Abstract
Colorectal cancer (CRC) is a malignant tumor with a high incidence, ranking first among gastrointestinal malignancies. We investigated the impact of polyphyllin I (PPI), a natural compound found in Paris polyphylla, on CRC. PPI has been documented to exhibit anticancer activity against various tumors. This study aimed to assess the effects of PPI on colorectal cancer and explore its potential mechanisms. Our research demonstrated that PPI inhibited proliferation, promoted apoptosis, and induced G2 cell-cycle arrest in a dose-dependent manner. Additionally, our results indicated that PPI suppressed Notch signaling by downregulating the Notch1 receptor, its ligand Jagged1, and the downstream target Hes1 expression. Furthermore, we confirmed the antitumor effect of PPI on patient-derived organoids. In conclusion, our study indicates that PPI impedes the growth of colon cancer by suppressing the Notch signaling pathway.
Keywords: Polyphyllin I, Colorectal cancer, Organoid, Proliferation, Apoptosis, Cell cycle arrest
1. Introduction
Colorectal cancer (CRC) is one of the most frequent tumors of the gastrointestinal tract and is one of the leading causes of cancer-related deaths [1,2]. Globally, the incidence of CRC is rapidly increasing due to abnormal lifestyles and unhealthy diets such as smoking, excessive alcohol consumption, and too much animal fats [3]. People in the family who have been diagnosed with CRC have a high risk of developing CRC [4]. In 2020, there were 1.93 million newly diagnosed cases of CRC and 935,000 fatalities globally [5]. The current CRC treatments usually include multiple combinations of tumor resection, chemotherapy, targeted therapy, and immunotherapy [6]. However, such therapies often have only partial success in most cancer patients due to chemotherapy resistance and severe side effects, highlighting the need for the new economic and effective anti-cancer agents [7,8].
Polyphyllin I (PPI) is a basic active ingredient isolated from the Chinese herbal medicine Paris polyphylla. Several independent studies have reported that PPI exhibits activity against cancer cells, such as non-small-cell lung cancer [9], human osteosarcoma [10] and melanoma [11]. Previous studies have showed that PPI also exerted chemosensitizing effects in many cancers [12,13]. Besides, Huang et al. found that PPI had a good protective effect on myocardial ischemia/reperfusion damage via controlling the inflammatory response and oxidative stress [14]. Wang et al. reported that Polyphyllin I could obviously improve ankle synovitis in Collagen-Induced Arthritic mice [15]. However, studies about the role of PPI in CRC are limited and the potential bioactivities and mechanisms of PPI in CRC remain unclear.
The Notch signaling pathway is one of the important pathways that determine cell fate. It has a feature of conservation and affects multiple physiological or pathological functions of cells such as proliferation, apoptosis, differentiation, stem cell maintenance, and cancer metastasis [16,17]. The Notch signaling system is activated by ligand binding, resulting in a conformational change in the Notch receptor that initiates sequential signaling [18]. Abnormal Notch signaling has been found in many tumors and exerts a complicated tumor-promoting or tumor-suppressing role according to the tumor microenvironment [19]. In recent years, it had been confirmed that the Notch signaling played an extremely essential role in the progression of CRC. Notch1 and its target gene Hes1 were overexpressed in most advanced colon tumors, but rarely expressed in early-stage or benign tumors [20]. Therefore, selectively targeting Notch ligands or receptors as an underlying therapeutic method is essential for the treatment of malignant tumors.
In this research, we investigated the influences of PPI on various biological behaviors such as proliferation and apoptosis of CRC cells or organoids, and the potential mechanism of PPI-mediated suppression of CRC was identified.
2. Materials and methods
2.1. Reagents and antibodies
Polyphyllin I (molecular formula: C44H70O16, purity ≥98 %) was obtained from Yuanye Bio-Technology Co., Ltd (Shanghai, China) and initially dissolved in DMSO (KeyGEN Biotech Corp., Ltd, Nanjing, China) to a final concentration of 80 mM and kept at −20 °C. Antibodies against Bax (ab32503), Bcl-2 (ab196495), P53 (ab26), CDK2 (ab32147), Notch1 (ab52627), Jagged1 (ab7771) and Hes1 (ab108937) were acquired from Abcam (Cambridge, UK). β-Actin (JTM6921) was from Jinting Bio-Technology Co., Ltd (Nanjing, China). The dilution ratio for all antibodies was 1:1000.
2.2. Cell culture
HCT8 cells and HCT116 cells were provided by the central laboratory of Nanjing Hospital of Traditional Chinese Medicine (Jiangsu, China). Cells were cultured in RPMI-1640 complete medium (Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) involving 10 % FBS (Gibco) and 1 % penicillin/streptomycin in 37 °C humidified chambers with 5 % carbon dioxide and subcultured every 2–3 days.
2.3. Cell viability assay
Cell counting kit 8 (CCK-8, Lianke Biotech Corp., Ltd, Hangzhou, China) was performed to detect changes in cell proliferation. Seeded and cultured HCT8 and HCT116 cells (1 × 105 cells/mL) in 96-well plates overnight. RPMI-1640 complete medium with various concentrations of PPI (0, 1.25, 2.5, 5, 10, 20 μM) were added into each well respectively. After 12 h, 24 h, and 48 h of intervention, the morphology changes of the cultured cells were examined and photographed under an inverted fluorescence microscope (Nikon, Tokyo, Japan). Then, the cells were added with 10 % CCK8 and incubated for 2 h in a 37 °C incubator. Employing a microplate reader (Biotek, Winooski, VT, USA) to test the absorbance at 450 nm. The cell survival rate was calculated as follows: cell survival rate (%) = (absorbance value of the treatment group)/(absorbance value of the untreated group) × 100 %.
2.4. Transmission electron microscopy (TEM) detection
The 2.5 % glutaraldehyde fixed the HCT8 cells treated with 5 μM PPI for 24 h. The cells were washed twice with PBS before the fixation with 2 % osmium tetroxide for 2 h. After being dehydrated with ethanol, they were embedded in resin which was then sliced into ultra-thin slices and stained with 3 % uranyl acetate and lead citrate for 5 min. Finally, the observation and recording of ultrastructural changes in cell slices were performed under a TEM (Hitachi, Japan).
2.5. Cell apoptosis assay
Cell apoptosis was evaluated with Annexin V-PE/7-AAD apoptosis detection kit (Bestbio Technology Co., Ltd, Shanghai, China). Briefly, cells were placed and cultured in 60 mm dishes for 24 h and then intervened with PPI at different concentrations (0, 2.5, 5, and 10 μM). Next, the cells were trypsinized, washed twice with ice-cold PBS, and stained using Annexin V-PE/7-AAD at 4 °C in the dark. Flow cytometry (Beckman Coulter, CA, USA) was employed to perform the data acquisition and analysis of the cell apoptosis.
2.6. Cell cycle analysis
HCT8 cells in RPMI-1640 complete medium were seeded in 60 mm dishes at a concentration of 1 × 106 cells/dish overnight and treated with various concentration gradients of PPI for 24 h. After that, cells of each dish were collected by trypsinization and fixed with 75 % cold ethanol overnight. Cells were then incubated with 20 μL RNase in a 37 °C water bath and with 400 μL PI at 4 °C for half an hour respectively. The analysis of cell cycle distribution was completed by flow cytometry (Beckman Coulter) and Modfit LT software (Verity Software House, Topsham, USA).
2.7. Western blotting analysis
After treatment, the total protein from HCT8 cells was obtained by lysing the cells in RIPA buffer (Jinting Bio-Technology Co., Ltd, Nanjing, China). Using a BCA protein assay reagent kit (Pierce, Thermo Fisher Scientific, Inc.) to quantify the protein concentration. The protein extract was isolated by sodium dodecyl sulfate-polyacrylamide gel (SDS–PAGE) and then moved to the PVDF membrane, which was then blocked with 5 % nonfat milk in TBST for 1.5 h. This membrane was incubated with the primary antibodies containing Notch1, Jagged1, Hes1, Bax, Bcl-2, P53, CDK2, and β-actin at 4 °C overnight, and then incubated further with horseradish peroxidase-conjugated antibodies (Jinting Bio-Technology Co., Ltd) for 2 h. The protein expression was detected with chemiluminescence reagents (Millipore, Waltham, MA, USA).
2.8. Reverse transcription PCR (RT-PCR) analysis
HCT8 cells were treated with different concentrations of PPI (0–10 μM) at the exponential growth phase for 24 h. Trizol reagent (Life, Thermo Fisher Scientific, Inc.) was employed to lyse cells and acquire total RNA on the ice. Then, total RNA was reverse transcribed into cDNA through a PrimeScript™ 1st Strand cDNA Synthesis Kit (Takara Bio, Inc., Otsu, Japan). Bio-Rad PCR machine and SYBRGreen PCR kit (Takara Bio, Inc.) were utilized to execute PCR. The primer sequences for the genes used in this research were exhibited in Table 1. The thermocycling conditions were as follows: 94 °C for 5 min, followed by 40 cycles at 94 °C for 30 s, 55 °C for 45 s, 72 °C for 30 s and 72 °C for 7 min. GAPDH was the internal control. Relative mRNA levels were analyzed using the 2−ΔΔCt method [21].
Table 1.
Primer sequences in this research.
| Name | Forward primer 5′- 3′ | Reverse primer 3′- 5′ |
|---|---|---|
| Notch1 | TACGTGTGCACCTGCCGGG | CGTTTCTGCAGGGGCTGGGG |
| Jagged1 | TCGCTGTATCTGTCCACCTG | AGTCACTGGCACGGTTGTAG |
| Hes1 | ATGACGGCTGCGCTGAGCAC | TAACGCCCTCGCACGTGAC |
| GAPDH | GTCGGAGTCAACGGATTTGG | AAAAGCAGCCCTGGTGACC |
2.9. Patients and samples
Tumor tissues and healthy colorectal tissues of CRC patients were acquired from surgical resection in Nanjing Hospital of Traditional Chinese Medicine. Written informed consent was obtained from all patients before the collection of samples. All tissues were obtained from patients who did not receive chemotherapy or radiation before operation. The tumor tissues were all pathologically analyzed at the department of pathology and all were colorectal carcinoma. The Ethics Committee of Nanjing Hospital of Traditional Chinese Medicine had reviewed and passed the experimental research.
2.10. Colorectal cancer organoid culture and viability assay
Human intestinal crypts from either tumor tissue or adjacent healthy tissue were extracted with collagenase (Sigma-Aldrich, Shanghai, China) and embedded in Matrigel, as reported previously by Sato et al. [22]. Organoid pellets were seeded in 48-well plates and then incubated in a DMEM/F12 complete medium with A-8301 (Tocris Bioscience, Shanghai, China), EGF (Sino, Shanghai, China), R-spondin 1 (Sino), Noggin (Sino), Gentamicin/amphotericin B (Gibco), Glutamax (Gibco), HEPES (Gibco), Normocin (InvivoGen, CA, USA), Nicotinamide (Sigma-Aldrich), B27 (Invitrogen, Thermo Fisher Scientific), N2 (Invitrogen), N-Acetylcysteine (Invitrogen). The medium was refreshed every two days, and the organoids were passaged every 10–14 days by mechanical disruption with a pipette tip.
The experiment consisted of 7 groups. After the organoids were seeded in 96-well plates overnight, PPI (0 μM, 5 μM, 10 μM, 20 μM, 40 μM, 80 μM, 160 μM) was added to each group of organoids for 24 h, respectively. Treated organoids were subjected to morphological analysis using an inverted fluorescence microscope (Nikon). Cell viability was measured utilizing a CCK8 kit (Lianke Biotech Corp., Ltd) on the basis of the instruction manual after PPI treatment.
2.11. Statistical analysis
All data analysis came from GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA). The results were expressed as mean ± standard deviation (mean ± SD). The One-way ANOVA test was used as a statistical method to evaluate the statistical differences between groups. Each experiment was repeated at least three times. A difference was considered to be significant when p < 0.05.
3. Results
3.1. Polyphyllin I inhibited the proliferation of the colorectal cancer cells
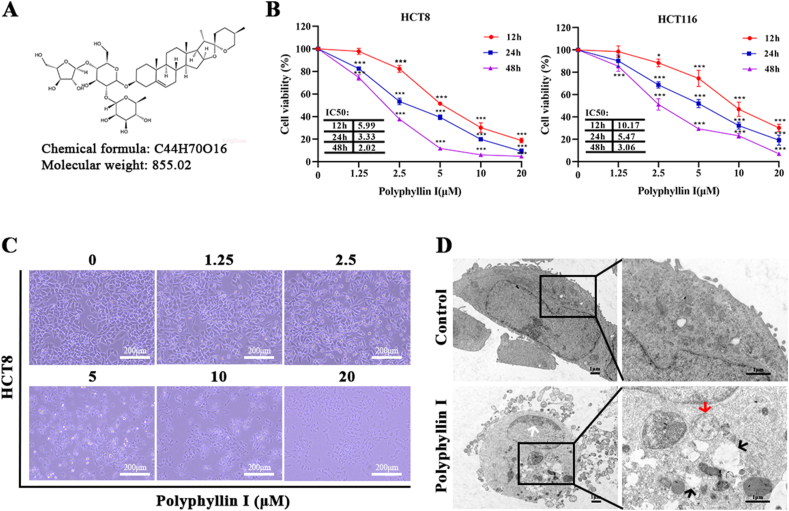
The chemical structure of PPI is presented in Fig. 1A. Two CRC cell lines, including HCT8 and HCT116, were originally used to investigate the suppressive effect on proliferation induced by PPI in vitro. After treatment with PPI for 12, 24, or 48 h, the IC50 values were 5.99, 3.33, or 2.02 μM in HCT8 cells and 10.17, 5.47, or 3.06 μM in HCT116 cells (Fig. 1B). The depressant effect of PPI on the proliferation of HCT8 and HCT116 cells gradually strengthened with the prolongation of time and the increase of dose. Under the same time and concentration of PPI intervention, the IC50 value of HCT8 cells was lower than that of HCT116 cells, indicating that the HCT116 cell line was less sensitive to PPI compared with the HCT8 (Fig. 1B). In summary, based on the IC50 values, the HCT8 cell line which was more sensitive to PPI was chosen for subsequent experiments. HCT8 cells were intervened with 0, 2.5, 5, 10, and 20 μM PPI for a day. We observed that with the increase of PPI dose, HCT8 cells gradually shed from the surface, lost the ability to attach, and the cell proliferation ability decreased (Fig. 1C). In cells intervened with PPI (concentration of 5 μM), the integrity of the cell membrane was destroyed and numerous vacuolar-like structures increased in the cytoplasm under the transmission electron microscopy. Other ultrastructure changes also included shrinkage of cell volume, margination of chromatin, and damage of organelles structure (Fig. 1D). Taken together, these above data intensively implied that PPI might exhibit profound inhibitory activity against human CRC cells.
Fig. 1.
Anti-proliferative effect of Polyphyllin I on CRC cells. A. Chemical structure of PPI. B. Under PPI (0, 1.25, 2.5, 5, 10, and 20 μM) treatment for 12, 24, and 48 h, HCT8 and HCT116 cells viability was measured by CCK8 assay. C. Under PPI (0, 1.25, 2.5, 5, 10, and 20 μM) treatment for 24 h, the morphological changes of the HCT8 cells were observed at × 100 magnification. Scale bar, 50 μM. D. Under 5 μM PPI treatment for 24 h, the ultrastructure of HCT8 cells was observed with TEM. The white arrow indicates the margination of chromatin. The black arrows indicate cell vacuolization. The red arrow indicates mitochondrial edema and dissolution of cristae within the mitochondria. Scale bar, 1 μM. Data were presented as the means ± SD, n = 4, *P < 0.05, ***P < 0.001 vs Control.
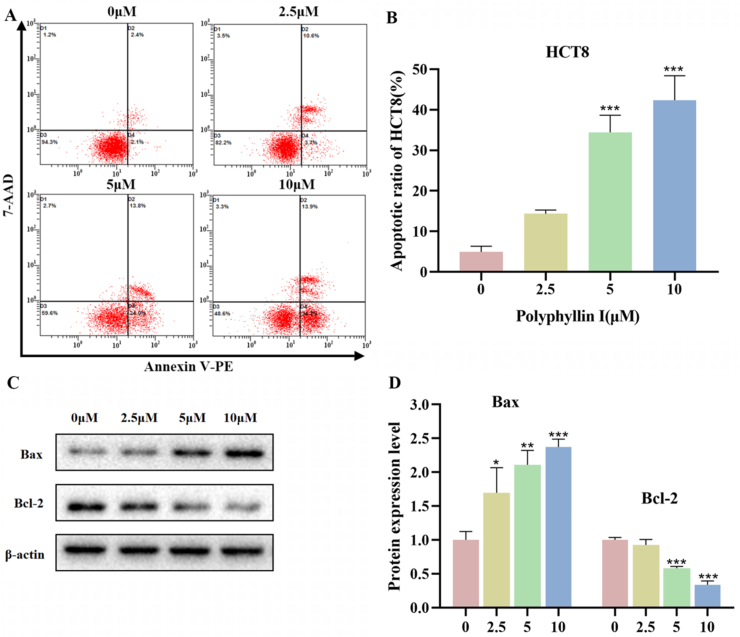
3.2. Polyphyllin I promoted the apoptosis of HCT8 cells through the regulation of protein expression of Bax and Bcl-2 in HCT8 cells
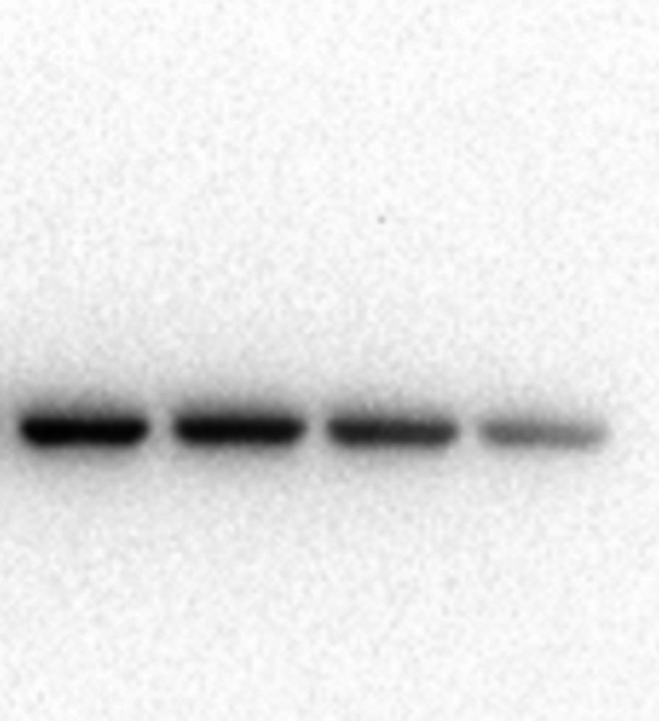
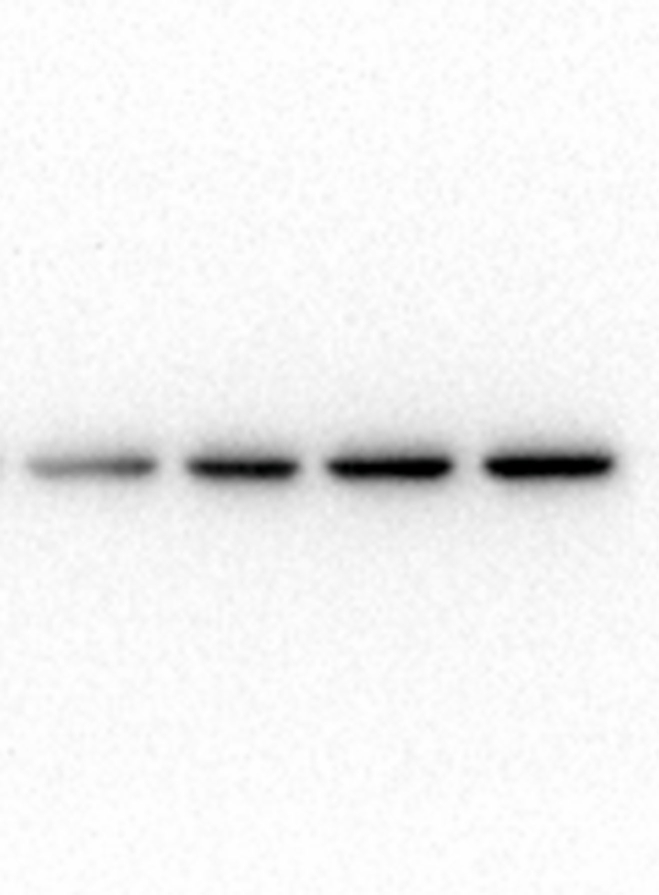
To examine the role of PPI-induced cytotoxicity on apoptosis, HCT8 cells were identified via dual-staining with Annexin V-PE and 7-AAD. As shown in Fig. 2A and B, apoptosis percentages in HCT8 cells after treatment with 0, 2.5, 5, and 10 μmol/L PPI for 24 h were 4.93 % ± 1.14 %, 14.37 % ± 0.74 %, 34.43 % ± 3.45 %, and 42.37 % ± 4.91 %. The results demonstrated that the apoptotic ratio of HCT8 cells was increased and the impact was dose-dependent. Moreover, to assess more exactly the role of PPI in the apoptotic pathway, Bcl-2 and Bax proteins were tested with Western blot analysis. Down-regulation of Bcl-2, as well as up-regulation of Bax, was observed following PPI treatment (Fig. 2C and D). Thus, it could be summarized that as the PPI dose increased, so did the total apoptosis ratio.
Fig. 2.
Polyphyllin I induced apoptosis of HCT8 cells. A. HCT8 cells treated with 5 μM PPI for 24 h were analyzed by flow cytometry. B. The percentage of apoptotic HCT8 cells. C. HCT8 cells were treated with 5 μM PPI for 24 h, and the protein levels of Bax and Bcl-2 were detected by Western blot analysis. D. Quantification of Bax and Bcl-2 expression levels in HCT8 cells using Image-Pro Plus 6.0 software. Data were presented as the means ± SD, n = 3, *P < 0.05, **P < 0.01, ***P < 0.001 vs Control.
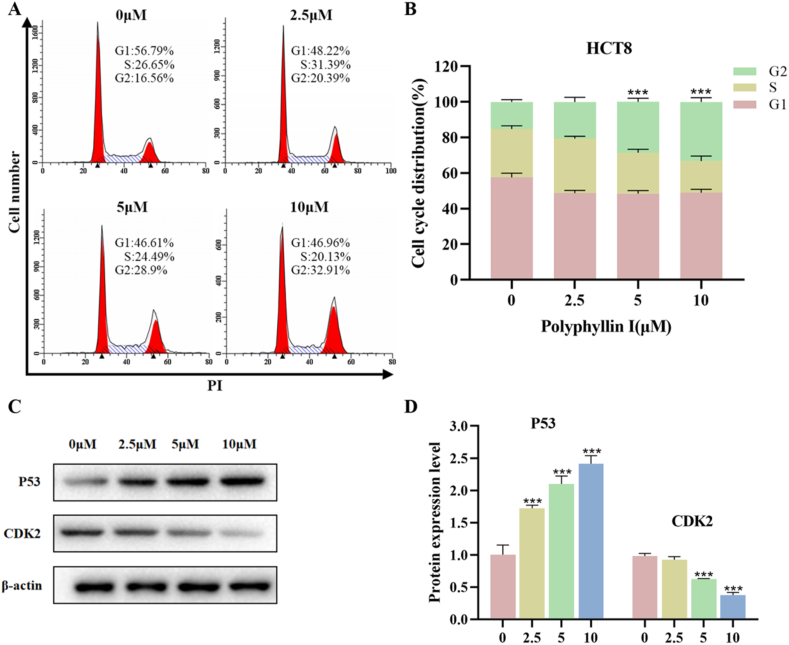
3.3. Polyphyllin I induced HCT8 cell cycle arrest at the G2 phase
We performed flow cytometry to analyze the HCT8 cell cycle. The percentage of HCT8 cells in the G2 phase increased from 16.56 % (without PPI) to 32.91 % (in the presence of 10 μM PPI), as shown in Fig. 3A and B. To study the mechanism of PPI-induced cell cycle arrest, we used Western blot analysis to detect cell cycle-related proteins. We found that P53 protein expression increased and CDK2 protein expression decreased in the HCT8 cell line after treatment with different concentrations of PPI (2.5, 5, and 10 μM) for one day (Fig. 3C and D). Therefore, these data suggest that PPI can trigger cell cycle arrest at the G2 phase in HCT8 cells.
Fig. 3.
Polyphyllin I induced cell cycle arrest in HCT8 cells. A. Under 5 μM PPI treatment for 24 h, flow cytometry was used to analyze the distribution percentages of different phases of the HCT8 cell cycle. B. The percentage of cells at different phases of the cycle was displayed as a bar graph. C. Under 5 μM PPI treatment for 24 h, the expressions of cell cycle-regulated proteins were measured by Western blot. D. Quantification of P53 and CDK2 expression levels in HCT8 cells with Image-Pro Plus 6.0 software. Data were presented as the means ± SD, n = 3, ***P < 0.001 vs Control.
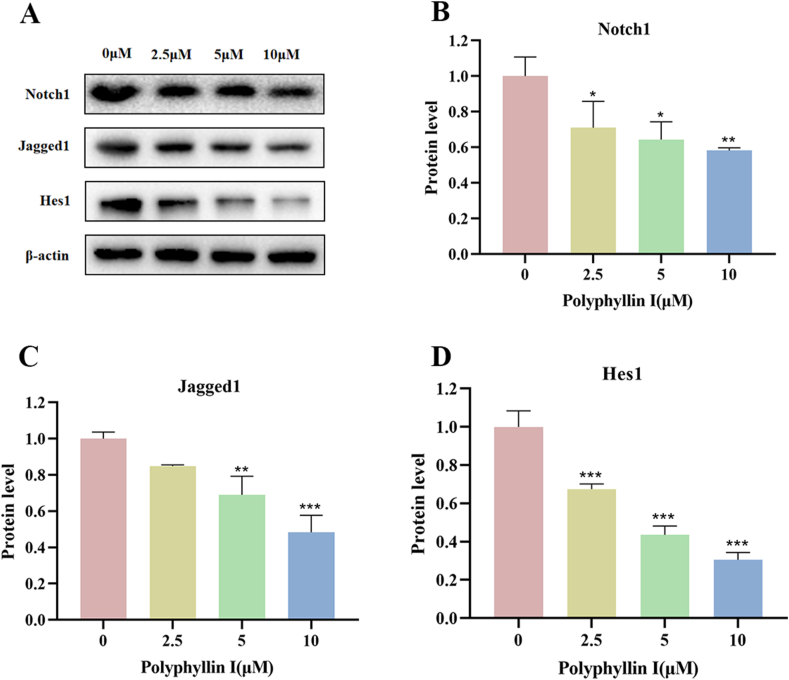
3.4. Polyphyllin I downregulated the expression of notch signaling pathway-related proteins in HCT8 cells
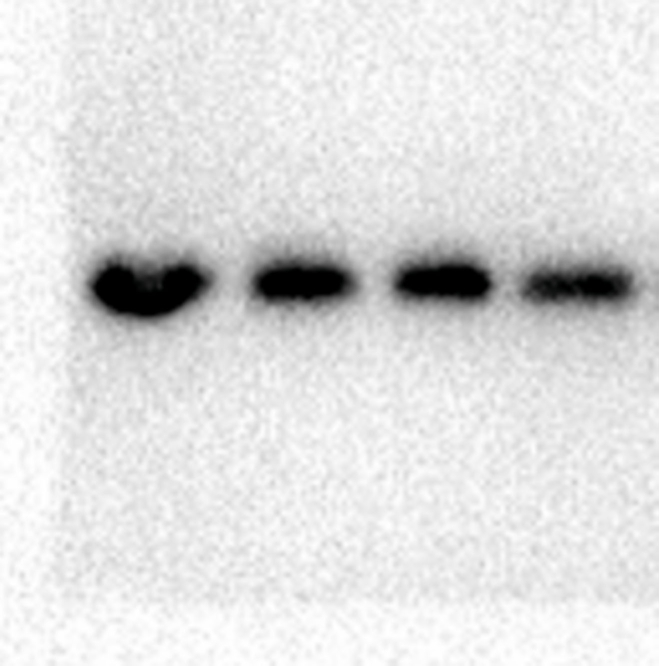
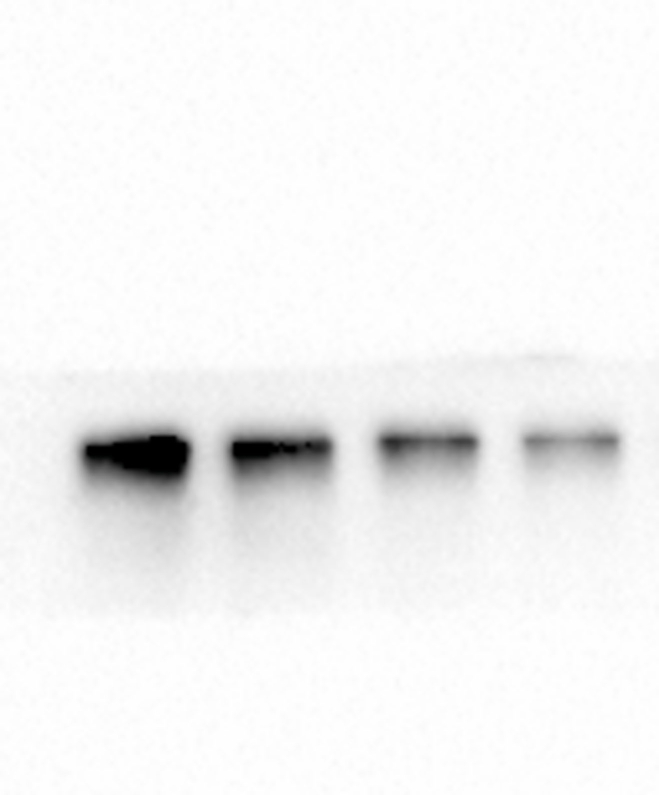
The above experiments demonstrated that PPI inhibited various vital biological processes, such as cell proliferation, cell cycle progression, and apoptosis. The Notch signaling pathway is crucial in various types of tumors. We speculated that the Notch signaling pathway might be the underlying target of PPI. To further elucidate whether the effects of PPI on cells were related to the Notch signaling pathway, we tested the expression changes of key proteins in this pathway via Western blot. We discovered that the protein expressions of Notch1, Jagged1, and Hes1 decreased in the 2.5 μM, 5 μM, and 10 μM groups compared to the 0 μM group (Fig. 4A–D). These data indicate that PPI suppresses the Notch pathway in a dose-dependent manner in HCT8 cells.
Fig. 4.
Polyphyllin I downregulated the expressions of Notch signaling pathway-related proteins in HCT-8 cells. A. After treating HCT8 cells with 5 μM PPI for 24 h, the expression levels of Notch1, Jagged1, and Hes1 in cells were detected via Western blot. β-Actin was employed as an internal control. B-D. Protein expressions were evaluated via histogram analyses. Data were presented as the means ± SD, n = 3, *P < 0.05, **P < 0.01, ***P < 0.001 vs Control.
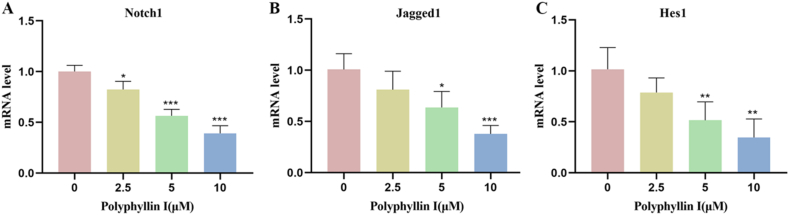
3.5. Polyphyllin I downregulated the mRNA expression of Notch1, Jagged1, and Hes1 in HCT8 cells
Subsequently, to verify the influence of the Notch signaling pathway on colorectal cancer, we examined the mRNA expressions of Notch1, Jagged1, and Hes1 using polymerase chain reaction analysis. Consistent with the protein level results, upon treating colorectal cancer HCT8 cells with different doses of PPI, we found that the mRNA expressions of these three genes were significantly reduced (Fig. 5A–C). These results suggest that PPI may exert its antitumor activity by partially inhibiting the Notch signaling pathway in colorectal cancer.
Fig. 5.
Polyphyllin I treatment downregulated the expressions of Notch signaling pathway-related genes in HCT8 cells. A. After treating HCT8 cells with 5 μM PPI for 24 h, mRNA expression of the Notch signaling receptor Notch1 was detected. B. After treating HCT8 cells with 5 μM PPI for 24 h, mRNA expression of the Notch signaling ligand Jagged1 was detected. C. After treating HCT8 cells with 5 μM PPI for 24 h, mRNA expression of the Notch signaling downstream target gene Hes1 was detected. Data were presented as the means ± SD, n = 3, *P < 0.05, **P < 0.01, ***P < 0.001 vs Control.
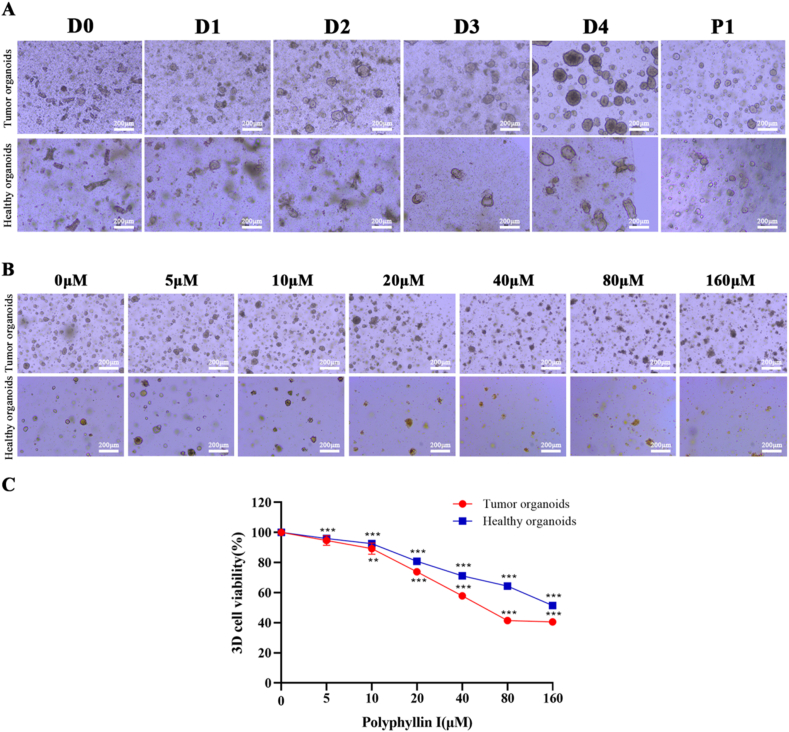
3.6. Polyphyllin I inhibited CRC patient-derived organoids
Morphological analysis showed that the patient-derived organoids grown from intestinal crypts became gradually thin-walled cystic structures, thick-walled cystic structures, or compact organoids over time (Fig. 6A), as previously described [23]. Human organoid cultures from healthy and tumor tissues were treated with PPI concentrations ranging from 5 to 160 μM for 24 h. We found that membrane integrity was disrupted in the experimental group while the 3D organoids preserved intact structures of the membrane in the untreated group, and PPI progressively weakened organoid numbers and size (Fig. 6B). In fact, the dose-dependent cell death was confirmed by the CCK8 assay and these data supported the conclusion that the organoids from healthy tissue were significantly less sensitive than organoids from tumor tissue (Fig. 6C).
Fig. 6.
Polyphyllin I inhibited CRC organoid growth. A. Chronological development of CRC patient-derived organoids in Matrigel droplet. B. Organoids were treated with PPI (5, 10, 20, 40, 80, and 160 μM) for 24 h, and the morphology was assessed at × 100 magnification by light microscopy. Scale bar, 50 μm. C. Cells viability was quantified by CCK8 assay. Data were presented as the means ± SD, n = 3, **P < 0.01, ***P < 0.001 vs Control.
4. Discussion
CRC has become an important clinical issue that requires new treatment methods. Natural products from medicinal plants are important resources for fighting tumors [24]. It has been recorded that the anti-cancer effects of PPI in tumors are mediated through different mechanisms that consist of autophagy, angiogenesis, and expression of malignant tumor-related genes [9,[25], [26], [27]]. In this study, we revealed that PPI reduced the viability of HCT8 and HCT116 cells in a time- and dose-dependent manner. By comparison with the value of IC50, it was observed that PPI had a stronger inhibitory effect on the HCT8 cells than the HCT116 cells under the same time and concentration of PPI intervention. We speculate that this may be related to the differential expression of proteins or genes affected by PPI in the two cell lines. In the subsequent research, we used HCT8 cells as a representative for further research. Moreover, we investigated the effects of PPI on human CRC organoids. Organoids are three-dimensional culture models based on stem cells that reproduce the cell growth environment, preserve genetic characteristics, and better mimic patient tumors. Therefore, they are considered to be the best in vitro models [28]. Our research showed the influence of PPI on the suppression of organoid morphology cultured from cancer patients, which corroborated the inhibiting effect of PPI. In contrast, PPI did not affect the growth of organoids from healthy tissues with low toxicity. By flow cytometry analysis, we found that PPI could promote apoptosis in HCT8 cells. Higher organisms exhibit internal and external pathways of apoptosis. Bcl-2 family is involved in the internal or mitochondrial apoptotic pathway. The family proteins are divided into proapoptotic members containing Bax and Bak, and antiapoptotic members containing Bcl-2 and Bcl-xl, which play a vital part in the regulation of apoptosis [29]. Liu et al. found that PPI could trigger mitochondria-mediated apoptosis in glioma cells through increasing the expression of Bax and reducing Bcl-2 expression, indicating that PPI induced apoptosis through mitochondria-mediated pathways [30]. In line with these reports, our research results exhibited that the expression of Bax increased and the expression of Bcl-2 decreased in HCT8 cells treated with PPI. In addition, we determined that PPI also aroused cells to be blocked at the G2 phase. Previous studies had shown that PPI could induce cell cycle arrest at the G2/M phase in human gastric carcinoma cells and G0/G1 phase in prostate cancer cells [31,32].
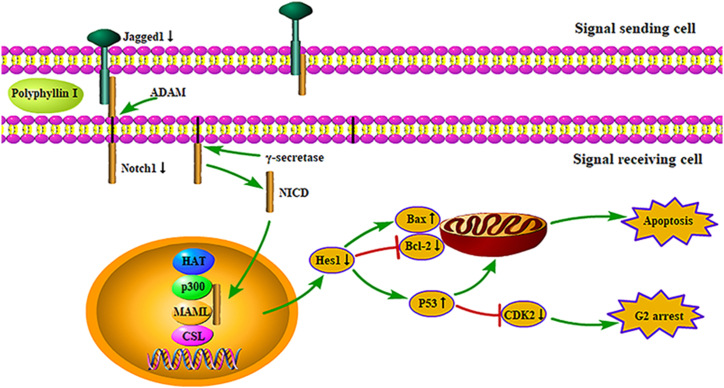
The Notch signaling pathway is quite vital in the occurrence and development of tumors. Abnormal activation of the signaling pathway leads to a series of abnormal biological modifications, which eventually leads to cell malignancies [33]. In mammals, the Notch signaling system molecules consist of five ligands (Jagged1-2, Delta-like1, 3, and 4) and four transmembrane receptors (Notch1-4), as well as some downstream target genes that comprise Hey1, Hes1, Deltex [34]. The Notch receptors undergo an initial cleavage in the Golgi apparatus and are then transported to the plasma membrane. When the Notch ligands bind to the receptors, the receptors are cleavaged by the metalloprotease (ADAM) and γ-secretase protein complex. Then intracellular domain (NICD) translocates into the nucleus and binds to the transcription factor CSL to form a complex. Then mastermind-like proteins (MAML), p300, and histone acetyltransferase (HAT) will be connected to CSL to form the MAML-CSL-HAT-P300 complex. Finally, the expression of the target genes is induced [34,35]. A variety of studies about colorectal cancer had shown that the Notch signaling was involved in the occurrence and development of CRC, and these researches highlighted the significant impact of the Notch signaling on tumor angiogenesis [36], cellular invasion and proliferation [37], stem cell function and phenotype [38], as well as the tumor immune microenvironment [37]. Animal experiments had demonstrated that the inhibition of the Notch pathway could reduce intestinal mucosal inflammation and maintain the stability of intestinal epithelial cells in GOLM1-deficient mice, thereby preventing the occurrence of colitis and CRC [39]. Liao et al. confirmed that Notch1 was overexpressed in cancer tissue and cell lines, and CRC cells were suppressed by genetic method to knock down Notch1 or Notch signaling pathway inhibitor, which hinted that activation of the Notch pathway was connected with the occurrence of CRC [40]. Therefore, targeting the Notch signaling pathway is potentially a valid mechanism for the treatment of CRC. Besides, the abnormally activated Notch signaling pathway was related to several other cancers including lung cancer [41] and gastric cancer [42]. However, in addition to carcinogenic effects related to cancer progression and metastasis, the Notch pathway also acted as a cancer inhibitor, depending on the context of cancer cells. Consistent with the results of our research, the expressions of Notch receptors, ligands, and target genes were decreased by PPI in HCT8 cells (RT-qPCR and WB). We observed that Notch signaling regulated the cell cycle and cell apoptosis via regulating Bax, Bcl-2, P53, and CDK2 proteins (Fig. 7).
Fig. 7.
Schematic diagram of the possible molecular mechanism of PPI-induced apoptosis in HCT8 cells.
5. Conclusion
In conclusion, the current research suggested that PPI restrained the growth of colorectal tumors in vitro and was the first to demonstrate that treatment with PPI was able to significantly inhibit patient-derived CRC organoids. PPI promoted cell apoptosis and arrested cell cycle progress via suppressing the expressions of Notch1, Jagged1, and Hes1. Altogether, PPI could be considered as a potential candidate for the management of CRC.
Funding
This work was supported by the Jiangsu Special Funds for Key Research and Development Program (BE2021611), the Natural Science Foundation of Nanjing University of Chinese Medicine (XZR2021044).
Ethics approval and consent to participate
The experimental protocol was approved by the Ethics Board of Nanjing Hospital of Chinese Medicine (KY2022266). The patients/participants provided their written informed consent to participate in this study.
Patient consent for publication
Patient consent for publication was covered by the informed consent document.
Availability of data
Data are available from corresponding authors, please contact fanzm@njucm.edu.cn.
CRediT authorship contribution statement
Yu Wang: Writing – review & editing. Hao Ge: Writing – original draft. Yi Zhang: Methodology. Pei Wang: Supervision. Haoran Zhao: Conceptualization. Lu Wang: Conceptualization. Zhimin Fan: Validation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors thank Central laboratory of Nanjing Hospital of Traditional Chinese Medicine, for providing the research platform of the current study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e37226.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
figs5.
figs6.
figs7.
figs8.
figs9.
figs10.
figs11.
figs12.
figs13.
figs14.
References
- 1.Bray F., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Sun X., et al. Colon cancer-related genes identification and function study based on single-cell multi-omics integration. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.789587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawk H.W., et al. Scaphium affine ethanol extract induces Anoikis by regulating the EGFR/Akt pathway in HCT116 colorectal cancer cells. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.621346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiong L.L., et al. Anti-colorectal cancer effects of scutellarin revealed by genomic and proteomic analysis. Chin. Med. 2020;15:28. doi: 10.1186/s13020-020-00307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu L.F., et al. Personalized immunotherapy in colorectal cancers: where do we stand? Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.769305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan A., et al. Immunotherapy in colorectal cancer: current achievements and future perspective. Int. J. Biol. Sci. 2021;17(14):3837–3849. doi: 10.7150/ijbs.64077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen K., et al. New benzimidazole acridine derivative induces human colon cancer cell apoptosis in vitro via the ROS-JNK signaling pathway. Acta Pharmacol. Sin. 2015;36(9):1074–1084. doi: 10.1038/aps.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alomrani A., et al. The use of chitosan-coated flexible liposomes as a remarkable carrier to enhance the antitumor efficacy of 5-fluorouracil against colorectal cancer. Saudi Pharmaceut. J. 2019;27(5):603–611. doi: 10.1016/j.jsps.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y., et al. Polyphyllin I activates AMPK to suppress the growth of non-small-cell lung cancer via induction of autophagy. Arch. Biochem. Biophys. 2020;687 doi: 10.1016/j.abb.2020.108285. [DOI] [PubMed] [Google Scholar]
- 10.Chang J., et al. Polyphyllin I suppresses human osteosarcoma growth by inactivation of Wnt/beta-catenin pathway in vitro and in vivo. Sci. Rep. 2017;7(1):7605. doi: 10.1038/s41598-017-07194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long J., Pi X. Polyphyllin I promoted melanoma cells autophagy and apoptosis via PI3K/Akt/mTOR signaling pathway. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/5149417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng F., et al. Polyphyllin I and VII potentiate the chemosensitivity of A549/DDP cells to cisplatin by enhancing apoptosis, reversing EMT and suppressing the CIP2A/AKT/mTOR signaling axis. Oncol. Lett. 2019;18(5):5428–5436. doi: 10.3892/ol.2019.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin L.T., et al. Paris polyphylla inhibits colorectal cancer cells via inducing autophagy and enhancing the efficacy of chemotherapeutic drug doxorubicin. Molecules. 2019;24(11) doi: 10.3390/molecules24112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang R., et al. The protective effect of polyphyllin I on myocardial ischemia/reperfusion injury in rats. Ann. Transl. Med. 2020;8(10):644. doi: 10.21037/atm-20-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q., et al. Polyphyllin I ameliorates collagen-induced arthritis by suppressing the inflammation response in macrophages through the NF-kappaB pathway. Front. Immunol. 2018;9:2091. doi: 10.3389/fimmu.2018.02091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brzozowa-Zasada M., et al. Notch and its oncogenic activity in human malignancies. Eur. Surg. 2017;49(5):199–209. doi: 10.1007/s10353-017-0491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiu M.X., Liu Y.M., Kuang B.H. The oncogenic role of Jagged1/Notch signaling in cancer. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110416. [DOI] [PubMed] [Google Scholar]
- 18.Misiorek J.O., et al. Context matters: NOTCH signatures and pathway in cancer progression and metastasis. Cells. 2021;10(1) doi: 10.3390/cells10010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katoh M, Katoh M. Precision medicine for human cancers with Notch signaling dysregulation (Review) Int J Mol Med. 2020 Feb;45(2):279–297. doi: 10.3892/ijmm.2019.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyagi A., Sharma A.K., Damodaran C. A review on notch signaling and colorectal cancer. Cells. 2020;9(6) doi: 10.3390/cells9061549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Sato T., et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141(5):1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 23.Hong H.K., et al. Establishment of patient-derived organotypic tumor spheroid models for tumor microenvironment modeling. Cancer Med. 2021;10(16):5589–5598. doi: 10.1002/cam4.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mothana R.A., et al. Comparative evaluation of cytotoxic, antimicrobial and antioxidant activities of the crude extracts of three Plectranthus species grown in Saudi Arabia. Saudi Pharmaceut. J. 2019;27(2):162–170. doi: 10.1016/j.jsps.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L., et al. Inhibition of EZH2 via activation of SAPK/JNK and reduction of p65 and DNMT1 as a novel mechanism in inhibition of human lung cancer cells by polyphyllin I. J. Exp. Clin. Cancer Res. 2016;35(1):112. doi: 10.1186/s13046-016-0388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo Q., et al. Role of the death receptor and endoplasmic reticulum stress signaling pathways in polyphyllin I-regulated apoptosis of human hepatocellular carcinoma HepG2 cells. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/5241941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W., et al. Inhibitory effects of Paris saponin I, II, Ⅵ and Ⅶ on HUVEC cells through regulation of VEGFR2, PI3K/AKT/mTOR, Src/eNOS, PLCgamma/ERK/MERK, and JAK2-STAT3 pathways. Biomed. Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110750. [DOI] [PubMed] [Google Scholar]
- 28.Elbadawi M., et al. Anti-inflammatory and tight junction protective activity of the herbal preparation STW 5-II on mouse intestinal organoids. Phytomedicine. 2021;88 doi: 10.1016/j.phymed.2021.153589. [DOI] [PubMed] [Google Scholar]
- 29.Watson A.J. Apoptosis and colorectal cancer. Gut. 2004;53(11):1701–1709. doi: 10.1136/gut.2004.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J., et al. Polyphyllin I induces G2/M phase arrest and apoptosis in U251 human glioma cells via mitochondrial dysfunction and the JNK signaling pathway. Acta Biochim. Biophys. Sin. 2017;49(6):479–486. doi: 10.1093/abbs/gmx033. [DOI] [PubMed] [Google Scholar]
- 31.He J., et al. Polyphyllin I induces autophagy and cell cycle arrest via inhibiting PDK1/Akt/mTOR signal and downregulating cyclin B1 in human gastric carcinoma HGC-27 cells. Biomed. Pharmacother. 2019;117 doi: 10.1016/j.biopha.2019.109189. [DOI] [PubMed] [Google Scholar]
- 32.Zhang D., et al. Polyphyllin I induces cell cycle arrest in prostate cancer cells via the upregulation of IL6 and P21 expression. Medicine (Baltim.) 2019;98(44) doi: 10.1097/MD.0000000000017743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaik J.P., et al. Frequent activation of notch signaling pathway in colorectal cancers and its implication in patient survival outcome. JAMA Oncol. 2020;2020 doi: 10.1155/2020/6768942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koveitypour Z., et al. Signaling pathways involved in colorectal cancer progression. Cell Biosci. 2019;9:97. doi: 10.1186/s13578-019-0361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang F., et al. Mutations in the notch signalling pathway are associated with enhanced anti-tumour immunity in colorectal cancer. J. Cell Mol. Med. 2020;24(20):12176–12187. doi: 10.1111/jcmm.15867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao M., et al. Classical angiogenic signaling pathways and novel anti-angiogenic strategies for colorectal cancer. Curr. Issues Mol. Biol. 2022;44(10):4447–4471. doi: 10.3390/cimb44100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang F., et al. Notch signaling mutations increase intra-tumor chemokine expression and predict response to immunotherapy in colorectal cancer. BMC Cancer. 2022;22(1):933. doi: 10.1186/s12885-022-10032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lan G., et al. Notch pathway is involved in the suppression of colorectal cancer by embryonic stem cell microenvironment. OncoTargets Ther. 2019;12:2869–2878. doi: 10.2147/OTT.S199046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pu Y., et al. GOLM1 restricts colitis and colon tumorigenesis by ensuring Notch signaling equilibrium in intestinal homeostasis. Signal Transduct. Targeted Ther. 2021;6(1):148. doi: 10.1038/s41392-021-00535-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao W., et al. Antitumor activity of Notch-1 inhibition in human colorectal carcinoma cells. Oncol. Rep. 2018;39(3):1063–1071. doi: 10.3892/or.2017.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J., et al. Analysis of molecular mechanism of YiqiChutan formula regulating DLL4-notch signaling to inhibit angiogenesis in lung cancer. BioMed Res. Int. 2021;2021 doi: 10.1155/2021/8875503. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Wang M., et al. Penicilazaphilone C. A new azaphilone, induces apoptosis in gastric cancer by blocking the notch signaling pathway. Front. Oncol. 2020;10:116. doi: 10.3389/fonc.2020.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from corresponding authors, please contact fanzm@njucm.edu.cn.