Abstract
The 2-((4-(chloromethyl)benzoyl)oxy)benzoic acid (4CH2Cl) is a potential analgesic compound derived from salicylic acid and 4-chloromethyl benzoyl chloride. Characterization required 4CH2Cl for the formulation of tablet dosage forms. This study aims investigate the effect of SSG, PVP-K30, and the combination of SSG*PVP K-30 on the formulation of 4CH2Cl tablets. Additionally, this study aimed to obtain the optimum 4CH2Cl tablet composition. The experiment followed the two-factor simplex lattice design and direct compression method. The analgesic activity of 4CH2Cl in the optimal tablet was investigated using the hot-plate methods. The ANOVA of linear models is acceptable and the polynomial coefficients of quadratic models are similar to those of linear models. The coefficient of the linear model shows that SSG and PVP K-30 increase the Carr index (16.26; 20.61), Hausner ratio (1.19; 1.29), hardness (4.19; 9.39), friability (0.48; 0.67), disintegration time (0.34; 7.50), and drug release (85.29; 97.69). The coefficient of the quadratic model shows that SSG*PVP K-30 increased the Carr index (1.90), Hausner ratio (0.04), hardness (1.88), friability (0.06), and drug release (4.56), and decreased disintegration time (−0.30). SSG and PVP K-30 increased Carr index, Hausner ratio, hardness, friability, disintegration time, and drug release. The combination of SSG*PVP K-30 has the same effect, except that the disintegration time decreased. The optimum tablet formula is 4CH2Cl (300 mg), Ne (75 mg), SSG (33.60 mg), PVP K-30 (22.40 mg), MCC (40 mg), and SDL (up to 800 mg). 4CH2Cl tablets can be a candidate and choice for new analgesic drugs in the future.
Keywords: 4CH2Cl, Tablet, Formulation, Analgesic, Simplex lattice design
Graphical abstract
Highlights
-
•
2-((4-(chloromethyl)benzoyl)oxy)benzoic acid (4CH2Cl).
-
•
Characterization of 4CH2Cl.
-
•
Formulation of 4CH2Cl tablet dosage form.
-
•
Optimum composition of 4CH2Cl tablet.
-
•
4CH2Cl tablet as analgesic.
1. Introduction
4CH2Cl is a salicylic acid derivative originating from salicylic acid and 4-chloromethyl benzoyl chloride. 4CH2Cl has analgesic potential and relatively low gastric ulceration side effects [Sugiarno, 2016; Tamayanti et al., 2016]. Tablets are the best choice for obtaining the solid 4CH2Cl compound with an exact measurable dosage and relatively simple administration (mainly orally). Caroline and colleagues have reported the Pharmacokinetic parameters of this compound, namely: maximum concentration in blood plasma (Cmax 0.53 μg/mL), time to reach maximum concentration in blood plasma (tmax 16.91 min), elimination rate (Kel. 0.02 min−1), time is eliminated in half (t1/2 el. 41.72), and partition coefficient (log P 3.73). The 4CH2Cl powder is white and forms aggregates when stored at ambient temperature (Sugiarno, 2016; Tamayanti et al., 2016).
The novelty of this study is in characterizing and formulating 4CH2Cl as a new analgesic compound in tablet dosage form using excipients that match the characteristics of the 4CH2Cl. The crucial excipients are insulators, disintegrants, and solubilizers. Neusilin (Ne) is insulating agent is used to prevent 4CH2Cl particle aggregation. Sodium starch glycolate (SSG) is a disintegrant that increases the tablet’s ability to disintegrate into granules or powders, supporting the dissolution process during oral administration. SSG is a relatively affordable and easily obtained disintegrating agent. Additionally, SSG particles are round, and the surface morphology is smooth (Hadinugroho et al., 2022b; Sheskey et al., 2017)]. These characteristics are very supportive for making tablets by direct compression. The action of SSG involves particles swelling when hydrated, thereby pushing the surrounding particles to separate from each other (Hadinugroho et al., 2022b; Markl and Zeitler, 2017). PVP K-30 is a solubilizer that increases the solubility of 4CH2Cl by reducing the difference in polarity between the media and 4CH2Cl particles, thus reducing the lipophilicity of 4CH2Cl. A low concentration of PVP K-30 significantly increases the solubility of 4CH2Cl. PVP K-30 is easily soluble and forms a gel before dissolving. PVP K-30 particles have a smooth surface morphology, supporting the manufacture of tablets by direct compression. Additionally, PVP K-30 is a potent solubilizer, making it effective and efficient (Kurakula and Rao, 2020; Sheskey et al., 2017). In general, the current manuscript presents an adjustment on the formula from previous studies on 2-((3-(chloromethyl)benzoyl)oxy)benzoic acid (3CH2Cl) tablets, using tablet excipients with characteristics adjusted to 4CH2Cl to make it more effective and efficient (Hadinugroho et al., 2022a; Hadinugroho et al., 2022a), Based on the yield synthesis experiment, 4CH2Cl is more abundant than 3CH2Cl, which suggests that 4CH2Cl could be more economical for mass production in tablet dosage form.
This study aimed to investigate the effect of SSG, PVP-K30, and the combination of SSG*PVP K-30 on the formulation of 4CH2Cl tablets. Additionally, this study aimed to obtain the optimum 4CH2Cl tablet composition. The experiment followed a two-factor simplex lattice design. The optimization factors are the concentration of SSG and PVP K-30. The optimization responses are the Carr index, Hausner ratio, hardness, friability, disintegrating time, and drug release. The optimization response and the optimum formula are analyzed using optimization software. The optimum tablet was used to test analgesic activity with a hot plate. This study can provide a new choice of analgesic tablets.
2. Material and methods
2.1. Raw materials and chemicals
The quality of the materials used in this study was analytical grade (p.a), pharmaceutical grade (p.g), and food grade (f.g). Salicylic acid (p.g.) (PT. Brataco, Indonesia), 4-chloromethyl benzoyl chloride (p.a.) (Sigma-Aldrich, GmbH, USA), pyridine (p.a.) (Merck KgaA, Darmstadt, Germany), neusilin (p.g.) (Gangwal Chemicals, India), sodium starch glycolate (p.g.) (JRS Pharma, India), polyvinyl pyrrolidone K-30 (BASF Corp., Germany), microcrystalline cellulose (p.g.) (Flocel 102, Gujarat Microwax PVT. LTD, India), spray-dried lactose (p.g.) (Foremost Farm, USA), sodium hydroxide (p.a.) (Merck KgaA, Darmstadt, Germany), potassium dihydrogen phosphate (p.a.) (Merck KgaA, Darmstadt, Germany), ethanol (p.a.) (Merck KgaA, Darmstadt, Germany), and distilled water (f.g.) (Brataco Chemical, Indonesia).
2.2. Synthesis of 4CH2Cl
The synthesis of 4CH2Cl adopts the previously described methods (Caroline et al., 2019b; Hadinugroho et al., 2022a; Tjahjono et al., 2021). Salicylic acid (1.8 mmol) and 4-chloromethyl benzoyl chloride (7.2 mmol) were mixed in an Erlenmeyer flask. Pyridine (1.7 x 10−6 mmol) and acetone (14.8 x 10−6 mmol) were added to form a wet mass of 4CH2Cl. This mixture was then irradiated with microwaves for 5 min using a Millstone Organic Synthesis Unit (MicroSYNTH) to produce solid 4CH2Cl. Unreacted salicylic acid was identified using ferric chloride (FeCl3) and precipitated with ethanol-water to separate and purify the 4CH2Cl compound. The 4CH2Cl was characterized by Fourier transform infrared (FTIR), nuclear magnetic resonance (NMR), thin-layer chromatography (TLC), UV spectrophotometry, and melting point analysis.
2.3. Fourier transform infrared spectroscopy
The structure of 4CH2Cl was analyzed by Fourier transform infrared spectroscopy (UATR PerkinElmer Spectrum Version 10.4.3), and recorded at a wave number of 4000-450 cm−1.
2.4. Nuclear magnetic resonance
1H and 13C NMR of 4CH2Cl was analyzed by liquid state NMR spectroscopy (JEOL RESONANCE 500 MHz, Japan) with acetone as solvent.
2.5. Thin layer chromatography
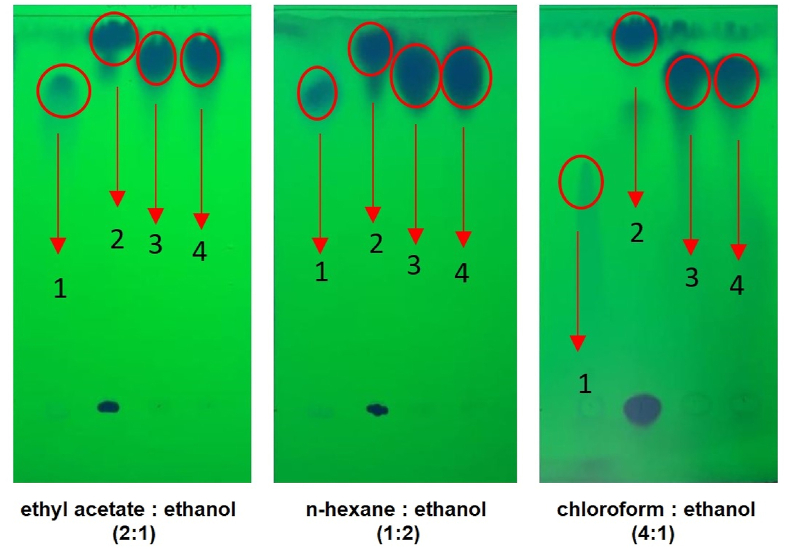
The 4CH2Cl (50 mg) was dissolved in ethanol (50 mL) and spotted (5 μL) on a 60 F254 silica gel plate (Merck, Germany) as the stationary phase. The plate was eluted in the chamber containing the saturated mobile phase. The mobile phase (v/v) used was ethyl acetate: ethanol (2:1); n-hexane: ethanol (1:2); and chloroform: methanol (4:1). The dry plate was observed with a UV lamp at 254 nm to determine the spot and the retention value (Rf). SA and 4-chloromethyl benzoyl chloride were used as a comparison.
2.6. Spectrophotometer
The wavelength of 4CH2Cl by spectrophotometer determined the 4CH2Cl. A solution of 4CH2Cl in ethanol (20 g/mL) was observed with a UV–VIS spectrophotometer (Hitachi U-1100, Jepang) at 200–400 nm and determined the maximum wavelength.
2.7. Melting point
The melting point of 4CH2Cl was determined using an Optimelt MPA 100 apparatus (USA). The temperature was set from 140 °C to 170 °C at a rate of 1 °C per minute. Approximately 2 mm of 4CH2Cl powder was placed in a capillary tube, which was then inserted into the melting point apparatus, and the melting point was recorded.
2.8. Tablet manufacturing
The tablet formulation contained 4CH2Cl (300 mg), Ne (75 mg), MCC (40 mg), and SDL (up to 800 mg). Table 1 presents the PVP K-30 and SSG quantities, with a production capacity of 120 g tablet mass or 150 tablets. 4CH2Cl and Ne were mixed homogeneously in a mortar and pestle. The other ingredients (SSG, PVP K-30, MCC, and SDLwere added and blended in a cubic mixer (Erweka, Germany) at 100 rpm for 2 min. The mixture’s compressibility was assessed, and the tablet mass was compressed into 800 mg tablets using a single-punch tablet press (Jenn Chian Machinery, Taiwan). The tablets were evaluated for hardness, friability, and disintegration time. A dissolution test was conducted to determine drug release.
Table 1.
The detailed composition of each formula.
| Material | Ta | Tb | Tc | To |
|---|---|---|---|---|
| 4CH2Cl [mg] | 300.00 | 300.00 | 300.00 | 300.00 |
| Ne [mg] | 75.00 | 75.00 | 75.00 | 75.00 |
| MCC [mg] | 40.00 | 40.00 | 40.00 | 40.00 |
| SSG [mg] | 40.00 | 32.00 | 24.00 | 33.60 |
| PVP K-30 [mg] | 16.00 | 24.00 | 32.00 | 22.40 |
| SDL [mg] | 329.00 | 329.00 | 329.00 | 329.00 |
The selection of the dose of 4CH2Cl 300 mg per tablet follows the dose of 3CH2Cl tablets, which is a salicylate derivative that has been reported previously in previous experiments (Hadinugroho et al., 2022a). Pharmacokinetic data of Cmax and Kel in rats show similarities between 4CH2Cl and 3CH2Cl. 4CH2Cl has a Cmax of 0.53 μg/mL and Kel of 0.020 min−1, while 3CH2Cl has a Cmax of 0.57 μg/mL and Kel of 0.018 min−1 (Caroline et al., 2019a; Sugiarno, 2016). Dose calculations were also performed and presented in the supplementary material Chapter S1.
2.9. Compressibility
Tablet mass was placed in a known-weight 100 mL measuring tube, and the initial weight was recorded. The tube was placed on a tapped density volumeter (Erweka, Germany) and tapped 500 times. Volumes before and after tapping were recorded to calculate bulk density (Bd) and tapped density (Td). The Carr index and Hausner ratio were calculated using Equations Equation 1, Equation 2), respectively (Aulton and Taylor, 2017; Hadinugroho et al., 2022b).
| Equation 1 |
| Equation 2 |
2.10. Hardness
Hardness was tested on six randomly selected tablets using an hardness tester (Erweka, Germany). Tablets were horizontally pressed with a metal block until they cracked, and the hardness was displayed on the apparatus monitor (Aulton and Taylor, 2017; The United States Pharmacopeial Convention, 2018).
2.11. Friability
Several tablets (10) were taken randomly because the weight per tablet was more than 650 mg or the total weight of 10 tablets was more than 6500 mg. Each tablet was dust-free, and selected tablets were weighed (W). All tablets were placed and rotated on a drum friability tester (Erweka, Germany) (25 rpm, 4 min). Each tablet was dust-free again and weighed (W'). Friability is determined according to Equation (3) (Aulton and Taylor, 2017; The United States Pharmacopeial Convention, 2018).
| Equation 3 |
2.12. Disintegration time
Six tablets were randomly selected from 18 tablets (Aulton and Taylor, 2017; The United States Pharmacopeial Convention, 2018). The tablets were placed in each holder of the disintegration tester (Erweka Z3, Germany). The tube moves reversibly vertically in the distilled water medium chamber (37 °C; 900 mL). The longest time, where the tablets disintegrated completely from 6 tablets in the holder, is defined as the disintegration time.
2.13. Dissolution
The tablets were dissolved using 900 mL of phosphate buffer medium pH 6.8 (37 ± 0.5 °C; 50 rpm). The test was carried out using the basket method for 60 min (Bertocchi et al., 2005; Hadinugroho et al., 2022a; The United States Pharmacopeial Convention, 2018; Zupančič-Bozič et al., 1997). The concentration of dissolved 4CH2Cl was determined by UV–VIS spectrophotometer (Hitachi U-1900, Japan) with a wavelength of about 242 nm.
2.14. Optimization of 4CH2Cl tablets
The optimization method used is a two-factor simplex lattice. We investigated the optimal concentration of SSG (24–40 mg) and PVP K-30 (16–32 mg). The optimization responses are the Carr index, Hausner ratio, hardness, friability, disintegrating time, and drug release. Preliminary experiments with a comparison of SSG (mg) and PVP K-30 (mg) were Ta (40:16), Tb (32:24), and Tc (24:32). Response processing using optimization software (Design Expert ver 10) and statistical analysis using ANOVA linear and quadratic model approaches (Hadinugroho et al., 2022a).
2.15. Release kinetics of 4CH2Cl from tablets
The release kinetics of 4CH2Cl tablets from three formulas were analyzed by DDSolver software. The release kinetics model used is Higuchi, Korsmeyer-Peppas, and first order (Equations Equation 4, Equation 5, Equation 6, Equation 7)) (Craciun et al., 2019; Kaleemullah et al., 2017; Panotopoulos and Haidar, 2018; Wahab et al., 2011):
| Equation 4 |
Ct: drug dissolved at time [g], Co: initial drug [g], and Ko: constant drug release [h−1].
| Equation 5 |
Ct: drug dissolved at time [g], KH: Higuchi constant [g/h1/2], and t: time [h].
| Equation 6 |
Ct/C∞: released drug fraction [g], Kk: Korsmeyer-Peppas constant [g.h], and n: diffusion exponential.
| Equation 7 |
(1-m): insoluble drug portion [g], Ti: lag time before dissolution, a: initial drug [g], b: parameter of curve slope.
2.16. Animal models and analgesic activity
Nine male BALB/C mice (1–2 months old; 20–25 g) were sourced from the Pharma-Veterinary Center of Surabaya, Indonesia. The mice were kept in a room with controlled temperature (20–24 °C) and 65% relative humidity, on a 12-h light/dark cycle. They were acclimatized for 7 days before the study began. All animal procedures followed Institutional Animal Care and Use Committee (IACUC) guidelines and received ethical approval by preclinical ethic committee Faculty of Veterinary Sciences, Gadjah Mada University, Yogyakarta-Indonesia No. 001/ECFKH/Eks/2022. The mice had free access to food and water throughout the 24-day period, including the acclimatization phase, with temperature recorded twice daily. The mice were assigned to three groups (n = 3 per group): control (untreated), ASA (Comparison), and 4CH2Cl (“To” group). ASA (Bayer, Leverkusen, Germany) or 4CH2Cl was given orally at a single dose of 60 mg/kg B, based on previously published methods (Tjahjono et al., 2024). The untreated groups were given 3% Pulvis Gummi Arabicum as a placebo. Analgesic activity experiment using the writhing test method. Pain induction was created by intraperitoneal injection of 0.6% acetic acid (0.01 mL/g body weight), according to previously published methods (Caroline et al., 2019a; Tjahjono et al., 2021). The successful induction response of pain induction was demonstrated by the mice writhing, which was observed for 10 min.
3. Result
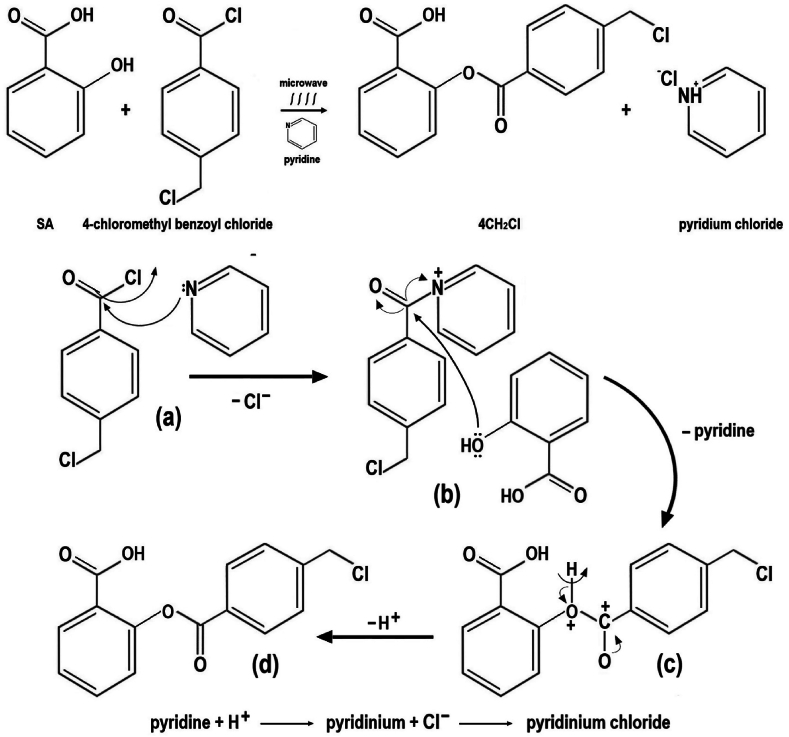
3.1. 4CH2Cl synthesis reaction mechanism
4CH2Cl synthesis reaction mechanism are presented in Fig. 1. The reaction begins with the attack of pyridine on Cl on 4-chloromethyl benzoyl chloride. Cl− is released and pyridine binds to 4-chloromethyl benzoyl chloride (a). The compound created is more electrophilic than 4-chloromethyl benzoyl chloride. OH on the phenolic of SA attacks the C atom at C=O of the compound and bonds to create a new compound (b). The pyridine ion is released and bonds with Cl−, called pyridine chloride. Simultaneously, the H atom at OH of the phenolic comes from SA is released so that the O atom loses an electron and bonds with the C atom at C=O of 4-chloromethyl benzoyl chloride, which loses an electron (c). The O atom at C=O, which comes from 4-chloromethyl benzoyl chloride, gains an electron so that a double bond occurs and 4CH2Cl is created (d).
Fig. 1.
4CH2Cl synthesis reaction mechanism.
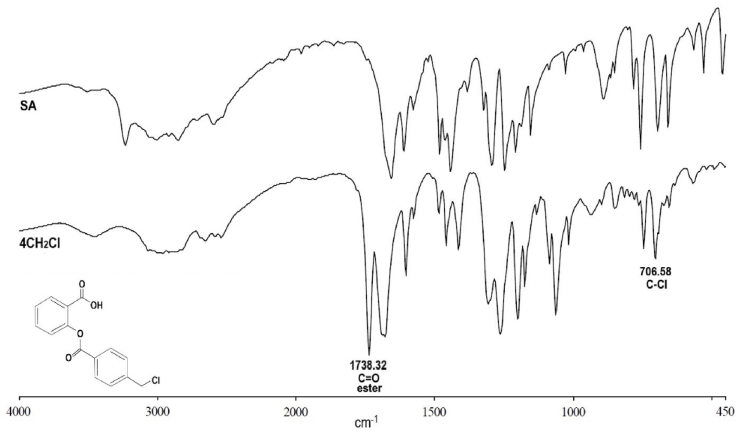
3.2. Fourier transform infrared spectroscopy
The infrared spectra of 4CH2Cl and SA are shown in Fig. 2. The 4CH2Cl spectra showed the carbonyl ester peak (C=O) appeared at a wavelength of 1738.32 cm−1 and the carboxylate peak (C=O) appeared at 1682.37 cm−1. The peak of C-O appears at wavelengths 1309.45 cm−1, 1264.38 cm−1, and 1201.42 cm−1. The peak of C-Cl appears at a wavelength of 706.58 cm−1. The SA spectra showed the carboxylate peak (C=O) appeared at 1658.14 cm−1. The peak of C-O appears at wavelengths 1295.17 cm−1, 1249.16 cm−1, and 1210.21 cm−1
Fig. 2.
FTIR spectrum of 4CH2Cl and SA. The presence of the ester carbonyl group (C=O) and C-Cl in 4CH2Cl is a specific group and is not present in SA.
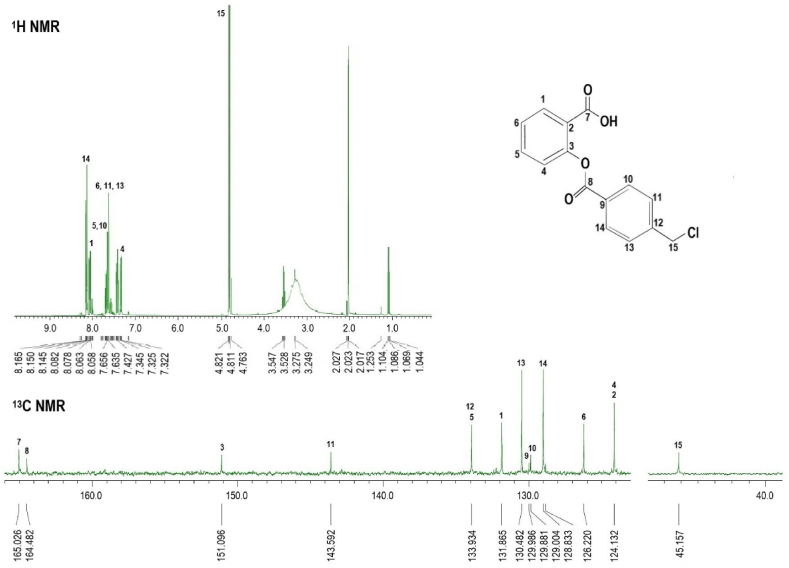
3.3. Nuclear magnetic resonance
The NMR analysis was performed as a follow-up infrared analysis to confirm the success of the synthesis. The NMR spectra of 4CH2Cl are shown in Fig. 3. The 1H NMR spectrum of 4CH2Cl shows C-H peaks at δ = 8.145 ppm (14), δ = 8.078 ppm (1), δ = 7.565 ppm (5.10), and δ = 7.635 ppm (6)(11)(13). CH-Cl peaks at δ = 4821 ppm (15).
Fig. 3.
1H and 13C NMR spectrum of 4CH2Cl. The presence of carbonyl ester group (C=O) (δ = 164.482 ppm) and C-Cl (δ = 45.157 ppm) are specific groups.
The 13C NMR spectra of 4CH2Cl show a carbonyl ester peak (C=O) at δ = 164.482 ppm (8); carboxylate peak (C=O) at δ = 165.026 ppm (7); C-Cl peak at δ = 45.157 ppm (15). C-H peak at δ = 151.96 ppm (3); δ = 143.592 ppm (11); δ = 133.934 ppm (5)(12); δ = 131.865 ppm (1); δ = 130,482 ppm (13); δ = 129.986 ppm (9); δ = 129.881 ppm (10); δ = 129.004 ppm (14); δ = 126.220 ppm (6); and δ = 124.132 ppm (2)(4).
3.4. Thin layer chromatography
The chromatograph of 4CH2Cl, SA, and 4-chloromethyl benzoyl chloride is shown in Fig. 4. The Rf value of 4CH2Cl with ethyl acetate: ethanol (1:2) is 0.82(3)(4); n-hexane: ethanol (1:2) is 0.81 (3)(4); and chloroform: ethanol (4:1) is 0.86 (3)(4). The Rf value of SA with ethyl acetate: ethanol (1:2) is 0.80 (1); n-hexane: ethanol (1:2) is 0.72 (1); and chloroform: ethanol (4:1) is 0.69 (1). The Rf value of 4-chloromethyl benzoyl chloride with ethyl acetate: ethanol (1:2) is 0.95 (2); n-hexane: ethanol (1:2) is 0.90 (2); and chloroform: ethanol (4:1) is 0.98 (2).
Fig. 4.
Chromatogram of thin layer chromatography with multiple mobile phases. Spot 1 is SA, spot 2 is 4-chloromethyl, and spot 3, 4 is 4CH2Cl.
3.5. Spectrophotometer
The UV spectra of 4CH2Cl are shown in Fig. 5. Screening the maximum wavelength of the 4CH2Cl peak is about 242 nm. The maximum wavelength of SA is about 212 nm. The difference in maximum wavelength shows that the chemical structure of 4CH2Cl is different from that of SA.
Fig. 5.
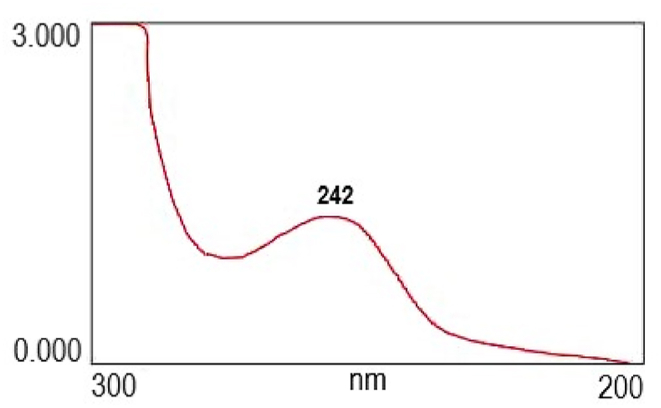
UV spectra of 4CH2Cl. The maximum wavelength of 4CH2Cl is at 242 nm.
3.6. Melting point
The melting point of 4CH2Cl is 157–159 °C. It is similar to the melting point of SA (158–160 °C).
3.7. Compressibility of 4CH2Cl tablets mass
Compressibility is determined as the ratio between the Carr index and the Hausner ratio. The values of each parameter for the three produced tablet masses are shown in Table 2. The value of the quadratic polynomial coefficient of the Carr index and Hausner ratio are PVP K-30 (20.45) (1.29), SSG (16.10) (1.19), and SSG*PVP K-30 (1.90) (0.04). The ANOVA results from the Carr index and Hausner ratio of linear models are acceptable, and the polynomial coefficients of quadratic models are similar to those of linear models (Table 2).
Table 2.
Detail evaluate of tablets mass, tablets, and dissolution of 4CH2Cl.
| Tablet |
SSG |
PVP K-30 |
Carr index |
Hausner ratio |
Hardness |
Friability |
Disintegrating time |
Drug release |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | [mg] | [mg] | [%] | SD | SD | [kp] | SD | [%] | SD | [min.] | SD | [%] | SD | |
| Ta | 40.00 | 16.00 | 16.10 | 0.13 | 1.19 | 0.01 | 4.03 | 0.04 | 0.47 | 0.10 | 0.59 | 0.10 | 84.91 | 0.92 |
| Tb | 32.00 | 24.00 | 18.75 | 0.28 | 1.25 | 0.01 | 7.10 | 0.07 | 0.57 | 0.11 | 1.37 | 0.09 | 92.25 | 0.53 |
| Tc | 24.00 | 32.00 | 20.45 | 0.23 | 1.29 | 0.01 | 9.23 | 0.14 | 0.66 | 0.12 | 7.75 | 0.13 | 97.31 | 0.66 |
| Tp | 33.60 | 22.40 | 18.00 | – | 1.24 | – | 6.56 | – | 0.55 | – | 3.20 | – | 90.25 | – |
| To | 33.60 | 22.40 | 18.10 | 0.10 | 1.24 | 0.02 | 6.51 | 0.22 | 0.54 | 0.14 | 3.30 | 0.16 | 90.30 | 0.54 |
Tp (prediction tablet): composition and quality prediction follow software design expert.
To (optimum tablet): the composition follows the software design expert, and the quality value is the result of the verification experiment.
3.8. The hardness of 4CH2Cl tablets
The hardness of 4CH2Cl tablets is presented in Table 2. The hardness of Tc tablets is higher than that of Ta and Tb tablets. Tablet hardness occurs due to the interlocking between the particles of the tablet mass. The value of the quadratic polynomial coefficient of PVP K-30 (9.23), SSG (4.03), and SSG*PVP K-30 (1.88). The ANOVA results from the hardness of linear models are acceptable, and the polynomial coefficients of quadratic models are similar to those of linear models (Table 2).
3.9. Friability of 4CH2Cl tablets
The friability is presented in Table 2. The most friable tablets are Tc, Tb, and Ta tablets. The value of the quadratic polynomial coefficient of PVP K-30 (0.66), SSG (0.47), and SSG*PVP K-30 (0.06). The friability ANOVA of the linear model was accepted, and the polynomial coefficients of quadratic models are similar to those of linear models (Table 2).
3.10. Disintegration time of 4CH2Cl tablets
The disintegration time of 4CH2Cl tablets is presented in Table 2. The value of the quadratic polynomial coefficient of PVP K-30 (7.75), SSG (0.59), and SSG*PVP K-30 (−0.30). The disintegration time ANOVA of the linear model was accepted, and the polynomial coefficients were similar to that of the quadratic model (Table 2).
3.11. Dissolution of 4CH2Cl tablets
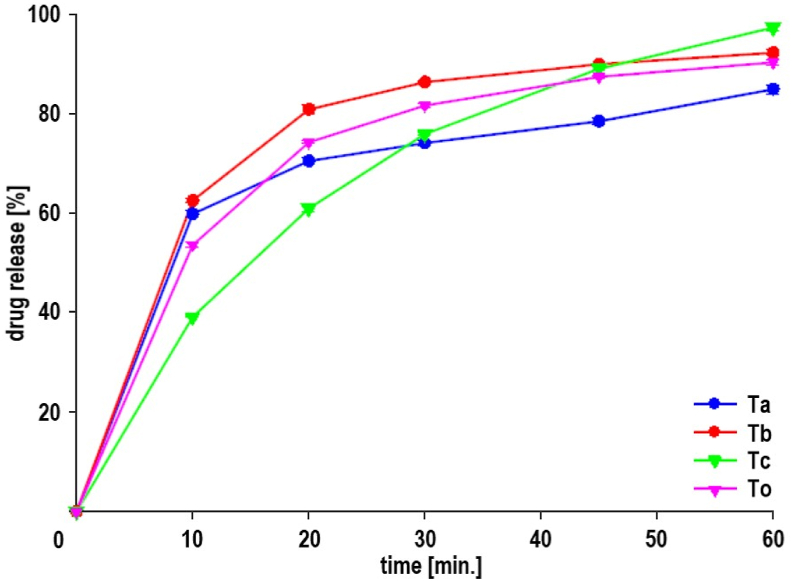
The release and dissolution profiles of 4CH2Cl are presented in Table 2 and Fig. 6. Dissolution data processing uses a standard curve for phosphate buffer pH 6.8 with equation Y = 0.1384 + 0.0714.X. The order of tablets with the highest release of 4CH2Cl was Tc, Tb, and Ta. The value of the quadratic polynomial coefficient of PVP K-30 (97.31), SSG (84.91), and SSG*PVP K-30 (4.56). The drug release ANOVA of the linear model was accepted, and the polynomial coefficients were similar to the quadratic model (Table 3).
Fig. 6.
The dissolution profile of Ta, Tb, Tc, and To tablets. The compositions of SSG [mg] and PVP K-30 [mg] are Ta (40.00 : 16.00), Tb (32.00 : 24.00), Tc (24.00 : 32.00), and To (33.60 : 22.40).
Table 3.
Statistic of tablets mass, tablets, and dissolution of 4CH2Cl.
| Parameter | ANOVA |
Polynomial |
To |
||||
|---|---|---|---|---|---|---|---|
| R-Squared | Adj R-Squared | Pred R-Squared | Adeq Precision | linier | quadratic | t | |
| Carr index | 0.9844 | 0.9687 | 0.7887 | 13.7368 | Y = 16.26 A + 20.61 B | Y = 16.10 A + 20.45 B + 1.90 A*B | 1.732 |
| Hausner ratio | 0.9868 | 0.9737 | 0.8224 | 15.0000 | Y = 1.19 A + 1.29 B | Y = 1.19 A + 1.29 B + 0.04 A*B | 0.378 |
| Hardness | 0.9892 | 0.9784 | 0.8545 | 16.5957 | Y = 4.19 A + 9.39 B | Y = 4.03 A + 9.23 B + 1.88 A*B | 0.388 |
| Friability | 0.9918 | 0.9835 | 0.8887 | 19.0000 | Y = 0.48 A + 0.67 B | Y = 0.47 A + 0.66 B + 0.06 A*B | 0.085 |
| Disintegrating time | 0.9856 | 0.9712 | 0.8053 | 14.3200 | Y = 0.34 A + 7.50 B | Y = 0.59 A + 7.75 B - 0.30 A*B | 1.076 |
| Drug release | 0.9889 | 0.9777 | 0.8496 | 16.3158 | Y = 85.29 A + 97.69 B | Y = 84.91 A + 97.31 B + 4.56 A*B | 0.172 |
3.12. Optimization of 4CH2Cl tablets
The experimental design followed a 2-factor simplex lattice (SSG and PVP K-30). The linear and quadratic statistical model predicts the effects of SSG, PVP K-30, and SSG*PVP K-30 (Table 3 and Supplementary Material Table S1). The ANOVA evaluated the acceptability of the linear model by considering the values of R-Squared, Adj R-Squared, Pred R-Squared, and Adeq Precision. The linear model is accepted if the R-Square and Pred R-Square have a difference of less than 0.2, Adeq Precision of more than 4, and R-Square is close to one. All optimization response parameters meet the specifications so that the coefficients are accepted. The coefficient quadratic model profile is similar to the linear model so that the combined coefficient of the two can be used to predict the effect of SSG*PVP K-30. The optimum tablet (To) was determined according to a numerical linear model. The prediction of the optimum tablet composition (Tp) and the verification results are presented in Table 3.
3.13. The release model kinetics of 4CH2Cl from tablets
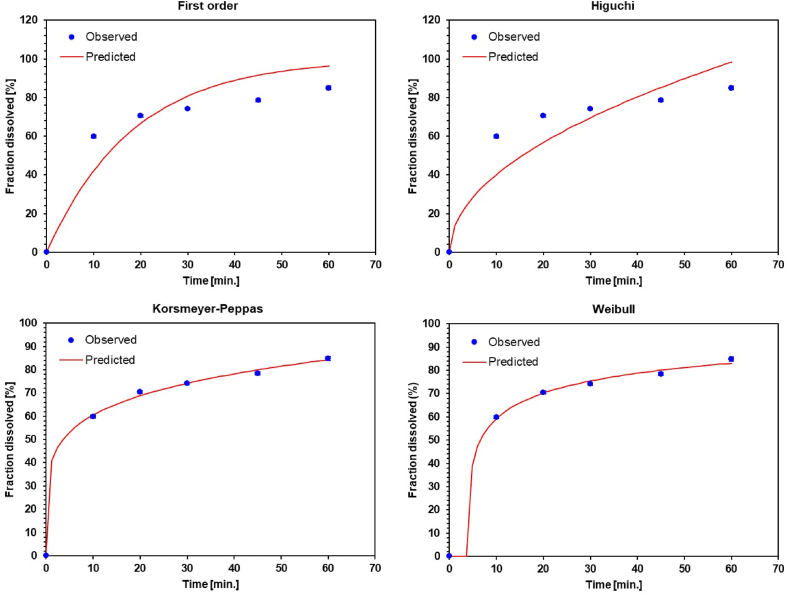
The analysis of the release 4CH2Cl was performed using DDSolver as shown in Table 3 and Fig. 7 (for details, see Supplementary Material in Figs. S1–S4). The analysis parameters are Rsqr_adj, MSE_root, and Akaike Information Criterion (AIC).
Fig. 7.
Release kinetics model of 4CH2Cl from Ta, Tb, Tc, and To tablets. The compositions of SSG [mg] and PVP K-30 [mg] are Ta (40.00 : 16.00) (Korsmeyer-Peppas), Tb (32.00 : 24.00) (Weibull), Tc (24.00 : 32.00) (First order, and To (33.60 : 22.40) (Weibull).
3.14. 4CH2Cl tablet inhibits acetic acid induced pain in mice
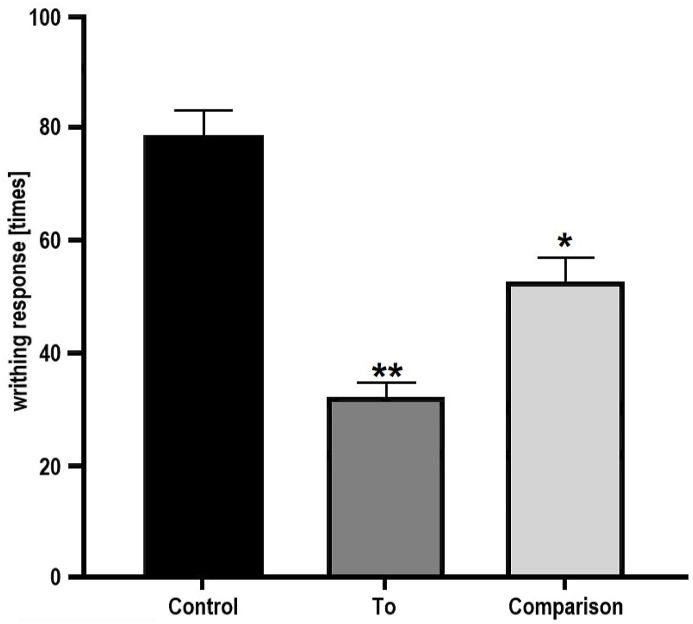
Both 4CH2Cl (32.22 ± 2.25 s response time) and ASA groups (52.83 ± 3.87 s response time) showed reduced writhing frequency (P < 0.05) when compared with the placebo group (78.33 ± 4.16 s response time). The 4CH2Cl test group also gave less writhing frequency than the ASA group (p = 0.05) (Fig. 8).
Fig. 8.
Analgesic activity of 4CH2Cl. Placebo are mices given tablets containing excipients as control. ASA are mices given ASA tablets as a comparison. 4CH2Cl are mices given 4CH2Cl tablets (To).
4. Discussion
The success of the synthesis was chemically confirmed using infrared and magnetic resonance. The infrared spectra showed the specific peak 4CH2Cl that SA did not have was C=O and C-Cl (Coates, 2006). The specific peak 13C NMR of 4CH2Cl show a carbonyl ester peak (C=O) at δ = 164.482 ppm and C-Cl peak at δ = 45.157 ppm (Flemming, 2016; Kupriyanova et al., 2021). On the chromatogram, the difference in the Rf value of 4CH2Cl with SA and 4-chloromethyl benzoyl chloride indicates a change in chemical structure, resulting in a change in the compound’s polarity. The Rf value for each mobile phase can provide information on the polarity of 4CH2Cl to support the formulation of pharmaceutical preparations. The spectrophotometer wavelength of 4CH2Cl is longer than SA because 4CH2Cl contains two benzene chromophores that can absorb UV light (Dachriyanus, 2004; Suhartati, 2017). This wavelength provides a reference for quantitative analysis of 4CH2Cl concentrations in various pharmaceutical dosage forms. The value of melting point indicates that 4CH2Cl is relatively stable at ambient temperature, around 25°C. In addition, the melting point is considered in determining the method of preparing pharmaceutical dosage forms.
The 4CH2Cl tablets contain Ne, SSG, PVP K-30, MCC, and SDL. Ne acts as an insulator between the 4CH2Cl particles so that the particles do not aggregate together. The hydrophobicity of Ne in 4CH2Cl improves the flow properties that support the tablet manufacturing process (Juneja et al., 2014; Lou et al., 2013; Shete et al., 2019). SSG acts as a disintegrating agent, accelerating the tablet disintegration. The SSG particles swell when hydrated, so the SSG particles push against the surrounding particles, and the tablet disintegrates (Hadinugroho et al., 2022b; Markl and Zeitler, 2017; Sheskey et al., 2017). PVP K-30, as a solubilizer, accelerates tablet hydration in the disintegration and dissolution processes (Kurakula and Rao, 2020; Sheskey et al., 2017). PVP K-30 accelerates SSG particles’ hydration, so the tablet disintegrates quickly. In addition, PVP K-30 accelerates the solubility of 4CH2Cl particles, thereby increasing the concentration of dissolving 4CH2Cl. MCC is used as a tablet filler because MCC has good compactibility. SDL is a filler because SDL has high density and good compactibility (Chaerunisaa et al., 2020; Thoorens et al., 2014; Yasmin et al., 2020). The combination of MCC and SDL can produce tablets with proportional thickness and diameter.
Analysis of tablet mass (Ta, Tb, and Tc) yielded a Carr index value between 16 until 20% and a Hausner ratio of less than 1.25 (Aulton and Taylor, 2017). This value means that the three tablet masses are good enough to flow, and the particle arrangement is quickly stable when subjected to mechanical forces. The tablet mass can move freely and fill the tablet machine die so that the tablet weight is achieved and consistent. Based on the value of the quadratic polynomial coefficient, the most influential parameter on the Carr index and Hausner ratio are PVP K-30, SSG, and SSG*PVP K-30. PVP K-30 particles are rounded and hollow and have a flat surface. The hollow PVP K-30 particles break easily to form fragments when subjected to mechanical movement, so the tablet mass requires a lot of activity to achieve a compressed particle arrangement. SSG particles are rounded and have a flat surface. The variation of the rounded shape involves a lot of mechanical movement for the arrangement of the tablet mass particles to become a compress. The lowest combination of SSG*PVP K-30 increases the Carr-index and Hausner ratio because PVP K-30 fragments can fill the porosity of the tablet mass so that a little mechanical movement can form a compressed tablet mass arrangement. Rounded shape with a flat surface of both particles, the tablet mass has good flow properties.
The tablet hardness specifications in this experiment were 4–8 kp. The materials that increase tablet hardness the most are PVP K-30, SSG, and SSG*PVP K-30. The PVP K-30 material has hygroscopic properties. When the tablet mass is compressed, the PVP K-30 particles become moist due to the heat of compression. The moisture derived from PVP K-30 particles can bind to the deformation of the tablet mass particles. When the tablet mass is compressed, the deformation of the SSG particles creates a narrow porosity in the interlocking deformation of the tablet mass particles. The lowest SSG*PVP K-30 increases tablet hardness. PVP K-30 fragment causes wide porosity in the interlocking deformation of tablet mass particles.
The tablet friability specifications in this experiment was ≤1% (The United States Pharmacopeial Convention, 2018). Tc tablets are more friable than Ta and Tb tablets. Tc tablets are the most friability but have the highest hardness. The friability occurs on the tablet’s surface, releasing particles or fines. The interlocking particles on the tablet surface are removed when mechanical motion is applied. The materials that increased the tablet friability the most were PVP K-30, SSG, and SSG*PVP K-30. The PVP K-30 materials could raise the friability because the PVP K-30 fragments could not adhere firmly to the tablet surface. The variation in the rounded shape of SSG resulted in many interlocking porosity deformation particles on the tablet surface. The combination of SSG*PVP K-30 slightly increased tablet friability due to the strong interlocking of particle deformation on the tablet surface. The interlocking strength is obtained from the moisture of the PVP K-30 fragments when rubbing against each other produces heat so that the particles' deformation on the tablet’s surface is not easily separated.
All tablets had a disintegration time of fewer than 30 min (The United States Pharmacopeial Convention, 2018). The SSG particles could swell when hydrated so that the surrounding tablet mass particles are pushed and separated from each other. The materials that increase the disintegration time the most are PVP K-30 and SSG. The combination of SSG*PVP K-30 reduces the disintegration time. The PVP K-30 dominates the increase in disintegration time because the PVP K-30 particles are hydrated to form a viscous gel to bind the particles, and the old tablet disintegrates. When the PVP K-30 gel is saturated and remains hydrated, the PVP K-30 dissolves. In this condition, the SSG particles swell, and the tablet disintegrates. The SSG particles swell depending on hydration rate and SSG concentration. When the hydration of SSG is slow, the old SSG particles swell, and the disintegration time of the tablets is long. When the concentration of SSG particles in the tablet is low, the SSG starts from the tablet’s surface. The driving force against the particles begins from the surface towards the tablet core so that the tablet disintegrates gradually. The SSG*PVP K-30 reduces disintegration time because PVP K-30 is easily soluble and accelerates SSG's hydration, so the SSG particles swell immediately, and the tablet disintegrates.
The 4CH2Cl is a new compound still being developed for dosage forms and has yet to be registered in regulations. The dissolution specification is at least 70% (Q) 4CH2Cl is released within 45 min. All tablets in this experiment within 45 min released above 75% (Q + 5). The presence of soluble and semipolar PVP K-30 accelerates the hydration of 4CH2Cl particles and dissolution (Kurakula and Rao, 2020). The materials that increase the dissolution the most, according to the quadratic model, are PVP K-30, SSG, and SSG*PVP K-30. The PVP K-30 dominates the dissolution of 4CH2Cl because of its soluble and semipolar nature. The PVP K-30 accelerates the hydration of the tablet and its constituent particles so that the tablet disintegrates quickly, and the concentration of 4CH2Cl is high in the dissolution medium. SSG’s presence accelerates the separated tablet mass particles, including 4CH2Cl particles. The interaction of 4CH2Cl particles with the dissolution media causes 4CH2Cl to dissolve. The combination of SSG*PVP K-30 increases the release of 4CH2Cl because PVP K-30 accelerates the hydration of SSG particles so that the tablets are immediately crushed into granules and powders. The PVP K-30 dissolves in the dissolution media accelerates hydration and dissolves 4CH2Cl particles.
The 4CH2Cl release kinetics model was determined by the highest Rsqr_adj, the lowest MSE_root, and the lowest AIC. Rsqr_adj describes the relationship between the dissolution time and the concentration of the dissolved. The MSE_root acts as the correlation analysis controller. The AIC determined the suitability of the release kinetics model equation (Gu et al., 2018; Mircioiu et al., 2019; Siswanto et al., 2015; Zhang et al., 2010).
The Ta tablets followed the kinetics of the Korsmeyer-Peppas model with the release of 4CH2Cl by Fickian diffusion (n = 0.18). The high concentration of SSG causes 4CH2Cl particles and other particles to be trapped in the swollen SSG particles. Diffusion of 4CH2Cl through the pathway created by soluble PVP K-30 particles. The Tb and To tablets followed the Weibull kinetic model with a rapid release of 4CH2Cl. The PVP K-30, easily soluble in dissolution media, accelerates hydration and dissolution of 4CH2Cl particles. The presence of SSG accelerates the disintegration of tablets into granules/powder so that the surface area for dissolving 4CH2Cl particles is wider. The Tc tablets follow the first-order kinetics model with the release of 4CH2Cl by diffusion through the porosity. Irregular PVP K-30 fragments cause a large amount of tablet or granule porosity. The diffusion mechanism of 4CH2Cl particles occurs through the porosity of the granules.
The three experimental groups of analgesic activity showed significantly different activity responses (P < 0.05). The effectiveness of 4CH2Cl pain inhibitors (Fig. 7) shows that the presence of excipients in To tablets does not interfere with the analgesic activity of 4CH2Cl.
5. Conclusion
SSG and PVP K-30 increased Carr index, Hausner ratio, hardness, friability, disintegration time, and drug release. The combination of SSG*PVP K-30 has the same effect, except that the disintegration time decreased. The optimum tablet formula is 4CH2Cl (300 mg), Ne (75 mg), SSG (33.60 mg), PVP K-30 (22.40 mg), MCC (40 mg), and SDL (up to 800 mg). The tablet characteristics have Carr index (18.10), Hausner ratio (1.24), hardness (6.51 Kp), friability (0.54%), disintegration time (3.30 min), and drug release (9.30%). 4CH2Cl tablets can be a candidate and choice for new analgesic drugs in the future.
Declaration of human and animal rights
The Research Ethics Commission of the Faculty of Veterinary Medicine, Gadjah Mada University Yogyakarta, Indonesia, stated that experiments using experimental animals (mice) had met the ethical requirements with a certificate No.001/EC-FKH/Ex./2022 dated January 14, 2022.
Availability of data and material
In addition to the tables and figures in the article, the research results present supporting data on the Supplementary Material: Tablet dosage calculation; the release of 4CH2Cl from the tablets; statistical analysis of 4CH2Cl tablets; the kinetics profile of the release of 4CH2Cl from Ta, Tb, Tc, and To. tablets.
Credit author statement
Wuryanto Hadinugroho: Designed the experiments, performed the experiments, analyzed and interpreted the data, wrote the manuscript. Yudy Tjahjono, Kuncoro Foe, Senny Yesery Esar, Caroline Caroline, Maria Annabela Jessica, Hendy Wijaya: performed the experiments, analyzed, and interpreted the data.
Declaration of competing interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Acknowledgment
Thanks to the Research and Community Service Institute of Widya Mandala Surabaya Catholic University, Surabaya, Indonesia, for supporting grants (5230/WM01/N/2021). Thanks to Angela Tiffany, Meidelin Ribka Abiati, Sherlilyta Stiara Dewi, and Khaterine Irene Phuk for their assistance during the experiment.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crphar.2024.100200.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
The authors do not have permission to share data.
References
- Aulton M.E., Taylor K.M.G. Churchill Livingstone Elsevier; New York: 2017. Aulton's Pharmaceutics the Design and Manufacture of Medicines, BMC Public Health. [Google Scholar]
- Bertocchi P., Antoniella E., Valvo L., Alimonti S., Memoli A. Diclofenac sodium multisource prolonged release tablets - a comparative study on the dissolution profiles. J. Pharm. Biomed. Anal. 2005;37:679–685. doi: 10.1016/j.jpba.2004.11.041. [DOI] [PubMed] [Google Scholar]
- Caroline, Foe K., Yesery Esar S., Soewandi A., Wihadmadyatami H., Widharna R.M., Tamayanti W.D., Kasih E., Tjahjono Y. Evaluation of analgesic and antiplatelet activity of 2-((3-(chloromethyl)benzoyl)oxy)benzoic acid. Prostag. Other Lipid Mediat. 2019;145 doi: 10.1016/j.prostaglandins.2019.106364. [DOI] [PubMed] [Google Scholar]
- Caroline Nathania, Foe K., Esar S.Y., Jessica M.A. Characterization of pharmacokinetics of 2-((3-(chloromethyl)benzoyl)oxy) benzoic acid in rats by using hplc-dad method. Int. J. Appl. Pharm. 2019;11:279–283. doi: 10.22159/ijap.2019v11i5.34536. [DOI] [Google Scholar]
- Chaerunisaa A.Y., Sriwidodo S., Abdassah M. Microcrystalline cellulose as pharmaceutical excipient. Pharm. Formul. Des. - Recent Pract. 2020 doi: 10.5772/intechopen.88092. [DOI] [Google Scholar]
- Coates J. Interpretation of infrared spectra, A practical approach. Encycl. Anal. Chem. 2006:10815–10837. doi: 10.1002/9780470027318.a5606. [DOI] [Google Scholar]
- Craciun A.M., Barhalescu M.L., Agop M., Ochiuz L. Theoretical modeling of long-time drug release from nitrosalicyl-imine-chitosan hydrogels through multifractal logistic type laws. Comput. Math. Methods Med. 2019;2009:1–10. doi: 10.1155/2019/4091464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dachriyanus . Spectroscopic Structural Analysis of Organic Compounds. Institute of Information and Communication Technology Development (LPTIK) Andalas University; Padang: 2004. [Google Scholar]
- Flemming C., Jr. Quantitative Analysis of Acetylsalicylic Acid by Q- NMR (Quantitative-Nuclear Magnetic Resonance Spectroscopy) 2016. Theses. [Google Scholar]
- Gu Y., Wei H.L., Balikhin M.M. Nonlinear predictive model selection and model averaging using information criteria. Syst. Sci. Control Eng. 2018;6:319–328. doi: 10.1080/21642583.2018.1496042. [DOI] [Google Scholar]
- Hadinugroho W., Foe K., Tjahjono Y., Caroline C., Yesery Esar S., Wijaya H., Annabella Jessica M. Tablet formulation of 2-((3-(chloromethyl)benzoyl)oxy)benzoic acid by linear and quadratic models. ACS Omega. 2022;7:34045–34053. doi: 10.1021/acsomega.2c03147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadinugroho W., Martodihardjo S., Fudholi A., Riyanto S. Preparation of citric acid-locust bean gum (CA-LBG) for the disintegrating agent of tablet dosage forms. J. Pharm. Innov. 2022;17:1160–1175. doi: 10.1007/s12247-021-09591-0. [DOI] [Google Scholar]
- Juneja P., Kaur B., Odeku O.A., Singh I. Development of corn starch-neusilin UFL2 conjugate as tablet superdisintegrant: formulation and evaluation of fast disintegrating tablets. J. Drug Deliv. 2014;2014:1–13. doi: 10.1155/2014/827035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaleemullah M., Jiyauddin K., Thiban E., Rasha S., Al-Dhalli S., Budiasih S., Gamal O.E., Fadli A., Eddy Y. Development and evaluation of Ketoprofen sustained release matrix tablet using Hibiscus rosa-sinensis leaves mucilage. Saudi Pharmaceut. J. 2017;25:770–779. doi: 10.1016/j.jsps.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupriyanova G., Rafalskiy V., Mershiev I., Moiseeva E. NMR spectroscopy reveals acetylsalicylic acid metabolites in the human urine for drug compliance monitoring. PLoS One. 2021;16:1–12. doi: 10.1371/journal.pone.0247102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurakula M., Rao G.S.N.K. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): as excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020;60 doi: 10.1016/j.jddst.2020.102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H., Liu M., Wang L., Mishra S.R., Qu W., Johnson J., Brunson E., Almoazen H. Development of a mini-tablet of co-grinded prednisone-neusilin complex for pediatric use. AAPS PharmSciTech. 2013;14:950–958. doi: 10.1208/s12249-013-9981-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markl D., Zeitler J.A. A review of disintegration mechanisms and measurement techniques. Pharm. Res. (N. Y.) 2017;34:890–917. doi: 10.1007/s11095-017-2129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mircioiu C., Voicu V., Anuta V., Tudose A., Celia C., Paolino D., Fresta M., Sandulovici R., Mircioiu I. Mathematical modeling of release kinetics from supramolecular drug delivery systems. Pharmaceutics. 2019;11:1–18. doi: 10.3390/pharmaceutics11030140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panotopoulos G.P., Haidar Z.S. Mathematical modeling for pharmacokinetic predictions from controlled drug release nano systems: a comparative parametric study. Biomed. Pharmacol. J. 2018;11:1801–1806. doi: 10.13005/bpj/1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheskey P.J., Walter C.G., Cable C.G. eighth ed. Pharmaceutical Press and American Pharmacists Association; London-Washington DC: 2017. Handbook of Pharmaceutical Excipients. [Google Scholar]
- Shete A., Salunkhe A., Yadav A., Sakhare S., Doijad R. Neusilin based liquisolid compacts of albendazole: design, development, characterization and in vitro anthelmintic activity. Marmara Pharm. J. 2019;23:441–456. doi: 10.12991/jrp.2019.151. [DOI] [Google Scholar]
- Siswanto A., Fudholi A., Nugroho A.K., Martono S. In vitro release modeling of aspirin floating tablets using ddsolver. Indones. J. Pharm. 2015;26:94. doi: 10.14499/indonesianjpharm26iss2pp94. [DOI] [Google Scholar]
- Sugiarno F.D. 2-(4-chloromethyl)benzoyl)oxy)benzoate in male white rats (Rattus norvegicus) Theses; 2016. [Google Scholar]
- Suhartati T. Anugrah Utama Raharja; Bandar Lampung: 2017. Basic of UV-VIS spectrophotometry and mass spectromistry for structural determination of organic compounds. [Google Scholar]
- Tamayanti W.D., Widharna R.M., Caroline C., Soekarjo B. Uji aktivitas analgesik asam 2-(3-(Klorometil)Benzoiloksi)Benzoat dan asam 2-(4-(Klorometil)Benzoiloksi)Benzoat pada tikus wistar jantan dengan metode plantar test. J. Pharm. Sci. Community. 2016;13:15–22. doi: 10.24071/jpsc.131125. [DOI] [Google Scholar]
- The United States Pharmacopeial Convention . Twinbrook Parkway; Rockville: 2018. Pharmacopeia 41-National Formulary 36. [Google Scholar]
- Thoorens G., Krier F., Leclercq B., Carlin B., Evrard B. Microcrystalline cellulose, a direct compression binder in a quality by design environment - a review. Int. J. Pharm. 2014;473:64–72. doi: 10.1016/j.ijpharm.2014.06.055. [DOI] [PubMed] [Google Scholar]
- Tjahjono Y., Caroline, Foe K., Wijaya H., Dewi B.D.N., Karnati S., Esar S.Y., Karel P., Partana F.R., Henrikus M.A., Wiyanto C.A., Wilianto Y.R., Hadinugroho W., Nugraha J., Nugrahaningsih D.A.A., Kusindarta D.L., Wihadmadyatami H. 2-(3-(Chloromethyl)benzoyloxy)benzoic Acid reduces prostaglandin E-2 concentration, NOX2 and NFKB expression, ROS production, and COX-2 expression in lipopolysaccharide-induced mice. Prostag. Other Lipid Mediat. 2024;174 doi: 10.1016/j.prostaglandins.2024.106866. [DOI] [PubMed] [Google Scholar]
- Tjahjono Y., Karnati S., Foe K., Anggara E., Gunawan Y.N., Wijaya H., Steven, Suyono H., Esar S.Y., Hadinugroho W., Wihadmadyatami H., Ergun S., Widharna R.M., Caroline . 2021. Prostaglandins and Other Lipid Mediators Acid in LPS-Induced Rat Model 154. [DOI] [PubMed] [Google Scholar]
- Wahab A., Khan G.M., Akhlaq M., Khan N.R., Hussain A., Khan M.F., Khan H. Formulation and evaluation of controlled release matrices of ketoprofen and influence of different co-excipients on the release mechanism. Pharmazie. 2011;66:677–683. doi: 10.1691/ph.2011.1040. [DOI] [PubMed] [Google Scholar]
- Yasmin R., Shoaib M.H., Ahmed F.R., Qazi F., Ali H., Zafar F. Aceclofenac fast dispersible tablet formulations: effect of different concentration levels of Avicel PH102 on the compactional, mechanical and drug release characteristics. PLoS One. 2020;15 doi: 10.1371/journal.pone.0223201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Huo M., Zhou J., Zou A., Li W., Yao C., Xie S. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12:263–271. doi: 10.1208/s12248-010-9185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupančič-Bozič D., Vrečer F., Kozjek F. Optimization of diclofenac sodium dissolution from sustained release formulations using an artificial neural network. Eur. J. Pharmaceut. Sci. 1997;5:163–169. doi: 10.1016/S0928-0987(97)00273-X. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
In addition to the tables and figures in the article, the research results present supporting data on the Supplementary Material: Tablet dosage calculation; the release of 4CH2Cl from the tablets; statistical analysis of 4CH2Cl tablets; the kinetics profile of the release of 4CH2Cl from Ta, Tb, Tc, and To. tablets.
The authors do not have permission to share data.