Abstract
Background:
The lack of complete protection against leishmaniasis and the challenges of anti-leishmaniasis drug treatment have made the treatment process more difficult. This study aimed to develop a new strategy for preparing a vaccine against cutaneous leishmaniasis using some of the antigenic proteins of the Leishmania parasite.
Methods:
This study was carried out in 2022 at Shahid Chamran University of Ahvaz, Ahvaz, Iran. After preparing suitable epitopes of the Leishmania parasite and examining their antiparasitic properties, the process of making a fusion vaccine was performed and with the help of various bioinformatics tools, physicochemical and structural properties as well as immunological and simulation properties were studied and finally optimized. Construction and cloning were performed in the E.coli K12 system and finally, the docking process was performed with Toll-like receptors (TLRs), major histocompatibility complex I (MHC-I), and MHC-II receptors. With the help of selected epitopes of the Leishmania parasite, which had a high percentage of population coverage, a stable, antigenic, and non-allergenic chimeric vaccine was predicted.
Results:
The results of the structural analysis of the TLR5\vaccine complex and simulation of its molecular dynamics showed a sufficiently stable binding. It also showed good potential for stimulation and production of active B cells and memory, as well as the potential for CD8+ T, CD4+ T cell production and development of Th2 and Th1-induced immune responses.
Conclusion:
Computational results showed that the designed immunogenic structure has the potential to adequately stimulate cellular and humoral immune responses against Leishmania parasitic disease. As a result of evaluating the effectiveness of the candidate vaccine through in vivo and in vitro immunological tests, it can be suggested as a vaccine against Leishmania major.
Keywords: Leishmania, Chimeric vaccine, Bioinformatics, Immunogenicity
Introduction
Leishmaniasis is a disease with worldwide distribution, which is spread by infection with the Leishmania parasite (1). Currently, anti-Leishmania drugs are expensive and have serious side effects and drug resistance (2). Current control of the disease relies on chemotherapy to reduce disease (3) and vector control to reduce transmission. However, elimination of this disease is not possible without providing appropriate and dominant immunity in the native population against parasites (4). Therefore, safer treatment processes such as vaccine design can help significantly in the process of disease control, but there is still no protective vaccine in normal use for humans with this disease. Therefore, it is necessary to make an effective vaccine (5).
Leishmania spp. produce many different secretory proteins with antigenic properties such as kinetoplastid membrane protein-11 (KMP-11), Leishmania eukaryotic initiation factor (LeIF), glycoprotein 63 (GP63), p36/LACK, and A2 that can elicit an immune response in the host. The dominant Th1 response activates the production of interferon-gamma (IFN-γ) macrophages to kill parasites by producing nitric oxide (6). On the other hand, the involvement of CD8+ T cells as a powerful immune arm in the control of leishmaniasis has been proven in the immune response against various species of Leishmania. IFN-γ production by these cells alters the Th2 response to Th1 from the outset (7). IFN-γ production by CD4+ T cells also controls disease in primary infections (8). IFN-γ production was shown in secondary infection of mice immune to L. major due to CD8+ T cells. Numerous reports of Leishmania antigens that elicit CD8+ T cell responses such as P8 and gp46, KMP11, cysteine protease B (CPB), nucleosomal histones, the L. major amastigote class I nuclease (LmaCIN), L. major stress-inducible protein 1 (LmSTI1) and TSA have been published in various studies. This means that CD8+ T lymphocytes can be important in protecting against Leishmania infections. Epitope vaccines have extraordinary benefits, especially the ability to direct the immune system to CD8+ T cell responses. Recently, many of these secretory proteins have been used in various vaccines, but their results have not been very satisfactory (9). Therefore, in this study, several of these proteins such as thiol-specific antioxidant (TSA), lipophosphoglycan-3 (LPG3), GP63, CPB, cysteine protease C (CPC), LMSTI-1, LeIF, KMP-11, and FliC were used to prepare efficient epitopes for the vaccine. It should be mentioned that some of these, such as the KMP-11 antigen, are also involved in parasite cell motility (10), and KMP-11 is present in all species of the kinetoplastid family, which can induce an appropriate cellular immune response (11).
In designing a strong vaccine, efficient stimulation of immune responses is one of the main goals of vaccination (12). Therefore, in this research work, bioinformatics tools were used to develop a polypeptide consisting of different epitopes including, TSA, LPG3, GP63, CPB, CPC, LMSTI-1, LeIF, KMP-11, and fliC of L. major parasite as an immunogenic vaccine against leishmaniasis.
Methods
Preparation of the desired protein sequence from NCBI
This study was carried out in 2022 at Shahid Chamran University of Ahvaz, Ahvaz, Iran. For obtaining sequences of nine L. major proteins including, TSA (ABX11567.1), LPG3 (XP_001466598.1), GP63 (ACL01096.2), CPB (CAJ02287.1), CPC (CBZ12414.1), LMSTI-1 (CAJ02290.1) and LeIF (CBZ12060). 1) KMP-11 (XP_001469033.1) and fliC (P72151.2) NCBI database (https://www.ncbi.nlm.nih.gov/) was used and the sequences were retrieved based on their access numbers.
Abundance prediction of linear B-cell and T-cell epitopes
At this stage, by using the epitope sequences in FASTA format and with the help of the IEDB server (https://www.iedb.org/), the frequency of epitopes used to prepare this vaccine in different world populations was predicted (13).
Design of vaccine structure and prediction of the second structure
Selected major histocompatibility complex (MHC-I), MHC-II, and B cell epitopes along with some linker proteins were used in vaccine design and adjuvants were added to the amino terminus of vaccines. Administration of TLR agonists as adjuvants to vaccine candidates elicits strong T-cell responses and antibodies. To analyze the secondary structure of the vaccine structure, Prabi ( https://prabi.ibcp.fr/ ) and Psipred (http://bioinf.cs.ucl.ac.uk/psipred/) servers were used.
In assessing the antigenicity of the entire vaccine structure, VaxiJen v2.0 (http://www.ddgpharmfac.net/vaxijen/VaxiJen/VaxiJen.html) servers and for validation, AntigenPro ( https://scratch.proteomics.ics.uci.edu/explanation.html#ANTIGENpro) servers were used.
The physicochemical properties, solubility, and surface availability of amino acids of the candidate vaccine were evaluated via Expasy-ProtParam (https://www.expasy.org/), PEPCALC (https://pepcalc.com/), and IEDB server.
Then, 3Drefine (https://3drefine.mu.hekademeia.org/) and Pymol (https://pymol.org/2/) servers were used to structurally modify and visualize the vaccine. Vaccine validation was also spatially verified through PROCHECK (https://www.ebi.ac.uk/thornton-srv/software/PROCHECK/) and ProSA (https://prosa.services.came.sbg.ac.at/prosa.php) servers.
Docking
Molecular binding to predict affinity between vaccine structure and some human TLRs and MHC-I, and MHC-II, was performed by ClusPro 2.0 server (https://cluspro.bu.edu/) after preparation by Swiss-PdbViewer (SPDBV), Discovery Studio, and Molegro Virtual Docker (MVD) software and their interactions were illustrated by Pymol and PDBSUM software (14).
Normal state analysis was performed to evaluate and analyze the structural stability of the selected docking complex through the iMODS web server (https://imods.iqfr.csic.es/).
Insilico's safety simulation was performed using a C-ImmSim server (https://kraken.iac.rm.cnr.it/C-IMMSIM/index.php) to confirm immunization and immune response against the selected vaccine (15).
For maximum expression of the vaccine in the host, the codon of the candidate vaccine was optimized using the Java Codon Compatibility Tool (JCat) (http://www.jcat.de/) to increase the translation rate of the vaccine in the E. coli K12 system.
Results
Predictive results of linear B cell and T cell epitopes
Fig. 1, is related to BiPred prediction and determines the antibody epitope prediction B cells, which is completely consistent with how the epitopes are placed in the vaccine structure in the diagram of vaccine sequence analysis by the IEDB server. Also, the IEDB MHC-II webserver was used to predict Helper T lymphocyte (HTL) epitopes specific for human alleles, the human leukocyte antigen (HLA) (Table 1).
Fig. 1:
Prediction of linear vaccine epitopes by IEDB server
Table 1:
Predictive results of epitopes related to MHC-I and MHC-II by using the IEDB server
| PROTEIN | VAXIJEN SCORE | BCELL EPITOPE | VAXIJEN SCORE | MHC-I EPITOPE | VAXIJEN SCORE | MHC-II EPITOPE | PROTEIN | VAXIJEN SCORE | BCELL EPITOPE |
|---|---|---|---|---|---|---|---|---|---|
| TSA | 0.6132, 0.8973 | NAKINCPAPPFEEVALMPNGSFKKISLAA, PANWKKGAPTMKPEPKASVEGY | 0.5334, 1.5613 | MLADKTKSIA, VLFFYPLDF | 0.6717, 1.3854 | FYPLDFTFVCPTEII, KWVVLFFYPLDFTFV | TSA | 0.6132, 0.8973 | NAKINCPAPPFEEVALMPNGSFKKISLAA, PANWKKGAPTMKPEPKASVEGY |
| LPG3 | 1.0507, 1.1385 | SAGDGRGT, EAIANGKQVENPAPSGHTHLKKPAYTKF | 0.8966, 1.2089 | KMLDILVNSL, MLDILVNSL | 0.7892, 1.4979 | STNILQRIRDLALQS, WVVLFFYPLDFTFVC | LPG3 | 1.0507, 1.1385 | SAGDGRGT, EAIANGKQVENPAPSGHTHLKKPAYTKF |
| GP63 | 1.1482, 0.7758 | DILTNEKRD, IASRYDQ | 1.1333, 1.0811 | FTDPSLAGV, EVMPWGQNA | 1.0258, 0.7223 | PPYWQYFTDPSLAGV, GLRVELSTVSNAFEG | GP63 | 1.1482, 0.7758 | DILTNEKRD, IASRYDQ |
| CPB | 0.9767, 2.9436 | RNMNGTVFTEKSYPYVSGNGDVPECSNSSELAPGA, DNGCGG | 1.0335, 0.934 | TGYPVSVHV, GLMLQAFEWV | 1.1943, 1.4140 | NGPISIAVDASSFMS, GARIDGYVSMESSER | CPB | 0.9767, 2.9436 | RNMNGTVFTEKSYPYVSGNGDVPECSNSSELAPGA, DNGCGG |
| CPC | 1.4454, 1.5386 | SKAKGQ, TSYSVKGEKE | 0.9557, 1.0592 | FLGGHAVKL, FVAEVNSKA | 1.4368, 0.5984 | FVAEVNSKAKGQWTA, GGHAVKLVGWGTQDG | CPC | 1.4454, 1.5386 | SKAKGQ, TSYSVKGEKE |
| LMSTI-1 | 0.9533, 1.3055 | ELKNKGNEEFSAGRYVEA, ECEKEHQKAVE | 0.7393, 0.5194, 0.5070 | AMDDPEIAA, NEEFSAGRY, TSYSNRAAAY | 0.9975, 0.5668 | RLYMEDQRFALTLM, PKLSLLMLQPDYVKM | LMSTI-1 | 0.9533, 1.3055 | ELKNKGNEEFSAGRYVEA, ECEKEHQKAVE |
| LEIF | 0.6395, 0.5344 | LVTPNEAKACEAHFRQGNEVRS, VTRRRVRSMGKLIKV | 1.0246, 0.5395 | KINDVVWVKI, QVNDTSAVV | 0.8648, 1.0609 | AGRYVEAVNYFSKAI, RIQEYMKDSGISSKI | LEIF | 0.6395, 0.5344 | LVTPNEAKACEAHFRQGNEVRS, VTRRRVRSMGKLIKV |
| KMP-11 | 0.8123 | EEFNRKMQEQNAKFFADKPDESTLSPEMKEH YEKFERMIKEHTEKFNKKMHEHSEHFKQK | 0.7709, 0.6506 | KMQEQNAKF, TTYEEFSAKL | 1.5050, 1.3416 | NAKFFADKPDESTLS, EQNAKFFADKPDEST | KMP-11 | 0.8123 | EEFNRKMQEQNAKFFADKPDESTLSPEMKEH YEKFERMIKEHTEKFNKKMHEHSEHFKQK |
| fliC | - | 0.7035, 0.8097 | KMDGAIPNL, YVQLNSPTA | 0.6095, 0.5518 | QRIRDLALQSANGSN, SLNTQRNLNASSNDL | fliC | - |
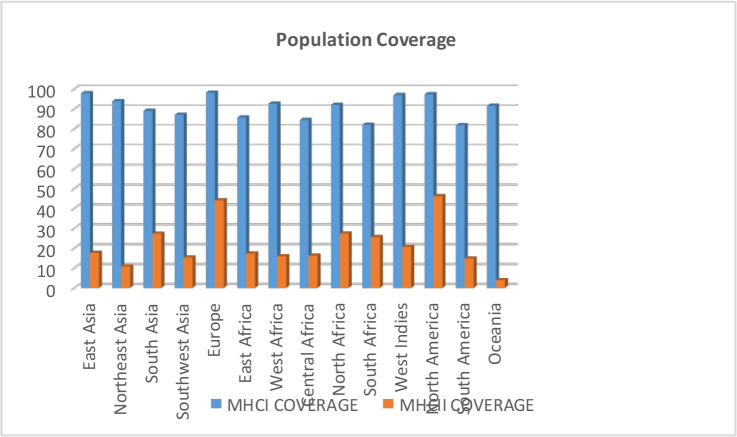
Population coverage of vaccine epitopes results
This calculation is based on HLA genotypic frequencies, assuming a non-linking imbalance between HLA loci. Vaccine epitopes designed with the IEDB population coverage tool were calculated. CD8+ T cell and CD4+ T cell epitopes were examined for all populations of the world with “region-country-ethnicity” (Fig. 2).
Fig. 2:
Population coverage results of vaccine epitopes
Vaccine immunogenic structure results
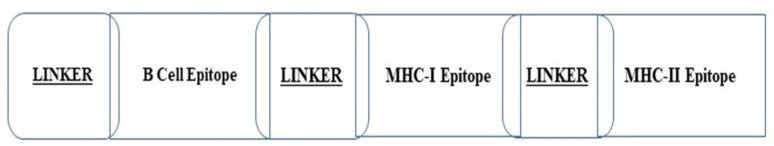
The chimer vaccine protein was generated from the epitopes screened in Table 1, in which the B cell epitopes were first put together and ligated together by the EAAAK linker. Then, with the help of the GPGPG linker, the epitopes derived from MHC-I were also connected, and finally, by the AAY linker, a connection was established between the two sections containing MHC-I and MHC-II epitopes (Fig. 3).
Fig. 3:
Schematic diagram of the designed vaccine structure
Evaluation of allergenicity and antigenicity of the designed vaccine
The antigenicity of the selected epitopes was investigated and confirmed by the VAXIJEN server. Moreover, their allergenicity is evaluated with the help of ALLerTPv.2.0 and ALLERTOP software, and the cases that had an allergic risk were removed and the rest were used in the construction of the vaccine structure. Moreover, all selected epitopes in Table 1 do not possess allergenic properties.
Physicochemical properties and solubility and available surface results
According to the results of Protoparam, Pepcalc, and Prosol and the amount of the available surface, the instability coefficient of the vaccine was 35.71 and the corresponding structure contained 736 amino acids equivalent to 2208 nucleic acids and had a stable composition and a suitable half-life of one hour in mammals. It also had a molecular weight of 82179.11, a gravity of −0.425, an aliphatic coefficient of 69.08, and an isoelectric pH of 5.58 (Fig. 4).
Fig. 4:
Access level of the vaccine designed by the IEDB server
The second structure prediction results
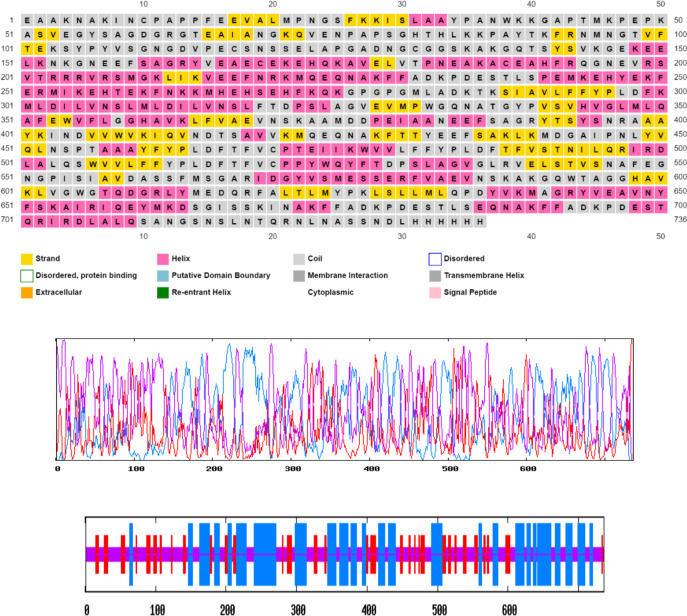
In terms of two-dimensional and three-dimensional structure, the relevant vaccine had 20.79% random screw and 20.79% wide strand and also had a beta screw of 7.61% (Fig. 5).
Fig. 5:
Graphical representation of secondary structure prediction of the multi-epitope vaccine. Here, the β-strands, α-helix, and random coils are indicated, and evaluation of the composition of the vaccine produced in terms of two-dimensional and three-dimensional structure with the help of software, A) psiPred and B) Prabi
Third structure modification and validation of the vaccine results
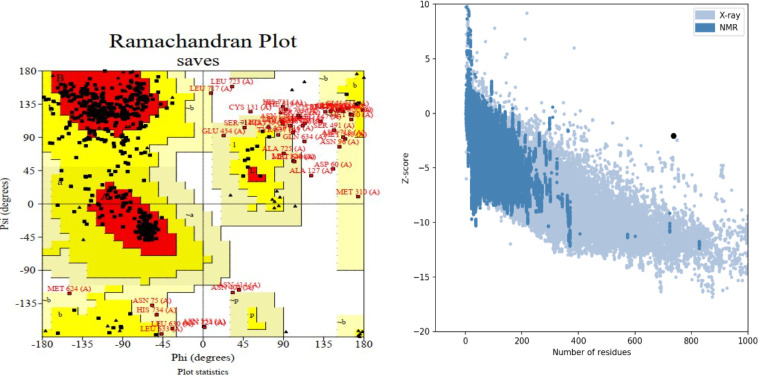
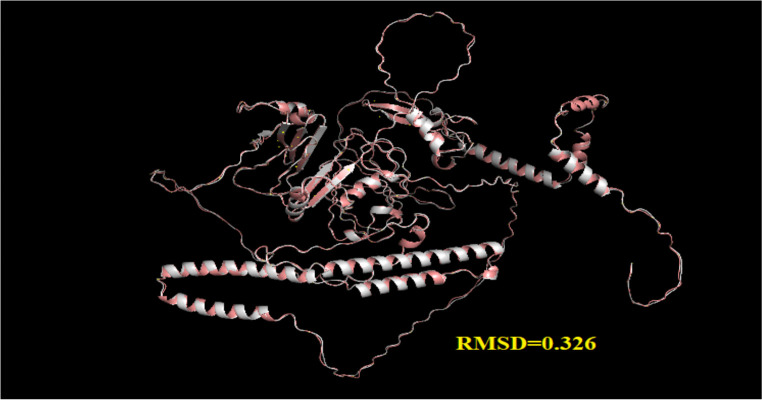
The best model was selected from different models of the ALPHAFOLD server (https://alphafold.ebi.ac.uk/). This structure scored −7 on the ProSA server and was within the range of natural compounds obtained from X-RAY technology. After correction by the 3DRefine server, it was evaluated through the pro check server, in which according to the Ramachandran diagram, about 97.1% of its amino acids were in an acceptable position (Fig. 6). In addition, the results of vaccine modification using the 3DREFINE server and visualizing by PYMOL with RMSD= 0.326 showed the improvement of the model after modification (Fig. 7).
Fig. 6:
Structural validation of the tertiary structure of the vaccine construct. A) Represents the Ramachandran plot of the refined model where the most favored, allowed regions are 97.1% using the Prochek server. B) Indicates ProSA-web validation of 3D structure showing Z-score (− 4.54)
Fig. 7:
Comparative image through PAYMOL alignment software between the two original and modified models vaccine after modifying and calculating the amount of RMSD between them
Docking results
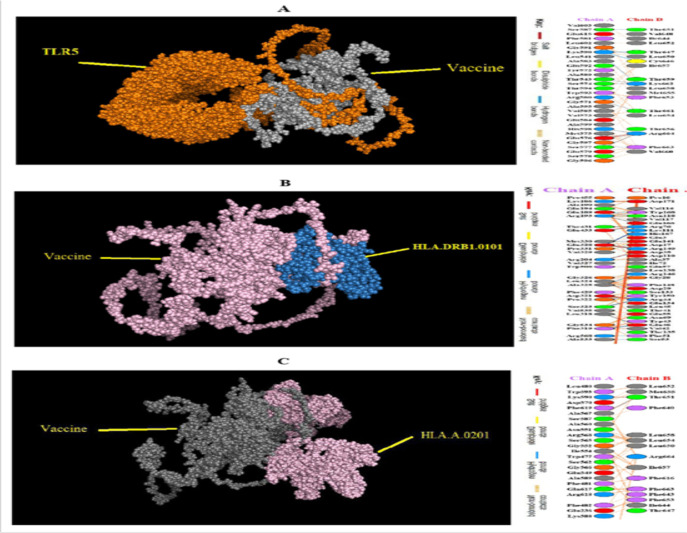
In this study, TLR5 was selected for the docking process due to its immunomodulatory ability to stimulate IFN-g as well as activation of type I IFN responses. Epitopes selected as CD4+ epitopes were able to stimulate both Th1 and Th2 cytokines. Complexes with the lowest energy scores were selected. TLR5 had the lowest energy level of 2174.1 - and the most stable state of connection with the vaccine. Interactions between vaccines designed with different receptors and residues and the type of links involved in these interactions were analyzed and obtained by the PDBsum server (Table 2) (Fig. 8).
Table 2:
Energy levels of vaccine/recipient docking complexes
| RECEPTOR | TLR1 | TLR2 | TLR3 | TLR4 | TLR5 | TLR8 |
|---|---|---|---|---|---|---|
| Lowest Energy | −1378.5 | −1642.8 | −1645.0 | −1899.8 | −2174.1 | −15281.0 |
Fig. 8:
Residual interactions between vaccine structures and and molecules of A) TLR5, B) HLA.DRB1.0101, and C) HLA.A.0201, which shows hydrogen bonds and other bonds between the residues of the molecule and the residues of the vaccine
Normal state analysis to determine the structural stability of the ligand/receptor complex (molecular dynamic simulation) results
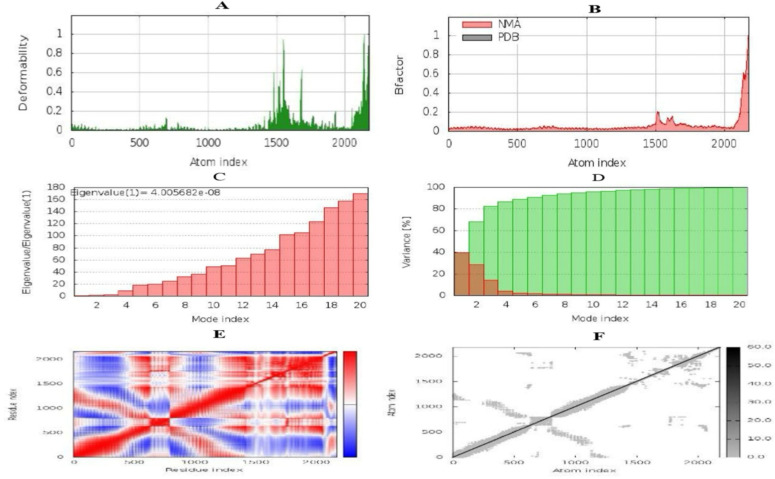
During the biophysical stability analysis and molecular dynamic simulations through the iMODS server (https://imods.iqfr.csic.es/), the deformation of the main iMOD chain was obtained, and it is shown in Fig. 9A. The hinge area tends to deform. The values of calculated factor B are proportional to the square root of the mean, and factor B measures the fluctuations of the atomic position and the unpredictability of each atom (Fig. 9B). Fig. 9C shows the eigenvalues and is closely related to the energy required to smooth the structure. This result reflected the stability of the dock complex shown by the eigenvalue. The specific value of the vaccine\TLR5 complex was 4.005682e-08, which indicates less energy for the deformation of the structure indicates the stability of the complex, and activates immunity to eliminate antigens. The variance is inversely related to the eigenvalue. In Fig. 9D, the individual variance with red and green represents the cumulative variance. The covariance map shows the relationship between the pairs of residues because the colors red, blue, and white represent the pairs of correlated, anti-correlated, and unrelated residues, respectively (Fig. 9E). The elastic model network illustrates spring formation between the corresponding pair of atoms. The gray color dots show spring formation; the thicker the gray color, the stronger the spring between corresponding pairs (Fig. 9F).
Fig. 9:
Molecular dynamics simulation, normal mode analysis, and receptor-ligand interactions using the iMODS server. A) This graph represents the deformability potential of each residue. The higher the peak, the higher will be the deformability. B) B-factor. C) Eigenvalue of vaccine-TLR5 complex. This graph shows the eigenvalue of the vaccine-TLR5, which interprets the ease of deforming the structure. The lower the eigenvalue, the higher the chance of deformation. D) Variance. E) Covariance matrix. Most of the region is red so it shows the correlated motion of residues; whereas, the blue region shows uncorrelated motion between residues. F) Elastic network model. In this graph, most of the atoms form stronger springs
In-silico immune simulation results
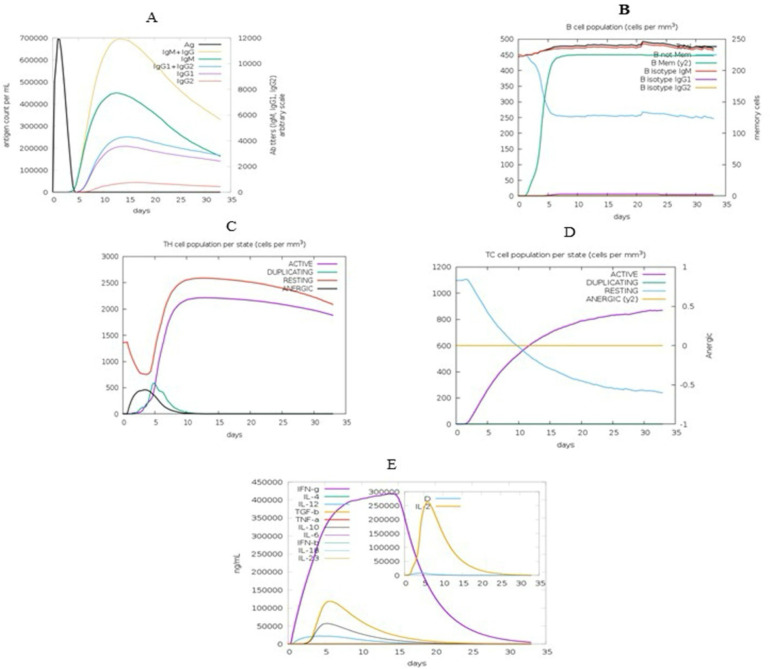
The results of the C-ImmSim server matched the actual immune responses and were associated with increased secondary response generation. The initial response was characterized by an increase in high IgM levels. In the secondary and tertiary responses, the increase in B and T cell populations was detected. Fig. 10A and B show an increase in the levels of IgG1, IgG2, and IgM. According to Figures 10C and D, the increase in the population of memory and immune cells after vaccine injection returns to the initial level after a certain period, which indicates the efficacy of the candidate vaccine (Fig. 10C–D). Moreover, high levels of cytokines such as IFN-γ, interleukin-12 (IL-12), transforming growth factor-beta (TGF-β), and IL-4 were seen in Fig. 10E. These parameters indicated good immunogenicity of the vaccine (Fig. 10).
Fig. 10:
Image of the results provided by the C-ImmSim server. The in-silico immune response induced by vaccine-TLR5. A) Production of several immunoglobulin subclasses (colored lines) in response to vaccine injection. B) B-cell richness after three injections. C) Evolution of T helper cells (Th cells) after the injections. D) Evolution of cytotoxic T cells (TC cells) after the injections. E) Production of cytokines and interleukins
Codon optimization and cloning preparation
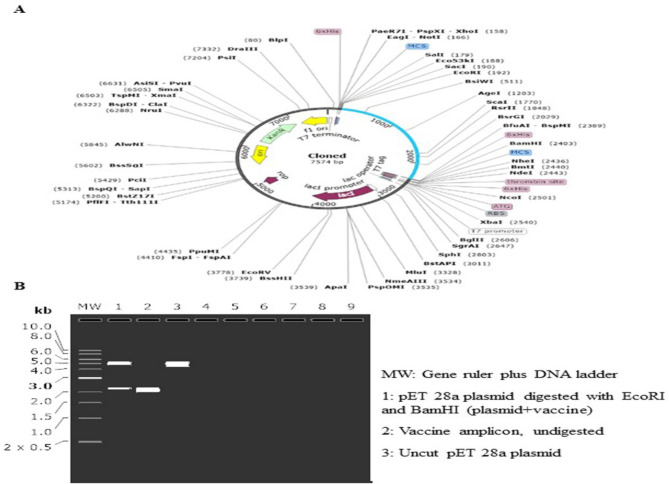
According to the JCat server's results, the optimized sequences' CAI values were 0.991, and its content had 48.143% of GC content in E. coli. Then, the simulation process was performed via Snap Gene software using the gene sequence structure of the designed vaccine and the vector and the cloned structure of the recombinant vector and the gene sequence of the designed vaccine. The restriction enzymes of EcoRI and BamHI were used for virtual cloning. Finally, an adapted codon sequence was inserted into the pET28a (+) vector for designing a recombinant plasmid using SnapGene software (Fig. 11).
Fig. 11:
A) In-silico cloning of fusion protein sequence into pET28a (+) vector using EcoRI and BamHI restriction enzymes. Blue-colored semicircle showing fusion protein sequence and black-colored semi-circles indicating pET28a (+) vector backbone. B) Image of vaccine cloning and electrophoresis process using SnapGene software
Discussion
High cost and drug resistance are among the shortcomings of chemotherapy in treating leishmaniasis (16). Advances in molecular immunology and the identification of dominant immune epitopes have led to fundamental changes in these experimental methods (17). Immunoinformatics tools are used to develop and design vaccines that reduce the negative aspects of experimental methods such as the predictable ethical aspects of laboratory work and experimental studies, which are costly and time-consuming (18).
In the humoral immune response, the presentation of antibodies is an essential part of vaccine design. Therefore, the identification of B cell epitopes can be useful (19). Because Leishmania sp. is an intracellular parasite, cytotoxic T-cell lymphocytes (CTLs) play an important role in its protective response. Bioinformatics software with access to Leishmania genomic and proteomic information can provide useful information about the characteristics of the candidate vaccine and improve its properties (20). Therefore, researchers can design more effective vaccines (21).
In the present study, bioinformatics and structural vaccination methods were important for designing fusion protein vaccines consisting of TSA, LPG3, GP63, CPB, CPC, LMSTI-1, LEIF, and KMP with the help of a suitable linker to maintain the biological activity of the domains. In the present study, a single-sequence linker (GPGPG) was used to link the two B-cell and T-cell vaccine epitopes, and a linker (EAAAK) was used at the beginning of the vaccine sequence to prevent unwanted connections and twists in the sequence (22). Based on the results of physicochemical parameters, solubility, and availability of the studied vaccine, the calculated PI index indicates the acidic nature of the vaccine, and this advantage plays an important role due to its role in amphibian/hydrophobic balance in the vaccine design process. The instability index provides an estimate of the stability of the protein in the test tube. The instability index of the vaccine with a coefficient of 35.71 indicates its stability. The aliphatic index indicates that the protein is occupied by aliphatic side chains. The mean gravity of the studied vaccine (0.425) indicates the optimal solubility of the vaccine in water. Also, the high aliphatic index of the studied vaccine (69.08) indicates the possibility of stability in a wide temperature range.
In evaluating the secondary structure of the vaccine, it was found that the biological function of the recombinant proteins is affected by their three-dimensional structure. The three-dimensional structure of the protein can be useful in protein function, dynamics, and interaction with ligands, and other proteins. Based on the validation results, the highest quality of the three-dimensional structure model of chimeric protein and the final selected model belonged to the best ALPHAFOLD model, which was modified with the help of 3DREFINE, and its structural quality was improved. The results of the Ramachandran diagram by Procheck server showed more than 90% of the amino acid position is favorable. During the results of the ProSA server with a score of −2.7, it was found that the final model is in the range of natural compounds obtained from X-RAY.
One of the critical steps in vaccine design is the prediction of antigen epitopes because the interaction between epitopes and MHC molecules can lead to cellular immunity (23). On the other hand, the immune response is important in controlling infection since the development of a specific T helper (Th) 1 response, based on the production of cytokines, such as interferon-gamma (IFN-γ), can protect the mammalian host from infection by parasites. Leishmaniasis is present in mammalian hosts as forced amastigotes within the macrophage. Macrophage activation is required to kill organisms in vivo, and IFN-γ, as the major macrophage activator, mediates defense against leishmaniasis (24). The designed vaccine induced IFN-γ and IL-2, IL-10, and IL-23 and increased IgM, IgG1, and IgG2 levels, indicating the vaccine's ability to stimulate the immune response. Analysis of the vaccine using the oxygen allotope tool showed that it has strong antigenic and nonallergenic properties.
Binding and binding analysis between vaccines and immune receptors were performed well and the best complex model was selected from them. Analysis and visualization of the best model of fusion receptor protein complexes and identification of important residues with various bonds involved by the PDBsum server indicated stable vaccine and receptor binding. Stability analysis of the structure of vaccine complexes with effective immune receptors was performed by the IMODE server in the host E. coli (25). To achieve a high level of expression of recombinant vaccine protein in E. coli (strain K12), codon optimization was performed by JCAT. The CAI (0.991) and GC (48.143%) indices obtained from JCAT showed that the vaccine could clone properly and express the E. coli host.
All these processes were performed in an insilico environment, but their final approval requires laboratory and clinical processes.
Conclusion
Collectively, our computational findings provide evidence that this predicted vaccine may hold potential as a therapeutic target for L. major disease.
Acknowledgements
The authors thank the professors of Shahid Chamran University of Ahvaz for their guidance in conducting research and writing the manuscript.
Footnotes
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Heydarpour F, Sari AA, Mohebali M, et al. Incidence and disability-adjusted life years (Dalys) attributable to leishmaniasis in Iran, 2013. Ethiop J Health Sci. 2016; 26: 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polonio T, Efferth T. Leishmaniasis: drug resistance and natural products. Int J Mol Med. 2008; 22(3): 277–286. [PubMed] [Google Scholar]
- 3.Hodiamont CJ, Kager PA, Bart A, et al. Species-directed therapy for leishmaniasis in returning travelers: a comprehensive guide. PLoS Negl Trop Dis. 2014; 8(5):e2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engwerda CR, Matlashewski G. Development of Leishmania vaccines in the era of visceral leishmaniasis elimination. Trans R Soc Trop Med Hyg. 2015; 109(7):423–4. [DOI] [PubMed] [Google Scholar]
- 5.Vakili B, Eslami M, Hatam GR, et al. Immunoinformatics-aided design of a potential multi-epitope peptide vaccine against Leishmania infantum. Int J Biol Macromol. 2018; 120(Pt A): 1127–1139. [DOI] [PubMed] [Google Scholar]
- 6.Chacko A, Joseph M, Feltis T, Morris SK. Case report: Successful treatment of cutaneous leishmaniasis with topical paramomycin in a child after treatment failure with systemic fluconazole. Am J Trop Med Hyg. 2016; 95(4):793–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uzonna JE, Joyce KL, Scott P. Low dose Leishmania major promotes a transient T helper cell type 2 response that is down-regulated by interferon γ–producing CD8+ T cells. J Exp Med. 2004; 199(11):1559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herath S, Kropf P, Müller I. Cross-talk between CD8+ and CD4+ T cells in experimental cutaneous leishmaniasis: CD8+ T cells are required for optimal IFN-γ production by CD4+ T cells. Parasite Immunol. 2003; 25(11–12): 559–67. [DOI] [PubMed] [Google Scholar]
- 9.Iborra S, Soto M, Carrión J, et al. Vaccination with a plasmid DNA cocktail encoding the nucleosomal histones of Leishmania confers protection against murine cutaneous leishmaniosis. Vaccine. 2004; 22(29–30):3865–76. [DOI] [PubMed] [Google Scholar]
- 10.Farajnia S, Mahboudi F, Ajdari S, et al. Alimohammadian, Mononuclear cells from patients recovered from cutaneous leishmaniasis respond to Leishmania major amastigote class I nuclease with a predominant Th1-like response. Clin Exp Immunol. 2005; 139(3): 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Méndez S, Gurunathan S, Kamhawi S, et al. The potency and durability of DNA-and protein-based vaccines against Leishmania major evaluated using low-dose, intradermal challenge. J Immunol. 2001; 166(8): 5122–5128. [DOI] [PubMed] [Google Scholar]
- 12.Thomson SA, Khanna R, Gardner J, et al. Minimal epitopes expressed in a recombinant polyepitope protein are processed and presented to CD8+ cytotoxic T cells: implications for vaccine design. Proc Natl Acad Sci U S A. 1995; 92(13): 5845–5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain K, Jain NK. Vaccines for visceral leishmaniasis: A review. J Immunol Methods. 2015; 422: 1–12. [DOI] [PubMed] [Google Scholar]
- 14.Kar T, Narsaria U, Basak S, et al. A candidate multi-epitope vaccine against SARS-CoV-2. Sci Rep. 2020; 10(1): 10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atapour A, Mokarram P, MostafaviPour Z, et al. Designing a fusion protein vaccine against HCV: an in silico approach. Int J Pept Res Ther. 2019; 25: 861–872. [Google Scholar]
- 16.Gradoni L, Soteriadou K, Louzir H, et al. Drug regimens for visceral leishmaniasis in Mediterranean countries. Trop Med Int Health. 2008; 13(10):1272–6. [DOI] [PubMed] [Google Scholar]
- 17.Ghorbani M, Farhoudi R. Leishmaniasis in humans: drug or vaccine therapy? Drug Des Devel Ther. 2017;12:25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponte-Sucre A, Gamarro F, Dujardin JC, et al. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl Trop Dis. 2017; 11(12): e0006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oli AN, Obialor WO, Ifeanyichukwu MO, et al. Immunoinformatics and vaccine development: an overview. Immunotargets Ther. 2020; 9:13–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahboobi M, Sedighian H, Malekara E, et al. Harnessing an integrative in silico approach to engage highly immunogenic peptides in an antigen design against epsilon toxin (ETX) of Clostridium perfringens. Int J Pept Res Ther. 2021; 27: 1019–1026. [Google Scholar]
- 21.Irvine DJ, Read BJ. Shaping humoral immunity to vaccines through antigen-displaying nanoparticles. Curr Opin Immunol. 2020; 65: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agallou M, Margaroni M, Kotsakis SD, et al. A canine-directed chimeric multi-epitope vaccine induced protective immune responses in BALB/c Mice infected with Leishmania infantum. Vaccines (Basel). 2020; 8(3): 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.María RR, Arturo CJ, Alicia JA, et al. The impact of bioinformatics on vaccine design and development. Vaccines. 2017; 2: 3–6.29263864 [Google Scholar]
- 24.Khatoon N, Pandey RK, Prajapati VK. Exploring Leishmania secretory proteins to design B and T cell multi-epitope subunit vaccine using immunoinformatics approach. Sci Rep. 2017; 7: 8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan MA, Ami JQ, Faisal K, et al. An immunoinformatic approach driven by experimental proteomics: in silico design of a subunit candidate vaccine targeting secretory proteins of Leishmania donovani amastigotes. Parasit Vectors. 2020; 13(1): 196. [DOI] [PMC free article] [PubMed] [Google Scholar]