Abstract
Background:
As an ecological and ethical method employed for poultry farming, free-range chicken keeping is growing and is important in fostering rural farmers' livelihoods and community prosperity. This study aimed to determine the nature and prevalence of helminth infections in native poultry in Zabol, Iran.
Methods:
Between 2022 and 2023, 160 chickens were acquired and slaughtered ethically, and then their gastrointestinal tracts were taken and formalin preserved in Zabol, Iran. The parasites were isolated from gastrointestinal mucus in the laboratory and characterized using a microscope and specific methods such as Acetocarmine staining.
Results:
Among 160 dissected birds, 92 (57.5%) were presented with gastrointestinal parasites, with 64 (40%) containing cestodes, 16 (10%) containing nematodes, and 12 (7.5%) infected by both. Observations were made of seven different species. Ascaridia galli (A. galli) (10%), Subulura brumpti (7.5%), and Heterakis gallinarum (3.12%) represent the three main nematode species. While Raillietina tetragona (R. tetragona) (33.12%) was the most prevalent cestode, R. echinobothrida (6.25%), R. magninumida (5%), and Cotugnia digonopora (3.12%) were less common.
Conclusion:
The study revealed that free-range chickens in the arid Zabol region had a high prevalence of gastrointestinal parasites. There is a strong correlation between chicken foraging behavior and exposure to contaminated soil, with certain nematodes and cestodes constituting the most prevalent, negatively affecting bird growth, productivity, and health. Therefore, improving poultry welfare by applying parasite control measures and implementing appropriate management strategies is essential.
Keywords: Free-range chicken, Gastrointestinal parasites, Helminths, Iran
Introduction
Today, keeping free-range chickens promotes sustainable and ethical methods for poultry farming worldwide. Throughout many cultures and regions, this traditional method offers numerous benefits to the environment and to chicken welfare (1). Foraging for natural foods and expressing their natural behaviors in open spaces makes chickens more vigorous and healthier (2). However, free-range chickens are more exposed to larval helminths and eggs by interacting with soil, plants, and insects (3). Foraging chickens can ingest these infectious stages, with roundworms (nematodes) and tapeworms (cestodes) being common helminth parasites associated with free-range chickens (4).
Parasite infections can cause reduced feeding efficiency, weight loss, reduced egg production, and general weakness in poultry (5–7). Particularly in young or immunocompromised birds, helminth infections can result in anemia, malnutrition, and even mortality. Free-range chickens must undergo regular health monitoring and preventive measures to mitigate helminth parasitic infections (8). It may be necessary to implement preventive measures such as sanitation and rotational grazing to prevent the buildup of larvae of helminths in the environment. In addition to using chemicals exclusively to control helminth populations, strategies such as hygiene practices and herbal remedies remarkably contribute to the control of helminth populations (9).
Different methods are used to diagnose gastrointestinal helminths in animals, each with advantages and disadvantages. Fecal samples are traditionally examined microscopically for diagnosis (10–12). Helminth eggs, larvae, and cysts can be detected using flotation and sedimentation techniques (13). Counting eggs using the McMaster method is commonly used to measure infection severity (14). ELISA and immunofluorescence assays are serological methods to detect antibodies related to helminth infections (15). When egg counts are low, or eggs are not shed in feces during pre-patent periods, these tests are beneficial for diagnosing infections. The polymerase chain reaction (PCR) is a highly sensitive and specific method for detecting helminths (16, 17). In epidemiological studies and the tracking of drug resistance, PCR identifies helminth species and strains by amplifying specific DNA sequences.
Due to recent developments in nanotechnology, nanobiosensors can detect helminth infections rapidly and precisely (18, 19). Using nanoparticles, these biosensors can detect helminth antigens or DNA in various samples, including blood, saliva, and feces. Compared to conventional methods, these are highly sensitive and provide results in less time (20). Diagnostic methods are often chosen based on resource availability, suspected helminth species, and clinical settings. Often, the most accurate diagnosis is achieved by combining multiple methods.
A high level of parasitic infection has been documented in several animal species living in Iran, particularly in the Zabol region (21). A significant burden of parasite infections is prevalent in this area, affecting livestock and wildlife alike (22). Zabol's climatic conditions and farming practices cause these parasites to spread and persist. Sheep, goats, and cattle are susceptible to infections with gastrointestinal helminths, contributing to substantial economic losses (23, 24). Although studies have been conducted on free-range poultry helminth infections in other regions of Iran (25–27), this study in Zabol is unique and essential because it aimed to provide insight into a still unstudied area and offers alternative solutions specific to the region.
Materials and Methods
Ethical statement
Examining gastrointestinal helminths in Zabol's free-range poultry aligns with the ethical code IR.UM.RCE.1400.087, emphasizing humane research, animal welfare, and responsible practices.
Study area
Zabol is located in the Sistan and Baluchestan province in southeastern Iran. Its geographical coordinates are 31.0294° N latitude and 61.4974° E longitude. The climate is arid during the summers and mild during the winters. Due to its barren surroundings and desert landscapes, the city experiences hot, dry weather throughout the year.
Study animals and sampling
The study population consisted of 160 free-ranging poultry from farms in the area, between 2022 and 2023. These birds spend their days foraging in the surrounding environment and spend the night in small poultry houses or backyards. In addition to household waste, grains may be incorporated into their diet. One hundred sixty birds were collected at random throughout the study area according to the formula, considering the prevalence of 72% for gastrointestinal parasites in birds.
According to ethical guidelines, the birds' gastrointestinal tracts, including the ceca, were removed from the vent to the rectum after slaughter. The gastrointestinal tract was placed in a 10% formalin solution before transferring to the laboratory for examination.
Laboratory examination of the gastrointestinal tract was conducted by longitudinally opening the intestines with scissors. Following this, parasites adhering to the mucous layer of the intestinal wall were scraped off with a spatula. After scraping, the contents were washed in water and sieved before being transferred to Petri dishes. Recovered nematodes were placed in a 5% glycerin and 70% alcohol mixture, and recovered cestodes were placed in 10% formalin.
The separated worms were described and classified using taxonomic keys. Before identification, the nematodes were cleared with lactophenol (28), and the cestodes were stained with acetocarmine (29).
Results
Of the 160 dissected birds, 92 (57.5%) had gastrointestinal parasites. Among them, 64 (40%) were parasitized with cestodes, 16 (10%) with nematodes, and 12 (7.5%) were concurrently infected with cestodes and nematodes.
Seven species of gastrointestinal worms were identified. Of the nematodes, A. galli (10%) and S. brumpti (7.5%) were the most common, and of the cestode species, R. tetragona (33.12%) was the most common (Table 1) (Figs. 1 and 2).
Table 1:
The number, prevalence, abundance, mean intensity ± SD, and range of gastrointestinal helminths found in 160 free-range chickens in Zabol, Iran
| Helminth/Host | Infestation number | Prevalence (%) | abundance | Intensity ± SD | Range | |
|---|---|---|---|---|---|---|
| Nematodes | Ascaridia galli | 32 | 10 | 0.34 | 2 ± 0.81 | 1–4 |
| Subulura brumpti | 17 | 7.5 | 0.18 | 1.4 ± 0.65 | 1–3 | |
| Heterakis gallinarum | 14 | 3.12 | 0.15 | 2.8 ± 0.83 | 2–4 | |
| Cestodes | Raillietina tetragona | 86 | 33.12 | 0.93 | 1.62 ± 0.87 | 1–4 |
| Raillietina echinobothrida | 20 | 6.25 | 0.21 | 2 ± 0.66 | 1–3 | |
| Raillietina magninumida | 10 | 5 | 0.1 | 1.25 ± 0.46 | 1–2 | |
| Cotugnia digonopora | 6 | 3.12 | 0.06 | 1.2 ± 0.44 | 1–2 |
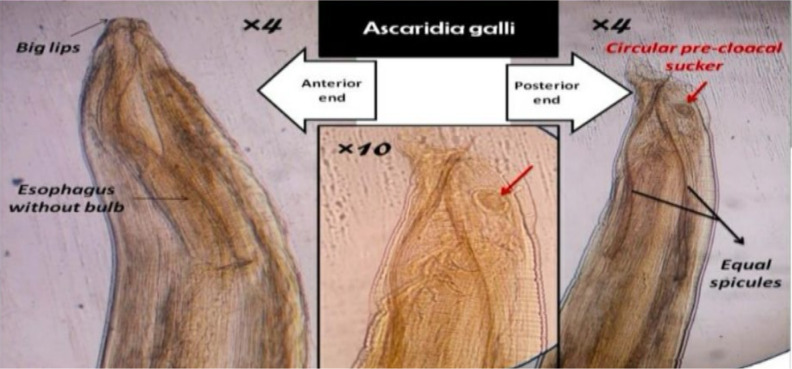
Fig. 1:
see above the anterior and posterior ends of the nematode A. galli at 40x and 100x magnification (4x and 10x objective lenses). The images showcase several diagnostic features, including the lips, circular precloacal sucker, and the esophagus. (Original image)
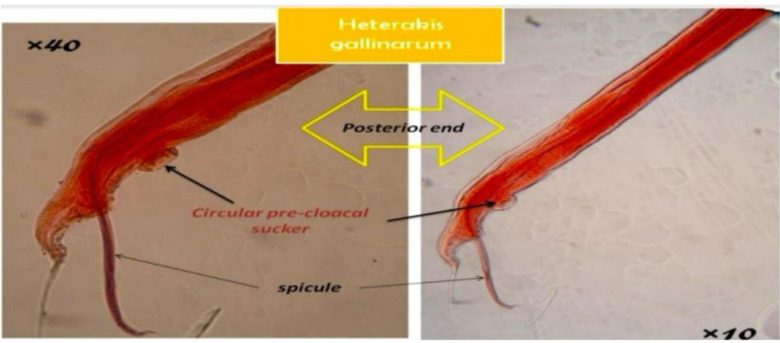
Fig. 2:
See above the posterior end of the nematode H. gallinarum at 100x and 400x magnification (10x and 40x objective lenses). The images highlight several diagnostic features, including the spicules, and the circular precloacal sucker. (Original image)
Discussion
The present research indicates a high prevalence of gastrointestinal parasites in free-range chickens that ingest soil, insects, and earthworms contaminated with parasites. In this survey, 57.5% of Zabul chickens were infected with gastrointestinal helminth. The results obtained are lower than those of Ebrahimi et al. (26), with a prevalence of 51% in Mashhad, and by Eslami et al. (27), with a prevalence of 96% in Golestan province. Variations in the prevalence of gastrointestinal worms in poultry can be attributed to several factors, including the type of poultry management system, sample size, parasite control methods, the seasons, bird ages, environments that have different intermediate host capabilities, birds with different resistances, and a variety of environmental conditions (30, 31).
A. galli was reported as the most prevalent nematode, with a prevalence of 10% in this study. Ingestion of eggs containing larvae, which are resistant to external factors, is the primary transmission mode of A. galli. The results of several studies indicate A. galli to be the most prevalent helminth infection: Ebrahimi et al. (26) reported 29% in Mashhad, Iran; Islami et al. (27) found 56% in Golestan, Ashenafi et al. (32) observed 55.26% in Ethiopia; Edilo Jorge Sarba et al. (33) documented 69.8% in Ethiopia; Matur et al. (34) noted 51.6% in Nigeria. A. galli can negatively affect bird health through food sharing, resulting in growth retardation, decreased productivity, and potential damage to the intestinal mucosa (35). Ascaridiosis is caused by irritation and inflammation of the mucosa caused by the parasite moving into the proventriculus after penetrating the intestinal lumen.
In this study, S. brumpti was found to have a prevalence of 5.5%, the second highest after A. galli. There were significantly more cases of this nematode in Zabol than in Mashhad or Golestan previously studied. Considering that S. brumpti's vector is a beetle, which was likely more abundant in dry areas like Zabol, this may indicate that the parasite was more prevalent due to its abundance in arid regions like Zabol. Infection with S. brumpti in chickens can cause minimal damage to the ceca but negatively affect the health and productivity of the birds.
H. gallinarum, another common nematode species, was prevalent in this study in 3.12% of the samples. Several studies have shown that H. gallinarum has the highest prevalence after A. galli, including Ebrahimi et al. (26), Ashenafi et al. (32), Mature et al. (34), Eslami et al. (27). Among the potential effects of H. gallinarum on poultry are delayed growth, enteritis, diarrhea, and likely mortality among juveniles.
For several reasons, it is important to know the nature and prevalence of these parasites. First and foremost, it allows the development of effective management and control strategies (36, 37). These strategies are essential to ensuring flock health and free-range farming sustainability (38). Developing these strategies is essential to flock health. Second, healthier chickens are more productive and profitable for farmers, positively impacting their livelihoods and communities. As a third consideration, there is the potential for zoonotic transmission, as parasites that spread to humans threaten public health (39). In addition to providing safe farming practices, comprehensive knowledge of these parasites enhances food security by reducing the chances that parasites will be transmitted through poultry products and, therefore, enhancing food security (40, 41).
Several limitations should be considered when interpreting the findings of this study. However, it provides valuable insights into the prevalence of helminth infections in native poultry in Zabol, Iran. In addition to identifying and characterizing parasites using Acetocarmine staining in conjunction with microscopic examination, these methods have limitations in terms of their sensitivity and specificity. Due to these constraints, the study could have missed or misidentified many parasites.
This study's limitations should be addressed by increasing the sample size and geographical scope in future research. Longitudinal studies would be beneficial for documenting seasonal changes in parasite prevalence over time and observing seasonal trends and long-term variations in them. A further improvement in the accuracy of parasite identification could be achieved by incorporating advanced diagnostic techniques. Researchers might see a revolution in understanding and managing parasites in free-range poultry farming thanks to these advancements in research methods.
Conclusion
Despite the arid climate, the research findings indicate a significant presence of gastrointestinal parasites in free-range chickens in the Zabol region. These parasites have detrimental effects on the birds' health, growth, and productivity; therefore, it is imperative to implement effective parasite control strategies and management practices to enhance the welfare of poultry in this area.
Acknowledgements
This work was supported by the Research Council of Ferdowsi University of Mashhad and conducted as research project No. 56573.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest regarding this manuscript's publication and/or funding.
References
- 1.Rajkumar U, Rama Rao S, Raju M, Chatterjee R. Backyard poultry farming for sustained production and enhanced nutritional and livelihood security with special reference to India: a review. Trop Anim Health Prod. 2021;53(1):176. [DOI] [PubMed] [Google Scholar]
- 2.Hafez HM, Attia YA. Challenges to the poultry industry: current perspectives and strategic future after the COVID-19 outbreak. Front Vet Sci. 2020;7:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cupo KL, Beckstead RB. Heterakis gallinarum, the cecal nematode of gallinaceous birds: a critical review. Avian Dis. 2019;63(3):381–8. [DOI] [PubMed] [Google Scholar]
- 4.Cornell KA, Smith OM, Crespo R, et al. Prevalence patterns for enteric parasites of chickens managed in open environments of the Western United States. Avian Dis. 2022;66(1):60–8. [DOI] [PubMed] [Google Scholar]
- 5.Ghafouri SA, Ghaniei A, Sadr S, et al. Anticoccidial effects of tannin-based herbal formulation (Artemisia annua, Quercus infectoria, and Allium sativum) against coccidiosis in broilers. J Parasit Dis. 2023;47(4):820–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarbiat B, Jansson D, Wall H, Tydén E, Höglund J. Effect of a targeted treatment strategy against Ascaridia galli on egg production, egg quality and bird health in a laying hen farm. Vet Parasitol. 2020;286:109238. [DOI] [PubMed] [Google Scholar]
- 7.Lozano J, Anaya A, Palomero Salinero A, et al. Gastrointestinal parasites of free-range chickens: A worldwide issue. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca Veterinary Medicine. 2019;76(2):110–17. [Google Scholar]
- 8.Mujyambere V, Adomako K, Olympio SO, et al. Local chickens in East African region: Their production and potential. Poult Sci. 2022;101(1):101547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jamil M, Aleem MT, Shaukat A, et al. Medicinal plants as an alternative to control poultry parasitic diseases. Life (Basel). 2022;12(3):449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demelash K, Abebaw M, Negash A, Alene B, Zemene M, Tilahun M. A review on diagnostic techniques in veterinary helminthlogy. Natural Science. 2016;14(7):109–18. [Google Scholar]
- 11.Incani RN, Ferrer E, Hoek D, et al. Diagnosis of intestinal parasites in a rural community of Venezuela: Advantages and disadvantages of using microscopy or RT-PCR. Acta Trop. 2017;167:64–70. [DOI] [PubMed] [Google Scholar]
- 12.Verocai GG, Chaudhry UN, Lejeune M. Diagnostic methods for detecting internal parasites of livestock. Vet Clin North Am Food Anim Pract. 2020;36(1):125–43. [DOI] [PubMed] [Google Scholar]
- 13.Inês EdJ, Figueiredo Pacheco FT, Carneiro Pinto M, et al. Concordance between the zinc sulphate flotation and centrifugal sedimentation methods for the diagnosis of intestinal parasites. Biomedica. 2016;36(4):519–24. [DOI] [PubMed] [Google Scholar]
- 14.Daş G, Klauser S, Stehr M, Tuchscherer A, Metges CC. Accuracy and precision of McMaster and Mini-FLOTAC egg counting techniques using egg-spiked faeces of chickens and two different flotation fluids. Vet Parasitol. 2020;283:109158. [DOI] [PubMed] [Google Scholar]
- 15.Roose S, Vande Velde F, Vlaminck J, Geldhof P, Levecke B. Serological diagnosis of soil-transmitted helminth (Ascaris, Trichuris and hookworm) infections: A scoping review. PLoS Negl Trop Dis. 2024;18(4):e0012049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basuni M, Muhi J, Othman N, et al. A pentaplex real-time polymerase chain reaction assay for detection of four species of soil-transmitted helminths. Am J Trop Med Hyg. 2011;84(2):338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Connell EM, Nutman TB. Molecular diagnostics for soil-transmitted helminths. Am J Trop Med Hyg. 2016;95(3):508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hajjafari A, Sadr S, Santucciu C, et al. Advances in Detecting Cystic Echinococcosis in Intermediate Hosts and New Diagnostic Tools: A Literature Review. Vet Sci. 2024;11(6):227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadr S, Lotfalizadeh N, Abbasi AM, et al. Challenges and prospective of enhancing hydatid cyst chemotherapy by nanotechnology and the future of nanobiosensors for diagnosis. Trop Med Infect Dis. 2023;8(11):494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadr S, Lotfalizadeh N, Ghafouri SA, Delrobaei M, Komeili N, Hajjafari A. Nanotechnology innovations for increasing the productivity of poultry and the prospective of nanobiosensors. Vet Med Sci. 2023;9(5):2118–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nourollahi Fard SR, Akhtardanesh B, Sadr S, Khedri J, Radfar MH, Shadmehr M. Gastrointestinal helminths infection of free-roaming cats (Felis catus) in Southeast Iran. Vet Med Sci. 2024;10(3):e1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahnama M, Siamardi AA, Alipour Eskandani M, et al. Exploring the prevalence and impact of Ligula intestinalis infection across fish species in Sistan region, Iran. J Parasit Dis. 2024:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatam-Nahavandi K, Carmena D, Rezaeian M, et al. Gastrointestinal parasites of domestic mammalian hosts in Southeastern Iran. Vet Sci. 2023;10(4):261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirzaei M, Dahmardeh E. The prevalence of Eimeria species in sheep in Zabol city, Iran. Sci Res Iran Vet J. 2016;11(4):98–105. [Google Scholar]
- 25.Rezaei F, Hashemnia M, Chalechale A, Seidi S, Gholizadeh M. Prevalence of ectoparasites in free-range backyard chickens, domestic pigeons (Columba livia domestica) and turkeys of Kermanshah province, west of Iran. J Parasit Dis. 2016;40:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebrahimi M, Asadpour M, Khodaverdi M, Borji H. Prevalence and distribution of gastrointestinal helminths in free range chickens in Mashhad, northeast of Iran. Sci Parasitol. 2014;15(1–4):38–42. [Google Scholar]
- 27.Eslami A, Ghaemi P, Rahbari S. Parasitic infections of free-range chickens from Golestan Province, Iran. Iran J Parasitol. 2009;4(3):10–14. [Google Scholar]
- 28.Mishra S, Kamra A, Chawla G. Processing of nematodes for identification. S, and Johansen, C.(eds) 2000. 1999:51–55. [Google Scholar]
- 29.Wardle RA. On the technique of cestode study. Parasitology. 1932;24(2):241–52. [Google Scholar]
- 30.Tsegaye AA, Miretie AA. Chicken Ascariasis and Heterakiasis: Prevalence and associated risk factors, in Gondar City, Northwest Ethiopia. Vet Med (Auckl). 2021;12:217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shifaw A, Feyera T, Walkden-Brown SW, et al. Global and regional prevalence of helminth infection in chickens over time: a systematic review and meta-analysis. Poult Sci. 2021;100(5):101082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashenafi H, Eshetu Y. Study on gastrointestinal helminths of local chickens in Central Ethiopia. Revue Méd Vét. 2004;155(10): 504–7. [Google Scholar]
- 33.Sarba EJ, Bayu MD, Gebremedhin EZ, et al. Gastrointestinal helminths of backyard chickens in selected areas of West Shoa Zone Central, Ethiopia. Vet Parasitol Reg Stud Reports. 2019;15:100265. [DOI] [PubMed] [Google Scholar]
- 34.Matur B, Dawam N, Malann Y. Gastrointestinal helminth parasites of local and exotic chickens slaughtered in Gwagwalada, Abuja (FCT), Nigeria. NY Sci J. 2010;3(5):96–9. [Google Scholar]
- 35.Adedokun SA, Olojede OC. Optimizing gastrointestinal integrity in poultry: the role of nutrients and feed additives. Front Vet Sci. 2019;5:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell D, Bari M, Rault JL. Free-range egg production: its implications for hen welfare. Animal Production Science. 2021; 61(10):848–855 [Google Scholar]
- 37.Sánchez-Casanova R, Sarmiento-Franco L, Phillips C, Zulkifli I. Do free-range systems have potential to improve broiler welfare in the tropics? Worlds Poult Sci J. 2020;76(1):34–48. [Google Scholar]
- 38.Bonnefous C, Collin A, Guilloteau LA, et al. Welfare issues and potential solutions for laying hens in free range and organic production systems: A review based on literature and interviews. Front Vet Sci. 2022;9:952922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarr A, Galal L, Boumediene F, et al. Seroprevalence and risk factors of Toxoplasma gondii infection in free-range chickens in Senegal, West Africa. Vector Borne Zoonotic Dis. 2020;20(1):15–21. [DOI] [PubMed] [Google Scholar]
- 40.Jacob JP, Pescatore AJ, Anderson KE, McCrea B, Shaw DP. Impact of free-range poultry production systems on animal health, human health, productivity, environment, food safety, and animal welfare issues. 2018. [Google Scholar]
- 41.Kijlstra A, Meerburg B, Bos A. Food safety in free-range and organic livestock systems: Risk management and responsibility. J Food Prot. 2009;72(12):2629–37. [DOI] [PubMed] [Google Scholar]