Abstract
Significant levels of adenovirus (Ad)-mediated gene transfer occur only in immature muscle or in regenerating muscle, indicating that a developmentally regulated event plays a major role in limiting transgene expression in mature skeletal muscle. We have previously shown that in developing mouse muscle, expression of the primary Ad receptor CAR is severely downregulated during muscle maturation. To evaluate how global expression of CAR throughout muscle affects Ad vector (AdV)-mediated gene transfer into mature skeletal muscle, we produced transgenic mice that express the CAR cDNA under the control of the muscle-specific creatine kinase promoter. Five-month-old transgenic mice were compared to their nontransgenic littermates for their susceptibility to AdV transduction. In CAR transgenics that had been injected in the tibialis anterior muscle with AdVCMVlacZ, increased gene transfer was demonstrated by the increase in the number of transduced muscle fibers (433 ± 121 in transgenic mice versus 8 ± 4 in nontransgenic littermates) as well as the 25-fold increase in overall β-galactosidase activity. Even when the reporter gene was driven by a more efficient promoter (the cytomegalovirus enhancer–chicken β-actin gene promoter), differential transducibility was still evident (893 ± 149 versus 153 ± 30 fibers; P < 0.001). Furthermore, a fivefold decrease in the titer of injected AdV still resulted in significant transduction of muscle (253 ± 130 versus 14 ± 4 fibers). The dramatic enhancement in AdV-mediated gene transfer to mature skeletal muscle that is observed in the CAR transgenics indicates that prior modulation of the level of CAR expression can overcome the poor AdV transducibility of mature skeletal muscle and significant transduction can be obtained at low titers of AdV.
Virus vectors used for gene transfer to skeletal muscle must be able to infect postmitotic cells. In addition, replacement therapy of genetic diseases such as Duchenne muscular dystrophy requires a large viral insert capacity in order to accommodate large cDNAs (e.g., the 13.9-kb dystrophin cDNA). Adenovirus vectors (AdV) fulfill both criteria and have proven to be useful in gene therapy applications directed at muscle (16). However, a major constraint in the use of AdV is that efficient gene transfer occurs only in immature muscle or in regenerating muscle (2, 9, 14, 17). The low level of transducibility of mature skeletal muscle may be partly due to inefficient binding of adenovirus (Ad) particles to the cell surface.
The entry of AdV into cells involves two different types of receptors: a high-affinity primary receptor, the coxsackievirus and Ad receptor CAR (3, 28), and lower-affinity secondary receptors that consist of the αV-containing integrins (αVβ3 and αVβ5) (23, 31) and perhaps also α5β1 (7). The structural features of Ad capsid that are implicated in AdV binding to the cell surface are the fiber protein and the penton base protein. AdV binds to its primary receptor through the knob domain at the tip of the fiber protein projecting from the Ad capsid (5, 18, 26, 27). Analysis of the crystal structure of the CAR-Ad fiber knob complex has recently shown that three CAR monomers bind each knob trimer (5). The N-terminal portion of CAR (amino acids 25 to 125, the immunoglobulin V domain) is sufficient for binding knob in solution and acts as a potent inhibitor of viral infection for cells in culture (11).
The AdV penton base consists of five identical RGD-containing subunits (25) that mediate binding to the heterodimeric cell surface receptors, integrins. Once bound, AdV is internalized via clathrin-coated pits (12). Not only does recombinant penton base protein block internalization of AdV, but RGD-containing peptides also inhibit AdV-mediated transduction (31). However, prior incubation of cells with the penton base protein does not prevent AdV attachment to the cell surface (31). Thus, the first stage of AdV infection has been viewed as consisting of two sequential steps involving an attachment receptor (CAR) and an internalization receptor (integrins). At high input doses of AdV (a high multiplicity of infection), cells that do not express CAR can still be infected, albeit with a much lower transduction efficiency. Under these conditions the low-affinity binding of penton base to integrins may be sufficient for some CAR-independent binding and uptake of AdV. Conversely there is some evidence that CAR can mediate AdV uptake through an integrin-independent pathway, as AdV in which the RGD site in the penton base has been ablated can still be internalized, at a rate dependent upon the fiber receptor concentration (10).
The decrease in gene transfer that occurs with maturation of skeletal muscle suggests that a developmentally regulated event plays a major role in limiting transgene expression in mature skeletal muscle. Our earlier studies had shown that although αVβ3 and αVβ5 levels decrease by about 70% during myogenesis and maturation of muscle fibers in the mouse (1), their lower levels could not account for the 95% decrease in AdV transduction between the neonatal period and 4 to 6 weeks of age (2). We subsequently showed that in developing mouse muscle, expression of the primary Ad receptor CAR is severely downregulated during muscle maturation, with CAR transcripts being barely detectable in the adult muscle (24). Furthermore, we demonstrated that forced expression of CAR in mouse myoblasts, followed by transfer of these myoblasts to syngeneic host muscle, resulted in the formation of myofibers with increased susceptibility to AdV transduction (24). These results suggested that CAR expression limits the susceptibility of myofibers to AdV transduction. The myoblast transfer experiments, however, did not permit us to address certain issues. For example, a relatively small number of CAR-expressing myofibers were obtained by this approach, making it impossible to determine the level of CAR expression required to increase the susceptibility of individual myofibers to AdV transduction. In addition, the majority of CAR-expressing myofibers with increased susceptibility to AdV transduction were of smaller diameter than nontransduced fibers. This raised the possibility that fibers with increased susceptibility to AdV transduction were forming predominantly from injected myoblasts and may not have been fully representative of myofibers in intact, mature skeletal muscle. Several factors, including the extensive basal lamina surrounding mature myofibers, may limit the access of exogenously introduced virus to the muscle fiber plasma membrane. To evaluate how global expression of CAR throughout muscle affects AdV-mediated gene transfer into mature skeletal muscle, we produced transgenic mice that express the CAR cDNA in a muscle-specific manner. With these mice, we evaluated the transducibility of mature skeletal muscle by AdV. The continued expression of CAR on muscle plasma membranes dramatically improved the extent of AdV-mediated gene transfer to skeletal muscle of 5- to 6-month-old transgenic mice.
MATERIALS AND METHODS
Production and genotyping of transgenic mice.
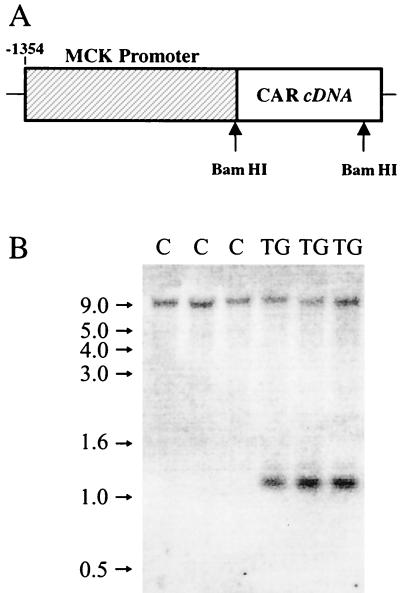
A cDNA containing the full-length coding sequence for Mus musculus CAR mRNA (GenBank accession number Y10320) that was generated by reverse transcriptase PCR (RT-PCR) was described previously (24). The full-length CAR cDNA (nucleotides 1 to 1098) was cloned downstream of regulatory sequences of a muscle-specific creatine kinase (MCK) gene (GenBank accession number AF188002). This fragment, which spans the region from −1,354 to +1 bp from the transcription initiation site, has previously been described (19) and contains the MCK E1 enhancer and promoter, but not the E2 enhancer found in the first intron of the gene. Transgenic mice were generated by pronuclear microinjection. Founder mice were identified by genotyping of tail DNA for the presence of MCK-CAR fusion sequences. The founders were bred with B6C3F1 mice to verify transgene expression by Northern and Western blot analyses. A single founder expressed CAR mRNA and protein. Subsequently, all mice were genotyped for the presence of CAR cDNA by Southern blot analysis of tail DNA following digestion with BamHI, which yielded an ∼1-kb fragment (Fig. 1). Hemizygous CAR transgenic mice between the ages of 5 and 6 months were used for AdV transduction experiments. These animals were normal in development, growth, and behavior.
FIG. 1.
Depiction of the construct used to generate the transgenic mice (A) and results of genotyping of the mice by Southern blot analysis (B). (B) Genomic DNA was digested with the restriction enzyme BamHI, electrophoresed on a 1% agarose gel, transferred to a nylon membrane, and hybridized with CAR cDNA. A BamHI fragment of ∼1 kb is present in transgenic mice (TG) but absent in control, nontransgenic littermates (C). The high-molecular-weight fragment (∼9 kb) that is visible in all lanes represents the endogenous CAR gene that cross-hybridizes with the CAR cDNA. Numbers on the left indicate the position of molecular weight markers (1-kb ladder) included in a flanking lane.
Analysis of CAR transgene expression.
To determine the level of CAR expression, skeletal muscle was homogenized in TRIzol (Life Technologies, Burlington, Ontario, Canada) to extract total RNA according to the manufacturer's instructions. Northern blot analysis was performed as described previously (24) using total RNA (10 μg per sample) that was electrophoresed on a formaldehyde agarose gel, followed by hybridization to the CAR cDNA probe. Equal loading of samples was verified by hybridization with a cDNA probe for the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase.
For Western blot analysis, tissues were excised from mice and homogenized in sample buffer as previously described (24). Protein samples (5 or 10 μg as indicated in figure legends) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 10% (wt/vol) acrylamide gels, followed by electrotransfer to nitrocellulose as described previously (24). The production, purification, and characterization of the polyclonal antibody against CAR has been previously described in detail (24).
In vivo AdV transduction.
The recombinant AdVCMVlacZ has been described previously (2). The AdV recombinant containing an Escherichia coli lacZ reporter gene driven by the cytomegalovirus (CMV) enhancer–chicken β-actin gene promoter was a kind gift of James Wilson (Philadelphia, Pa.) (34). The AdV preparations were purified by two centrifugations over discontinuous cesium chloride gradients (2). The virus band was collected, diluted in phosphate-buffered saline, and desalted on a Sephadex G25 column (Pharmacia, Montreal, Quebec, Canada). All injections were performed with freshly purified AdV, and within each experiment, all groups of animals received the same preparation of AdV at the indicated titer. For all preparations of AdV, the ratio of total particles (as determined by optical density) to infectious titer (as determined by total cytopathic effect on 293 A cells) was between 50:1 and 100:1. Immunosuppression was carried out as described previously (22). All mice received daily subcutaneous injections of FK506 (5 mg/kg of body weight) starting 2 days prior to AdV administration and continuing until they were euthanized. Hind limbs (tibialis anterior muscles) of adult mice (5 to 6 months old) were injected percutaneously with a single deposit of 25 μl of recombinant AdV at a titer of 1012 particles/ml. In some animals, the contralateral tibialis anterior was injected with AdV at a titer of 2 × 1011 particles/ml. Eight days after AdV injection, the animals were euthanized and AdV-transduced muscle fibers were identified by histochemistry for β-galactosidase activity. The number of β-galactosidase-positive fibers in the entire tibialis anterior and extensor digitorum longus (EDL) muscles was quantitated by counting the positive fibers under light microscopy. For quantitation of β-galactosidase activity, 60 sections of 10-μm thickness each were prepared from the region immediately adjacent to the one that had been sampled for histochemistry. The frozen muscle sections were homogenized in 100 mM phosphate buffer (pH 7.8) and 0.2% Triton X-100 for chemiluminescent detection of β-galactosidase according to the manufacturer's instructions (Galactolight; Tropix, Inc., Bedford, Mass.). A BioOrbit luminometer (Turku, Finland) was used to measure light emission. A standard curve was generated by serial dilutions of pure β-galactosidase (Boehringer Mannheim, Laval, Quebec, Canada), and the muscle β-galactosidase activity was converted to nanograms of enzyme. Differences between groups were determined either by t test (unpaired) for the AdVCMVlacZ-injected groups or by analysis of variance (ANOVA) for all other groups, with statistical significances being defined as P values of <0.05.
RESULTS
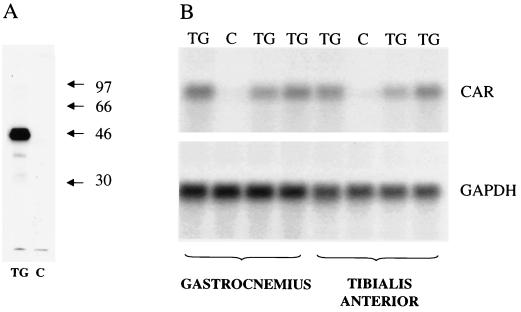
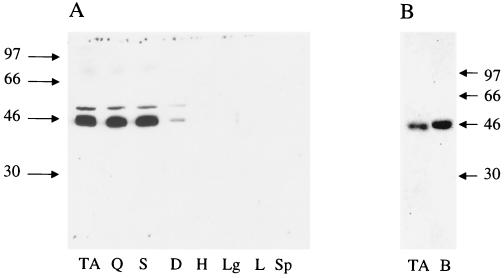
We had previously demonstrated that high-level, muscle-specific transgene expression can be obtained using 5′ regulatory sequences from the MCK gene (19). In this study we used the same 1,354-bp fragment from the MCK gene to regulate the expression of a full-length CAR cDNA in transgenic mice (Fig. 1). Transgenic mice were produced that expressed moderately high levels of CAR in skeletal muscle as assessed by Northern and Western blotting. As shown in Fig. 2, little or no expression of the endogenous CAR transcript or protein was detected in the adult control, nontransgenic mouse littermates, confirming previous results (4, 29), including our own (24). In contrast, the CAR transgene that was under the control of the MCK1354 promoter had sustained expression even in adult tissue. Furthermore, of several adult tissues tested, significant expression of CAR was only detectable in skeletal muscle (Fig. 3A). To some extent this is a question of antibody sensitivity, as on prolonged exposure, traces of CAR expression could be seen in liver and heart tissue (data not shown). Failure to detect more substantial expression of CAR protein in lung, liver, and heart is somewhat surprising as CAR transcripts are readily detectable in these tissues in nontransgenic mice (4, 28), and some protein accumulation would be expected from the endogenous gene. Clearly, CAR expression in muscles of adult transgenic mice far exceeds levels of endogenous expression seen in any adult tissue tested. However, expression of CAR in muscles of the transgenic mice is of the same order of magnitude as expression from the endogenous gene in 10-day-postnatal mouse brain (Fig. 3B).
FIG. 2.
Expression of CAR in transgenic mice and control, nontransgenic littermates as analyzed by Western (A) and Northern (B) blotting. (A) Samples (10 μg of protein each) of gastrocnemius muscle from an adult transgenic mouse (TG) and a control nontransgenic littermate (C) were run on an SDS–10% polyacrylamide gel. Proteins in the gel were transferred to nitrocellulose and incubated with antiserum to the extracellular domain of mouse CAR. A major band corresponding to CAR is visible at ∼46 kDa in the transgenic muscle but absent in control muscle. (B) Samples (10 μg of total RNA each) from the gastrocnemius and tibialis anterior muscles of three transgenic mice (TG) and one control nontransgenic littermate (C) were electrophoresed on a formaldehyde agarose gel and hybridized with CAR cDNA and glyceraldehyde-3-phosphate dehydrogenase cDNA probes as indicated.
FIG. 3.
(A) Expression of CAR in various tissues of adult (5-month-old) transgenic mice. Samples (10 μg of protein each) of tissue dissolved in Laemmli sample buffer were loaded in each lane of an SDS–10% PAGE gel. Proteins in the gel were transferred to nitrocellulose and incubated with antiserum to the extracellular domain of mouse CAR. From left to right, the samples were from tibialis anterior (TA), quadriceps (Q), soleus (S), diaphragm (D), heart (H), lung (Lg), liver (L), and spleen (Sp) tissue. Numbers on the left indicate the position of molecular weight markers included in a flanking lane. (B) Comparison of the level of expression of CAR in tibialis anterior muscle from a 5-month-old transgenic mouse (lane TA) with expression in brain of a 10-day-old control, nontransgenic littermate (lane B). Samples (5 μg of protein each) of tissue dissolved in Laemmli sample buffer were loaded in each lane of an SDS–10% PAGE gel. Proteins in the gel were transferred to nitrocellulose and incubated with antiserum to the extracellular domain of mouse CAR. Numbers on the right of the panel indicate the position of molecular weight markers included in a flanking lane.
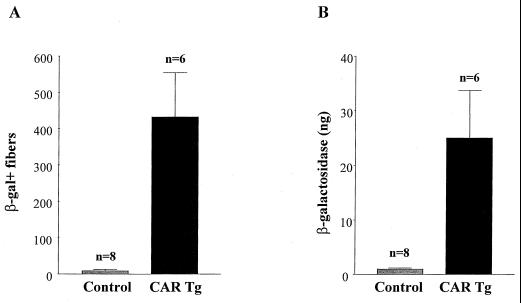
The transducibility by recombinant AdV of adult mouse skeletal muscle is much lower than that of skeletal muscle of neonates (2, 9, 14). This decrease in transducibility is evident as early as 2 weeks of age. To determine the susceptibility of the CAR transgenic mice to transduction by AdV, 5- to 6-month-old transgenic mice were compared to their nontransgenic littermates that served as controls. Animals were injected with a recombinant (E1 and E3 deleted) AdV carrying a CMVlacZ expression cassette. To minimize immune reaction to transgene expression, animals were treated with FK506. Euthanasia was performed 8 days later; the injected muscles were sectioned and stained histochemically for β-galactosidase activity. Muscle samples from CAR transgenic mice had a significantly higher number of β-galactosidase-positive fibers than those of nontransgenic controls (Fig. 4A). In accordance with previous data, nontransgenic adult mice had 8 ± 4 positive fibers; in contrast, the CAR transgenics had 433 ± 121 positive fibers (P = 0.0015; unpaired t test). The increase in the number of transduced fibers was accompanied by a similar increase in overall β-galactosidase activity (Fig. 4B).
FIG. 4.
AdV transducibility of CAR transgenics by AdVCMVlacZ. (A) Quantitation of the number of β-galactosidase-positive fibers, following a single injection of AdVCMVlacZ (1012 particles/ml) into the tibialis anterior muscle of 5- to 6-month-old hemizygous CAR transgenic mice compared to control, nontransgenic littermates (P = 0.0015; unpaired t test). (B) The muscles that had been examined in the results shown in panel A were sectioned, and β-galactosidase activity was determined as described in Materials and Methods. Results are means ± standard error.
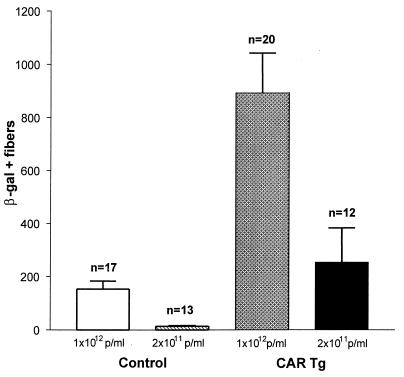
Ad-mediated gene expression in adult muscle is affected by the nature of the promoter that is used to regulate the transferred gene. It had previously been shown that the use of a hybrid promoter comprising the chicken β-actin promoter and the CMV enhancer resulted in better gene expression in adult skeletal muscle than that obtained with the CMV promoter (15). To test whether sustained expression of CAR in adult skeletal muscle influences the transducibility by AdV carrying the lacZ gene under the control of this alternative promoter, 5-month-old animals were injected in the tibialis anterior muscle with an AdV recombinant containing an E. coli lacZ reporter gene driven by the CMV enhancer–chicken β-actin gene promoter. Even with this more-efficient promoter, there was differential transducibility of skeletal muscle that depended on CAR expression: although nontransgenic littermates had an average of 153 ± 30 positive fibers, CAR transgenics had 893 ± 149 positive fibers (P < 0.001; ANOVA) (Fig. 5). In one animal, a single 25-μl injection of AdV resulted in the transduction of the entire tibialis anterior and EDL muscles (3,000 muscle fibers) (Fig. 6A).
FIG. 5.
AdV transducibility of CAR transgenics by AdV expressing lacZ under the control of the CMV enhancer–β-actin promoter. Quantitation of the number of β-galactosidase-positive fibers following a single 25-μl injection of either 1 × 1012 or 2 × 1011 particles/ml into the tibialis anterior muscle of 5- to 6-month-old hemizygous CAR transgenic mice compared to control, nontransgenic littermates. At the higher dose, differences between the control group and the CAR transgenics were significant (P < 0.001; ANOVA). Note that transducibility obtained with the CAR transgenics injected with the lower dose is similar to that with controls receiving the higher dose of AdV (differences between these two groups are not significant by ANOVA, followed by the Bonferroni posttest). Results are means ± standard error.
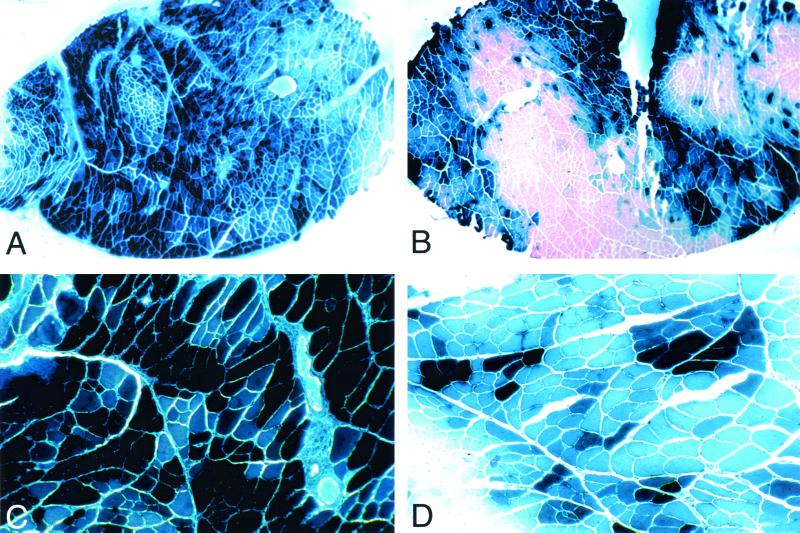
FIG. 6.
AdV transducibility of CAR transgenics by AdV expressing lacZ under the control of the CMV enhancer–β-actin promoter. Photomicrographs of mouse tibialis anterior muscle showing histochemical staining of muscle fibers for β-galactosidase activity subsequent to a single injection of AdV at a titer of 1 × 1012 (A and C) or 2 × 1011 (B and D) particles/ml in the contralateral muscle are shown. Note that at the higher dose of AdV, all fibers express β-galactosidase.
We hypothesized that the presence of increased numbers of AdV attachment receptors might obviate the need to inject large doses of AdV to obtain a meaningful number of transduced muscle fibers. The mice that had been injected in the above experiment with AdV at 1 × 1012 particles/ml also received AdV in the contralateral tibialis anterior muscle at the lower titer of 2 × 1011 particles/ml (injectate containing a total of 5 × 109 particles). Increased transduction due to expression of CAR was obtained even at these lower titers (Fig. 5 and 6B) with an average of 253 ± 130 fibers being positive in the CAR transgenics as opposed to 14 ± 4 fibers in the nontransgenic littermates.
DISCUSSION
A major issue in gene therapy is efficient and widespread delivery of the therapeutic gene to the target tissue. AdV-mediated gene transfer occurs inefficiently in adult skeletal muscle. The transcript for the primary AdV receptor CAR is undetectable by Northern blot analysis of skeletal muscle tissue of human (3, 28), mouse (4, 24, 28), and rat (8) origin. Even when sensitive detection methods are used, such as RT-PCR (24) and competitive RT-PCR (8) to estimate the abundance of the CAR mRNA, the levels of CAR transcript are extremely low in adult mouse skeletal muscle. Thus, the lack of CAR may be a major impediment to efficient gene transfer to mature skeletal muscle.
Forced expression of exogenous CAR has recently been used to facilitate the entry of AdV serotype 5 into a number of cell types that are not generally susceptible to AdV, such as primary fibroblasts (13), lymphocytes (20), myoblasts (24), and tumor cells (21). However, it was unclear whether CAR could effect an increase in gene transfer in vivo where presumably greater barriers exist that can hinder the interaction between AdV and the cell surface. In this regard, mature skeletal muscle fibers are surrounded by a well-developed basal lamina that could theoretically limit the access of AdV to the muscle fiber plasmalemma. In order to address the question of whether the absence of CAR is a major limiting factor in AdV-mediated gene transfer to mature skeletal muscle in vivo, we produced transgenic mice that maintained a relatively high level of CAR expression in their skeletal muscle. In the present study, when AdVCMVlacZ was injected intramuscularly at a single site into the tibialis anterior of 5- to 6-month-old animals, the number of β-galactosidase-positive fibers was consistently higher in the CAR-expressing transgenics than in their nontransgenic littermates (Fig. 5). The dramatic enhancement in AdV transducibility indicates that upregulation of CAR can overcome local constraints to tissue penetration by the AdV in these relatively old animals.
The experimental results obtained in vivo measure the transducibility of the tissue: the final readout is a consequence of both the entry of AdV and the subsequent expression of the expression cassette contained within the virus vector. Thus, a low level of transducibility as determined by low levels of transgene expression may result from lack of virus attachment-internalization and/or transcriptional inefficiency within specific tissues of the transgene promoter that is used. We specifically examined this issue by comparing in CAR transgenic mice and control littermates the transduction efficiency that was obtained when the E. coli lacZ reporter gene was placed under the control of the hybrid CMV–β-actin promoter. As expected, this transcriptional unit is expressed at higher levels in mature skeletal muscle (15), resulting in a higher number of β-galactosidase-positive fibers in the muscles of control mice (153 ± 30) than in the muscles of those injected with AdVCMVlacZ (8 ± 4) (Fig. 4 and 5). Remarkably, the presence of CAR also influenced the levels of transducibility that were attained in this experiment, with an average sixfold increase in the number of β-galactosidase-positive fibers in the muscles of CAR transgenics. In addition, in one transgenic mouse, a single injection of 2.5 × 1010 viral particles resulted in the transduction of the entire tibialis anterior and EDL muscles, achieving the same extent of transduction that is usually only observed in neonatal mice (Fig. 6). In this context, the efficiency of AdV-mediated gene transfer has been ascribed to the presence of myoblasts in the developing muscle (30). Moreover, it was suggested that multinucleated myofibers were incapable of being transduced by AdV (29). However, our results clearly demonstrate that under appropriate biological conditions, extremely efficient AdV transduction of mature skeletal muscle can be achieved. Furthermore, these results also suggest that modulation of CAR levels can significantly decrease the dose of administered vector needed to obtain acceptable levels of gene transfer for therapeutic purposes.
A potential means of circumventing the poor transducibility of adult skeletal muscle would be the use of adenovirus vectors engineered to have modified tissue tropism. One such vector is AdZ.F(pK7), in which lysine moieties have been incorporated into the AdV fiber protein to target surface receptors containing heparan sulfate (32, 33). Although no significant difference was observed in transduction efficiency between AdV with wild-type fiber protein (AdZ) and AdZ.F(pK7) in neonatal mice injected in the hind limb, there was a fourfold increase in efficiency in the adult mice (4 to 5 months of age) that were injected in the EDL muscle (6). Curiously, in the neonates, unlike the relatively even distribution of AdZ, the fibers that were transduced with AdZ.F(pK7) were those at the periphery of muscle fascicles and the perimysial connective tissue (6). In the adult, only a proportion of muscle fibers were transduced, perhaps as a consequence of the occupancy of the receptors by endogenous ligands and components of the extracellular matrix. In a separate study by van Deutekom and colleagues, the efficiency of transduction of adult normal and dystrophic muscle with AdZ.F(pK7) was shown to be significantly lower than what is commonly obtained with wild-type fiber-containing AdV in neonate skeletal muscle (29). In this regard, our results clearly show that upregulation of CAR can lead to complete transduction of the tibialis anterior and EDL of adult 5- to 6-month-old mice (Fig. 6A).
We produced transgenic mice that express CAR in order to address specific issues in AdV-mediated gene transfer to adult skeletal muscle (lack of AdV receptors and presence of physical barriers). The dramatic enhancement in AdV-mediated gene transfer to mature skeletal muscle that is observed with these CAR transgenics indicates that prior modulation of the level of CAR expression results in extremely efficient AdV transducibility of mature skeletal muscle. In the context of gene therapy directed to human muscle, a transient increase in CAR expression could be achieved either through activation of the transcription of endogenous CAR gene or as part of a two-step gene therapy protocol, by regulatable expression of CAR delivered through a different viral vector such as the adeno-associated virus.
ACKNOWLEDGMENTS
J.N., N.L., H.L., and P.C.H. contributed equally to this work.
We acknowledge the expert technical assistance of C. Allen, C. Guérin, U. Klutzny, S. Prescott, and N. Rieger.
This work was supported by funding from the Muscular Dystrophy Association (United States), Deutsche Forschungsgemeinschaft (Bonn, Germany), and the Duchenne Parents Project of Germany (aktion benni & co e.V.). J.N. is a Research Scholar of the Fonds de la recherche en santé du Québec.
REFERENCES
- 1.Acsadi G, Jani A, Huard J, Blaschuk K, Massie B, Holland P, Lochmuller H, Karpati G. Cultured human myoblasts and myotubes show markedly different transducibility by replication-defective adenovirus recombinants. Gene Ther. 1994;1:338–340. [PubMed] [Google Scholar]
- 2.Acsadi G, Jani A, Massie B, Simoneau M, Holland P, Blaschuk K, Karpati G. A differential efficiency of adenovirus-mediated in vivo gene transfer into skeletal muscle cells of different maturity. Hum Mol Genet. 1994;3:579–584. doi: 10.1093/hmg/3.4.579. [DOI] [PubMed] [Google Scholar]
- 3.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 4.Bergelson J M, Krithivas A, Celi L, Droguett G, Horwitz M S, Wickham T, Crowell R L, Finberg R W. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J Virol. 1998;72:415–419. doi: 10.1128/jvi.72.1.415-419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bewley C M, Springer K, Zhang Y B, Freimuth P, Flanagan J M. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science. 1999;286:1579–1583. doi: 10.1126/science.286.5444.1579. [DOI] [PubMed] [Google Scholar]
- 6.Bouri K, Feero W G, Myerburg M M, Wickham T J, Kovesdi I, Hoffman E P, Clemens P R. Polylysine modification of adenoviral fiber protein enhances muscle cell transduction. Hum Gene Ther. 1999;10:1633–1640. doi: 10.1089/10430349950017635. [DOI] [PubMed] [Google Scholar]
- 7.Davison E, Diaz R M, Hart I R, Santis G, Marshall J F. Integrin α5β1-mediated adenovirus infection is enhanced by the integrin-activating antibody TS2/16. J Virol. 1997;71:6204–6207. doi: 10.1128/jvi.71.8.6204-6207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fechner H, Haack A, Wang H, Wang X, Eizema K, Pauschinger M, Schoemaker R, Veghel R, Houtsmuller A, Schultheiss H P, Lamers J, Poller W. Expression of coxsackie adenovirus receptor and αv-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther. 1999;6:1520–1535. doi: 10.1038/sj.gt.3301030. [DOI] [PubMed] [Google Scholar]
- 9.Feero W G, Rosenblatt J D, Huard J, Watkins S C, Epperly M, Clemens P R, Kochanek S, Glorioso J C, Partridge T A, Hoffman E P. Viral gene delivery to skeletal muscle: insights on maturation-dependent loss of fiber infectivity for adenovirus and herpes simplex type 1 viral vectors. Hum Gene Ther. 1997;8:371–380. doi: 10.1089/hum.1997.8.4-371. [DOI] [PubMed] [Google Scholar]
- 10.Freimuth P. A human cell line selected for resistance to adenovirus infection has reduced levels of the virus receptor. J Virol. 1996;70:4081–4085. doi: 10.1128/jvi.70.6.4081-4085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freimuth P, Springer K, Berard C, Hainfeld J, Bewley M, Flanagan J. Coxsackievirus and adenovirus receptor amino-terminal immunoglobulin V-related domain binds adenovirus type 2 and fiber knob from adenovirus type 12. J Virol. 1999;73:1392–1398. doi: 10.1128/jvi.73.2.1392-1398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greber U F, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 13.Hidaka C, Milano E, Leopold P L, Bergelson J M, Hackett N R, Finberg R W, Wickham T J, Kovesdi I, Roelvink P, Crystal R G. CAR-dependent and CAR-independent pathways of adenovirus vector-mediated gene transfer and expression in human fibroblasts. J Clin Investig. 1999;103:579–587. doi: 10.1172/JCI5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huard J, Lochmuller H, Acsadi G, Jani A, Holland P, Guerin C, Massie B, Karpati G. Differential short-term transduction efficiency of adult versus newborn mouse tissues by adenoviral recombinants. Exp Mol Pathol. 1995;62:131–143. doi: 10.1006/exmp.1995.1015. [DOI] [PubMed] [Google Scholar]
- 15.Ishii A, Hagiwara Y, Saito Y, Yamamoto K, Yuasa K, Sato Y, Arahata K, Shoji S, Nonaka I, Saito I, Nabeshima Y, Takeda S. Effective adenovirus-mediated gene expression in adult murine skeletal muscle. Muscle Nerve. 1999;22:592–599. doi: 10.1002/(sici)1097-4598(199905)22:5<592::aid-mus7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Karpati G, Gilbert R, Petrof B J, Nalbantoglu J. Gene therapy research for Duchenne and Becker muscular dystrophies. Curr Opin Neurol. 1997;10:430–435. doi: 10.1097/00019052-199710000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Kass-Eisler A, Falck-Pedersen E, Elfenbein D H, Alvira M, Buttrick P M, Leinwand L A. The impact of developmental stage, route of administration and the immune system on adenovirus-mediated gene transfer. Gene Ther. 1994;1:395–402. [PubMed] [Google Scholar]
- 18.Kirby I, Davison E, Beavil A J, Soh C P, Wickham T J, Roelvink P W, Kovesdi I, Sutton B J, Santis G. Identification of contact residues and definition of the CAR-binding site of adenovirus type 5 fiber protein. J Virol. 2000;74:2804–2813. doi: 10.1128/jvi.74.6.2804-2813.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larochelle N, Lochmuller H, Zhao J, Jani A, Hallauer P, Hastings K E, Massie B, Prescott S, Petrof B J, Karpati G, Nalbantoglu J. Efficient muscle-specific transgene expression after adenovirus-mediated gene transfer in mice using a 1.35 kb muscle creatine kinase promoter/enhancer. Gene Ther. 1997;4:465–472. doi: 10.1038/sj.gt.3300414. [DOI] [PubMed] [Google Scholar]
- 20.Leon R P, Hedlund T, Meech S J, Li S, Schaack J, Hunger S P, Duke R C, DeGregori J. Adenoviral-mediated gene transfer in lymphocytes. Proc Natl Acad Sci USA. 1998;95:13159–13164. doi: 10.1073/pnas.95.22.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li D, Duan L, Freimuth P, O'Malley B W., Jr Variability of adenovirus receptor density influences gene transfer efficiency and therapeutic response in head and neck cancer. Clin Cancer Res. 1999;5:4175–4181. [PubMed] [Google Scholar]
- 22.Lochmuller H, Petrof B J, Pari G, Larochelle N, Dodelet V, Wang Q, Allen C, Prescott S, Massie B, Nalbantoglu J, Karpati G. Transient immunosuppression by FK506 permits a sustained high-level dystrophin expression after adenovirus-mediated dystrophin minigene transfer to skeletal muscles of adult dystrophic (mdx) mice. Gene Ther. 1996;3:706–716. [PubMed] [Google Scholar]
- 23.Mathias P, Wickham T, Moore M, Nemerow G. Multiple adenovirus serotypes use αv integrins for infection. J Virol. 1994;68:6811–6814. doi: 10.1128/jvi.68.10.6811-6814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nalbantoglu J, Pari G, Karpati G, Holland P C. Expression of the primary coxsackie and adenovirus receptor is downregulated during skeletal muscle maturation and limits the efficacy of adenovirus-mediated gene delivery to muscle cells. Hum Gene Ther. 1999;10:1009–1019. doi: 10.1089/10430349950018409. [DOI] [PubMed] [Google Scholar]
- 25.Neumann R, Chroboczek J, Jacrot B. Determination of the nucleotide sequence for the penton-base gene of human adenovirus type 5. Gene. 1988;69:153–157. doi: 10.1016/0378-1119(88)90389-7. [DOI] [PubMed] [Google Scholar]
- 26.Roelvink P W, Mi L G, Einfeld D A, Kovesdi I, Wickham T J. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science. 1999;286:1568–1571. doi: 10.1126/science.286.5444.1568. [DOI] [PubMed] [Google Scholar]
- 27.Santis G, Legrand V, Hong S S, Davison E, Kirby I, Imler J L, Finberg R W, Bergelson J M, Mehtali M, Boulanger P. Molecular determinants of adenovirus serotype 5 fiber binding to its cellular receptor CAR. J Gen Virol. 1999;80:1519–1527. doi: 10.1099/0022-1317-80-6-1519. [DOI] [PubMed] [Google Scholar]
- 28.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Deutekom J C, Cao B, Pruchnic R, Wickham T J, Kovesdi I, Huard J. Extended tropism of an adenoviral vector does not circumvent the maturation-dependent transducibility of mouse skeletal muscle. J Gene Med. 1999;1:393–399. doi: 10.1002/(SICI)1521-2254(199911/12)1:6<393::AID-JGM65>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 30.van Deutekom J C, Floyd S S, Booth D K, Oligino T, Krisky D, Marconi P, Glorioso J C, Huard J. Implications of maturation for viral gene delivery to skeletal muscle. Neuromuscul Disord. 1998;8:135–148. doi: 10.1016/s0960-8966(98)00019-4. [DOI] [PubMed] [Google Scholar]
- 31.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 32.Wickham T J, Roelvink P W, Brough D E, Kovesdi I. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nat Biotechnol. 1996;14:1570–1573. doi: 10.1038/nbt1196-1570. [DOI] [PubMed] [Google Scholar]
- 33.Wickham T J, Tzeng E, Shears L L, Roelvink P W, Li Y, Lee G M, Brough D E, Lizonova A, Kovesdi I. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Haecker S E, Su Q, Wilson J M. Immunology of gene therapy with adenoviral vectors in mouse skeletal muscle. Hum Mol Genet. 1996;5:1703–1712. doi: 10.1093/hmg/5.11.1703. [DOI] [PubMed] [Google Scholar]