ABSTRACT
Background
Individualizing induction therapy based on immunological risk is crucial for optimizing outcomes in kidney transplantation.
Methods
A retrospective analysis included 157 first live-donor non-sensitized kidney transplant recipients (KTRs). Within this cohort, 96 individuals exhibited low human leukocyte antigen (HLA) matching (5–6 HLA mismatches). The low HLA match subgroup was categorized into 52 KTRs receiving basiliximab alone and 44 recipients treated with a combined single ATG dose of 1.5 mg/kg and basiliximab. The primary endpoint was early acute cellular rejection (ACR) within 6 months post-transplant while secondary outcomes encompassed infection rates, renal allograft function, length of stay (LOS) and readmissions post-transplant.
Results
The incidence of early ACR was decreased for low HLA match KTRs, who received ATG–basiliximab, when compared with low HLA-matched KTRs who received basiliximab alone (9.1% vs 23.9%, P = .067). Age was a predictor for rejection, and subgroup analysis showed consistent rejection reduction across age groups. No significant differences were observed in admission for transplant LOS or in peri-operative complications, nor in infections rate including BK and cytomegalovirus viremia, allograft function and number of readmissions post-transplant up to 6 months post-transplant.
Conclusion
In non-sensitized first live-donor KTRs with low HLA matching, a dual ATG–basiliximab induction approach significantly reduced early ACR without compromising safety.
Keywords: anti-thymocyte globulin (ATG), basiliximab, HLA match, induction treatment, kidney transplantation
KEY LEARNING POINTS.
This study presents a novel approach to individualizing induction therapy in kidney transplantation.
This study adds:
This study highlights the efficacy of a combined induction regimen comprising basiliximab and a single dose of anti-thymocyte globulin (ATG) compared with basiliximab alone in kidney transplant recipients with low human leukocyte antigen (HLA) match.
The primary outcome, early acute cellular rejection within 6 months post-transplant, was significantly reduced with the dual ATG–basiliximab approach.
Importantly, the dual induction strategy did not compromise safety, as evidenced by comparable rates of infections, renal allograft function and peri-operative complications.
Potential impact:
This research underscores the potential of tailored induction therapies to optimize outcomes in kidney transplantation.
INTRODUCTION
Induction immunosuppression in renal transplantation plays a pivotal role in preventing acute rejection episodes and enhancing long-term graft survival. Among the various induction agents available, anti-thymocyte globulin (ATG) and basiliximab have emerged as key players, each with its unique mechanisms of action and clinical efficacy [1].
ATG, a polyclonal antibody derived from rabbit or horse sources, exerts its immunosuppressive effects by targeting T lymphocytes, making it an effective agent in preventing acute rejection. On the other hand, basiliximab, a monoclonal interleukin-2 receptor antagonist, offers a targeted approach by specifically inhibiting the activation of T cells. Both agents have demonstrated efficacy in reducing the incidence of acute rejection, but their comparative advantages and disadvantages remain the subject of investigation [1]. Research has indicated a lower incidence of rejection when employing ATG, recognized as a more robust induction therapy [2–5]. However, it is noteworthy that this approach is associated with an elevated risk of infections in the short term [6] and an increased susceptibility to malignancies in the long term [7, 8].
Tailoring immunosuppressive therapy based on individualized immunological risk is a crucial approach to enhancing the success of organ transplantation and minimizing complications. Each transplant recipient possesses a unique immunological profile that influences the risk of rejection versus infection. This tailored approach involves assessing factors such as the recipient's immunological history, human leukocyte antigen (HLA) sensitization, HLA matching, presence of donor-specific antibodies and overall status of the immune system [9]. Additionally, donor characteristics such as donor type (live versus deceased) [10], age and peri-transplant ischemic injury leading to delayed or slow graft function are considered [11]. The degree of HLA matching influences the risk of both acute and chronic rejection and plays a pivotal role in determining the immunosuppressive protocol for renal transplantation [12, 13].
Due to the prevalent utilization of living unrelated donor (LURD) transplants in Israel [14] , a substantial proportion of non-sensitized recipients have a low level of HLA matching (5–6 HLA mismatch). Our observations revealed a heightened acute rejection rate in this particular population when employing basiliximab induction. In response to this, we have modified our induction protocol, opting for a combination of single ATG dose and basiliximab. The primary objective was to diminish the incidence of acute rejection, and notably, we have significantly decreased the ATG dosage to mitigate the potential adverse effects associated with higher doses of ATG.
In this study, we compare the 6-month outcomes between basiliximab induction alone and a dual ATG–basiliximab approach in this population. Specifically, our focus is on the acute rejection rate, infection rates, including urinary tract infections (UTI), bacteremia, BK and cytomegalovirus (CMV) viremia, as well as renal allograft function. Additionally, we compare the length of stay (LOS) for transplant admissions and the overall number of readmissions post-transplant between the two groups.
MATERIALS AND METHODS
Study population and design
Clinical and biochemical data retrieval was conducted with the assistance of MDClone© software, a data extraction and synthesis tool intricately connected to the medical records of patients treated within our institution (http://www.mdclone.com). To ensure data accuracy and reliability, the information collected through MDClone underwent rigorous manual assessment and validation. Supplementary details were obtained from relevant clinical records. The study received approval from the local ethics committee (Institutional Review Board approval number: SMC-7053-20).
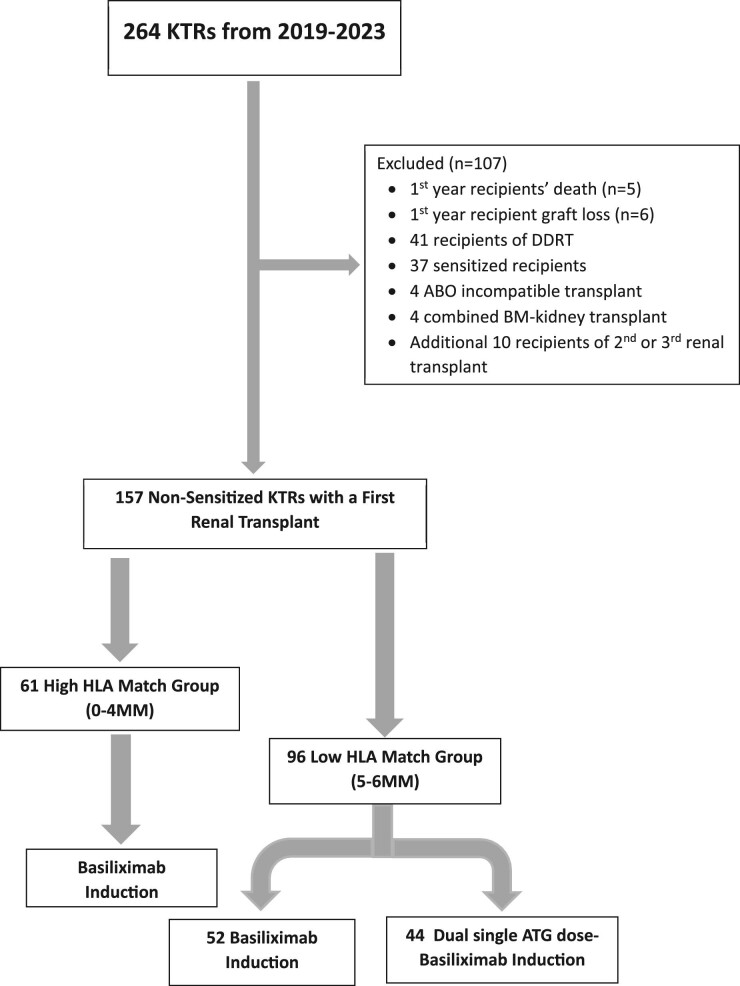
The original dataset comprised 264 transplant recipients who underwent renal transplantation at Sheba Medical Center between August 2019 and August 2023. Among them, 234 underwent their first renal transplant, while 30 underwent a second to fourth renal transplant. Subsequently, six recipients with first-year graft loss and five who passed away during the initial post-transplant year were excluded from the analysis. Furthermore, 41 recipients of deceased donor renal transplants, 37 sensitized recipients with antibodies to HLA (with and without donor-specific antibodies), 4 who underwent ABO incompatible renal transplants, 4 recipients who underwent a combined bone marrow–kidney transplant and an additional 10 recipients who underwent their second or third renal transplant were excluded from the study.
The final study cohort comprised 157 non-sensitized kidney transplant recipients (KTRs) who underwent their first live-donor renal transplant at Sheba Medical Center. Of these, 61 fell within the 0–4 HLA mismatch range with their donors, constituting the high HLA match group, while 96 had 5–6 HLA mismatches, categorizing them as the low HLA match group. All recipients in the high HLA match group received induction therapy with basiliximab. In the low HLA match group, 52 received basiliximab and 44 were subjected to a combined single ATG dose–basiliximab regimen for induction, as illustrated in Fig. 1.
Figure 1:
CONSORT diagram.
Induction therapy
The basiliximab induction protocol involved administering 20 mg intravenously in accordance with the manufacturer's guidelines. This entailed the first infusion prior to graft reperfusion, followed by a second infusion on Day 4. The manufacturer did not recommend any premedication or dose adjustments.
For the combined ATG–basiliximab induction, a single dose of 1.5 mg/kg body weight of rabbit ATG was administered intravenously intraoperatively before graft reperfusion. Subsequently, two additional doses of 20 mg basiliximab were given postoperatively on Day 0 and Day 4, as described above.
HLA analysis
Blood samples from potential donors and recipients were dispatched to the Tissue Typing Laboratory at Sheba Medical Center for HLA typing prior to transplantation. Utilizing the NGS-go MX6-1 kit, the next-generation sequencing (NGS) method was employed to perform HLA genotyping for six specific loci (A, B, C, DRB1, DQB1 and DPB1). Subsequent to the analysis of sequencing data (FASTQ files) using NGSengine software from GenDx in the Netherlands, results pertaining to HLA-A, HLA-B and HLA-DRB1 alleles were compiled. Comparison of HLA typing data for each allele between donor and recipient pairs facilitated the determination of a 0–6 HLA match/mismatch based on allele-level compatibility across all six loci.
Maintenance immunosuppression
At our medical center, the standard maintenance immunosuppression regimen for KTRs comprises a calcineurin inhibitor (usually tacrolimus), an anti-metabolite [usually a mycophenolate-based drug, mainly mycophenolic acid (MPA)], and prednisone, as described previously [15]. For KTRs with a low immunological risk of rejection, early steroid withdrawal (ESW) is implemented 5–8 days after transplant, and the maintenance regimen thus consists of tacrolimus and MPA. Conversion to a mammalian target of rapamycin (mTOR) inhibitor (sirolimus or everolimus) is instituted according to the patient's risk of malignancy and lack of tolerance to CNIs.
Primary and secondary outcomes
The primary endpoint of this study was the rate of early acute cellular rejection (ACR), whether biopsy-proven or clinically proven. Clinically proven ACR was determined by a rapid increase in serum creatinine (Scr) without evidence of a prerenal/nephrotoxic injury, highly suggestive of rejection, which was clinically confirmed by a rapid response to intravenous steroid pulse. Early ACR, for the purposes of this study, was specifically defined as any cellular rejection episode occurring within the initial 6 months post-transplantation. In our study cohort, we identified a total of 20 cases of early ACR: 13 were confirmed by biopsy, while 7 were diagnosed clinically. Additionally, we assessed several secondary outcomes, including the UTI and bacteremia rate during the first month and from 1–6 months post-transplant, and the presence of CMV and BK virus viremia. CMV and BK viremia were identified through serum polymerase chain reaction, with a threshold of above 1000 copies/mL for CMV and above 500 copies/mL for BK, all within the first 6 months post-transplantation. Other secondary measures encompassed renal allograft function at the 6-month post-transplant mark, admission for transplant LOS and the total number of readmissions post-transplant during the initial 6 months post-transplant.
Data extraction and study assessments
The following information was extracted from electronic patient records: age, gender, etiology of end-stage renal disease (ESRD), dialysis pre-transplant, donor type, age and gender, donor–recipient's degree of HLA mismatch (HLA MM) and donor/recipient CMV status, transplant date and number, induction type, ESW status, presence of delayed or slowed graft function, relevant medical history [specifically smoking status, hypertension, congestive heart failure (CHF), ischemic heart disease (IHD) , pre-transplant diabetes and pre-transplant immunosuppressive therapy], perioperative complications and the occurrence of early ACR.
The following clinical and biochemical parameters were retrieved in an automated fashion from MDClone: admission for transplant LOS, number of admissions post the transplant admission until 6 months post-transplant, average 1–6 months post-transplant systolic and diastolic blood pressures, weight and body mass index (BMI), average Scr 25–40 days post-transplant (Scr 1 m), average Scr 2.5–3.5 months post-transplant (Scr 3 m) and average Scr 5–7 months post-transplant (Scr 6 m). Average tacrolimus trough blood levels were retrieved 0–3, 0–7 and 0–30 days post-transplant and 1–6 months post-transplant. Additional data retrieved between 3–6 months post-transplant: average hemoglobin (Hb), average absolute lymphocyte count, average absolute neutrophil count, average platelets (PLT) count, average glucose and HbA1C, average uric acid, average albumin and globulin, average C-reactive protein (CRP) and average protein/creatinine ratio. Infections data obtained included: CMV and BK viremia 1–6 months post-transplant, urine and blood culture results 0–1 and 1–6 months post-transplant. Use of the following medications between 1–6 months post-transplant was automatically obtained from MDClone: MPA, mTOR inhibitor, beta blockers (BB), calcium channel blockers (CCB) and renin–angiotensin–aldosterone system (RAAS) inhibitors.
Statistical analysis
All demographic, clinical and biochemical covariates of interest were systematically tabulated and compared. Categorical variables were compared using the Chi-squared test, with Fisher's exact test employed in cases of small cell counts. Continuous variables underwent preliminary normality testing using the Shapiro–Wilk test, alongside assessments for equality of variances. Subsequently, normally distributed variables were compared using t-tests or analysis of variance, while non-normally distributed variables were subjected to non-parametric tests.
For the primary outcome of post-transplant ACR, a logistic multivariable model was employed. Initially, univariate models were evaluated, with variables displaying significance (P < .05) or deemed clinically relevant progressing to multivariate modeling. Statistical analyses were performed using the SPSS software package.
RESULTS
Characteristics of the cohort of non-sensitized KTRs who had a first live-donor kidney transplant and received basiliximab for induction
For the 113 KTRs (Table 1A), mean transplant age was 53 ± 14.5 years, 84 (74.3%) were males and 81 (71.1%) were on renal replacement therapy before the transplant. Ninety (79.6%) had an LURD transplant while 23 (20.4%) had a living related donor (LRD) transplant. Past medical histories of hypertension, diabetes, IHD and CHF were present in 102 (90.3%), 35 (31%), 25 (22.1%) and 6 (5.3%) KTRs, respectively. Mean donor age was 41.2 ± 9.9 years and 75 (67.6%) of the donors were males. Donor/recipient CMV status was positive/positive in 86 (76.1%) and positive/negative in 12 (10.6%) KTRs. Mean admission for transplant LOS was 9.2 ± 3.5 days. Early ACR occurred in 17 (15%) of the cohort with 9 (52.9%) of the ACR occurred within the first post-transplant week. Other clinical characteristics including ESRD etiology, time on pre-transplant dialysis, rate of pre-transplant immunosuppressive therapy, perioperative complications, rate of delayed or slow graft function and of ESW, and number of readmissions post-transplant up to 6 months post-transplant are detailed in Table 1A. For all other biochemical and clinical characteristics including vital signs, Scr and estimated glomerular filtration rate (eGFR) up to 6 months post-transplant and other laboratory results, rate of infections including presence of CMV and BK viremia, UTI and bacteremia up to 6 months post-transplant and medications use, see Table 1B.
Table 1A:
Demographic and clinical characteristics of KTRs who received basiliximab for induction stratified by degree of HLA mismatch.
| Variable | Total cohort (n = 113) | High HLA match group 0–4 MM (n = 61) | Low HLA match group 5–6 MM (n = 52) | P-value |
|---|---|---|---|---|
| RTR characteristics | ||||
| Transplant age, years (mean ± SD) | 53 ± 14.5 | 53.4 ± 15.9 | 52.5 ± 12.8 | .742 |
| Male sex, n (%) | 84 (74.3) | 45 (73.8) | 39 (75.0) | .881 |
| ESRD etiology, n (%) | ||||
| DN | 17 (15.0) | 10 (16.4) | 7 (13.5) | .703 |
| GN | 29 (25.7) | 16 (26.2) | 13 (25.0) | |
| Nephrosclerosis | 14 (12.4) | 7 (11.5) | 7 (13.5) | |
| PCKD | 14 (12.4) | 6 (9.8) | 8 (15.4) | |
| Other | 21 (18.6) | 14 (23.0) | 7 (13.5) | |
| Unknown | 18 (15.9) | 8 (13.1) | 10 (19.2) | |
| Pre-transplant dialysis | 81 (71.7) | 44 (72.1) | 37 (71.2) | .909 |
| Time on dialysis (years) [median (IQR)] | 1.2 (0.5–2.5) | 1.1 (0.4–2.4) | 1.3 (1.3–2.7) | .233 |
| Smoking status | ||||
| Current smokers | 8 (7.1) | 3 (4.9) | 5 (9.6) | .548 |
| Past smokers | 34 (30.1) | 20 (32.8) | 14 (26.9) | |
| No | 71 (62.8) | 38 (62.3) | 33 (63.5) | |
| Medical history, n (%) | ||||
| HTN | 102 (90.3) | 55 (90.2) | 47 (90.4) | .969 |
| Pre-transplant diabetes | 35 (31.0) | 19 (31.1) | 16 (30.8) | .965 |
| IHD | 25 (22.1) | 12 (19.7) | 13 (25.0) | .496 |
| CHF | 6 (5.3) | 2 (3.3) | 4 (7.7) | .411 |
| Pre-transplant immunosuppressive therapy | 21 (18.6) | 12 (19.7) | 9 (17.3) | .747 |
| Donor characteristics | ||||
| Type, n (%) | ||||
| LURD | 90 (79.6) | 38 (62.3) | 52 (100) | <.001** |
| LRD | 23 (20.40) | 23 (37.7) | ||
| Donor age, years (mean ± SD) | 41.2 ± 9.9 | 39.9 ± 9.3 | 42.9 ± 10.5 | .113 |
| Male sex, n (%) | 75 (67.6) | 36 (60.0) | 39 (76.5) | .065 |
| CMV D/R, n (%) | ||||
| Pos/Pos | 86 (76.1) | 46 (75.4) | 40 (76.9) | .634 |
| Pos/Neg | 12 (10.6) | 5 (8.2) | 7 (13.5) | |
| Neg/Pos | 12 (10.6) | 8 (13.1) | 4 (7.7) | |
| Neg/Neg | 3 (2.7) | 2 (3.3) | 1 (1.9) | |
| Peritransplant data | ||||
| Admission for transplant LOS (mean ± SD) | 9.2 ± 3.5 | 9.4 ± 4.5 | 9.0 ± 1.8 | .464 |
| Perioperative complications, n (%) | ||||
| Infectious | 9 (8) | 4 (6.5) | 5 (9.6) | .923 |
| Cardiovascular | 6 (5.3) | 3 (4.9) | 3 (5.8) | |
| Surgical | 7 (6.2) | 5 (8.2) | 2 (3.8) | |
| Obstructive uropathy | 3 (2.7) | 2 (3.3) | 1 (1.9) | |
| Other | 3 (2.7) | 1 (1.6) | 2 (3.8) | |
| Delayed graft function, n (%) | 3 (2.7) | 2 (3.3) | 1 (1.9) | 1 |
| Slow graft function, n (%) | 7 (6.2) | 3 (4.9) | 4 (7.7) | .701 |
| Early ACR, n (%) | 17 (15.0) | 5 (8.2) | 12 (23.1) | .027* |
| Days from transplant to ACR (n = 17), n (%) | ||||
| 2–7 | 9 (52.9) | 2 (40.0) | 7 (59.0) | .677 |
| ≥8 | 8 (47.1) | 3 (60.0) | 5 (41.0 ) | |
| ESW, n (%) | 55 (48.7) | 36 (59.0) | 19 (36.5) | .017* |
| Number of admissions (up to 6 months post-transplant) [median (IQR)] | 0 (0–1) | 0 (0–1.5) | 1 (0–1) | .998 |
*P < .05; **P < .01.
DN, diabetic nephropathy; GN, glomerulonephritis; HTN, hypertension; IQR, interquartile range; PCKD, polycystic kidney disease; RTR, renal transplant recipients; SD, standard deviation/R, donor/recipient; Pos, positive; Neg, negative.
Table 1B:
Biochemical and clinical characteristics of KTRs who received basiliximab for induction stratified by degree of HLA mismatch.
| Variable | Total cohort (n = 113) | High HLA match group 0–4 MM (n = 61) | Low HLA match group 5–6 MM (n = 52) | P-value |
|---|---|---|---|---|
| Vital signs and other clinical parameters, 1–6m, average (mean ± SD) | ||||
| SBP (mmHg) | 130.9 ± 14.8 | 130.1 ± 13.8 | 131.8 ± 16.1 | .543 |
| DBP (mmHg) | 76.6 ± 7.0 | 76.5 ± 6.5 | 76.6 ± 7.5 | .953 |
| Weight (kg) | 77.4 ± 15.2 | 76.7 ± 13.6 | 78.0 ± 16.8 | .66 |
| BMI (kg/m2) | 26.6 ± 4.3 | 26.8 ± 3.3 | 26.4 ± 5.1 | .718 |
| Serum creatinin (mg/dL) and eGFR (CKD-EPI) post-transplanta (mean ± SD) | ||||
| Scr 1 m, average | 1.4 ± 0.5 | 1.3 ± 0.4 | 1.4 ± 0.5 | .681 |
| eGFR 1 m, average | 59.4 ± 17.6 | 59.1 ± 17.3 | 59.7 ± 18.0 | .858 |
| Scr 3 m, average | 1.4 ± 0.4 | 1.3 ± 3.5 | 1.4 ± 0.5 | .53 |
| eGFR 3 m, average | 60.4 ± 17.7 | 60.0 ± 16.0 | 60.8 ± 19.5 | .81 |
| Scr 6 m, average | 1.3 ± 0.4 | 1.3 ± 0.4 | 1.4 ± 0.5 | .622 |
| eGFR 6 m, average | 62.7 ± 17.2 | 62.8 ± 16.0 | 62.6 ± 18.6 | .951 |
| Other laboratory results | ||||
| Tacrolimus level 0–7 days, max (μg/L) (mean ± SD) | 14.5 ± 5.4 | 14.3 ± 5.5 | 14.7 ± 5.4 | .739 |
| Tacrolimus level 0–7 days, min (μg/L) (mean ± SD) | 6.2 ± 2.2 | 6.4 ± 2.6 | 6.0 ± 1.6 | .401 |
| Tacrolimus level 0–7 days, average (μg/L) (mean ± SD) | 9.6 ± 2.7 | 9.5 ± 2.9 | 9.6 ± 2.5 | .852 |
| Tacrolimus level 0–30 days, average (μg/L) (mean ± SD) | 10.8 ± 1.7 | 10.9 ± 1.9 | 10.6 ± 1.3 | .286 |
| Tacrolimus level 1–6 m, average (μg/L) (mean ± SD) | 8.9 ± 1.1 | 8.7 ± 1.2 | 9.2 ± 1.0 | .014* |
| Hb 3–6 m, average (g/dL) (mean ± SD) | 13.0 ± 1.6 | 13.1 ± 1.5 | 12.9 ± 1.7 | .649 |
| Lympho 3–6 m, average (K/μL) (mean ± SD) | 1.5 ± 0.5 | 1.4 ± 0.5 | 1.5 ± 0.6 | .488 |
| Neut 3–6 m, average (K/μL) (mean ± SD) | 4.2 ± 1.4 | 4.2 ± 1.1 | 4.3 ± 1.6 | .634 |
| PLT 3–6 m, average (K/μL) (mean ± SD) | 193.2 ± 58.9 | 190.3 ± 59.1 | 196.3 ± 59.1 | .606 |
| Glucose 3–6 m, average (mg/dL) (mean ± SD) | 121.0 ± 32.6 | 118.7 ± 32.6 | 123.4 ± 32.7 | .463 |
| HbA1C 3–6 m, average (g/dL) (mean ± SD) | 5.9 ± 1.1 | 5.8 ± 0.9 | 6.1 ± 1.1 | .28 |
| Uric acid 3–6 m, average (mg/dL) (mean ± SD) | 6.0 ± 1.4 | 6.0 ± 1.1 | 6.0 ± 1.6 | .828 |
| Albumin 3–6 m, average (g/dL) (mean ± SD) | 4.1 ± 0.3 | 4.1 ± 0.3 | 4.1 ± 0.3 | .479 |
| Globulin 3–6 m, average (g/dL) (mean ± SD) | 2.6 ± 0.3 | 2.6 ± 0.3 | 2.5 ± 0.3 | .193 |
| CRP 3–6 m, average [median (IQR)] | 4.5 (2.2–11.2) | 4.7 (2.4–9.6) | 4.6 (2.1–11.9) | .874 |
| Urine protein/creatinine 3–6 m, average [median (IQR)] | 0.2 (0.1–0.3) | 0.2 (0.1–0.3) | 0.2 (0.1–0.3) | .332 |
| Infections, n (%) | ||||
| CMV positive (>1000 copies/mL) 1–6 m | 1 (0.9) | 1 (1.6) | 0 | 1 |
| BK positive (>500 copies/ml) 1–6 m | 16 (14.2) | 8 (13.1) | 8 (15.4) | .73 |
| Positive urine culture 0–1 m | 14 (12.4) | 8 (13.1) | 6 (11.5) | .8 |
| Positive urine culture 1–6 m | 18 (15.9) | 7 (11.5) | 11 (21.2) | .161 |
| Positive blood culture 0–1 m | 1 (0.9) | 1 (1.6) | 0 | .345 |
| Positive blood culture 1–6 m | 1(0.9) | 1 (1.6) | 0 | 1 |
| Medications, n (%) | ||||
| MPA 1–6 m | 99 (87.6) | 51 (83.3) | 48 (92.3) | .162 |
| mTOR inhibitor 1–6 m | 2 (1.8) | 0 | 2 (3.8) | .21 |
| BB 1–6 m | 68 (60.2) | 35 (57.4) | 33 (63.5) | .51 |
| CCB 1–6 m | 56 (49.6) | 29 (47.5) | 27 (51.9) | .642 |
| RAAS inhibition 1–6 m | 19 (16.8) | 7 (11.5) | 12 (23.1) | .1 |
One-month average, values measured 25–40 days post-transplant, 3-month average, values measured 2.5–3.5 months post-transplant; 6-month average, values measured 5–7 months post-transplant.
eGFR was calculated according to the following CKD-EPI formula: eGFR = 141 * min (Scr/k, 1)α * max(Scr/k, 1) – 1.209 * 0.993Age * 1.018 * 1.159 (if Black), where Scr, standardized serum creatinine; k = 0.7 if female, 0.9 if male; α = –0.329 if female, –0.411 if male; min = the minimum of Scr/k of 1; max = the maximum of Scr/k or 1.
* P < .05.
m, month; BB, beta blockers; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; DBP, diastolic blood pressure; IQR, interquartile range; Lympho, lymphocytes; Neut, neutrophils; PLT, platelets; SBP, systolic blood pressure; SD, standard deviation.
Univariate comparison of non-sensitized KTRs who had a first live-donor kidney transplant and received basiliximab for induction with high HLA match (0–4 MM) vs those with low HLA match (5–6 MM)
The KTRs cohort was divided into two groups, 61 (54%) with high HLA match (0–4 MM) and 52 (46%) with low HLA match (5–6 MM). In the high HLA match group 23 (37.7%) had an LRD transplant and 38 (62.3%) had an LURD transplant, while in the low HLA match group all had an LURD transplant (P < .001). A higher rate of donor males was present in the low compared with the high HLA match group [39 (76.5%) and 36 (60%), respectively, P = .065]. Rate of early ACR was significantly higher in the high compared with the low HLA match group [12 (23.1%) and 5 (8.2%), respectively, P = .027]. ESW was more prevalent in the low as opposed to the high HLA match group (59% vs 36.5%, P = .017). All other comparisons of characteristics are shown in Table 1A.
Mean tacrolimus trough blood level at 1–6 months post-transplant was significantly higher in the low compared with the high HLA match group (9.2 ± 1.0 vs 8.7 ± 1.2 μg/L, P = .014), while mean tacrolimus trough blood levels were not significantly different between the groups at 0–7 days and 0–30 days post-transplant. For all other variables which were not significantly different between the two groups including CMV and BK viremia from 1–6 months post-transplant, UTI and bacteremia rate from 0–1 months and from 1–6 months post-transplant, see Table 1B.
Characteristics of the cohort of a non-sensitized KTRs who had a first live-donor kidney transplant with low HLA match (5–6 MM)
For the 96 KTRs (Table 2A), mean transplant age was 52.7 ± 12.5 years, 69 (71.9%) were males and 74 (77.1%) were on renal replacement therapy before the transplant. Past medical histories of hypertension, diabetes, IHD and CHF were present in 87 (90.6%), 29 (30.2%), 24 (25.0%) and 5 (5.2%) KTRs, respectively. Mean donor age was 42.9 ± 9.5 years and 67 (70.5%) of the donors were males. Donor/recipient CMV status was positive/positive in 72 (75.0%) and positive/negative in 15 (15.6%) KTRs. Mean admission for transplant LOS was 9.3 ± 2.8 days. Early ACR occurred in 16 (16.7%) of the cohort with 9 (56.3%) of the ACR occurring within the first post-transplant week. Other clinical characteristics are detailed in Table 2A. For all other biochemical and clinical characteristics including vital signs, Scr and eGFR up to 6 months post-transplant and other laboratory results, rate of infections including presence of CMV and BK viremia, UTI and bacteremia up to 6 months post-transplant and medications use, see Table 2B.
Table 2A:
Demographic and clinical characteristics of low HLA match (5–6 MM) KTRs who received basiliximab versus combined ATG–basiliximab for induction.
| Variable | Total cohort (n = 96) | Basiliximab (n = 52) | ATG–basilixiamb (n = 44) | P-value |
|---|---|---|---|---|
| RTR characteristics | ||||
| Transplant age, years (mean ± SD) | 52.7 ± 12.5 | 52.5 ± 12.8 | 53.0 ± 12.2 | .84 |
| Male sex, n (%) | 69 (71.9) | 39 (75.0) | 30 (68.2) | .459 |
| ESRD etiology, n (%) | ||||
| DN | 15 (15.6) | 7 (13.5) | 8 (18.2) | .267 |
| GN | 22 (22.9) | 13 (25.0) | 9 (20.5) | |
| Nephrosclerosis | 8 (8.3) | 7 (13.5) | 1 (2.3) | |
| PCKD | 18 (18.8) | 8 (15.4) | 10 (22.7) | |
| Other | 17 (17.7) | 7 (13.5) | 10 (22.7) | |
| Unknown | 16 (16.7) | 10 (19.2) | 6 (13.6) | |
| Pre-transplant dialysis, n (%) | 74 (77.1) | 37 (71.2) | 55 (84.1) | .133 |
| Time on dialysis (years) [median (IQR)] | 1.5 (0.6–3.1) | 1.3 (1.3–2.7) | 1.8 (0.7–3.3) | .675 |
| Smoking status, n (%) | ||||
| Current smokers | 10 (10.4) | 5 (9.6) | 5 (11.4) | .949 |
| Past smokers | 25 (26.0) | 14 (26.9) | 11 (25.0) | |
| No | 61 (63.5) | 33 (63.5) | 28 (63.6) | |
| Medical history, n (%) | ||||
| Hypertension | 87 (90.6) | 47 (90.4) | 40 (90.9) | 1 |
| Pre-transplant diabetes | 29 (30.2) | 16 (30.8) | 13 (29.5) | .896 |
| IHD | 24 (25.0) | 13 (25.0) | 11 (25.0) | 1 |
| CHF | 5 (5.2) | 4 (7.7) | 1 (2.3) | .371 |
| Pre-transplant immunosuppressive therapy | 14 (14.6) | 9 (17.3) | 5 (11.4) | .411 |
| Donor characteristics | ||||
| Donation age, years (mean ± SD) | 42.9 ± 9.5 | 42.9 ± 10.5 | 42.9 ± 8.3 | .979 |
| Male sex, n (%) | 67 (70.5) | 39 (76.5) | 28 (63.6) | .171 |
| CMV D/R, n (%) | ||||
| Pos/Pos | 72 (75.0) | 40 (76.9) | 32 (72.7) | .73 |
| Pos/Neg | 15 (15.6) | 7 (13.5) | 8 (18.2) | |
| Neg/Pos | 8 (8.3) | 4 (7.7) | 4 (9.1) | |
| Neg/Neg | 1 (1.0) | 1 (1.9) | 0 | |
| Peritransplant data | ||||
| Admission for transplant LOS (mean ± SD) | 9.3 ± 2.8 | 9.0 ± 1.8 | 9.6 ± 3.6 | .278 |
| Perioperative complications, n (%) | ||||
| Infectious | 11 (11.45) | 5 (9.6) | 6 (13.6) | .935 |
| Cardiovascular | 6 (6.25) | 3 (5.8) | 3 (6.8) | |
| Surgical | 5 (5.2) | 2 (3.8) | 3 (6.8) | |
| Obstructive uropathy | 1 (1.04) | 1 (1.9) | 0 | |
| Other | 4 (4.2) | 2 (3.8) | 2 (4.5) | |
| Delayed graft function, n (%) | 5 (5.3) | 1 (1.9) | 4 (9.1) | .176 |
| Slow graft function, n (%) | 12 (12.5) | 4 (7.7) | 8 (18.2) | .122 |
| Early ACR, n (%) | 16 (16.7) | 12 (23.1) | 4 (9.1) | .067 |
| Days from transplant to ACR (n = 17), n (%) | ||||
| 2–7 | 9 (56.3) | 7 (59.0) | 2 (50.0) | .464 |
| ≥8 | 7 (43.7) | 5 (41.0 ) | 2 (50.0) | |
| ESW | 44 (45.8) | 19 (36.5) | 25 (56.8) | .047* |
| Number of admissions (up to 6 months post-transplant) | 0 (0–1) | 1 (0–1) | 0 (0–1) | .218 |
P < .05.
DN, diabetic nephropathy; GN, glomerulonephritis; IQR, interquartile range; PCKD, polycystic kidney disease; RTR, renal transplant recipients; SD, standard deviation; D, donor; R, recipient; Pos, positive; Neg, negative.
Table 2B:
Biochemical and clinical characteristics of low HLA match (5–6 MM) KTRs who received basiliximab versus combined ATG–basiliximab for induction.
| Variable | Total cohort (n = 96) | Basiliximab (n = 52) | ATG–basilixiamb (n = 44) | P-value |
|---|---|---|---|---|
| Vital signs and other clinical parameters, 1–6 m average (mean ± SD) | ||||
| SBP (mmHg) | 132.4 ± 14.6 | 131.8 ± 16.1 | 133.1 ± 12.6 | .673 |
| DBP (mmHg) | 78.0 ± 7.6 | 76.6 ± 7.5 | 79.8 ± 7.5 | .048 |
| Weight (kg) | 78.2 ± 15.7 | 78.0 ± 16.8 | 78.4 ± 14.5 | .913 |
| BMI (kg/m2) | 26.4 ± 4.6 | 26.4 ± 5.1 | 26.4 ± 3.8 | .992 |
| Serum creatinine (mg/dL) and eGFR (CKD-EPI) post-transplant (mean ± SD) | ||||
| Scr 1 m, average | 1.4 ± 0.5 | 1.4 ± 0.5 | 1.3 ± 0.3 | .544 |
| eGFR 1 m, average | 59.6 ± 17.3 | 59.7 ± 18.0 | 59.6 ± 16.6 | .973 |
| Scr 3 m, average | 1.3 ± 0.4 | 1.4 ± 0.5 | 1.3 ± 0.3 | .127 |
| eGFR 3 m, average | 61.4 ± 17.6 | 60.8 ± 19.5 | 62.1 ± 14.7 | .73 |
| Scr 6 m, average | 1.3 ± 0.3 | 1.4 ± 0.5 | 1.2 ± 0.3 | .153 |
| eGFR 6 m, average | 63.6 ± 17.5 | 62.6 ± 18.6 | 64.9 ± 16.2 | .508 |
| Other laboratory results | ||||
| Tacrolimus level 0–3 days, max (μg/L) (mean ± SD) | 11.7 ± 5.7 | 13.0 ± 6.2 | 9.9 ± 4.5 | .02* |
| Tacrolimus level 0–3 days, min (μg/L) (mean ± SD) | 8.1 ± 4.0 | 8.9 ± 4.5 | 7.0 ± 2.9 | .185 |
| Tacrolimus level 0–3 days, average (μg/L) (mean ± SD) | 9.8 ± 4.5 | 10.9 ± 5.0 | 8.4 ± 3.5 | .006** |
| Tacrolimus level 0–7 days, max (μg/L) (mean ± SD) | 13.5 ± 4.9 | 14.7 ± 5.4 | 11.8 ± 3.8 | .005** |
| Tacrolimus level 0–7 days, min (μg/L) (mean ± SD) | 5.8 ± 1.7 | 6.0 ± 1.6 | 5.5 ± 1.9 | .348 |
| Tacrolimus level 0–7 days, average (μg/L) (mean ± SD) | 9.0 ± 2.4 | 9.6 ± 2.5 | 8.2 ± 2.1 | .006** |
| Tacrolimus level 0–30 days, average (μg/L) (mean ± SD) | 10.2 ± 1.5 | 10.6 ± 1.3 | 9.8 ± 1.5 | .015** |
| Tacrolimus level 1–6 m, average (μg/L) (mean ± SD) | 9.3 ± 1.6 | 9.2 ± 1.0 | 9.5 ± 2.1 | .34 |
| Hb 3–6m, average (g/dL) (mean ± SD) | 12.9 ± 1.6 | 12.9 ± 1.7 | 12.8 ± 1.5 | .71 |
| Lympho 3–6m, average (K/μL) (mean ± SD) | 1.4 ± 0.5 | 1.5 ± 0.6 | 1.3 ± 0.4 | .07 |
| Neut 3–6 m, average (K/μL) (mean ± SD) | 4.3 ± 1.7 | 4.3 ± 1.6 | 4.2 ± 1.8 | .754 |
| PLT 3–6 m, average (K/μL) (mean ± SD) | 201.8 ± 58.0 | 196.3 ± 59.1 | 210.2 ± 56.1 | .277 |
| Glucose 3–6 m, average (mg/dL) (mean ± SD) | 118.4 ± 29.4 | 123.4 ± 32.7 | 110.8 ± 21.8 | .053 |
| HbA1C 3–6 m, average (g/dL) (mean ± SD) | 5.9 ± 1.2 | 6.1 ± 1.1 | 5.7 ± 1.1 | .19 |
| Uric acid 3–6 m, average (mg/dL) (mean ± SD) | 6.1 ± 1.5 | 6.0 ± 1.6 | 6.3 ± 1.3 | .283 |
| Albumin 3–6 m, average (g/dL) (mean ± SD) | 4.2 ± 0.3 | 4.1 ± 0.3 | 4.2 ± 0.2 | .752 |
| Globulin 3–6 m, average (g/dL) (mean ± SD) | 2.5 ± 0.3 | 2.5 ± 0.3 | 2.5 ± 0.3 | .682 |
| CRP 3–6m, average (mg/L) [median (IQR)] | 4.3 (1.9–12.0) | 4.6 (2.1–11.9) | 4.2 (1.6–12.3) | .301 |
| Urine protein/creatinine 3–6 m, average [median (IQR)] | 0.2 (0.1–0.3) | 0.2 (0.1–0.3) | 0.1 (0.1–0.3) | .659 |
| Infections, n (%) | ||||
| CMV positive (>1000 copies/mL) 1–6 m | 2 (2.1) | 0 | 2 (4.5) | .207 |
| BK positive (>500 copies/mL) 1–6 m | 13 (13.5) | 8 (15.4) | 5 (11.4) | .566 |
| Positive urine culture 0–1 m | 12 (12.5) | 6 (11.5) | 6 (13.6) | .757 |
| Positive urine culture 1–6 m | 17 (17.7) | 11 (21.2) | 6 (13.6) | .336 |
| Positive blood culture 0–1 m | 2 (2.1) | 0 | 2 (4.5) | .207 |
| Positive blood culture 1–6 m | 0 | 0 | 0 | |
| Medications, n (%) | ||||
| MPA 1–6 m | 90 (93.8) | 51 (98.1) | 39 (88.6) | .09 |
| mTOR inhibitor 1–6 m | 3 (3.1) | 2 (3.8) | 1 (2.3) | 1 |
| BB 1–6 m | 58 (60.4) | 33 (63.5) | 25 (56.8) | .507 |
| CCB 1–6 m | 47 (49.0) | 27 (51.9) | 20 (45.5) | .528 |
| RAAS inhibition 1–6 m | 15 (15.6) | 12 (23.1) | 3 (6.8) | .029* |
One-month average, values measured 25–40 days post-transplant, 3-month average, values measured 2.5–3.5 months post-transplant; 6-month average, values measured 5–7 months post-transplant.
eGFR was calculated according to the following CKD-EPI formula: eGFR = 141 * min (Scr/k, 1)α * max(Scr/k, 1) – 1.209 * 0.993Age * 1.018 * 1.159 (if Black), where Scr, standardized serum creatinine; k = 0.7 if female, 0.9 if male; α = –0.329 if female, –0.411 if male; min = the minimum of Scr/k of 1; max = the maximum of Scr/k or 1.
*P < .05; **P < .01.
m, month; BB, beta blockers; BMI, body mass index; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; DBP, diastolic blood pressure; IQR, interquartile range; Lympho, lymphocytes; MPA, mycophenolic acid; mTOR, mammalian target of rapamycin; Neut, neutrophils; PLT, platelets; SBP, systolic blood pressure; SD, standard deviation.
Univariate comparison of non-sensitized KTRs who had a first live-donor kidney transplant with low HLA match (5–6 MM) who received basiliximab vs combined ATG–basiliximab for induction
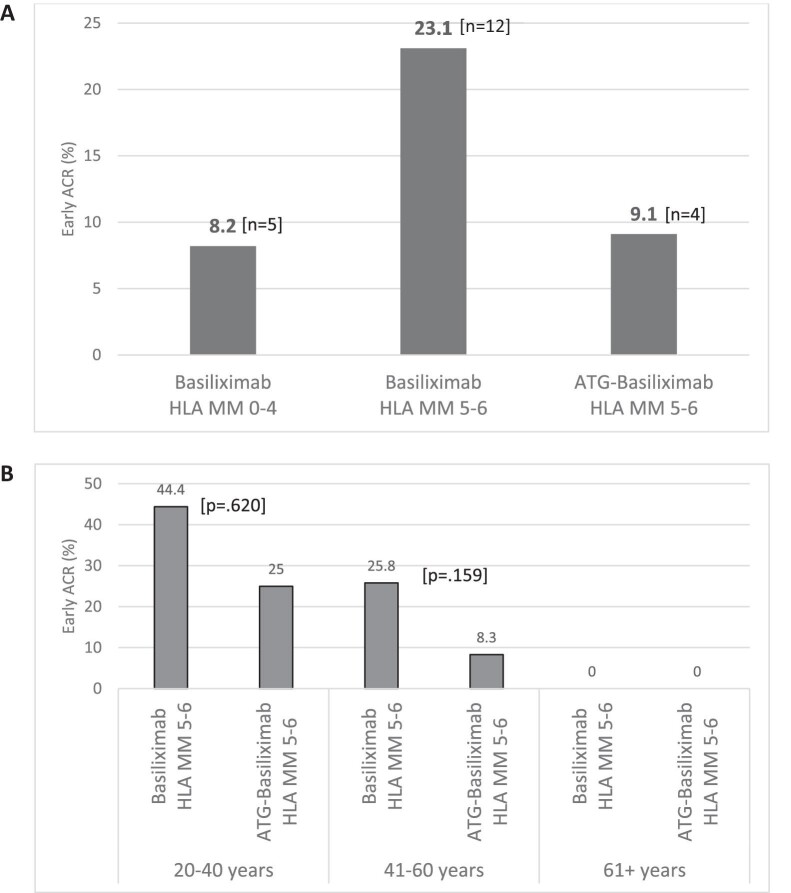
The KTRs cohort was divided into two groups, 52 (54.2%) who received basiliximab and 44 (45.8%) who were treated with combined ATG–basiliximab for induction. Early ACR rate dramatically reduced upon transition to ATG–basiliximab regimen from 12 (23.1%) to 4 (9.1%), P = .067 (Fig. 2A). ESW was more prevalent in the combined ATG–basiliximab as opposed to the basiliximab group (56.8% vs 36.5%, P = .047). All other comparisons of characteristics are shown in Table 2A.
Figure 2:
(A) Early ACR rates in non-sensitized KTRs with first kidney transplants, contrasting high HLA match KTRs with basiliximab and low HLA matches KTRs with basiliximab and ATG–basiliximab induction. (B) Early ACR rates in low HLA match KTRs receiving basiliximab alone vs ATG–basiliximab induction across age categories.
Mean and maximum tacrolimus trough blood levels at 0–3 and 0–7 days post-transplant and mean tacrolimus trough blood level at 0–30 days post-transplant were significantly higher in the basiliximab versus combined ATG–basiliximab group. Use of RAAS inhibition medications was more prevalent in the basiliximab as opposed to the ATG–basiliximab group. For all other variables that were not significantly different between the two groups including CMV and BK viremia from 1–6 months post-transplant, UTI and bacteremia rate from 0–1 months and from 1–6 months post-transplant, see Table 2B.
Logistic regression analysis for early post-transplant ACR in KTRs with low HLA match (5–6 MM)
In a multivariable logistic regression analysis, the odds for early ACR were 8% lower for every 1-year increase in age [odds ratio 0.92, 95% confidence interval (CI) 0.87–0.97, P = .002]. Induction therapy was not found to be an independent predictor for early ACR (Table 3A).
Table 3A:
Multivariate logistic regression analysis for early post-transplant ACR in KTRs with low HLA match (5–6 MM) (n = 96).
| Effect | Odds ratio (95% CI) | P-value |
|---|---|---|
| Age at transplant, per 1 year increase | 0.92 (0.87–0.97) | .002** |
| Male vs female | 3.24 (0.65–16.1) | .151 |
| Combined ATG–basiliximab vs basiliximab induction | 0.30 (0.08–1.11) | .07 |
**P < .01.
Subgroup analysis of early post-transplant ACR in KTRs with low HLA match (5–6 MM) across various age groups
The low HLA group was stratified into three distinct age subgroups: 20–40 years (n = 17), 41–60 years (n = 55) and >60 years of age (n = 24). The rejection rate demonstrated a notable decline from 44.4% to 25% in the 20–40 years age subgroup and from 25.8% to 8.3% in the 41–60 years age subgroup. However, the P-values did not reach statistical significance, likely due to the limited number of recipients in each subgroup. No rejections were observed in the >60 years subgroup (Fig. 2B and Table 3B).
Table 3B:
Univariate analysis for early ACR rate in low HLA match (5–6 MM) group divided into age subgroups.
| Age subgroups | All low HLA match with early ACR, n (%) | Low HLA match with basiliximab early ACR, n (%) | Low HLA match with ATG–basilixiamb early ACR, n (%) | P-value |
|---|---|---|---|---|
| 20–40 years (n = 17) | 6 (35.3) | 4 (44.4) | 2 (25.0) | .62 |
| 41–60 years (n = 55) | 10 (18.2) | 8 (25.8) | 2 (8.3) | .159 |
| >60 years (n = 24) | 0 | 0 | 0 |
DISCUSSION
Tailoring immunosuppressive therapy to match the immunological risk of transplant recipients poses a significant challenge. In addressing this issue, we have introduced a distinctive induction regimen that combines two well-established agents, ATG and basiliximab. This approach was specifically designed for a population of KTRs who were non-sensitized, had undergone their first kidney transplant and exhibited a low HLA match (5–6 MM).
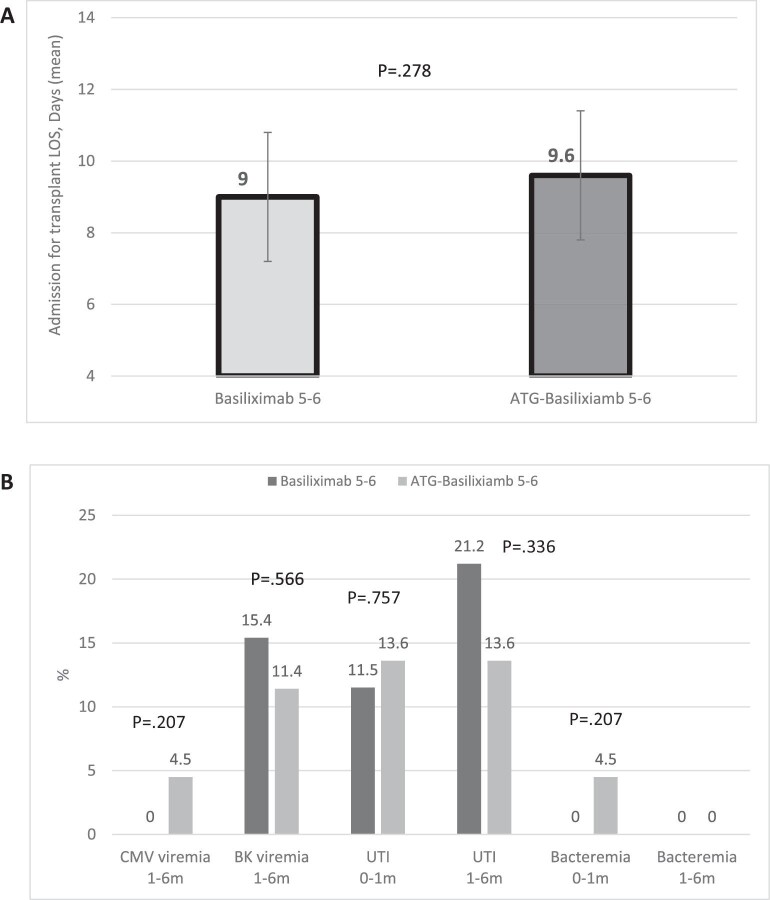
Our findings reveal a noteworthy reduction in early ACR after changing from basiliximab to the combined ATG–basiliximab induction regimen (Fig. 2A). Although age emerged as a predominant predictor for rejection, limiting our ability to demonstrate the significance of induction therapy in a multivariable analysis, an examination of various age subgroups unveiled an even more substantial reduction in rejection rates within each group (Fig. 2B). Crucially, our study did not observe any uptick of transplant admission LOS (Fig. 3A) or perioperative complications, encompassing delayed or slow graft function, infectious complications, cardiovascular issues or any other complications. Furthermore, no increase in the incidence of post-transplant infections (Fig. 3B), or number of admissions post the transplant admission up to 6 months post-transplant was observed. Renal allograft function within the initial 6 months post-transplant demonstrated no discernible differences. Our results robustly advocate for the use of the combined ATG–basiliximab regimen for induction in this specific KTRs population, highlighting its potential safety in other KTRs.
Figure 3:
(A) Admission for transplant LOS in low HLA match KTRs with basiliximab vs ATG–basiliximab induction. (B) Infections rate up to 6 months post-transplant in low HLA match KTRs with basiliximab vs ATG–basiliximab induction.
Mitigating the incidence of acute rejection is crucial for enhancing both short- and long-term outcomes. Acute rejection is associated with worsening renal allograft function, and an augmented risk of subsequent acute and chronic rejection, thereby exacerbating long-term allograft dysfunction [16–20]. Furthermore, the potent immunosuppressive agents administered to address acute rejection are linked to an elevated short-term risk of infections [6] and a heightened long-term risk of malignancies [7, 8]. We observed an impressive reduction in rejection rate (from 23.1% to 9.1%) when switching induction therapy from basiliximab to dual ATG–basiliximab. Notably, this 9.1% rejection rate is comparable to the 8.2% rejection rate observed in the high HLA match group that received basiliximab (Fig. 2A). The diminished rejection rate was attained despite the notably lower tacrolimus 12-h trough levels observed in the combined ATG–basiliximab group during the initial 3 days, 1 week and 1 month following transplantation (Table 2B). Additionally, ESW was more frequently encountered in this group, with a prevalence of 56.8% compared with 36.5% in the basiliximab group (P = .047) (Table 2A). Furthermore, among the age subgroups of 20–40 and 41–60 years, the decline in rejection rate was even higher (from 44.4% to 25% and from 25.8% to 8.3%, respectively) (Table 3B).
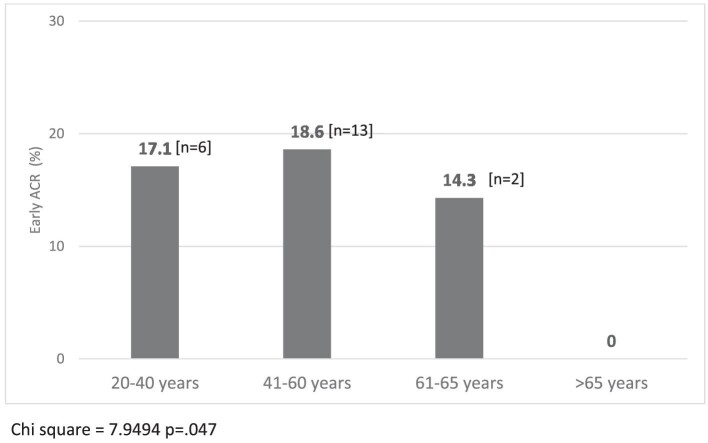
Studies exploring the association between age and rejection have revealed nuanced patterns that underscore the impact of age on graft outcomes. Younger recipients often face an increased risk of acute rejection following renal transplantation which may be attributed to a more robust immune system and increased immunogenicity, predisposing them to a more vigorous rejection response. Conversely, while older age is generally associated with a less reactive immune system, comorbidities commonly associated with aging can complicate the post-transplant course [21–23]. Our findings reveal that recipient age independently predicts rejection, with the odds of rejection decreasing by 8% for every additional year of age in a multivariate analysis (P = .002, Table 3A). Within our total cohort of 157 non-sensitized KTRs undergoing their first live-donor kidney transplant, the rejection rates were 17.1%, 18.6%, 14.3% and 0% for age subgroups 20–40, 41–60, 61–65 and >65 years, respectively (P = .047) (Fig. 4). In the subset of 96 KTRs with low HLA match (5–6 MM), rejection rates were 35.3%, 18.2% and 0% for age subgroups 20–40, 41–60 and >60 years, respectively (P = .01, Table 3B). Optimization of immunosuppressive approaches based on age-related factors, such as immune senescence or the heightened immune activity observed in younger recipients, should be further explored aimed at optimizing graft outcomes across diverse age groups.
Figure 4:
Early ACR rates in non-sensitized KTRs with first kidney transplants across age categories.
Several studies have demonstrated the superior efficacy of thymoglobulin compared with interleukin-2 receptor antibodies in kidney transplant outcomes [3] revealing a lower incidence of acute rejection at 1 year post-transplant [2, 24] and a significant reduction in delayed graft function [4]. On the other hand, some studies in low immunological risk recipients have found both induction therapies to be equally effective in terms of graft and patient survival, as well as preventing acute rejection. However, ATG is associated with a higher incidence of adverse events, including CMV infection, leukopenia and thrombocytopenia [6, 25].
The optimal ATG dose remains a topic of debate, with changes observed over time. Notably, an increased ATG dose has been linked to a higher rate of complications [26]. A single high ATG dose of 9 mg/kg has been associated with significant hemodynamic and pulmonary side effects during drug infusion [4], while ATG doses lower than 5 mg/kg have been associated with a heightened risk of rejection [27]. Currently, the trend is towards shorter treatment durations (3–5 days) and lower doses (4.5–6 mg/kg) compared with older regimens which used up to 10 mg/kg for thymoglobulin [28]. In this context, a single thymoglobulin dose of 1.5 mg/kg in live-donor renal transplants has shown favorable outcomes without increasing the risk of infections or malignancies [29].
Against this backdrop, the implementation of a dual induction regimen, combining ATG and basiliximab, particularly with a single 1.5 mg/kg dose of ATG, emerges as a promising approach. This dual action targets different pathways in the immune response, potentially providing more comprehensive suppression of rejection-related immune activity, resulting in enhanced immunosuppressive efficacy, reduced rejection risk, and improved long-term graft function. Importantly, using a single 1.5 mg/kg dose of ATG in this dual induction regimen may help mitigate adverse events associated with higher doses, including infection and cytokine release syndrome, contributing to a more favorable safety profile. Additionally, from a cost-effectiveness standpoint, the dual induction regimen with a single 1.5 mg/kg ATG dose appears to strike a balance compared with other intensive induction strategies.
Our study acknowledges several limitations. While our unique induction regimen is designed for non-sensitized individuals with a low HLA match (5–6 MM) in their first kidney transplant, generalizing findings to a diverse transplant population may be limited. Uncertainties arise from the lack of consensus on the optimal ATG dose, considering variations in dosing strategies and changes over time. The study's focus on short-term outcomes within the initial 6 months post-transplant provides valuable insights, but an extended follow-up is crucial for a comprehensive evaluation. The lack of histological confirmation for some of the diagnosed rejections, retrospective design and a relatively small sample size are additional limitations. Despite these constraints, our study highlights the potential benefits of the dual low ATG dose–basiliximab induction regimen, emphasizing the need for further research and personalized induction strategies for diverse transplant cohorts.
Representing a pioneering use of the dual low ATG dose–basiliximab induction regimen, our research demonstrates its efficacy in lowering acute rejection rates without an increase in perioperative or infectious complications. In conclusion, our findings underscore the potential advantages of this induction regimen for non-sensitized KTRs undergoing their initial transplant with a l HLA match. The observed reduction in early acute rejection rates and the favorable safety profile provide encouraging evidence for the efficacy of this innovative induction approach.
Contributor Information
Tammy Hod, Renal Transplant Center, Sheba Medical Center, Tel Hashomer, Israel; Faculty of Medicine, Tel-Aviv University, Tel Aviv, Israel.
Shmuel Levinger, Faculty of Medicine, Tel-Aviv University, Tel Aviv, Israel.
Enosh Askenasy, Renal Transplant Center, Sheba Medical Center, Tel Hashomer, Israel; Faculty of Medicine, Tel-Aviv University, Tel Aviv, Israel.
Maya Siman-Tov, Department of Emergency and Disaster Management, School of Public Health, Faculty of Medicine, Tel-Aviv University, Tel Aviv, Israel.
Yana Davidov, Faculty of Medicine, Tel-Aviv University, Tel Aviv, Israel; Liver Disease Center, Sheba Medical Center, Tel Hashomer, Israel.
Ronen Ghinea, Renal Transplant Center, Sheba Medical Center, Tel Hashomer, Israel; Faculty of Medicine, Tel-Aviv University, Tel Aviv, Israel; Department of Surgery B, Sheba Medical Center, Tel Hashomer, Israel.
Niv Pencovich, Renal Transplant Center, Sheba Medical Center, Tel Hashomer, Israel; Faculty of Medicine, Tel-Aviv University, Tel Aviv, Israel; Department of Surgery B, Sheba Medical Center, Tel Hashomer, Israel.
Ido Nachmani, Faculty of Medicine, Tel-Aviv University, Tel Aviv, Israel; Department of Surgery B, Sheba Medical Center, Tel Hashomer, Israel.
Eytan Mor, Renal Transplant Center, Sheba Medical Center, Tel Hashomer, Israel; Faculty of Medicine, Tel-Aviv University, Tel Aviv, Israel; Department of Surgery B, Sheba Medical Center, Tel Hashomer, Israel.
FUNDING
There were no funding sources.
AUTHORS’ CONTRIBUTIONS
T.H.: conception and design, data acquisition, data interpretation, writing, revising; S.L.: data acquisition; E.A.: data acquisition; M.S.-T.: statistical analysis; Y.D.: data interpretation; R.G.: data acquisition; N.P.: data interpretation, revising; I.N.: conception and design; E.M.: data interpretation, revising.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
REFERENCES
- 1. Hardinger KL, Brennan DC, Klein CL. Selection of induction therapy in kidney transplantation. Transpl Int 2013;26:662–72. 10.1111/tri.12043 [DOI] [PubMed] [Google Scholar]
- 2. Brennan DC, Daller JA, Lake KD et al. ; Thymoglobulin Induction Study Group . Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med 2006;355:1967–77. 10.1056/NEJMoa060068 [DOI] [PubMed] [Google Scholar]
- 3. Willoughby LM, Schnitzler MA, Brennan DC et al. Early outcomes of thymoglobulin and basiliximab induction in kidney transplantation: application of statistical approaches to reduce bias in observational comparisons. Transplantation 2009;87:1520–9. 10.1097/TP.0b013e3181a484d7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kyllonen LE, Eklund BH, Pesonen EJ et al. Single bolus antithymocyte globulin versus basiliximab induction in kidney transplantation with cyclosporine triple immunosuppression: efficacy and safety. Transplantation 2007;84:75–82. 10.1097/01.tp.0000268084.64888.f3 [DOI] [PubMed] [Google Scholar]
- 5. Brennan DC, Schnitzler MA. Long-term results of rabbit antithymocyte globulin and basiliximab induction. N Engl J Med 2008;359:1736–8. 10.1056/NEJMc0805714 [DOI] [PubMed] [Google Scholar]
- 6. Mourad G, Rostaing L, Legendre C et al. Sequential protocols using basiliximab versus antithymocyte globulins in renal-transplant patients receiving mycophenolate mofetil and steroids. Transplantation 2004;78:584–90. 10.1097/01.TP.0000129812.68794.CC [DOI] [PubMed] [Google Scholar]
- 7. Bustami RT, Ojo AO, Wolfe RA et al. Immunosuppression and the risk of post-transplant malignancy among cadaveric first kidney transplant recipients. Am J Transplant 2004;4:87–93. 10.1046/j.1600-6135.2003.00274.x [DOI] [PubMed] [Google Scholar]
- 8. Cherikh WS, Kauffman HM, McBride MA et al. Association of the type of induction immunosuppression with posttransplant lymphoproliferative disorder, graft survival, and patient survival after primary kidney transplantation. Transplantation 2003;76:1289–93. 10.1097/01.TP.0000100826.58738.2B [DOI] [PubMed] [Google Scholar]
- 9. Scott N, Bendavid A, Davidov Y et al. The Clinical manifestation of immunosuppressive therapy as a tool to improve immune monitoring in renal transplant recipients. Kidney Blood Press Res 2023;48:445–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bestard O, Thaunat O, Bellini MI et al. Alloimmune risk stratification for kidney transplant rejection. Transpl Int 2022;35:10138. 10.3389/ti.2022.10138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Postalcioglu M, Kaze AD, Byun BC et al. Association of cold ischemia time with acute renal transplant rejection. Transplantation 2018;102:1188–94. 10.1097/TP.0000000000002106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karatas M, Okut G, Simsek C et al. Outcomes of 6 human leukocyte antigen-mismatched living donor kidney transplant: a study with biopsy amendment. Exp Clin Transplant 2022;20:149–52. 10.6002/ect.MESOT2021.P73 [DOI] [PubMed] [Google Scholar]
- 13. Wiebe C, Nickerson PW. Role of HLA molecular mismatch in clinical practice. Hum Immunol 2022;83:219–24. 10.1016/j.humimm.2021.11.005 [DOI] [PubMed] [Google Scholar]
- 14. Vital A, Siman-Tov M, Shlomai G et al. Assessing health-related quality of life in non-directed versus directed kidney donors: implications for the promotion of non-directed donation. Transpl Int 2024;37:12417. 10.3389/ti.2024.12417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hod T, Ben-David A, Olmer L et al. BNT162b2 third booster dose significantly increases the humoral response assessed by both RBD IgG and neutralizing antibodies in renal transplant recipients. Transpl Int 2022;35:10239. 10.3389/ti.2022.10239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El-Zoghby ZM, Stegall MD, Lager DJ et al. Identifying specific causes of kidney allograft loss. Am J Transplant 2009;9:527–35. 10.1111/j.1600-6143.2008.02519.x [DOI] [PubMed] [Google Scholar]
- 17. Bouatou Y, Viglietti D, Pievani D et al. Response to treatment and long-term outcomes in kidney transplant recipients with acute T cell-mediated rejection. Am J Transplant 2019;19:1972–88. 10.1111/ajt.15299 [DOI] [PubMed] [Google Scholar]
- 18. Mehta R, Bhusal S, Randhawa P et al. Short-term adverse effects of early subclinical allograft inflammation in kidney transplant recipients with a rapid steroid withdrawal protocol. Am J Transplant 2018;18:1710–7. 10.1111/ajt.14627 [DOI] [PubMed] [Google Scholar]
- 19. Hoffman W, Mehta R, Jorgensen DR et al. The impact of early clinical and subclinical T cell-mediated rejection after kidney transplantation. Transplantation 2019;103:1457–67. 10.1097/TP.0000000000002560 [DOI] [PubMed] [Google Scholar]
- 20. Nankivell BJ, Agrawal N, Sharma A et al. The clinical and pathological significance of borderline T cell-mediated rejection. Am J Transplant 2019;19:1452–63. 10.1111/ajt.15197 [DOI] [PubMed] [Google Scholar]
- 21. Long JJ, Motter JD, Jackson KR et al. Characterizing the risk of human leukocyte antigen-incompatible living donor kidney transplantation in older recipients. Am J Transplant 2023;23:1980–9. 10.1016/j.ajt.2023.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jo SJ, Park JB, Lee KW. Prediction of very early subclinical rejection with machine learning in kidney transplantation. Sci Rep 2023;13:22387. 10.1038/s41598-023-50066-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hod T, Goldfarb-Rumyantzev AS. Clinical issues in renal transplantation in the elderly. Clin Transplant 2015;29:167–75. 10.1111/ctr.12481 [DOI] [PubMed] [Google Scholar]
- 24. Patlolla V, Zhong X, Reed GW et al. Efficacy of anti-IL-2 receptor antibodies compared to no induction and to antilymphocyte antibodies in renal transplantation. Am J Transplant 2007;7:1832–41. 10.1111/j.1600-6143.2007.01860.x [DOI] [PubMed] [Google Scholar]
- 25. Lebranchu Y, Bridoux F, Buchler M et al. Immunoprophylaxis with basiliximab compared with antithymocyte globulin in renal transplant patients receiving MMF-containing triple therapy. Am J Transplant 2002;2:48–56. 10.1034/j.1600-6143.2002.020109.x [DOI] [PubMed] [Google Scholar]
- 26. Nafar M, Dalili N, Poor-Reza-Gholi F et al. The appropriate dose of thymoglobulin induction therapy in kidney transplantation. Clin Transplant 2017;31. 10.1111/ctr.12977 [DOI] [PubMed] [Google Scholar]
- 27. Mehta RB, Shimko K, Zhang X et al. Rabbit antithymocyte globulin dose and early subclinical and clinical rejections in kidney transplantation. Clin Transplant 2022;36:e14582. 10.1111/ctr.14582 [DOI] [PubMed] [Google Scholar]
- 28. Ducloux D, Bamoulid J, Daguindau E et al. Antithymocytes globulins: time to revisit its use in kidney transplantation? Int Rev Immunol 2018;37:183–91. 10.1080/08830185.2018.1455194 [DOI] [PubMed] [Google Scholar]
- 29. Schenker P, Ozturk A, Vonend O et al. Single-dose thymoglobulin induction in living-donor renal transplantation. Ann Transplant 2011;16:50–8. 10.12659/AOT.881865 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.