Abstract
Background:
Anopheles stephensi is a significant malaria vector in Pakistan, and understanding its feeding behavior is necessary to control the spread of malaria. However, limited information is available on the host preferences of A. stephensi in Pakistan. Therefore, we aimed to explore the feeding behavior of A. stephensi, a malaria vector, in the District Khyber, Khyber Pakhtunkhwa, Pakistan.
Methods:
A total of 7462 mosquitoes were collected between March and September 2021, with 1674 (22.4%) identified as A. stephensi (952 female and 722 male). Among the female A. stephensi, 495 (52%) were blood-fed. DNA was extracted from the blood-fed female A. stephensi mosquitoes using the Ammonium Acetate Precipitation Method followed by PCR analysis, blood meal sources were identified. Nested PCR on 191 pooled samples was used to detect Plasmodium falciparum and Plasmodium vivax.
Results:
Cattle blood meals were predominant (73%), followed by human (20%) and chicken (7%), with no dog blood meals detected. All individual mosquito samples were negative for Plasmodium falciparum, while two pooled samples (out of 191) tested positive for P. vivax.
Conclusion:
A. stephensi in Khyber District primarily displayed anthropophagic feeding behavior, with a small portion of the population infected with P. vivax. The results underscore the importance of targeted vector control strategies, environmental management, community engagement and continuous monitoring to suppress malaria transmission.
Keywords: Anopheles stephensi, Plasmodium vivax, Plasmodium falciparum, Pakistan
Introduction
Mosquitoes, belonging to the family Culicidae, are known to transmit various diseases, including malaria, dengue, and filariasis, which significantly impact human health and socio-economic development. These vectors require important nutrients for egg production, obtained through the acquisition and digestion of protein-rich blood (1). The concept of blood feeding in insects emerged when plant-sucking insects accidentally bit vertebrates, leading to the development of a digestive physiology that allows for metabolic absorption. During the ovarian cycle, female mosquitoes also transmit pathogens such as dengue and malaria to animals through blood feeding (2) .
The evolution of blood feeding in insects led to a mutually influential parasitic development between the vertebrate host and the insect, particularly when blood became the primary source of nutrients for egg production. During the ovarian cycle, female mosquitoes also transmit pathogens such as dengue and malaria to animals through blood feeding (3,4). During blood feeding, insects rely on host-specific cues to accurately identify their preferred host in a heterogeneous environment. Host preference is a critical feature that influences the reproductive success of blood-feeding insects, including mosquitoes that transmit pathogens such as dengue and malaria to animals during the ovarian cycle (5,6).
In Pakistan, malaria is a major health issue, with Plasmodium vivax and P. falciparum being the primary transmitted species. Anopheles stephensi, a species of mosquito, has been identified as one of the main vectors for malaria transmission in the country. The feeding behavior of A. stephensi is intricately linked to the transmission of malaria (7,8). When an infected mosquito bites a human, it unwittingly injects malaria parasites, known as Plasmodium, into the bloodstream. These parasites multiply within the liver before invading red blood cells, causing the debilitating symptoms of malaria.
The parasite then completes its life cycle by returning to the mosquito during its next blood meal, potentially perpetuating the transmission (9). Understanding the feeding preferences of A. stephensi is necessary in this complex cycle. It helps determine whether they primarily feed on humans, perpetuating the malaria cycle, or prefer other animal hosts, acting as potential dead ends for transmission (4,6). Unraveling their blood meal choices provides critical information about the human-mosquito interface and the vulnerability of communities to malaria outbreaks (10).
We investigated the feeding behavior and host preference of A. stephensi, analyzes factors influencing host preference. Besides, we examined the role of A. stephensi in malaria transmission in District Khyber, Pakistan. Directly detecting Plasmodium within the mosquito population provides a snapshot of the current malaria landscape, informing control efforts and improving public health outcome
Methods
Study Area
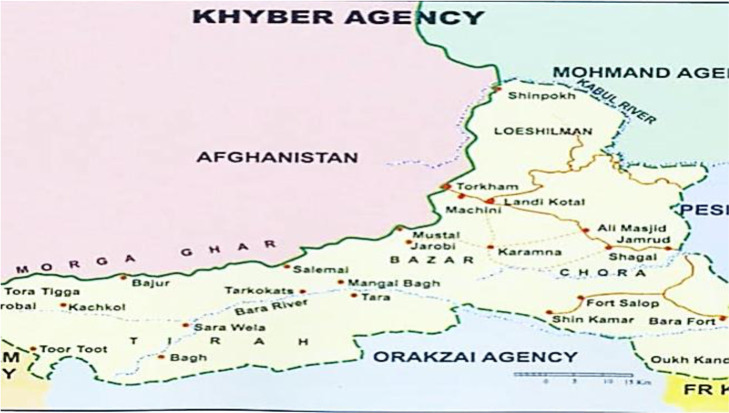
The study area was District Khyber, in the Northern region of Pakistan, bordering Afghanistan, Peshawar City, Orakzai, and Kurram districts. With a total area of 2,576 square kilometers and only 8.22% forest cover, Khyber features a rugged and barren mountainous landscape with narrow valleys. Summers are hot (May to August), while winters are cold (November to January) (Fig. 1).
Fig. 1:
Map of Khyber district, Khyber Pakhtunkhwa (https://cmdo.org.pk/khyber-agency/).
Data collection
From Mar to Sep 2021, 7,462 mosquitoes were such as A. stephensi (1674), Armigeres (2,100), Culex pipiens (1,565), Aedes agyptii (1,468) and A. culicifacies (565) during daytime hours using the flit method. Collections occurred within a closed room draped in white sheets. After sealing the room to restrict air movement, a fast-acting volatile insecticide was applied, and the room remained closed for 5 minutes. Collected mosquitoes were subsequently aspirated from the sheets and placed in 3 ml blood collection tubes containing silica gel. The mosquitoes were identified on the basis of morphological characters using species identification keys given in Fauna of British India by Barraud (1934) and Christopher (1935) (11–13). Only A. stephensi (22.43%) was used for studying the blood feeding behavior and malaria detection.
DNA Extraction
DNA extraction from the collected mosquito samples involved pooling the blood-fed A. stephensi mosquitoes into groups of five, followed by DNA extraction from the head, thorax, and abdomen of the female mosquitoes using the standard ammonium acetate precipitation method (14).
PCR Amplification
To detect host blood in mosquitoes from extracted DNA, four different host specific primers (Table 1) were used for PCR amplification (15,16).
Table 1:
Host specific primers used for the detection DNA from the blood of A. stephensi
| Host Specie |
Primer sequence
F: forward 5′–3′ and R: reverse 5′–3′ |
Size of amplified product (bp) |
|---|---|---|
| Human | 5′-TTCGGCGCATGAGCTGGAGTCC-3′ F | |
| 5′-TATGCGGGGAAACGCCATATCG-3′ R | 228 | |
| Bovine | 5′-GCCATATACTCTCCTTGGTGACA-3′ F | |
| 5′-GTAGGCTTGGGAATAGTACGA-3 R | 271 | |
| Dog | 5′-GAACTAGGTCAGCCCGGTACTT-3′ F | |
| 5′-CGGAGCACCAATTATTAACGGC-3′ R | 153 | |
| Chicken | 5′GGGACACCCTCCCCCTTAATGACA-3′ F | |
| 5′GGAGGGCTGGAAGAAGGAGTG-3′ R | 266 |
To identify the blood meal source, a PCR reaction mixture of 20 μl was prepared, consisting of DNTPs (2 μl), Taq Buffer (2 μl), Taq Polymerase (0.3 μl), MgCl2 (1.2 μl), host-specific Primers (1 μl each for forward and reversed primer), DNA (1 μl), and distilled water (11.5 μl).
Detection of malarial parasites
To detect malarial parasites, a nested PCR technique was used with outer and inner primers specific for the Plasmodium genus, P. falciparum and P. vivax. Two rounds of PCR were performed, first using outer primers specific for the Plasmodium genus (rPLU_6 and rPLU_5), followed by inner primers specific for P. falciparum (rFAL_1 and rFAL_2) or P. vivax (rVIV_1 and rVIV_2) (Table 2).
Table 2:
These primers used for the detection of malarial parasites
| Primer name | Sequence | Size (bp) |
|---|---|---|
| rPLU_6 | 5′-TTAAAATTGTTGCAGTTAAAACG-3′ | 1200 |
| rPLU_5 | 5′-CTTGTTGTTGCCTTAAACTTC-3′ | 1200 |
| rFAL_1 | 5′-TTAAACTGGTTTGGGAAAACCAAATATATT-3′ | 205 |
| rFAL_2 | 5′-ACACAATGAACTCAATCATGACTACCCGTC-3′ | 205 |
| rVIV_1 | 5′-CGCTTCTAGCTTAATCCACATAACTGATAC-3′ | 120 |
| rVIV_2 | 5′-ACTTCCAAGCCGAAGCAAAGAAAGTCCTTA-3′ | 120 |
The thermal conditions for the primers are as follows: 95°C for 3 min, 40 cycles of 58°C for 30 seconds (for human), 51°C for 30 seconds (for bovine), 55°C for 30 seconds (for dog), and 59.5°C for 30 seconds (for chicken), followed by 72°C for 30 seconds and 72°C for 3 min (Table 2).
Gel Electrophoresis
The PCR products were visualized on a 1% ethidium bromide-stained agarose gel using a 100 bp DNA ladder marker in a 5X Tris-borate-EDTA running buffer. The gel was subjected to electrophoresis at 90 volts for 45 min, and the agarose gel was visualized under a UV Transilluminator. The image was then photographed.
Ethical Declaration
This study adhered to ethical guidelines for field mosquito collection. Mosquitoes were captured using light traps, a non-invasive method. No anesthetic or surgical procedures were employed on the mosquitoes. The research protocol was approved by Ethical committee of Institute of Zoological sciences, University of Peshawar, Pakistan
Results
Blood Meal Analysis of A. stephensi Mosquitoes
From March 2021 to September 2021 a total of 7462 mosquitoes were collected of which 1674 (22.43%) were A. stephensi. Among the collected mosquitoes, 722 (43.13%) were males, and 952 (56.86%) were females. Out of the 952 females, 495 (51.99%) were blood-fed, while 457 (48.00%) were unfed. Upon further analysis, out of the 495 blood-fed A. stephensi, host blood was detected in 490 specimens, with 5 showing no amplification product. Subsequently, all the blood samples underwent PCR analysis, revealing that 73% (357/490) of the specimens contained cattle blood, characterized by a band size of 271 bp, while 20% (98) were found to have human blood, and 7% (34) had chicken blood. Notably, none of the specimens were found to have dog blood (Table 3 and Fig. 2–4).
Table 3:
Blood Meal Analysis of Anopheles stephensi Mosquitoes. N=495
| Host category | No. of blood fed Anopheles stephensi n(%) |
|---|---|
| Cattle | 357 (73) |
| Human | 98 (20) |
| Chicken | 34 (7) |
| Dog | 0 (0) |
| Total known | 490 (100) |
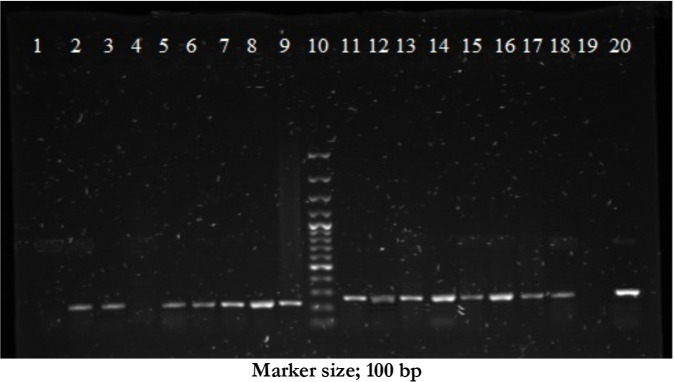
Fig. 2:
Gel showing 228 bp band size for human blood (row 2, 3, 5–8 test samples, row 1 and 4 negative samples, row 9 positive control from human blood, row 10 100 bp ladder marker) and 271 bp band size for cattle (row 11- 18 positive samples, row 19 negative sample, row 20 positive control detected in mosquitoes from cow blood) detected in mosquitoes
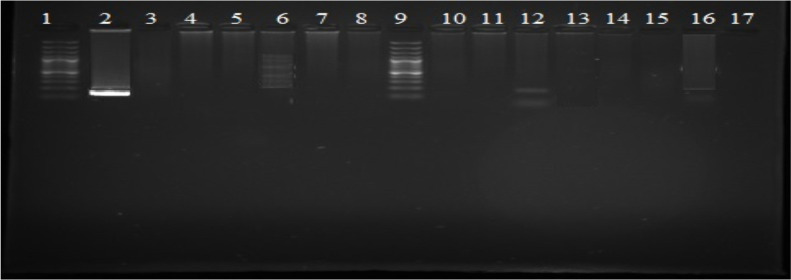
Fig. 4:
Gel showing no detection of 153 bp band size for dog blood (row 2 positive control from dog blood, row 1 and 9 100 bp ladder marker) in mosquitoes
Fig. 3:
Gel showing 266 bp band size for chicken blood (row 5–8 negative samples and row 20 positive control from chicken blood, row 1 and 11 100 bp ladder marker) detected in mosquitoes
Molecular identification of Plasmodium species from blood meals sources
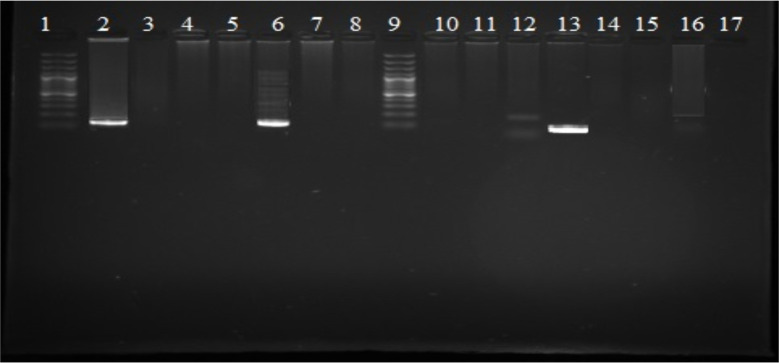
All A. stephensi pools were negative for P. falciparum, as none of the samples showed an amplified DNA band size of 205 bp. However, two pools were found positive for P. vivax, showing an amplified band size of 120 bp (Fig. 5).
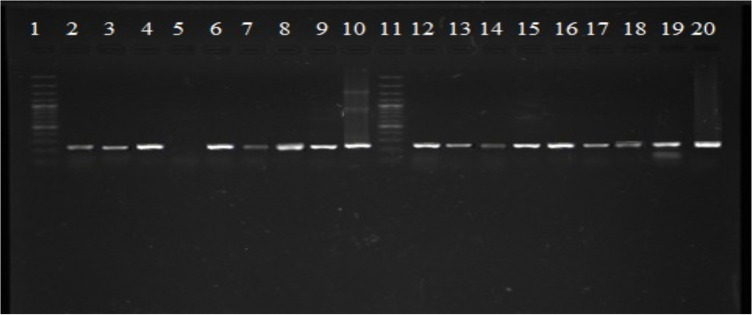
Fig. 5:
Gel showing detection of P. vivax (120 bp) in Anopheles stephensi collected from District Khyber (row 6, 13 positive samples, row 2 positive control of P. vivax DNA sampled from human beings row 1 and 9 100 bp ladder marker
Discussion
Feeding behavior is the act of acquiring nutrients from their host, and insects, such as mosquitoes, transfer disease-causing agents to their hosts during the act of feeding. Present study, focused on the feeding behavior of A. stephensi, a malaria vector in the merged tribal district of Khyber in Khyber Pakhtunkhwa, Pakistan. The observed feeding pattern of A. stephensi, with a preference for cattle but also feeding on humans and chickens, aligns with findings from other studies. A study on A. stephensi in Peninsular Malaysia also found that the mosquitoes fed on various blood sources, including humans, cattle, and pigs (17). In Peninsular Malaysia, Anopheles mosquitoes were found to have a preference for certain blood sources, such as pigs and cattle (18).
In present study detection of human blood in A. stephensi indicating that this mosquito species is both zoophagic and anthropophagic in nature. In comparison to other studies, the present study has confirmed the findings of Mehravaran et al in Jiroft, southeast Iran, who reported high anthropophagy in A. stephensi(19), and Kumari et al in Angul district, Orissa, India, found that A. stephensi and A. culicifacies were both zoophagic and anthropophagic (20).
Present study revealed that A. stephensi was a significant vector for P. vivax in the study area, with 20% of the specimen’s positive for the presence of the parasite. This highlights the importance of controlling the population of this mosquito species in the affected area to reduce the spread of malaria. In Africa Among engorged mosquitoes, cattle and goats emerged as the primary blood source for A. stephensi (98%) and A. gambiae (80%). although only A. stephensi showed minimal human feeding (2%). However, nearly half of the engorged A. stephensi mosquitoes (48%) yielded undetermined bloodmeal sources. Notably, only 0.5% of A. stephensi carried P. falciparum sporozoites, emphasizing their potential but limited role in natural malaria transmission (21). Similarly another study conducted in Goa, India, found two A. stephensi mosquitoes positive for P. falciparum sporozoites out of 831 female A. stephensi examined (22). In Kenya P. falciparum and P. vivax were detected in A. stephensi mosquitoes (23).
Our study also reports A. stephensi positive for the presence of P. vivax through PCR. Present study employed PCR for detection of blood meal in A. stephensi while Mehravaran et al. (2012) used ELISA kit for host blood detection. PCR is a much more sensitive technique than ELISA but both have shown anthropophagic behavior in A. stephensi (19). Present study emphasizes the importance of A. stephensi as a malaria vector in the merged tribal district of Khyber. Vector surveillance and control strategies can help decrease the mosquito vector population in the affected area, resulting in a decrease in human-mosquito interaction and, consequently, a decrease in the disease burden. Vector control is the most suitable method to decrease malaria morbidity and mortality in the local population, thus improving the life standard of the affected population in district Khyber.
Conclusion
This study provides valuable insights into the feeding behavior and Plasmodium detection in A. stephensi, highlighting its importance as a malaria vector in Khyber. The results reveal that A. stephensi is both zoophagic and anthropophagic, with one-fifth of blood-fed females having human blood meals (20%). Notably, P. vivax is a prevalent malaria-causing agent in the area, with 20% of the specimens testing positive for the parasite. To safeguard Khyber's residents, implementing targeted vector control strategies to decrease A. stephensi populations, reducing breeding sites through environmental management and community engagement, and continuously monitoring mosquito population dynamics and P. vivax prevalence are crucial in effectively suppressing malaria transmission.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project, King Saud University, Riyadh, Saudi Arabia for funding this work through project number–(RSP2024R197) and National Geospatial National Centre of GIS and space applications, grant/reference number NCGSA-RF-34-RS&GIS-17.
Footnotes
Conflict of Interest
The authors have declared that there is no conflict of interest regarding the publication of this article
References
- 1.Tjaden NB, Caminade C, Beierkuhnlein C, Thomas SM. Mosquito-borne diseases: advances in modelling climate-change impacts. Trends Parasitol. 2018; 34:227–245. [DOI] [PubMed] [Google Scholar]
- 2.Talyuli OA, Bottino-Rojas V, Polycarpo CR, Oliveira PL, Paiva-Silva GO. Non-immune traits triggered by blood intake impact vectorial competence. Front Physiol. 2021; 12:638033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brackney DE, LaReau JC, Smith RC. Frequency matters: How successive feeding episodes by blood-feeding insect vectors influences disease transmission. PLoS Pathog. 2021; 17(6):e1009590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Annu Rev Entomol. 2013; 58:433–453. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez C, Desquesnes M, Touratier L, Büscher P. Trypanosoma evansi: recent outbreaks in Europe. Vet Parasitol. 2010; 174:26–29. [DOI] [PubMed] [Google Scholar]
- 6.Verschut TA, Blažytė-Čereškienė L, Apšegaitė V, Mozūraitis R, Hambäck PA. Natal origin affects host preference and larval performance relationships in a tritrophic system. Ecol Evol. 2017; 7(7):2079–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan HAA, Akram W, Lee S. Resistance to selected pyrethroid insecticides in the malaria mosquito, Anopheles stephensi (Diptera: Culicidae), from Punjab, Pakistan. J Med Entomol. 2018; 55(3):735–738. [DOI] [PubMed] [Google Scholar]
- 8.Khan MI, Qureshi H, Bae SJ, Khattak AA, Anwar MS, et al. Malaria prevalence in Pakistan: A systematic review and meta-analysis (2006–2021). Heliyon. 2023; 9(4):e15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennink S, Kiesow MJ, Pradel G. The development of malaria parasites in the mosquito midgut. Cell Microbiol. 2016; 18(7):905–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas S, Ravishankaran S, Justin NJA, Asokan A, Mathai MT, et al. Resting and feeding preferences of Anopheles stephensi in an urban setting, perennial for malaria. Malar J. 2017; 16(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barraud P, Covell G, others. The Morphology of the Buccal Cavity in Anopheline and Culicine Mosquitoes. Indian J Med Res. 1928; 15:671–680. [Google Scholar]
- 12.Christophers SR. On the importance of larval characters in the classification of mosquitoes.8th ed. Calcutta: Office of the Superintendent of Government Printing, India; 1906. [Google Scholar]
- 13.Qutubuddin M. Mosquito studies in the Indian subregion. Part I. Taxonomy--a brief review. Pacif Insects. 1960; 2:133–147. [Google Scholar]
- 14.Nichols AF, Itoh T, Graham JA, Liu W, Yamaizumi M, et al. Human damage-specific DNA-binding protein p48: characterization of XPE mutations and regulation following UV irradiation. J Biol Chem. 2000; 275(28):21422–21428. [DOI] [PubMed] [Google Scholar]
- 15.Gunathilaka N, Denipitiya T, Hapugoda M, Abeyewickreme W, Wickremasinghe R. Determination of the foraging behaviour and blood meal source of malaria vector mosquitoes in Trincomalee District of Sri Lanka using a multiplex real time polymerase chain reaction assay. Malar J. 2016; 15:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kavunga-Membo H, Ilombe G, Masumu J, Matangila J, Imponge J, et al. Molecular identification of Plasmodium species in symptomatic children of Democratic Republic of Congo. Malar J. 2018; 17(1):334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeyaprakasam NK, Low VL, Liew JWK, Pramasivan S, Wan-Sulaiman WY, et al. Blood meal analysis of Anopheles vectors of simian malaria based on laboratory and field studies. Sci Rep. 2022; 12:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briggs AM, Hambly MG, Simão-Gurge RM, Garrison SM, Khaku Z, et al. Anopheles stephensi Feeding, Flight Behavior, and Infection With Malaria Parasites are Altered by Ingestion of Serotonin. Front Physiol. 2022; 13:911097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehravaran A, Vatandoost H, Oshaghi MA, Abai MR, Edalat H, et al. Ecology of Anopheles stephensi in a malarious area, southeast of Iran. Acta Med Iran. 2012;50(1):61–65. [PubMed] [Google Scholar]
- 20.Kumari S, Parida SK, Marai N, et al. Vectorial role of anopheles subpictus Grassi and anopheles culicifacies Giles in Angul District, Orissa, India. Southeast Asian J Trop Med Public Health. 2009; 40(4):713–9. [PubMed] [Google Scholar]
- 21.Emiru T, Getachew D, Murphy M, et al. Evidence for a role of Anopheles stephensi in the spread of drug and diagnosis-resistant malaria in Africa. Nat Med. 2023; 29(12):3203–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korgaonkar NS, Kumar A, Yadav RS, et al. Mosquito biting activity on humans & detection of Plasmodium falciparum infection in Anopheles stephensi in Goa, India. Indian J Med Res. 2012; 135(1):120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochomo EO, Milanoi S, Abong’o B, Onyango B, Muchoki M, et al. Detection of Anopheles stephensi Mosquitoes by Molecular Surveillance, Kenya. Emerg Infect Dis. 2023; 29(12):2498–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]