ABSTRACT
Background:
Hypertension remains one of the most important modifiable risk factors for stroke and heart disease. Anti-hypertensive medications are effective, but are often not used to maximum benefit. Sub-optimal dosing by prescribers and challenges with medication-taking for patients remain barriers to effective blood pressure control.
Objectives:
We aimed to systematically develop a theory-based complex intervention to support General Practitioners (GPs) and people with hypertension to maximise medication use to control blood pressure.
Methods:
We used the three-phase Behaviour Change Wheel (BCW) as the overarching intervention development framework. Collective Intelligence methodology was used to operationalise the stakeholder input to Phases 2 and 3 of the BCW. This took the form of a Collective Intelligence workshop with 19 stakeholders from diverse backgrounds including lived experience, general practice, nursing, pharmacy and health psychology. Techniques such as barrier identification, idea-writing and scenario-based design were used to generate possible intervention options. Intervention options were then selected and refined using the Acceptability, Practicability, Effectiveness, Affordability, Side-effects and Equity (APEASE) criteria and guidance from the MIAMI Public and Patient Involvement Panel.
Results:
The finalised MIAMI intervention consists of both GP and patient supports. GP supports include a 30-minute online training, information booklet and consultation guide (drop-down menu) embedded within the patient electronic health system. Patient supports include a pre-consultation plan, website, and a structured GP consultation with results from an Ambulatory Blood Pressure Monitor and urine chemical adherence test. The intervention components have been mapped to the intervention functions of the BCW and Behaviour Change Technique Ontology.
Conclusion:
Collective Intelligence offered a novel method to operationalise stakeholder input to Phases 2 and 3 of the BCW. The MIAMI intervention is now at pilot evaluation stage.
KEYWORDS: Hypertension, intervention development, behaviour change wheel, collective intelligence, complex intervention
SUSTAINABLE DEVELOPEMENT GOALS: SDG 3: Good health and well-being
Introduction
Hypertension is one of the most important risk factors for stroke and heart disease. International comparisons have shown that Ireland has relatively high levels of inadequately managed blood pressure (BP) (Zhou et al., 2019). Recent international guidelines have stated that ‘poor adherence to treatment – in addition to physician inertia – is the most important cause of poor BP control’ (McEvoy et al., 2024). Problems with medication taking, or ‘poor adherence’ are very common in hypertension, with rates between 30%–50% (Hameed & Dasgupta, 2019; Lawson et al., 2020; Tomaszewski et al., 2014; Vrijens et al., 2017) Improving how antihypertensive medications are used has the potential to quickly reduce BP and the related cardiovascular risk in most patients (McEvoy et al., 2024). When considered as a behaviour, medication-taking can be broadly broken into three components: initiation (which occurs when a person takes the first dose of the prescribed medication); implementation (the extent a patient’s actual dosing corresponds with the prescribed regime); and discontinuation (which occurs when the patient stops taking the medication) (Vrijens et al., 2012). A term related to these behaviours is persistence, which refers to the length of time between initiation and last dose (Vrijens et al., 2012). A number of interventions have been implemented to improve medication-taking behaviours in hypertension, and, while these have generally shown modest improvements, there is no consensus on what intervention ‘ingredients’ are most effective (Morrissey et al., 2017).
Inertia is the second key challenge identified in global blood pressure control (McEvoy et al., 2024). Clinical inertia is largely seen as failure to advance therapy when appropriate to do so, an issue that is particularly pertinent in the treatment of hypertension. Two Irish studies have confirmed that suboptimal dosing is a significant issue in Irish general practice populations of patients with resistant hypertension (Hayes et al., 2018) and post stroke and/or transient ischaemic attack (Doogue et al., 2020). However, in terms of the definition of ‘clinical inertia,’ it can be argued that failure to advance therapy is just one feature of a larger phenomenon. It has been suggested that ‘clinical inertia’ is not only related to escalation of therapy, but is a much wider concept which encompasses failure to improve care at many levels of healthcare (Khunti & Davies, 2017). In the context of hypertension care, we know that conversations around medication-taking behaviour are often omitted – a recent survey of 200 hypertension specialists in 30 countries found that the topic of medication-taking was suboptimally addressed, despite its importance (Burnier et al., 2021). A similar survey of healthcare professionals (n = 3196) working in primary care in Europe found that less than half of respondents ever asked their patients whether they have missed any doses of their medication (Clyne et al., 2016).
This avoidance of this topic in the patient-physician consultation may represent a ‘missing piece’ in the medication-taking puzzle, as it is well established that primary care clinicians can enable patients in their medication-taking practices (Chen et al., 2013; Kerse et al., 2004). A supportive discussion can identify which perceptual and practical barriers a patient may be facing in their medication taking. For example, a recent phenomenological hermeneutical study on patients with cardiac conditions’ experiences with medicines reported the theme of ‘unsuitable without adjustment.’ This qualitative data highlighted how discussion with the prescribing physician and changes to both the type and dose of medication may be required to make medication suitable to the patients’ lives (Fuller et al., 2021). Therefore, a systematically developed approach is needed to ensure that the hypertension consultation includes the topic of medication taking. This will help avoid that part of clinical inertia that is associated with assessing patient barriers and facilitators to medication taking and providing appropriate supports for long-term medication use.
A key objective of the present study was to develop a complex intervention that would support both General Practitioners (GPs) and people with hypertension to maximise medication use to control blood pressure. The field of complex intervention development has benefitted from recent advances in guidance, such as the updated Medical Research Council (MRC) framework (Skivington et al., 2021) and O’Cathain’s consensus exercise (O’Cathain et al., 2019). Both emphasise the importance of reviewing the evidence, drawing on existing theories, using development frameworks (such as the Behaviour Change Wheel, Michie et al., 2011), articulating programme theory, understanding context and involving stakeholders throughout the iterative development process.
To generate the evidence required for the intervention development, we conducted two systematic reviews and three primary studies; this work is outlined in Table 1. We also set up a ‘Public and Patient Involvement’ (PPI) panel of six people with lived experience of hypertension and primary care to guide the work.
Table 1.
Evidence sources used in the development phase of the MIAMI intervention.
| Source | Type of activity | Overview of findings | BCW step(s) informed |
|---|---|---|---|
| Prevalence of treatment-resistant hypertension after considering pseudo-resistance and morbidity: A cross-sectional study in Irish primary care (Hayes et al., 2018) | Primary cross-sectional research |
|
1, 2 |
| Medication adherence among patients with apparent treatment-resistant hypertension: Systematic review and meta-analysis (Durand et al., 2017) | Systematic review |
|
1, 2 |
| Medication adherence for resistant hypertension: Assessing theoretical predictors of adherence using direct and indirect adherence measures (Durand et al., 2018) | Primary cross-sectional research |
|
3, 4, 5 |
| A qualitative comparison of high and low adherers with apparent treatment-resistant hypertension (Durand et al, 2020) | Primary qualitative research |
|
3, 4, 5 |
| Effectiveness and content analysis of interventions to enhance medication adherence and blood pressure control in hypertension: A systematic review and meta-analysis (Morrissey et al., 2017) | Systematic review |
|
5, 7, 8 |
| PPI meetings | PPI meetings |
|
1, 2, 3, 4, 5, 7, 8 |
We selected the three-phase Behaviour Change Wheel (BCW) as the development framework for this work and used our evidence sources, relevant theory (Horne et al., 2019; Horne & Weinman, 1999) and PPI input to complete Phase 1, which involves identifying components of the target behaviour (who, what, where, when, and how often) to be addressed through the intervention using the COM-B (Capability, Opportunity, Motivation-Behaviour) model. Table 2 outlines the breakdown of the target behaviours and mapping of the target ‘modifiable factors.’
Table 2.
Phase 1 of the Behaviour Change Wheel.
| Medication taking | |
| Target behaviour | |
| Who | Person with hypertension |
| What | Taking medication as prescribed |
| When, where, how often | As part of daily routine |
| Target factor | COM-B component |
| Treatment beliefs | Reflective motivation |
| Habit | Automatic motivation |
| Pill burden | Physical opportunity |
| Inertia | |
| Target behaviour | |
| Who | GP |
| What |
|
| When, where, how often | As part of regular practice |
| Target factor | COM-B component |
| Familiarisation with dosing guidelines | Psychological capability |
| Capacity (and time) to have conversations about medication use | Social opportunity |
| Competing priorities in consultation | Reflective motivation |
| Familiarisation with medication-taking supports | Psychological capability |
Phases 2 and 3 of the BCW are ‘identifying intervention options’ and ‘identifying content and implementation options.’
Some intervention options were integrated into existing hypertension management protocols in the Irish context. For example, Ambulatory Blood Pressure Monitoring (ABPM), is recommended as a key part of the diagnosis and the ongoing management of blood pressure in primary care in Ireland and many other contexts internationally (Cepeda et al., 2023). Therefore, the intervention was embedded within general practice sites’ existing ABPM protocols. We also supplemented the ABPM protocol with a chemical adherence test that was carried out on patient urine samples provided around the time of ABPM administration. Chemical adherence testing is an emerging assessment procedure that screens for the presence of medications in patient urine samples (Lane et al., 2022). There is some evidence that this test can facilitate patient and provider conversations about medication that might help improve adherence to antihypertensives and reduce BP (Gupta et al., 2017).
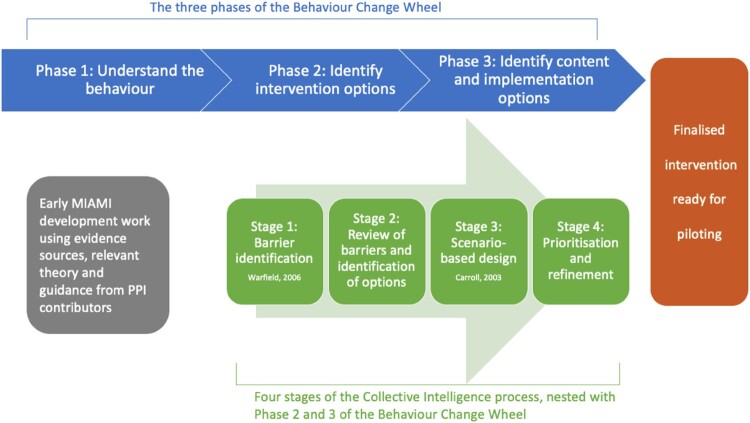
Stakeholder involvement is a critical component of Phases 2 and 3 of the BCW. In this context, ‘Collective Intelligence’ is a useful method for systematically operationalising stakeholder engagement. The term Collective Intelligence refers to the intelligence, or knowledge, that is generated from a group of people working together on a complex problem. The process of Collective Intelligence carefully delineates content and process roles. Responsibility for contributing ideas is assigned to experts, while the workshop facilitator takes responsibility for choosing and implementing selected methodologies. The methodologies are carefully selected for key tasks, including generating, clarifying, structuring, interpreting, and amending ideas. Emphasis is given to balancing behavioural and technical demands of group work while honouring design laws concerning variety, parsimony, and saliency (Broome & Chen, 1992). There must be enough variety in options to cover all needs, but possible paths and solutions must exist in harmony to avoid creating confusion or disrupting problem resolution (Klir & Ashby, 1991). In the context of interdisciplinary work, Collective Intelligence supports high quality idea generation and exchanges, as it includes a set of methods, tools, and a facilitated thought and action mapping process that helps groups to develop outcomes that integrate contributions from individuals with diverse views, backgrounds, and perspectives (Hogan et al., 2014). Collective Intelligence has been applied in a wide variety of situations to accomplish many different goals including developing a national well-being measurement framework (Hogan et al., 2015), designing an e-learning tool to support health practitioners caring for patients taking multiple medications (Hanlon et al., 2020) and identifying barriers and user needs from eHealth interventions for chronic pain (O’Reilly et al., 2022). Figure 1 illustrates the Collective Intelligence process nested within the BCW. The Collective Intelligence method is particularly compatible with the BCW emphasis on placing ‘no priority on an individual, group, or environmental perspective – intra-psychic and external factors all have equal status in controlling behaviour’ (Michie et al., 2011). Collective Intelligence explicitly assigns equal expert status to all stakeholders including patients, clinicians and behaviour change specialists; in identifying content and implementation options.
Figure 1.
The Collective Intelligence process nested within the overarching BCW framework (Carroll, 2003; Warfield, 2006).
This paper describes the systematic development of a complex intervention to support General Practitioners (GPs) and people with hypertension to maximise medication use to control BP, i.e. the MIAMI intervention. We particularly focus on the role of Collective Intelligence in informing Phase 2 and Phase 3 of the BCW approach to intervention development. This MIAMI intervention is currently being evaluated in a pilot cluster randomised controlled trial (RCT) that aims is to gather and analyse feasibility data to allow us to (1) refine the intervention, and (2) determine the feasibility of a definitive RCT. The full protocol has already been published (Morrissey et al., 2023).
Method
The development process is reported using the Guidance for Reporting of Intervention Development (GUIDED) checklist (Duncan et al., 2020) (see Appendix 1) and the intervention is reported using the Template for Intervention Description and Replication (TIDieR) checklist (Hoffmann et al., 2014) (Appendix 2).
Ethics
Ethical approval was granted from the Irish College of General Practitioners Research Ethics Committee (ICGP_REC_21_0050).
Participants
Nineteen stakeholders, with a variety of expertise, including lived experience, general practice, nursing, pharmacy and health psychology took part in the Collective Intelligence workshop. Table 3 outlines the backgrounds of participants. Patient representatives (people living with hypertension) were invited from the project PPI panel, as well as an open invitation in a local newspaper. The remaining participants were invited purposively to ensure diversity in backgrounds and perspectives. The workshop took place in April 2022 at the University of Galway. Informed consent was obtained from all participants.
Table 3.
Participant characteristics.
| Participant number | Background and expertise | Gender |
|---|---|---|
| 1 | Patient representative (MIAMI PPI panel member) | Man |
| 2 | Patient representative (MIAMI PPI panel member) | Man |
| 3 | Patient representative (MIAMI PPI panel member) | Man |
| 4 | Patient representative (MIAMI PPI panel member) | Woman |
| 5 | Patient representative (MIAMI PPI panel member) | Woman |
| 6 | Patient representative | Man |
| 7 | Patient representative | Man |
| 8 | Patient representative | Woman |
| 9 | Clinical pharmacologist | Man |
| 10 | GP and researcher in primary care | Man |
| 11 | GP and researcher in primary care | Man |
| 12 | GP | Man |
| 13 | GP trainee | Man |
| 14 | Nurse (primary care) | Woman |
| 15 | Research nurse in primary care | Woman |
| 16 | Dietician | Woman |
| 17 | Pharmacist | Woman |
| 18 | Researcher in health psychology | Woman |
| 19 | Researcher in health psychology | Woman |
Procedure
Stage 1: pre-workshop idea generation
The first stage of the Collective Intelligence process involved systematic analysis of barriers to meaningful and productive dialogue between patients and GPs around medication use in the context of hypertension. The 19 stakeholders were contacted in advance of the session by email, with a request to generate a set of barriers in response to the following trigger question: ‘What are barriers to patients and GPs bringing up and working through issues around how people with high blood pressure use their medication?’ By email submission, stakeholders identified 154 barriers. These responses were subsequently reviewed by the research team and categorised using the paired comparison method (Warfield & Cárdenas, 1994). The paired comparison method provides a simple way of summarising a group of individuals’ opinions, attitudes or beliefs about a topic in a systematic and objective manner. Responses are clustered into categories, based on conceptual similarity, so that they can be presented back to the group as an overview of the issues within each category, and the problem space as a whole. These categories provide a focus for initial discussions at the Collective Intelligence workshop.
Stage 2: review of barriers
On the day of the Collective Intelligence workshop, the workspace was arranged such that the ‘problem field’ (i.e. the categorised barriers received in advance of the session) were displayed on the walls. Participants were deliberately divided into four groups prior to the workshop, with a mix of stakeholder expertise and perspectives at each table. The workshop was facilitated by two experts in Collective Intelligence, who were not part of the project team. A short presentation providing an overview of the problem space as regards hypertension and medication was delivered to provide context for the activities that would be completed throughout the day.
Following the presentation, participants engaged in a review of the categories of barriers, with a view to generating options for overcoming these barriers. To generate options in response to barriers, small working groups of participants (4–5 persons each) engaged in idea generation in response to three assigned barrier categories. During this stage of the workshop, the ‘ideawriting’ technique was used (Wood & Roth, 1990). Each of the small working groups was asked to generate options in writing and to use open dialogue to explore the meaning of ideas generated. Five steps were involved in idea writing: (a) a stimulus question was presented to participants; (b) each participant worked alone to silently generate ideas in writing; (c) written sheets of ideas were exchanged within the working groups and individuals had the opportunity to add ideas as they read others’ papers; (d) unique ideas were discussed and clarified; and (e) each working group orally reported the ideas generated in a plenary session. This stage of work focused on the generation of options in response to barrier categories and allowed stakeholders to scope out a broad range of options in response to barriers before focusing attention on specific scenarios of application of the MIAMI intervention.
Stage 3: generation of user needs
The next stage involved using scenario-based design methods to generate specific user needs (Rosson & Carroll, 2002). In advance of the Collective Intelligence session, the research team and facilitation team worked together to design a set of scenarios that could be used as inspiration for group idea generation during the workshop. These were based on relevant theories and frameworks (e.g. the Common-Sense Self-Regulation Model Leventhal et al., 2003 and Necessity-Concerns Framework Horne & Weinman, 1999), the work the research team had conducted on Stage 1 of the BCW (see Table 2) and clinical experience. Following guidelines provided by Rosson & Carroll (Rosson & Carroll, 2002), design representations captured in the scenarios were concrete, flexible, and generative and did not specify fixed solutions. The scenarios that were designed by the facilitation and research team for this task presented challenges faced by both GPs and patients, to help workshop participants to orient themselves in each individual ‘actor’s’ problem space, so that they could imagine such a situation arising and consider what supports would be useful in each of these circumstances. An example of one of the scenarios used can be seen in Figure 2. The aim was to prompt user needs in relation to: (1) Information Needs; (2) Communication Needs; (3) Decision-Making Support; and, (4) Behavioural Support Needs. Participants were asked to consider the roles of each actor in each scenario and generate a list of needs, and the reasons for these needs. These needs were subsequently discussed by sub-groups and all ideas were collated by the workshop facilitation team. The identification of user needs generated through this scenario-based design stage, informed by the earlier Collective Intelligence analysis of barriers, and generation of targeted options, provided a strong basis for further design work.
Figure 2.
Scenario used in Stage 3.
Stage 4: prioritisation and refinement
As outlined in the introduction, the Collective Intelligence process was couched within the larger framework of the BCW. The targeted options produced at the Collective Intelligence workshop allowed us to identify possible intervention functions (Phase 2 of the BCW) but refinement, identification of content and operationalisation was required (Phase 3 of the BCW). This was conducted by the core research team and PPI panel. User needs and targeted options were tabulated by the research team and brought to PPI and wider study team meetings, where the priority of each need and utility of each option was discussed. This was informed by the Acceptability, Practicability, Effectiveness, Affordability, Side-effects and Equity (APEASE) criteria (Michie et al., 2011). If an option met the APEASE criteria and was considered acceptable and useful, it was chosen as an intervention component. Components were mapped to the intervention functions of the BCW and Behaviour Change Technique Ontology to inform the logic model of the intervention. Subsequent meetings focused on possible barriers and suggestions for improvement to each component. Several iterations of each component were worked through until the component was considered acceptable and useful to PPI panel members.
Results
Stages 1 & 2: pre-workshop idea generation and review of barriers
In response to the pre-workshop idea generation, 154 barriers to patients and GPs bringing up and working through issues around how people with high blood pressure use their medication were collated. Using the paired comparison method, these were summarised across 15 categories.
During the workshop, stakeholders generated a total of 157 options for overcoming these barriers. Table 4 presents an overview of the categories, sample barriers, and sample options for overcoming barriers. The full categorised sets of barriers and options can be found at https://osf.io/xusby/
Table 4.
Results of Stage 1 and Stage 2 of the Collective Intelligence process.
| Name and description of barrier | Examples of barrier | Example of options |
|---|---|---|
|
Understanding Barriers relating to issues of knowledge, awareness, and understanding of patients in relation to medication for high blood pressure |
|
|
|
Perceived necessity Barriers associated with the extent to which patients believe blood pressure medication to be necessary and important, or not |
|
|
|
Patient supports The absence of supports for patients who need to take medication for high blood pressure |
|
|
|
Negative beliefs about medication Barriers relating to patient’s concerns and fears about medication |
|
|
|
Resistance to diagnosis and treatment Beliefs and concerns that patients have in relation to receiving a diagnosis of, and beginning treatment for, high blood pressure. |
|
|
|
Personal circumstances and challenges Barriers addressed a range of external factors which can impinge on an individual’s ability to consistently take their blood pressure medication. |
|
|
|
GP assumptions Challenges specifically from the GP side of the interaction, such as false beliefs and assumptions. |
|
|
|
Dialogue and decision making Challenges relating to poor conversational dynamics which may impact on a patient’s decisions around their medication. |
|
|
|
Multimorbidity Challenges faced by patients in managing multiple conditions and prescriptions. |
|
|
|
GP prescribing worries Fears and concerns that GPs have in relation to prescribing blood pressure medication. |
|
|
|
GP consultation constraints Barriers associated with the lack of time available for consultations, and the range of negative implications this lack of time has. |
|
|
|
Routines and habits Barriers which impinge on patient’s ability to form habits and consistently take their medication. |
|
|
|
Perceived power imbalance Patients’ reluctance to engage in this discussion, or disclose their concerns, out of fear of being judged negatively by the GP. |
|
|
|
Cost A stand-alone category of barriers, negatively impacting on open conversations between GPs and patients in relation to taking blood pressure medication. |
|
|
|
Planning and organisation Challenges with planning skills. |
|
|
Stage 3: generation of user needs
Using scenario-based design, stakeholders also identified a set of user needs, under the four headings of (i) information needs, (ii) communication and collaboration needs, (iii) decision-making needs, and (iv) behavioural support needs.
Stakeholders identified 102 Information Needs (subsequently divided into 10 categories), 126 Communication and collaboration needs (subsequently divided into 8 categories), 65 Decision-making needs (subsequently divided into 6 categories) and 89 Behavioural support needs (subsequently divided into 8 categories). Table 5 provides the five most common needs within each category, along with a selection of illustrative quotes. The full set of categorised needs is provided at https://osf.io/xusby/
Table 5.
Results of stage 3 of the Collective Intelligence process.
| Categories of needs | Sample needs within the consultation |
|---|---|
| Information needs | |
| Patient understanding (22%) | ‘To understand why I need medication’ |
| Importance of controlling blood pressure (17%) | ‘Information on the consequences of not taking the medication’ |
| Forms of information delivery (13%) | ‘Written information to take home with me’ ‘An infographic to explain the risks and side effects’ |
| Understanding the patient perspective and concerns (13%) | ‘To know what concerns, worries the patient has with taking all of her medication’ ‘Information about the best way to support patient decision making’ |
| Accessibility and quality of information (9%) | ‘Easy access to leaflets, website and videos that can be recommended to patients’ ‘Clear and concise instructions about my tablet’ |
| Communication needs | |
| Empowering and understanding (27%) | ‘GP to acknowledge my difficulties’ ‘An action plan that reflects my needs + expectations, tailored to me/my lifestyle’ ‘To be an active participant in my options’ |
| Open communication (24%) | ‘Ask open questions around medication taking’ ‘Open, honest, person centred communication’ ‘To practice using clear language’ |
| Creating an environment for communication (24%) | ‘To avoid seeming rude or dismissive’ ‘Need the patient to feel that the decisions are collaborative’ |
| Tools and strategies (6%) | ‘To use tools to determine patient’s needs’ ‘Have a pre-consultation plan, so can have questions/concerns ready to discuss’ |
| Communication actors (6%) | ‘Involve the practice nurse in communication’ ‘My GP to send the pharmacist my prescription directly’ |
| Decision making needs | |
| Shared decision making (34%) | ‘Ask patient if she agrees with decision’ ‘Actively listen to each other’ ‘To balance my professional opinion with the desires of the patient’ |
| Informed decision making (23%) | ‘To ensure that patient has the information necessary to make an informed decision’ ‘Think about what I want from the doctor appointment’ ‘My GP to provide recommended resources e.g. websites/online apps’ |
| Tools to support decision making (17%) | ‘A list of prompts to guide my conversation – focused but open’ ‘A tool I can take home with me so that I can reflect and affirm my decision making’ |
| Plans and expectations (14%) | ‘Decide what is most important to work out first’ ‘At the appointment close, ask ‘so are you happy to try that?’ ‘An agreement on follow up plans’ |
| Testing (6%) | ‘The inclusion of the urine testing to be clear and safe’ ‘Further tests – 24 h blood pressure monitor’ |
| Behavioural support needs | |
| Tools and strategies (34%) | ‘Offer some tools to remind the patient to take meds’ ‘A app or test messaging service assist with reminders’ |
| Support from others (17%) | ‘To know of and be able to direct and signpost the patient to community/online supports (groups/information/class)’ ‘An easy way to communicate with pharmacists re. supporting patients’ ‘Talk to my family about my medicine needs’ |
| Follow-up (11%) | ‘To follow up regularly with the patient’ ‘To schedule a follow-up appointment to check on my progress and for support’ |
| Goals and roadmaps (10%) | ‘Agree a roadmap of actions with the patient’ ‘To be clear on what exactly I have to do’ |
| Medication and lifestyle (10%) | ‘Link medication-taking with other habits’ |
Stage 4: prioritisation and refinement
Through the use of the APEASE criteria, along with discussion with the PPI panel and wider research team, user needs were prioritised and targeted options were chosen to become intervention components. These were mapped to the intervention functions of the BCW and Behavioural Change Technique Ontology (Marques et al., 2023). The research team and PPI panel then worked together to operationalise and create the content for the intervention components. For example, the informational BP videos were co-created by the research team and PPI panel, where the PPI panel suggested content for the video, the research team drafted the video and the PPI panel gave several rounds of feedback with suggested modifications before approving the final version.
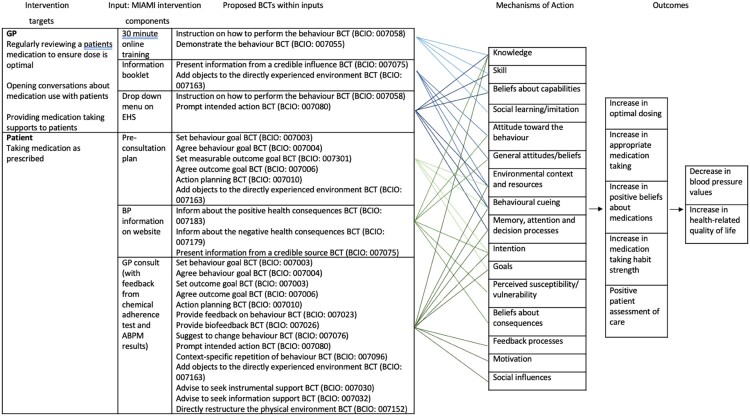
The finalised MIAMI intervention is described in Table 6. The logic model for the intervention is depicted in Figure 3, to graphically represent the intervention structures and processes, including proposed BCTs and associated mechanisms of action. Intervention components can be seen in detail in the intervention manual (https://osf.io/xusby/).
Table 6.
Continued
| Patient | |||
|---|---|---|---|
| Component | User need | Intervention function | Details |
| Pre-consultation plan | Communication need Decision need |
Environmental restructuring | Short document containing
|
| GP consultation | Communication need Decision need Behavioural need |
Environmental restructuring | Discussion of ABPM and urine test results. Use of pre-consultation plan to create shared action plan GP to review prescription and adjust to include single pill combinations and blister pack if appropriate GP to advise on habit creation GP to send text messages on dontforget.ie and Croí website if appropriate |
| Croí website | Information need | Education | Page on website containing (both text and video format)
|
Table 6.
Finalised MIAMI intervention.
| GP | |||
|---|---|---|---|
| Component | User need | Intervention function | Details |
| Training videos | Communication need Information need Behavioural need |
Education Training Modelling |
30 min training package(6 short videos and quiz) Part 1: Adherence to medication, including the extent of non-adherence, key factors associated with adherence, and types of non-adherence Part 2: Three case studies to illustrate how you might support patients’ medication adherence in different scenarios Part 3: Skills and strategies to support adherence Part 4: A case study illustrating the use of the MIAMI intervention |
| MIAMI booklet | Information need | Education | Booklet contains
|
| Drop-down menu on computer to guide consultation | Communication need Decision need Behavioural need |
Environmental restructuring | Drop-down menu on computer which contains a guide to the consultation.
Information about blood pressure and blood pressure medication is available on the Croí website: https://croi.ie/health/heart-conditions/high-blood-pressure/ |
Figure 3.
MIAMI logic model.
The TIDieR checklist is presented in Appendix 2.
Discussion
This paper comprehensively and transparently reports the development process of the MIAMI intervention, with the BCW as an overarching guidance framework and Collective Intelligence methodology used to operationalise stakeholder input. GUIDED and TiDIER checklists can be seen in Appendices 1 and 2. The finalised intervention provides supports to both GPs and people with hypertension to allow a useful and non-judgemental consultation about medication-taking. Full details of GP and patient components can be seen in Table 6.
One of the particularly novel aspects of this behavioural intervention was the use of the chemical adherence test as part of this complex behavioural intervention. There is increasing interest in using such tests as part of hypertension care (Lane et al., 2022); we have previously shown, in a pilot study, the feasibility, acceptability, and efficacy of routinely using these tests in Irish general practice (Hayes et al., 2019). There is similar confirmatory evidence from a UK opportunistic study of 191 patients with hypertension, all on at least one blood pressure lowering medication, that it is indeed feasible to carry these tests out in a general practice setting. However, this UK study suggested that it is not appropriate to carry out these tests routinely given the low prevalence of 4.7% of chemical non-adherence that was detected (mean age 76.2 ± 6.6 years) (Sheppard et al., 2022). The authors recommended that chemical urine testing be reserved for patients who are on multiple blood pressure lowering medications and whose blood pressure control is not optimal. This is the specific patient group for which the MIAMI intervention has been developed (Lane et al., 2022).
Another unique feature of this study was the use of the Collective Intelligence methodology within the BCW framework for behavioural intervention design. This provided an efficient method to collectively orient and engage a diverse group of stakeholders in this complex problem space. However, the BCW focuses on identifying and changing one specific behaviour and following a prescribed set of steps to identify intervention options. It was sometimes challenging to maintain this approach in the intervention development process, given the complexity of the multiple interdependent patient and provider behaviours involved in using medication appropriately. While Collective Intelligence provides a systematic and structured method to gather input from multiple stakeholders, it also provided the freedom to reconceptualise the nature of the problem and to generate solutions that are not easily rendered into specific techniques and related mechanisms of action. For example, the identification of the critical role of enhancing the more intangible ‘space between us’ factors (Lloyd, 2009) that might be variously referred to as therapeutic alliance (Elvins & Green, 2008) or patient-provider relationship (Drossman & Ruddy, 2020) meant that reducing the solutions to a set of specific behaviour change techniques and related mechanisms of action was challenging at time. While we do believe that Collective Intelligence aligns with the spirit of the BCW and PPI in research, in that it assigns equal expert status to all stakeholders including patients, clinicians and behaviour change specialists, it remains to be seen whether this method translates more widely to multiple health behaviour change design applications.
A key question that this study can address relates to the feasibility of addressing one specific kind of medication use for one single disease, as part of a behavioural intervention in a general practice consultation. Recently the powerful concept of Time Needed to Treat (TNT) has been developed (Johansson et al., 2023). Broadly speaking this refers to the time in a consultation that would be required to implement a treatment recommendation and serves to remind those developing interventions and guidelines for the general practice context that implementation of new disease-specific practices might often be unfeasible, if time constraints are not appropriately considered (Albarqouni et al., 2023). This is particularly the case given the reality of the extent of multimorbidity and related polypharmacy in older adulthood, where GPs are often faced with an already unmanageable range of guideline recommended practices to prioritise before patient support with medication-taking can be considered (Foley et al., 2021). By working closely with GPs throughout the project, the evaluation of the MIAMI intervention will keep these implementation considerations at the forefront of the final analyses.
In conclusion, there are several strengths to this intervention design, including the systematic development of the intervention, the diverse stakeholder involvement, and continuous PPI contributions throughout the entire programme of work. While this was sometimes challenging, it allowed the behavioural intervention development to align with the principles of co-production that are increasingly recognised as being essential to the design of sustainable and implementable health interventions (Batalden et al., 2016). The detailed description of the intervention development and content in this paper will enable future work to incorporate the study findings more accurately into evidence synthesis research and to carry out replication studies or extensions and refinements of the MIAMI intervention. The full pilot and feasibility evaluation of the MIAMI intervention, including a qualitative substudy and health economics costings, is underway and will be reported separately in a subsequent paper.
Acknowledgements
We would like to acknowledge and thank all the stakeholders who participated in the Collective Intelligence workshop. We would also like to thank our Trial Management Group for their work on this project – Dr Eamon Dolan, Prof Paddy Gillespie, Dr Anna Hobbins, Dr Lisa Hynes, Prof Bill McEvoy and Prof John Newell. The study was conducted in accordance with the Declaration of Helsinki and was approved by an Institutional Review Board/Ethics committee. See details under Methods. All participants gave explicit written consent for participation. ECM: conceptualisation, methodology, formal analysis, investigation, data curation, writing – original draft preparation, project administration, funding acquisition; OMH: methodology, formal analysis, investigation, data curation, writing – original draft preparation, project administration; MJH: methodology, formal analysis, investigation, data curation,, writing – review & editing; PJM: conceptualisation, methodology, supervision, writing – review & editing, funding acquisition; LOG: investigation, data curation, writing – review & editing, project administration; MB: conceptualisation, writing – review & editing, funding acquisition; MC: conceptualisation, methodology, writing – review & editing, funding acquisition; SD: conceptualisation, methodology, writing – review & editing, funding acquisition; HD: conceptualisation, writing – review & editing, funding acquisition; PH: conceptualisation, methodology, writing – review & editing, funding acquisition; PPI: conceptualisation, writing – review & editing; AWM: conceptualisation, methodology, supervision, writing – review & editing, funding acquisition; GJM: conceptualisation, methodology, supervision, writing – review & editing, funding acquisition.
Appendices.
Appendix 1. GUIDED checklist
| Item description | Explanation | Page in manuscript |
|---|---|---|
| 1. Report the context for which the intervention was developed. | Understanding the context in which an intervention was developed informs readers about the suitability and transferability of the intervention to the context in which they are considering evaluating, adapting or using the intervention. Context here can include place, organisational and wider sociopolitical factors that may influence the development and/or delivery of the intervention | 2,3 |
| 2. Report the purpose of the intervention development process. | Clearly describing the purpose of the intervention specifies what it sets out to achieve. The purpose may be informed by research priorities, for example those identified in systematic reviews, evidence gaps set out in practice guidance such as The National Institute for Health and Care Excellence or specific prioritisation exercises such as those undertaken with patients and practitioners through the James Lind Alliance. | 2,3 |
| 3. Report the target population for the intervention development process. | The target population is the population that will potentially benefit from the intervention – this may include patients, clinicians, and/or members of the public. If the target population is clearly described then readers will be able to understand the relevance of the intervention to their own research or practice. Health inequalities, gender and ethnicity are features of the target population that may be relevant to intervention development processes. | 2,3 |
| 4. Report how any published intervention development approach contributed to the development process | Many formal intervention development approaches exist and are used to guide the intervention development process (e.g. 6Squid (16) or The Person Based Approach to Intervention Development (17)). Where a formal intervention development approach is used, it is helpful to describe the process that was followed, including any deviations. More general approaches to intervention development also exist and have been categorised as follows (3):- Target Population-centred intervention development; evidence and theory-based intervention development; partnership intervention development; implementation-based intervention development; efficacy based intervention development; step or phased-based intervention development; and intervention-specific intervention development (3). These approaches do not always have specific guidance that describe their use. Nevertheless, it is helpful to give a rich description of how any published approach was operationalised | 6 |
| 5. Report how evidence from different sources informed the intervention development process. | Intervention development is often based on published evidence and/or primary data that has been collected to inform the intervention development process. It is useful to describe and reference all forms of evidence and data that have informed the development of the intervention because evidence bases can change rapidly, and to explain the manner in which the evidence and/or data was used. Understanding what evidence was and was not available at the time of intervention development can help readers to assess transferability to their current situation. | 4,5 Table 1 |
| 6. Report how/if published theory informed the intervention development process. | Reporting whether and how theory informed the intervention development process aids the reader’s understanding of the theoretical rationale that underpins the intervention. Though not mentioned in the e-Delphi or consensus meeting, it became increasingly apparent through the development of our guidance that this theory item could relate to either existing published theory or programme theory | 12 |
| 7. Report any use of components from an existing intervention in the current intervention development process. | Some interventions are developed with components that have been adopted from existing interventions. Clearly identifying components that have been adopted or adapted and acknowledging their original source helps the reader to understand and distinguish between the novel and adopted components of the new intervention. | 7 |
| 8. Report any guiding principles, people or factors that were prioritised when making decisions during the intervention development process. | Reporting any guiding principles that governed the development of the application helps the reader to understand the authors’ reasoning behind the decisions that were made. These could include the examples of particular populations who views are being considered when designing the intervention, the modality that is viewed as being most appropriate, design features considered important for the target population, or the potential for the intervention to be scaled up. | 10–13 |
| 9. Report how stakeholders contributed to the intervention development process. | Potential stakeholders can include patient and community representatives, local and national policy makers, health care providers and those paying for or commissioning health care. Each of these groups may influence the intervention development process in different ways. Specifying how differing groups of stakeholders contributed to the intervention development process helps the reader to understand how stakeholders were involved and the degree of influence they had on the overall process. Further detail on how to integrate stakeholder contributions within intervention reporting are available. | 10–13 |
| 10. Report how the intervention changed in content and format from the start of the intervention development process. | Intervention development is frequently an iterative process. The conclusion of the initial phase of intervention development does not necessarily mean that all uncertainties have been addressed. It is helpful to list remaining uncertainties such as the intervention intensity, mode of delivery, materials, procedures, or type of location that the intervention is most suitable for. This can guide other researchers to potential future areas of research and practitioners about uncertainties relevant to their healthcare context. | n/a – this is the first iteration of the intervention |
| 11. Report any changes to interventions required or likely to be required for subgroups | Specifying any changes that the intervention development team perceive are required for the intervention to be delivered or tailored to specific sub groups enables readers to understand the applicability of the intervention to their target population or context. These changes could include changes to personnel delivering the intervention, to the content of the intervention, or to the mode of delivery of the intervention. | n/a – we do not have enough data at the moment to make these sort of recommendations. |
| 12. Report important uncertainties at the end of the intervention development process. | Intervention development is frequently an iterative process. The conclusion of the initial phase of intervention development does not necessarily mean that all uncertainties have been addressed. It is helpful to list remaining uncertainties such as the intervention intensity, mode of delivery, materials, procedures, or type of location that the intervention is most suitable for. This can guide other researchers to potential future areas of research and practitioners about uncertainties relevant to their healthcare context. | 29 |
| 13. Follow TIDieR guidance when describing the developed intervention. | Interventions have been poorly reported for a number of years. In response to this, internationally recognised guidance has been published to support the high quality reporting of health care? Interventions and public health interventions. This guidance should therefore be followed when describing a developed intervention. | Appendix 2 |
| 14. Report the intervention development process in an open access format. | Unless reports of intervention development are available people considering using an intervention cannot understand the process that was undertaken and make a judgement about its appropriateness to their context. It also limits cumulative learning about intervention development methodology and observed consequences at later evaluation, translation and implementation stages. Reporting intervention development in an open access (Gold or Green) publishing format increases the accessibility and visibility of intervention development research and makes it more likely to be read and used. Potential platforms for open access publication of intervention development include open access journal publications, freely accessible funder reports or a study web-page that details the intervention development process | We intend to publish this paper in an OA journal. Study materials can be found at https://osf.io/xusby/ |
Appendix 2: TIDIeR checklist
| Item number | Item | |
|---|---|---|
| BRIEF NAME | ||
| 1. | Provide the name or a phrase that describes the intervention. | MIAMI |
| WHY | ||
| 2. | Describe any rationale, theory, or goal of the elements essential to the intervention. | The MIAMI intervention aims to support General Practitioners and people with hypertension to maximise medication use to control blood pressure |
| WHAT | ||
| 3. | Materials: Describe any physical or informational materials used in the intervention, including those provided to participants or used in intervention delivery or in training of intervention providers. Provide information on where the materials can be accessed (e.g. online appendix, URL). | MIAMI training videos (GP intervention component) MIAMI booklet (GP intervention component) – https://osf.io/xusby/ Pre-consultation plan (Patient intervention component) – https://osf.io/xusby/ Blood pressure webpage on Croí website (Patient intervention component) – https://croi.ie/heart/high-blood-pressure/ |
| 4. | Procedures: Describe each of the procedures, activities, and/or processes used in the intervention, including any enabling or support activities. | The MIAMI intervention will be delivered at a minimum of one GP appointment during a three-month period The MIAMI intervention is a structured set of supports for GPs and patients with hypertension to facilitate adequate information exchange within consultations about long-term antihypertensive medication use and adherence skill development. Full details are in Table 6. It will include the following, where indicated: Patients
|
| WHO PROVIDED | ||
| 5. | For each category of intervention provider (e.g. psychologist, nursing assistant), describe their expertise, background and any specific training given. | GPs – 30 min online training in how to structure a consultation around medication taking and adherence issues |
| HOW | ||
| 6. | Describe the modes of delivery (e.g. face-to-face or by some other mechanism, such as internet or telephone) of the intervention and whether it was provided individually or in a group. | GPs – Training provided online. GPs can access individually and undergo training at own time and pace. Patients – Face to face consultation with GP |
| WHERE | ||
| 7. | Describe the type(s) of location(s) where the intervention occurred, including any necessary infrastructure or relevant features. | GPs – online Patients – at GPs clinic |
| WHEN and HOW MUCH | ||
| 8. | Describe the number of times the intervention was delivered and over what period of time including the number of sessions, their schedule, and their duration, intensity or dose. | The MIAMI intervention will be delivered at a minimum of one GP appointment during a three-month period. Consultations typically last 15–20 min. |
| TAILORING | ||
| 9. | If the intervention was planned to be personalised, titrated or adapted, then describe what, why, when, and how. | GP component is not personalised. Patient component can be personalised by the GP – they will use their judgement and training to decide what adherence support a patient may need. Patients may decide themselves whether to being the pre-consultation plan to their GP appointment. |
| MODIFICATIONS | ||
| 10. | If the intervention was modified during the course of the study, describe the changes (what, why, when, and how). | NA (intervention not yet delivered) |
| HOW WELL | ||
| 11. | Planned: If intervention adherence or fidelity was assessed, describe how and by whom, and if any strategies were used to maintain or improve fidelity, describe them. | We are planning to assess fidelity during the pilot RCT through a checklist on the GP software. |
| 12. | Actual: If intervention adherence or fidelity was assessed, describe the extent to which the intervention was delivered as planned. | NA (intervention not yet delivered) |
Funding Statement
This work was supported by the Health Research Board [HRB-DIFA-2020-012].
Open Scholarship

This article has earned the Center for Open Science badge for Open Data. The data are openly accessible at https://osf.io/xusby/.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The materials and datasets generated and/or analysed during the current study are available in the OSF repository https://osf.io/xusby/.
References
- Albarqouni, L., Montori, V., Jørgensen, K. J., Ringsten, M., Bulbeck, H., & Johansson, M. (2023). Applying the time needed to treat to NICE guidelines on lifestyle interventions. BMJ Evidence-Based Medicine, 28, 354–355. [DOI] [PubMed] [Google Scholar]
- Batalden, M., Batalden, P., Margolis, P., Seid, M., Armstrong, G., Opipari-Arrigan, L., & Hartung, H. (2016). Coproduction of healthcare service. BMJ Quality & Safety, 25(7), 509–517. 10.1136/bmjqs-2015-004315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome, B. J., & Chen, M. (1992). Guidelines for computer-assisted group problem solving: Meeting the challenges of complex issues. Small Group Research, 23(2), 216–236. 10.1177/1046496492232005 [DOI] [Google Scholar]
- Burnier, M., Prejbisz, A., Weber, T., Azizi, M., Cunha, V., Versmissen, J, et al. , & Working Group on Cardiovascular Therapy and Adherence of the European Society of Hypertension. (2021). Hypertension healthcare professional beliefs and behaviour regarding patient medication adherence: A survey conducted among European Society of Hypertension Centres of Excellence. Blood Pressure, 30(5), 282–290. 10.1080/08037051.2021.1963209 [DOI] [PubMed] [Google Scholar]
- Carroll, J. M. (2003). Making use: Scenario-based design of human-computer interactions. MIT Press. [Google Scholar]
- Cepeda, M., Pham, P., & Shimbo, D. (2023). Status of ambulatory blood pressure monitoring and home blood pressure monitoring for the diagnosis and management of hypertension in the US: An up-to-date review. Hypertension Research, 46(3), 620–629. 10.1038/s41440-022-01137-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. C., Tseng, C. H., & Cheng, S. H. (2013). Continuity of care, medication adherence, and health care outcomes among patients with newly diagnosed type 2 diabetes: A longitudinal analysis. Medical Care, 51(3), 231–237. https://journals.lww.com/lww-medicalcare/Fulltext/2013/03000/Continuity_of_Care,_Medication_Adherence,_and.4.aspx [DOI] [PubMed] [Google Scholar]
- Clyne, W., Mshelia, C., McLachlan, S., Jones, P., de Geest, S., Ruppar, T., et al. , & Kardas, P. (2016). A multinational cross-sectional survey of the management of patient medication adherence by European healthcare professionals. BMJ Open, 6(2), e009610. 10.1136/bmjopen-2015-009610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doogue, R., McCann, D., Fitzgerald, N., Murphy, A. W., Glynn, L. G., & Hayes, P. (2020). Blood pressure control in patients with a previous stroke/transient ischaemic attack in primary care in Ireland: A cross sectional study. BMC Family Practice, 21(1), 139. 10.1186/s12875-020-01211-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drossman, D. A., & Ruddy, J. (2020). Improving patient-provider relationships to improve health care. Clinical Gastroenterology and Hepatology, 18(7), 1417–1426. 10.1016/j.cgh.2019.12.007 [DOI] [PubMed] [Google Scholar]
- Duncan, E., O’Cathain, A., Rousseau, N., Croot, L., Sworn, K., Turner, K.M., Yardley, L., & Hoddinott, P. (2020). Guidance for reporting intervention development studies in health research (GUIDED): An evidence-based consensus study. BMJ Open, 10(4), e0033516. 10.1136/bmjopen-2019-033516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand, H., Casey, M., Glynn, L. G., Hayes, P., Murphy, A. W., & Molloy, G. J. (2020). A qualitative comparison of high and low adherers with apparent treatment-resistant hypertension. Psychology, health and medicine, 25(1), 64–77. [DOI] [PubMed] [Google Scholar]
- Durand, H., Hayes, P., Harhen, B., Conneely, A., Finn, D. P., Casey, M., Murphy, A. W., & Molloy, G. J. (2018). Medication adherence for resistant hypertension: assessing theoretical predictors of adherence using direct and indirect adherence measures. British Journal of Health Psychology, 23(4), 949–966. [DOI] [PubMed] [Google Scholar]
- Durand, H., Hayes, P., Morrissey, E. C., Newell, J., Casey, M., Murphy, A. W., & Molloy, G J. (2017). Medication adherence among patients with apparent treatment-resistant hypertension: systematic review and meta-analysis. Journal of Hypertension, 35(12), 2346–2357. [DOI] [PubMed] [Google Scholar]
- Elvins, R., & Green, J. (2008). The conceptualization and measurement of therapeutic alliance: An empirical review. Clinical Psychology Review, 28(7), 1167–1187. 10.1016/j.cpr.2008.04.002 [DOI] [PubMed] [Google Scholar]
- Foley, L., Larkin, J., Lombard-Vance, R., Murphy, A. W., Hynes, L., Galvin, E., & Molloy, G. J. (2021). Prevalence and predictors of medication non-adherence among people living with multimorbidity: A systematic review and meta-analysis. BMJ Open, 11(9), e044987. 10.1136/bmjopen-2020-044987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller, J. M., Barenfeld, E., & Ekman, I. (2021). Why do patients struggle with their medicines? A phenomenological hermeneutical study of how patients experience medicines in their everyday lives. PLoS One, 16(8), e0255478. 10.1371/journal.pone.0255478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, P., Patel, P., Štrauch, B., Lai, F. Y., Akbarov, A., Gulsin, G. S., et al. , & Tomaszewski, M. (2017). Biochemical screening for nonadherence is associated with blood pressure reduction and improvement in adherence. Hypertension, 70(5), 1042–1048. 10.1161/HYPERTENSIONAHA.117.09631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed, M. A., & Dasgupta, I. (2019). Medication adherence and treatment-resistant hypertension: A review. Drugs in Context, 8, 212560. 10.7573/dic.212560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon, M., Hogan, M., Durand, H., Pilch, M., Harney, O., Molloy, G., & Murphy, A. W. (2020). Designing an e-learning tool to support health practitioners caring for patients taking multiple medications. HRB Open Research, 3, 59. 10.12688/hrbopenres.13110.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, P., Casey, M., Glynn, L. G., Molloy, G. J., Durand, H., O'Brien, E., Dolan, E., Newell, J., & Murphy, A. W. (2018). Prevalence of treatment-resistant hypertension after considering pseudo-resistance and morbidity: A cross-sectional study in Irish primary care. British Journal of General Practice, 68(671), e394. 10.3399/bjgp18X696221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, P., Casey, M., Glynn, L. G., Molloy, G. J., Durand, H., O'Brien, E., et al. , & Murphy, A. W., (2019). Measuring adherence to therapy in apparent treatment-resistant hypertension: A feasibility study in Irish primary care. British Journal of General Practice, 69(686), e621. 10.3399/bjgp19X705077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, T. C., Glasziou, P. P., Boutron, I., Milne, R., Perera, R., Moher, D., et al. , & Michie, S. (2014). Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ, 348(3), g1687. 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- Hogan, M., Harney, O., & Broome, B. (2014). Integrating argument mapping with systems thinking tools: Advancing applied systems science. In Okada A., Buckingham Shum S. J., & Sherborne T. (Eds.), Knowledge cartography: Software tools and mapping techniques (pp. 401–421). Springer. 10.1007/978-1-4471-6470-8_18 [DOI] [Google Scholar]
- Hogan, M. J., Johnston, H., Broome, B., McMoreland, C., Walsh, J., Smale, B., et al. , & Groarke, A. M. (2015). Consulting with citizens in the design of wellbeing measures and policies: Lessons from a systems science application. Social Indicators Research, 123(3), 857–877. 10.1007/s11205-014-0764-x [DOI] [Google Scholar]
- Horne, R., Cooper, V., Wileman, V., & Chan, A. (2019). Supporting adherence to medicines for long-term conditions. European Psychologist, 24(1), 82–96. [Google Scholar]
- Horne, R., & Weinman, J. (1999). Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. Journal of Psychosomatic Research, 47(6), 555–567. 10.1016/S0022-3999(99)00057-4 [DOI] [PubMed] [Google Scholar]
- Johansson, M., Guyatt, G., & Montori, V. (2023). Guidelines should consider clinicians’ time needed to treat. BMJ, 380, e072953. 10.1136/bmj-2022-072953 [DOI] [PubMed] [Google Scholar]
- Kerse, N., Buetow, S., Mainous, A. G., Young, G., Coster, G., & Arroll, B. (2004). Physician-patient relationship and medication compliance: A primary care investigation. The Annals of Family Medicine, 2(5), 455–461. 10.1370/afm.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khunti, K., & Davies, M. J. (2017). Clinical inertia – Time to reappraise the terminology? Primary Care Diabetes, 11(2), 105–106. 10.1016/j.pcd.2017.01.007 [DOI] [PubMed] [Google Scholar]
- Klir, G. J., & Ashby, W. R. (1991). Requisite variety and its implications for the control of complex systems. In Facets of Systems Science (pp. 405–417). Springer. https://link.springer.com/chapter/ 10.1007/978-1-4899-0718-9_28 [DOI] [Google Scholar]
- Lane, D., Lawson, A., Burns, A., Azizi, M., Burnier, M., Jones, D. J. L., et al. , & Endorsed by the European Society of Hypertension (ESH) Working Group on Cardiovascular Pharmacotherapy and Adherence. (2022). Nonadherence in hypertension: How to develop and implement chemical adherence testing. Hypertension, 79(1), 12–23. 10.1161/HYPERTENSIONAHA.121.17596 [DOI] [PubMed] [Google Scholar]
- Lawson, A. J., Hameed, M. A., Brown, R., Cappuccio, F.P., George, S., Hinton, T., et al. , & Dasgupta, I. (2020). Nonadherence to antihypertensive medications is related to pill burden in apparent treatment-resistant hypertensive individuals. Journal of Hypertension, 38(6), 1165–1173. 10.1097/HJH.0000000000002398 [DOI] [PubMed] [Google Scholar]
- Leventhal, H., Brissette, I., & Leventhal, E. A. (2003). The common-sense model of self-regulation of health and illness. Self-Regulation of Health and Illness Behavior, 1, 42–65. [Google Scholar]
- Lloyd, D. M. (2009). The space between us: A neurophilosophical framework for the investigation of human interpersonal space. Neuroscience & Biobehavioral Reviews, 33(3), 297–304. 10.1016/j.neubiorev.2008.09.007 [DOI] [PubMed] [Google Scholar]
- Marques, M. M., Wright, A. J., Corker, E., Johnston, M., West, R., Hastings, J., Zhang, L., & Michie, S. (2023). The behaviour change technique ontology: Transforming the behaviour change technique taxonomy v1. Wellcome Open Research, 8. 10.12688/wellcomeopenres.19363.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy, J. W., McCarthy, C. P., Bruno, R. M., Brouwers, S., Canavan, M. D., Ceconi, C., Christodorescu, R. M., Daskalopoulou, S. S., Ferro, C. J., Gerdts, E., Hanssen, H., Harris, J., Lauder, L., McManus, R. J., Molloy, G. J., Rahimi, K., Regitz-Zagrosek, V., Rossi, G. P., Sandset, E. C., & Touyz, R. M. (2024). 2024 ESC Guidelines for the management of elevated blood pressure and hypertension: Developed by the task force on the management of elevated blood pressure and hypertension of the European Society of Cardiology (ESC) and endorsed by the European Society of Endocrinology (ESE) and the European Stroke Organisation (ESO). European Heart Journal, ehae178. 10.1093/eurheartj/ehae178 [DOI]
- Michie, S., Van Stralen, M. M., & West, R. (2011). The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implementation Science, 6(1), 42. 10.1186/1748-5908-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey, E. C., Durand, H., Nieuwlaat, R., Navarro, T., Haynes, R. B., Walsh, J. C., & Molloy, G. J., (2017). Effectiveness and content analysis of interventions to enhance medication adherence and blood pressure control in hypertension: A systematic review and meta-analysis. Psychology & Health, 32(10), 1195–1232. 10.1080/08870446.2016.1273356 [DOI] [PubMed] [Google Scholar]
- Morrissey, E., Murphy, A., Murphy, P., O'Grady, L., Byrne, M., Casey, M. (2023). Supporting GPs and people with hypertension to maximise medication use to control blood pressure: Protocol for a pilot cluster RCT of the MIAMI intervention [version 1; peer review: awaiting peer review]. HRB Open Research, 6(6), 10.12688/hrbopenres.13661.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Cathain, A., Croot, L., Duncan, E., Rousseau, N., Sworn, K., Turner, K. M., Yardley, L., & Hoddinott, P. (2019). Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open, 9(8), e029954. 10.1136/bmjopen-2019-029954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly, P. M., Harney, O. M., Hogan, M. J., Mitchell, C., McGuire, B. E., & Slattery, B. (2022). Chronic pain self-management in middle-aged and older adults: A collective intelligence approach to identifying barriers and user needs in eHealth interventions. Digital Health, 8, 20552076221105484. 10.1177/20552076221105484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosson, M. B., & Carroll, J. M. (2002). Usability engineering: Scenario-based development of human-computer interaction. Morgan Kaufmann. [Google Scholar]
- Sheppard, J. P., Albasri, A., Gupta, P., Patel, P., Khunti, K., Martin, U., McManus, R. J., & Hobbs, F. D. R. (2022). Measuring adherence to antihypertensive medication using an objective test in older adults attending primary care: Cross-sectional study. Journal of Human Hypertension, 36(12), 1106–1112. 10.1038/s41371-021-00646-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skivington, K., Matthews, L., Simpson, S. A., Craig, P., Baird, J., Blazeby, J. M., et al. , & Moore, L. (2021). A new framework for developing and evaluating complex interventions: Update of Medical Research Council guidance. BMJ, 374, n2061. 10.1136/bmj.n2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszewski, M., White, C., Patel, P., Masca, N., Damani, R., Hepworth, J., et al. , & Williams, B. (2014). High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart, 100(11), 855–861. 10.1136/heartjnl-2013-305063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijens, B., Antoniou, S., Burnier, M., de la Sierra, A., & Volpe, M. (2017). Current situation of medication adherence in hypertension. Frontiers in Pharmacology, 8, 100. 10.3389/fphar.2017.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijens, B., De Geest, S., Hughes, D. A., Przemyslaw, K., Demonceau, J., Ruppar, T., Dobbels, F., Fargher, E., Morrison, V., Lewek, P., Matyjaszczyk, M., Mshelia, C., Clyne, W., Aronson, J. K., Urquhart, J., & ABC Project Team. (2012). A new taxonomy for describing and defining adherence to medications. British Journal of Clinical Pharmacology, 73(5), 691–705. 10.1111/j.1365-2125.2012.04167.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield, J. N. (2006). An introduction to systems science. World scientific. [Google Scholar]
- Warfield, J. N., & Cárdenas, A. R. (1994). A handbook of interactive management. Iowa State University Press Ames. [Google Scholar]
- Wood, W. C., & Roth, R. M. (1990). A workshop approach to acquiring knowledge from single and multiple experts (pp. 275–300).
- Zhou, B., Danaei, G., Stevens, G. A., Bixby, H., Taddei, C., Carrillo-Larco, R.M., et al. , & Ezzati, M. (2019). Long-term and recent trends in hypertension awareness, treatment, and control in 12 high-income countries: An analysis of 123 nationally representative surveys. The Lancet, 394(10199), 639–651. 10.1016/S0140-6736(19)31145-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The materials and datasets generated and/or analysed during the current study are available in the OSF repository https://osf.io/xusby/.