Abstract
Aim: To investigate the pemetrexed encapsulated polymeric mixed micelles (PMMs) against breast cancer treatment.
Methods: We meticulously optimized the formulation and conducted extensive characterizations, including photon correlation spectroscopy for micellization, advanced analytical techniques and in vitro cell line assessments.
Results: The PMM exhibited favorable characteristics, with a spherical morphology, hydrodynamic particle size of 19.58 ± 0.89 nm, polydispersity index of 0.245 ± 0.1, and a surface charge of -9.70 ± 0.61 mV. Encapsulation efficiency and drug payload reached 96.16 ± 0.37% and 4.5 ± 0.32%, respectively. Cytotoxicity analysis indicated superior efficacy of the PMM over the drug solution.
Conclusion: The PMM formulation exhibited controlled release of the drug, and demonstrated enhanced cytotoxicity against breast cancer cells, highlighting its therapeutic promise.
Keywords: : breast cancer, pemetrexed, pluronics, polymeric mixed micelles, TPGS
Graphical Abstract
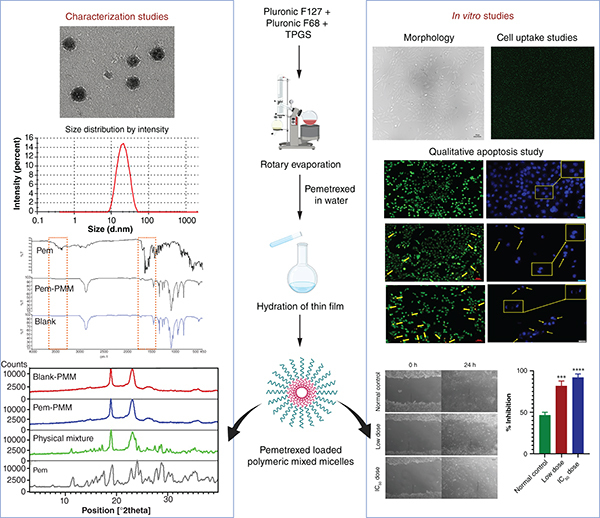
Plain language summary
Summary points.
Breast cancer is a profoundly complex malignancy characterized by extensive genetic and clinical diversity, displaying a wide array of clinicopathological and morphological attributes and demonstrating variable responses to various therapeutic modalities.
In this investigation, we delved into the intricate realm of medical science by examining the ternary amalgamation of Pluronic F127, Pluronic F68 and D-α-tocopheryl PEG 1000 succinate to encapsulate pemetrexed within a composite of polymeric mixed micelles.
Pemetrexed, a pharmacological agent, operates as an antimetabolite, effectively inhibiting critical enzymes such as thymidylate synthase, dihydrofolate reductase and glycinamide ribonucleotide formyltransferase, which play pivotal roles in DNA synthesis.
The meticulously prepared formulation underwent a process of optimization, followed by exhaustive characterization studies. The micellization phenomena were subjected to meticulous scrutiny using photon correlation spectroscopy.
We employed advanced analytical techniques including Fourier transform infrared spectroscopy, differential scanning calorimetry, powder x-ray diffraction, 1D proton nuclear magnetic resonance (NMR) spectroscopy, one-dimensional selective rotating-frame Overhauser effect NMR spectroscopy and 2D proton–proton rotating-frame Overhauser effect NMR spectroscopy to elucidate the intricate interplay between the formulation's excipients and the therapeutic agent.
We conducted rigorous assessments of drug and formulation dissolution behavior, along with in vitro studies on MCF-7 cell lines, to ascertain the formulation's efficacy against breast cancer.
Our in vitro drug-release investigation unveiled a sustained release profile, outperforming the drug solution, with release kinetics adhering to the Higuchi model.
NMR spectroscopy confirmed the presence of noncovalent interactions between the drug and the polymer components of the formulation.
Our comprehensive cytotoxicity analysis demonstrated that the meticulously developed formulation exhibits superior efficacy compared with the unadulterated drug solution.
The culmination of these findings unequivocally suggests that the polymeric mixed micelles represent a promising paradigm in the realm of drug-delivery systems, holding great potential for the treatment of breast cancer.
Cancer, an ailment characterized by aberrant and unchecked cellular proliferation, presents a formidable challenge due to its profound intricacies in cell and tissue biology, pathological manifestations, diversity and therapeutic responsiveness. The etiology of cancer is a complex interplay of multifarious factors [1]. Statistical data from 2020 underscore the grim reality, revealing that over 10 million lives were claimed by two predominant malignancies: breast and lung cancer [2]. Breast cancer (BC), in particular, emerges as a complex entity marked by both genetic and clinical heterogeneity, manifesting an array of clinicopathological and morphological attributes, all while exhibiting diverse responses to a myriad of therapeutic interventions [2].
BC, a prevalent affliction among women, boasts an incidence rate of 24.5% and carries a mortality burden of 15.5% [3]. This insidious malady, characterized by the unbridled proliferation of breast cells, typically culminates in the formation of discernible lumps or masses, often detectable through radiographic means. Left unchecked, it embarks on metastasis, disseminating to distant anatomical sites such as the bone, lung, brain and liver, further exacerbating its malignant potency. BC is broadly categorized into two distinctive subtypes, carcinomas and sarcomas, predicated upon their origins. Sarcomas arise from the connective tissues that provide structural support to the lobules and ducts of the mammary gland, while carcinomas originate within the terminal ducts of the breast. Therapeutic interventions for BC encompass surgical excision, chemotherapy and radiation therapy. Chemotherapy entails the administration of cytotoxic agents, which can be either specific or nonspecific in their mechanism of action, aiming to either exterminate cancer cells or arrest their proliferative capacity. Nonetheless, a notable drawback resides in the nonselectivity of these agents, resulting in collateral damage to healthy cells and tissues. In response to this limitation, concerted efforts are proceeding to enhance the safety and efficacy of cytotoxic agents through innovative drug-delivery methodologies, striving to usher in a new era of precision and targeted cancer treatment [4].
Pemetrexed (Pem) is an antimetabolite that inhibits the glycinamide ribonucleotide formyltransferase, dihydrofolate reductase and thymidylate synthase, which is essential for DNA synthesis [5,6]. Inhibition of these enzymes by the folate analog can be mechanistically significant against cancer. Antifolates are predominantly carried by the reduced folate carrier and bind with greater affinity to α-folate receptors, which are upregulated in various tumors. Pem has a greater affinity for the α-folate receptors than folate in L1210 cells and a similar affinity for reduced folate carriers as methotrexate. Pem was approved for non-small-cell lung carcinoma and malignant pleural mesothelioma by the US FDA. It is indicated as a solitary agent along with other anticancer drugs for treating colorectal, gastric, genitourinary and pancreatic cancers, and BCs [6]. Previously, various nanoformulations were reported to improve the oral permeability, stability, cellular uptake and therapeutic efficacy of Pem [7–9].
Polymeric micelles are self-aggregated assemblies of amphiphiles that can improve the therapeutic activity of chemotherapeutics [10]. Poloxamers or Pluronic® are a family of triblock copolymer amphiphiles composed of a hydrophilic palisade layer of poly(ethylene oxide) (PEO) and central hydrophobic poly(propylene oxide) (PPO). Pluronic F127 (F127) (EO102PO70EO102) is a triblock polymer with a longer hydrophilic palisade layer of PEO chains that provide stealth characteristics and facilitate escape from reticuloendothelial system uptake. Pluronic F68 (F68) is an amphiphilic polymer like F127 with central hydrophobic PPO and hydrophilic PEO chains. Various investigations reported that the PPO blocks incorporate into the cell membrane and disrupt the membrane packing, improving the drug molecules' permeability [11]. D-α-Tocopheryl PEG 1000 succinate (TPGS) is a hydrophilic analog of tocopherol and inhibits the P-glycoprotein (P-gp) efflux, thereby hindering the efflux of the drug from the cancer cells and exhibiting synergistic effects with Pluronics [12,13]. Mixed micelles of Pluronics and TPGS are extensively investigated by researchers for drug delivery. Recently, Srivastava and group fabricated nanomicelles of TPGS and Pluronics F127 for the delivery of the baicalein for BC therapy [14]. The synergistic action of the copolymers exhibited potent activity and increased efficacy against BC cells. In another investigation, this binary system was used to deliver paclitaxel and regorafenib to triple-negative BC, demonstrating enhanced paclitaxel therapeutic efficacy and overcoming multidrug resistance [15].
Recent investigations on mixed polymeric micelles were focused on binary mixtures [16–19], and information on ternary mixed micelles has not been widely explored. We explored the ternary system of F127, F68 and TPGS to formulate polymeric mixed micelles (PMMs) to deliver Pem to improve its therapeutic potential against BC. The prepared formulation was optimized, and the characterization studies were performed. The critical micellar concentration (CMC), hydrodynamic diameter, polydispersity index (PDI) and zeta potential of the PMM were further studied by photon correlation spectroscopy. Fourier transform infrared (FTIR), differential scanning calorimetry (DSC), powder x-ray diffraction (PXRD), 1D 1H, 1D selective and 2D nuclear magnetic resonance (NMR) techniques were explored further to understand the interactions between the excipients and the Pem. Also, the Pem and Pem-loaded PMM (Pem-PMM) dissolution behavior and in vitro cell line investigations were conducted to determine the Pem-PMM efficacy against BC.
Materials & methods
Pem was received as a gift sample from a local pharmaceutical company. F127, F68, TPGS, were procured from Sigma Aldrich (MA, USA). Acetonitrile (ACN) was acquired from Finar Chemicals Ltd (Ahmedabad, India). MCF-7 cell line was obtained from the National Centre for Cell Sciences (Pune, India). DMEM, RPMI media, fetal bovine serum (FBS), trypsin were purchased from HiMedia Pvt. Ltd (Mumbai, India). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 4′-6-diamino-2-phenylindole (DAPI), fluorescein isothiocyanate (FITC), acridine orange (AO), and ethidium bromide (EB) were purchased from Thermo Fisher Scientific Pvt. Ltd (Hyderabad, India). All the chemicals and reagents were used without further purification. Water was collected from an Ultra Clear® TP TWF ultrapure water system and filtered through 0.22 μm filters (Durapore® membrane, Millipore Sigma, MA, USA) used in all experiments.
Analytical method development of Pem
The analytical method of Pem was established on reverse-phase HPLC (RP-HPLC) (Shimadzu, Kyoto, Japan). Shimadzu Shim-Pack Solar C18 column (4.6 internal diameter × 250 mm, 5 μm particle size) was employed for the development of the method. A total of 20 μl injection volume was spiked into the column and analyzed at a wavelength of 245 nm. Triplicates of solution in the concentration range of 2–20 μg/ml were made and analyzed using RP-HPLC using ACN:10 mM ammonium formate (pH 4.5, 12:88) as mobile phase. The obtained chromatogram was studied using Lab Solutions software [20].
Determination of CMC
The CMC of F127/F68/TPGS was evaluated utilizing Malvern Zetasizer ZS90 (Malvern Instruments Ltd, Malvern, UK). The equimolar ratio of the surfactant concentrations was analyzed using the Zetasizer by subjecting them to a 5 mW Helium–Neon laser (scattering angle of 173°) at 25°C. The CMC plot was obtained from the graph between log concentration and intensity counts/min (Kilo counts per second, KCPS). The relation of CMC with the size of the micelles was also evaluated [21]. The experiment was replicated thrice to achieve statistical data.
Preparation of PMM
The thin-film hydration (TFH) method was employed to formulate Pem-PMM. PMM was prepared by dissolving appropriate amounts of F127, F68 and TPGS in ACN in a 100 ml round-bottom flask (RBF). The mixture was subjected to evaporation to produce a thin film using a rotary evaporator (Heidolph Instrument Pvt. Ltd, Schwabach, Germany), under reduced pressure at a suitable temperature (45°C) for 45 min. After the RBF was placed in a vacuum oven (NLVO1SE, Newtronic Lifecare Equipments Pvt. Ltd, Mumbai, India) overnight to eliminate residual ACN, the film was hydrated with a Pem solution (3 mg/10 ml) at 150 r.p.m. and 45°C for 45 min. The blank polymeric micelles were fabricated in the same process by hydrating the thin film with water instead of Pem solution. The obtained transparent solution was stored at 4°C for further use. The schematic illustration of the fabrication of PMM is shown in Supplementary Figure 1.
Lyophilization of the formulation
Freeze–thaw study
Different cryoprotectants (mannitol, trehalose, lactose) were added to the prepared micelles at 1% concentration before they were frozen in liquid nitrogen for sufficient time. The samples were thawed at room temperature and observed for the aggregates. Using a Zetasizer (NanoZS, Malvern), the particle diameter and PDI of the samples were evaluated prior to and after undergoing a freeze–thaw cycle.
Lyophilization
Filtered samples of the developed Pem-PMM formulation and blank PMM were subjected to 15 min of instant freezing with liquid nitrogen after being passed through 0.2 μm syringe filters. The frozen Pem-PMM was freeze-dried in a lyophilizer for 24 h at a suitable temperature (<-60°C) and vacuum for sublimation. The samples were subjected to an overnight placement in a vacuum oven to ensure the removal of residual moisture. Later, the materials were kept in a freezer at 4°C for further study.
Moisture content
Thermogravimetric analysis (TGA) was employed to calculate the percentage moisture content due to temperature increase. In brief, a weighted amount of the lyophilized Pem-PMM was heated at a constant rate of 20°C/min, up to 500°C, and the % weight loss was observed [22].
Physiochemical characterization of PMM
The morphological analysis was performed for the lyophilized formulation of Pem-PMM and blank PMM samples by depositing them onto double-sided adhesive carbon tape preceded by gold coating to increase the conductivity and then analyzing samples by means of field emission-scanning electron microscope (SEM) (Quanta 400 SEM, FEI Company, Cambridge, UK) at an accelerating voltage of 10 kV. The morphological analysis of the freshly prepared formulations of blank PMM and Pem-PMM was examined by transmission electron microscope (TEM) (JEM 1400, JEOL, MA, USA). The micelles were dripped on a carbon-coated grid and stained with 2% uranyl acetate. The stained samples were studied under TEM after drying [23].
Hydrodynamic diameter, PDI and surface charge of the blank PMM and Pem-PMM were determined at a 173° scattering angle using a Zetasizer (NanoZS) at 25°C. Measurements for all samples were conducted in triplicate (n = 3), and the values are presented as the mean value ± standard deviation [24].
The impact of dilution on stability was examined following a methodology outlined in a prior report [25]. The mixed micelles were diluted using phosphate-buffered saline (PBS) with a pH of 7.4. The diluted micelles were filtered through a 0.2 μm syringe filter, and size was checked by a Zetasizer (NanoZS). All the measurements were done in triplicate.
Attenuated Total Reflectance-Fourier Transform Infrared spectroscopic (ATR-FTIR) analysis (Spectrum 2, Perkin Elmer, CT, USA) was conducted to explore the drug and the excipient's interactions. The study involved scanning samples, including Pem, excipients, physical mixture, lyophilized Pem-PMM and blank PMM, across the 400–4000 cm-1 with a resolution of four [26].
PXRD analysis of the Pem, excipients, physical mixture, lyophilized Pem-PMM and blank PMM was done with the Empyrean series 3 x-ray diffractometer (Malvern Panalytical, Malvern, UK) having Cu-Kα radiation source, and the samples were scanned over the range of 2–40° at a speed of 0.0436°/s.
DSC was employed to record the thermograms for the Pem, Pem-PMM and blank PMM (STARe system, Mettler Toledo, Greifensee, Switzerland). The preweighed samples were put into aluminum pans and exposed to heating at a rate of 10°C/min from 25 to 300°C while continuously purging with nitrogen.
Lyophilized samples of blank PMM and Pem-PMM were assigned signals using a Bruker Avance 500 MHz spectrometer (Bruker Ltd, MA, USA). For recording 1D 1H NMR studies, the samples were added with D2O, and for recording 1D selective rotating-frame Overhauser effect spectroscopy (ROESY) and 2D ROESY investigations, with a mixture of H2O and D2O (9:1). The chemical shifts in the spectrum are expressed in p.p.m.
Stability studies
Short-term stability studies
To assess the stability, the prepared Pem-PMM was kept at room temperature, 4°C and 40°C. Triplicate samples were kept at each specified temperature and examined for changes in physical appearance (color changes, precipitation, particle size and PDI). Also, the stability of the Pem at 45°C was investigated. The stability of the Pem at 45°C was checked by developing a stability-indicating method. The Pem dissolved in equal ratios of ACN and water was subjected to 45°C for 1 h, and the sample was analyzed using RP-HPLC (Waters e2695, Waters Corporation, MA, USA) equipped with Photodiode array detector) with an Agilent Zobrax Eclipse Plus C18 column (Agilent Technologies, CA, USA) (250 mm × 4.6 mm, 5 μm). A gradient method using ammonium acetate (pH = 4.5):ACN was employed. The 10 μl injection of the sample was injected into the column and the chromatogram of the sample was compared with the standard.
Long-term stability studies
To test the samples' long-term stability, the lyophilized samples were kept at 4°C for 3 months, and the PXRD pattern of the lyophilized powder was analyzed. In addition, the particle size and PDI following reconstitution were assessed.
Drug loading & encapsulation efficiency
Drug loading (DL) and encapsulation efficiency (EE) were assessed through the same HPLC-based analytical method previously outlined. The formulated Pem-PMM underwent centrifugation at 12,000 r.p.m. for 20 min, after which the supernatant was collected. This supernatant was appropriately diluted with ACN and then quantified for drug content using HPLC analysis. DL% and EE% were determined using Equations 1 & 2 as outlined below [27].
| (Equation 1) |
| (Equation 2) |
In vitro drug-release study
The in vitro dissolution studies were executed with the dialysis sac technique. The assembly was kept under controlled conditions by providing nitrogen. The Pem-PMM and Pem solutions were kept in a seal-ended dialysis bag (HiMedia, cutoff 12,000–14,000 Da) and placed in a PBS physiological buffer medium containing 2% Tween® 80 (SRL Pvt. Ltd, Mumbai, India). The study was performed at constant stirring (100 r.p.m.) under sink conditions at 37°C. Fractions of 1 ml of solutions were removed at predetermined intervals, substituted with equal quantities of PBS buffer and examined by HPLC analysis using the parameters described in the analytical development of Pem in the Materials & Methods section [24]. The data gathered is presented as mean ± standard deviation (n = 3).
Hemolysis assay
The hemolysis rate of Pem, blank PMM and Pem-PMM was estimated to check the biocompatibility. Fresh blood was collected from Wistar rats, centrifugated at 3000 r.p.m. for 5 min and washed with PBS buffer until the supernatant was clear. The red blood corpuscles (RBCs) pellet was resuspended in PBS buffer, and 50 μl of these RBCs were added with 1 ml of the Pem, blank PMM and Pem-PMM at varied concentrations (100–0.0001 p.p.m.) followed by incubation at 37°C for 1 h. Intact RBCs were pelleted via centrifugation and the supernatant (100 μl) transferred into a 96-well plate, and its absorbance was measured at 540 nm using a microplate reader. All samples were tested in triplicate, and the percent hemolysis was determined relative to negative control (Triton™ X-100, Sigma Aldrich, MA, USA) and positive control (PBS buffer) [28].
In vitro cell line studies
MCF-7 cells were grown in DMEM supplemented with 10% FBS and a 1% penicillin–streptomycin mixture. The cells were then incubated for 24 h until reaching a confluence of over 80–90% in a humidified environment with 5% CO2 at 37°C.
In vitro cytotoxicity studies
Cytotoxicity analysis was done using MTT assay for Pem, Pem-PMM and blank PMM. MCF-7 cells were seeded into 96-well plates with DMEM and 10% FBS and incubated for 24 h. The media was substituted with DMEM having Pem, Pem-PMM and blank PMM, and the cells were subjected to 24 and 48 h treatment. After completion of the incubation period, 100 μl of MTT solution was introduced to each well and subjected to incubation for 4 h. Afterward, dimethyl sulfoxide was added to dissolve the purple formazan crystals. Meanwhile, the absorbance at 570 nm was quantified using a microplate reader, and an IC50 was determined [24].
Cell internalization study
The cell internalization investigation was conducted to demonstrate the internalization of PMM-loaded FITC into the MCF-7 cells. The cells were plated into 12-well plates at a density of 1 × 104 cells and provided with DMEM comprising 10% FBS. Subsequently, the cells were treated with a free dye solution and dye-loaded PMM, followed by a 6-h incubation period. Fluorescence imaging was then conducted using the Nikon Ti2 microscope (Tokyo, Japan).
Qualitative cell apoptosis assay
Apoptosis stands as a pivotal process often disrupted during tumorigenesis. The determined IC50 values, indicating the growth inhibition potential of the synthesized PMM as determined by cell viability assay, is an interest in exploring the PMM's ability to induce programmed cell death of cancer cells. Qualitative assays for cellular apoptosis, primarily conducted through the DAPI and AO/EB dual staining techniques, are commonly performed qualitative cell apoptosis assays.
AO/EB dual staining technique
The cell apoptosis assay was performed using the AO/EB staining technique. MCF-7 cells at a density of 1 × 104 cells were plated into each well of a 12-well plate and allowed to incubate for 24 h. Cells were treated with Pem-PMM containing IC50 and low-dose value, followed by incubation for 24 h. Subsequently, the media in each well was replaced with fresh media and allowed to incubate with dye solutions (20 μl) of AO and EB. The AO stains the live cells, whereas the destruction/deterioration of cell membranes is stained by EB.
DAPI staining technique
A 12-well plate was employed, where MCF-7 cells were seeded at a density of 1 × 105 cells per well and incubated for 24 h. Subsequent to this, the plated cells were treated with a low-dose and IC50 value concentration of Pem-PMM and incubated for 24 h. The cells were washed thrice using sterile PBS at pH 7.4 and fixed utilizing a 4% paraformaldehyde solution for 30 min. Subsequently, each well received an addition of 10 μl DAPI solution to enable nuclear staining and examined with a fluorescent microscope installed with a blue filter at a magnification of 20×.
Reactive oxygen species assay
2′,7′-dicholorofluorescein diacetate (DCFDA) is a lipophilic cationic fluorescent dye used to detect reactive oxygen species (ROS) generated in cells. When nonfluorescent DCFDA is introduced into cells, esterases deacetylate it, and when it combines with ROS, green fluorescence is produced. The MCF-7 cells were seeded into 12-well plates and allowed to incubate overnight. The cells were exposed to a low dose and IC50 of Pem-PMM the following day. Postincubation, the wells were added with DCFDA for 30 min before imaging with a fluorescence microscope (Nikon Eclipse TiS, Tokyo, Japan) at 20× magnification [29].
Morphological observations
To observe the morphology, the 1 × 104 MCF-7 cells were plated in 12-well plates, followed by overnight incubation. Postincubation, the cells were subjected to low, IC50 and high doses of Pem-PMM and incubated for 24 h. The morphological changes, such as alterations in the cell size and shape, were observed under an inverted microscope (Nikon Eclipse TiS, Tokyo, Japan).
Cell migration assay
The term ‘metastasis’ is the migration of cancer cells from their site of origin to another area of the body. An assay for wound healing was used to understand the impact of Pem-PMM on the migration of MCF-7 cells, an aggressive and highly invasive cancer cell line. The basis for this test was the evidence that when a fresh artificial wound, or ‘scratch’, forms on a confluent cell monolayer, the cells around the edges of the incision can move inward to close it shut until new cell–cell contacts are made. The application of antineoplastics inhibits cell migration, preventing the wound from closing. Hence, to evaluate the Pem-PMM effect on cell migration, scratches were made on MCF-7 cell monolayers with the aid of a sterile 200 μl pipette tip, followed by treatment with a low dose and IC50 of the Pem-PMM and the cells were incubated. The imaging of the cells' migration toward the wounds was done at 0 and 24 h using phase contrast microscopy.
Statistical analysis
All the experimentations were performed in triplicate and analyzed with Graph Pad Prism 8 software (CA, USA) by employing one-way analysis of variance with statistical significance values (where *represents p < 0.05; **represents p < 0.01; ***represents p < 0.001; ****represents p < 0.0001).
Results
PMMs are extensively studied nanostructured vehicles in the field of nanomedicine. They can be self-assembled into a nanostructure and can encapsulate both hydrophobic and hydrophilic drugs [30]. The hydrophilic shell surrounds the outer part of the micelle, while the hydrophobic core is located inside. These micelles offer various advantages, including small particle size, high efficiency in DL, excellent stability, sustained release of drugs, improved uptake by cells and enhanced therapeutic effects. Moreover, they can augment the solubility and stability of the encapsulated components within the formulation.
Analytical method development of HPLC
HPLC analytical method of Pem was developed using the fixed wavelength measurement of the dilution from 2–20 μg/ml at λmax 245 nm. The chromatogram was obtained at a retention time of 5.7 min, shown in Supplementary Figure 2. The linearity range was observed from 2 to 20 μg/ml, and the R2 value obtained was 0.999. The HPLC calibration curve was also recorded at 245 nm using the mobile phase of ACN and ammonium formate with a flow rate of 1 ml/min in the ratio of 12:88.
Determination of CMC
The CMC of the equimolar ratio of ternary systems was approximately 0.0004882% at 25°C. The graph depicted in Figure 1A illustrates the relationship between light scattering intensity and polymer concentration. Remarkably, the concentration of polymers is notably low, indicating the remarkable integrity and stability of the micelles, even when subjected to extreme dilution within the body. The hydrodynamic diameter has also been verified with DLS. The plot represents the intensity and size changes with concentration, and the micelles exhibited abrupt change near the CMC. The size is nearly constant at concentrations above CMC, as observed in Figure 1B.
Figure 1.
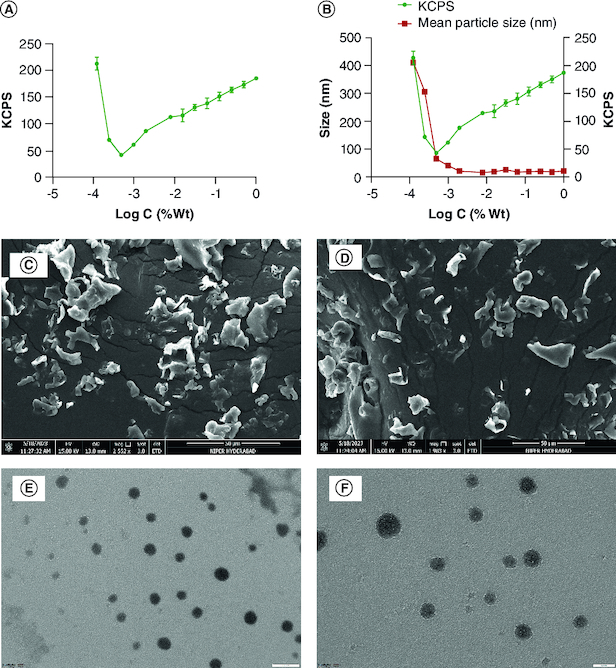
Characterization studies. (A) Critical micellar concentration determination using dynamic light scattering, and (B) correlation between KCPS, hydrodynamic size and concentration. Scanning electron microscope images of blank-PMM (C) and lyophilized Pem-PMM (D). TEM images of blank PMM (E) and Pem-PMM (F). All experiments were performed in triplicate.
KCPS: Kilo counts per second; PMM: Polymeric mixed micelle; Wt: Weight.
Preparation of PMM
PMMs hold great promise as nanocarriers, particularly in drug delivery for tumor targeting. In this investigation, we synthesized Pem-PMM for the specific purpose of treating BC. The choice of polymers for constructing these nanomicelles, namely F127, F68 and TPGS, was based on their widespread use due to their low CMC, stealth properties, synergistic effects and other favorable attributes. While there are various methods available for preparing PMMs, we opted for the TFH method, taking into account the physicochemical nature of the active pharmaceutical ingredient (API) and the desired characteristics of the final product. Different ratios of the F127, F68 and TPGS were used to fabricate the thin film. The 2:1:1 ratio of F127, F68 and TPGS exhibited smaller particle sizes with acceptable PDI, and this ratio continued for DL. The weighed amount of the Pem and excipients dissolved in the organic solvent, resulting in drug precipitation during film formation. To overcome this hurdle, the film was formed with excipients by dissolving in ACN, followed by hydration of the film with Pem solution (weighed amount of Pem in a specific quantity of water). Briefly, F127 (30 ± 0.05 mg), F68 (15 ± 0.05 mg) and TPGS (15 ± 0.05 mg) were dissolved in a sufficient amount of ACN in RBF. The solution was evaporated for 1 h in a rotary vacuum evaporator (Heidolph Instrument Pvt. Ltd), and kept at 50°C at 150 r.p.m. to form a thin film. The formed thin film was kept overnight in a vacuum dryer at 45°C to remove the residual solvent. Later, thin film was hydrated with 10 ml of Pem solution (3 mg/10 ml) at 45°C for 1 h to obtain Pem-PMM.
Lyophilization of the Pem-PMM
Freeze–thaw study
The freeze–thaw method is an easy and conventional method to select the cryoprotectant for the freeze-drying of the formulation. Freezing is the preliminary step for lyophilization. Factors like freezing time, concentration and type of cryoprotectant should be considered while selecting the cryoprotectant. A 1% concentration of trehalose, mannitol and lactose was added to the Pem-PMM, and a Pem-PMM without cryoprotectant was used for comparison, as shown in Supplementary Figure 3. Particle size analysis after the freezing–thawing showed that the lactose and mannitol exhibited a minimum difference in the size and PDI compared with the noncryoprotected samples. In contrast, trehalose exhibited much smaller particle sizes and higher PDI [31].
Lyophilization
The PMMs were lyophilized to improve the kinetic and thermodynamic behavior of the systems. The lyophilized powder was reconstituted in water, which exhibited particle size (19 nm) and PDI (0.23) as that of the freshly prepared Pem-PMM. The lactose-added Pem-PMM was also lyophilized in a similar manner.
Moisture content
The percentage moisture content was determined by using TGA. The percentage weight loss was used to analyze the percentage moisture content in the lyophilized sample. TGA thermogram exhibited 0.292% moisture at 100°C, and no significant weight loss was observed until 250°C, as shown in Supplementary Figure 4.
Physiochemical characterization of PMM
The SEM analysis was performed and studied to evaluate the microstructure of the lyophilized blank PMM and Pem-PMM powder, as shown in Figure 1C & D, respectively. The freeze-dried powder exhibited a granular surface with a porous structure. The heterogeneous particle size of the freeze-dried powder may be due to the lyophilization process, where sublimation occurs, leading to nonuniform size [32]. The TEM micrographs of the blank PMM and Pem-PMM exhibited a spherical shape, as represented in Figure 1E & F. The size of the PMM determined by TEM was comparable to that determined by the dynamic light scattering (DLS) (Zetasizer).
The particle diameter, PDI and surface charge of the PMM prepared under optimized conditions, with and without the Pem, were measured and are reported in Table 1. The hydrodynamic diameter of the Pem-PMM and blank PMM is illustrated in Supplementary Figure 5. The zeta potential quantifies the charge on the PMM surface, typically determined by laser Doppler anemometry/electrophoresis. The zeta potential was -9.70 ± 0.61 mV (pH = 7.2 ± 0.1), as shown in Table 1.
Table 1.
The particle size, polydispersity index and zeta potential of blank polymeric mixed micelles and pemetrexed-loaded polymeric mixed micelles. Data are reported as mean ± standard deviation (n = 3).
| Formulation | Mean particle size ± SD (n = 3) | Mean polydispersity index ± SD (n = 3) | Mean zeta potential ± SD (n = 3) |
|---|---|---|---|
| Blank polymeric mixed micelles | 20.90 ± 0.96 nm | 0.298 ± 0.1 | -9.37 ± 0.36 mV |
| Pemetrexed polymeric mixed micelles | 19.58 ± 0.89 nm | 0.245 ± 0.1 | -9.70 ± 0.61 mV |
SD: Standard deviation.
As micelles formation and stability are concentration-dependent, a study of the impact of dilution on the micelle size was performed. The particle size of the fabricated PMM showed uniformity against dilution with PBS up to 100-fold. The results demonstrated that most of the particles were in the range of 17.56 ± 0.37 to 19.53 ± 0.53 nm, showing that the prepared micelles are stable to 100-times dilution, as shown in Supplementary Table 1. The findings show that the micelles are stable to extreme dilution encountered in the in vivo conditions.
The FTIR analysis was performed to obtain insights into the structure and chemical functional groups of the Pem. Major characteristic absorption peaks for the Pem were noted at 1570 cm-1 (vPyrrole), 1678 (vC=O) cm-1, 1623 cm-1 (vN-H) and 3442 cm-1 (vN-H, vO-H). The absorption peaks of F127 and F68 were observed at 2891 cm-1 (vC-H aliphatic stretching), 1111 cm-1 (vC-O stretch) and 1343 cm-1 (vO-H, in-plane O–H bend). Major characteristic absorption peaks for TPGS were noted at 1736 cm-1 (vC=O of succinate ester), 1250 cm-1 (vC-O stretching), 2870 cm-1 (vC-H overlapping of –CH stretching) 1141 cm-1, and 1623 cm-1 (vN-H), 1458 cm-1 (vC-C, aromatic stretching), 3506 cm-1 (vO-H) and CH2 group of PEG chain at 1377.67 cm-1, respectively. The physical mixture of the Pem and the excipient did not exhibit any new peaks and peak shifts, demonstrating that the polymers and the Pem are compatible. The FTIR spectrum of excipients and physical mixture is illustrated in Supplementary Figure 6 [33]. FTIR analysis of the Pem, lyophilized Pem-PMM and blank PMM are illustrated in Figure 2A.
Figure 2.
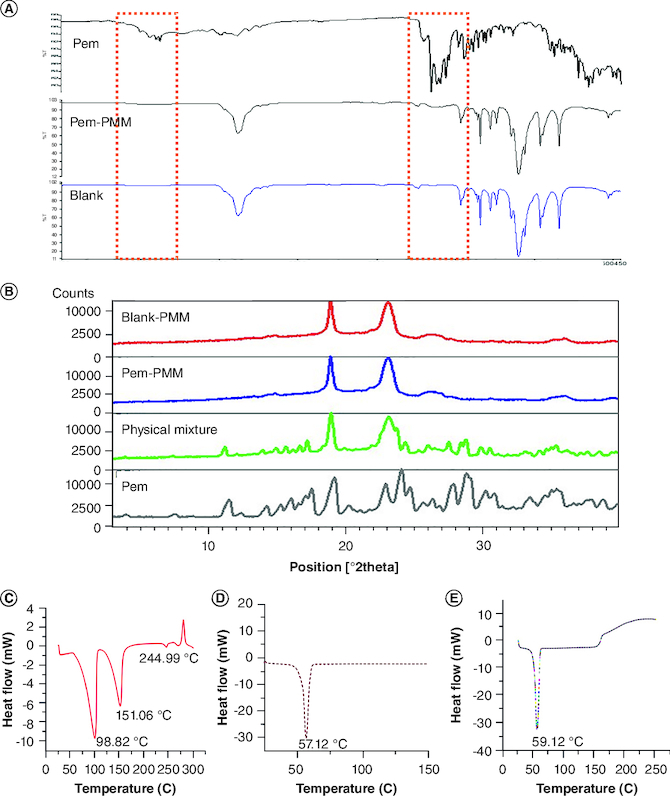
Fourier transform infrared analysis, powder x-ray diffraction and differential scanning calorimetric analysis of formulation components. (A) Fourier transform infrared spectra of Pem, blank PMM and Pem-PMM. (B) Powder x-ray diffraction diffractograms of blank PMM, Pem-PMM, physical mixture, and Pem. (C) DSC thermogram of Pem. (D) DSC thermogram of Pluronic F127. (E) DSC thermogram of Pem-PMM. All the experiments were performed in triplicate.
DSC: Differential scanning calorimetry; Pem: Pemetrexed; Pem-PMM: Pemetrexed-loaded polymeric mixed micelle; PMM: Polymeric mixed micelle.
The PXRD analysis of the Pem shows sharp, narrow and intense peaks at 7.82, 11.53, 19.37, 23.74 and 35.21°, which confirms the crystalline nature of the Pem. The F127 and F68 exhibited similar patterns of sharp peaks at 19.56 and 23.65° due to their semicrystallinity. TPGS showed two peaks at 19.19 and 23.25° of 2θ, conforming to the semicrystalline nature of the PEG chains of the TPGS [34]. The PXRD patterns of the Pem and excipients are shown in Supplementary Figure 7. The dispersion state of the Pem in the lyophilized Pem-PMM was studied by the PXRD to understand the changes in the crystallinity of the Pem after encapsulation in the PMM, and their diffractograms are shown in Figure 2B.
The DSC investigation was conducted to observe the thermal events of the Pem, Pem-PMM and blank PMM. The DSC thermograms of Pem, F127 and Pem-PMM are shown in Figure 2C–E. Pem exhibited endotherms at 98.82, 151.06 and 244.59°C and an exotherm at 279°C. The F127 exhibited melting at 57.12°C, as illustrated in Figure 2D. The crystalline nature of Pem was lost when loaded in the PMM, as indicated by the absence of a sharp endotherm at 244.90°C in Pem-PMM.
1H NMR spectrum of Pem was recorded in D2O using 1H NMR experiments, and the spectrum is shown in Figure 3A. The Pem spectrum showed the aliphatic and aromatic protons in accordance with the literature, with a water peak at 4.79 p.p.m. The calibrated spectra of the 1H NMR of the Pem, blank PMM and Pem-PMM exhibited a change in the chemical shifts, as illustrated in Figure 3B. Interpretation of the 1H NMR data shows that H4 protons belonging to the pyrrole, H7 and H8 protons of the benzene ring, and H5 and H6 of the aliphatic chains are shifted downfield in the micellar system. The difference in the chemical shift is shown in Figure 3C and the values are given in Supplementary Table 2. 1D selective ROESY was performed in water with 10% D2O with the reference compound, trimethyl propyl silane. Saturation of the specific proton of 8.3 p.p.m. (amine proton) exhibited nuclear Overhauser effect (NOE) with the 3.7 p.p.m. (alkyl group of Pluronics), as illustrated in Figure 3D. The partial spectrum of 2D ROESY exhibiting cross-peaks is illustrated in Figure 4A, and the full spectrum of 2D ROESY is represented in Supplementary Figure 8. The protons in the region of the 6–8 p.p.m. (H7, H8, H9) belong to the Pem and exhibited cross-peaks with the alkyl groups of the micellar system.
Figure 3.
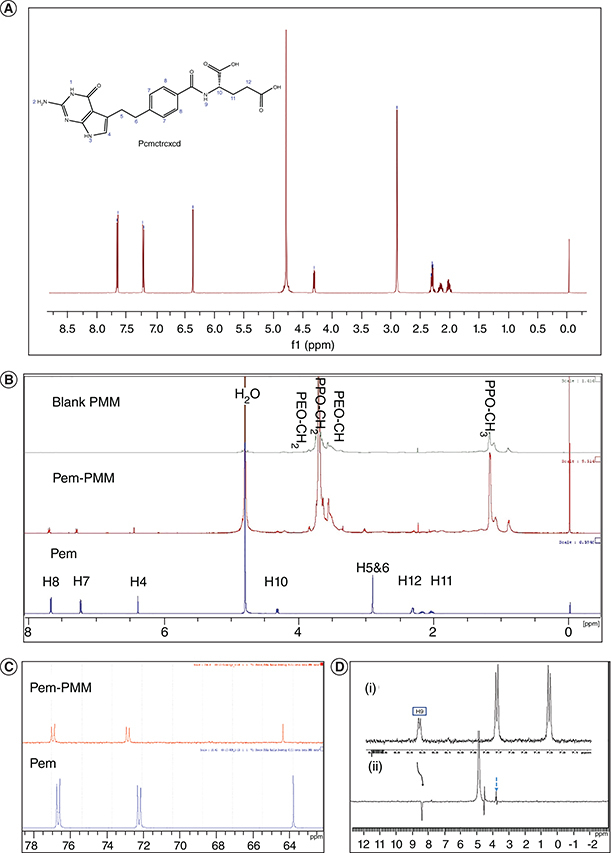
1D 500 MHz nuclear magnetic resonance spectra. (A) 1D 1H NMR spectrum of Pem. (B) 1D 1H NMR overlay spectra of Pem, Pem-PMM and blank PMM in D2O. (C) 1D 1H (partial) spectrum illustrating the Pem and Pem-PMM in D2O. (D) 1D selective rotating-frame Overhauser effect spectroscopy spectra showing (i) partial 1H spectrum of Pem-PMM and (ii) partial 1H NMR selective rotating-frame Overhauser effect spectroscopy illustrating the irradiation of peak at 8.35 of Pem-PMM spectra in H2O + D2O. All the experiments were performed in triplicate (n = 3).
NMR: Nuclear magnetic resonance; Pem: Pemetrexed; Pem-PMM: Pemetrexed-loaded polymeric mixed micelle; PMM: Polymeric mixed micelle.
Figure 4.
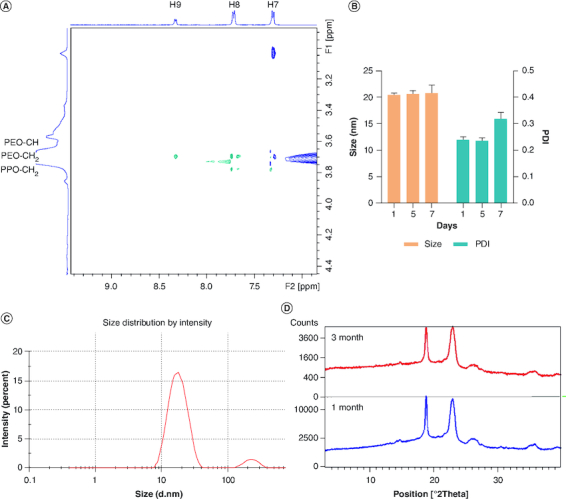
Characterization studies. (A) Partial 1H-1H 2D rotating-frame Overhauser effect spectroscopy spectrum illustrating cross-peaks in the Pem-PMM in H2O + D2O. (B) Stability analysis of Pem-PMM solution showing the effect of size and PDI for 7 days. (C) Particle size distribution of reconstituted Pem-PMM. (D) Overlay of powder x-ray diffraction analysis of lyophilized Pem-PMM formulation at 1 month and 3 months. All the experiments were performed in triplicate.
PDI: Polydispersity index; Pem-PMM: Pemetrexed-loaded polymeric mixed micelle.
The stability of the Pem at the PMM fabrication temperature (45°C) was investigated and the overlay chromatogram plot with the standard Pem is represented in Supplementary Figure 9. Pem did not exhibit any degradation products at 45°C. The short-term stability was performed for the Pem-PMM for 7 days at 4°C. The Pem-PMM exhibited stability for the initial 7 days with particle diameter and PDI of 20.28 ± 0.49 nm and 0.24 ± 0.01 on day 0, and 20.38 ± 1.61 nm and 0.35 ± 0.02 on day 7 with no visible signs of precipitation. The size and PDI data of 7-day stability samples are illustrated in Figure 4B. At room temperature, Pem-PMM showed precipitation after 3 days. Long-term stability studies were performed with lyophilized powder for 3 months. The reconstituted Pem-PMM solution exhibited no alteration of particle diameter (19.20 ± 0.09 nm) or PDI (0.25 ± 0.06), and lyophilized Pem-PMM exhibited no alteration in x-ray diffraction pattern, as shown in Figure 4C & D, respectively, demonstrating that the lyophilized Pem-PMM was stable at 4°C for the tested period.
DL & EE
The %EE and %DL were evaluated using the same HPLC method described in the previous section. The %EE and DL for the Pem-PMM were 96.16 ± 0.37% and 4.5 ± 0.32%. The encapsulation of the Pem in the PMM might be responsible for its high encapsulation.
In vitro drug-release study
Drug-release behaviors of Pem solution and Pem-PMM were studied in different pH conditions, that is, pH = 4.8, 6.8 and 7.4, under controlled conditions. The typical cumulative drug-release patterns under the physiological environment are shown in Figure 5A & B. We observed a significant degradation of the Pem in the dissolution media after 4 h. According to the previous reports on Pem stability, oxygen significantly impacts the stability of the Pem in solution form [35]. We attempted to apply stringent conditions for drug release by supplying nitrogen during dissolution studies. The Pem-PMM showed prolonged drug release at different pH relative to the Pem solution. More than 80% of the Pem release was shown in the initial 6 h from the Pem solution, whereas the Pem-PMM exhibited sustained release for 48 h at pH 6.8, and 7.4 and for 24 h at pH 4.0. The sustained release from the Pem-PMM is attributed to the encapsulation of the Pem in the PMM. The release data were subjected to drug-release kinetics to understand the mechanism of drug release from the Pem-PMM for pH 4.0, 6.8 and 7.4, respectively. Data fitting suggested a good fit for Higuchi kinetics for pH 4.0, pH 6.8 and 7.4, with an R2 of 0.8624, 0.9314 and 0.8775, respectively, as shown in Figure 5C, which implies that the Pem release is attributed to the swelling or erosion of the PMM. The equations and R2 values are given in Supplementary Table 3.
Figure 5.
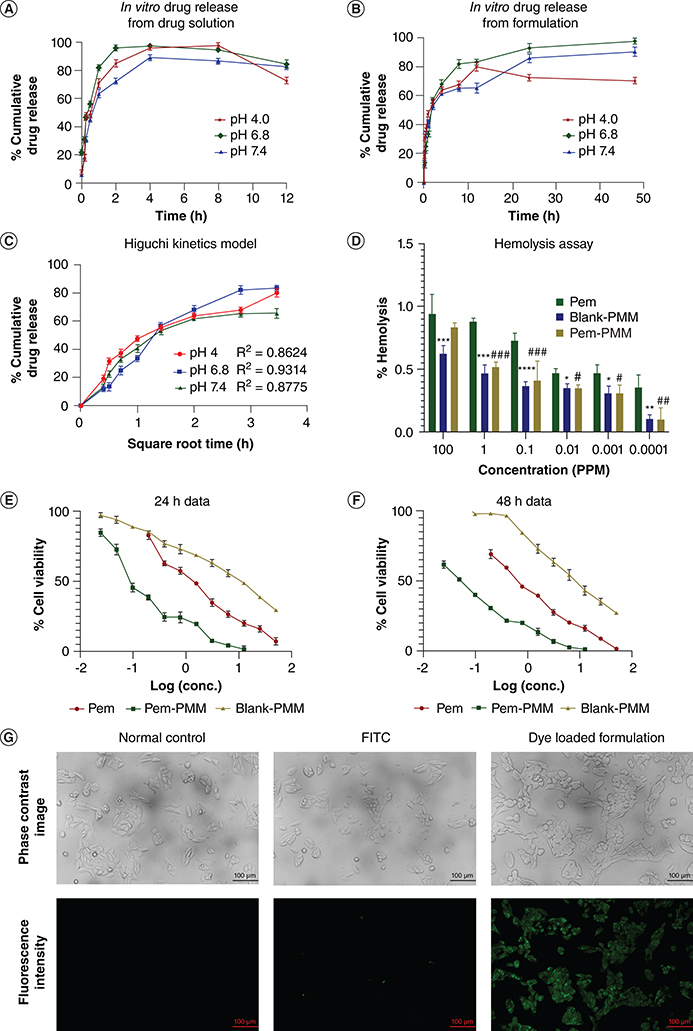
In vitro drug-release studies. (A) Pem solution at pH 4.0, 6.8 and 7.4. (B) Pem-PMM at pH 4.0, 6.8 and 7.4. (C) Higuchi kinetics at pH 4.0, 6.8 and 7.4. (D) Hemolysis assay of the Pem, blank PMM and Pem-PMM. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay of Pem, Pem-PMM and blank on MCF-7 cells at (E) 24 h and (F) 48 h. (G) Cell uptake studies of control, FITC and FITC-loaded PMM in MCF-7 cells. The data experiments were performed in triplicate and represented as mean ± standard deviation. */#represents p ≤ 0.05; **/##represents p ≤ 0.01; ***/###represents p ≤ 0.001; ****represents p ≤0.0001.
conc.: Concentration; FITC: Fluorescein isothiocyanate; Pem: Pemetrexed; Pem-PMM: Pemetrexed-loaded polymeric mixed micelle; PMM: Polymeric mixed micelle.
Hemolysis assay
Hemocompatibility is necessary for polymeric micelles as they directly interact with the blood components upon intravenous administration. The hemolytic activity is directly related to the hemoglobin released into the solution. In this assay, different concentrations of the Pem, blank PMM and Pem-PMM were treated with RBC and evaluated for hemolysis. The percentage of hemolysis is represented in Figure 5D. The prepared micelles exhibited negligible toxicity (<5%), showing the selected polymers' excellent biocompatibility. The highest concentration (100 p.p.m.) of the blank PMM and Pem-PMM exhibited less than 2.5% hemolysis.
Cell line studies
In vitro cell toxicity studies
The MTT assay of the Pem, Pem-PMM and blank PMM for 24 and 48 h is represented in Figure 5E & F, respectively. The in vitro cytotoxicity analysis in MCF-7 cells exhibited that the IC50 values of the Pem, Pem-PMM and blank PMM for 24 h were 1.555, 0.130829 and 11.109 μg/ml, and 48 h were 0.485309, 0.024 and 8.358 μg/ml, respectively.
Cell internalization studies
The cell internalization of PMM was imagined via fluorescence microscopy using a green filter. As the copolymers are nonfluorescent, fluorescent probe FITC was introduced into PMM. Figure 5G strongly indicates that dye-loaded PMM internalizes into cells and emits fluorescence; this might be facilitated by the endocytosis mechanism. The presence of TPGS in F127/F68/TPGS mixed micelles serves as an inhibitor for the Pgp-2 efflux pump, thereby aiding in the inhibition of the Pgp-2 efflux transport system.
Qualitative cell apoptosis assay
AO/EB staining studies
Dual staining of the control group exhibited a circular intact nucleus emitting green fluorescence, indicating live cells. Figure 6A shows MCF-7 cells exposed to Pem-PMM, revealing the existence of the early and late apoptotic cells showing crescent or granular nuclei and orange to red fluorescence, respectively. This result indicates that the encapsulation of Pem in the PMM retained the cytotoxic ability of the drug.
Figure 6.
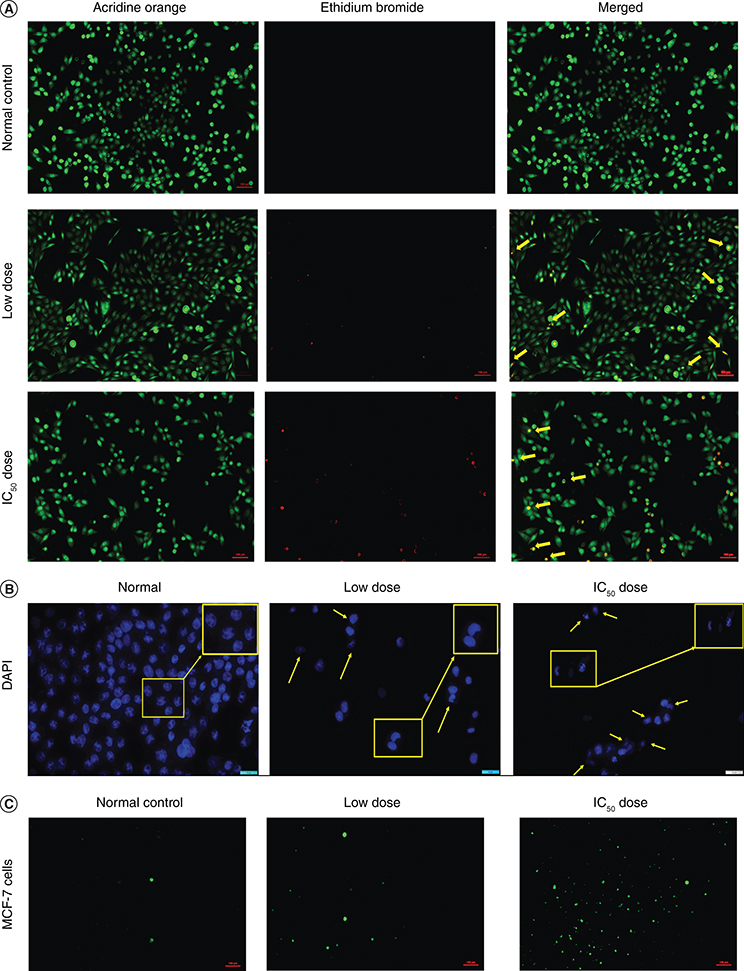
Qualitative apoptosis assay in MCF-7 cells. (A) Ethidium bromide/acridine orange dual staining of cells (10× magnification) after treatment with Pem-PMM. (B) DAPI staining of cells (40× magnification) after treatment with Pem-PMM. The yellow arrows indicate the morphological changes and nuclear fragmentation of the cells. The yellow boxes represent the few apoptotic cells at higher magnification. (C) Reactive oxygen species/2′,7′-dichlorofluorescein diacetate assay postincubation of MCF-7 cells (10× magnification) with Pem-PMM. All the experiments were performed in triplicate.
DAPI: 4′-6-diamino-2-phenylindole; Pem-PMM: Pem-loaded polymeric mixed micelle.
DAPI staining studies
Nuclear changes (condensed and bright nuclei) were seen in MCF-7 cells after being treated with Pem-PMM. DAPI nuclear staining revealed the nuclear fragmentation, morphological alterations and nuclear blebbing in the treated group, as emphasized with yellow arrows, which indicates the initiation of apoptosis. Figure 6B shows the MCF-7 cell nuclei stained with DAPI after treatment with Pem-PMM. On the contrary, control group cells were live with intact nuclei exhibiting faint fluorescence.
ROS/DCFDA assay
DCFDA was used to detect the generated ROS after treatment of the cells with the Pem-PMM. Figure 6C shows the green fluorescence exhibited by the MCF-7 cells after treatment with the Pem-PMM, which is attributed to the reaction between the deacetylated DCFDA and the generated ROS in the cells. The normal group exhibited negligible green fluorescence, while the treated groups exhibited green fluorescence, indicating the presence of ROS inside the cells due to the treatment with the Pem-PMM.
Morphological observations
The impact of Pem-PMM on MCF-7 cell morphology was appraised by imaging the cellular morphology using a phase contrast microscope. Figure 7A represents the morphological alterations in the cells after treatment. The alterations in the morphology and size of cells, cell shrinkage and reduction in the live cells number indicate the emergence of apoptosis in the MCF-7 cells.
Figure 7.
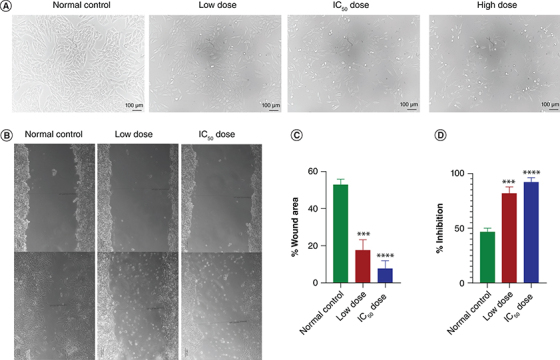
In vitro analysis of Pem-PMM in MCF-7 cells. (A) Morphological observations of MCF-7 cells treated with low dose, IC50 and high dose of Pem-PMM. The inhibitory effect of Pem-PMM on cell migration activity of MCF-7 cells. (B) Images of migrated MCF-7 cells, scale bar = 100 μm. Quantification of cell migration of MCF-7 cells after treatment with a low dose and IC50 of Pem-PMM. (C) Percentage wound area. (D) Percentage inhibition. The data experiments were performed in triplicate and represented as mean ± standard deviation.***p < 0.001; ****p < 0.0001 represents significant value.
Pem-PMM: Pem-loaded polymeric mixed micelle.
In vitro cell migration assay
To evaluate the metastasis potential, a cell culture wound closure assay was conducted, and the images are shown in Figure 7B. After 24 h, the scratch area was occupied with 52.92 ± 2.42% of normal control cells, whereas the treatment with low dose and IC50 dose of Pem-PMM represented significant inhibition of cell migration with 17.80 ± 4.46% and 7.96 ± 3.21% cells, respectively, in the wounded area, as shown in Figure 7C & D. With the above results, it is evident that the Pem-PMM can effectively inhibit the MCF-7 cells' invasion and metastasis.
Discussion
Nanotechnology-based drug delivery has shown tremendous progress in cancer research by reducing the side effects and improving the pharmacokinetics of chemotherapeutics. Polymer-based delivery systems have been utilized to improve the therapeutic efficacy of various bioactives. Controlling particle size facilitates escaping the reticuloendothelial system and improves the ‘enhanced permeation and retention effect’ improving intratumoral accumulation. Based on the physicochemical characteristics of the API, suitable nanoformulations must be chosen to vehiculate them to the intended site. The drawbacks of the API can be overcome by choosing the appropriate nanocarrier system, such as polymer–drug conjugates, micelles, etc. The polymeric micelles can be fabricated from various amphiphilic copolymers like PEG-PPO-PEG triblock copolymer, PEG-phosphatidylethanolamine, PEG-amino acids and PEG-carbonates [36–38]. PMM exhibits smaller particle sizes with improved drug permeation and has high drug encapsulation. They also improve the stability and therapeutic efficacy of the drug.
Numerous investigations have been conducted on combining Pluronics and TPGS due to synergistic effects [19,39,40]. TPGS enhances the stability of the nanocarriers, which may be attributed to the antioxidant effects, and acts as a P-gp efflux inhibitor. Mixed micelles of this system might alter the cell membrane fluidity, thus improving the permeation of Pem. Ternary systems combining the three surfactants possibly provide more significant advantages than binary mixtures or single copolymers. For instance, Fahmy et al. recently prepared a ternary complex of Pluronic P123, F68 and Labrasol for ocular fungal mycosis treatment [41]. Similarly, another study investigated F127/Pluronic P123/vitamin E TPGS mixed micelles for oral delivery of mangiferin and quercetin, elucidating their micellization and solubilization behavior [42]. These results support the superiority of the ternary system over the binary mixtures of the surfactants.
Pem is approved for the treatment of various cancers, including BC. It is a multitargeted antifolate that exhibits its action by disturbing the metabolic process involving folate, which is essential for cellular division. Contrary to the classical antimetabolites like methotrexate, which blocks selective enzymes, Pem blocks multiple sites, creating a combinatorial effect by inhibiting multiple enzymes in pyridine and purine biosynthetic pathways [43]. However, the dose-limited side effects, P-gp efflux and stability are some of the downsides of Pem. Hence, in the current investigation, we fabricated mixed micelles of F127/F68/TPGS to improve the efficacy of Pem in the treatment of BC.
The formation of micelles can be assessed using a range of analytical methods that rely on detecting abrupt changes in the physical characteristics of copolymers [44]. In this research study, we utilized DLS to ascertain the CMC within the ternary systems. DLS also referred to as ‘quasi-elastic light scattering or photon correlation spectroscopy’, stands as a swift and established analytical technique for measuring parameters like macromolecule size, PDI, molecular weight and more. The elementary tenet of DLS is rooted in the Brownian movement molecules in the solution, which causes alteration of the incident light at different intensities. The intensities are measured as a function of time, which can be incorporated into the Stokes–Einstein equation to predict the hydrodynamic particle size. The point at which there is an abrupt alteration in the intensity of scattering is considered the CMC. The intensity exhibits a linear increase after CMC, as the micelles number increases. The lower CMC ascertains the stability of the micelles toward extreme dilutions in the biological milieu [45,46].
Particle diameter impacts the biodistribution and circulation times of the nanocarriers in vivo. It was observed that the Pem loading did not affect the hydrodynamic diameter of the polymeric micelles, which might be due to the Pem loading in the corona of the PMM. Small particle size facilitates enhanced penetration into solid tumors [47]. The PDI serves as an indicator of the uniformity within the sample. The outcomes revealed that micelles generated using the TFH resulted in a narrow size distribution [48]. The PEO/PEG on the surface ensures steric stabilization, further inhibiting the aggregation and providing higher stability. The prepared micelles can be considered stealth vesicles exhibiting excellent stability in the biological milieu, avoiding rapid phagocyte clearance and preserving their circulation in the body. The rationale behind studying the impact of dilution on the size of the formulation lies in understanding how micelle formation and stability are influenced by concentration variations. By investigating this aspect, we aim to ascertain the robustness of our micelle system under conditions mimicking those encountered in vivo, where significant dilution can occur. The particle size of the fabricated PMM showed uniformity against dilution with PBS up to 100-fold. The results show that the PMMs are stable to extreme dilution encountered in the in vivo conditions.
FTIR was performed to obtain further insights into possible interactions between Pem and polymers. The physical mixture spectra did not exhibit extra peaks, indicating its compatibility. The characteristic peaks of Pem were clear in the FTIR spectrum, whereas the Pem peaks were obscured in the Pem-PMM spectra, and intensity was reduced as the Pem was entrapped within the micellar system. The indifference between the blank PMM and Pem-PMM spectra shows that the Pem is encapsulated in the formulation. These results are in line with the PXRD results. The PXRD spectra of the physical mixture exhibited the sharp crystalline peaks of the Pem and the excipients. In contrast, the lyophilized Pem-PMM exhibited the absence and reduction of the major peaks of the Pem. However, the minor peaks of PEO groups of the excipients were present in the x-ray diffraction patterns of the lyophilized Pem-PMM. This illustrates that the probable interactions among the excipients and the Pem might have prevented its crystallization. DSC investigations were carried out to understand the thermal events. In the Pem, endothermic transitions were observed at 98.82 and 151.06°C, which might be due to the dehydration, and the transition at 244.59°C might be attributed to the melting of the dehydrated form of the Pem. The exothermic peak at 279°C might be associated with the decomposition of the Pem. These peaks confirm the crystalline nature of the Pem [33]. The crystalline nature of Pem was lost when loaded in the PMM, as indicated by the absence of a sharp endotherm at 244.90°C in Pem-PMM. The molecular dispersion of the Pem in the micellar system might be responsible for the loss of endotherm in the Pem-PMM formulation.
NMR spectroscopy is a powerful characterization tool to probe the molecular structure and dynamics at the atomic level. 1H NMR spectra were recorded and interpreted to understand the encapsulation of Pem in the PMM. The precise location of the Pem in the micelles was further confirmed using selective 1D and 2D ROESY, which ascertains the interactions between the spatially close protons by showing a cross-peak in the 2D spectra attributed to the NOE. The difference in the chemical shift between the Pem and Pem-PMM may be attributed to the encapsulation of the Pem into the PMM. Deuterium in the solvent (D2O) undergoes fast exchange with amine (–NH2) and carboxylic (–COOH) protons in the sample. To overcome this, 1D selective ROESY and 2D ROESY experiments were recorded in water with 10% D2O with the reference compound, trimethyl propyl silane, after the suppression of water peak (4.79 p.p.m.) to investigate the NOE between the Pem and the nanocarrier system. 1D selective ROESY exhibited interactions between the Pem and the micellar system. 2D ROESY was performed to confirm the interactions between the polymer and the Pem. The protons in the region of 6–8 p.p.m. (H7, H8, H9) belong to the Pem and exhibited cross-peaks with the alkyl groups of the F127, which may be attributed to the interaction of the Pem with the Pluronics. This confirms the Pem loading within the micellar system.
Dissolution studies show that the Pem release from the Pem-PMM occurred in a controlled manner, which may be attributed to the encapsulation of the drug in the micelles. At the tumor pH, 80% of the Pem was released within the initial 24 h and aided in reducing tumor proliferation right after its delivery. The hemolytic test confirmed that Pem-PMM exhibited greater hemocompatibility than the free drug. F127 is reported to reduce the hemolytic activity of amphiphilic drugs, which may be due to the lower hemolytic potential of the formulated micellar system. The hemolysis assay demonstrated the excellent biocompatibility of the PMM [49].
Our rationale for selecting MCF-7 cell lines stems from their widespread use as a model for investigating BC therapies. Additionally, MCF-7 cells represent a pertinent model for hormone receptor-positive BC, a subtype that constitutes a significant portion of BC cases. MCF-7 cells were employed to investigate the effectiveness of the Pem-PMM. The polymers utilized in the formulation exhibit cytotoxicity at higher concentrations; to assess the safety profile of our nanocarrier, cytotoxicity analysis was performed, and cellular death was investigated. The results of our cytotoxicity assays revealed that the blank formulation exhibited an IC50 value of 11.109 μg/ml, indicating its cytotoxic effects on MCF-7 cells. Conversely, the Pem-PMM exhibited a substantially lower IC50 value of 0.130829 μg/ml at 24 h, demonstrating its cytotoxicity at a very low concentration. The eightfold difference in IC50 value between the Pem and Pem-PMM shows the enhanced efficacy of the formulation. The lower IC50 value of the Pem-PMM is probably due to the TPGS, which acts as a P-gp efflux inhibitor, thereby preventing efflux and promoting enhanced endocytosis. Furthermore, the inclusion of TPGS in our formulation serves a dual purpose. Not only does TPGS act as a stabilizer for the nanocarrier, but it also possesses inherent biological properties, including antioxidant and anticancer activities. TPGS has been shown to enhance drug-delivery efficacy by promoting apoptosis in cancer cells and overcoming multidrug resistance mechanisms, thereby augmenting the therapeutic potential of our nanocarrier. Our comprehensive in vitro cytotoxicity analysis demonstrated that the nanocarrier containing TPGS exhibited significant anticancer activity against MCF-7 cells. Also, previous investigations reported that Pluronic destabilizes the cell membrane and improves the permeability of the drug, which might be attributed to the enhanced activity of the formulation [50].
Cell internalization investigations were conducted to check the effectiveness of the PMM in delivering the Pem into the MCF-7 cells. The figures demonstrated higher cellular uptake of the PMM due to its smaller size and the presence of TPGS. Cao et al. demonstrated that the Pluronics/TPGS mixed micelles facilitate cell uptake via clathrin-dependent, cholesterol-independent and caveolae-independent pathways in melanoma cells [51]. This mechanism may be attributed to the higher cellular uptake of the dye-loaded PMM compared with the dye solution. Qualitative estimation of apoptosis was investigated after exposure to Pem-PMM via dual (AO/EB) staining and DAPI staining techniques. DAPI is a blue fluorescent nuclear staining dye that distinguishes apoptotic cells through observable nuclear alterations such as bright nuclei, condensed or fragmented chromatin and horseshoe-shaped nuclei. Similar results were reported by Chary et al. using F127/TPGS mixed micelles for the delivery of the venetoclax to cancer cells [27].
ROS plays an important role in the immune system and cell disruption. The treatment with Pem-PMM resulted in an excess onsite generation of ROS indicated by the green fluorescence formed by the reaction of generated ROS and formed fluorescent 2′,7′-dichlorofluorescein. The cells treated with Pem-PMM exhibited greater fluorescence than the normal group, which may be due to improved apoptosis-inducing potential, high entrapment of the Pem and improved permeation of the Pem into the cells. Morphological observations of MCF-7 cells after treatment with different doses (low, IC50 and high doses) of Pem-PMM revealed the disruption and shrinkage of the cell membrane. The Pem-PMM exhibited a concentration-dependent reduction in the cells. Cancerous cells proliferate and migrate with the aid of the extracellular matrix by intravasing into the systemic circulation, followed by their attachment at a distinct site, and lastly, extravasing by creating their own identity. This is termed metastatic progression, a primary reason for mortality in cancer patients. Pem-PMM showed hindrance to the migration, which might be attributed to the internalization of the Pem through the PMM into the MCF-7 cells. These results indicate that the Pem-PMM has the potential of averting metastasis in vitro.
Our study sheds light on the therapeutic potential of Pem-PMM as a promising therapeutic approach for treating BC. Comprehensive in vitro experiments demonstrated the enhanced cytotoxicity and cellular uptake of the Pem-PMM compared with the Pem alone, demonstrating its potential as a nanocarrier. Furthermore, our work investigated the interactions of the Pem with the micellar system through different analytical techniques. However, while the in vitro studies give the preliminary data, further in vivo studies are essential to validate the safety and efficacy of the Pem-PMM. The extrapolation of our results to clinical settings requires consideration of the tumor microenvironment, pharmacokinetics, etc., to have a comprehensive understanding of the potential of the Pem-PMM. Despite these downsides, our work contributes to expanding the pool of evidence highlighting the effectiveness of the nanocarrier systems for the treatment of cancer. By addressing these limitations, we can further elucidate the translational potential of the Pem-PMM in improving therapeutic outcomes in cancer treatment.
Conclusion
In this research study, we engineered PMM encapsulated with Pem to enhance the drug's effectiveness against BC. The formulation, Pem-PMM, was crafted using TFH and extensively evaluated through various analytical methods. The optimized PMM displayed a spherical morphology, boasting a particle size of 19.58 ± 0.89 nm, a PDI of 0.245 ± 0.1 and a surface charge of -9.70 ± 0.61 mV. %EE and %DL for Pem-PMM reached 96.16 ± 0.37% and 4.5 ± 0.09%, respectively. The presence of the drug within the micellar structure was verified through FTIR, PXRD and DSC analyses.
Furthermore, through 1D, selective 1D and 2D NMR studies, we confirmed the interactions between the Pem and the excipients employed in the formulation. To assess micellar stability upon dilution, we conducted a dilution stability evaluation, demonstrating the system's resilience. In vitro drug-dissolution investigations divulged a sustained release profile compared with the Pem solution, following Higuchi kinetics. Hemolysis assays indicated the biocompatibility of the micellar system with red blood cells. Most importantly, in vitro cytotoxicity analysis highlighted the superior efficacy of our prepared Pem-PMM compared with the pure drug solution. The IC50 value of the Pem-PMM was eightfold less than the Pem alone, attributed to the encapsulation of Pem within the micellar system. Qualitative cell uptake studies, apoptosis assays and cell migration assays further underscored the potential impact of the micellar system on BC cells. Collectively, these findings suggest that PMM holds great promise as a nanocarrier for BC treatment.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the Department of Pharmaceutics, National Institute of Pharmaceutical Education and Research, Hyderabad (Ministry of Chemical and Fertilizers, New Delhi, India) for providing extending facilities during this manuscript.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Nel J, Elkhoury K, Velot Éet al. Functionalized liposomes for targeted breast cancer drug delivery. Bioact. Mater. 24, 401–437 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumari NU, Pardhi E, Chary PS, Mehra NK. Exploring contemporary breakthroughs in utilizing vesicular nanocarriers for breast cancer therapy. Ther. Deliv. 15(4), 279–303 (2024). [DOI] [PubMed] [Google Scholar]
- 3.Dinakar YH, Rajana N, Kumari NU, Jain V, Mehra NK. Recent advances of multifunctional PLGA nanocarriers in the management of triple-negative breast cancer. AAPS PharmSciTech 24(8), 1–20 (2023). [DOI] [PubMed] [Google Scholar]
- 4.Rajana N, Mounika A, Chary PSet al. Multifunctional hybrid nanoparticles in diagnosis and therapy of breast cancer. J. Control. Rel. 352, 1024–1047 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Gridelli C, Maione P, Rossi Aet al. Pemetrexed in advanced non-small-cell lung cancer. Expert Opin. Drug Saf. 10(2), 311–317 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Felip E, Rosell R. Pemetrexed as second-line therapy for advanced non-small-cell lung cancer (NSCLC). Ther. Clin. Risk Manag. 4(3), 579–585 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soni K, Mujtaba A, Kohli K. Lipid drug conjugate nanoparticle as a potential nanocarrier for the oral delivery of pemetrexed diacid: formulation design, characterization, ex vivo, and in vivo assessment. Int. J. Biol. Macromol. 103, 139–151 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Pangeni R, Panthi VK, Yoon IS, Park JW. Preparation, characterization, and in vivo evaluation of an oral multiple nanoemulsive system for co-delivery of pemetrexed and quercetin. Pharmaceutics 10(3), 158 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ak G, Aksu D, Çapkın E, Sarı Ö, Kımız Geboloğlu I, Şanlıer ŞH. Delivery of pemetrexed by magnetic nanoparticles: design, characterization, in vitro and in vivo assessment. Prep. Biochem. Biotechnol. 50(3), 215–225 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Ghezzi M, Pescina S, Padula Cet al. Polymeric micelles in drug delivery: an insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Rel. 332, 312–336 (2021). [DOI] [PubMed] [Google Scholar]; • Reviews the polymeric micelles in cancer therapy.
- 11.Arranja A, Schroder AP, Schmutz M, Waton G, Schosseler F, Mendes E. Cytotoxicity and internalization of Pluronic micelles stabilized by core cross-linking. J. Control. Rel. 196, 87–95 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Gregoriou Y, Gregoriou G, Yilmaz Vet al. Resveratrol loaded polymeric micelles for theranostic targeting of breast cancer cells. Nanotheranostics 5(1), 113 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Developed a strategy for delivering Resveratrol using a newly synthesized nanocarrier with the potential for both diagnosis and treatment.
- 13.Mi Y, Liu X, Zhao J, Ding J, Feng SS. Multimodality treatment of cancer with herceptin conjugated, thermomagnetic iron oxides and docetaxel loaded nanoparticles of biodegradable polymers. Biomaterials 33(30), 7519–7529 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Srivastava S, Kumar A, Yadav PKet al. Formulation and performance evaluation of polymeric mixed micelles encapsulated with baicalein for breast cancer treatment. Drug Dev. Ind. Pharm. 47(9), 1512–1522 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Nezhadi S, Norouzi P, Rasouli A, Akbari Javar H, Ostad SN, Dorkoosh F. Co-delivery of paclitaxel and regorafenib by F127/TPGS mixed micelles for triple negative breast cancer treatment. J. Drug Deliv. Sci. Technol. 86, 104673 (2023). [Google Scholar]
- 16.Gao Y, Li LB, Zhai G. Preparation and characterization of Pluronic/TPGS mixed micelles for solubilization of camptothecin. Colloids Surf. B Biointerfaces 64(2), 194–199 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Patil KS, Hajare AA, Manjappa AS, More HN, Disouza JI. Design, development, in silico and in vitro characterization of docetaxel-loaded TPGS/Pluronic F 108 mixed micelles for improved cancer treatment. J. Drug Deliv. Sci. Technol. 65, 102685 (2021). [Google Scholar]
- 18.Shen C, Zhu J, Song Jet al. Formulation of Pluronic F127/TPGS mixed micelles to improve the oral absorption of glycyrrhizic acid. Drug Dev. Ind. Pharm. 46(7), 1100–1107 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Meng X, Liu J, Yu X, Li J, Lu X, Shen T. Pluronic F127 and D-α-tocopheryl polyethylene glycol succinate (TPGS) mixed micelles for targeting drug delivery across the blood–brain barrier. Sci. Rep. 7(1), 1–12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Fabrication of polymeric mixed micelles for delivery of drug to the brain.
- 20.Muralidharan S, Kumar JR, Dhanara SA. Development and validation of an high-performance liquid chromatographic, and a ultraviolet spectrophotometric method for determination of ambroxol hydrochloride in pharmaceutical preparations. J. Adv. Pharm. Technol. Res. 4(1), 65–68 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butt AM, Amin MCIM, Katas H, Sarisuta N, Witoonsaridsilp W, Benjakul R. In vitro characterization of Pluronic F127 and D-α-tocopheryl polyethylene glycol 1000 succinate mixed micelles as nanocarriers for targeted anticancer-drug delivery. J. Nanomater. 2012, 11 (2012). [Google Scholar]
- 22.Changsan N, Sawatdee S, Suedee R, Chunhachaichana C, Srichana T. Aqueous cannabidiol β-cyclodextrin complexed polymeric micelle nasal spray to attenuate in vitro and ex vivo SARS-CoV-2-induced cytokine storms. Int. J. Pharm. 640(4), 123035 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhavana V, Chary PS, Rajana Net al. Multimodal lemongrass oil based topical nanoemulgel ingrained with ferulic acid for wound healing activity. J. Mol. Liq. 389, 122870 (2023). [Google Scholar]
- 24.Rajana N, Chary PS, Pooja YSet al. Quality by design approach-based fabrication and evaluation of self-nanoemulsifying drug delivery system for improved delivery of venetoclax. Drug Deliv. Transl. Res. 14, 1–24 (2023). [DOI] [PubMed] [Google Scholar]
- 25.Zhai Y, Guo S, Liu Cet al. Preparation and in vitro evaluation of apigenin-loaded polymeric micelles. Colloids Surf. A Physicochem. Eng. Asp. 429, 24–30 (2013). [Google Scholar]
- 26.Kaviarasi B, Rajana N, Pooja YS, Rajalakshmi AN, Singh SB, Mehra NK. Investigating the effectiveness of difluprednate-loaded core-shell lipid-polymeric hybrid nanoparticles for ocular delivery. Int. J. Pharm. 640(4), 123006 (2023). [DOI] [PubMed] [Google Scholar]
- 27.Chary PS, Rajana N, Devabattula Get al. Design, fabrication and evaluation of stabilized polymeric mixed micelles for effective management in cancer therapy. Pharm. Res. 39(11), 2761–2780 (2022). [DOI] [PubMed] [Google Scholar]; •• Repurposing of the drug using polymeric mixed micelles.
- 28.Pooja YS, Rajana N, Yadav R, Naraharisetti LT, Godugu C, Mehra NK. Design, development, and evaluation of CDK-4/6 inhibitor loaded 4-carboxy phenyl boronic acid conjugated pH-sensitive chitosan lecithin nanoparticles in the management of breast cancer. Int. J. Biol. Macromol. 258(Part 1), 128821 (2024). [DOI] [PubMed] [Google Scholar]
- 29.Rajana N, Sandeep Chary P, Bhavana Vet al. Targeted delivery and apoptosis induction of CDK-4/6 inhibitor loaded folic acid decorated lipid-polymer hybrid nanoparticles in breast cancer cells. Int. J. Pharm. 651, 123787 (2024). [DOI] [PubMed] [Google Scholar]
- 30.Cavalcante CH, Fernandes RS, de Oliveira Silva Jet al. Doxorubicin-loaded pH-sensitive micelles: a promising alternative to enhance antitumor activity and reduce toxicity. Biomed. Pharmacother. 134, 111076 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Moretton MA, Chiappetta DA, Sosnik A. Cryoprotection-lyophilization and physical stabilization of rifampicin-loaded flower-like polymeric micelles. J. R. Soc. Interface 9(68), 487–502 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Tommaso C, Como C, Gurny R, Möller M. Investigations on the lyophilisation of MPEG-hexPLA micelle based pharmaceutical formulations. Eur. J. Pharm. Sci. 40(1), 38–47 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Vandana M, Sahoo SK. Reduced folate carrier independent internalization of PEGylated pemetrexed: a potential nanomedicinal approach for breast cancer therapy. Mol. Pharm. 9(10), 2828–2843 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Koulouktsi C, Nanaki S, Barmpalexis P, Kostoglou M, Bikiaris D. Preparation and characterization of alendronate depot microspheres based on novel poly(-ϵ-caprolactone)/vitamin E TPGS copolymers. Int. J. Pharm. 1(4), 100014 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Won DH, Park H, Ha ESet al. Effect of formulation factors and oxygen levels on the stability of aqueous injectable solution containing pemetrexed. Pharmaceutics 12(1), 1–17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Evaluation of critical micellar concentration of the copolymer using spectroscopic techniques.
- 36.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu. Rev. Med. 63, 185–198 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J. Hematol. Oncol. 14(1), 1–27 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghosh B, Biswas S. Polymeric micelles in cancer therapy: state of the art. J. Control. Rel. 332, 127–147 (2021). [DOI] [PubMed] [Google Scholar]
- 39.Gregoriou Y, Gregoriou G, Yilmaz Vet al. Resveratrol loaded polymeric micelles for theranostic targeting of breast cancer cells. Nanotheranostics 5(1), 113–124 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saxena V, Delwar Hussain M. Poloxamer 407/TPGS mixed micelles for delivery of gambogic acid to breast and multidrug-resistant cancer. Int. J. Nanomed. 7, 713 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fahmy AM, Hassan M, El-Setouhy DA, Tayel SA, Al-mahallawi AM. Voriconazole ternary micellar systems for the treatment of ocular mycosis: statistical optimization and in vivo evaluation. J. Pharm. Sci. 110(5), 2130–2138 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Thanitwatthanasak S, Sagis LMC, Chitprasert P. Pluronic F127/Pluronic P123/vitamin E TPGS mixed micelles for oral delivery of mangiferin and quercetin: mixture-design optimization, micellization, and solubilization behavior. J. Mol. Liq. 274, 223–238 (2019). [Google Scholar]
- 43.Hanauske A, Chen V, Paoletti P, Niyikiza C, Hanauske A-R. Pemetrexed disodium: a novel antifolate clinically active against multiple solid tumors. Oncologist 6(4), 363–373 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Fluksman A, Benny O. A robust method for critical micelle concentration determination using coumarin-6 as a fluorescent probe. Anal. Methods 11(30), 3810–3818 (2019). [Google Scholar]
- 45.Wei Z, Hao J, Yuan Set al. Paclitaxel-loaded Pluronic P123/F127 mixed polymeric micelles: formulation, optimization and in vitro characterization. Int. J. Pharm. 376(1–2), 176–185 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Topel Ö, Çakir BA, Budama L, Hoda N. Determination of critical micelle concentration of polybutadiene-block-poly(ethyleneoxide) diblock copolymer by fluorescence spectroscopy and dynamic light scattering. J. Mol. Liq. 177, 40–43 (2013). [Google Scholar]
- 47.Cabral H, Matsumoto Y, Mizuno Ket al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 6(12), 815–823 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Shen C, Zhu J, Song Jet al. Formulation of Pluronic F127/TPGS mixed micelles to improve the oral absorption of glycyrrhizic acid. Drug Dev. Ind. Pharm. 46(7), 1100–1107 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Amarnath Praphakar R, Sam Ebenezer R, Vignesh S, Shakila H, Rajan M. Versatile pH-responsive chitosan-g-polycaprolactone/maleic anhydride-isoniazid polymeric micelle to improve the bioavailability of tuberculosis multidrugs. ACS Appl. Bio Mater. 2(5), 1931–1943 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Demina T, Grozdova I, Krylova Oet al. Relationship between the structure of amphiphilic copolymers and their ability to disturb lipid bilayers. Biochemistry 44(10), 4042–4054 (2005). [DOI] [PubMed] [Google Scholar]
- 51.Cao X, Zhou X, Wang Yet al. Diblock- and triblock-copolymer based mixed micelles with high tumor penetration in vitro and in vivo. J. Mater. Chem. B 4(19), 3216–3224 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


