Abstract
Although numerous observational studies have reported on the association between alcohol consumption and cancer, insufficient studies have estimated the causality. Our study evaluated the causal relationship between various types of cancer according to the frequency of drinking and the amount of alcohol consumed. The research data were obtained from the publicly available MR-Base platform. The frequency and amount of drinking were selected as the exposure, and 16 cancer types were selected as the outcome. Two-sample summary data Mendelian randomization (2SMR) was conducted to examine the causality between alcohol consumption and cancer type. Additionally, for cancers suspected of pleiotropy, outliers were removed and re-analyzed through radial MR. The MR results using the inverse variance weighted (IVW) method were different before and after removing outliers. The biggest differences were found for esophageal cancer and biliary tract cancer. For esophageal cancer, after removing outliers (rs13102973, rs540606, rs650558), the OR (95% CI) was 3.44 (1.19–9.89), which was statistically significant (p = 0.02172). Even in biliary tract cancer, after removing outliers (rs13231886, rs58905411), the OR (95% CI) was 3.86 (0.89–16.859), which was of borderline statistical significance (p = 0.07223). The strongest association was found for esophageal cancer. For other cancers, the evidence was not sufficient to draw conclusions. More research is needed to understand the causality between drinking and cancer.
Keywords: alcohol consumption, causality, Mendelian randomization, cancer
1. Introduction
Heavy alcoholic beverage consumption is an established risk factor for poor health outcomes, and it is one of the leading causes of death [1]. Heavy alcohol consumption is a well-documented deleterious lifestyle behavior that activates the brain’s reward pathways, resulting in the release of dopamine [2]. Alcohol consumption is linked to cancer through several mechanisms: acetaldehyde, a byproduct of ethanol metabolism, causes DNA damage; oxidative stress from reactive oxygen species (ROS) leads to cellular damage; and immunosuppression and inflammation promote tumor growth. Additionally, alcohol increases estrogen levels, raising the risk of hormone-sensitive cancers, and induces folate deficiency, resulting in DNA instability. These pathways are supported by extensive literature, including reviews [3,4,5,6,7].
Evidence that drinking alcohol is a risk factor for cancer has been reported since the early 20th century [8]. According to the IARC Monographs reported in 1988, alcoholic beverages were described as “carcinogenic to humans”. A few decades later, an association between alcohol consumption with cancers of the oral cavity, pharynx, larynx, esophagus, liver, colorectum, and female breast were reported [9]. Alcohol consumption is known to elevate the risk of cancer by increasing the level of oxidized metabolite acetaldehyde, which is carcinogenic to humans [10]. However, alcohol consumption may simultaneously reduce the cancer risk by alternative mechanisms, such as by increasing insulin sensitivity through elevated adiponectin levels [11].
In observational studies, alcohol consumption was reported to be positively correlated with the risk of head and neck cancer, esophageal cancer, stomach cancer, liver cancer, and breast cancer [12,13,14,15], while non-Hodgkin’s lymphoma [14,15,16] is inversely related. Additionally, heavy drinking was associated with an increased risk of colorectal cancer, whereas light or moderate drinking was associated with a reduced risk [17]. Results from previous observational studies can be interpreted as identifying associations, but their causality interpretation is limited due to the influence of confounding variables. However, recently, through the Mendelian randomization research method, which looks at the relationship with cancer occurrence through the amount of alcohol exposure as if it were a genetic variant, it has become possible to interpret it from a causal point of view. Genetic factors are randomly assigned and determined at conception, so they are independent of confounding variables. The new research method developed using this perspective is called Mendelian randomization.
In this study, a two-sample Mendelian randomization study was conducted to investigate the causal relationship between drinking frequency/alcohol consumption and the occurrence of various cancers.
2. Materials and Methods
2.1. Data Source
The data of this study were obtained from the MR-Base platform (http://www.mrbase.org, accessed on 12 March 2023), which systematically integrates the GWAS summary datasets. For the exposure, we used data on drinking frequency [18] and drinking amount [19]. Alcohol consumption data were measured in units of weekly alcohol consumption. We selected GWAS datasets that specifically investigated alcohol consumption as the exposure and various types of cancer as the outcomes. This ensured that the genetic instruments (single nucleotide polymorphisms, or SNPs) used in the MR analysis were relevant and directly related to our research question. Aiming to minimize heterogeneity and population stratification bias, we selected GWAS datasets conducted in similar populations. For this study, we used data from predominantly Western populations.
2.2. Variables
The data for alcohol consumption were obtained from the GWAS conducted by Howe et al. [20] under the Within Family GWAS Consortium, which provided a comprehensive analysis of genetic associations with alcohol consumption. The dataset used for this study is available on the MR-Base platform (http://www.mrbase.org) under the dataset ID ieu-b-4834 [20]. This citation acknowledges both the original study and the data repository from which the data were accessed, ensuring proper credit is given to the researchers and the platform facilitating the data availability (MRcIEU). We used “Alcohol consumption” (ID: ieu-b-4834) and “Alcohol frequency” (ID: ukb-a-25) as search terms for our analysis. As an outcome, the cancer types were stomach, liver, lung, thyroid, cervix, breast, prostate, colorectal, bladder, larynx, lip/oral/pharynx, brain, biliary tract, and esophagus. For both exposure and outcome data, sample size, number of variants, first author, year, name of consortium, sex, and build reported in the MR-Base were extracted.
2.3. Statistical Analysis: Two-Sample Mendelian Randomization Analysis
We confirm that the exposure (alcohol consumption) and outcome (cancer risk) datasets used in our study were obtained from separate and independent sources. To further ensure the independence of the datasets, we cross-referenced participant identifiers and confirmed that there was no overlap in the individual participants included in both the exposure and outcome datasets.
After selecting each exposure and outcome using the MR-Base platform, each two-sample Mendelian randomization was analyzed. SNPs obtained from the GWAS of exposure and outcome were used with clumping to prune the SNPs for LD. If a particular exposure SNP was not present in an outcome dataset, proxy SNPs were used instead through LD tagging.
SNPs were selected based on a significance threshold of p < 5 × 10−8. This stringent threshold ensures that only SNPs with a strong association with the exposure were included, reducing the risk of including weak or non-informative instruments. To ensure the reliability of the selected SNPs as proxies for alcohol consumption and frequency, we applied several validation steps. First, we conducted a clumping procedure to remove the SNPs in linkage disequilibrium (LD) to avoid redundancy. Second, we cross-referenced the selected SNPs with the literature and database annotations to confirm their known associations with alcohol-related traits. Finally, we assessed the validity of these SNPs in our MR analysis by evaluating their relevance and strength as instruments through standard MR diagnostics.
2.4. Outlier Detection
Outliers were identified using the radial MR framework, which provides a measure of heterogeneity through the radial distance statistic. Specifically, we used the radial Q-statistic to quantify the influence of each genetic variant on the overall Mendelian randomization analysis. A genetic variant was classified as an outlier if its radial Q-statistic exceeded a threshold of 3 standard deviations from the mean radial Q-statistic of all variants. This threshold was chosen to balance sensitivity and specificity in detecting influential outliers without overfitting the model. Removing outliers is crucial to reduce the impact of variants that disproportionately affect the MR estimates due to pleiotropy or other biases. The radial MR method allows us to pinpoint these influential variants more precisely, ensuring the robustness and reliability of our results. By setting a threshold based on the standard deviation of the radial Q-statistic, we aimed to systematically and objectively identify and remove variants that could skew the analysis.
In this case, the default value 0.8 was used as the Minimum LD R2 value. For the MAF threshold for aligning palindromes, a default value of 0.3 was used. In this analysis, four methods were selected for two-sample Mendelian randomization: inverse variance weighted, MR-Egger, weighted median, and weighted mode. If the p-value of MR-Egger was significant, the pleiotropy test was performed through the intercept test. In addition, when an outlier was suspected, additional analysis was performed through radial Mendelian randomization. In this case, summary statistics were downloaded from the MR-Base platform and additional analysis was performed through R analysis. That is, the results of beta values were converted to odds ratios and 95% confidence intervals and presented as independent figures.
3. Results
Table 1 shows the characteristics of the exposure data and outcome data used in this study. In this study, the exposure data were alcohol consumption. Outcome data were extracted from all 16 cancers. All data were from the European Population with HG19/GRCh37 Build.
Table 1.
General characteristics of study data.
| Trait Name | Sample Size | Number of Variants | First Author | Year | Consortium | Sex | |
|---|---|---|---|---|---|---|---|
| Exposure | |||||||
| ukb-a-25 | Alcohol intake frequency | 336,965 | 10,894,599 | Neale BM [21] | 2017 | Neale lab | M, F |
| ieu-b-4834 | Alcohol consumption | 83,626 | 7,914,362 | Howe LJ [20] | 2022 | WFGC | M, F |
| Outcome | Cancer site | ||||||
| finn-b-c3-stomach | Stomach | NA | 16,380,305 | NA | 2021 | Finngen [22] | M, F |
| finn-b-C3-liver | Liver | NA | 16,380,303 | NA | 2021 | Finngen [22] | M, F |
| ieu-b-4955 | Lung | 374,687 | 11,078,115 | Burrows [23] | 2021 | UK Biobank | M, F |
| finn-b-c3-colon | Colon | NA | 16,380,317 | NA | 2021 | Finngen [22] | M, F |
| finn-b-c3-thyroid | Thyroid | NA | 16,380,316 | NA | 2021 | Finngen [22] | M, F |
| finn-b-c3-cervix | Cervix | NA | 16,378,927 | NA | 2021 | Finngen [22] | M, F |
| ieu-a-1168 | Breast | 33,832 | 13,011,123 | Michailidou K [24] | 2015 | BCAC | F |
| finn-b-c3-prostate | Prostate | NA | 16,377,987 | NA | 2021 | Finngen [22] | M |
| finn-b-c3-colorectal | Colorectal | NA | 16,380,321 | NA | 2021 | Finngen [22] | M, F |
| finn-b-c3-bladder | Bladder | NA | 16,380,305 | NA | 2021 | Finngen [22] | M, F |
| finn-b-c3-larynx | Larynx | NA | 16,380,304 | NA | 2021 | Finngen [22] | M, F |
| finn-b-c3-lip-oral | Lip/Oral/Pharynx | NA | 16,380,304 | NA | 2021 | Finngen [22] | M, F |
| ieu-b-94 | Oral cavity | 4151 | 7,510,833 | Lesseur | 2016 | OOCOC | M, F |
| finn-b-c3-brain | Brain | NA | 16,380,308 | NA | 2021 | Finngen [22] | M, F |
| finn-b-c3-Biliary tract | Biliary tract | NA | 16,380,304 | NA | 2021 | Finngen [22] | M, F |
| finn-b-c3-oesphagus | Oesphagus | NA | 16,380,466 | NA | 2021 | Finngen [22] | M, F |
WFGC: Within Family GWAS Consortium. BACA: Breast Cancer Association Consortium; OOCOC: Oncoarray Oral Cavity and Oropharyngeal Cancer.
Supplemental Materials Tables S1 and S2 show the two-sample MR results before removing outliers between alcohol consumption, alcohol intake frequency as an exposure and the 16 cancer types. The MR results by the inverse variance weighted method were not significant in all cancers except stomach cancer, which showed borderline significant results (p = 0.0486).
In Table 2 and Table 3 we made a comparison between the two-sample MR results before removing outlier SNPs and after removing outlier SNPs for both alcohol consumption and alcohol intake frequency as an exposure. The MR results by the IVW method were different before and after removing outliers. The largest differences were found for esophageal cancer and biliary tract cancer. For esophageal cancer, after removing outliers (rs13102973, rs540606, rs650558), the OR (95% CI) was 3.44 (1.19–9.89), which was statistically significant (p = 0.0217). Even in biliary tract cancer, after removing outliers (rs13231886, rs58905411), the OR (95% CI) was 3.86 (0.89–16.859), which was of borderline statistical significance (p = 0.0722).
Table 2.
Two-sample Mendelian randomization results between alcohol consumption and cancer site: comparison before and after outlier removal.
| Cancer Site | Sample Size | Cases | Before Removing Outliers | After Removing Outliers | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |||
| Stomach | 174,006 | 633 | 1.06 (0.56–1.98) | 0.86300 | 1.06 (0.73–1.54) | 0.7739 |
| Liver | 218,488 | 304 | 1.14 (0.46–2.83) | 0.7704 | 0.73 (0.27–1.94) | 0.4401 |
| Lung | 217,165 | 2,671 | 0.99 (0.99–1.00) | 0.4408 | 0.99 (0.99–1.00) | 0.4584 |
| Colon | 174,006 | 1,803 | 1.13 (0.77–1.66) | 0.5098 | 0.85 (0.62–1.16) | 0.2978 |
| Thyroid | 174,006 | 989 | 1.18 (0.70–1.97) | 0.5616 | 2.39 (2.07–2.74) | ≤0.0001 |
| Cervix | 99,321 | 1,648 | 0.98 (0.62–1.55) | 0.9260 | 0.98 (0.73–1.32) | 0.9096 |
| Breast | 115,178 | 15,748 | 0.93 (0.81–1.63) | 0.2660 | 0.90 (0.82–0.99) | 0.0159 |
| Prostate | 74,865 | 6311 | 1.09 (0.80–1.48) | 0.5630 | 1.05 (0.86–1.26) | 0.6568 |
| Colorectal | 215,770 | 3022 | 0.94 (0.70–1.27) | 0.6844 | 0.87 (0.72–1.06) | 0.2467 |
| Bladder | 174,006 | 1115 | 1.29 (0.80–2.09) | 0.2906 | 1.17 (0.99–1.38) | 0.1062 |
| Larynx | 218,612 | 180 | 2.12 (0.44–10.13) | 0.3357 | NA | NA |
| Lip-Oral-Pharynx | 218,666 | 126 | 1.16 (0.28–4.74) | 0.8317 | 1.53 (0.64–3.62) | 0.3661 |
| Oral cavity | 4151 | 1223 | 2.33 (0.91–5.92) | 0.07685 | NA | NA |
| Brain | 218,328 | 464 | 0.98 (0.45–2.13) | 0.9604 | 1.04 (0.65–1.67) | 0.8965 |
| Biliary tract | 174,006 | 109 | 0.15 (0.01–3.97) | 0.30220 | 0.60 (0.28–1.27) | 0.3423 |
| Oesophagus | 218,560 | 232 | 1.61 (0.58–4.48) | 0.3590 | 1.83 (0.90–3.75) | 0.0449 |
Alcohol consumption (id: ieu-b-4834).
Table 3.
Comparison of the two sample Mendelian randomization results between alcohol intake frequency and cancer sites.
| Cancer Site | Sample Size | Cases | Before Removing Outliers | After Removing Outliers | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |||
| Stomach | 174,006 | 633 | 0.48 (0.23–1.00) | 0.05 | 1.77 (0.97–3.23) | 0.0036 |
| Liver | 218,488 | 304 | 0.96 (0.34–2.71) | 0.9386 | 0.82 (0.33–2.07) | 0.6813 |
| Lung | 217,165 | 1627 | 0.92 (0.58–1.45) | 0.7169 | 1.08 (0.73–1.58) | 0.7088 |
| Colon | 174,006 | 1803 | 1.11 (0.768–1.616) | 5.69 × 10−1 | 1.83 (0.99–3.35) | 0.0022 |
| Thyroid | 174,006 | 989 | 1.12 (0.57–2.18) | 0.7411 | 1.15 (0.65–2.04) | 0.6219 |
| Cervix | 99,321 | 1648 | 0.99 (0.54–1.80) | 0.9665 | 0.85 (0.55–1.30) | 0.4488 |
| Breast | 115,178 | 8401 | 0.84 (0.61–1.15) | 0.2786 | 0.93 (0.84–1.02) | 0.1189 |
| Prostate | 88,902 | 6311 | 0.99 (0.71–1.38) | 0.9552 | 0.93 (0.72–1.21) | 0.6027 |
| Colorectal | 215,770 | 3022 | 0.79 (0.57–1.11) | 0.1822 | 0.85 (0.67–1.09) | 0.1666 |
| Bladder | 174,006 | 1115 | 1.19 (0.69–2.05) | 0.5414 | 1.08 (0.88–1.30) | 0.5002 |
| Larynx | 218,612 | 180 | 0.59 (0.15–1.27) | 0.4409 | 0.69 (0.19–2.45) | 0.5743 |
| Lip-Oral-Pharynx | 218,666 | 126 | 1.14 (0.20–6.64) | 0.8808 | 1.02 (0.28–3.78) | 0.7621 |
| Oral cavity | 4151 | 1223 | 2.32 (0.91–5.92) | 0.0768 | NA | NA |
| Brain | 218,328 | 464 | 1.35 (0.58–3.14) | 0.4905 | 1.16 (0.57–2.36) | 0.6839 |
| Biliary tract | 174,006 | 109 | 1.44 (0.25–8.43) | 0.6776 | 3.86 (0.89–16.85) | 0.0722 |
| Oesophagus | 218,560 | 232 | 2.58 (0.74–9.00) | 0.1380 | 3.44 (1.19–9.89) | 0.0217 |
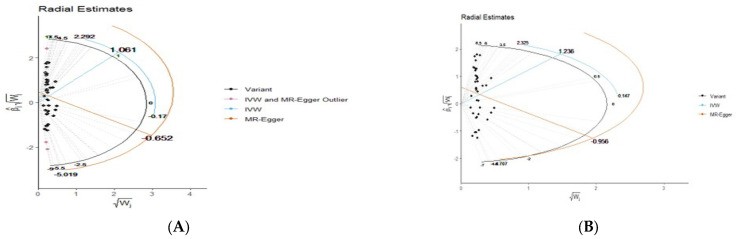
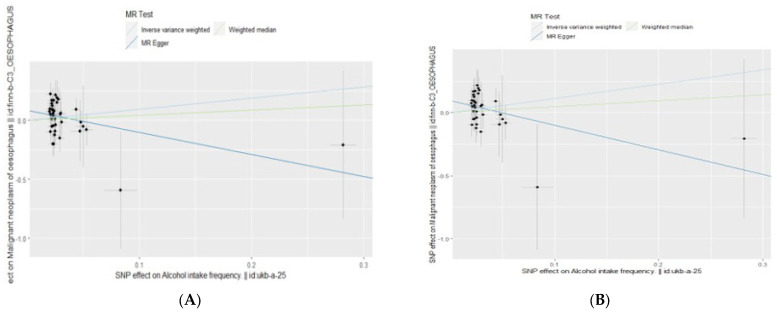
Figure 1A shows the existence of outliers through radial MR. The orange color in Figure 1A indicates outliers. Figure 1B is the radial MR result after removing the outliers. Figure 2A,B compares the scatter plot before and after removing three outliers from esophageal cancer.
Figure 1.
(A) Radial MR results for esophageal cancer and outlier discovery (pink colored dots). (B) Radial MR results for esophageal cancer after removing outliers.
Figure 2.
(A) Scatter plot for esophageal cancer before removing outliers. (B) Radial MR results for esophageal cancer after removing outliers.
4. Discussion
In this study, two-sample MR analysis was conducted using the MR-Base platform aiming to estimate the causal relationship between alcohol consumption and cancer risk, which is still controversial throughout previously reported observational studies [6,7]. One alcohol-related exposure and 16 cancer types were analyzed as outcomes. This study used Western data for both exposure and outcome.
Results of the study IVW method results were different before and after removing outliers. The biggest differences were found in esophageal cancer and biliary tract cancer. For esophageal cancer, after removing outliers (rs13102973, rs540606, rs650558), the OR (95% CI) was 3.44 (1.19–9.89), which was statistically significant (p = 0.02172). As an in-depth analysis, since there were 41 SNPs in esophageal cancer, three outliers were found as a result of additional radial MR. That is, this is an analysis of the number of esophageal cancers in 232 people. If the sample of esophageal cancer becomes larger, it is highly likely to be a significant result.
A review of published Western studies on alcohol consumption and cancer is as follows. Genetically predicted alcohol intake was statistically significantly associated with lung cancer in the International Lung Cancer Consortium (OR 1.94; 95% CI 1.41–2.68; p = 4.68 × 10−5), but UK Biobank (OR 1.12, 95% CI 0.65–1.93, p = 0.686) did not show a similar relationship [18]. Alcohol consumption has also been reported to be associated with breast cancer risk in a dose-response manner, with an 8% to 12% increase in risk for every 10 g/day increase in alcohol consumption [25,26]. The detrimental effects of heavy drinking on colorectal cancer were suggested by studies using the COLCA1/COLCA2 and ALDH2 genotypes as indicators of alcohol exposure [17].
Conversely, light alcohol consumption was not associated with an increased risk of colorectal cancer [26] in some studies. Another MR study found no association between alcohol intake and the incidence of prostate cancer [27]. However, there was a statistically significant relationship between alcohol intake and head and neck cancer [28]. However, in this study, there was no statistically significant association between alcohol consumption and other sites of cancer. That is, we found no evidence to support a relationship between alcohol consumption and overall or site-specific cancer risk.
The association between alcohol consumption and cancer risk has been examined extensively in observational studies, revealing varying patterns across different types of cancer. Notably, alcohol consumption has consistently shown positive correlations with the risk of head and neck cancer, esophageal cancer, stomach cancer, liver cancer, and breast cancer [14]. Conversely, other studies have suggested an inverse relationship between alcohol consumption and the risk of kidney cancer [29] and non-Hodgkin’s lymphoma [30,31]. Furthermore, the relationship between alcohol consumption and colorectal cancer risk appears to vary by drinking pattern. Heavy alcohol consumption has been associated with an increased risk of colorectal cancer, whereas light or moderate drinking has been linked to a reduced risk [17]. Larsson et al. also conducted a Mendelian randomization (MR) analysis examining the associations between smoking, alcohol consumption, and cancer risk. They found that genetically predicted alcohol consumption was statistically significantly associated with an increased risk of lung cancer but was not associated with other site-specific cancers or overall cancer risk [18]. In contrast, our study employed a radial MR analysis to compare the results before and after removing outlier SNPs, thereby providing more robust causal estimates [30].
Given that much of the current evidence comes from observational epidemiological studies, an assessment of the causal relationship of these findings is necessary.
The possible mechanisms of alcohol consumption on cancer development are well known. Among the ADH and ALDH enzymes that affect the metabolism of alcohol that enter the body after drinking, the gene that affects ALDH is ALDH2, and it is located on chromosome 12. This gene is an Asian specific gene and is mainly distributed in East Asian countries. In Asians, in the case of a specific type (AA) of ALDH2, facial flushing occurs after drinking, and nausea and vomiting occur. Studies have shown that these subjects are at higher risk of developing esophageal cancer if they continue to drink. On the other hand, in the case of Westerners, it is known that the alcohol-related gene is located on chromosome 4. The main cause of cancer is acetaldehyde, one of the toxins produced by the breakdown of alcohol. Once ingested, alcohol is metabolized by enzymes including alcohol dehydrogenase (ADH), cytochrome P-450 2E1 (CYP2E1) and bacterial catalase to produce acetaldehyde [31]. Acetaldehyde rapidly binds to DNA and proteins and produces DNA adducts, leading to DNA mutations, DNA crosslinking and chromosomal abnormalities [32,33]. Because acetaldehyde is highly reactive with DNA, it can bind to DNA and form DNA adducts that alter its physical conformation and potentially block DNA synthesis and repair [27,28,30]. These DNA adducts are particularly genotoxic because they can cause DNA point mutations, double-strand breaks, sister chromatid exchanges, and conformational changes to chromosomes [32,33].
The limitation of this study is that it is difficult to apply the research results to East Asians. Genetic variations such as the ALDH2 polymorphism, which is more prevalent in East Asian populations, may show different effects on the cancer risk caused by alcohol consumption. Regarding heterogeneity among cancers, cancers have variable etiologies even within the same organ site. Thus, our study was not able to fully account for the heterogeneity with cancer types, potentially obscuring subtype-specific associations. Also, we acknowledge the possibility of horizontal pleiotropy, where genetic variants used as instruments might affect cancer risk through pathways other than alcohol consumption.
We used the methods used to detect and mitigate pleiotropic effects, such as MR-Egger regression, and the use of multiple instruments.
Since our study used diverse data sources, study designs and population characteristics between the studies used for MR analysis can introduce inconsistencies and affect the comparability of results. Due to these limitations, it is crucial to interpret the findings with caution and we emphasize the need for further research, including studies with larger sample sizes and more diverse populations.
5. Conclusions
In conclusion, the strongest association was shown for esophageal cancer. For the other cancers, the evidence was not sufficient to draw any conclusions. More research is needed to understand the causality between drinking and cancer. The absence of a significant causal relationship with drinking alcohol in most cancers except for esophageal cancer does not mean that drinking is not harmful. Clearly, in observational studies, it is true that drinking a lot of alcohol was associated with a higher risk of developing various cancers. It is true that alcohol itself is not causal to the occurrence of cancer, but the act of drinking itself increases the risk of cancer, since drinking tends to be accompanied by specific situations. Ultimately, from a public health point of view, it is important to avoid excessive drinking. Future studies should focus on elucidating the underlying biological mechanisms, particularly for esophageal and biliary tract cancers. Additionally, examining the role of genetic predisposition in modulating these risks can provide deeper insights into personalized prevention strategies. Overall, our study underscores the importance of integrating genetic data with epidemiological research to inform public health recommendations and clinical interventions aimed at reducing alcohol-related cancer burdens.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/epidemiologia5030043/s1, Table S1: Two sample Mendelian Randomization between alcohol consumption and cancer sites (id:ieu-b-4834); Table S2: Two sample Mendelian Randomization between alcohol intake frequency and cancer sites (ukb-a-25).
Author Contributions
Y.J. designed the study and revised the manuscript. Y.J. oversaw the data analysis and wrote the manuscript. M.R. and J.-W.S. edited the article. Y.J. conducted the statistical analysis. M.R. and J.-W.S. critically revised the final manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was reviewed and approved by the Ewha Womans University Seoul Hospital Institutional Review Board (IRB File No: SEUMC 2021-08-026).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used in this study are publicly available and can be accessed by anyone. All datasets analyzed during this study are open at MR-Base platform (http://www.mrbase.org).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. RS-2023-00210888). This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI21C1243).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Poikolainen K. Alcohol and mortality: A review. J. Clin. Epidemiol. 1995;48:455–465. doi: 10.1016/0895-4356(94)00174-O. [DOI] [PubMed] [Google Scholar]
- 2.Volkow N.D., Wang G.J., Baler R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seitz H.K., Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer. 2007;7:599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 4.Annunziata G., Ciampaglia R., Schisano C. Alcohol-induced oxidative stress and its impact on liver injury. Nutrients. 2018;10:65. [Google Scholar]
- 5.Manthey J., Rehm J., Gmel G. The burden of alcohol use disorders in 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2017;4:987–996. doi: 10.1016/S2215-0366(18)30337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W.Y., Rosner B., Hankinson S.E., Colditz G.A., Willett W.C. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306:1884–1890. doi: 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balbo S., Meng L., Bliss R.L., Jensen J.A., Hatsukami D.K., Hecht S.S., Upadhyaya P. Folate deficiency and alcohol-induced cancer: Implications for DNA methylation. Cancer Res. 2009;69:9365–9372. [Google Scholar]
- 8.Lamy L. Clinical and statistical study of 134 cases of cancer of the oesophagus and of the cardia. Arch. Mal. App. Dig. 1910;4:451–475. [Google Scholar]
- 9.Secretan B., Straif K., Baan R., Grosse Y., El Ghissassi F., Bouvard V., Benbrahim-Tallaa L., Guha N., Freeman C., Galichet L., et al. A review of human carcinogens—Part E: Tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–1034. doi: 10.1016/S1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO) Global Status Report on Alcohol and Health 2018. World Health Organization; Geneva, Switzerland: 2018. [Google Scholar]
- 11.Brien S.E., Ronksley P.E., Turner B.J., Mukamal K.J., Ghali W.A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: Systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636. doi: 10.1136/bmj.d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen N.E., Beral V., Casabonne D., Kan S.W., Reeves G.K., Brown A., Green J. Moderate alcohol intake and cancer incidence in women. J. Natl. Cancer Inst. 2009;101:296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 13.Jung S., Wang M., Anderson K., Baglietto L., Bergkvist L., Bernstein L., Brandt P.A.v.D., Brinton L., Buring J.E., Eliassen A.H., et al. Alcohol consumption and breast cancer risk by estrogen receptor status: In a pooled analysis of 20 studies. Int. J. Epidemiol. 2016;45:916–928. doi: 10.1093/ije/dyv156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagnardi V., Rota M., Botteri E., Tramacere I., Islami F., Fedirko V., Scotti L., Jenab M., Turati F., Pasquali E., et al. Alcohol consumption and site-specific cancer risk: A comprehensive dose-response meta-analysis. Br. J. Cancer. 2015;112:580–593. doi: 10.1038/bjc.2014.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNabb S., Harrison T.A., Albanes D., Berndt S.I., Brenner H., Caan B.J., Campbell P.T., Cao Y., Chang-Claude J., Chan A., et al. Meta-analysis of 16 studies of the association of alcohol with colorectal cancer. Int. J. Cancer. 2020;146:861–873. doi: 10.1002/ijc.32377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walters R.K., Polimanti R., Johnson E.C., McClintick J.N., Adams M.J., Adkins A.E., Aliev F., Bacanu S.-A., Batzler A., Bertelsen S., et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat. Neurosci. 2018;21:1656–1669. doi: 10.1038/s41593-018-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue M. Association between alcohol consumption and colorectal cancer risk. Curr. Nutr. Rep. 2013;2:71–73. doi: 10.1007/s13668-012-0033-z. [DOI] [Google Scholar]
- 18.Larsson S.C., Carter P., Kar S., Vithayathil M., Mason A.M., Michaëlsson K., Burgess S. Smoking, alcohol consumption, and cancer: A mendelian randomisation study in UK Biobank and international genetic consortia participants. PLoS Med. 2020;17:e1003178. doi: 10.1371/journal.pmed.1003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong J., Yang L., Deng Y.Q., Yan S.Y., Gu J.M., Li B.H., Zi H., Ming D.J., Zeng X.T., Wang Y.B. The causal association between smoking, alcohol consumption and risk of bladder cancer: A univariable and multivariable Mendelian randomization study. Int. J. Cancer. 2022;151:2136–2143. doi: 10.1002/ijc.34228. [DOI] [PubMed] [Google Scholar]
- 20.Howe L.J., Nivard M.G., Morris T.T., Chen Z., Lin K., Mills M.C., Millwood I., Walters R. Within-sibship GWAS of 25 phenotypes improves estimates of direct genetic effects. Nat. Genet. 2022;54:581–592. doi: 10.1038/s41588-022-01062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neale BM, Neale Lab. Alcohol Intake Frequency (Dataset: ukb-a-25) [Data Set]. IEU Open GWAS Project. 2017. [(accessed on 26 November 2022)]. Available online: https://gwas.mrcieu.ac.uk/datasets/ukb-a-25/
- 22.Finngen Consortium. Datasets for Various Traits, Including Stomach Cancer (finn-b-c3-stomach), Liver Cancer (finn-b-C3-liver), Colon Cancer (finn-b-c3-colon), Thyroid Cancer (finn-b-c3-thyroid), Cervix Cancer (finn-b-c3-cervix), Prostate Cancer (finn-b-c3-prostate), Colorectal Cancer (finn-b-c3-colorectal), Bladder Cancer (finn-b-c3-bladder), Larynx Cancer (finn-b-c3-larynx), Lip-Oral Cancer (finn-b-c3-lip-oral), Brain Cancer (finn-b-c3-brain), Biliary Tract Cancer (finn-b-c3-Biliary tract), Oesphagus Cancer (finn-b-c3-oesphagus) [Data Sets]. IEU Open GWAS Project. 2021. [(accessed on 26 November 2022)]. Available online: https://gwas.mrcieu.ac.uk.
- 23.Burrows, UK Biobank. Lung Cancer (Dataset: ieu-b-4955) [Data Set]. IEU Open GWAS Project. 2021. [(accessed on 26 November 2022)]. Available online: https://gwas.mrcieu.ac.uk/datasets/ieu-b-4955/
- 24.Michailidou K. BCAC. Breast Cancer (GWAS) (Dataset: ieu-a-1168) [Data Set]. IEU Open GWAS Project. 2015. [(accessed on 26 November 2022)]. Available online: https://gwas.mrcieu.ac.uk/datasets/ieu-a-1168/
- 25.Zhou X., Wang L., Xiao J., Sun J., Yu L., Zhang H., Meng X., Yuan S., Timofeeva M., Law P.J., et al. Alcohol consumption, DNA methylation and colorectal cancer risk: Results from pooled cohort studies and Mendelian randomization analysis. Int. J. Cancer. 2022;151:83–94. doi: 10.1002/ijc.33945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Q., Xie W., Wang Y., Chong F., Song M., Li T., Xu L., Song C. Alcohol Consumption by Beverage Type and Risk of Breast Cancer: A Dose-Response Meta-Analysis of Prospective Cohort Studies. Alcohol Alcohol. 2020;55:246–253. doi: 10.1093/alcalc/agaa012. [DOI] [PubMed] [Google Scholar]
- 27.Brunner C., Davies N.M., Martin R.M., Eeles R., Easton D., Kote-Jarai Z., Al Olama A.A., Benlloch S., Muir K., Giles G., et al. Alcohol consumption and prostate cancer incidence and progression: A Mendelian randomisation study. Int. J. Cancer. 2017;140:75–85. doi: 10.1002/ijc.30436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boccia S., Hashibe M., Galliì P., De Feo E., Asakage T., Hashimoto T., Hiraki A., Katoh T., Nomura T., Yokoyama A., et al. Aldehyde dehydrogenase 2 and head and neck cancer: A meta-analysis implementing a Mendelian randomization approach. Cancer Epidemiol. Biomark. Prev. 2009;18:248–254. doi: 10.1158/1055-9965.EPI-08-0462. [DOI] [PubMed] [Google Scholar]
- 29.Lesseur, Oncoarray Oral Cavity and Oropharyngeal Cancer. Oral Cavity Cancer (Dataset: ieu-b-94) [Data Set]. IEU Open GWAS Project. 2016. [(accessed on 26 November 2022)]. Available online: https://gwas.mrcieu.ac.uk/datasets/ieu-b-94/
- 30.Mahabir S., Leitzmann M.F., Virtanen M.J., Virtamo J., Pietinen P., Albanes D., Taylor P.R. Prospective study of alcohol drinking and renal cell cancer risk in a cohort of Finnish male smokers. Cancer Epidemiol. Biomark. Prev. 2005;14:170–175. doi: 10.1158/1055-9965.170.14.1. [DOI] [PubMed] [Google Scholar]
- 31.Tramacere I., Pelucchi C., Bonifazi M., Bagnardi V., Rota M., Bellocco R., Scotti L., Islami F., Corrao G., Boffetta P., et al. Alcohol drinking and non-Hodgkin lymphoma risk: A systematic review and a meta-analysis. Ann. Oncol. 2012;23:2791–2798. doi: 10.1093/annonc/mds013. [DOI] [PubMed] [Google Scholar]
- 32.Terry M.B., Gammon M.D., Zhang F.F., Knight J.A., Wang Q., Britton J.A., Teitelbaum S.L., Neugut A.I., Santella R.M. ADH3 genotype, alcohol intake and breast cancer risk. Carcinogenesis. 2006;27:840–847. doi: 10.1093/carcin/bgi285. [DOI] [PubMed] [Google Scholar]
- 33.Lorenti Garcia C., Mechilli M., Proietti De Santis L., Schinoppi A., Kobos K., Palitti F. Relationship between DNA lesions, DNA repair and chromosomal damage induced by acetaldehyde. Mutat. Res. 2009;662:3–9. doi: 10.1016/j.mrfmmm.2008.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study are publicly available and can be accessed by anyone. All datasets analyzed during this study are open at MR-Base platform (http://www.mrbase.org).