Abstract
BACKGROUND
Spinal cord stimulation (SCS) has demonstrated potential as a therapy to enhance motor functional recovery after spinal cord injury (SCI). Epidural SCS for motor recovery is traditionally performed via the dorsal electrode. While ventral epidural stimulation may provide more direct and specific stimulation of the ventral motor neurons involved in motor control, it is largely unstudied, and its role in motor recovery after SCI is unclear. In order to profile the safety and feasibility of ventral epidural spinal stimulation (VSS), the authors present a patient who underwent VSS following a corpectomy to treat SCI related to metastatic epidural cord compression.
OBSERVATIONS
A patient underwent transpedicular corpectomy for spinal cord decompression, as well as the placement of 2 ventral epidural electrodes, followed by concurrent physical therapy and ventral epidural stimulation. He was nonambulatory preoperatively but was able to walk over 300 feet with the assistance of a rolling walker at the conclusion of the 3-week study period. VSS was noted to produce improvements in muscle contraction when stimulation was on.
LESSONS
VSS appears to be safe, feasible, and well tolerated. VSS, as compared to standard-of-care therapy for SCI, can be used in conjunction with physical therapy and may lead to improvements in motor function.
Keywords: ventral spinal cord, spinal cord stimulation, electrical stimulation
ABBREVIATIONS: CT = computed tomography, DSS = dorsal spinal stimulation, EMG = electromyography, MH = medial hamstring, MRI = magnetic resonance imaging, PT = physical therapy, RF = rectus femoris, SCI = spinal cord injury, SCS = spinal cord stimulation, SOL = soleus, TA = tibialis anterior, VSS = ventral epidural spinal stimulation.
Spinal cord injury (SCI), whether traumatic or nontraumatic, is a devastating condition that leads to impairments in motor, sensory, and autonomic function from which there is often minimal recovery in humans. One method of treatment is spinal cord stimulation (SCS), that is, the therapeutic delivery of electrical impulses to the spinal cord through implanted electrodes. Recent studies in patients with SCI have demonstrated that SCS, in combination with motor rehabilitation, can modulate the spinal circuitry, leading to the recovery of motor function (e.g., standing and gait) as well as improvements in autonomic nervous system functionality (e.g., cardiovascular and bladder control).1–5 The mechanism underlying the functional benefits of SCS in SCI is not entirely clear, but available evidence suggests that it may arise from a combination of enhancements in 1) neuron survival and differentiation, 2) axonal growth and elongation, and 3) synaptogenesis and dendrite stability, all of which are known to be regulated by neural activity in the setting of undue stress or injury to the central nervous system.6
SCS following SCI, aimed at functional recovery, is conventionally achieved by placing an epidural lead dorsally1–5 or through less invasive means such as transcutaneous spinal stimulation.7– 10 While the dorsal aspect of the spinal cord is anatomically more accessible for epidural lead placement, lower motor neuron cell bodies are localized ventrally within the spinal gray matter, with axons projecting out to innervate the vast majority of somatic skeletal muscles. This positional aspect of motor innervation suggests that dorsal spinal stimulation (DSS) may be limited in specificity for the stimulation of motor neurons, instead activating a multitude of sensory, proprioceptive, and motor pathways concomitantly.11 This, in turn, may lead to inadvertent activation of nontargeted neural circuitries, reduced motor control, and nonnatural movement patterns.12, 13 DSS is thought to activate central pattern generators directly or indirectly through the activation of sensory or proprioceptive pathways.5, 14, 15 In contrast, ventral epidural spinal stimulation (VSS), wherein an epidural stimulator array is placed on the ventral aspect of the spinal cord, has been shown to activate unique circuits that may differ from those activated by DSS.16 Sharpe and Jackson compared the electromyography (EMG) responses to intraspinal as well as both dorsal and ventral epidural and subdural stimulation of the cervical enlargement in anesthetized monkeys. They concluded that the motor effects of SCS were mediated by both direct activation of motor neurons and indirect activation mediated by descending projections, afferent inputs, and/or local spinal interneuron circuits, with predominantly direct effects being seen with stimulation of the ventral surface and predominantly indirect effects being seen with dorsal stimulation. Thus, the motor neurons stimulated most directly by VSS are not necessarily inaccessible to DSS stimulation, but stimulation specificity for motor neurons appears to be lower with DSS.16
Our collaborative research group has demonstrated the recovery of upper-limb function following VSS therapy in a rat model of chronic cervical SCI, suggesting that 1) it is a safe alternative to other modalities of stimulation, namely dorsal epidural, transcutaneous, or intraspinal; 2) it may allow for lower stimulation amplitudes compared to dorsal stimulation modalities; and 3) it confers the ability to specifically target discrete pools of motor neurons within the ventral aspect of the spinal cord. The above studies and theory suggest that VSS possesses substantial therapeutic potential for motor recovery after SCI. Still, the feasibility, safety, and efficacy of VSS in humans with SCI remain unexplored, largely because the placement of an electrode in the ventral epidural space is technically challenging from a posterior approach because of the location of the spinal cord and nerve roots. However, patients with SCI related to fractures, tumors, or other pathologies for which corpectomy is indicated as a standard-of-care treatment present a unique opportunity for the study of VSS, as once the corpectomy is complete, the surgeon possesses relatively unobstructed access to the ventral epidural space, allowing for the placement of a ventral epidural array.
Here, we present a case involving a patient with a T10 SCI related to metastatic epidural spinal cord compression. The patient underwent resection of the tumor via a transpedicular corpectomy, followed by the immediate placement of 2 ventral epidural electrodes at the time of surgery. After the surgery, the patient underwent physical therapy (PT) in the presence of VSS 3 times weekly for 3 weeks, after which the SCS electrodes were removed. The patient tolerated the stimulation well and experienced substantial improvement in lower-extremity strength and balance function. This report supports the safety and feasibility of ventral epidural SCS in humans. Furthermore, it illustrates differences in motor activity in the presence or absence of ventral epidural stimulation.
Illustrative Case
Presentation
A 36-year-old male with no significant past medical history presented with 2 weeks of progressive thoracic back pain, as well as numbness and tingling in his bilateral lower extremities. He had initially presented to a chiropractor with the above complaints and eventually underwent thoracic spine magnetic resonance imaging (MRI), which demonstrated a large peridiaphragmatic, retroperitoneal malignancy with extension into the T9 and T10 vertebral bodies. A pathological fracture of T10 was seen with retropulsion and spinal cord compression. He was sent to the emergency room and admitted to the hospital at that time. Motor strength was normal throughout, and reflexes were normal at the time of initial presentation. Presurgery strength testing was done on the standard 5/5 muscle strength scale and retroactively classified on the American Spinal Injury Association Impairment Scale. Computed tomography (CT) of the chest, abdomen, and pelvis revealed a mass with a diameter of 7.6 cm located at the cecum, along with abdominopelvic lymphadenopathy, hypoechoic masses in the liver suggestive of metastases, and multiple lung nodules suggestive of metastases. He underwent an ultrasound-guided biopsy of one of the liver masses, the final pathology of which was possibly consistent with metastatic carcinoma with enteric differentiation. While awaiting final pathology results from the liver biopsy, the patient experienced rapid neurological deterioration, with 2/5 strength throughout his left lower extremity and 4/5 strength throughout his right lower extremity. Repeat thoracic spine MRI demonstrated worsened spinal cord compression. It was recommended that he undergo surgical decompression and stabilization.
Surgery
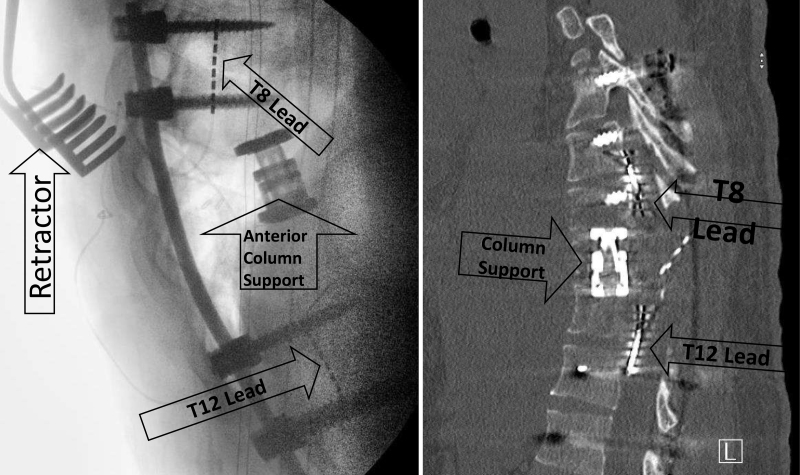
The patient was taken to the operating room and underwent T10 transpedicular corpectomy, T9–11 decompressive laminectomy, resection of the epidural tumor, and T7–L3 posterior instrumentation with arthrodesis. He also underwent the placement of two 8-contact spinal cord stimulator electrodes (SC-2316-50E, Boston Scientific) within the ventral epidural space. Validation of the electrodes was performed using the Boston Scientific testing cables (SC-4116) and Spectra WaveWriter trial kits (SC-6500-62). The electrodes were placed cranial (T8) and caudal (T12) to the injury site in a vertical and linear fashion (Fig. 1). The electrodes were tunneled out of the wound through separate stab incisions and secured to the skin with sutures so that they could be removed later without the need for an additional operation.
FIG. 1.
Left: An intraoperative lateral fluoroscopic image demonstrates the placement of the electrodes above and below the site of injury, as well as an anterior column support cage. Right: Thoracic spine CT shows the epidural leads present within the ventral epidural space cranial and caudal to the site of SCI. Arrows indicate surgical device placement.
VSS Therapy
During the VSS therapy period, the patient underwent PT sessions 3 times a week. Trials included an unstimulated phase and a stimulated phase. During stimulation, biphasic submotor-threshold SCS was performed without causing discomfort to the patient. Although the patient was blind to stimulator status, that is, stimulation on or off, he may have been able to feel the stimulation while it was turned on. The spinal stimulation parameters included a pulse width of 250 μsec, a frequency of either 2 Hz (probe) or 30 Hz (therapeutic), and an amplitude ranging between 1 and 8 mA.17
Electrophysiology Assessments
Trigno Avanti wireless surface EMG electrodes (Delsys Inc.) were placed longitudinally over the left and right rectus femoris (RF), medial hamstring (MH), tibialis anterior (TA), and soleus (SOL) muscles. EMG data were amplified using a Trigno Avanti amplifier (Delsys Inc.). The tests were performed in the supine position with the patient’s legs placed in the Exolab apparatus (Antex Lab LLC), supporting the hip, knee, and ankle joints at 155°, 90°, and 90°, respectively. These joint angle positions were selected to isolate knee and ankle joint movements. Four calibrated, 2-sided load cells (FSH00007, FUTEK) measured forces generated by the left and right knee extensors and plantar flexors independently. EMG and force signals were recorded at a sampling frequency of 2000 Hz using a PowerLab data acquisition system (ADInstruments). Once the patient regained the ability to stand, force measurements were performed on a force plate (AccuSway force platform, AMTI) utilizing a custom-built apparatus (Knee Assist) designed to facilitate minimally assisted standing.
Summary of Patient Outcomes
During the first postoperative week, evoked responses in the distal bilateral lower extremities were minimal, even with relatively high stimulation intensities (> 6 mA), and the patient was able to generate very little force in the lower extremities without stimulation. In the presence of VSS, the patient demonstrated increased force generation in the tested lower-extremity muscles. In the second postoperative week, the patient was able to generate higher force in the bilateral lower-extremity musculature in the absence of stimulation, and this force continued to increase in the presence of stimulation. During attempted standing in the second postoperative week, the patient demonstrated increased EMG signals in the plantar flexors (indicating improved standing) in the presence of stimulation compared with off-stimulation times. Overall, stimulation resulted in increased EMG signal and force generation in the flexor muscles of the bilateral lower extremities, particularly when compared with off-stimulation times.
At 3 weeks postoperatively, the electrodes were removed, and the patient continued PT. Overall, the stimulation was well tolerated, and the patient did not experience any complications related to the stimulation or the surgery itself.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
This is the first published case of human ventral epidural SCS and demonstrates the safety and feasibility of the technique for the treatment of SCI. Prior to and immediately following surgical intervention, the patient was nonambulatory and exhibited pronounced weakness in the lower extremities (Fig. 2). Over the course of 3 weeks of VSS, the patient demonstrated progressive improvement in lower-extremity function. Finally, at the conclusion of the 3-week stimulation period and at the time of spinal cord stimulator removal, the patient was able to ambulate 100 meters with the assistance of a rolling walker.
FIG. 2.
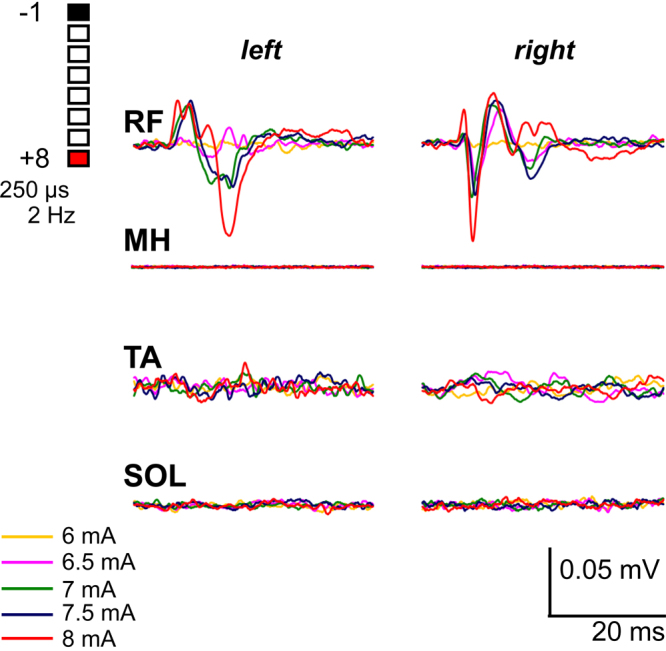
One week postoperative EMG data for spinally evoked potentials in the RF, MH, TA, and SOL muscles. The muscle electrical activity was measured in mV in the time window of 40 msec. Note the bilateral symmetry and varying response levels across the different muscle groups, with RF being responsive and all other muscles lacking a substantial response despite high stimulation intensities (> 6 mA).
Lessons
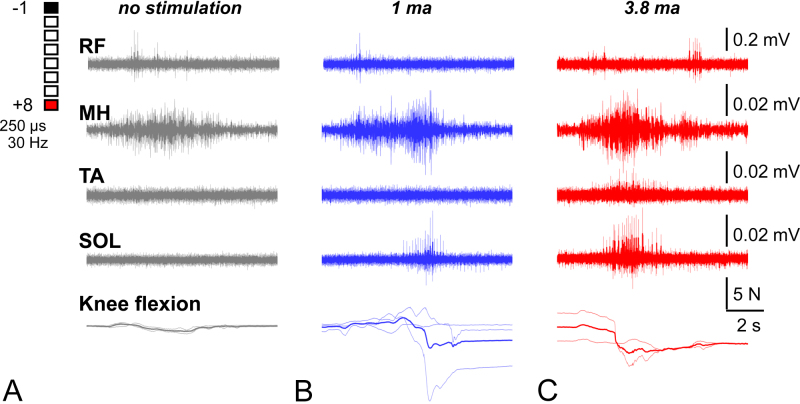
The patient tolerated stimulation well and experienced no complications related to the stimulation, establishing a safety profile for the therapy. Because improvements in motor function are frequently observed following surgical interventions for SCI, it remains uncertain whether the observed functional improvements in this case were augmented by the use of VSS or whether they were solely a consequence of the surgical procedure performed. Nonetheless, VSS induced objective improvements in muscle activity and force generated by the leg muscles as compared to the movements performed in the same period without stimulation (Fig. 3).
FIG. 3.
One-week postoperative EMG activity of selected lower-limb muscles and knee flexion force during voluntary efforts in the presence of varying VSS intensities: none (A), 1 mA (B), and 3.8 mA (C). Amplitude scales for EMG signals are provided on the far right. The lowest row depicts the associated knee flexion force (N) over a 2-second period. The unstimulated group shows the weakest activity and force, whereas the 1-mA and 3.8-mA stimulation groups show strong and consistent muscle activation, with the 1-mA group showing larger variability in force. We also observed a simultaneous burst of activity from the SOL during MH activation in both stimulation groups. Note that the scale of observed electrical activity has a magnitude of 0.01 mV, which is much smaller than the typical electrical activity (> 1 mV) observed in concerted muscular events.17
In week 1 following surgery (first session of PT), the patient was unable to flex his knee until the VSS was applied, after which he was able to move his knee with clearly observed activity in the MH and SOL muscles (Fig. 3). Interestingly, the higher stimulation level provided a more consistent result in terms of knee flexion, suggesting a dose-dependent relationship between electrical stimulation and motor activation.
In the second postoperative week, the patient was able to perform knee flexion without VSS. During knee flexion in the presence of VSS, the results mirrored those observed in week 1. Specifically, there was more intense muscle activity observed in the presence of VSS compared to no stimulation and a distinct activation of the MH muscle. Notably, the magnitude of the EMG signal in tested muscles during the second week was approximately an order of magnitude larger than in the initial postoperative week, and force generation was also increased (Fig. 4).
FIG. 4.
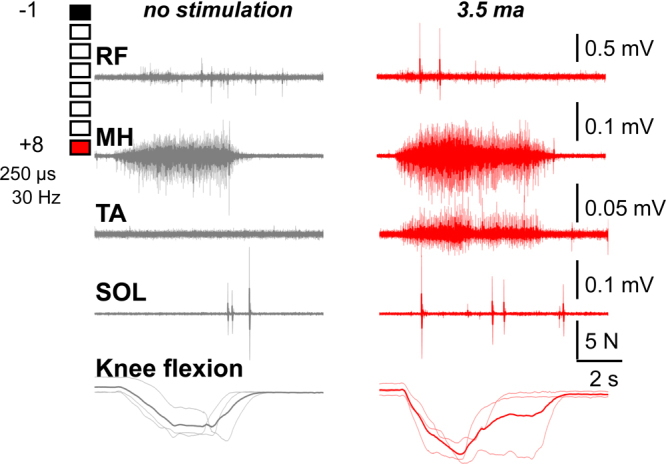
Two weeks postoperative EMG activity and generated force from the left leg muscles during attempts to perform left knee flexion in conditions of no stimulation (left) or 3.5 mA stimulation (right). The lowest row depicts the associated knee flexion force (in N) over a 2-second period. The MH is active, and knee flexion occurs both with and without stimulation. There is greater intensity, TA coactivation, and force generation with stimulation. The SOL and RF show millisecond-long bursts of activity that are more frequent during stimulation. Note that the magnitude of intensity is approximately 1 order of magnitude larger than in the previous week.
In week 2, a limits of stability test was conducted.7 In neurologically intact adults, the key postural muscle engaged during standing is the SOL, with minimal activity in the TA, and additional contributions from the MH and RF.18, 19 Body weight shifts activate leg muscles depending on the direction of movement.7 Our observations in this case closely aligned with these findings: the SOL and MH were active during shifts forward, while the TA and RF were active during shifts backward. VSS promoted tonic EMG activity in the SOL (Fig. 5). In contrast, TA and RF activity in the presence of VSS was characterized by a burst pattern, indicating functional postural adjustment, as opposed to their tonic activity indicative of impeded postural control without stimulation (Fig. 5). These findings further shine light on the potential therapeutic mechanism of action of VSS, namely being able to accelerate the initiation and maintenance of muscular activity in otherwise significantly weakened patients.
FIG. 5.
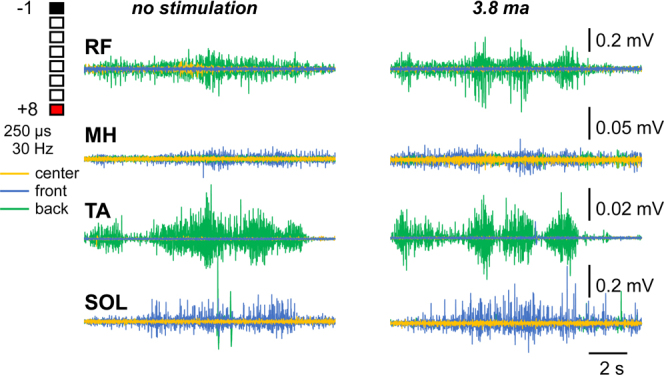
Two weeks postoperative EMG activity of the right leg muscles during quiet standing and body weight shifts performed without (left) and in the presence (right) of 3.8 mA stimulation. Front shifts primarily recruited the SOL and, to a lesser extent, the MH. Backward shifts recruited the RF and TA. The pattern of activation was constant in the SOL (slow-twitch dominant) versus clustered in bursts in the RF and TA (fast-twitch dominant). In center standing, there was minimal activation across all muscles, with larger contributions from the SOL and MH.
Our report demonstrates the safety and feasibility of VSS in a patient with SCI, while also illustrating how VSS results in objective improvements in muscle activity. One significant limitation of the current approach to VSS is the invasiveness required for electrode placement. However, many minimally invasive techniques have emerged that allow access to the ventral space for the treatment of various pathologies. One such treatment is full endoscopic spine surgery, an approach that provides endoscopic access to the ventral aspect of the spinal epidural space via a natural transforaminal route20 and could conceivably be utilized for minimally invasive placement of a SCS electrode into the ventral epidural space. Endoscopic spine surgery can be performed with local anesthetics, allowing for faster recovery and an enhanced patient experience.21 Advances in minimally invasive surgical techniques (e.g., full endoscopic spine surgery), combined with the positive outcomes documented in this study, support VSS as an increasingly realistic strategy for future SCS approaches, particularly in promoting motor recovery after SCI.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Sayenko, Barber. Acquisition of data: Steele, Martin, Sayenko, Barber. Analysis and interpretation of data: Afridi, Steele, Sayenko, Barber. Drafting the article: Afridi, Sayenko, Barber. Critically revising the article: Afridi, Sayenko, Barber. Reviewed submitted version of manuscript: Afridi, Sayenko, Barber. Approved the final version of the manuscript on behalf of all authors: Afridi. Statistical analysis: Afridi. Administrative/technical/material support: Afridi, Sayenko. Study supervision: Barber.
Supplemental Information
Previous Presentations
Portions of this work were presented as an oral presentation at the AANS Annual Meeting, Los Angeles, CA, April 20–24, 2023.
Correspondence
Abdullah K. Afridi: Texas A&M School of Engineering Medicine, Houston, TX. abdullaha@tamu.edu.
References
- 1.Harkema S, Gerasimenko Y, Hodes J, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377(9781):1938-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angeli CA, Boakye M, Morton RA, et al. Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med. 2018;379(13):1244-1250. [DOI] [PubMed] [Google Scholar]
- 3.Gill ML, Grahn PJ, Calvert JS, et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med. 2018;24(11):1677-1682. [DOI] [PubMed] [Google Scholar]
- 4.Grahn PJ, Lavrov IA, Sayenko DG, et al. Enabling task-specific volitional motor functions via spinal cord neuromodulation in a human with paraplegia. Mayo Clin Proc. 2017;92(4):544-554. [DOI] [PubMed] [Google Scholar]
- 5.Taccola G, Sayenko D, Gad P, Gerasimenko Y, Edgerton VR. And yet it moves: recovery of volitional control after spinal cord injury. Prog Neurobiol. 2018;160:64-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borodinsky LN, Belgacem YH, Swapna I. Electrical activity as a developmental regulator in the formation of spinal cord circuits. Curr Opin Neurobiol. 2012;22(4):624-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sayenko DG, Rath M, Ferguson AR, et al. Self-assisted standing enabled by non-invasive spinal stimulation after spinal cord injury. J Neurotrauma. 2019;36(9):1435-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerasimenko Y, Gorodnichev R, Moshonkina T, Sayenko D, Gad P, Edgerton VR. Transcutaneous electrical spinal-cord stimulation in humans. Ann Phys Rehabil Med. 2015;58(4):225-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sayenko DG, Atkinson DA, Dy CJ, et al. Spinal segment-specific transcutaneous stimulation differentially shapes activation pattern among motor pools in humans. J Appl Physiol (1985). 2015;118(11):1364-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sayenko DG, Atkinson DA, Floyd TC, et al. Effects of paired transcutaneous electrical stimulation delivered at single and dual sites over lumbosacral spinal cord. Neurosci Lett. 2015;609:229-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogan MK, Barber SM, Rao Z, et al. A wireless spinal stimulation system for ventral activation of the rat cervical spinal cord. Sci Rep. 2021;11(1):14900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barra B, Roux C, Kaeser M, et al. Selective recruitment of arm motoneurons in nonhuman primates using epidural electrical stimulation of the cervical spinal cord. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:1424-1427. [DOI] [PubMed] [Google Scholar]
- 13.Cho N, Squair JW, Bloch J, Courtine G. Neurorestorative interventions involving bioelectronic implants after spinal cord injury. Bioelectron Med. 2019;5(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7(3):269-277. [DOI] [PubMed] [Google Scholar]
- 15.Greiner N, Barra B, Schiavone G, et al. Recruitment of upper-limb motoneurons with epidural electrical stimulation of the cervical spinal cord. Nat Commun. 2021;12(1):435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharpe AN, Jackson A. Upper-limb muscle responses to epidural, subdural and intraspinal stimulation of the cervical spinal cord. J Neural Eng. 2014;11(1):016005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamane M, Aoki M, Sasaki Y, Hayashi T. Feedforward coactivation of trunk muscles during rapid shoulder movements. JSES Int. 2022;6(4):660-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masani K, Sayenko DG, Vette AH. What triggers the continuous muscle activity during upright standing? Gait Posture. 2013;37(1):72-77. [DOI] [PubMed] [Google Scholar]
- 19.Sayenko DG, Masani K, Vette AH, Alekhina MI, Popovic MR, Nakazawa K. Effects of balance training with visual feedback during mechanically unperturbed standing on postural corrective responses. Gait Posture. 2012;35(2):339-344. [DOI] [PubMed] [Google Scholar]
- 20.Lee SH, Musharbash FN. Uniportal, transforaminal endoscopic thoracic discectomy: review and technical note. Neurospine. 2023;20(1):19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner R, Telfeian AE, Iprenburg M, et al. Transforaminal endoscopic foraminoplasty and discectomy for the treatment of a thoracic disc herniation. World Neurosurg. 2016;90:194-198. [DOI] [PubMed] [Google Scholar]