Abstract
Human dirofilariasis is a rare anthropo-zoonotic disease, mainly detected in Southern and Eastern Europe, Asia Minor, Central Asia, and Sri Lanka. An increasing number of autochthonous Dirofilaria spp. infections has been recently reported in the areas previously considered free of the disease, including northern Europe and the Baltic States. A rare autochthonous case of scrotal dirofilariasis detected in Lithuania was described. Here, a 42-year-old male presented with a 1 cm nodule, limited in the scrotum. A nodule excision was performed. On histological examination, a degenerating roundworm with the features of Dirofilaria spp. (multilayered cuticle, well-developed musculature, focally preserved longitudinal ridges) was detected in the abscess cavity. No additional treatment was needed. Twenty-four publications reporting 28 male genitalia dirofilariasis cases in European countries have been identified.
Keywords: Dirofilaria, Human dirofilariasis, Male genital tumors
Introduction
Human dirofilariasis is a rare anthropo-zoonotic disease commonly caused by Dirofilaria repens and D. immitis (1,2). While D. immitis has a worldwide distribution, D. repens is detected in the Old World (Europe, Asia, Africa), with endemic regions in southern and eastern Europe, Asia Minor, Central Asia, and Sri Lanka (2–4). Lately, an increasing number of autochthonous D. repens infections has been reported in the areas recently considered free of the disease, such as northern Europe and the Baltic States (5–8).
Domestic and wild carnivores are the definitive hosts and natural reservoirs of Dirofilaria spp., whose infective larvas are transmitted by blood-sucking mosquitoes of the Culicidae family, mainly by genera Anopheles, Aedes, and Culex (1,3,9,10). Humans might be accidentally infected by mosquitoes that fed previously on animals presenting with microfilaremia (5). Due to the specific immune response in humans, Dirofilaria usually does not mature sufficiently to produce microfilaria; therefore, humans become dead-end hosts in the epizootic chain (11). Nevertheless, symptomatic human dirofilariasis often becomes a diagnostic challenge and needs to be differentiated from other diseases with similar clinical presentation.
Pulmonary, subcutaneous, and ocular dirofilariasis are the three most common clinical forms reported in the literature (5). D immitis cause human pulmonary dirofilariasis, manifesting as a small solitary coin lesion in the chest X-ray, which is often initially misdiagnosed as malignancy (3,12). The ocular infection, usually initiating urgent medical attention, is caused by D. repens and presents as a nodule in the eyelid and orbital area or a migrating worm in the subconjunctival tissue or vitreous body (3,6,13,14). Subcutaneous tissue is commonly affected by D. repens and rarely by D. immitis, presenting with single or multiple nodules most often located in the upper half of the body (1,3,14). Male genital dirofilariasis is extremely rare with less than 30 cases reported in the literature. Scrotal, epididymal, testicular, or spermatic cord nodules may imitate malignant tumors and often requires surgical treatment, while the correct diagnosis is determined only after histological examination (1,6,15–36).
Here we present a rare autochthonous case of scrotal dirofilariasis detected in Lithuania. In addition, the literature reporting male genitalia dirofilariasis in Europe is reviewed, discussing the aspects and challenges of this unusual helminthiasis.
Case report
The patient gave a written informed consent for the publication of identifiable details, which include case history, details within the text and associated images.
A 42-year-old male was referred to the urologist with a 1 cm nodule in the scrotum, which was not painful and slightly alternating in size. The patient observed the lesion for four months before seeking medical consultation. He did not report any other symptoms or comorbidities and denied recent travels to foreign countries.
Medical examination showed a solid subcutaneous nodule limited in the scrotum and not attached to the testicular structures. Blood laboratory parameters were within normal values. Even though the benign scrotal tumor was suspected, to achieve the final diagnosis, a nodule excision was performed.
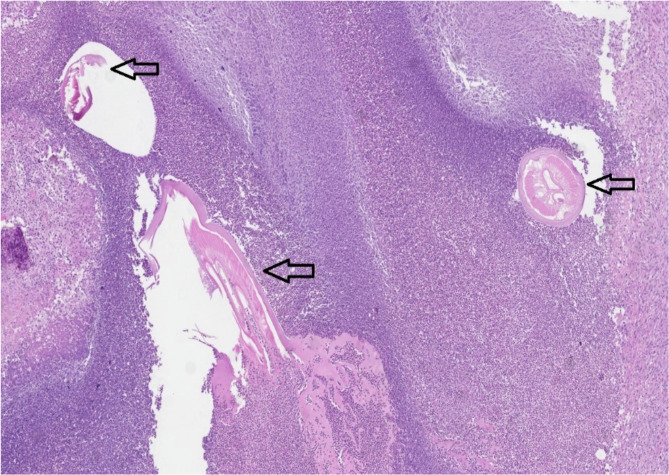
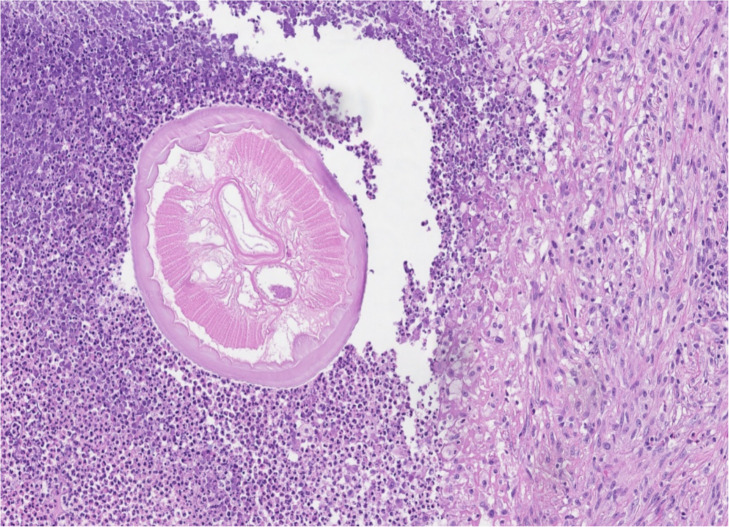
On histological examination mass consisted of areas of fibrotic tissue with prominent inflammatory infiltration surrounding abscess cavity. In the background of debris and neutrophils in the abscess cavity, few sections of degenerating roundworm were present (Fig. 1). One section with more preserved histologic details retained features of Dirofilaria spp. (multilayered cuticle, well developed musculature, focally preserved longitudinal ridges) (Fig. 2).
Fig. 1:
Disintegrating sections of round worm (arrows) in the background of neutrophils and debris (Original)
Fig. 2:
Cross-section of round worm with more preserved histological details typical for Dirofilaria spp. (Original)
The postoperative course was uneventful, and the patient rapidly recovered. No other specific treatment was administered in this case.
Discussion
Dirofilariasis in male genitalia is a rare zoonosis, with only 28 cases in Europe and 2 cases in Lithuania reported in the English literature (Table 1).
Table 1:
Published cases of dirofilariasis in male genitalia in Europe
| Author, year of publication | Year of the case | Country | Travel anamnesis | Age | Localization | Parasite | Diagnostics | Treatment |
|---|---|---|---|---|---|---|---|---|
| Pampiglione et al.(15), 1996 | 1994 | Greece | n.d. | 3 y | Scrotum | D. repens | Histology | n.d. |
| Pampiglione et al.(16), 1998 | 1997 | Hungary | Italy (5 years | 37 y | Spermatic cord | D. repens | Histology | Orcho-funiculectomy |
| Elek et al.(17), 2000 | ||||||||
| Pampiglione et al.(18), 2001 | 1997 | Italy | n.d. | 52 y | Epididymis | D. repens | Histology | n.d. |
| 1998 | Adult | Spermatic cord | ||||||
| 1999 | 36 y | Epdidymis | ||||||
| 1999 | 44 y | Spermatic cord | ||||||
| Angeli et al.(19), 2007 | 1999 | Italy | n.d. | 28 y | Epididymis, deferent duct | D. repens | Histology | Surgery (not specified) |
| Džamić et al. (20), 2009 | 2001–2008 | Serbia | no | 40 y | Epididymis | D. repens | Histology | Surgery (not specified) |
| no | 19 y | Scrotum-testis | D. repens | |||||
| Fleck et al.(21), 2009 | n.d. | Germany | Tunisia (2.5 years ago) | 28 y | Epididymis | D. repens | Histology, serology (high titers of antibodies), IgE (370 IU/mL) | Tumor excision |
| D’Amuri et al. (22), 2012 | n.d. | Italy | n.d. | 45 y | Spermatic cord | D. repens | Histology | Orcho-funiculectomy |
| Leccia et al. (23), 2013 | 2006 | France | no | 29 y | Epididymis | D. repens | Histology, hyperleukocytosis without eosinophilia | Tumor excision |
| 2010 | no | 66 y | Spermatic cord | Histology | Tumor excision | |||
| Kallampallil et al.(24), 2013 | n.d. | United Kingdom | n.d. | 13 y | Epididymis | D. immitis | Histology | Orchiectomy |
| Krajina et al.(25), 2015 | n.d. | Croatia | n.d. | 21 y | Epididymis | D. repens | Histology, serology | Tumor excision |
| Bertozzi et al.(26), 2015 | n.d. | Italy | n.d. | 3 y | Scrotum | D. repens | Histology, molecular tests, hyperleukocytosis | Tumor excision |
| Fuehrer et al.(1), 2016 | 2009 | Austria | Namibia | 61 y | Epididymis | D. repens | Histology, PCR eosinophilia 9% | n.d. |
| Tumolskaya et al.(27), 2016 | 2015 | Russia (Moscow) | n.d. | 14 mo | Scrotum | D. immitis | Histology, PCR. | Tumor excision |
| Kaftandjiev et al.(28), 2016 | n.d. | Bulgaria | n.d. | 31 y | Epididymis | Dirofilaria spp. | Histology | Tumor excision |
| Tripi et al.(29), 2016 | n.d. | Italy | no | 11 mo | Scrotum | D. repens, | US (“filarial dance”), histology | Tumor excision |
| Pigac et al.(30), 2016 | n.d. | Croatia | no | 3 y | Scrotum | Dirofilaria spp. | Histology | Tumor excision |
| Bausch et al.(31), 2017 | n.d. | Switzerland | India | 54 y | Epididymis | D. repens | Histology, PCR unsuccessful | Tumor excision |
| Velev et al.(33), 2019 | n.d. | Bulgaria | n.d. | 11 y | Epididymis | D. repens | Histology | Tumor excision |
| Sabūnas et al.(6), 2019 | 2015 | Lithuania | no | 79 y | Penis | D. repens | Histology | Surgery (not specified) |
| Boldiš et al.(32), 2020 | 2017 | Slovakia | Denmark, Germany | 48 y | Epididymis | D. repens | Histology, PCR, eosinophilia (16.6% → 3.2% (after 9 months of observation)) | Tumor excision |
| Nagy et al.(34), 2021 | 2019 | Slovakia | Turkey | 73 y | Epididymis | D. repens | US (“filarial dance”), histology | Albendazole 14 days, after a10 day break, further 7 days at a dose of 2×400 mg per day. Subsequent orchiectomy |
| Pansini et al.(35), 2022 | 2021 | Italy | no | 11 y | Scrotum | D. repens | US (“filarial dance”), histology | Tumor excision |
| Ugolini et al.(36), 2022 | 2017 | Italy | n.d. | 13 y | Testicular tunics | D. repens | US (“filarial dance”), MRI, histology, PCR | Tumor excision |
Abbreviations: n.d. – no data, no – no travelling to foreign countries, y – years, mo – months, US – ultrasound, PCR – polymerase chain reaction, MRI – magnetic resonance imaging
Due to unspecific and usually not severe symptoms and similarity to other conditions, many cases of human dirofilariasis may remain underdiagnosed and unreported.
The life cycle of Dirofilaria consists of five larval stages. After mating in carnivores (e. g., dogs), adult females produce microfilariae (first-stage larvae) and release them in the peripheral blood. Aedes, Anopheles, or Culex mosquitoes, the vectors and intermediate hosts of Dirofilaria, ingest the microfilariae while feeding on an infected animal. Microfilariae then migrate to the Malpighian tubules of the insect, where they molt into the second and third (infective) larval stages. The latter actively moves from Malpighian tubules to the proboscis. The infective larva is transmitted when the mosquito with the third-stage larva bites a potential definitive host, including a human. In the mammalian host, the third-stage larva migrates to the subcutaneous tissue (or other location), molts two more times, and matures into adults, which are theoretically able to mate and produce microfilariae (5,6,29). Importantly an intracellular bacterial endosymbiont of the genus Wolbachia (Rickettsiaceae) is found in all filarioid stages of Dirofilaria species. Wolbachia spp. is essential for successful embryogenesis, molting, development, fertility, and survival of the adult helminth (6). The antigen sets of Dirofilaria itself and its endosymbiont Wolbachia stimulate inflammation and induce specific immune responses in humans, so the complete development of Dirofilaria into a sexually mature adult is uncommon (3,6). Therefore, clinical human dirofilariasis is rare, and if the larva survives, usually only a single pre-adult or adult nematode, sometimes already disintegrated, can be found in the histological specimens (3).
Two main factors determine the geographic distribution of D. immitis and D. repens: a sufficient number of dogs infected with productive adult Dirofilaria spp. and the presence of the mosquito species capable of transmitting the nematodes (3). In Europe, historically, dirofilariasis was considered endemic in the Mediterranean region (Italy, Southern France, Greece) and South-Eastern Europe (Ukraine, Belarus, Russia), with the most human cases reported in Italy (1,2,13,14,37). Due to the climate changes, extending mosquito breeding season, facilitated pet traveling, and under-diagnosis of infected dogs, Dirofilaria spp. distribution is expanding rapidly [5,9,10]. Recently, new endemic areas in Austria, Croatia, and Hungary have been confirmed (37). Currently, dirofilariasis is an emerging zoonosis in Baltic and Nordic countries (6–8,38). In the Baltic countries, the first canine D. repens was detected in Latvia in 2008, followed by Lithuania in 2010, and Estonia in 2012 (8). In 2016, 24% of 125 veterinarians who worked in the Baltic (Estonia, Latvia, and Lithuania) and the Nordic countries (Denmark, Finland, Iceland, Norway, and Sweden) countries and participated in a questionnaire study reported having encountered one or more autochthonous canine cases of dirofilariasis (38). In recent research, 2.7% of 2280 blood samples from pet and shelter dogs in Lithuania were found positive for the presence of D. repens microfilariae (6). In another study, the D. repens DNA was detected in 37.7% of 77 sled dogs tested in Lithuania (7). The first human dirofilariasis cases in the Baltic-Nordic region have been diagnosed in Latvia (2010), in Lithuania (2011), and in Finland (2015) (8). Since then, before our case, nine human D. repens dirofilariasis have been diagnosed in Lithuania, including one penis dirofilariasis in 2015 (6). Of note, many canine and human Dirofilariasis cases may remain unrecorded due to the benign clinical presentation or even asymptomatic course of the infection. Nonetheless, the currently available epidemiological data shows that the numbers of human dirofilariasis in Lithuania and the Baltic region are likely to increase in the upcoming decade (6,8).
Dirofilariasis in male genitalia usually presents as a single solid nodule, which appears after a 6–9-month incubation period since the mosquito bite, and might slowly migrate or change size (6,29,30). Some authors suggested that D. repens might have a genital tropism in response to sex hormones (2). Mosquito bite usually remains unnoticed and not reported by the patient. Only sometimes, the parasite migration from the bite site, which lasts weeks or months, might be symptomatic and cause swelling, erythema, irritation, and itching until Dirofilaria stops migrating and forms a nodule of around 1 cm in size (5,17,32).
Dirofilariasis of male genitalia is often misinterpreted as a benign or malignant tumor; therefore, in most cases, surgical excision of the lesion or radical orchiectomy was performed (6,16,17,19–36). Rarely, an intense local immune response might develop, causing symptoms of an abscess, including elevated body temperature and eosinophilia (5). In pediatric case reported by Bertozzi et al., dirofilariasis mimicked an acute scrotum, which led to scrotum surgical exploration (26).
In human dirofilariasis, the alterations in laboratory parameters are absent or unspecific. Mild eosinophilia and elevated IgE levels have been found in several reported cases (1,21,32). Nevertheless, the normal blood cell counts or IgE levels do not exclude dirofilariasis. Imaging techniques, such as ultrasound, might be used for differential diagnostics. In several cases, an ultrasound examination performed with a high-resolution probe showed a worm-like tubular coiled structure with a parallel echogenic wall in continuous movement after the mechanical stimulation (“filarial dance sign”) (29,34–36). Such specific radiological signs are often absent, as Dirofilaria in the subcutaneous nodule is rarely vivid, and its morphology might be already destroyed due to immune reactions (5,26).
The conclusive diagnosis of dirofilariasis is primarily made by histological evaluation of the excised nodule (5). Granulomatous chronic-type tissue reaction with eosinophils, plasma cells, histiocytes, and foreign body giant cells infiltration surrounding the nematode is usually found (16,17,21,27). Occasionally, acute inflammation and abscess formation, hemorrhages, or necrotizing vasculitis might be observed (3,5). Nevertheless, the differentiation among Dirofilaria species might be challenging or sometimes even not possible in case of nematode destruction. If no conclusive morphological diagnosis can be made, PCR from the intact worm, fresh tissue, or paraffin-embedded tissue is used. Due to its high sensitivity, PCR is an invaluable method when the structure of the parasite is altered, even when only limited amount of DNA is available. Nevertheless, PCR for Dirofilaria genes is usually accessible only in specialized centers (1,5,27,32,36). Similarly, one of the primary limitations of our case-report is the absence of DNA analysis of the specimen. While the initial histological examination was conducted due to a suspected malignancy, the parasite was discovered incidentally. As a result, DNA analysis was not part of our original diagnostic approach. We acknowledge that DNA examination could have provided additional valuable insights into the identification and characterization of the parasite. Future studies should incorporate DNA analysis to enhance the understanding and diagnostic accuracy of similar cases.
As a definite diagnosis of dirofilariasis in male genitalia is almost exceptionally made after the removal of the nodule and histological evaluation, surgery is considered a treatment of choice in such cases. Nevertheless, there are reports that the nodule might heal itself without intervention (19). As microfilaremia is extremely rare, systemic treatment is rarely used in humans, so little is known about the benefits of antifilarial medication. In most cases, radical orchiectomies are performed due to suspicion of malignancy, especially if the nodule of the testis, epididymis, or spermatic cord is detected (16,17,22,24). Sometimes testicles could be preserved if dirofilariasis were suspected, and additional examinations, including a biopsy or serological tests, were performed before the surgery.
Conclusion
From the clinical point of view, due to the benign character and uncomplicated treatment of human dirofilariasis, it is considered a low-priority infection. Nonetheless, differential diagnostics, especially the rejection of malignancies, is crucial. Moreover, each human dirofilariasis case is of interest from an epidemiological perspective. Therefore, the awareness of Dirofilaria spp. as a possible causative agent of nodules or tumors in male genitalia is noteworthy.
Acknowledgements
The authors declare that there was no financial support for this case report
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Fuehrer HP, Auer H, Leschnik M, et al. Dirofilaria in Humans, Dogs, and Vectors in Austria (1978–2014)-From Imported Pathogens to the Endemicity of Dirofilaria repens. PLoS Negl Trop Dis. 2016;10(5):e0004547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pampiglione S, Rivasi F. Human dirofilariasis due to Dirofilaria (Nochtiella) repens: an update of world literature from 1995 to 2000. Parassitologia. 2000;42(3–4):231–54. [PubMed] [Google Scholar]
- 3.Simón F, Siles-Lucas M, Morchón R, et al. Human and animal dirofilariasis: the emergence of a zoonotic mosaic. Clin Microbiol Rev. 2012;25(3):507–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balendran T, Yatawara L, Wickramasinghe S. Human Dirofilariasis Caused by Dirofilaria repens in Sri Lanka from 1962 to 2020. Acta Parasitol. 2022;67(2):628–39. [DOI] [PubMed] [Google Scholar]
- 5.Capelli G, Genchi C, Baneth G, et al. Recent advances on Dirofilaria repens in dogs and humans in Europe. Parasit Vectors. 2018;11(1):663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabūnas V, Radzijevskaja J, Sakalauskas P, et al. Dirofilaria repens in dogs and humans in Lithuania. Parasit Vectors. 2019;12(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alsarraf M, Levytska V, Mierzejewska EJ, et al. Emerging risk of Dirofilaria spp.. infection in Northeastern Europe: high prevalence of Dirofilaria repens in sled dog kennels from the Baltic countries. Sci Rep. 2021;11(1):1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deksne G, Jokelainen P, Oborina V, et al. The Zoonotic Parasite Dirofilaria repens Emerged in the Baltic Countries Estonia, Latvia, and Lithuania in 2008–2012 and Became Established and Endemic in a Decade. Vector Borne Zoonotic Dis. 2021;21(1):1–5. [DOI] [PubMed] [Google Scholar]
- 9.Genchi C, Rinaldi L, Mortarino M, et al. Climate and Dirofilaria infection in Europe. Vet Parasitol. 2009;163(4):286–92. [DOI] [PubMed] [Google Scholar]
- 10.Otranto D, Cantacessi C, Dantas-Torres F, et al. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part II: Helminths and arthropods. Vet Parasitol. 2015;213(1–2):24–37. [DOI] [PubMed] [Google Scholar]
- 11.Ermakova LA, Nagorny SA, Krivorotova EY, et al. Dirofilaria repens in the Russian Federation: current epidemiology, diagnosis, and treatment from a federal reference center perspective. Int J Infect Dis. 2014;23:47–52. [DOI] [PubMed] [Google Scholar]
- 12.Grapatsas K, Kayser G, Passlick B, et al. Pulmonary coin lesion mimicking lung cancer reveals an unexpected finding: Dirofilaria immitis. J Thorac Dis. 2018;10(6):3879–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tasić-Otašević SA, Trenkić Božinović MS, Gabrielli SV, et al. Canine and human Dirofilaria infections in the Balkan Peninsula. Vet Parasitol. 2015;209(3–4):151–6. [DOI] [PubMed] [Google Scholar]
- 14.Sałamatin RV, Pavlikovska TM, Sagach OS, et al. Human dirofilariasis due to Dirofilaria repens in Ukraine, an emergent zoonosis: epidemiological report of 1465 cases. Acta Parasitol. 2013;58(4):592–8. [DOI] [PubMed] [Google Scholar]
- 15.Pampiglione S, Canestri Trotti G, Rivasi F, et al. Human dirofilariasis in Greece: a review of reported cases and a description of a new, subcutaneous case. Ann Trop Med Parasitol. 1996;90(3):319–28. [DOI] [PubMed] [Google Scholar]
- 16.Pampiglione S, Elek G, Pálfi P, et al. Human Dirofilaria repens infection in Hungary: a case in the spermatic cord and a review of the literature. Acta Vet Hung. 1999;47(1):77–83. [DOI] [PubMed] [Google Scholar]
- 17.Elek G, Minik K, Pajor L, et al. New human Dirofilarioses in Hungary. Pathol Oncol Res. 2000;6(2):141–5. [DOI] [PubMed] [Google Scholar]
- 18.Pampiglione S, Rivasi F, Angeli G, et al. Dirofilariasis due to Dirofilaria repens in Italy, an emergent zoonosis: report of 60 new cases. Histopathology. 2001;38(4):344–54. [DOI] [PubMed] [Google Scholar]
- 19.Angeli L, Tiberio R, Zuccoli R, et al. Human dirofilariasis: 10 new cases in Piedmont, Italy. Int J Dermatol. 2007;46(8):844–7. [DOI] [PubMed] [Google Scholar]
- 20.Dzamić AM, Colović IV, Arsić-Arsenijević VS, et al. Human Dirofilaria repens infection in Serbia. J Helminthol. 2009;83(2):129–37. [DOI] [PubMed] [Google Scholar]
- 21.Fleck R, Kurz W, Quade B, et al. Human dirofilariasis due to Dirofilaria repens mimicking a scrotal tumor. Urology. 2009;73(1):209.e1–3. [DOI] [PubMed] [Google Scholar]
- 22.D’Amuri A, Senatore SA, Carlà TG, et al. Cutaneous dirofilariasis resulting in orchiectomy. J Cutan Pathol. 2012;39(2):304–5. [DOI] [PubMed] [Google Scholar]
- 23.Leccia N, Patouraux S, Carpentier X, et al. Pseudo-tumor of the scrotum, a rare clinical presentation of dirofilariasis: a report of two autochtonous cases due to Dirofilaria repens. Pathog Glob Health. 2012;106(6):370–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kallampallil J, Wood SJ, O’Dempsey T, et al. Nematode infection mimicking paratesticular malignancy. BMJ Case Rep. 2013;2013:bcr2013200775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krajina V, Pavoković D. Epididymal dirofilariasis – case report. Eur Urol Suppl. 2015;14(6):e1236. [Google Scholar]
- 26.Bertozzi M, Rinaldi VE, Prestipino M, et al. Dirofilariasis Mimicking an Acute Scrotum. Pediatr Emerg Care. 2015;31(10):715–6. [DOI] [PubMed] [Google Scholar]
- 27.Tumolskaya NI, Pozio E, Rakova VM, et al. Dirofilaria immitis in a child from the Russian Federation. Parasite. 2016;23:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaftandjiev IT, Harizanov RN. Rare case of epididymal dirofilariasis. QJM. 2016;109(5):351–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tripi F, Scarlata F, Verde V, et al. Human Dirofilariasis Presenting as Scrotal Mass. J Urol Nephrol Open Access. 2016; 3(1): 1–4. [Google Scholar]
- 30.Pigac B, Masic S, Masic V. Subcutaneous scrotal dirofilariasis in a 3- year- old boy. Pediatr Urol Case Rep. 2016;3(4):110–110. [Google Scholar]
- 31.Bausch K, Bosl M, Matter M, et al. When you hear hoof beats … consider zebras - A diagnostic challenge. Travel Med Infect Dis. 2017;19:73–74. [DOI] [PubMed] [Google Scholar]
- 32.Boldiš V, Ondriska F, Bošák V, et al. Pseudo-Tumor of the Epididymis, a Rare Clinical Presentation of Human Dirofilaria repens Infection: a Report of Autochthonous Case of Dirofilariasis in Southwestern Slovakia. Acta Parasitol. 2020;65(2):550–3. [DOI] [PubMed] [Google Scholar]
- 33.Velev V, Pelov T, Garev T, et al. Epididymal Dirofilariasis in a Child: First Case Report from Bulgaria. Med Princ Pract. 2019;28(1):96–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagy V, Nagyová D. A rare clinical presentation of human Dirofilaria repens infection as a pseudo-tumour of the epididymis - Case Report. Ann Agric Environ Med. 2021;28(2):348–51. [DOI] [PubMed] [Google Scholar]
- 35.Pansini A, Magenes VC, Casini F, et al. Testicular Dirofilariasis in an Italian 11-Year-old Child. Pediatr Infect Dis J. 2022;41(12):e539–e540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ugolini S, Lima M, Maffi M, et al. Dirofilaria repens Testicular Infection in Child, Italy. Emerg Infect Dis. 2022;28(12):2569–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Genchi C, Kramer L. Subcutaneous dirofilariosis (Dirofilaria repens): an infection spreading throughout the old world. Parasit Vectors. 2017;10(Suppl 2):517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiškina V, Jokelainen P. Vector-borne parasitic infections in dogs in the Baltic and Nordic countries: A questionnaire study to veterinarians on canine babesiosis and infections with Dirofilaria immitis and Dirofilaria repens. Vet Parasitol. 2017;244:7–11. [DOI] [PubMed] [Google Scholar]