Abstract
Background
Unexpectedly low natriuretic peptide (NP) levels in proportion to heart failure severity are often observed in obese individuals. However, the magnitude of NP elevation in response to acute cardiac stress in obesity has not yet been extensively studied. This study aimed to determine the impact of obesity on the increase in plasma NP in response to cardiac hemodynamic stress during acute coronary syndrome (ACS) attacks.
Methods and Results
The study population included 557 consecutive patients with ACS for whom data were collected during emergency cardiac catheterization. To determine the possible impact of body mass index (BMI) on the relationship between left ventricular ejection fraction (LVEF) and plasma B-type NP (BNP) levels, the study population was divided into two groups (Group 1: BMI <25, Group 2: BMI ≥25 [kg/m2]). Both BMI and LVEF were significantly and negatively correlated with BNP. Although a significant negative correlation between LVEF and BNP was observed in both groups, the regression line of Group 2 was significantly less steep than that of Group 1. Accordingly, BNP/LVEF ratio in Group 2, which indicates the extent of BNP increase in response to LVEF change, was significantly lower than that in Group 1.
Conclusions
Blunted increase in plasma BNP in response to cardiac hemodynamic stress during ACS attacks was observed in obese individuals. In addition to the relatively low plasma BNP levels at baseline in obese individuals, the blunted response of BNP elevation to ACS attacks may have important pathophysiological implications for hemodynamic regulation and myocardial energy metabolism.
Keywords: Acute coronary syndrome, Natriuretic peptides, Obesity
1. Introduction
Natriuretic peptides (NP), such as A-type natriuretic peptide (ANP) and B-type natriuretic peptide (BNP), are hormones produced and secreted in the heart in response to cardiac stress [1], [2]. The plasma levels of NP serve as critical biomarkers of the severity of heart failure in various cardiovascular diseases [3]. ANP is primarily produced and secreted in the atria, whereas BNP is mainly produced and secreted in the ventricles [4]. Since BNP production and secretion are stimulated by cardiac overload, including end-diastolic wall stress, plasma BNP levels are closely correlated with disease severity and have been recognized as biochemical markers of ventricular dysfunction [1], [2], [5], [6], [7]. Likewise, acute ischemic insult to the heart leads to an immediate increase in circulating BNP levels, the magnitude of which is proportional to the severity of ischemia [8]. Accordingly, plasma BNP levels have also been reported to increase in acute coronary syndrome (ACS), such as acute myocardial infarction (AMI) and unstable angina pectoris (UAP) [9], [10], and can therefore be a significant prognostic marker [6], [11].
Increasing evidence suggests that baseline plasma NP levels are low in obese individuals without heart failure [12], [13], [14]. Furthermore, unexpectedly low NP levels in proportion to the severity of heart failure are often observed in obese patients (also known as “NP handicap”) [15], [16], [17], [18], [19]. Considering previous studies, including our own, which suggest that NP regulate the energy metabolism, improved insulin resistance and the promotion of glucose utilization in addition to the classical actions of hemodynamic regulation [20], [21], [22], [23], [24], [25], [26], “NP handicap” may be unfavorable, particularly for patients with ACS in whom glucose is a preferential substrate for the cardiac energy metabolism [27], [28].
Although BNP plays an essential role as an “emergency” cardiac hormone against ventricular overload [29], the impact of obesity on the response of plasma BNP to cardiac stress during the acute phase of cardiovascular diseases, typically ACS, has not yet been extensively investigated. In this human clinical study, we investigated whether there was a blunted increase in plasma BNP levels in response to cardiac overload during ischemic attacks of ACS in obese individuals, which may contribute to impaired myocardial glucose utilization.
2. Methods
2.1. Study patients
The study population comprised 976 consecutive patients who underwent emergency cardiac catheterization at our institution for the evaluation and treatment of ACS between February 2012 and October 2023. ACS was defined as the presence of AMI or UAP, as described previously [27], [30]. All patients with ACS underwent cardiac catheterization within 24 h of onset [27], [30]. Patients were excluded if their plasma BNP data were not available, if they were receiving dialysis, if they were in cardiopulmonary arrest just before or at the time of data sampling, or if they did not undergo left ventriculography. As all patients underwent left ventriculography in the present study, the cohort consisted of individuals with relatively stable hemodynamics and oxygenation. Therefore, there were no cases of cardiogenic shock (i.e., Killip class IV) or severe cases requiring mechanical circulatory support during cardiac catheterization in the present study. If a patient was enrolled more than once during the study period, only data from the initial inclusion were included in the analyses. The ethics committee of the Jikei University School of Medicine approved the study protocol (27-103[7988]) and we complied with the routine ethical regulations of our institution. All clinical investigations were conducted in accordance with the principles of the Declaration of Helsinki. As this was a retrospective study, instead of obtaining informed consent from each patient, we posted a notice about the study design and contact information in a public location in our institution according to our routine ethical regulations. In this public notification, we ensured that patients had the opportunity to refuse to participate (opt-put) in the study.
2.2. Data collection
The clinical characteristics of the patients were retrospectively collected from hospital medical records. Blood samples and hemodynamic data were collected during cardiac catheterization [26], [27], [30]. Plasma and serum biochemical analyses, including the BNP levels, were performed in the central laboratory of our hospital during the study period [26], [27], [30]. Some patients had comorbid cardiovascular diseases such as valvular disease, arrhythmia, cardiomyopathy, and other conditions. Hypertension and diabetes mellitus were defined as described previously [26], [27], [30]. The estimated glomerular filtration rate (eGFR) was calculated as previously described [26], [27], [30]. The left ventricular ejection fraction (LVEF), left ventricular end-diastolic pressure (LVEDP), left ventricular end-systolic volume (LVESV), and left ventricular end-diastolic volume (LVEDV) were measured at the time of left ventriculography [26], [27], [30].
2.3. Statistical analyses
Continuous variables are expressed as median ± interquartile range. Differences between the two groups were evaluated using the Mann-Whitney U-test. Correlations between two variables (BNP, eGFR, hemoglobin, C-reactive protein [CRP], age, body mass index [BMI], and LVEF) were assessed using Spearman’s rank correlation coefficient. Multiple regression analysis was performed to compare multiple values. To exclude the possibility of spurious correlations in the Spearman’s rank correlation coefficient, partial correlation analyses were also performed. In the analysis, we created an indicator variable coded 0/1 for variables with two categories, such as sex. To examine whether BMI levels had a statistically significant impact on the relationship between plasma BNP levels and LVEF, a multiple linear regression analysis was employed in which the dependent variable was plasma BNP levels, and the explanatory variables were LVEF, BMI levels, and the interaction term between LVEF and BMI (LVEF × BMI) [31]. We present scatter plots by BMI levels to illustrate the modification in the relationship between plasma BNP levels and LVEF. In the analysis of the BNP/LVEF ratio, a common logarithmic conversion (log) was performed on the ratio values because of exponential change. Statistical analyses were performed using the SPSS Statistics software (version 29.0, SPSS Inc., Chicago, IL, USA). Statistical significance was set at P < 0.05.
Path analysis based on covariance structure analysis was used to elucidate the direct contributions of age, sex, BMI, eGFR, hemoglobin (Hb), CRP, and LVEF to BNP. In other words, a path model was proposed to investigate the relationship between the clinical factors in this study population, and specifically to identify the possible impact of LVEF and BMI on BNP by considering the relationship between BNP and other factors. This analysis compared the power of multiple independent variables that confounded each other [26], [27]. Path analysis was performed using the IBM SPSS AMOS software program (version 29.0, Amos Development Corporation, Meadville, PA, USA), as previously described [26], [27], [30]. Briefly, the model defines hierarchical regression models between clinical factors and BNP levels. For every regression, the total variance in the dependent variable is theorized to be affected by either the independent variables included in the model or the extraneous variables (e). When obtaining the critical ratios of the differences between the parameters, AMOS was used to display the matrix, which included a row and column for each parameter of the model [26], [27]. The obtained structural equation models (SEMs) were tested and confirmed at a significance level of P < 0.05.
3. Results
3.1. Characteristics of the study patients
The clinical characteristics of 557 patients are presented in Table 1. The median BMI was 24.4 kg/m2 (interquartile range [IQR] 22.1-27.1 kg/m2), the median plasma BNP level was 26.5 pg/mL (IQR 11.2-74.8 pg/mL), the median LVEDP was 15 mmHg (IQR 11-20 mmHg), and the median LVEF was 56.7% (IQR 49.8-63.0%).
Table 1.
Clinical characteristics
| n = 557 | Median (IQR) or Number (%) |
|---|---|
| Age, years old | 60 (52-70) |
| Gender; Male (%) | 474 (85.1%) |
| BMI, kg/m2 | 24.4 (22.1-27.1) |
| Diabetes mellitus (%) | 154 (27.6%) |
| Hypertension (%) | 373 (67.0%) |
| Dyslipidemia (%) | 388 (69.7%) |
| Smoking (%) | 377 (67.7%) |
| Laboratory findings | |
| BNP, pg/mL | 26.5 (11.2-74.8) |
| Albumin, g/dL | 4.0 (3.8-4.3) |
| eGFR, ml/min/1.73m2 | 78.0 (67.0-90.0) |
| Hb, g/dL | 14.2 (13.1-15.0) |
| HbA1c, % | 5.8 (5.6-6.4) |
| TG, mg/dL | 81 (51-139) |
| LDL-C, mg/dL | 120 (94-145) |
| HDL-C, mg/dL | 50 (42-61) |
| CRP, mg/dL | 0.08 (0.04-0.28) |
| Hemodynamic findings | |
| Blood Pressure, mmHg | |
| Systolic | 137 (122-154) |
| Diastolic | 79 (69-88) |
| Mean | 105 (93-114) |
| LVEDP, mmHg | 15 (11-20) |
| LVEDV, mL | 105.5 (87.5-125.1) |
| LVEDVI, mL/m2 | 59.8 (50.2-69.7) |
| LVESV, mL | 45.3 (35.1-57.1) |
| LVESVI, mL/m2 | 25.2 (20.2-31.8) |
| LVEF, % | 56.7 (49.8-63.0) |
| Diagnosis | |
| Myocardial infarction (%) | 277 (49.7%) |
| Unstable angina (%) | 280 (50.3%) |
| Rhythm | |
| Sinus rhythm | 537 (96.4%) |
| Atrial fibrillation | 12 (2.2%) |
| Others | 8 (1.4%) |
| Medications | |
| ACE inhibitors (%) | 19 (3.4%) |
| ARBs (%) | 159 (28.5%) |
| ARNIs (%) | 2 (0.4%) |
| Beta blockers (%) | 83 (14.9%) |
| Calcium channel blockers (%) | 191 (34.3%) |
| Diuretics (%) | 34 (6.1%) |
Abbreviations: ACE = angiotensin converting enzyme; ARB = angiotensin II type I-receptor blockers; ARNI = angiotensin receptor neprilysin inhibitor; BMI = body mass index; BNP = B-type natriuretic peptide; CRP = C-reactive protein; eGFR = estimated glomerular filtration rate; Hb = hemoglobin; HbA1c = hemoglobin A1c; HDL-C = high-density lipoprotein; IQR = interquartile range; LDL-C = low-density lipoprotein; LVEDP = left ventricular end-diastolic pressure; LVEDV = left ventricular end-diastolic volume; LVEDVI = left ventricular end-diastolic volume index; LVEF = left ventricular ejection fraction; LVESV = left ventricular end-systolic volume; LVESVI = left ventricular end-systolic volume index; TG = triglycerides.
3.2. Correlations of BMI or LVEF with BNP
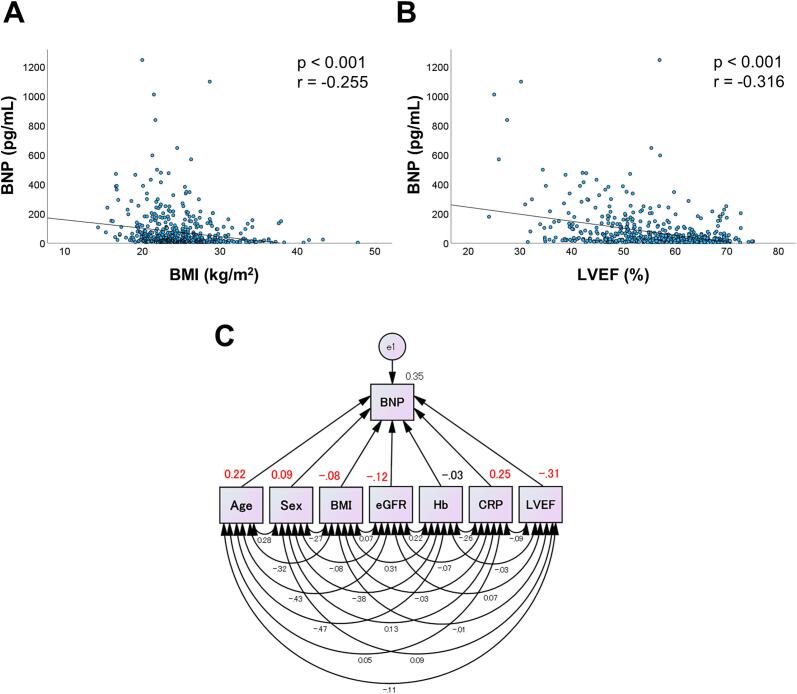
Figures 1A and 1B show Spearman’s rank correlation coefficients between plasma BNP levels and BMI (Figure 1A) and LVEF (Figure 1B). Both BMI and LVEF were significantly and negatively correlated with BNP levels (P < 0.001, each).
Fig. 1.
Both BMI and LVEF were negatively correlated with BNP. Correlations between BNP levels and BMI (A) and between BNP levels and LVEF (B) in the entire study population (n=557 each) are shown. P-values and correlation coefficient values (r) are noted. (C) Path diagrams against plasma BNP levels are shown. A path model was theoretically proposed to clarify the contribution of various parameters to the BNP. The path has a coefficient that shows the standardized coefficient of a regressing independent variable on the dependent variable of the relevant path. These variables indicate the standardized regression coefficients (direct effects). The underlined portions indicate significant values. Sex was coded 0/1 as an indicator variable (male = 0, female = 1). BMI = body mass index; BNP = B-type natriuretic peptide; CRP = C-reactive protein; e = extraneous variable; eGFR = estimated glomerular filtration rate; Hb = hemoglobin; LVEF = left ventricular ejection fraction.
3.3. The multiple regression analysis to determine the factors associated with BNP
Multiple regression analysis was performed to assess independent determinants of plasma BNP levels (Table 2). LVEF was negatively correlated with BNP level (P < 0.001), as expected, whereas BMI was also negatively correlated with BNP level (P < 0.05). Age, sex (male = 0, female = 1), and CRP level were positively correlated, whereas eGFR was negatively correlated with BNP levels.
Table 2.
Multiple regression analyses to identify the clinical factors influencing the plasma BNP levels
| R2=0.348 | Non-standard coefficient |
Standard regression coefficients | Test statistic | P-value | 95% CI | VIF | |
|---|---|---|---|---|---|---|---|
| Regression coefficient | Standard error | ||||||
| Age | 2.258 | 0.457 | 0.218 | 4.946 | < 0.001 | 1.361 to 3.155 | 1.633 |
| Sex (M0, F1) | 32.404 | 13.659 | 0.091 | 2.372 | 0.018 | 5.573 to 59.234 | 1.235 |
| BMI | -2.307 | 1.120 | -0.077 | -2.060 | 0.040 | -4.508 to -0.107 | 1.185 |
| eGFR | -0.835 | 0.277 | -0.116 | -3.017 | 0.003 | -1.379 to -0.291 | 1.244 |
| Hb | -2.460 | 3.335 | -0.032 | -0.738 | 0.461 | -9.010 to 4.091 | 1.535 |
| CRP | 20.403 | 2.923 | 0.252 | 6.981 | < 0.001 | 14.662 to 26.143 | 1.100 |
| LVEF | -4.171 | 0.472 | -0.312 | -8.840 | < 0.001 | -5.098 to -3.244 | 1.049 |
R2: adjusted coefficient of determination, CI: confidence interval, VIF: variance inflation factor.
Abbreviations: F = female; M = male; other abbreviation as in Table 1.
3.4. The concept of the proposed path model
To eliminate any confounding biases and clarify the possible correlations between BMI, LVEF and BNP more directly, a path model based on covariance structure analysis was proposed (Figure 1C). As a matter of logic, the theoretical path model was created by positioning the levels of various clinical factors that potentially influence the BNP levels, such as, age, sex (male = 0, female = 1), BMI, eGFR, Hb, CRP, and LVEF in parallel while carefully considering the correlations between each pair of factors. The association between clinical factors is linked by 2-way arrows [26], [27]. The paths between variables were drawn from independent to dependent variables with directional arrows for each regression model [26], [27].
3.5. The results of the path model
The precise results of the path model are presented in Supplementary Table 1. Consistent with the results of the multiple regression analyses, LVEF and BMI were significantly and negatively associated with plasma BNP levels (path from LVEF to BNP: β = -0.31, P < 0.001; from BMI to BNP: β = -0.08, P < 0.04).
3.6. Factors that potentially mediate the interactions among the BMI, LVEF, and BNP levels
Partial correlation analyses were performed to clarify the factors that might have a substantial impact on the interactions between BMI, LVEF, and plasma BNP levels (Supplementary Table 2). As expected, a significant negative correlation between BNP and LVEF was consistently observed even when various clinical factors were considered as control variables. Similarly, a significant negative correlation between BNP and BMI was consistently observed after considering various factors as control variables, although this correlation failed to reach statistical significance when age was used as a control variable (P = 0.055). These data indicate that the negative correlations between BNP and LVEF or BMI were independent of various other clinical factors and that there were no spurious correlations.
3.7. The impact of obesity on the relationship between LVEF and BNP
To determine the possible impact of BMI on the relationship between LVEF and plasma BNP, the study population was divided into two groups based on BMI with a cutoff value of 25 kg/m2. A BMI ≥25 kg/m2 is defined as obese by the Japan Society for the Study of Obesity and overweight according to the World Health Organization [18]. The clinical characteristics of Group 1 (BMI < 25 kg/m2) and Group 2 (BMI ≥ 25 kg/m2) are shown in Table 3. The median BMI was 22.4 kg/m2 in Group 1 and 27.4 kg/m2 in Group 2. Plasma BNP levels in Group 2 were significantly lower than those in Group 1, as expected. Importantly, LVEF values were comparable between the two groups.
Table 3.
Clinical characteristics of the two groups divided by BMI levels
| Group | Group 1 | Group 2 | |
|---|---|---|---|
| BMI<25 | 25≤BMI | P-value | |
| n | 307 | 250 | |
| Myocardial infarction (%) | 152 (49.5%) | 125 (50.0%) | 0.909 |
| Unstable angina (%) | 155 (50.5%) | 125 (50.0%) | 0.909 |
| BMI, kg/m2 | 22.4 (20.7-23.6) | 27.4 (25.9-29.6) | <0.001 |
| Age, years old | 64 (53-73) | 58 (50-65) | <0.001 |
| Gender; Male (%) | 243 (79.2%) | 231 (92.4%) | <0.001 |
| BNP, pg/mL | 33.1 (12.7-94.6) | 21.5 (10.2-62.0) | 0.003 |
| eGFR, ml/min/1.73m2 | 77.0 (66.5-89.1) | 79.4 (68.2-91.1) | 0.216 |
| Hb, g/dL | 13.8 (12.7-14.7) | 14.6 (13.6-15.3) | <0.001 |
| CRP, mg/dL | 0.06 (0.03-0.22) | 0.12 (0.05-0.32) | <0.001 |
| LVEF, % | 57.2 (49.8-63.7) | 56.3 (49.3-61.8) | 0.206 |
Abbreviation as in Table 1.
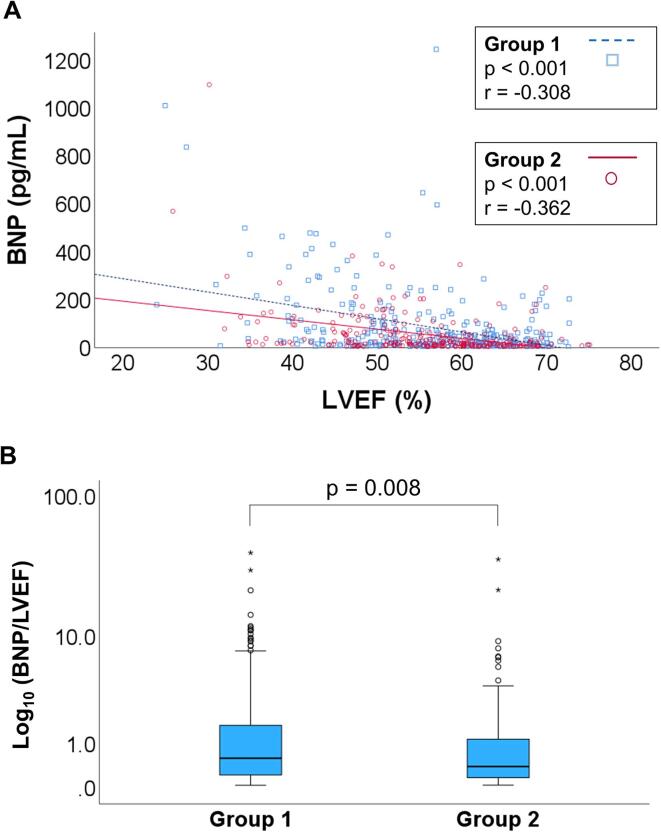
Figure 2A shows Spearman’s rank correlation coefficients between plasma BNP levels and LVEF in Groups 1 and 2. Although a significant negative correlation between LVEF and BNP was observed in both groups (P < 0.001, each), the regression line of Group 2 appeared less steep than that of Group 1, thus suggesting that plasma BNP elevation in response to LVEF decline is attenuated in obese individuals.
Fig. 2.
Blunted response of BNP increase to impaired cardiac contraction in obesity. (A) Correlations between LVEF and BNP in Group 1 (BMI <25, n = 307) (blue squares) versus Group 2 (BMI ≥25, n = 250) (red circles) are shown. The P values and correlation coefficient values (r) were noted. (B) The BNP/LVEF ratios in Group 1 (BMI <25, n = 307) and Group 2 (BMI ≥25, n = 250) are shown. Data are presented as medians, and boxes represent the interquartile ranges. Abbreviations as in Figure 1.
Multiple regression analysis was performed to determine whether BMI had a statistically significant impact on the relationship between LVEF and plasma BNP levels (Table 4). Statistical analysis included LVEF, BMI, and its interaction term (LVEF × BMI) as independent predictors of plasma BNP levels as the dependent variable. As a result, each independent parameter was statistically significant (P < 0.04), suggesting that the relationship between LVEF and plasma BNP levels was significantly modified by BMI.
Table 4.
Multiple regression analysis for influence of BMI on a relationship between BNP and LVEF.
| Explanatory variable (n = 557) | β-Coefficient | 95% CI | P-value |
|---|---|---|---|
| LVEF | -11.645 | -17.906, -5.385 | <0.001 |
| BMI | -21.508 | -35.850, -7.165 | 0.003 |
| LVEF × BMI | 0.278 | 0.027, 0.529 | 0.030 |
| Constant | 872.043 | 514.231, 1229.855 | <0.001 |
Objective variable: BNP (R2: 0.173)
Explanatory variable used in the equation: LVEF, BMI, LVEF × BMI
Abbreviation as in Table 1.
Furthermore, we compared the BNP/LVEF ratio between Groups 1 and 2 to assess the degree of plasma BNP elevation in response to the decline in LVEF (Figure 2B). We found that the ratio in Group 2 was significantly lower than that in Group 1 (P = 0.008), indicating a blunted response of the plasma BNP increase to impaired cardiac contraction during an ACS attack in obese individuals. In addition, the same analysis was performed exclusively for patients with AMI, which represents a more severe form of ACS with a greater cardiac overload. The clinical characteristics of the patients with AMI are shown in Supplementary Table 3. The BNP/LVEF ratio was consistently and significantly lower in Group 2 than in Group 1, even in patients with AMI (Supplementary Figure 1). To determine whether the degree of blunted BNP elevation response in the high BMI group varies depending on the severity of pulmonary congestion and thus the severity of heart failure, further analyses were performed with a focus on LVEDP values, one of the objective indicators of pulmonary congestion. The study population was divided into two groups based on LVEDP, using a cutoff value of 15 mmHg. LVEDP ≥15 mmHg is considered to be significantly elevated according to multiple guidelines/statements, including the guidelines from the Japanese Circulation Society [32] and the Scientific Statement from the American Heart Association [33]. Incidentally, the median LVEDP of the study subjects was 15 mmHg (Table 1). The BNP/LVEF ratio in Group 2 was significantly lower than that in Group 1 in subjects with elevated LVEDP, although a similar but non-significant trend was observed in subjects with non-elevated LVEDP (Supplementary Figure 2). These data suggest that the blunted response of the plasma BNP level increase in obese individuals becomes more pronounced in more severe cases with greater pulmonary congestion.
4. Discussion
In this clinical study of ACS, both BMI and LVEF were inversely correlated with plasma BNP level. Specifically, the BNP levels in the obese group were significantly lower than those in the non-obese group. It is noteworthy that the magnitude of plasma BNP elevation in response to reduced cardiac contractility was smaller in the obese group, suggesting that there is a blunted increase in plasma BNP levels in response to cardiac hemodynamic stress during the acute phase of an ACS attack in obese individuals. The present study is significant not only because it observed lower BNP levels in obese patients, but also because it shows that plasma BNP elevation in response to impaired EF is significantly attenuated in obese patients. This indicates a distinct trajectory of BNP levels in this population during ischemic attacks of ACS.
Several reports have indicated that plasma NP levels are lower in obese individuals with [15], [16], [17], [18], [19] or without [12], [13], [14] heart failure. However, only a few studies have investigated the relatively rapid reactivity of plasma NP levels to changes in cardiac hemodynamics in obesity. One study showed a lack of an ANP elevation response to saline load in obese individuals [34], and another showed reduced BNP concentrations despite elevated LVEDP in obese patients [35]. The results of both studies support our current findings; however, to our knowledge, this is the first report to compare the degree of plasma BNP elevation in response to cardiac stress during the acute ischemic phase of ACS based on obesity levels.
The precise mechanisms underlying unexpectedly low NP levels in obese individuals are yet to be fully established. The commonly suggested mechanism is increasing NP clearance in obese individuals because of the high expression of NP clearance receptors (NPR-C), which are abundant in adipose tissues [12], [23], [36], [37], and/or higher activities of neprilysin, which is responsible for the degradation of NP [38]. However, this does not appear to be the primary mechanism of the present findings considering the results of a previous study that showed that the concentrations of not only BNP, but also N-terminal pro-BNP (NT-proBNP), which is insensitive to NPR-C and neprilysin, are reduced in obese individuals despite the elevation of LVEDP [35]. It is unlikely that NPR-C and/or neprilysin are suddenly activated during ACS attacks. A more plausible mechanism could be the impaired production of “biologically active” NP in obesity [14], [24], [39], leading to a blunted increase in plasma BNP in response to ACS attacks. Further studies are required to determine whether impaired NP production/secretion in obese individuals is responsible for the blunted NP elevation response to ACS attacks by measuring NT-proBNP or mid-regional pro-ANP (MR-proANP) (recently reported as a more sensitive marker [40]), both of which are unaffected by NPR-C and neprilysin.
In addition to the classical actions of hemodynamic regulation on the renal and cardiovascular systems, increasing evidence suggests that NPs also regulate energy balance and glucose homeostasis through interorgan metabolic cross-talk with adipose tissues [20], [21], [22], [23], [24], [25], [26], [36], [37]. Our recent basic research showed that NP ameliorated both systemic and myocardial insulin resistance in diet-induced obesity [24], [25]. Furthermore, our previous clinical study showed that acute action of BNP promoted glucose utilization and substantially improved insulin resistance in patients with ACS [27]. This indicates the previously underappreciated role of NP in glucose metabolism, which counteracts increased insulin resistance during ACS attacks. Considering our series of research findings, the blunted NP elevation response in obese individuals is likely unfavorable owing to the further increase in insulin resistance during ischemic attacks of ACS, in which myocardial glucose utilization and metabolism become critical for ATP generation and cardiomyocyte survival [27], [28].
Study limitations
In the present study, we did not investigate prognosis. The clinical significance of this study would be enhanced by further investigation into whether patients with a blunted increase in plasma BNP actually manifest any long-term impairment of their cardiac function (i.e., lack of reverse remodeling), as well as a higher incidence of cardiovascular events (e.g., heart failure readmission and/or mortality). In this context, it would be interesting to see the impact of agents that increase biologically active NP levels (i.e., sacubitril/valsartan) on the clinical course of patients with a blunted BNP elevation [41]. Given that all of the subjects in the present study underwent left ventriculography, it is likely that the majority had relatively stable hemodynamics and oxygenation, suggesting that this cohort is not ideally suited for assessing both short-term and long-term prognoses. Further studies are needed to investigate the prognosis according to cardiac function data obtained from other modalities, such as echocardiography, in a broader range of populations under more severe conditions. Moreover, investigating the individual impact of BMI, BNP, and LVEF on the prognosis is an important focus for research that holds significant clinical implications. Another limitation is that we did not investigate the effect of sex on the increase in plasma BNP levels. A recent study indicated the presence of sex-associated differences in the regulation of the NP levels [3], [42]. The sample in the current study was predominantly male (85.1%). Thus, further studies with a larger number of female patients are warranted. Finally, it would be interesting to determine whether similar findings occur during the acute phase of other cardiovascular diseases such as general acute decompensated heart failure.
5. Conclusions
In addition to the generally low plasma BNP levels at baseline in obese individuals, there was a blunted increase in plasma BNP in response to cardiac hemodynamic stress during the acute phase of an ACS attack in this patient population. Therefore, in obese patients, it is important to recognize that plasma BNP levels are unexpectedly low relative to the extent of myocardial damage caused by ACS, and care must be taken to avoid underestimation of disease severity. In other words, when treating obese patients with reduced LVEF, it is important to exercise caution even if plasma BNP levels are not substantially elevated. Likewise, relative NP deficiency due to a blunted increase in response to cardiac hemodynamic stress during ACS attacks may further exacerbate insulin resistance and impair myocardial glucose metabolism, which is crucial during ACS attacks, thereby promoting the progression of ACS pathology. Based on the finding that the blunted response of the BNP increase becomes more pronounced in more severe cases, where NP elevation is required to play a compensatory role, the relative deficiency of NP in patients with high BMI could potentially result in a poor prognosis. The administration of agents that increase biologically active NP levels during the acute phase of ACS may therefore have therapeutic benefits, particularly in obese individuals [24], [25], [27], [41].
Funding
This study was supported in part by Grants-in-Aid for the Ministry of Education, Culture, Sports, Science and Technology [JP23K07563] (to Dr. Nagoshi), [JP24K19041] (to Dr. Oi), [JP24K19067] (to Dr. Kimura), [JP24K11300] (to Dr. Kashiwagi), [JP22K08113] (to Dr. Yoshimura) and SENSHIN Medical Research Foundation (to Dr. Nagoshi). Outside this study, Dr. Nagoshi received research funds from Nippon Boehringer Ingelheim Co., Ltd. and the speaker’s honorarium from Kowa Pharmaceutical Co., Ltd., AstraZeneca K.K., Nippon Boehringer Ingelheim Co., Ltd., Bayer Yakuhin Ltd., Mochida Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd., TOA EIYO Ltd., Sumitomo Pharma Co., Ltd., Viatris Pharm Japan Inc., SEKISUI Medical Co., Ltd., and Takeda Pharmaceutical Co., Ltd. Dr. Yoshimura received research funds from Otsuka Pharmaceutical Co. Ltd. and Mochida Pharmaceutical Co. Ltd. and the speaker's honorarium from Otsuka Pharmaceutical Co. Ltd., Novartis Pharma K.K., Daiichi Sankyo Co. Ltd., Mochida Pharmaceutical Co. Ltd., Astellas Pharma Inc., Bayer Yakuhin Ltd., Viatris Inc., AstraZeneca K.K., and Novo Nordisk Pharma Ltd.
CRediT authorship contribution statement
Toraaki Okuyama: Writing – original draft, Visualization, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Tomohisa Nagoshi: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. Nana Hiraki: Writing – review & editing, Resources, Investigation, Data curation. Toshikazu D. Tanaka: Writing – review & editing, Visualization. Yuhei Oi: Resources, Investigation, Funding acquisition, Formal analysis, Data curation. Haruka Kimura: Resources, Investigation, Funding acquisition, Formal analysis, Data curation. Yusuke Kashiwagi: Resources, Investigation, Funding acquisition, Formal analysis, Data curation. Kazuo Ogawa: Resources, Investigation, Formal analysis, Data curation. Kosuke Minai: Software, Resources, Investigation, Data curation. Takayuki Ogawa: Resources, Investigation, Data curation. Makoto Kawai: Writing – review & editing, Validation, Software, Methodology, Formal analysis. Michihiro Yoshimura: Writing – review & editing, Visualization, Validation, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2024.101508.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Yoshimura M., Yasue H., Okumura K., Ogawa H., Jougasaki M., Mukoyama M., et al. Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation. 1993;87:464–469. doi: 10.1161/01.cir.87.2.464. [DOI] [PubMed] [Google Scholar]
- 2.Yasue H., Yoshimura M., Sumida H., Kikuta K., Kugiyama K., Jougasaki M., et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90:195–203. doi: 10.1161/01.cir.90.1.195. [DOI] [PubMed] [Google Scholar]
- 3.Harada E., Mizuno Y., Ishii M., Ishida T., Yamada T., Kugimiya F., et al. beta-Blockers are associated with increased B-type natriuretic peptide levels differently in men and women in heart failure with preserved ejection fraction. Am J. Physiol. Heart Circ. Physiol. 2022;323:H276–H284. doi: 10.1152/ajpheart.00029.2022. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa Y., Nishikimi T., Kuwahara K. Atrial and brain natriuretic peptides: hormones secreted from the heart. Peptides. 2019;111:18–25. doi: 10.1016/j.peptides.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Madamanchi C., Alhosaini H., Sumida A., Runge M.S. Obesity and natriuretic peptides, BNP and NT-proBNP: mechanisms and diagnostic implications for heart failure. Int. J. Cardiol. 2014;176:611–617. doi: 10.1016/j.ijcard.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki S., Yoshimura M., Nakayama M., Mizuno Y., Harada E., Ito T., et al. Plasma level of B-type natriuretic peptide as a prognostic marker after acute myocardial infarction: a long-term follow-up analysis. Circulation. 2004;110:1387–1391. doi: 10.1161/01.CIR.0000141295.60857.30. [DOI] [PubMed] [Google Scholar]
- 7.Nagoshi T. Close linkage between natriuretic peptides and obesity- impact of sex on the interorgan metabolic crosstalk. Circ. J. 2021;85:655–656. doi: 10.1253/circj.CJ-21-0202. [DOI] [PubMed] [Google Scholar]
- 8.Sabatine M.S., Morrow D.A., de Lemos J.A., Omland T., Desai M.Y., Tanasijevic M., et al. Acute changes in circulating natriuretic peptide levels in relation to myocardial ischemia. J. Am Coll. Cardiol. 2004;44:1988–1995. doi: 10.1016/j.jacc.2004.07.057. [DOI] [PubMed] [Google Scholar]
- 9.Morita E., Yasue H., Yoshimura M., Ogawa H., Jougasaki M., Matsumura T., et al. Increased plasma levels of brain natriuretic peptide in patients with acute myocardial infarction. Circulation. 1993;88:82–91. doi: 10.1161/01.cir.88.1.82. [DOI] [PubMed] [Google Scholar]
- 10.Kikuta K., Yasue H., Yoshimura M., Morita E., Sumida H., Kato H., et al. Increased plasma levels of B-type natriuretic peptide in patients with unstable angina. Am Heart J. 1996;132:101–107. doi: 10.1016/s0002-8703(96)90396-8. [DOI] [PubMed] [Google Scholar]
- 11.Omland T., Persson A., Ng L., O'Brien R., Karlsson T., Herlitz J., et al. N-terminal pro-B-type natriuretic peptide and long-term mortality in acute coronary syndromes. Circulation. 2002;106:2913–2918. doi: 10.1161/01.cir.0000041661.63285.ae. [DOI] [PubMed] [Google Scholar]
- 12.Wang T.J., Larson M.G., Levy D., Benjamin E.J., Leip E.P., Wilson P.W., et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 13.Wang T.J., Larson M.G., Keyes M.J., Levy D., Benjamin E.J., Vasan R.S. Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation. 2007;115:1345–1353. doi: 10.1161/CIRCULATIONAHA.106.655142. [DOI] [PubMed] [Google Scholar]
- 14.Parcha V., Patel N., Musunuru K., Margulies K.B., Cappola T.P., Halade G., et al. Natriuretic peptide deficiency in obese individuals: mechanistic insights from healthy organ donor cohort. J. Am Coll. Cardiol. 2021;77:3138–3140. doi: 10.1016/S0735-1097(21)04493-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachmann K.N., Gupta D.K., Xu M., Brittain E., Farber-Eger E., Arora P., et al. Unexpectedly low natriuretic peptide levels in patients with heart failure. JACC Heart Fail. 2021;9:192–200. doi: 10.1016/j.jchf.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn V.S., Knutsdottir H., Peterson T.E., Kikuchi D., Vungarala S., Kass D.A., et al. Relationship between myocardial NPPB expression and serum NT-proBNP in heart failure with preserved ejection fraction. JACC Heart Fail. 2024 doi: 10.1016/j.jchf.2024.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehra M.R., Uber P.A., Park M.H., Scott R.L., Ventura H.O., Harris B.C., et al. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J. Am Coll. Cardiol. 2004;43:1590–1595. doi: 10.1016/j.jacc.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 18.Horwich T.B., Hamilton M.A., Fonarow G.C. B-type natriuretic peptide levels in obese patients with advanced heart failure. J. Am Coll. Cardiol. 2006;47:85–90. doi: 10.1016/j.jacc.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 19.Ndumele C.E., Matsushita K., Sang Y., Lazo M., Agarwal S.K., Nambi V., et al. NT-proBNP and heart failure risk among individuals with and without obesity: the ARIC study. Circulation. 2016;133:631–638. doi: 10.1161/CIRCULATIONAHA.115.017298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins S. A heart-adipose tissue connection in the regulation of energy metabolism. Nat. Rev. Endocrinol. 2014;10:157–163. doi: 10.1038/nrendo.2013.234. [DOI] [PubMed] [Google Scholar]
- 21.Coue M., Moro C. Natriuretic peptide control of energy balance and glucose homeostasis. Biochimie. 2016;124:84–91. doi: 10.1016/j.biochi.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Bordicchia M., Liu D., Amri E.Z., Ailhaud G., Dessi-Fulgheri P., Zhang C., et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyashita K., Itoh H., Tsujimoto H., Tamura N., Fukunaga Y., Sone M., et al. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58:2880–2892. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oi Y., Nagoshi T., Kimura H., Tanaka Y., Yoshii A., Yasutake R., et al. Exogenous ANP treatment ameliorates myocardial insulin resistance and protects against ischemia-reperfusion injury in diet-induced obesity. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23158373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura H., Nagoshi T., Oi Y., Yoshii A., Tanaka Y., Takahashi H., et al. Treatment with atrial natriuretic peptide induces adipose tissue browning and exerts thermogenic actions in vivo. Sci. Rep. 2021;11:17466. doi: 10.1038/s41598-021-96970-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang R., Nagoshi T., Kimura H., Tanaka T.D., Yoshii A., Inoue Y., et al. Possible association between body temperature and B-type natriuretic peptide in patients with cardiovascular diseases. J Card Fail. 2021;27:75–82. doi: 10.1016/j.cardfail.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Uno G., Nagoshi T., Yoshii A., Inoue Y., Tanaka Y., Kimura H., et al. Collaborative activities of noradrenaline and natriuretic peptide for glucose utilization in patients with acute coronary syndrome. Sci Rep. 2019;9:7822. doi: 10.1038/s41598-019-44216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagoshi T., Yoshimura M., Rosano G.M., Lopaschuk G.D., Mochizuki S. Optimization of cardiac metabolism in heart failure. Curr. Pharm Des. 2011;17:3846–3853. doi: 10.2174/138161211798357773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagawa O., Ogawa Y., Itoh H., Suga S., Komatsu Y., Kishimoto I., et al. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. Evidence for brain natriuretic peptide as an “emergency” cardiac hormone against ventricular overload. J. Clin. Invest. 1995;96:1280–1287. doi: 10.1172/JCI118162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiraki N., Nagoshi T., Okuyama T., Tanaka T.D., Oi Y., Kashiwagi Y., et al. Inhibitory action of B-type natriuretic peptide on adrenocorticotropic hormone in patients with acute coronary syndrome. Am J. Physiol. Heart Circ. Physiol. 2023;325:H856–H865. doi: 10.1152/ajpheart.00315.2023. [DOI] [PubMed] [Google Scholar]
- 31.Inoue T., Kawai M., Nakane T., Nojiri A., Minai K., Komukai K., et al. Influence of low-grade inflammation on plasma B-type natriuretic peptide levels. Intern. Med. 2010;49:2659–2668. doi: 10.2169/internalmedicine.49.4211. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura T., Hirata Y., Ise T., Iwano H., Izutani H., Kinugawa K., et al. JCS/JSCVS/JCC/CVIT 2023 guideline focused update on indication and operation of PCPS/ECMO/IMPELLA. Circ. J. 2024;88:1010–1046. doi: 10.1253/circj.CJ-23-0698. [DOI] [PubMed] [Google Scholar]
- 33.Geller B.J., Sinha S.S., Kapur N.K., Bakitas M., Balsam L.B., Chikwe J., et al. Escalating and de-escalating temporary mechanical circulatory support in cardiogenic shock: a scientific statement from the american heart association. Circulation. 2022;146:e50–e68. doi: 10.1161/CIR.0000000000001076. [DOI] [PubMed] [Google Scholar]
- 34.Licata G., Volpe M., Scaglione R., Rubattu S. Salt-regulating hormones in young normotensive obese subjects. Effects of saline load. Hypertension. 1994;23:I20–I24. doi: 10.1161/01.hyp.23.1_suppl.i20. [DOI] [PubMed] [Google Scholar]
- 35.Taylor J.A., Christenson R.H., Rao K., Jorge M., Gottlieb S.S. B-type natriuretic peptide and N-terminal pro B-type natriuretic peptide are depressed in obesity despite higher left ventricular end diastolic pressures. Am Heart J. 2006;152:1071–1076. doi: 10.1016/j.ahj.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Coue M., Barquissau V., Morigny P., Louche K., Lefort C., Mairal A., et al. Natriuretic peptides promote glucose uptake in a cGMP-dependent manner in human adipocytes. Sci. Rep. 2018;8:1097. doi: 10.1038/s41598-018-19619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu W., Shi F., Liu D., Ceddia R.P., Gaffin R., Wei W., et al. Enhancing natriuretic peptide signaling in adipose tissue, but not in muscle, protects against diet-induced obesity and insulin resistance. Sci. Signal. 2017;10 doi: 10.1126/scisignal.aam6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Standeven K.F., Hess K., Carter A.M., Rice G.I., Cordell P.A., Balmforth A.J., et al. Neprilysin, obesity and the metabolic syndrome. Int. J. Obes (Lond). 2011;35:1031–1040. doi: 10.1038/ijo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arora P., Wu C., Hamid T., Arora G., Agha O., Allen K., et al. Acute metabolic influences on the natriuretic peptide system in humans. J. Am Coll Cardiol. 2016;67:804–812. doi: 10.1016/j.jacc.2015.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan E.S.J., Chan S.P., Liew O.W., Chong J.P.C., Gerard Leong K.T., Daniel Yeo P.S., et al. Differential associations of A-/B-type natriuretic peptides with cardiac structure, function, and prognosis in heart failure. JACC Heart Fail. 2024;12:461–474. doi: 10.1016/j.jchf.2023.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Ishii M., Kaikita K., Sato K., Sueta D., Fujisue K., Arima Y., et al. Cardioprotective effects of LCZ696 (Sacubitril/Valsartan) after experimental acute myocardial infarction. JACC Basic Transl. Sci. 2017;2:655–668. doi: 10.1016/j.jacbts.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shetty N.S., Patel N., Gaonkar M., Li P., Arora G., Arora P. Natriuretic peptide normative levels and deficiency: the national health and nutrition examination survey. JACC Heart Fail. 2024;12:50–63. doi: 10.1016/j.jchf.2023.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.