in the various agro-ecological zones. The current study aims to explore the potential use of cashew apple by-products (CABP) in ruminants’ nutrition in the West Africa region as alternative feedstuff.
2. Materials and methods
2.1. Study area
The two selected areas, Sudanian Zone (SZ) and Sudano-Guinea Zone (SGZ), are the most suitable areas for cashew cultivation in West Africa and are both cultivated in the Republic of Benin. The municipalities of Parakou and Ketou, located in SZ and SGZ, respectively, were chosen for collecting samples. Parakou is in the North-East of Benin, located between the latitudes 8°55′ and 10°53′N, and the longitudes 2° and 3°50′E. This area is characterized by two seasons (dry: November to May; rainy: May to October) with annual rainfall varying between 900 and 1000 mm. The average relative humidity and temperature values are 54.9 % and 27.5 °C, respectively. Ketou is in the South-East of Benin between the latitudes 7°10′0″ N and the longitudes 2°34′60’’E with 1100–1400 mm of rainfall within two rainy seasons (April to July and October to November) and two dry seasons (August to September and December to March). The average relative humidity and temperature values are 66.3 % and 26.9 °C, respectively [23].
2.2. Samples collection
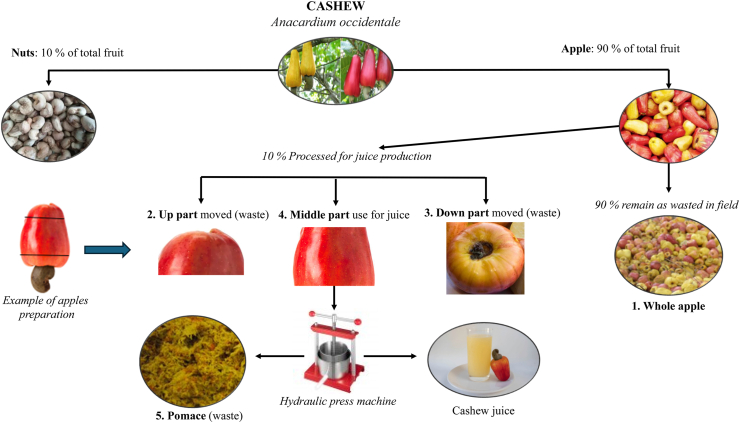
Cashew apples were collected separately according to variety (red and yellow) and cultivation area (SZ and SGZ) after harvesting the nuts. All the samples were transported within 1 h to the Laboratory of Ecology, Health, and Animal Production (Faculty of Agronomy, University of Parakou) to be processed in accordance with the local juice processing technique. A steel knife was used to move the extremities parts of apples (up and down), whereas a hydraulic press machine (Tianyu Youdo Machine, model UDZL-W33, dimensions 330_330_600 mm, Kaifeng, China) was used to press the middle parts, generating juice and pomace. The by-products obtained through this process were: upper part, lower part, and pomace. In addition, the whole apples and the unpressed middle part were also collected from the apples left over in the cashew plantation field after the harvesting of the nuts. The biggest particles were cut into small pieces for fast drying in a dried oven at 65 °C. The cashew apples processing and its by-products generation is presented in Fig. 1. All the samples were sent to the Laboratory of Feed Evaluation (Department of Veterinary Medicine and Animal Production, University of Napoli Federico II, Italy), where they were ground at 1.0 mm for chemical composition analysis and in vitro fermentation.
Fig. 1.
Cashew apple processing into juice in West Africa region and No. 1 to 5 samples collected.
2.3. Chemical composition assessment
The AOAC method was used to determine residual dry matter at 103 °C (DM, ID: 2001.12), crude protein (CP, ID: 978.04), ether extract (EE, ID: 920.39), and ash (ID: 930.05) [24]. Neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) were also quantified, according to Van Soest [25]. The total sugars (TS) and free sugars (FS) were quantified only for whole apple and pomace [26], the most representative by-products in terms of quantity available. These by-products were infused with distilled water at 1:40 and 1:80 ratio for FS and TS, respectively. The mixture of FS was left at room temperature (20 °C) for 30 min while 20 ml of HCl (1:1) was added to each flask of TS and incubated in a thermostatic bath at 70 °C for 3 h followed by three drops of 1 % phenolphthalein to cool solution. Before the filtration, 5 ml of Carrez solution I and II were added every 10 min to both FS and TS flasks. The filtrated solutions were titrated using Fehling's solution (A and B) and 1 % of methylene blue. Then the FS and TS were determined as (f x d)/(a - 0.1) where f is the factor due to the power of sugar (being 3.35 for reducing sugars and 5.15 for total sugars), d is the dilution factor, and a is the volume (ml) of the solution used during the titration.
2.4. Determination of polyphenols
Polyphenol extraction was carried out as follows [27]: 0.1 g of each sample was added to 1.0 mL of methanol/water solution (70:30 v/v) and vortexed for 1 min, then centrifuged for 10 min (14800 rpm, 4 °C), the supernatants filtered using 0.45 μm PTFE filters, and diluted for HPLC analyses. The chromatographic separation was performed (HPLC SHIMADZU equipped with UV/VIS SPD-20° detector; Prominence, USA) [27]. The identified phenolic compounds were: gallic acid, protocatechuic acid, chlorogenic acid, procyanidin B2, 4-hydroxibenzoic acid, epicatechin, p-coumaric acid, sinapic acid, trans-ferulic acid, naringin, quercetin, and trans-cinnamic acid. Two unidentified phenolic compounds corresponding to ‘Unknown 1’ and ‘Unknown 2’ were quantified as the equivalent of gallic acid. The retention times of identified polyphenols varied from 4 min for gallic acid to 28 min for trans-cinnamic acid. The analyses were conducted in three replicates and the results were expressed as μg/g of dry weight using a calibration curve with the corresponding pure compounds.
2.5. In vitro fermentation
For the in vitro trial, 1.0027 ± 0.0010 g of each sample with 3 replications each were incubated at 39 °C under anaerobic conditions in 120 ml serum bottles containing 74 ml of medium solution, 4 ml of reducing agent and 10 ml of sheep rumen liquor [28]. Three bottles without substrate (blanks) were also incubated to correct the fermentation parameters. In total 63 bottles were incubated. The medium solution and reducing agent used were prepared following the methodology proposed by Lowe et al. [29] and approved by Theodorou et al. [30] as.
-
•Medium solution: 2.5 L (H2O) + 0.5 mL(micromineral solution) + 1 L (buffer solution) + 1 l (macro-mineral solution) + 5 mL (resazurin solution, 0.1 g/100 mL H2O).
-
•Micromineral solution: 100 mL (H2O) + 13.2 g CaCl2⋅2H2O + 10 g MnCl2⋅4H2O + 1 g CoCl2⋅6H2O + 8 g FeCl3⋅6H2O.
-
•Buffer solution: 1 L (H2O) + 4 g NH4HCO3 + 35 g NaHCO3
-
•Macro-mineral solution: 1 L (H2O) + 4.65 g Na2HPO4⋅2H2O + 6.2 g KH2PO4 + 0.6 g MgSO4.7H2O
-
•
-
•
Reducing agent: 190 mL (H2O) + 1.25 g cysteine HCl + 8 mL 1M NaOH + 1.25 g Na2S⋅9H2O.
Regarding the rumen liquor, it was collected in the morning at an EU-authorized slaughterhouse (EU Council, 2004) from three adults males of Merinizzata sheep raised on natural pasture and transported to the laboratory within 1 h in pre-warmed thermos (39 °C). Before use, the rumen liquor was measured for pH, verifying it was adequate for microorganisms’ activity (6.08 ± 0.15). At the laboratory, the rumen contents were mixed, filtered through cheese cloths and flushed under CO2 to guarantee the anaerobiosis All the steps of experimental trial were approved by the Ethical Animal Care and Use Committee of University of Napoli Federico II (Prot. 2019/0013729 of February 08, 2019). The pressure and the gas produced were recorded 21 times with a manual pressure transducer (Cole and Palmer Instrument Co, Vernon Hills, IL, USA). After 48 h the fermentation was stopped by cooling the bottles at 4 °C and the pH values per bottle were measured (pH-meter ThermoOrion 720 A+, Fort Collins, CO, USA). The fermentation liquors were sampled and stored at −16 °C for volatile fatty acid (VFA) detection. Pre-weighed sintered glass crucibles (Schott Duran, Mainz, Germany, porosity #2) were used to collect the undegraded material by filtration. Then, they were oven-dried (103 °C) and burned (550 °C) to calculate the organic matter degraded (dOM, %) and the cumulative volume of gas related to incubated OM (OMCV, mL/g).
2.6. Volatile fatty acid detection
The fermentation liquors were centrifuged twice at 12,000×g for 10 min (Universal 32R centrifuge, Hettich FurnTech Division DIY, Melle- Neuenkirchen, Germany) for VFA determination [31]. One milliliter of supernatant was mixed with 1 ml of 0.06 mol oxalic acid to inject in gas chromatography (ThermoQuest 8000top Italia SpA, Rodano, Milan, Italy) equipped with a fused silica capillary column (30 m, 0.25 mm ID, 0.25 μm film thickness), with an external standard solution (acetic, propionic, butyric, iso- butyric, valeric and iso-valeric acids). Equation (1) was utilized to quantify in percentages the branched-chain fatty acids (BCFA) produced during the fermentation [31].
| (1) |
2.7. Data processing
The metabolizable energy (ME, MJ/kg; equation (2)) was estimated using the chemical composition data and in vitro fermentation parameters [32]:
| (2) |
where CP, EE, and GP are respectively crude protein, ether extract, and G24 the volume of gas recorded after 24 h of incubation by 200 mg of DM.
To estimate the fermentation kinetics, for each incubated bottle, the gas production profiles were fitted to a sigmoidal model (equation (3)) [33].
| (3) |
where G is the total gas produced at the end of incubation time (ml/g of incubated OM) at time t (h), A is the asymptotic gas production (ml/g), B is the time at which one half of A is reached (h), and C is the curve switch. Then maximum fermentation rate (Rmax, ml/h) and the time at which its occurs (Tmax, h) were calculated using equations (4), (5)) respectively [34]:
| (4) |
| (5) |
2.8. Statistical analysis
All the data were applied in JMP® software (Version 14 SW, SAS Institute Inc., Cary, NC, USA, 1989–2019) to statistically evaluate the following fixed effect: cultivation zone, variety, and the by-products. The t-test and Tukey's HSD test were used. equation (6) was utilized as statistical model fixing the significance level at 5 %.
| (6) |
where Y is the experimental data, μ the general mean, Z the effect of cultivation zone (i = Sudanian and Sudano-Guinea), V the variety effect (j = red and yellow), B the by-products (k = whole, up, down, middle, and pomace). Only Z B interaction was considered, because the other interaction including variety effect resulted not statistically significant. The correlations between chemical composition, in vitro fermentation parameters, and polyphenol content were also performed (JMP® 1989–2019).
3. Results
3.1. Chemical characterization and nutritive value
The chemical composition of cashew apple by-products (CABP) is presented for the five by-products in the two sampling areas as the average of the two varieties (Table 1). The results showed that the variety effect was significant only for ADF, ADL and ME (p < 0.05), while the zone and by-products effects were highly significant (p < 0.0001) for all the parameters. DM ranged between 26.10 % in SZ pomace and 7.41 % in SGZ down part. A similar trend was observed in the two zones (pomace > up > whole > middle > down), with values always being higher in SZ. Ash content in both areas showed the highest value (p < 0.05) in the down part and the lowest in pomace. The content of CP, EE, NDF and ADF was higher in pomace; all these parameters were always higher in SGZ compared to SZ. Regarding ADL, the content was high in all samples of both areas, ranged between 8.03 and 17.47 % DM, especially in pomace (11.52 and 17.47 % DM, in SZ and SGZ, respectively). The non-structural carbohydrates (NSC) showed high values in all samples, with the highest values in whole and up part and the lowest level in pomace; different rankings in the two areas resulted. For sugar content (data not showed), significant differences (p < 0.001) were observed in the two analyzed by-products, whole and pomace. In particular, total sugar and free sugar (TS and FS, % DM) were higher in the whole apples (SZ: 57.51 and 65.00; SGZ: 54.21 and 53.58, for TS and FS, respectively) compared to pomace (SZ: 43.00 and 44.93; SGZ: 42.731 and 30.15, for TS and FS respectively). Estimated ME ranged between 6.90 and 7.77 MJ/kg DM with any significance (p > 0.05) among the principal factors. The Zone*By-product interaction was significant only for ash, NDF, and ADF.
Table 1.
Chemical composition of cashew apple by-products from Sudanian and Sudano-Guinea zones of West Africa.
| Item |
DM |
CP |
EE |
Ash |
NDF |
ADF |
ADL |
NSC |
ME |
|---|---|---|---|---|---|---|---|---|---|
| % | % DM | MJ/kg | |||||||
| Sudanian Zone | |||||||||
| Whole | 14.46C | 6.87BC | 1.85C | 2.95B | 16.13B | 16.60C | 8.68AB | 72.19AB | 7.23 |
| Up | 19.75B | 6.17C | 1.81C | 2.44BC | 11.26C | 17.04C | 9.80AB | 78.32A | 6.90 |
| Down | 12.76C | 8.49AB | 2.63B | 3.89A | 18.47B | 20.28B | 10.40AB | 66.56B | 7.51 |
| Middle | 14.88C | 6.04C | 1.49C | 2.60BC | 10.40C | 14.94C | 8.03B | 79.46A | 7.00 |
| Pomace | 26.10A | 9.48A | 3.94A | 2.11C | 31.66A | 30.14A | 11.52A | 52.79C | 7.02 |
| Sudano-Guinea Zone | |||||||||
| Whole | 11.24BC | 8.77C | 2.33C | 2.38B | 18.28C | 22.14C | 12.38B | 68.24A | 7.40 |
| Up | 16.38B | 8.68C | 2.35C | 2.26B | 18.36C | 21.39C | 11.60B | 68.34A | 7.77 |
| Down | 7.41C | 10.15B | 3.11B | 3.11A | 23.82B | 28.63B | 13.49B | 59.81B | 7.53 |
| Middle | 8.80C | 8.33C | 3.03B | 2.41B | 20.97BC | 21.02C | 10.83B | 65.26AB | 7.15 |
| Pomace | 22.98A | 14.03A | 4.94A | 1.60C | 34.12A | 33.54A | 17.47A | 45.29C | 7.36 |
| P value | |||||||||
| Z | <0.0001 | <0.0001 | 0.0007 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0315 |
| Var | 0.5685 | 0.8624 | 0.5628 | 0.0517 | 0.4614 | <0.0001 | <0.0001 | 0.6192 | 0.0120 |
| BP | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0002 | <0.0001 | 0.3441 |
| Z BP | 0.6594 | 0.1100 | 0.4426 | 0.0083 | 0.0002 | 0.0406 | 0.3244 | 0.0255 | 0.3468 |
| MSE | 6.2940 | 1.2746 | 0.4498 | 0.0315 | 2.9881 | 2.4680 | 3.9229 | 1.3326 | 0.2997 |
DM: dry matter, CP: crude protein, EE: ether extract, NDF: neutral detergent fiber, ADF: acid detergent fiber, ADL: acid detergent lignin, ME: estimated metabolizable energy, Var: variety, Z: zone, BP: by-product, NSC: non-structural carbohydrate (NSC = 100 – (%NDF + %CP + %EE + %Ash), Z BP: Zone By-products interaction. MSE: mean square error. Along the column, for each zone, different letters indicate the statistical differences (p < 0.05).
3.2. In vitro fermentation characteristics
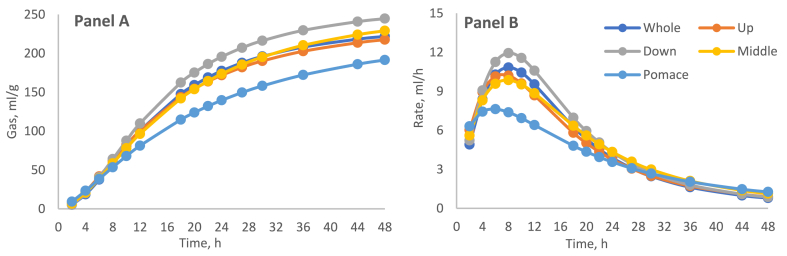
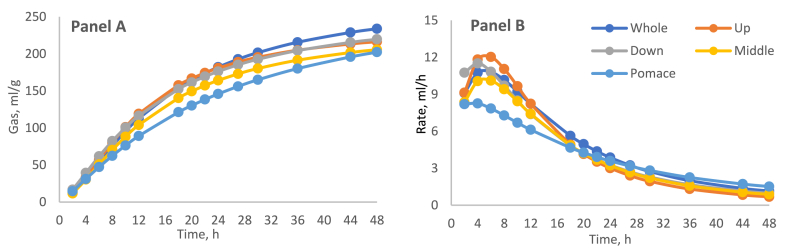
The in vitro fermentation characteristics in the five by-products of the two sampling areas (shown as the average of the two varieties) were statistically influenced by the factors considered (Table 2). The pH recorded at the end of the fermentation trial was different in terms of cultivation zone (6.42 and 6.56, for SZ and SGZ respectively; p = 0.003) whereas no differences were observed among by-products. On the other hand, the OM degradability (dOM) and the time at which the maximum fermentation rate occurs (Tmax) were significantly different (p < 0.0001) between the cultivation zones. The by-products factor showed a significant effect (p < 0.0001) in all parameters except pH, A, and Tmax. In particular, the highest dOM was obtained in the SZ middle part (73.73 %) and SGZ whole apples (61.62 %), while the pomace showed the lowest degradability in both zones (51.70 and 43.63 %, SZ, and SGZ respectively). Moreover, the pomace had the lowest Rmax in both zones (7.97 mL/h and 7.79 mL/h, for SZ and SGZ, respectively), whereas the down and up parts showed the highest (11.78 and 13.08 mL/h, in SZ and SGZ, respectively). The fermentation process of CABP showed that the gas productions of both zones were more similar from 2 h to 10 h of incubation; after this time in SZ, down part increased the gas production and in SGZ, middle part decreased the gas production (Fig. 2, Fig. 3, Panel A). For most samples, the fermentation rate reached the maximum around 8 h for SZ and before (around 5 h) for SGZ and then decreased gradually until the stop at 48 h (Fig. 2, Fig. 3, Panel B). Pomaces in both zones showed the slowest fermentation rate profile.
Table 2.
In vitro fermentation characteristics of cashew apple by-products from Sudanian and Sudano-Guinea zones of West Africa.
| Item |
pH |
dOM |
OMCV |
Yield |
A |
B |
Tmax |
Rmax |
|---|---|---|---|---|---|---|---|---|
| % | mL/g | mL/g | mL/g | h | h | mL/h | ||
| Sudanian Zone | ||||||||
| Whole | 6.54 | 67.68BC | 222.8 | 327.8 | 243.3 | 14.40B | 7.56 | 11.40 |
| Up | 6.55 | 70.10B | 235.6 | 338.8 | 257.6 | 14.82B | 7.96 | 11.71 |
| Down | 6.48 | 64.96C | 237.1 | 367.2 | 267.9 | 14.44B | 6.64 | 11.78 |
| Middle | 6.40 | 73.73A | 229.1 | 311.7 | 267.2 | 16.81A | 7.84 | 9.92 |
| Pomace | 6.43 | 51.70D | 193.9 | 347.5 | 249.7 | 19.52A | 6.63 | 7.97 |
| Sudano-Guinea Zone | ||||||||
| Whole | 6.56 | 61.62AB | 235.7 | 384.7AB | 278.7AB | 15.59B | 5.03 | 11.00AB |
| Up | 6.51 | 59.44BC | 227.3 | 378.5AB | 238.0B | 12.00B | 4.93 | 13.08A |
| Down | 6.63 | 51.28D | 223.4 | 439.2A | 258.8AB | 14.13B | 3.82 | 11.51AB |
| Middle | 6.58 | 58.57BC | 211.5 | 360.6B | 236.0B | 13.73B | 5.10 | 10.71AB |
| Pomace | 6.64 | 43.63E | 180.8 | 429.3AB | 286.9A | 24.10A | 2.29 | 7.79B |
| P value | ||||||||
| Z | 0.0031 | <0.0001 | 0.4169 | <0.0001 | 0.3769 | 0.874 | <0.0001 | 0.6522 |
| Var | 0.9240 | 0.0772 | <0.0001 | 0.0005 | 0.3770 | 0.184 | 0.0158 | 0.0914 |
| BP | 0.5972 | <0.0001 | <0.0001 | <0.0001 | 0.197 | <0.0001 | 0.0693 | 0.002 |
| Z BP | 0.0721 | 0.0002 | 0.2628 | 0.7714 | 0.0014 | 0.0014 | 0.8685 | 0.7961 |
| MSE | 0.0080 | 2.9021 | 29.47 | 139.9 | 310.1 | 2.618 | 1.8159 | 2.5934 |
dOM: organic matter degradability, OMCV: cumulative volume of gas related to incubated organic matter, Yield: cumulative volume of gas related to degraded organic matter, A: potential gas production, B: time at which A/2 was reached, Tmax: time at which maximum rate was reached, Rmax: maximum fermentation rate, Var: variety, Z: zone, BP: by-product, Z BP: Zone By-products interaction. MSE: mean square error. Along the column, for each zone, different letters indicate the statistical differences (p < 0.05).
Fig. 2.
In vitro gas production profile (Panel A) and fermentation rate (Panel B) of cashew apple by-products from Sudanian zone of West Africa.
Fig. 3.
In vitro gas production profile (Panel A) and fermentation rate (Panel B) of cashew apple by-products from Sudano-Guinea Zone of West Africa.
The in vitro volatile fatty acids determined at 48 h in the five by-products of the two cultivation zones show significant differences for all the factors considered and most of the parameters determined (Table 3). The total volatile fatty acid (VFA) was higher in SZ for all by-products compared to their corresponding values in SGZ. VFA varied from 46.47 mmol (pomace) to 60.97 mmol (middle) in SZ and from 42.70 mmol (pomace) to 54.50 mmol (up) in SGZ mainly contributed by acetate and propionate production for both zones. In addition, pomace showed the lowest for most volatile fatty acid except branched chain fatty acid (BCFA) and acetate-to-propionate ratio for both zones.
Table 3.
In vitro fermentation end-products of cashew apples by-products from Sudanian and Sudano-Guinea zones of West Africa.
| Item |
VFA |
Ace |
Pro |
Iso-But |
But |
Iso-Val |
Val |
BCFA |
Ace/Pro |
|---|---|---|---|---|---|---|---|---|---|
| Mmol/g iOM | % | ||||||||
| Sudanian Zone | |||||||||
| Whole | 53.81ABC | 28.35 | 20.20B | 0.20 | 4.00B | 0.22 | 0.84BC | 0.88AB | 1.61AB |
| Up | 54.50AB | 29.54 | 23.91A | 0.19 | 4.96B | 0.21 | 1.03AB | 0.77B | 1.40B |
| Down | 53.20BC | 27.92 | 18.37B | 0.22 | 5.83B | 0.24 | 0.62CD | 0.96AB | 1.73AB |
| Middle | 60.97A | 28.56 | 21.53AB | 0.24 | 9.21A | 0.22 | 1.22A | 1.01A | 1.52B |
| Pomace | 46.47C | 27.10 | 14.61C | 0.21 | 3.7B | 0.20 | 0.37D | 0.96AB | 2.03A |
| Sudano-Guinea Zone | |||||||||
| Whole | 50.08BC | 27.79AB | 15.34BC | 0.19 | 5.90 | 0.21 | 0.64 | 0.95B | 2.15B |
| Up | 54.50AB | 29.70A | 19.98A | 0.23 | 3.94 | 0.21 | 0.44 | 0.97AB | 1.81B |
| Down | 46.60CD | 29.27A | 12.40C | 0.22 | 3.99 | 0.20 | 0.51 | 1.11AB | 3.01A |
| Middle | 48.50BCD | 26.21AB | 16.29B | 0.22 | 4.98 | 0.20 | 0.61 | 1.01AB | 1.92B |
| Pomace | 42.70D | 24.63B | 13.38BC | 0.19 | 3.80 | 0.25 | 0.44 | 1.17A | 2.06B |
| P value | |||||||||
| Z | <0.0001 | 0.1308 | <0.0001 | 0.6381 | 0.0026 | 0.5110 | <0.0001 | <0.0001 | <0.0001 |
| Var | 0.7435 | 0.9996 | 0.1248 | 0.0117 | 0.1277 | 0.0054 | 0.0011 | 0.5807 | 0.0286 |
| BP | <0.0001 | 0.0004 | <0.0001 | 0.0077 | <0.0001 | 0.6606 | <0.0001 | 0.0001 | <0.0001 |
| Z BP | 0.0525 | 0.0906 | 0.0201 | 0.0453 | <0.0001 | 0.0998 | <0.0001 | 0.5269 | <0.0001 |
| MSE | 15.474 | 3.7922 | 3.1574 | 0.0005 | 1.7043 | 0.0019 | 0.0186 | 0.0117 | 0.0587 |
VFA: total volatile fatty acid, Ace: acetate, Pro: propionate, Iso-But: iso-butyrate, But: butyrate, Iso-Val: iso-valerate, Val: valerate, BCFA: branched chain fatty acid, Ace/Pro: acetate to propionate ratio. Var: variety, Z: zone, BP: by-product Z BP: Zone By-products interaction. MSE: mean square error. Along the column, for each zone, different letters indicate the statistical differences (p < 0.05).
The correlation between chemical composition and in vitro fermentation characteristics showed many significant values (Table 4). Except for butyrate and Rmax, the main chemical composition parameters (CP, EE, NDF, ADF, and ADL) were correlated to the in vitro fermentation ones, and the significance of correlation coefficient varied from 0.001 to 0.05. They were positively correlated to pH, acetate, and BCFA, Ace/Pro ratio and the time to reach ½ of potential gas production (B), while they were negatively correlated to dOM, OMCV, propionate, valerate, VFA production and Tmax.
Table 4.
Correlation between chemical composition and in vitro fermentation data (n = 20).
| Items | CP | EE | NDF | ADF | ADL |
|---|---|---|---|---|---|
| pH | 0.7066*** | 0.6027** | 0.5262* | 0.5669** | 0.6967*** |
| VFA | −0.8179*** | −0.8030*** | −0.8279*** | −0.7602*** | −0.6373** |
| Acetate | 0.7229*** | 0.6896*** | 0.7013*** | 0.7440*** | 0.6176** |
| Propionate | −0.5773** | −0.4768* | −0.5869** | −0.6813** | −0.5300* |
| Iso-Butyrate | 0.5933** | 0.5730** | 0.6123** | 0.6709** | 0.5726** |
| Butyrate | −0.3925NS | −0.4616* | −0.3342NS | −0.2789NS | −0.2723NS |
| Iso-Valerate | 0.7276*** | 0.7340*** | 0.5620* | 0.4593* | 0.4354 NS |
| Valerate | −0.6655** | −0.7018*** | −0.7007*** | −0.7144*** | −0.6432** |
| BCFA | 0.9148*** | 0.8753*** | 0.7968*** | 0.7734*** | 0.7133*** |
| Acetate/Propionate | 0.6134** | 0.5276* | 0.5789** | 0.6978*** | 0.5736** |
| dOM | −0.8843*** | −0.8413*** | −0.9416*** | −0.9166*** | −0.7334*** |
| OMCV | −0.4858* | −0.5870** | −0.6669** | −0.5942** | −0.4546* |
| Tmax | −0.7829*** | −0.6561** | −0.6964*** | −0.7657*** | −0.7238*** |
| Rmax | −0.2030NS | −0.3064NS | −0.4761* | −0.4085NS | −0.2136NS |
| B | 0.4899* | 0.5055* | 0.6144** | 0.5721** | 0.4446* |
CP: crude protein, EE: ether extract, NDF: neutral detergent fiber, ADF: acid detergent fiber, ADL: acid detergent lignin, VFA: total volatile fatty acid, BCFA: branched chain fatty acid, dOM: organic matter degradability, OMCV: cumulative gas volume related to incubated organic matter, Tmax: time at which maximum rate was reached, Rmax: maximum fermentation rate, B: time at which A/2 was reached. ***, **, *, NS: p < 0.001, p < 0.01, p < 0.05, not significant, respectively.
3.3. Polyphenols
The polyphenol content determined in pomace and whole cashew apples of SZ and SGZ are reported in Table 5. A very highly significant effect was observed as regards zone and by-products rather than variety. Naringin and protocatechuic acid were the most abundant polyphenols from both zones followed by gallic acid. Two ‘unknown’ compounds were detected in SZ, and only one in SGZ; both were quite abundant. Conversely, chlorogenic acid was the least abundant phenolic compound in the whole by-product and was not found in the whole by-product from SZ, whereas 4-hydroxybenzoic and p-coumaric acid were the least abundant compounds found in the pomace from SZ and SGZ, respectively. Gallic acid, protocatechuic acid and chlorogenic acid were significantly (p < 0.05) more abundant in the pomace compared to the whole by-product from SZ. Consequently, the total polyphenol content in pomace from SZ was significantly higher than in the whole by-product (3813 vs. 2736 μg/g DW, p = 0.0002). A less clear distribution of polyphenols across the by-products from SGZ was observed, with protocatechuic acid content being 2.5-fold lower in pomace compared to the whole by-product. Therefore, a similar content of total polyphenol was found in whole and pomace by-products from SGZ (1755 vs. 1374 μg/g DW, p > 0.05).
Table 5.
Polyphenol content in pomace and whole cashew apples from Sudanian and Sudano-Guinea zones of West Africa.
| Sudanian Zone |
Sudano-Guinea Zone |
P value |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| μg/g dry weight | Whole | Pomace | Whole | Pomace | Z | V | B | Z*B | MSE |
| Gallic acid | 178.72 | 103.63 | 172.05 | 140.86 | NS | ** | NS | NS | 10.4 |
| Protocatechuic acid | 553.1 | 696.1 | 934.4A | 378.9B | NS | NS | NS | ** | 495 |
| Chlorogenic acid | ND | 30.66 | 2.30B | 8.80A | *** | NS | *** | . | 2.48 |
| Procyanidin B2 | 28.74B | 50.38A | 7.91 | 22.74 | *** | NS | *** | NS | 0.37 |
| 4-hydroxy benzoic acid | 16.97B | 25.69A | 5.68B | 10.52A | *** | ** | *** | NS | 0.58 |
| Epicatechin | 21.46B | 97.00A | 22.00B | 109.1A | NS | ** | *** | NS | 5.89 |
| p-coumaric acid | 7.70B | 26.97A | 5.98 | 8.00 | *** | * | *** | *** | 0.10 |
| Sinapic acid | 14.45B | 34.34A | 9.64B | 13.12A | *** | *** | *** | *** | 0.48 |
| trans-ferulic acid | 16.09B | 44.28A | 12.43 | 10.34 | *** | ** | *** | *** | 1.40 |
| Unknown 1 | 246.5B | 672.8A | 120.27 | 136.4 | *** | * | *** | *** | 28.8 |
| Naringin | 1206B | 1477A | 415.2 | 469.8 | *** | NS | ** | * | 188 |
| Unknown 2 | 364.3B | 439.3A | ND | ND | – | * | * | – | 4.5 |
| Quercetin | 28.50B | 48.30A | 32.02 | 43.93 | NS | NS | *** | NS | 2.07 |
| trans-cinnamic acid | 53.82B | 81.17A | 14.6B | 37.05A | *** | NS | *** | NS | 0.46 |
| Total | 2,736B | 3,813A | 1755 | 1374 | *** | NS | * | *** | 967 |
Z: zone, B: By-products, V: Variety, Z B: Zone By-products interaction. Along the row, for each zone, different letters indicate the statistical differences (p < 0.05). ***, **, *, NS: p < 0.001, p < 0.01, p < 0.05, not significant, respectively. MSE: mean square error.
The correlation analyses to evaluate the effect of polyphenols on chemical composition and in vitro fermentation characteristics gave the following significant results.
-
•
Epicatechin with chemical composition data: EE (+0.842; p < 0.01), structural carbohydrates (+0.874 and + 0.906, p < 0.01, with NDF and ADF, respectively) and ash (−0.785; p < 0.05) and in vitro data as well: dOM and OMCV (−0.775, −0.742; p < 0.05, respectively);
-
•
Quercetin with EE (+0.821; p < 0.05) and ADF (+0.760, p < 0.05);
-
•
Gallic acid with ME (−0.775; p < 0.05) and Iso-Val (−0.768; p < 0.05);
-
•
Naringin with potential gas production (−0.825; p < 0.05);
-
•
Procyanidin B2 with pH (−0.728; p < 0.05);
-
•
Total polyphenols with pH (−0.790; p < 0.05) and potential gas production (−0.840; p < 0.01);
-
•
Protocatechuic and Chlorogenic acid with butyrate (+0.866; p < 0.01) and iso-butyrate (+0.747; p < 0.05), respectively.
In general, the polyphenols present in CABP had a little effect on chemical nutrient utilization and fermentation parameters.
4. Discussion
4.1. Chemical composition
The aim of the current study was to characterize the cashew apple by-products (CABP) left in the field and during the juice production to promote their use in the nutrition of West Africa ruminants as an alternative feed principally during the dry season. The natural pasture, varying according to the season, is the main feed resource for ruminants in West Africa. Unsurprisingly, the decrease in natural pasture during the dry season generally has a negative effect on the performance of the animals. As part of the inventory of alternative feed, the current study collecting five cashew by-products from red and yellow varieties in Sudanian and Sudano-Guinea areas of Benin and expected differences in nutrients due to genetics, environmental conditions, and processing methods. In general, by-products collected in Sudanian Zone (SZ) compared to Sudano-Guinea Zone (SGZ) showed differences for most chemical parameters, probably due to the different rainfall and soil conditions. The DM content was relatively low in all samples, highlighting the difficulty in consider these by-products for long-term use, as also reported in other research [35,36]. The DM content was slightly higher and less variable in SZ as compared to SGZ, and the highest value was found in pomace. The pomace also showed higher values of crude protein, lipids, and structural carbohydrates. The high structural carbohydrates content in pomace might be due to the low moisture after the juice has been extracted. The CP content observed in other publications [19,37,38] on dehydrated cashew apple pomace and bagasse (ranging between 13 and 15 % DM) is similar to the CP content found in SGZ pomace (14 % DM) and higher than the other by-products from SGZ and all of SZ. CP level up to 19 % DM was reported in the cashew apple waste of Indian varieties [39,40]. The ash and structural carbohydrates reported by other authors [37,38] on dehydrated cashew apple pomace and bagasse were higher than our findings. The metabolizable energy, estimated from chemical composition and in vitro data, was similar for both zones and between by-products. In other West Africa feedstuffs, a comparable metabolizable energy content is reported in sun-dried yellow and red cashew pulp meal [41], partly due to the high level of ether extract (10 % DM), which could affect the in vitro rumen fermentation. The differences observed in our results and the literature on CABP would be due to the differences in the parts of by-products considered, processing techniques and conditions in areas of cultivation. Based on CP and ME content, CABP are comparable to pineapples, citrus pulp, sugar beet pulp, and sugar beet root for CP content and almond hulls, lettuce leave, apple pomace, banana stem, and jackfruit waste for ME content [15,38,42]. This similarity could help to collect and pool fruit by-products when they are available in the same area according to the feeding objective (protein source vs energy source).
4.2. In vitro fermentation characteristics
In the rumen, the normal pH is maintained at 5.5–6.5 with a possible fluctuation to 2.5–3 depending on the acid produced during the fermentation which is related to the kind of feed. At the end of the fermentation process of cashew by-products, the pH values varied between 6.40 and 6.64 which confirms the effectiveness of the trial [31]. High OM degradability was observed for the middle part and whole apple in both zones, whereas low values were found in the pomace of both regions. The same trend was observed for the fermentation kinetics and volatile fatty acids production, except for the branched-chain and acetate to propionate ratio. The high level of structural carbohydrates, specifically lignin present in the pomaces, could explain the low in vitro fermentation pattern. On the other hand, the whole and middle part of cashew apples had the greatest content of non-structural carbohydrates (NSC) and sugar (FS and TS) which favored the growth of microorganisms, fiber attack and the production of volatile fatty acid. However, the effects of lignified cell walls on feed digestibility and end fermentation products were widely reported [43,44]. In addition, we should also consider that lignin is one of the arrays of polyphenols present in plant biomass with potential antioxidant activity [45]. Furthermore, the low dOM and VFA of pomace in both zones could be explained by the low NSC which was extracted more from the juice limiting the quota of energy more easily available in the rumen for the activity of microorganisms. Microorganisms in the rumen utilize carbohydrates (NSC and fiber) for their own growth, the resulting end-products (VFA) are then utilized by the animal for maintenance and production (meat or milk). Our results related to dOM in pomaces are consistent with other data reported on cashew apple waste [38]. On the other hand, the dOM of cashew whole apples, and other parts (up, down and middle part) were comparable to previous data reported for different by-products such as peels, core, and pomace from pineapples [42] and yellow, black, and red maca varieties [46]. The in vitro fermentation parameters (OM degradability, gas and VFA production) that we found fall within the range of fruit and vegetable by-products [15] also tested using the in vitro fermentation technique. The similarities would probably lead to the chemical composition which include lower NDF and EE content and larger availability of free sugar of fruit and vegetable waste compared to the valuable, conventional ruminants’ feedstuffs.
4.3. Polyphenols
Polyphenols are metabolites developed in the plant kingdom to confront environmental constraints such as insects, herbivores and heat stress. Polyphenols can have a positive or negative affect on animals' health, physiology, and quality of their products [47]. It was reported that polyphenols have antioxidant, immunomodulatory, antimutagenic, and anti-inflammatory effects and may reduce antibiotic use in livestock production [48] thereby guaranteeing the safety of food (meat and milk) for humans. The current study revealed that naringin, protocatechuic, and gallic acid are polyphenols found in abundance in CABP. The total polyphenols of the whole and the pomace from SZ were around two or three times higher than the same by-products from SGZ, respectively. The differences are due to the heat stress characterized by the high temperatures and the low annual rainfall in Sudanian Zone (SZ) which increase the concentration of polyphenols in the apples. Naringin found in the endocarp of citrus fruits is responsible for an unacceptable bitter taste [49] and is probably the cause of astringent and bitter taste of cashew apples. However, the quantity of naringin in fruit or fruit by-products depends on their maturity. Therefore, the presence of a high level of naringin in the animals' diet will probably increase feed bitterness and reduce its intake. Naringin and quercetin from the flavonoid group of polyphenols have been shown to increase the total of volatile fatty acid and decrease methane production through the reduction of the protozoa and methanogen population in the rumen with no negative effect on rumen fermentation [47,50]. Regarding tannins, which are part of non-flavonoid group, are divided into two groups - condensed tannins and hydrolysable tannins. The condensed tannins are polymers of flavan-3-ol subunits (subunits of procyanidins: catechin and epicatechin; subunits of prodelphinidins: gallocatechin and epigallocatechin) whereas hydrolysable tannins are water-soluble molecules composed of a glucose core esterified with gallic acid or ellagic acid. Recently, tannins have received more attention as regards the nutrition of ruminants due to their ability to bind dietary protein to the attack of microorganisms in rumen (protein bypass). Thus, the use of nitrogen (N) increases and reduces ruminal ammonia formation and N excretion from urine to feces [51,52]. Tannins can also bind with carbohydrates to reduce the palatability of ruminants' feed, feed intake and thereby methane (CH4) production. However, hydrolysable tannins have been reported to be more effective than condensed tannins in decreasing CH4 production, maintaining nutrient digestibility and VFA production [52]. As an example, the effect of gallic acid on CH4 emissions and N excretion in beef cattle diet containing alfalfa silage was tested and it was shown that feeding gallic acid has the potential to decrease the environmental impact of ruminants (low CH4 and ammonia emission) without negative effect on degradability, VFA production and animal performance [53]. The same result was found in vitro with 75 mg/g DM of gallic acid or ellagic acid in ruminants' diet [52]. However, plant species, fruit maturity status and the cultivation area affect the total amount and individual polyphenols in fruit by-products, while their bioactivity through ruminants’ digestion tracts also depends on their molecular weight, conjugation with other derivatives or the hydrolysis of enzymes.
5. Conclusions
The investigation of cashew apple by-products (CABP) in the Sudanian and Sudano-Guinea zones of West Africa region revealed that they can be a potential alternative foodstuff for ruminants during the dry season. Differences between the cultivation zones were more pronounced than differences between the varieties. The chemical variables assessed in by-products from Sudano-Guinea Zone were slightly higher than those from Sudanian Zone. Pomace showed the lowest dOM and volatile fatty acid production due to the high content of structural carbohydrates, specifically lignin. In addition, CABP contains naringin, protocatechuic and gallic acid as main polyphenols which are probably the source of the bitter and astringent taste. However, the polyphenols present in CABP had a minor effect on chemical nutrient use and rumen fermentation parameters. So, CABP could be collected in each cultivation zone separating pomace to other by-products without variety consideration. However, future research must focus on preservation techniques such as ensiling and in vivo trials regarding how to enhance CABP self-life and evaluating the acceptability and the effect on the performance and health of animals. Extraction of polyphenols in CABP may also be considered to produce feed ingredients that may mitigate methane formation, thus supporting sustainable ruminant production. According to these preliminary results, we could consider cashew apple by-products a promising eco-sustainable feed in ruminant nutrition and an alternative ingredient in West Africa mainly during the dry season.
Ethical statement
The animal study protocol was approved by the Ethical Animal Care and Use Committee of the University of Napoli Federico II (Prot. 2019/0013729 of February 08, 2019).
Additional information
No addition information for this paper.
Funding
This study was carried out within the AGRITECH National Research Center and received funding from the European Union Next-Generation (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR) – MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4 – D.D. 1032 June 17, 2022, CN00000022). This manuscript reflects only the authors’ views and opinions, neither the European Union nor the European Commission can be considered responsible for them.
Data availability statement
The data are available from the corresponding author and will be made available on request.
CRediT authorship contribution statement
Dieu donné Kiatti: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Bossima Ivan Koura: Visualization, Validation, Resources, Methodology, Investigation, Conceptualization. Alessandro Vastolo: Writing – review & editing, Validation, Software, Methodology, Conceptualization. Manuela Flavia Chiacchio: Visualization, Methodology, Formal analysis. Paola Vitaglione: Visualization, Resources, Methodology. Luc Hippolyte Dossa: Visualization, Validation, Methodology. Monica Isabella Cutrignelli: Visualization, Validation, Resources, Methodology, Investigation, Conceptualization. Serena Calabrò: Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Thanks to the International Agreement for Co-Operation between the University of Naples, Federico II (Department of Veterinary Medicine and Animal Production) and the National University of Agriculture (School of Animal Production and Health). DR 2632 del 27/6/22 (Resp. Prof. Serena Calabrò).
References
- 1.Runjala S., Kella L. Cashew apple (Anacardium occidentale L.) therapeutic benefits, processing and product development: an overview. Pharm. Innov. 2017;6(7):260–264. [Google Scholar]
- 2.Das I., Arora A. Post-harvest processing technology for cashew apple - a review. J. Food Eng. 2017;194:87–98. doi: 10.1016/j.jfoodeng.2016.09.011. [DOI] [Google Scholar]
- 3.FAOSTAT. Food and Agriculture Organization of the United Nations. available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 25 February 2022).
- 4.Talasila U., Shaik K.B. Quality, spoilage and preservation of cashew apple juice: a review. J. Food Sci. Technol. 2013;52:54–62. doi: 10.1007/s13197-013-0931-0. [DOI] [Google Scholar]
- 5.Cruz Reina L.J., Durán-Aranguren D.D., Forero-Rojas L.F., Tarapuez-Viveros L.F., Durán-Sequeda D., Carazzone C., Sierra R. Chemical composition and bioactive compounds of cashew (Anacardium occidentale) apple juice and bagasse from Colombian varieties. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e09528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aidoo R., Kwofie E.M., Ngadi M.O. Circularity of cashew apples: Examining the product-process pathways, techno-functional, nutritional/phytomolecular qualities for food applications. ACS Food Sci. Technol. 2022;2(7):1051–1066. doi: 10.1021/acsfoodscitech.2c00093. [DOI] [Google Scholar]
- 7.Oliveira N.N., Mothé C.G., Mothé M.G., Oliveira L.G. Cashew nut and cashew apple: a scientific and technological monitoring worldwide review. J. Food Sci. Technol. 2020;57(1):12–21. doi: 10.1007/s13197-019-04051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nair K.P.P. The Agronomy and Economy of Important Tree Crops of the Developing World. Elsevier; Amsterdam: 2010. Cinchona (cinchona sp) pp. 111–129. [Google Scholar]
- 9.Kannan V., Rangarajan V., Manjare S.D., Pathak P.V. Microbial production of value-added products from cashew apples - an economical boost to cashew farmers. J. Pure Appl. Microbiol. 2021;15(4):1816–1832. doi: 10.22207/JPAM.15.4.71. [DOI] [Google Scholar]
- 10.Asmawati A., Marianah M., Atoum M.F.M., Sari D.A., Iqrar I., Hussain Z., Setyobudi R.H., Nurhayati N. The potential of cashew apple waste as a slimming agent. Jordan J. Biol. Sci. 2022;15(5):887–892. [Google Scholar]
- 11.Emelike N.J.T., Ebere C.O. Effect of treatments on the tannin content and quality assesment of cashew apple juice and the kernel. Eur J. Food Sci. Technol. 2016;4:25–36. [Google Scholar]
- 12.Dheeraj, Srivastava A., Mishra A. Mitigation of cashew apple fruits astringency. Environ Sustain. 2023;6:312–329. doi: 10.1007/s42398-023-00276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padonou S.W., Houssou P., Agbobatinkpo P., Olou D., Klotoe H.A., Todohoue C., Guédou M., Adegbola P., Aboh A., Montcho K., Dossou J., Mensah G.A. vol. 13. 2016. pp. 1–27. (Fiche technique: La presse hydraulique pour la production du jus de pomme d’anacarde, MAEP). [Google Scholar]
- 14.Goula A.M., Lazarides H.N. Integrated processes can turn industrial food waste into valuable food by-products and/or ingredients: the cases of olive mill and pomegranate wastes. J. Food Eng. 2015;167:45–50. doi: 10.1016/j.jfoodeng.2015.01.003. [DOI] [Google Scholar]
- 15.García-Rodríguez J., Ranilla M.J., France J., Alaiz-Moretón H., Carro M.D., López S. Chemical composition, in vitro digestibility and rumen fermentation kinetics of agro-industrial by-products. Animals. 2019;9(861):1–13. doi: 10.3390/ani9110861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cristina dos Santos Lima F., Luiz Honorato da Silva F., Palmeira Gomes J., Mariano da Silva Neto J. Chemical composition of the cashew apple bagasse and potential use for ethanol production. Adv. Chem. Eng. Sci. 2012;2:519–523. doi: 10.4236/aces.2012.24064. [DOI] [Google Scholar]
- 17.Koura B.I., Vastolo A., Kiatti D., Cutrignelli M.I., Houinato M., Calabrò S. Nutritional value of climate-resilient forage species sustaining peri-urban dairy cow production in the coastal grasslands of Benin (West Africa) Animals. 2022;12(24):1–16. doi: 10.3390/ani12243550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira A.C.H., Neiva J.N.M., Rodriguez N.M., Lobo R.N.B., Vasconcelos V.R. Nutritive value of elephantgrass silage added by-products from the cashew juice industry. Rev. Bras. Zootec. 2004;33(6):1380–1385. [Google Scholar]
- 19.Rodrigues M.R.C., Rondina D., Araujo A.A., Arruda I.J., Silva L.M., Nunes- Pinheiro D.C., Fernandes A.A.O. Use of dehydrated cashew apple pomace (Anacardium occidentale) the feeding of lambs weaning puberty: metabolic responses and sex hormone. Ciencia Animal. 2010;20:17–26. [Google Scholar]
- 20.Okpanachi U., Agu C.I., Igoche L.E., Oyedapo F.A. Potentials of two varieties of cashew apple pulp as feedstuff for ruminants. Niger. J. Anim. Prod. 2018;45(4):203–209. [Google Scholar]
- 21.Okpanachi U., Ayoade J.A., Tuleun C.D. Composition and anti-nutritional factors (Phyto-Nutrients) present in both red and yellow varieties of sun-dried cashew pulp. Am. J. Food Sci. Heal. 2016;2:45–48. https://www.researchgate.net/publication/335665454 [Google Scholar]
- 22.Filho M.R.R., Soto-Blanco B. Poisoning by cashew apple (Anacardium occidentale L.) in cattle. Acta Sci. Vet. 2012;40:1–5. [Google Scholar]
- 23.Gnanglè C.P., Glèlè Kakaï R., Assogbadjo A.E., Vodounnon S., Yabi J.A., Sokpon N. Tendances Climatiques Passées, Modelisation, Perception et Adaptations Locale au Bénin. Climatologie. 2011;8:27–40. doi: 10.4267/climatologie.259. [DOI] [Google Scholar]
- 24.AOAC . eighteenth ed. vol. 2. Assoc Off Anal Chem; Arlington, VA, USA: 2005. (Official Methods of Analysis). [Google Scholar]
- 25.Van Soest P.J., Robertson J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber, and non starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- 26.AOAC Official method 925.35 sucrose in fruit and fruit product. Assoc Off Anal Chem. Arlington, VA, USA. 2000 [Google Scholar]
- 27.Chiacchio M.F., Tagliamonte S., Visconti A., Ferracane R., Mustafa A., Vitaglione P. Baobab-fruit shell and fibrous filaments are sources of antioxidant dietary fibers. Molecules. 2022;27:5563. doi: 10.3390/molecules27175563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theodorou M.K., Williams B.A., Dhanoa M.S., McAllan A.B., France J.A. Simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994;48:185–197. [Google Scholar]
- 29.Lowe S.E., Theodorou M.K., Trincv A.P.J., Hespell R.B. Growth of anaerobic rumen fungi on defined and semi-defined media lacking rumen fluid. J. General Microbio. 1985;131:2225–2229. doi: 10.1099/00221287-131-9-2225. [DOI] [Google Scholar]
- 30.Theodorou M.K., Williams B.A., Dhanoa M.S., McAllan A.B., France J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994;48:185–197. [Google Scholar]
- 31.Calabrò S., Oteri M., Vastolo A., Cutrignelli M.I., Todaro M., Chiofalo B., Gresta F. Amaranthus grain as a new ingredient in diets for dairy cows: productive, qualitative, and in vitro fermentation traits. J. Sci. Food Agric. 2022;102:4121–4130. doi: 10.1002/jsfa.11761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menke K.H., Stengass H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988;28:7–55. [Google Scholar]
- 33.Groot J.C., Cone J.W., Williams B.A., Debersaques F.M., Lantinga E.A. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feedstuff. Anim. Feed Sci. Technol. 1996;64:77–89. doi: 10.1016/S0377-8401(96)01012-7. [DOI] [Google Scholar]
- 34.Bauer E., Williams B.A., Voigt C., Mosenthin R., Verstegen M.W.A. Microbial activities of faeces from unweaned and adult pigs, in relation to selected fermentable carbohydrates. J. Anim. Sci. 2001;73:313–332. doi: 10.1017/s135772980005829x. [DOI] [Google Scholar]
- 35.Murugan S., Kumar S., Rajan J.K., Varshney L., Kumar V. Cashew apple (anacardium occidentale): evaluation of physical and chemical composition Indian J. Nat. Sci. 2015;5:4255–4259. [Google Scholar]
- 36.Tai V.A., Tuan B.Q., Thuy V.T.T., Trach N.X. Use of cashew apple fruit silage in the cattle fattening diet. Livest. Res. Rural Dev. 2020;32:1–5. [Google Scholar]
- 37.Araújo A.R., Costa J.B., Rogério M.C.P., Carneiro M.S.S., Muniz L.C., Fontenele R.M., Silva V.L. Dehydrated cashew apple in different grinding sizes to sheep. Acta Scientiarum Ani.l Sci. 2022;44:1–7. doi: 10.4025/actascianimsci.v44i1.54398. [DOI] [Google Scholar]
- 38.Raseel K., Chacko B., Sunanda C., Dildeep V., Abraham J. Nutrient evaluation of energy rich unconventional feeds available in Wayanad. Int. J. Sci. Nat. 2018;9:117–118. [Google Scholar]
- 39.Bhamare K.S., Dildeep V., Senthil Murugan S., Chavan S.J. Nutritive evaluation of Cashew apple waste in broilers. Int. J. Sci. Nat. 2016;7(3):629–632. [Google Scholar]
- 40.Sreekutty P.S., Senthil Murugan S., Dildeep V., Chacko B., Balusamy C. Chemical composition of cashew apple waste. Shanlax Int. J. Vet. Sci. 2017;5:2321–6387. [Google Scholar]
- 41.Okpanachi U., Attah S., Oyewole B.O. Effects of varieties of dried cashew pulp on in vitro fermentation parameters and volume of gas produced at different incubation time. Agric. Food. 2016;4:530–536. [Google Scholar]
- 42.Kiatti D., Vastolo A., Koura B.I., Vitaglione P., Cutrignelli M.I., Calabrò S. The chemical characteristics and in vitro degradability of pineapple by-products as potential feed for ruminants. Animals. 2023;13(20):1–15. doi: 10.3390/ani13203238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pragna P., Chauhan S.S., Sejian V., Leury B.J., Dunshea F.R. Climate change and goat production: enteric methane emission and its mitigation. Animals. 2018;8(12):235. doi: 10.3390/ani8120235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giamouri E., Zisis F., Mitsiopoulou C., Christodoulou C., Pappas A.C., Simitzis P.E., Kamilaris C., Galliou F., Manios T., Mavrommatis A., Tsiplakou E. Sustainable strategies for greenhouse gas emission reduction in small ruminants farming. Sustain. Times. 2023;15(5):4118. doi: 10.3390/su15054118. [DOI] [Google Scholar]
- 45.Okonkwo C.E., Hussain S.Z., Onyeaka H., Adeyanju A.A., Nwonuma C.O., Bashir A.A., Farooq A., Zhou C. Lignin polyphenol: from biomass to innovative food applications, and influence on gut microflora. Ind. Crops Prod. 2023;206(3) [Google Scholar]
- 46.Vastolo A., Calabrò S., Carotenuto D., Cutrignelli M.I., Kiatti D., Tafuri S., Ciani F. Maca (lepidium meyenii): in vitro evaluation of rumen fermentation and oxidative stress. Fermentation. 2023;9(6):568. doi: 10.3390/fermentation9060568. [DOI] [Google Scholar]
- 47.Formato M., Cimmino G., Brahmi-Chendouh N., Piccolella S., Pacifico S. Polyphenols for livestock feed: sustainable perspectives for animal husbandry? Molecules. 2022;27(22):7752. doi: 10.3390/molecules27227752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bešlo D., Došlić G., Agić D., Rastija V., Šperanda M., Gantner V., Lučić B. Polyphenols in ruminant nutrition and their effects on reproduction. Antioxidants. 2022;11(5):1–22. doi: 10.3390/antiox11050970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tian M., Wang J., Hayat K., Lu H., Cong L., Huang M. Fabrication of pea protein–naringin Pickering emulsion to mask the bitterness of naringin. Int. J. Food Sci. Technol. 2023;58:3838–3849. [Google Scholar]
- 50.Shilpa V.S., Shams R., Dash K.K., Pandey V.K., Dar A.H., Mukarram S.A., Harsányi E., Kovács B. Phytochemical properties, extraction, and pharmacological benefits of naringin: a review. Molecules. 2023;28:5623. doi: 10.3390/molecules28155623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yanza Y.R., Fitri A., Suwignyo B., Elfahmi, Hidayatik N., Kumalasari N.R., Irawan A., Jayanegara A. The utilisation of tannin extract as a dietary additive in ruminant nutrition: a meta-analysis. Animals. 2021;11(11):3317. doi: 10.3390/ani11113317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manoni M., Terranova M., Amelchanka S., Pinotti L., Silacci P., Tretola M. Effect of ellagic and gallic acid on the mitigation of methane production and ammonia formation in an in vitro model of short-term rumen fermentation. Anim. Feed Sci. Technol. 2023;305:1–9. [Google Scholar]
- 53.Aboagye I.A., Oba M., Koenig K.M., Zhao G.Y., Beauchemin K.A. Use of gallic acid and hydrolyzable tannins to reduce methane emission and nitrogen excretion in beef cattle fed a diet containing alfalfa silage. J. Anim. Sci. 2019;97(5):2230–2244. doi: 10.1093/jas/skz101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author and will be made available on request.