Abstract
Purpose of the Review
This article reviews common imaging modalities used in diagnosis and management of acute stroke. Each modality is discussed individually and clinical scenarios are presented to demonstrate how to apply these modalities in decision-making.
Recent Findings
Advances in neuroimaging provide unprecedented accuracy in determining tissue viability as well as tissue fate in acute stroke. In addition, advances in machine learning have led to the creation of decision support tools to improve the interpretability of these studies.
Summary
Noncontrast head computed tomography (CT) remains the most commonly used initial imaging tool to evaluate stroke. Its exquisite sensitivity for hemorrhage, rapid acquisition, and widespread availability make it the ideal first study. CT angiography (CTA), the most common follow-up study after noncontrast head CT, is used primarily to identify intracranial large vessel occlusions and cervical carotid or vertebral artery disease. CTA is highly sensitive and can improve accuracy of patient selection for endovascular therapy through delineations of ischemic core. CT perfusion is widely used in endovascular therapy trials and benefits from multiple commercially available machine-learning packages that perform automated postprocessing and interpretation. Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) can provide valuable insights for outcomes prognostication as well as stroke etiology. Optical coherence tomography (OCT), positron emission tomography (PET), single-photon emission computerized tomography (SPECT) offer similar insights. In the clinical scenarios presented, we demonstrate how multimodal imaging approaches can be tailored to gain mechanistic insights for a range of cerebrovascular pathologies.
Neuroimaging is playing an increasingly central role in the diagnosis and management of patients with suspected stroke. Recent advances have resulted in a shift away from traditional time-based definitions of treatment eligibility towards tissue- and imaging-based definitions in acute ischemic stroke (AIS). With these shifts, there is an increased importance for neurologists to understand and interpret neuroimaging studies and make use of available artificial intelligence (AI) decision support tools. In this review, we discuss some of the more commonly used imaging modalities for patients with stroke and provide clinical scenarios to demonstrate when and how these studies can inform their care.
Computed Tomography
Noncontrast head CT (NCHCT) remains the study of choice for the initial evaluation of patients with suspected stroke. NCHCT is widely available, inexpensive, rapidly obtained, and can be deployed in mobile stroke units. Newer CT scanners with up to 640 detector rows can examine the whole brain in less than 1 second. NCHCT average effective radiation dose is 2 mSv, with values in literature ranging from 0.9 to 4.0 mSv.1 Because the clinical presentation of AIS and intracranial hemorrhage (ICH) can be indistinguishable, the exquisite sensitivity of NCHCT for ICH makes it an invaluable tool. Whereas high detector scanners are the latest innovation, standard CT scanners accomplish the majority of acute stroke management at equal efficacy. An acute ICH will appear hyperdense (bright) as compared to normal brain parenchyma at 40–60 Hounsfield units (HU). The appearance may increase to 60–80 HUs within the first few hours, then attenuate over time at a rate of −0.7 to 1.5 HU/d.2 NCHCT is less sensitive for detecting subacute or chronic hematomas.
In addition to ruling out hemorrhage, NCHCT can reveal valuable insights for patients with AIS, including signs of early ischemic changes, chronic infarcts, or the hyperdense artery sign. In the first 6 hours of symptom onset, subtle early ischemic changes (EICs) can appear as loss of gray–white differentiation, cortical swelling, or mass effect.3 EICs later appear as a more distinct hypodensity, as the infarction becomes irreversible and cytotoxic edema develops. Interrater reliability for any early infarction sign varies between a kappa value of 0.14 and 0.78.4 The Alberta Stroke Program Early CT Score (ASPECTS) is a widely used 10-point scale for the evaluation of early ischemic changes in the middle cerebral artery (MCA) territory.5 A window width and level setting of 40/40 on NCHCT can accentuate the contrast for brain parenchyma to better visualize EICs. EICs are not contraindications to reperfusion, but extensive early ischemic changes are associated with poor outcomes and with greater risk of fatal or symptomatic hemorrhage with thrombolytics. Chronic infarct on NCHCT, well-defined hypodensity or encephalomalacia in an arterial territory, can be informative for stroke etiology and help guide management. The hyperdense artery sign, defined as notable hyperdensity within an artery compared to an isodense, normal appearance of an artery, can be highly specific for the presence of an intraarterial thrombus.
Whereas NCHCT has a low sensitivity for initial diagnosis of AIS, it has high specificity for irreversible ischemic injury. NCHCT sensitivity for infarction of follow-up imaging varies from 20% to 87% depending on the quality of imaging and training and experience of the reader.4
CT Angiography
CT angiography (CTA) of the head and neck is a fast and reliable diagnostic study for extra- and intracranial occlusive disease. Spiral CT and increasing detector arrays have improved the resolution of imaging, decreased scan time, and increased scanning volumes. Quicker scans reduce movement artifact and the amount of contrast needed. CTA average effective radiation dose is 5 mSv, with reported range from 0.8 to 20 mSv.6 CTA sensitivity and specificity has been reported as 100% and 63%, respectively, in extracarotid disease7 and up to 100% for intracranial disease.8 Maximum intensity projection and 3D reconstructions provide rapid detection of clot length, distal stenosis, and leptomeningeal collaterals. CTA source images can be used to detect brain tissue infarction. Diminished contrast enhancement on 40/40 window distal to the large vessel occlusion (LVO) likely represents hypoperfused brain parenchyma that will be irreversibly damaged without restoration of flow.9 CTA source images can improve the sensitivity and accuracy of final infarct volume and correlate closely with MRI diffusion-weighted images.10
CTA should not delay the administration of intravenous (IV) thrombolytics, and stroke protocols often specify administration of IV tissue plasminogen activator (tPA) bolus and initiation of drip prior to CTA. CTA is usually performed following the NCHCT and tPA administration, if applicable, to identify major arterial occlusions in patients who may be candidates for endovascular thrombectomy (EVT). CTA requires good IV access and adequate renal function due to the contrast bolus. Although often debated, accumulating evidence suggests no association between contrast administration and acute kidney injury.11
In acute hemorrhagic stroke, CTA can accurately diagnose cerebral aneurysms down to 2 mm in diameter.12 Similarly, CTA has diagnostic utility for arteriovenous malformations and venous thrombosis. Hematoma growth within the first 3 hours occurs in approximately 30% of patients with ICH, which is predictive of a poor prognosis.13 CTA spot sign, a leak of contrast media hyperdense to the surround hematoma, can predict hematoma growth, morbidity, and mortality.14
CT Perfusion
CT perfusion (CTP) is a CT technique meant to measure cerebral tissue perfusion. CTP effective radiation doses have ranged between 1.1 and 5 mSv.6 Whereas many parameters may be calculated with this approach, commonly derived maps include relative cerebral blood flow (rCBF), relative cerebral blood volume (rCBV), time to maximum of residue function (Tmax), and mean transit time (MTT).15 CTP images are acquired by the administration of a venous bolus of iodinated contrast followed by repeated CT acquisitions over the same region of brain over a period of time. From these images, the transit of contrast into the arteries, out into the capillaries and parenchyma, and finally into the veins is captured, and based on these time–density contrast curves, the above measures are calculated.
In AIS, CTP is used to predict the extent of irreversible brain injury (termed “ischemic core”) as well as the volume of tissue at risk of irreversible injury (termed “penumbra”).16 Ischemic core predictions are usually made from relative cerebral blood flow (rCBF) or relative cerebral blood volume (rCBV) changes relative to the contralateral hemisphere and penumbral predictions off of MTT or Tmax. CBF and CBV have been extensively validated, with 24-hour MRI and CT, and a relative reduction by 30% in the CBF is the most commonly used threshold to define infarct core.17 However, because infarct expansion can occur without rapid reperfusion, the validation of this measure is imperfect. CBF definitions of infarct core are known to over- and underestimate core in different situations, particularly in patients undergoing rapid reperfusion after imaging, and those who present very early in their infarct course. Both MTT and Tmax measure the delay in perfusion to brain tissue. A range of thresholds for these 2 measures have been tested, and most commonly validated against delayed MRI in patients without successful reperfusion. The most commonly used measurement to detect the volume of tissue at risk, and the one that is believed to be most accurate, is Tmax >6 seconds. RAPID, Viz.ai, Olea, GE, Philips, Siemens Syngo, and MIStar are a few of the commonly used software packages to perform automated determinations of these measures from raw CTP images.15 A mismatch ratio can calculated by the volume of penumbra (e.g., Tmax >6 seconds) divided by the volume of core (e.g., rCBF <30% compared to the contralateral hemisphere). Such automated measurements were used in both late-window EVT trials and were incorporated into the recent guidelines.18 Based on these metrics, an absence of mismatch CBF and Tmax would indicate that treatment is unlikely to benefit the patient.
Although CTP has evolved rapidly, limitations persist including patient motion artifacts, partial averaging effect of selected regions of interest for arterial input and venous outflow, and postprocessing variability. These limitations can lead to differences in parameter maps across different vendor maps. The requirement for CTP to identify EVT candidates in delayed or early time windows remains a question of ongoing study.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is superior to NCHCT for the detection of ischemia at earlier time points as well as smaller or posterior fossa lesions. On the other hand, MRI is expensive with limited availability and contraindicated in certain patients with metallic foreign bodies or older generation implantable devices. Standard sequences (T1-weighted, T2-weighted, and fluid-attenuated inversion recovery [FLAIR]) are relatively insensitive for detection of hyperacute ischemia, but diffusion-weighted imaging (DWI) is highly sensitive, specific, and accurate (88%–100%, 95%–100%, and 95% respectively).19 DWI can detect early ischemia within minutes, appearing as a hyperintense signal from restricted water diffusion in brain tissue. DWI is an exponential function of the random Brownian motion of water in tissue, with a linear component based on a T2-weighted signal and a brighter (i.e., more restricted) diffusibility signal. Apparent diffusion coefficient (ADC) maps can help distinguish between true restricted diffusion (DWI bright, ADC dark, T2 bright) and T2 shine-through (DWI bright, ADC bright, T2 bright). DWI signal intensity is maximal at 40 hours and normalizes within 2 to several weeks; ADC signal intensity is maximal at 28 hours, pseudonormalizes at 10 days, and then converts to bright. AIS signs can also be seen on other MRI sequences: absent arterial flow void on T2, “blooming” hypointense artery on gradient recalled echo (GRE) due to acute intravascular thrombus, and intravascular hyperintensity on FLAIR, indicative of slow or collateral flow. GRE or susceptibility-weighted imaging (SWI) sequences can evaluate for the presence of acute or chronic hemorrhages and cerebral veins, which appear dark.
In ICH, MRI appearance changes by hematoma age.20 Although the MRI physics related to hemorrhage and blood products are complex, the 5 generally accepted stages of hemorrhage are hyperacute (<24 hours, oxyhemoglobin, T1 isointense, T2 bright), acute (1–3 days, deoxyhemoglobin, T1 isointense, T2 dark), early subacute (>3 days, intracellular methemoglobin, T1 bright, T2 dark), late subacute (>7 days, extracellular methemoglobin, T1 bright, T2 bright), and chronic (>14 days, hemosiderin, T1 dark, T2 dark). An intraparenchymal hematoma volume may be misrepresented on GRE, as the signal is amplified and may appear larger than the amount of blood present. Subarachnoid or subdural hemorrhages can be readily detected on FLAIR sequence, in which CSF signal is suppressed. HARM sign (hyperintense acute reperfusion marker) on postcontrast FLAIR indicates early blood-brain barrier disruption and is associated with reperfusion and increased risk of hemorrhagic transformation, often after thrombolytic therapy.21
Recently, MRI has been shown to identify patients with unknown time of onset of AIS. In the WAKE-UP trial, patients who had restricted diffusion DWI changes without FLAIR changes were randomized to receive IV tPA.22 A DWI-positive lesion without corresponding FLAIR change is suggestive that the stroke occurred within 6 hours, and this study showed benefit for thrombolysis in the cohort with this imaging signature relative to placebo.
MRI also provides information regarding stroke etiology. In ischemic stroke, involvement of multiple vascular territories or a cortical wedge-shaped infarct can suggest cardioembolic etiology, where borderzone infarcts between 2 vascular territories can suggest arterial stenosis.23 In hemorrhagic stroke, lobar, cortical, or corticosubcortical microbleeds or superficial siderosis can suggest cerebral amyloid angiopathy and smaller hematoma within a DWI lesion with minimal edema can suggest hemorrhagic transformation over primary hematoma.
MR Angiography
Magnetic resonance angiography (MRA) is a powerful diagnostic modality for the detection of vessel stenosis or occlusions, but more time-consuming than CTA and not available for emergent use 24/7 in all hospitals. It can be used in place of CTA in the acute stroke setting for detection of LVO in patients with IV contrast allergy or acute renal failure. It often is used for vessel imaging during the subacute or chronic infraction phase in conjunction with MRI studies. Given concern of gadolinium retention in patients with chronic kidney disease or on dialysis and risk of nephrogenic systemic fibrosis,24 MRA of the head and neck can be obtained with or without contrast, the latter utilizing time-of-flight technique. In contrast-enhanced MRA, the vascular anatomy is outlined with blood containing the bolus injection of gadolinium. In time-of-flight MRA, the vascular signal is flow dependent on the direction and velocity of blood, is more prone to artifact, and can overestimate the degree of stenosis.
Despite its limitations, MRA rivals other imaging modalities for the diagnosis of stenoses, occlusions, and dissections. MRA sensitivity and specificity for carotid occlusions in 100% in most studies.25 If MRA shows no stenosis or less than 70% stenosis, no further diagnostic evaluation is typically warranted. However, if MRA shows greater than 70% stenosis, further evaluation is suggested with another modality such as duplex ultrasonography or conventional angiography. A newer application of contrast-enhanced MRI vascular imaging is black-blood imaging, also known as spatial presaturation MRI.26 In black-blood MRI, the lumen of the vessel is converted to low signal intensity, providing higher image clarity to the vessel wall and its various components including lipid deposits, fibrous caps, calcium, and thrombus. Dissections can be seen as increased signal of the vessel wall or surrounding with lumen narrowing on T1-weighted images with fat suppression and absent or decreased visualization of the vessel.27 False lumens with intimal flaps are best seen on T2-weighted images. In the setting of negative MRA and high clinical suspicion, CTA should be obtained to rule out a dissection. MRA has 95% sensitivity in detecting intracranial aneurysms, with false-positives and negatives often located at the skull base and MCA.28 MRA also has an up to 69%–100% sensitivity and 75%–100% specificity to detect an aneurysm after subarachnoid hemorrhage, with lower sensitivity with smaller aneurysms. However, aneurysms smaller than 5 mm may be missed.
MR Perfusion
Magnetic resonance perfusion (MRP), like CTP, can produce perfusion maps of the relative CBV, CBF, Tmax, and MTT. MRP postprocessing images can be generated within minutes upon bolus injection of gadolinium and acquisition of SWI and T2-weighted sequences. MRP can also be obtained without contrast using arterial spin labeling, and gives comparable diagnostic information of perfusion defects in acute infarcts, tissue reperfusion after recanalization, and hyperperfusion of reperfused subacute infarcts.29 A greater number of patients with modest ischemia who do not reach the threshold of tissue compromise to cause DWI positivity may be detected with MRP through regions of relative hypoperfusion. Furthermore, MRI diffusion–perfusion mismatch can be used to select patients for extended time window reperfusion, as in the DEFUSE-3 trial.30
MR or CT Venography
MR venography (MRV) or CT venography (CTV) can be obtained in cases of suspected venous occlusion.31 MRV with contrast is the accepted choice of imaging in the diagnosis of sagittal sinus thrombosis. MRI with MRV has a sensitivity of 95% in the detection of cerebral venous sinus thrombosis. The specificity of MRV can vary depending on the manner in which it has been acquired. Recent studies of contrast-enhanced 4D MRV have demonstrated sensitivity of 97% and specificity of 99% for this technique.32 MRV findings of cerebral thrombosis include lack of high flow signal from the sinus and direct visualization of the thrombus, including isolated cortical thrombosis. Venous congestion, infarction, and hemorrhage can be also visualized on MRI, the latter of which has the typical MRI appearance of blood based on the stage of blood breakdown product. Thrombosed sinuses or veins may also be seen on T2- and T1-weighted sequences with a sensitivity of 91% and 71%, respectively.
NCHCT and CTV can also be obtained, but these studies are not as sensitive in diagnosing cerebral venous thrombosis. A thrombosed vein or sinus will appear hyperdense (“dense cord”) on NCHCT. In comparison, the “empty delta” will be present on the NCHCT with contrast. CTV is not sensitive to detect cortical venous thrombosis.
Optical Coherence Tomography
Optical coherence tomography (OCT) is a minimally invasive emerging technology already in wide use in cardiology that provides high-resolution imaging of intravascular pathology by measuring optical scattering in tissues through low-coherence interferometry.33 Despite an imaging depth of only a few millimeters, OCT yields significantly higher resolution images than ultrasound, providing micron level of detail in the evaluation of vascular pathologies that involve vessel wall layers. OCT has been used successfully in the evaluation of extracranial pathologies including atherosclerosis and dissection.34 As an adjunctive imaging modality during endoluminal stenting, OCT can be used to identify stent apposition to the parent vessel and potential leaks.35 In addition, OCT can identify intraluminal stenosis or thrombosis, neovascularization, ulceration, and lipid deposition. Other putative applications include risk stratification for cerebral aneurysms. As OCT imaging devices develop and become safer for intracranial deployment, this imaging modality may play a greater role in characterization of intracranial atherosclerotic disease as well. OCT is an emergent imaging field with respect to endovascular visualization and will likely play a more prominent role in the coming years.
Ultrasonography
Ultrasonography remains the gold standard for noninvasive real-time monitoring of cerebral blood flow. Ultrasonography including Duplex ultrasonography and transcranial Doppler ultrasonography (TCD) is inexpensive, readily available in many hospitals, and safe, given that it does not involve radiation or contrast. Ultrasonography is, however, highly operator-dependent, and its results can have significant variability across different imaging laboratories. Ultrasound images are formed through the backscattering or reflection of sound waves detected by the probe from tissues with different acoustic properties.36 Images are generated using a technique called brightness modulated (B-mode) that determines the amplitude of sequentially arriving returned echoes. The grayscale images are displayed as either white (strong) or dark (weak), based on the depth of the returned echoes. Blood flow can also be displayed as color images within the B-mode (color-coded Doppler flow imaging). B-flow, harmonic imaging, Doppler velocity spectral display, and 3D ultrasonography are additional techniques and modalities to examine the vessels and their pathologies.37,38
Ultrasound has a broad spectrum of diagnostic and therapeutic applications. Ultrasound is commonly used to study extracranial carotid stenosis and plaque morphology. The degree of carotid stenosis can be investigated through peak systolic velocity, end-diastolic velocity (EDV), and the systolic internal carotid artery/common carotid artery velocity ratio by B-mode ultrasound.39 Dissections and inflammatory disease including Takayasu arteritis of the anterior and posterior circulation can also be detected and monitored with ultrasonography.40,41
In acute stroke, ultrasonography can provide real-time information about stenosis and occlusion and collateral circulation and monitor vasomotor reactivity, embolization, and recanalization following thrombolytic therapy.42 Vasomotor reactivity testing, emboli detection, and right-to-left shunt detection help ascertain stroke mechanisms, plan treatment, and determine prognosis. TCD can detect and monitor for vasospasm after spontaneous subarachnoid hemorrhage, monitor elevated intracranial pressure through waveform changes, and confirm cerebral circulatory arrest. In addition, high-frequency ultrasound and ultrasound with microbubbles (sonolysis) can potentially enhance the efficacy of thrombolysis and achieve recanalization in the absence of thrombolysis.43 Research into disruption of the blood-brain barrier, enhancement of gene therapy delivery, and targeted drug delivery with ultrasound is ongoing.
Positron Emission Tomography/Single-photon Emission Computerized Tomography
Positron emission tomography (PET) and single-photon emission computerized tomography (SPECT) are nuclear medicine imaging modalities with great impact on research in the field of stroke. PET can be used to assess the pathophysiologic changes induced during ischemia, measuring the physiologic parameters and imaging the distribution of molecular markers.44 In stroke, 15O-labeled water PET can provide information when going from penumbra to ischemic tissue through abnormal glucose and oxygen metabolism.45 Radioligands can also be used as early markers of irreversible damage, which can be used to predict the size of final infarcts. With PET, the reserve capacity of CBF can be tested in carotid atherosclerotic disease, which is helpful in planning for future interventions.46 Using an acetazolamide challenge, SPECT can also evaluate decreased vascular reserve, which can be used to predict development of ischemia in patients undergoing endarterectomy. SPECT can characterize atherosclerotic plaque content, including oxidized low-density lipoprotein and apoptotic bodies.47 Reperfusion can be re-demonstrated on nuclear studies.48 Furthermore, PET may also provide details on metabolic and molecular alterations during neuroinflammation after stroke.
Digital Subtraction Angiography
Digital subtraction angiography (DSA) remains the gold standard for luminal imaging, providing exquisite spatial and temporal resolution of the cervical and cerebral circulation. It allows the neurointerventionalist to accurately diagnose and tailor treatments such as revascularization, angioplasty, stenting, and coiling to the patient. Large series have reported an approximately 1% risk of stroke with the procedure, dissection, puncture site complications, and iodine-related renal dysfunction.49 Additional limitations include operator-dependent image quality, limited availability, and cost. Other articles in this issue provide additional discussion on the role of DSA in stroke.
Artificial Intelligence/Machine Learning
Artificial intelligence (AI) has been making headways into medical imaging, and acute stroke may be one of its first widespread applications. As discussed above, the challenge with acute stroke imaging interpretation is the need for immediate reads, 24/7, at every hospital that receives patients with stroke. Current human resources to perform these reads at the expert level is lacking. As thrombolysis and EVT time windows broaden, this expertise gap will only become more pronounced. Machine learning (ML) offers an attractive solution as a decision support tool to aid the neurologist evaluation by directing attention towards regions of the imaging of particular relevance and rendering a suggested diagnosis.50
ML algorithms are already in relatively widespread, and rapidly expanding, use for the evaluation of patients for EVT.30,51,52 There are a number of software platforms available including eASPECTS (Brainomix), RAPID (IschemaView), and Viz.ai. These packages offer automated CT analysis with color-coded maps of the ischemic core and penumbra and angiographic vessel analysis. ML algorithms have also been developed to reliably identify the presence or absence of a LVO and determine the extent of infarction from CTA.9 AI can be further applied and used for acute prognosis prediction including risk of ICH.53 As the number of patients evaluated for acute stroke therapies increases, the role of ML is likely to continue to grow.
Illustrative Clinical Scenarios
Evaluation of ICH
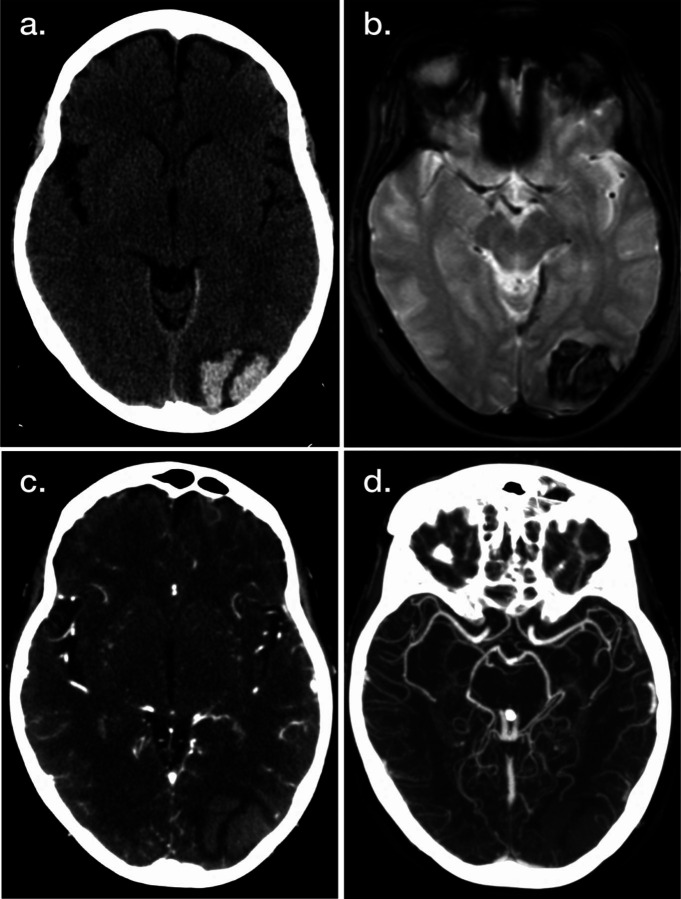
In many centers evaluating patients with possible acute stroke, including mobile stroke units, NCHCT remains the initial imaging study of choice. As discussed above, its speed as well as exquisite sensitivity for acute ICH enable rapid delivery of thrombolytic therapy in eligible patients, as ICH can be confidently excluded with a normal NCHCT. In patients who are discovered to have ICH, as shown in Figure 1, NCHCT also provides additional information, as the pattern and location of the acute hemorrhage can give clues as to the etiology. Hypertensive hemorrhages are commonly located in predictable brain regions including the pons, cerebellar nuclei, basal ganglia, and thalamus. Hemorrhages outside these territories (Figure 1A) may be associated with vascular malformations, amyloid angiopathy, or other etiologies. In patients with these less common etiologies, additional evaluation with MRI (Figure 1B) and CTA (Figure 1, C and D) can be helpful to identify arteriovenous malformations, cerebral aneurysms, cavernous malformations, hemorrhagic tumors, and other etiologies. CTA can also inform the likelihood of hematoma expansion. Note however that these additional modalities are not 100% sensitive to rule out these alternate etiologies, and at times additional imaging such as CTV or MRV, DSA, or delayed repeated MRI is required to complete the imaging evaluation.
Figure 1. Illustrative Example of Neuroimaging Applications for Intracranial Hemorrhage (ICH).
Noncontrast head CT (A), MRI gradient recalled echo (B), CT angiography (CTA) (C), and maximum intensity projection CTA (D) images demonstrate multiple representations of acute ICH.
Acute Decision-Making for Thrombolysis and Thrombectomy
The past several years have witnessed a sea change in eligibility for acute reperfusion therapies in AIS, for both thrombolytic as well as mechanical thrombectomy approaches. The neuroimaging evaluation is supplanting previous time-based definitions, particularly in the case of mechanical thrombectomy, but with recent and upcoming trials, possibly for thrombolysis as well. NCHCT, the commonly performed initial imaging study, is the only imaging study required prior to treatment with IV rtPA, by ruling out ICH.18 While ancillary studies such as MRI may provide additional information on likelihood of post-tPA hemorrhage by identifying microhemorrhages, current guidelines do not support its routine use for this purpose. Because the treatment effect of thrombolysis is highly time-dependent, the routine usage of CTP and MRP prior to treatment is similarly not recommended.
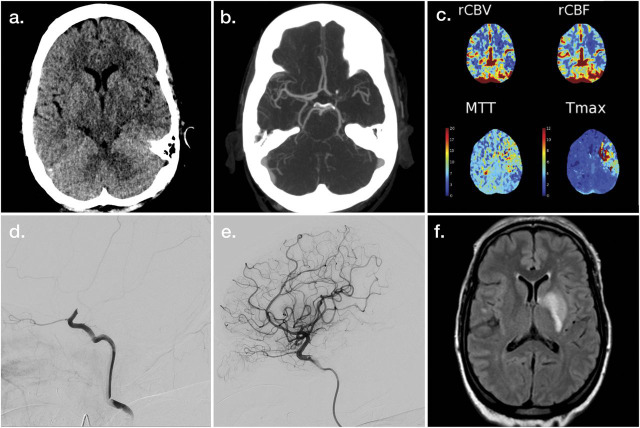
Once eligibility for thrombolysis has been determined and treatment delivered if appropriate, the next consideration is for mechanical thrombectomy. To this end, NCHCT continues to serve a valuable purpose. While it may not be the most sensitive technique to evaluate for acute ischemic changes, trained observers can consistently and reliably identify areas of irreversible injury, and quantified scales using NCHCT data are closely correlated with 90-day clinical outcomes in patients treated with thrombectomy.54 In Figure 2A, there is perceptible asymmetry of the lentiform regions of the left hemisphere compared to the right, and the remainder of the hemispheric regions (not shown) appeared symmetric, without evidence of ischemic changes. CTA is a highly effective subsequent imaging study to evaluate for intracranial occlusions (Figure 2B). Its required use has been questioned in the past, as prior publications demonstrated that patients with clinical deficits totaling a National Institutes of Health Stroke Scale ≥10 were 80% likely to harbor an intracranial occlusion.55 This approach was used in the IST-3 trial, and 89/434 (21%) of the patients randomized to endovascular therapy did not undergo mechanical thrombectomy, in large part because no accessible thrombus was present. In the 2019 AIS guidelines from the American Heart Association/American Stroke Association, noninvasive vascular imaging (i.e., CTA) is recommended in the initial imaging evaluations for patients who otherwise meet criteria.18
Figure 2. Illustrative Example of Neuroimaging Applications for Large Vessel Occlusion Acute Ischemic Stroke.
Noncontrast head CT (A), maximum intensity projection CT angiography (CTA) (B), postprocessed CT perfusion maps (C), and left carotid artery injection digital subtraction angiography (DSA) (D) demonstrate findings associated with acute left internal carotid artery terminus occlusion. Postrecanalization images are provided as left internal carotid artery injection DSA (E) and fluid-attenuated inversion recovery MRI (F). MTT = mean transit time; rCBF = relative cerebral blood flow; rCBV = relative cerebral blood volume; Tmax = time to maximum of residue function.
The critical question when evaluating a patient with LVO for possible benefit from EVT is the extent of ischemic core and the extent of the penumbra. CTP (Figure 1C) is an increasingly widely used tool to measure these, relying on calculated maps based on the transit of contrast through cerebral tissue. CTP, with automated postprocessing software packages to perform these calculations, and criteria based on strict cutoffs from these predicted volumes, has been shown to identify a cohort of patients who benefit from endovascular reperfusion (DAWN, DEFUSE-3)30,56 many hours after symptom onset. It is worth noting, however, that even patients who do not fulfill these criteria may benefit, and that the predicted ischemic core and penumbra regions may be inaccurate, in unpredictable ways.57,58
Rapid revascularization is the goal of treatment in patients with large vessel occlusion with reversibly injured tissue (Figure 2, D and E). In these cases, the final infarct volume (Figure 2F) should ideally correlate closely with the predicted initial ischemic core (Figure 1, A and C).
Evaluation of Carotid Stenosis
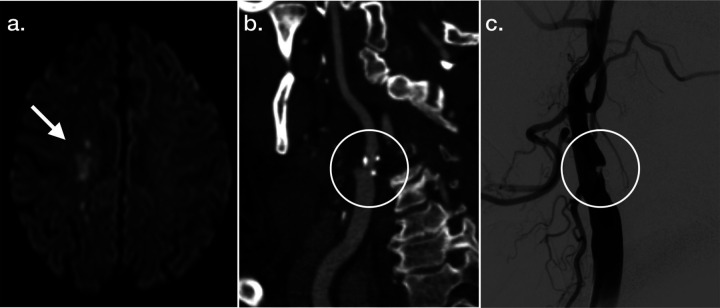
Extracranial carotid artery stenosis is one of the few acute stroke etiologies that can be directly addressed with a surgical procedure, and imaging plays a key role in its diagnosis as well as the criteria to define treatment eligibility. The pattern of infarction as seen on MRI (Figure 3A) can help suggest the etiology, and in this case, infarctions in the MCA/anterior cerebral artery or MCA/posterior cerebral artery borderzone frequently occur with vascular stenoses. Despite advances in our understanding of plaque morphology, and differential risks of recurrent stroke associated with varying atheroma compositions, guidelines for revascularization as well as the reimbursement of the procedure are tied directly to imaging-based measurements of degree of stenosis, for both symptomatic and asymptomatic lesions.59 Today, noninvasive imaging techniques including CTA (Figure 3B) can provide highly accurate and easily visualizable depictions of these stenosis, which can mirror the findings seen on gold standard DSA (Figure 3C).
Figure 3. Illustrative Example of Neuroimaging Applications in Carotid Stenosis.
MRI diffusion-weighted imaging (A), 3D reconstructed CT angiography (B), and right common carotid artery injection digital subtraction angiography (C) images are provided characterizing features associated with right internal carotid artery stenosis.
Evaluation of Intracranial Stenosis
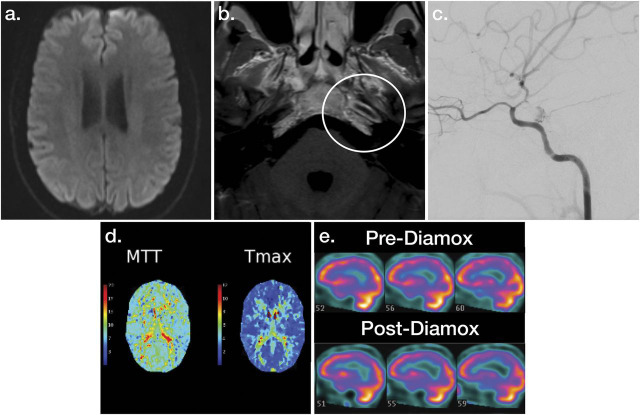
Stenosis of the intracranial vessels can result from a wide range of etiologies, including inflammatory disorders (i.e., vasculitis), atherosclerosis, moyamoya syndrome, dissection, and others; imaging plays a key role in the diagnosis and physiologic characterization of these disorders. Infarction that results from intracranial stenosis can occur as a result of hypoperfusion to cerebral tissues distal to the stenosis, occlusion of a branch vessel originating within the area of stenosis, or downstream thrombo-embolus originating from the area of vascular injury and stenosis. In the case of hypoperfusion, patients may present with transient or longer-lasting focal symptoms indicative of regional cerebral dysfunction, without conventional imaging evidence of established infarction in the acute setting (Figure 4A). Contrast-enhanced MRI, with techniques to focus on the vessel wall, can demonstrate wall thickening and enhancement of the affected or adjacent regions, a finding that can be helpful in narrowing the differential diagnosis of this condition (Figure 4B). DSA, with its superior spatial and temporal resolution, remains the gold standard for the visualization of the degree of intracranial luminal compromise, but more importantly it also displays changes in CBF downstream of the lesion (Figure 4C). Because cerebral perfusion is dynamic, clinical symptomatology can fluctuate, and performing conventional perfusion studies that capture a snapshot in time such as CTP can misrepresent the potential vulnerability to ischemia, by over- or underestimating hypoperfusion (Figure 4D). In these situations, a dynamic imaging modality that incorporates a challenge to the cerebrovascular reserve can help to define regions of brain tissue at risk of ischemia. One relatively widely available such study is SPECT with acetazolamide. Images are acquired at baseline and then after administration of acetazolamide and changes (decreases, in particular) of radionucleotide uptake in different brain regions can be qualitatively assessed (Figure 4E). SPECT imaging in patients with cerebrovascular stenotic disease has been validated against gold standard O-15-H2O PET.60
Figure 4. Illustrative Example of Neuroimaging Evaluations for Intracranial Stenotic Disease.
MRI diffusion-weighted imaging (A), contrast-enhanced T1 MRI (B), left internal carotid artery injection digital subtraction angiography (C), postprocessed CT perfusion maps (D), and acetazolamide SPECT (E) images demonstrate features associated with left intracranial carotid artery stenosis. MTT = mean transit time; Tmax = time to maximum of residue function.
Modern neuroimaging is able to determine tissue viability and predict tissue fate with improved accuracy. With these advancements have come expanded roles for thrombolysis and thrombectomy in greater proportions of patients. As these treatment options grow, the role of the neurologist in understanding and interpreting neuroimaging studies in a rapid and confident fashion is becoming increasingly crucial.
Glossary
- ADC
apparent diffusion coefficient
- AI
artificial intelligence
- AIS
acute ischemic stroke
- ASPECTS
Alberta Stroke Program Early CT Score
- CBF
cerebral blood flow
- CBV
cerebral blood volume
- CT
computed tomography
- CTA
CT angiography
- CTP
CT perfusion
- CTV
CT venography
- DSA
digital subtraction angiography
- DWI
diffusion-weighted imaging
- EDV
end-diastolic velocity
- EIC
early ischemic change
- EVT
endovascular thrombectomy
- FLAIR
fluid-attenuated inversion recovery
- GRE
gradient recalled echo
- ICH
intracranial hemorrhage
- LVO
large vessel occlusion
- MCA
middle cerebral artery
- ML
machine learning
- MRA
magnetic resonance angiography
- MRP
magnetic resonance perfusion
- MRV
magnetic resonance venography
- MTT
mean transit time
- NCHCT
noncontrast head CT
- OCT
optical coherence tomography
- PET
positron emission tomography
- rCBF
relative cerebral blood flow
- rCBV
relative cerebral blood volume
- SPECT
single-photon emission computerized tomography
- SWI
susceptibility-weighted imaging
- TCD
transcranial Doppler ultrasonography
- Tmax
time to maximum of residue function
- tPA
tissue plasminogen activator
Appendix. Authors
Study Funding
American Academy of Neurology/Society of Vascular and Interventional Neurology Interventional Neurology Career Development Award (PI: S.A. Sheth).
Disclosure
A. Czap is funded by a grant from the NIH (2T32NS007412-21). S. Sheth served at the Neuroimaging Core Laboratory for Penumbra COMPLETE registry; performs neuroimaging in his clinical practice and bills for this procedure; and is funded by grants from the NIH (U18EB029353 and R01NS121154), American Academy of Neurology/Society of Vascular and Interventional Neurology, and the Translational Research Institute for Space Health (19-19BRASH-2-0030). Go to Neurology.org/N for full disclosures.
References
- 1.Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248(1):254-263. [DOI] [PubMed] [Google Scholar]
- 2.Osborn AG. Diagnostic imaging. Brain, 2nd ed. Amirsys; 2010. [Google Scholar]
- 3.von Kummer R, Bourquain H, Bastianello S, et al. . Early prediction of irreversible brain damage after ischemic stroke at CT. Radiology. 2001;219(1):95-100. [DOI] [PubMed] [Google Scholar]
- 4.Wardlaw JM, Mielke O. Early signs of brain infarction at CT: observer reliability and outcome after thrombolytic treatment: systematic review. Radiology. 2005;235(2):444-453. [DOI] [PubMed] [Google Scholar]
- 5.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy: Alberta Stroke Programme Early CT Score. Lancet. 2000;355(9216):1670-1674. [DOI] [PubMed] [Google Scholar]
- 6.Cohnen M, Wittsack HJ, Assadi S, et al. Radiation exposure of patients in comprehensive computed tomography of the head in acute stroke. AJNR Am J Neuroradiol. 2006;27(8):1741-1745. [PMC free article] [PubMed] [Google Scholar]
- 7.Josephson SA, Bryant SO, Mak HK, Johnston SC, et al. . Evaluation of carotid stenosis using CT angiography in the initial evaluation of stroke and TIA. Neurology. 2004;63(3):457-460. [DOI] [PubMed] [Google Scholar]
- 8.Nguygen-Huynh MN, Wintermark M, English J, et al. . How accurate is CT angiography in evaluate intracranial atherosclerotic disease? Stroke. 2008;39(4):1184-1188. [DOI] [PubMed] [Google Scholar]
- 9.Sheth SA, Lopez-Rivera V, Barman A, Grotta JC, et al. . Machine learning-enabled automated determination of acute ischemic core from computed tomography angiography. Stroke. 2019;50(11):3093-3100. [DOI] [PubMed] [Google Scholar]
- 10.Bal S, Bhatia R, Menon BK, Shobha N, et al. . Time dependence of reliability of noncontrast computed tomography in comparison to computed tomography angiography source image in acute ischemic stroke. Int J Stroke. 2015;10(1):55-60. [DOI] [PubMed] [Google Scholar]
- 11.Brinjikji W, Demchuk AM, Murad MH, Rabinstein AA, et al. . Neurons over nephrons: systematic review and meta-analysis of contrast-induced nephropathy in patients with acute stroke. Stroke. 2017;48(7):1862-1868. [DOI] [PubMed] [Google Scholar]
- 12.Yang ZL, Ni QQ, Schoepf UJ, De Cecco CN. Small intracranial aneurysms: diagnostic accuracy of CT angiography. Radiology. 2017;285(3):941-952. [DOI] [PubMed] [Google Scholar]
- 13.Dowlatshahi D, Demchuk AM, Flaherty ML, et al. . Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76(14):1238-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, et al. . Prediction of haematoma growth and outcome in patients with intracerebral hemorrhage using the CT angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol. 2012;11(4):307-314. [DOI] [PubMed] [Google Scholar]
- 15.Vagal A, Wintermark M, Nael K, Bivard A, et al. . Automated CT perfusion imaging for acute ischemic stroke: pearls and pitfalls for real-world use. Neurology. 2019;93(20):888-898. [DOI] [PubMed] [Google Scholar]
- 16.Donahue J, Wintermark M. Perfusion CT and acute stroke imaging: foundations, applications, and literature review. J Neuroradiol. 2015;42(1):21-29. [DOI] [PubMed] [Google Scholar]
- 17.Demeestere J, Wouters A, Christensen S, Lemmens R, Lansberg MG. Review of perfusion imaging in acute ischemic stroke: from time to tissue. Stroke. 2020;51(3):1017-1024. [DOI] [PubMed] [Google Scholar]
- 18.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, et al. . Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. [DOI] [PubMed] [Google Scholar]
- 19.Grotta JC, Adams HP. Stroke: pathophysiology, diagnosis, and management. In: Magnetic Resonance Imaging of Cerebrovascular Disease, 6th ed. Elsevier; 2016. [Google Scholar]
- 20.Kidwell CS, Wintermark M. Imaging of intracranial haemorrhage. Lancet Neurol. 2008;7(3):256-267. [DOI] [PubMed] [Google Scholar]
- 21.Kidwell CS, Latour L, Saver JL, et al. . Thrombolytic toxicity: blood brain barrier disruption in human ischemic stroke. Cerebrovasc Dis. 2008;25(4):338-343. [DOI] [PubMed] [Google Scholar]
- 22.Thomalla G, Simonsen CZ, Boutitie F, et al. . WAKE-UP Investigators. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379(7):611-622. [DOI] [PubMed] [Google Scholar]
- 23.Kang DW, Chalela JA, Ezzeddine MA, et al. . Association of ischemic lesion patterns on early diffusion-weighted imaging with TOAST stroke subtypes. Arch Neurol. 2003;60(12):1730-1734. [DOI] [PubMed] [Google Scholar]
- 24.Do C, DeAguero J, Brearley A, et al. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360. 2020;1(6):561-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Debrey SM, Yu H, Lynch JK, et al. . Diagnostic accuracy of magnetic resonance angiography for internal carotid artery disease: a systematic review and meta-analysis. Stroke. 2008;39(8):2237-2248. [DOI] [PubMed] [Google Scholar]
- 26.Al-Smadi AS, Abdalla RN, Elmokadem AH, Shaibani A, et al. . Diagnostic accuracy of high-resolution black-blood MRI in the evaluation of intracranial large-vessel arterial occlusions. AJNR Am J Neuroradiol. 2019;40(6):954-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stringaris K, Liberopoulos K, Giaka E, et al. . Three-dimensional time-of-flight MR angiography and MR imaging versus conventional angiography in carotid artery dissections. Int Angiol. 1996;15(1):20-25. [PubMed] [Google Scholar]
- 28.Sailer AM, Wagemans BA, Nelemans PJ, et al. . Diagnosing intracranial aneurysms with MR angiography: systematic review and meta-analysis. Stroke. 2014;45(1):119-126. [DOI] [PubMed] [Google Scholar]
- 29.Alsop DC, Detre JA. Multisection cerebral blood flow MR imaging with continuous arterial spin labeling. Radiology. 1998;208(2):410-416. [DOI] [PubMed] [Google Scholar]
- 30.Albers GW, Marks MP, Kemp S, et al. . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saposnik G, Barinagarrementeria F, Brown RD Jr, et al. . Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192. [DOI] [PubMed] [Google Scholar]
- 32.Meckel S, Reisinger C, Bremerich J, et al. Cerebral venous thrombosis: diagnostic accuracy of combined, dynamic and static, contrast-enhanced 4D MR venography. AJNR Am J Neuroradiol. 2010;31(3):527-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen CJ, Kumar JS, Chen SH, Ding D, et al. . Optical coherence tomography: future applications in cerebrovascular imaging. Stroke 2018;49(4):1044-1050. [DOI] [PubMed] [Google Scholar]
- 34.Cilingiroglu M, Hakeem A, Feldman M, Wholey M. Optical coherence tomography imaging in asymptomatic patients with carotid artery stenosis. Cardiovasc Revasc Med. 2013;14(1):53-56. [DOI] [PubMed] [Google Scholar]
- 35.Reimers B, Nikas D, Stabile E, Favero L, et al. . Preliminary experience with optical coherence tomography imaging to evaluate carotid artery stents: safety, feasibility and techniques. EuroIntervention. 2011;7(1):98-105. [DOI] [PubMed] [Google Scholar]
- 36.Edelman SK. Understanding Ultrasound Physics, 2nd ed. ESP, Inc.; 1997. [Google Scholar]
- 37.Seidel G, Algermissen C, Christoph A, et al. . Harmonic imaging of the human brain: visualization of brain perfusion with ultrasound. Stroke. 2000;31(1):151-154. [DOI] [PubMed] [Google Scholar]
- 38.Wessels T, Harrer J, Stetter S, et al. . Three-dimensional assessment of extracranial Doppler sonography in carotid artery stenosis compared with digital subtraction angiography. Stroke. 2004;35(8):1847-1851. [DOI] [PubMed] [Google Scholar]
- 39.Grant EG, Benson CB, Moneta GL, et al. . Carotid artery stenosis: gray-scale and Doppler US diagnosis: Society of Radiologists in Ultrasound consensus conference. Radiology. 2003;229(2):340-346. [DOI] [PubMed] [Google Scholar]
- 40.Wessels T, Mosso M, Krings T, et al. . Extracranial and intracranial vertebral artery dissection: long-term clinical and duplex sonographic follow-up. J Clin Ultrasound. 2008;36(8):472-479. [DOI] [PubMed] [Google Scholar]
- 41.Park S, Chung J, Lee J, et al. . Carotid artery involvement in Takayasu's arteritis: evaluation of the activity by ultrasonography. J Ultrasound Med. 2001;20(4):371-378. [DOI] [PubMed] [Google Scholar]
- 42.Basic identification criteria of Doppler microembolic signals: consensus committee of the ninth international cerebral hemodynamic symposium. Stroke. 1995;26(6):1123. [PubMed] [Google Scholar]
- 43.Saqqur M, Tsivgoulis G, Nicoli F, et al. . The role of sonolysis and sonothrombolysis in acute ischemic stroke: a systematic review and meta-analysis of randomized controlled trials and case-control studies. J Neuroimaging. 2014;24(3):209-220. [DOI] [PubMed] [Google Scholar]
- 44.Powers WJ, Zazulia AR. PET in cerebrovascular disease. PET Clin. 2010;5(1):83106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baron JC. Mapping the ischaemic penumbra with PET: implications for acute stroke treatment. Cerebrovasc Dis. 1999;9(4):193-201. [DOI] [PubMed] [Google Scholar]
- 46.Matsubara S, Moroi J, Suzuki A, Sasaki M, et al. . Analysis of cerebral perfusion and metabolism assessed with positron emission tomography before and after carotid artery stenting: clinical article. J Neurosurg. 2009;111(1):28-36. [DOI] [PubMed] [Google Scholar]
- 47.Jaffer FA, Libby P, Weissleder R. Molecular and cellular imaging of atherosclerosis: emerging applications. J Am Coll Cardiol. 2006;47(7):1328-1338. [DOI] [PubMed] [Google Scholar]
- 48.Grotta JC, Alexandrov AV. tPA-associated reperfusion after acute stroke demonstrated by SPECT. Stroke. 1998;29(2):429-432. [DOI] [PubMed] [Google Scholar]
- 49.Kaufmann TJ, Huston J III, Mandrekar JN, et al. . Complications of diagnostic cerebral angiography: evaluation of 19,826 consecutive patients. Radiology. 2007;243(3):812-819. [DOI] [PubMed] [Google Scholar]
- 50.Murray NM, Unberath M, Hager GD, Hui FK. Artificial intelligence to diagnose ischemic stroke and identify large vessel occlusions: a systematic review. J Neurointerv Surg. 2020;12(2):156-164. [DOI] [PubMed] [Google Scholar]
- 51.Nagel S, Sinha D, Day D, et al. . e-ASPECTS software is non-inferior to neuroradiologists in applying the ASPECT score to computed tomography scans of acute ischemic stroke patients. Int J Stroke. 2017;12(6):615-622. [DOI] [PubMed] [Google Scholar]
- 52.Herweh C, Ringleb PA, Rauch G, et al. . Performance of e-ASPECTS software in comparison to that of stroke physicians on assessing CT scans of acute ischemic stroke patients. Int J Stroke. 2016;11(4):438-445. [DOI] [PubMed] [Google Scholar]
- 53.Bentley P, Ganesalingam J, Carlton Jones AL, et al. . Prediction of stroke thrombolysis outcome using CT brain machine learning. Neuroimage Clin. 2014;4:635-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoo AJ, Zaidat OO, Chaudhry ZA, Berkhemer OA, et al. . Impact of pretreatment noncontrast CT Alberta Stroke Program Early CT Score on clinical outcome after intra-arterial stroke therapy. Stroke. 2014;45(3):746-751. [DOI] [PubMed] [Google Scholar]
- 55.Fischer U, Arnold M, Nedeltchev K, et al. . NIHSS score and arteriographic findings in acute ischemic stroke. Stroke. 2005;36(10):2121-2125. [DOI] [PubMed] [Google Scholar]
- 56.Nogueira RG, Jadhav AP, Haussen DC, et al. . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. [DOI] [PubMed] [Google Scholar]
- 57.Lee S, Yoo AJ, Marquering HA, et al. . CLEAN investigators. Accuracy of "at risk" tissue predictions using CT perfusion in acute large vessel occlusions. J Neuroimaging. 2019;29(3):371-375. [DOI] [PubMed] [Google Scholar]
- 58.Boned S, Padroni M, Rubiera M, et al. . Admission CT perfusion may overestimate initial infarct core: the ghost infarct core concept. J Neurointerv Surg. 2017;9(1):66-69. [DOI] [PubMed] [Google Scholar]
- 59.Abbott AL, Paraskevas KI, Kakkos SK, et al. . Systematic review of guidelines for the management of asymptomatic and symptomatic carotid stenosis. Stroke. 2015;46(11):3288-3301. [DOI] [PubMed] [Google Scholar]
- 60.Ogasawara K, Ito H, Sasoh M, et al. . Quantitative measurement of regional cerebrovascular reactivity to acetazolamide using 123I-N-isopropyl-p-Iodoamphetamine autoradiography with SPECT: validation study using H2 15O with PSET. J Nucl Med. 2003;44(4):520-525. [PubMed] [Google Scholar]