ABSTRACT
Akkermansia sp are common members of the human gut microbiota. Multiple reports have emerged linking the abundance of A. muciniphila to health benefits and disease risk in humans and animals. This review highlights findings linking Akkermansia species in the gastrointestinal (GI) tract to health outcomes across a spectrum of disorders, encompassing those that affect the digestive, respiratory, urinary, and central nervous systems. The mechanism through which Akkermansia exerts a beneficial versus a detrimental effect on health is likely dependent on the genetic makeup of the host metabolic capacity and immunomodulatory properties of the strain, the competition or cooperation with other members of the host microbiota, as well as synergy with co-administered therapies.
KEYWORDS: Akkermansia muciniphila, massiliensis, biwaensis, ignis, durhamii, microbiota, obesity, diabetes, neurological disease, cancer, immunotherapy, liver, infections, sepsis, asthma, nephropathy, ALS, AD, PD, ASD, MS, helminth, GVHD, IBD, influenza, tuberculosis, Clostridium difficile, Salmonella typhimurium, epilepsy, NAFLD, CKD, LCMV
Introduction
The microbe Akkermansia muciniphila was first isolated two decades ago1 and has emerged as an important member of the complex microbial community in the gastrointestinal tract.2 Over the last decade, multiple reports have linked the abundance of A. muciniphila to health benefits and disease risk.
The genus Akkermansia was named after the Dutch microbiologist Dr. Antoon Akkermans, and the species designation muciniphila was used to highlight the ability of the first species isolated to thrive using mucin as the sole carbon and energy source. A. muciniphila strain MucT represents the first human isolate belonging to the Verrucomicrobiota phylum. This phylum mainly comprises bacteria from marine environments and animal feces. In addition to muciniphila, the Akkermansia genus includes the species massiliensis,3 biwaensis,4 ignis5 and durhamii,6 as well as additional potential species within clade AmIII.7 The genomes of A. muciniphila are ~ 10% smaller than those of other Akkermansia species.6 Based on metagenomic sampling, approximately half of human fecal samples contain detectable levels of Akkermansia. Among those, A. muciniphila is the most common species, representing ~ 70% of all isolates.6,8 Akkermansia muciniphila is subdivided into subsp muciniphila (AMIa) and communis (AMIb).6 Whether the genetic differences between Akkermansia species and subspecies contribute to their impact on human health remains to be determined.
The application of animal models to understand the impact of Akkermansia on human health
Akkermansia can ameliorate disease in animal models
Introducing Akkermansia into animals can ameliorate disease and modulate the immune system toward an anti-inflammatory response. For instance, repeated administration of A. muciniphila MucT has been reported to protect mice from infection by Clostridioides difficile,9 Listeria monocytogenes,10 Fusobacterium nucleatum,11 Porphyromonas gingivalis12,13 and Salmonella typhimurium.14 Pasteurized A. muciniphila protects against S. typhimurium infection,14 and the outer membrane protein Amuc_1100 is sufficient to protect against P. gingivalis periodontitis.13 In the case of F. nucleatum, Akkermansia can directly block the expression of an F. nucleatum virulence factor, thus inhibiting the activation of the NFkB pathway in gingival epithelial cells.11 Common mechanisms proposed to explain Akkermansia protective role in these infection models include increases in anti-inflammatory responses mediated by IL-10, and expression of tight junction proteins that limit pathogen translocation across epithelial barriers. It is important to consider that these studies focused exclusively on mouse models and involved multiple inoculations of Akkermansia.
Mouse models of diseases of impaired lipid homeostasis such as atherosclerosis,15 acute and chronic hyperlipidemia,16 nonalcoholic fatty liver disease (NAFLD)17–19 and nonalcoholic steatohepatitis (NASH)20 also point to a beneficial role for Akkermansia. Viable A. muciniphila MucT was required to diminish inflammation in acute and chronic hyperlipidemia16 and atherosclerosis.15 For the latter, an increase in the expression of tight junction proteins and reduction in proinflammatory cytokines and macrophage infiltration lead to decreased metabolic endotoxemia-induced inflammation and improved gut barrier.15 In a mouse model of hyper triglyceridemia (CREBH-null), administration of A. muciniphila increased the expression of LDL receptors, reduced hepatic endoplasmic reticulum stress, and lowered triglycerides, dampening inflammatory responses.16 The protection against NAFLD in mouse models involves a decline in hepatic enzymes in the serum,17,19 decreased IL-6 in the liver,17 reduced expression of NLRP3 and TLR4/NF-kB, and changes in the microbiota composition.19 A third study in NAFLD mouse models proposed a synergistic role with Bifidobacterium bifidum for activating hepatic farnesol X receptors and suppressing those in the colon, plus increased tight junction protein expression.18 The prevention of hepatic inflammation was also reported for a mouse model of NASH, where Akkermansia administration decreased TLR2 expression, reduced hepatic M1 macrophages and inflammatory γδT and γδT17 cells.20 One of these studies in liver disease proposed that Amuc_1100 was sufficient to mimic the effects of live Akkermansia.19
Overall, these studies suggest that Akkermansia modulation of host responses after repeated administration, especially through dampening of inflammation, can be protective in the context of infection with several pathogens and during hepatic or lipid metabolism-linked diseases. In some studies, viable Akkermansia was not required for this effect, with Amuc_1100 being able to mimic the response to viable bacteria in NAFLD.
Akkermansia abundance negatively impacts health in animal models
In specific contexts, Akkermansia can have a detrimental impact on some mouse models of disease. A. muciniphila can bloom upon infection with lymphocytic choriomeningitis virus or following antibiotic treatment in an immune-compromised host, e.g., graft versus host disease (GVHD). In the first case, LCMV infection induces anorexia, followed by an increase in A. muciniphila abundance, attenuating CD8+ T cell response to the virus.21 Because Akkermansia proliferates robustly in host mucins, its abundance is independent of nutrients consumed by the host, and thus, the relative levels of Akkermansia increase during fasting.21 Similarly, during hematopoietic stem cell transplantation, Akkermansia levels increase as the host’s appetite declines following chemical and radiation therapy. As overall mucolytic activities increase, the intestinal barrier becomes compromised, which increases the risk of neutropenic fevers and GVHD.22
The lack of fiber in the diet restricts the growth of glycan-consuming commensals, which cannot rely on mucin and related glycoproteins as the sole carbon and nitrogen source. Akkermansia bloom in BALB/c mice fed a fiber-free diet led to thinning of the mucus layer, increased type I and II cytokines, and IgE coating of commensals. This can result in an increased risk of allergic reactions to food allergens.23 Moreover, an intervention study in animals on a fiber-free diet with A. muciniphila supports the premise that the bacterium’s mucin-degrading capabilities can negatively impact immune health. A. muciniphila-induced thinning of the mucus layer increased the vulnerability of mice to C. rodentium infections.24
Overall, these animal studies suggest that the impact of Akkermansia supplementation on its host’s health is modulated by diet and immune status. The complex interactions between hosts, their microbiota, diet, and exposure to microbial pathovars can impact Akkermansia’s potential as a probiotic. In addition, understanding the impact of diverse Akkermansia species and the proposed mechanism should be considered.
Akkermansia’s impact on human health as inferred by clinical associations
Diseases where Akkermansia abundance correlates with positive health outcomes, and Akkermansia can ameliorate disease in animal models and humans
Since the initial study ascribing a protective role for A. muciniphila in metabolic disorders, multiple reports linking Akkermansia to positive health outcomes have emerged. The relative abundance of A. muciniphila in animal models25 and humans26 decreases during diabetes or obesity. Moreover, oral administration of Akkermansia mitigates disease in animals25–32 and has proven beneficial in preliminary clinical studies, improving insulin sensitivity, plasma cholesterol levels, and human inflammation markers.33 Foundational studies by DeVos and Cani found that live but not heat-killed A. muciniphila can reverse metabolic disorders by increasing endocannabinoids that control inflammation, gut peptide secretion, and gut barrier function in mice.25 Since then, pasteurized A. muciniphila27, A. muciniphila Extracellular Vesicles (EVs),26,27,29 Amuc_1100,27,34 and P9 (a protein capable of inducing glucagon-like peptide-1)31 have been shown to be sufficient to improve obesity markers. In vitro studies have suggested that TLR2 is activated by Amuc_1100,27 and phosphorylation of the kinase AMPK occurs after exposure to EVs,26 hinting at potential mechanisms of action. Mucins may modulate some of these activities, as A. muciniphila cultivated in synthetic media, as opposed to mucin-containing media, exhibited greater efficacy in enhancing intestinal barrier function and ameliorating metabolic disorders in mice.30
It should be noted that these intervention studies have exclusively concentrated on the A. muciniphila strain MucT. There is strong evidence of heterogeneity among Akkermansia species and A. muciniphila subspecies.6,35 How these may diverge in their impact on host physiology is poorly understood.
Diseases where Akkermansia abundance correlates with positive health outcomes, and it can ameliorate disease in animal models
Recent observations indicate that the severity of a broad spectrum of diseases could be potentially impacted by Akkermansia abundance. It includes responsiveness to cancer immunotherapies, clinical depression, asthma, sepsis, irritable bowel syndrome, osteoporosis, alcoholic steatohepatitis, chronic kidney disease, and tuberculosis.
In cancer, a revolutionary study demonstrated that a fecal microbiota transplant (FMT) supplemented with A. muciniphila, strain CSUR P2261, improved patients’ response to immune checkpoint inhibition (ICI) with anti-PD1 antibodies.36 In a mouse model of subcutaneous sarcoma, A. muciniphila enhanced IL-12-dependent recruitment of CCR9+CXCR3+CD4+ central memory T cells to tumors and reduced the number of regulatory T cells (Tregs) in the tumor-infiltrating lymphocytes.36 Since then, there have been reports of lower Akkermansia abundance in patients with non-small cell lung (NSCLC)37 and colorectal38 cancers. Additionally, Akkermansia is enriched in the gut microbiota of patients who respond to immunotherapy in renal cell cancer (RCC) and NSCLC cases.36,39,40 However, the level of Akkermansia is an important variable as high abundance (>4.66%) is associated with shorter survival as much as with no Akkermansia.39 In addition, the positive impact on survival is more robust in those colonized with A. muciniphila subspecies AmIa (subsp. muciniphila).6 Positive outcomes require the rest of the microbiota, as indicated by the 50% success rate in tumor response to ICI inhibition in mouse models after FMTs from NSCLC donors supplemented with Akkermansia.39 In an animal lung cancer model, A. muciniphila MucT inhibited tumorigenesis, and it was detected in the blood immediately after gavage and cleared by six hours, suggesting a non-GI mechanism of action in this experimental system.41 In colitis-associated colorectal cancer (CAC) animal models, pasteurized Akkermansia and Amuc_1100 can delay tumorigenesis by promoting the expansion and activation of cytotoxic T cells (CTLs) in the colon and mesenteric lymph nodes.42 Additional anti-tumor mechanisms include the enrichment of M1-like macrophages in the colorectal cancer tumor microenvironment (TME) in an NLRP3-dependent manner.38 EVs and Amuc_2172, an acetyltransferase that promotes the secretion of HSP70 in cancer cells, are proposed to promote CD8+ T cell activity.43 Finally, oral administration of live or pasteurized A. muciniphila, or recombinant Amuc_1100, can improve the outcome of IL-2 therapy in B16F10 and CT26 (subcutaneous melanoma and colorectal cancer) tumor-bearing mice. Amuc_1100 increased the CTLs and decreased Tregs in the TME.44 In summary, Akkermansia can enhance cancer immunotherapies by modulating immune cell infiltration into the TME.
Asthma is a condition in which the inflammation of the airways and an excess of mucus impact lung function. There appears to be a negative correlation between Akkermansia abundance in the gut and the severity of asthma in humans.45,46 Mouse models of allergen-induced respiratory airway disease support a protective role for Akkermansia. The protective effect applies only to pasteurized and live A. muciniphila, not heat-killed or supernatants.45,47 It is associated with decreased eosinophils in bronchoalveolar lavage (BAL), less secretion of Th2 cytokines in the lung, which are essential for IgE production, and a lymphocyte profile in lung tissues consistent with anti-inflammatory responses.45 These findings were supported by reports describing a decrease in airway hyper-responsiveness in lung mast cells, lower house dust mites-specific IgE, suppression of Th2 cytokines and eotaxin in bronchial lavages and increase in cecal SCFA as well as changes in the abundance of SCFA-associated genera in the gut.47
The relative levels of Akkermansia abundance have been reported to decrease in patients with sepsis and corresponding mouse models of the disease. Oral supplementation of A. muciniphila to mouse models of sepsis indicated that live, but not heat-killed, A. muciniphila provided protection. A peptide enriched in Akkermansia cultures supernatants and in the cecum and plasma of germ-free mice colonized with A. muciniphila, Arg-Lys-His (RKH), is responsible for binding TLR 4 and inhibiting systemic inflammation in sepsis mice and piglet models.48 Akkermansia‘s role in dampening sepsis is yet another example of how this microbe can modulate immunity systemically.
Irritable Bowel Syndrome (IBS) is a prevalent disorder affecting the gut-brain axis, characterized by symptoms such as abdominal pain, bloating, diarrhea, and constipation. In IBS patients who underwent FMT, the engraftment of Akkermansia correlated negatively with pain intensity, suggesting a potential role of Akkermansia in pain modulation in IBS.49 In a separate study involving IBS patients who had not responded to standard treatment for over a year and were treated by FMT, increased levels of Akkermansia were observed in individuals who showed lower IBS severity scores.50 Consistent with these findings, pasteurized A. muciniphila MucT reduced symptoms in a mouse model of colonic hypersensitivity.51 In the “Neonatal maternal separation” model, administration of Akkermansia led to an increase in ZO-1 expression with reduced epithelial permeability and improved anxiety measurements in a Citrobacter infection model. In both cases, a moderate increase in IL-22 expression was observed. Additionally, in vitro capacity was demonstrated to neuromodulate nociceptors in the colon.51
Depression stands as the foremost cause of disability globally, with mounting evidence highlighting a role for the microbiota, including studies in germ-free mice.52 Akkermansia levels were found to be lower in patients with depressive conditions, such as bipolar disorder,53 first episode of depression,54 and late-life depression.55 In contrast, in a cohort that compared severe versus mild depression, Akkermansia positively correlated with the severity of the disease.56 Similarly, Akkermansia was found to be enriched in atypical versus typical depression.57 In two studies with mouse models of stress-induced depression, Akkermansia was diminished in animals with more severe symptoms.58,59 These findings suggest that the association between this microbe and disease status may vary across subtypes of depression. However, these studies await confirmation in larger cohorts as the association between Akkermansia abundance with subtypes of depression was derived from a relatively small number of patients, both for atypical depression (15 atypical, 44 typical, 19 controls)57 and severity of disease (mild, n = 7, moderate, n = 18, severe, n = 14)56 subgroups. Nonetheless, multiple studies in mouse models support the role of A. muciniphila in dampening depression.60–69 Either A. muciniphila MucT,60,62,65,66,68 from clade AMIa (subspecies muciniphila), or A. muciniphila strain GP01,69 from clade AMIb (subspecies communis),6 are sufficient for this effect. Potential mechanisms of action include an increase in serotonin (5-HT) in the circulatory, digestive, and neurological systems; inhibition of serotonin transporter (SERT) expression in the gut, reduction in pro-inflammatory cytokines, upregulation of Brain-derived neurotrophic factor (BDNF); and modulation of the abundance or activities of other members of the microbiota. Live bacteria, EVs,62 or Amuc_1100,61,64,67,68 have been positively associated with an antidepressant effect. It has been proposed that Amuc_1100 binding to TLR2 increases intestinal serotonin. A truncated form of Amuc_1100 lacking the first 80 amino acids and with a higher affinity for TLR2 can exert antidepressant activity in animals.64
The degradation of bone tissue characterizes osteoporosis (OP). Osteopenia denotes a bone mineral density reduction before reaching the OP threshold. Akkermansia abundance tends to decrease in the gut microbiota of individuals with osteopenia70 and is further reduced in OP.71 Akkermansia levels are also diminished in Sprague Dawley rats with OP.72 Oral administration of A. muciniphila MucT to ovariectomized C57BL/6 mice shielded these mice from bone loss.73 Both viable A. muciniphila and EVs were protective, but pasteurized bacteria were not.73,74 The authors proposed that nanovesicles may penetrate and accumulate within bone tissue to exert beneficial effects, but how vesicles would access these sites and inhibit bone loss is unclear.
Patients with alcoholic steatohepatitis (ASH) display lower levels of Akkermansia.75 In a mouse model of ethanol-induced hepatic injury, administration of A. muciniphila prevented and ameliorated steatosis and infiltration of MPO+ neutrophils.75,76 Because Akkermansia was not detected in the hepatic tissue, its protective role was proposed to depend on increased expression of tight junction proteins, with a consequent reduction in endotoxin translocation.76
Chronic Kidney Disease (CKD) is a pathological condition characterized by progressive kidney damage, potentially culminating in renal failure. The abundance of Akkermansia correlates negatively with CKD.77 Supplementation with Akkermansia (strain GDMCC 1.1346) improved renal function parameters such as urine protein levels, serum creatinine, and blood urea nitrogen (BUN) serum levels in nephrectomized Sprague-Dawley rats. Investigators observed suppression of epithelial-mesenchymal transition (EMT) and decreased IL1B, IL10, and LPS in circulation.78
Lastly, tuberculosis is a bacterial infection caused by Mycobacterium tuberculosis that causes severe lung damage. A. muciniphila abundance is reduced in patients with active tuberculosis.79 Specific type I interferon receptor 1 (encoded by IFNAR1) alleles contribute to an enhanced immune response (higher tumor necrosis factor), decrease levels of A. muciniphila, and lead to more severe disease in transgenic mouse models and humans. Oral supplementation of A. muciniphila MucT in mouse models of tuberculosis reduces infection, lessens pathology, and reduces circulating TNF. Akkermansia exerts its beneficial impact through palmitoleic acid-mediated epigenetic inhibition of TNF.79
Diseases where Akkermansia abundance correlates with negative health outcomes, but it can lessen disease in animal models
Several reports suggest that Akkermansia is associated with a higher risk or severity of disease in humans. Unexpectedly, in some instances, supplementation with Akkermansia is protective in animal models of the same diseases. Examples include infections caused by helminths, influenza, severe fever with thrombocytopenia syndrome virus (SFTSV), and neurological diseases such as Alzheimer’s, Amyotrophic Lateral Sclerosis, Autism Spectrum Disorder, Epilepsy, and Multiple Sclerosis.
Increased Akkermansia levels have been reported in humans80 and mice81 infected with nematodes. However, oral gavage with live or pasteurized A. muciniphila MucT in mice infected with the roundworm Trichinella spiralis enhanced pathogen clearance via TLR2 independent of type II immunity.82 Similarly, infection of mice with the influenza virus leads to an increase in Akkermansia levels. But paradoxically, if mice are first gavaged with A. muciniphila MucT, the animals display lower pulmonary viral titers, reduced proinflammatory cytokine expression, and enhanced levels of type I and type II interferons upon infection with influenza.83 In humans infected with SFTSV, Akkermansia levels were higher in patients who survived and inversely correlated with inflammatory markers in serum. In animal models of SFTSV infection, oral gavage with A. muciniphila MucT mitigated infection, increasing the percentage of mice survival. A. muciniphila produces harmaline, which induces changes in the bile acid profile via BAAT (bile acid-CoA: amino acid N-acyltransferase) expression, thereby offering protection against SFTSV by suppressing NF-KB-mediated systemic inflammation through the transmembrane G-protein coupled receptor-5 (TGR5) pathway.84
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by a decline in cognitive function, memory loss, and behavioral changes. Reports in distinct human populations found increased Akkermansia abundance in individuals with AD.85–88 However, in a mouse model of AD (APP/PS1), oral administration of A. muciniphila GP01 (clade AMIb) improved impaired cognition and anxiety-related behaviors, which paralleled Aβ plaque formation in the cerebral cortex and improved glucose tolerance, intestinal barrier function, and dyslipidemia.89 Similar conclusions were made in two different animal models, AD-like Sprague-Dawley rat models injected by AlCl3 and D-galactose,90 and AlCl3 exposed zebrafish models.91 In both cases, administration of live or pasteurized A. muciniphila, respectively, reduced markers of AD. Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder that affects nerve cells in the brain and spinal cord. When comparing individuals with ALS to their spouses within one year of diagnosis, Akkermansia levels were elevated only in ALS patients.92 In mice expressing an ALS-associated human mutated superoxide dismutase 1 (SOD1.G93A), there is a decrease in the Akkermansia relative abundance as the disease progresses compared to wild-type littermates. Two Akkermansia strains (muciniphila MucT and ATCC BAA-2869 (isolated from a squirrel) can improve motor symptoms when given to SOD1.G93A mice. This improvement in ALS correlated with increased nicotinamide levels in the serum and CSF, which could improve mitochondrial function.93 Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder characterized by challenges in social interaction, communication, and repetitive behaviors. The association between Akkermansia abundance and ASD depends on the population assessed; it decreased in cohorts in Australia94 and China95 but increased in Ecuador.96 The size of the ASD cohorts also varied, which might explain the discrepancies encountered (23 ASD in Australia, 25 ASD in Ecuador, 48 ASD in China). When tested in a mouse model of ASD (C57BL/6J VPA-treated), live A. muciniphila MucT activated dopaminergic neurons, improving social deficits.97 Cerebral palsy (CP) and epilepsy are two interacting diseases with symptoms that appear in early childhood and denote a disruption in neuronal activity in the brain. Akkermansia levels are also increased in children with CP and epilepsy.98 An extreme ketogenic diet is an effective treatment for patients who fail to respond to anticonvulsant drugs. In mouse models of epilepsy (6-Hz and Kcna1-/-), a ketogenic diet alters the gut microbiota, increases Akkermansia relative abundance, and protects against seizures. A. muciniphila MucT strain co-administered with Parabacteroides confers seizure protection with increased GABA/glutamate in the hippocampus and decreased circulating gamma-glutamyl amino acids.99 Multiple Sclerosis (MS) is a chronic autoimmune disease that affects the central nervous system. Earlier studies established a positive correlation between Akkermansia abundance and MS diagnosis.100,101 In contrast, a later study indicated higher levels of Akkermansia were linked to lower disability scores in relapsing-remitting (RRMS) and progressive MS.102 It has also been reported that Akkermansia-specific IgG increases in the cerebrospinal fluid of RRMS compared to other neurological diseases.103 Similarly, A. muciniphila significantly increases the differentiation of peripheral blood mononuclear cells into Th1 lymphocytes in vitro, but T lymphocyte differentiation does not occur in multiple lymphoid tissues when administered to germ-free mice.100 Subsequent studies ascribed a positive impact of Akkermansia on MS symptoms using either A. muciniphila MucT or A. muciniphila strains (Prog-BWH-J5, RRMS-BWH-H3, Prog-BWH-I7) isolated from patients.102,104 A marked surge in Akkermansia levels occurs at the peak of the disease in the EAE (experimental autoimmune encephalomyelitis) mouse model and untreated MS patients, which is proposed to be mediated by the microRNA miR-30d. This bloom correlates with an expansion of Tregs, which ameliorates disease in the EAE model.104 In addition, Akkermansia strain BWH-H3 reduced RORγT+ γδ T cells and IL-17–producing γδ T cells, which are pro-inflammatory.102 Interestingly, a comprehensive prospective study in infants who did not develop neurological disorders such as ADHD, ASD, Speech, and intellectual disability in childhood found Akkermansia was enriched in healthy children as well as metabolites such as 3,4-dihydroxy-phenyl-propionic acid.105 Indeed, considering published observations, it’s plausible to interpret that the increase in Akkermansia abundance may represent a response to these neurological disorders rather than being a causative factor.
Diseases where the results are inconsistent for supporting a protective role for Akkermansia: the role of genetic diversity and animal models as confounders
Inflammatory Bowel Disease (IBD) encompasses a group of chronic inflammatory conditions of the digestive tract, represented mainly by Crohn’s disease (CD) and ulcerative colitis (UC). In patients with IBD, some studies report a negative correlation between A. muciniphila levels and IBD,106,107 while others did not find an association.108,109 Evidence indicates that the type of A. muciniphila subspecies present may differentially affect protection from CD and UC. Lower levels of subspecies muciniphila (AmIa) are associated with cases of CD and UC, but lower levels of subspecies communis (AmIb) are associated with UC cases only.6 The impact of Akkermansia on protection from IBD is controversial. In a mouse model of early-stage IBD (deletion of the transcription factor Hnf4a in intestinal epithelial cells), spikes in Akkermansia levels were associated with spontaneous episodes of colitis and elevated intestinal inflammation.5 However, the strain in these mice represented a new Akkermansia species, A. ignis.6 NLRP6 (NOD‐like receptor family pyrin domain containing 6) restricts A. muciniphila colonization. NLRP6 deficient animals are enriched for A. muciniphila and display higher intestinal inflammation, and IL10-/- NLRP6 -/- mice develop spontaneous colitis in a facility where IL10-/- mice do not. Furthermore, when gavaged weekly, a murine isolate of Akkermansia induced colitis in IL10-/- mice.110 However, in a different model of experimental colitis induced by Dextran Sulfate Sodium (DSS), repeated gavages with various Akkermansia strains, including the mouse strain “139”,111 MucT,42,111,112 and another murine isolate of A. muciniphila113 were all protective. Differences in the expansion of Tregs and lipocalin-2 levels have been reported for different Akkermansia strains.111 EVs,112 pasteurized A. muciniphila MucT, and Amuc_110042 could recapitulate the effect of live Akkermansia. The mechanisms underlying the protection of colitis likely involve improvement of gut epithelial integrity, a boost in the number of type 3 innate immune cells,113 and a reduction in infiltrating macrophages and CD8+ cytotoxic T cells in the colon.42 In summary, Akkermansia levels can modulate the risk of IBD. However, specific Akkermansia species or strains may have no effect, be protective, or exacerbate disease depending on other variables such as the host’s genetic makeup, repeated stimulation, immune status, and diet.
Diseases where Akkermansia abundance correlates with negative health outcomes, and disease is worsened in animal models
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by the loss of dopamine-producing neurons in the brain. Akkermansia is increased in the microbiota of geographically distant humans with PD.114–118 Akkermansia-induced mitochondrial Ca2+ overload leads to the generation of reactive oxygen species and the aggregation of pathogenic αSynuclein (αSyn) in a neuroendocrine cell line. Akkermansia supplementation in mice induced αSyn aggregation in enteroendocrine cells, but this was not linked to more severe motor deficiency.119 Patients undergoing hematopoietic stem cell transplantation can develop neutropenic fevers and be at an increased risk for developing Graft vs Host Disease (GVHD). The risk for neutropenic fevers is highest in patients with an increased abundance of mucin-degrading bacteria, including Akkermansia. This effect can be reproduced in mice undergoing either chemical or radiation-induced immune ablation and is linked to a drastic reduction in food consumption.120 Under conditions of caloric restriction, some fiber-degrading bacteria turn to mucins as a nutrient source, a process exacerbated by Akkermansia. Indeed, administration of a murine isolate of A. muciniphila (strain MDA-JAX AM001) to Akkermansia-free mice subjected to caloric restriction led to a thinning of the mucus layer in the GI. Treatment of these animals with an antibiotic targeting Akkermansia preserves the mucus layer, reduces inflammation markers, and prevents hypothermia.120 This highlights the potential therapeutic implications of modulating Akkermansia abundance to mitigate the adverse effects of chemotherapy and radiotherapy in cancer patients.
Immunoglobulin A nephropathy (IgAN), a predominant cause of renal failure, is an autoimmune disorder characterized by the deposition of IgA-dominant immune complexes in the glomerular mesangium. Akkermansia and other mucin-degraders are increased in the microbiota of patients with IgA nephropathy.121 Incubation of IgA subclass 1 (IgA1) with A. muciniphila induces recognition by autoantibodies in the serum of these patients. Mice expressing human IgA1 and the human Fc α receptor I (α1KI-CD89tg) developed an aggravated IgA nephropathy when colonized by A. muciniphila MucT. A. muciniphila deglycosylated IgA1, which enhanced its translocation across the mouse gut to the kidney, where it forms part of immune deposits. Additionally, two risk loci for IgAN are SNPs in the alpha-defensin genes DEFA5 and DEFA6, which modulate A. muciniphila growth.121 This is an example of a combination of variance host genetics and Akkermansia abundance that can exacerbate the pathology of IgAN.
Conclusions and perspectives
The accelerating number of studies linking Akkermansia to potential beneficial effects on humans across various disease categories presses the question of the mechanisms that enable this bacterium to impact so many aspects of human health. An emerging theme is that immunomodulation at the GI, potentially through the strengthening of gut barrier functions, can impact local and systemic inflammation. Other proposed mechanisms can contribute to overall immunomodulation, from the skewing of Treg cells106 to the direct secretion of TLR activators.19,27,64 Given that the GI is highly enervated, and most lymphocytes travel through the GI lymphatics, it is unsurprising that the microbiota and Akkermansia sp. would have a broader impact on immune homeostasis.
Given its potential impact as an immunomodulatory role and that viable Akkermansia is not always required to exert beneficial effects, this microbe presents opportunities as a therapeutic. For Type II diabetes, Akkermansia benefits are supported by a randomized, double-blind, placebo-controlled pilot study,33 with potential further investigations involving P9 and Amuc_1100. Even in this, the first and most studied disease, the impact of long-term administration of Akkermansia is unclear.
Animal models show evidence of Akkermansia intervention’s beneficial effects in several neurological diseases. Further studies must focus on determining if there are groups of patients, e.g., who harbor low Akkermansia levels or the “wrong” species, who would benefit from Akkermansia administration. Similarly, confirmatory studies in pre-clinical models and potential intervention studies in humans are needed for chronic kidney disease (CKD) and aging-related diseases like OP and atherosclerosis. In instances where protection against infection was observed, most studies were conducted in mouse models, highlighting the need for research on human subjects. In liver disease and asthma, Akkermansia shows promise as a protective factor in animal models, suggesting potential interventions in humans. In oncology and immunotherapies, confirmatory studies in animal models are essential to determine synergy with other therapeutics before clinical translation. In addition, new approaches are being developed to subcategorize dysbiotic states for more targeted microbial interventions. An example is the assessment of the levels of Akkermansia in combination with species-interacting groups (SIGs), which can correlate with overall survival in lung cancers.39,122
For other ailments, like IBD and other chronic GI inflammatory conditions, the impact of Akkermansia appears to be model-dependent and heavily influenced by diet (e.g., low-fiber content), immunosuppression, and food allergies. Mucin consumption by Akkermansia is predicted to negatively impact gut barrier function, particularly in the context of diets low on fiber or in low food consumption. Another limitation is that most in vitro studies of Akkermansia impact of cellular function have relied on culturing the microbe on porcine gastric mucins (Type II or III), which differ from human intestinal mucins.1,11,12,27,38,44,47,51,119,123 Mucin complexity also varies along the GI, with MUC5AC and MUC6 expressed in the stomach, and MUC2 the main mucin in the small intestine and colon.124 Colonic mucins present increased sulfation compared to gastric mucins. While Akkermansia can use gastric porcine O-glycans, it grows poorly on sulfated colonic mucins.125 Further studies involving the use of human-derived colonic mucins will aid in the elucidation of Akkermansia mucin metabolism in vivo.
Under some circumstances, Akkermansia levels are associated with increased disease severity in humans. Paradoxically, oral supplementation with Akkermansia has proven beneficial in conditions like SFTSV, Alzheimer’s disease, and helminth infections. Understanding the variables underlying disparate outcomes between exposure to endogenous levels of Akkermansia and the oral introduction of regular doses of Akkermansia products will help design more effective probiotic formulations.
New areas that warrant further study, considering the potential negative impact of Akkermansia, are its role in eating disorders and the effect of diet in Akkermansia interventions. Broadly, in other areas where the microbiome emerges as a modulator of health, the interaction between Akkermansia and other microbes should help better predict the impact of the community on the host’s physiology. Furthermore, establishing Akkermansia as a probiotic requires defining dosing and understanding the rules of competition between native strains and supplemented ones. The increased evidence of genetic diversity among Akkermansia could further refine the choice of species and strain(s) regarding therapeutic potential. The species A. massiliensis and A. biwaensis harbor higher gene content compared to A. muciniphila, which suggests that potentially new beneficial effects may be discovered.
Another critical area of development is determining the mechanisms of Akkermansia immunomodulation. For instance, while activation of TLR2 by Akkermansia has been proposed as responsible for strengthening epithelial tight junctions, this has not been tested formally in mice with tissue-specific TLR2 genetic ablations. Similarly, recent advances in the genetic manipulation of Akkermansia should allow for rigorous testing of whether proteins like Amuc_1100, P9, and Amuc_2172 have the same immunomodulatory traits in vivo as has been predicted from purified proteins.126 Lastly, Akkermansia’s impact on host physiology is likely intertwined with that of other microbiota members, as exemplified by cross-feeding interactions with Phocaeicola vulgatus SNUG 40,005127 and synergistic protective effects with Parabacteroides against colitis.113
While considerable enthusiasm surrounds the potential health benefits of Akkermansia (as suggested by earlier studies), the literature reviewed indicates that caution is necessary before categorizing Akkermansia unequivocally as a “good bug” without considering the broader context. There is a risk that some of the numerous reports of positive associations between Akkermansia abundance and health status have been impacted by publication bias. In this review, we highlighted the conditions and diseases associated with Akkermansia, differentiating between those supported by correlations alone and those including intervention studies. Additionally, we separated the findings based on studies conducted in animal models or human subjects to clarify the status of Akkermansia research in different areas (Figure 1). There is no prospect of implementing a “one size fits all” therapeutic approach, as the effects of Akkermansia depend on many factors, such as the status of the mucus barrier, GI inflammation, and especially the metabolic or neurological imbalance present in an individual. The mechanism through which Akkermansia would exert a beneficial versus a detrimental effect on health would be reliant on the genetics of the strain, the capacity to engraft, its mucin-utilization capabilities, the secreted proteins and metabolites, the immunomodulatory properties, the competition or cooperation with members of the host microbiota, as well as synergy with co-administered treatments.
Figure 1.
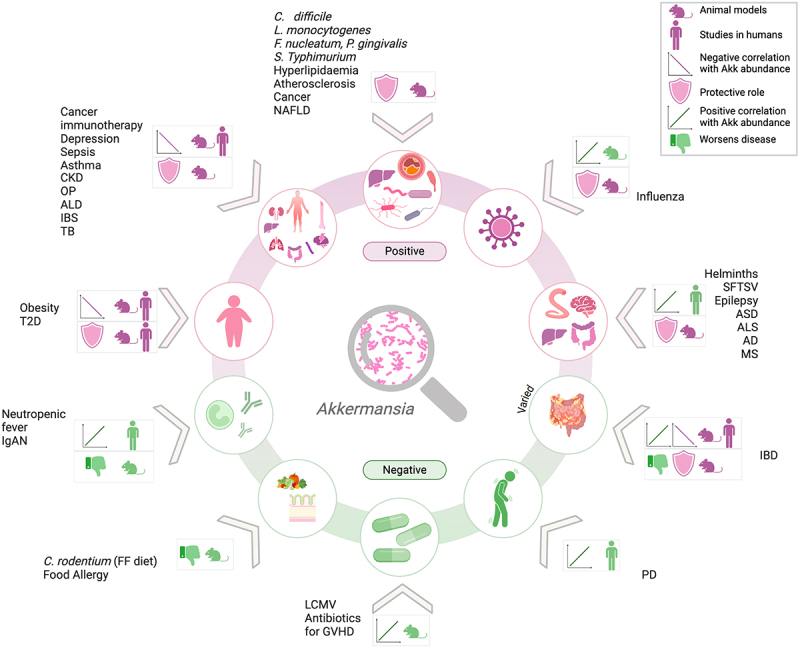
Akkermansia orchestrates a spectrum of health outcomes. Graphic summary of the studies reviewed indicating the outcome of correlation (green indicates negative, purple indicates positive) and the intervention studies performed in animal models and/or human cohorts. Acronyms in the figure: CKD, chronic kidney disease; OP, osteoporosis; ALD, alcoholic liver disease; IBS, irritable bowel syndrome; TB, tuberculosis; C. difficile, Clostridioles difficile; L. monocytogenes, Listeria monocytogenes; F. nucleatum, Fusobacterium nucleatum; P. gingivalis, Porphyromonas gingivalis; S. typhimurium, Salmonella typhimurium; NAFLD, nonalcoholic fatty liver disease; SFTSV, severe fever with thrombocytopenia syndrome virus; ASD, autism spectrum disorder; ALS, amyotrophic lateral sclerosis; AD, alzheimer’s disease; MS, multiple sclerosis; IBD, inflammatory bowel disease; PD, parkinson’s disease; LCMV, lymphocytic choriomeningitis virus; GVHD, graft versus host disease; C. rodentium, Citrobacter rodentium; FF, fiber free; IgAN, immunoglobulin a nephropathy; T2D, type 2 diabetes.
Funding Statement
The work was supported by grants from the NIH [AI142376-05] and the HHMI Emerging Infections Program.
Disclosure statement
Raphael Valdivia is a co-founder of Bloom Sciences (San Diego, CA).
Data availability statement
There is no research data in this paper.
References
- 1.Derrien M, Vaughan EE, Plugge CM, de Vos WM.. Akkermansia muciniphila gen. nov. sp. nov. a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54(5):1469–17. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 2.Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S. Intestinal integrity and akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microbiol. 2007;73(23):7767–7770. doi: 10.1128/AEM.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ndongo S, Armstrong N, Raoult D, Fournier P-E. Reclassification of eight akkermansia muciniphila strains and description of akkermansia massiliensis sp. nov. And Candidatus akkermansia timonensis, isolated from human feces. Sci Rep. 2022;12(1):21747. doi: 10.1038/s41598-022-25873-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi Y, Kawahara T, Inoue S, Kohda N. Akkermansia biwaensis sp. nov. an anaerobic mucin-degrading bacterium isolated from human faeces. Int J Syst Evol Microbiol. 2023; 73(1. doi: 10.1099/ijsem.0.005697. [DOI] [PubMed] [Google Scholar]
- 5.Kelly C, Jawahar J, Davey L, Everitt JI, Galanko JA, Anderson C, Avendano JE, McCann JR, Sartor RB, Valdivia RH, et al. Spontaneous episodic inflammation in the intestines of mice lacking HNF4A is driven by microbiota and associated with early life microbiota alterations. mBio. 2023;14(4):e0150423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mueller KD, Panzetta ME, Davey L, McCann JR, Rawls JF, Flores GE, Valdivia RH. Pangenomic analysis identifies correlations between akkermansia species and subspecies and human health outcomes. Microbiome Res Rep. 2024;3:33. doi: 10.20517/mrr.2024.09. [DOI] [Google Scholar]
- 7.Guo X, Li S, Zhang J, Wu F, Li X, Wu D, Zhang M, Ou Z, Jie Z, Yan Q, et al. Genome sequencing of 39 akkermansia muciniphila isolates reveals its population structure, genomic and functional diverisity, and global distribution in mammalian gut microbiotas. BMC Genomics. 2017;18(1):800. doi: 10.1186/s12864-017-4195-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karcher N, Nigro E, Punčochář M, Blanco-Míguez A, Ciciani M, Manghi P, Zolfo M, Cumbo F, Manara S, Golzato D, et al. Genomic diversity and ecology of human-associated akkermansia species in the gut microbiome revealed by extensive metagenomic assembly. Genome Biol. 2021;22(1):209. doi: 10.1186/s13059-021-02427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z, Xu Q, Gu S, Chen Y, Lv L, Zheng B, Wang Q, Wang K, Wang S, Xia J, et al. Akkermansia muciniphila ameliorates clostridioides difficile infection in mice by modulating the intestinal microbiome and metabolites. Front Microbiol. 2022;13:841920. doi: 10.3389/fmicb.2022.841920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keane JM, Las Heras V, Pinheiro J, FitzGerald JA, Núñez-Sánchez MA, Hueston CM, O’Mahony L, Cotter PD, Hill C, Melgar S, et al. Akkermansia muciniphila reduces susceptibility to listeria monocytogenes infection in mice fed a high-fat diet. Gut Microbes. 2023;15(1):2229948. doi: 10.1080/19490976.2023.2229948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song B, Xian W, Sun Y, Gou L, Guo Q, Zhou X, Ren B, Cheng L. Akkermansia muciniphila inhibited the periodontitis caused by Fusobacterium nucleatum. NPJ Biofilms Microbiomes. 2023;9(1):49. doi: 10.1038/s41522-023-00417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huck O, Mulhall H, Rubin G, Kizelnik Z, Iyer R, Perpich JD, Haque N, Cani PD, de Vos WM, Amar S, et al. Akkermansia muciniphila reduces porphyromonas gingivalis-induced inflammation and periodontal bone destruction. J Clin Periodontol. 2020;47(2):202–212. doi: 10.1111/jcpe.13214. [DOI] [PubMed] [Google Scholar]
- 13.Mulhall H, DiChiara JM, Deragon M, Iyer R, Huck O, Amar S. Akkermansia muciniphila and Its pili-like protein Amuc_1100 modulate macrophage polarization in experimental periodontitis. Infect Immun. 2020; 89(1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Liu H, Liu H, Teng Y, Qin N, Ren X, Xia X. Live and pasteurized akkermansia muciniphila decrease susceptibility to Salmonella Typhimurium infection in mice. J Adv Res. 2023;52:89–102. doi: 10.1016/j.jare.2023.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in apoe −/− mice. Circulation. 2016;133(24):2434–2446. [DOI] [PubMed] [Google Scholar]
- 16.Shen J, Tong X, Sud N, Khound R, Song Y, Maldonado-Gomez MX, Walter J, Su Q. Low-density lipoprotein receptor signaling mediates the triglyceride-lowering action of akkermansia muciniphila in genetic-induced hyperlipidemia. Arterioscler Thromb Vasc Biol. 2016;36(7):1448–1456. doi: 10.1161/ATVBAHA.116.307597. [DOI] [PubMed] [Google Scholar]
- 17.Kim S, Lee Y, Kim Y, Seo Y, Lee H, Ha J, Lee J, Choi Y, Oh H, Yoon Y, et al. Akkermansia muciniphila prevents fatty liver disease, decreases serum triglycerides, and maintains gut homeostasis. Appl Environ Microbiol. 2020; 86(7. doi: 10.1128/AEM.03004-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nian F, Wu L, Xia Q, Tian P, Ding C, Lu X. Akkermansia muciniphila and bifidobacterium bifidum prevent NAFLD by regulating FXR expression and gut microbiota. J Clin Transl Hepatol. 2023;11(4):763–776. doi: 10.14218/JCTH.2022.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qu D, Chen M, Zhu H, Liu X, Cui Y, Zhou W, Zhang M. Akkermansia muciniphila and its outer membrane protein Amuc_1100 prevent high-fat diet-induced nonalcoholic fatty liver disease in mice. Biochem Biophys Res Commun. 2023;684:149131. doi: 10.1016/j.bbrc.2023.149131. [DOI] [PubMed] [Google Scholar]
- 20.Han Y, Ling Q, Wu L, Wang X, Wang Z, Chen J, Zheng Z, Zhou Z, Jia L, Li L, et al. Akkermansia muciniphila inhibits nonalcoholic steatohepatitis by orchestrating TLR2-activated γδT17 cell and macrophage polarization. Gut Microbes. 2023;15(1):2221485. doi: 10.1080/19490976.2023.2221485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labarta-Bajo L, Gramalla-Schmitz A, Gerner RR, Kazane KR, Humphrey G, Schwartz T, Sanders K, Swafford A, Knight R, Raffatellu M, et al. CD8 T cells drive anorexia, dysbiosis, and blooms of a commensal with immunosuppressive potential after viral infection. Proc Natl Acad Sci USA. 2020;117(40):24998–25007. doi: 10.1073/pnas.2003656117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shono Y, Docampo MD, Peled JU, Perobelli SM, Velardi E, Tsai JJ, Slingerland AE, Smith OM, Young LF, Gupta J, et al. Increased gvhd-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med. 2016;8(339):339ra71. doi: 10.1126/scitranslmed.aaf2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parrish A, Boudaud M, Grant ET, Willieme S, Neumann M, Wolter M, Craig SZ, De Sciscio A, Cosma A, Hunewald O, et al. Akkermansia muciniphila exacerbates food allergy in fibre-deprived mice. Nat Microbiol. 2023;8(10):1863–1879. doi: 10.1038/s41564-023-01464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolter M, Grant ET, Boudaud M, Pudlo NA, Pereira GV, Eaton KA, Martens EC, Desai MS. Diet-driven differential response of Akkermansia muciniphila modulates pathogen susceptibility. Mol Syst Biol. 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, et al. Cross-talk between akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110(22):9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chelakkot C, Choi Y, Kim D-K, Park HT, Ghim J, Kwon Y, Jeon J, Kim M-S, Jee Y-K, Gho YS, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 2018;50(2):e450. doi: 10.1038/emm.2017.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23(1):107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 28.Everard A, Plovier H, Rastelli M, Van Hul M, de Wouters d’Oplinter A, Geurts L, Druart C, Robine S, Delzenne NM, Muccioli GG, et al. Intestinal epithelial N-acylphosphatidylethanolamine phospholipase D links dietary fat to metabolic adaptations in obesity and steatosis. Nat Commun. 2019;10(1):457. doi: 10.1038/s41467-018-08051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashrafian F, Shahriary A, Behrouzi A, Moradi HR, Keshavarz Azizi Raftar S, Lari A, Hadifar S, Yaghoubfar R, Ahmadi Badi S, Khatami S, et al. Akkermansia muciniphila-derived extracellular vesicles as a mucosal delivery vector for amelioration of obesity in mice. Front Microbiol. 2019;10:2155. doi: 10.3389/fmicb.2019.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin J, Noh J-R, Chang D-H, Kim Y-H, Kim MH, Lee ES, Cho S, Ku BJ, Rhee M-S, Kim B-C, et al. Elucidation of Akkermansia muciniphila probiotic traits driven by mucin depletion. Front Microbiol. 2019;10:1137. doi: 10.3389/fmicb.2019.01137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon HS, Cho CH, Yun MS, Jang SJ, You HJ, Kim J-H, Han D, Cha KH, Moon SH, Lee K, et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat Microbiol. 2021;6(5):563–573. doi: 10.1038/s41564-021-00880-5. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Zhong Z, Wang B, Xia X, Yao W, Huang L, Wang Y, Ding W. Early-life high-fat diet-induced obesity programs hippocampal development and cognitive functions via regulation of gut commensal akkermansia muciniphila. Neuropsychopharmacology. 2019;44(12):2054–2064. doi: 10.1038/s41386-019-0437-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, et al. Supplementation with akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25(7):1096–1103. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng X, Huang W, Li Q, Chen Y, Wu L, Dong Y, Huang X, He X, Ou Z, Peng Y, et al. Membrane protein Amuc_1100 derived from Akkermansia muciniphila Facilitates Lipolysis and browning via activating the AC3/PKA/HSL pathway. Microbiol Spectr. 2023;11(2):e0432322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becken B, Davey L, Middleton DR, Mueller KD, Sharma A, Holmes ZC, Dallow E, Remick B, Barton GM, David LA, et al. Genotypic and phenotypic diversity among human isolates of akkermansia muciniphila. mBio. 2021;12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP. et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 37.Feng C, Li N, Gao G, He Q, Kwok L-Y, Zhang H. Dynamic changes of the gut microbiota and its functional metagenomic potential during the development of non-small cell lung cancer. Int J Mol Sci. 2024; 25(7. doi: 10.3390/ijms25073768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan L, Xu C, Ge Q, Lin Y, Wong CC, Qi Y, Ye B, Lian Q, Zhuo W, Si J, et al. A. Muciniphila suppresses colorectal tumorigenesis by inducing TLR2/NLRP3-mediated M1-like TAMs. Cancer Immunol Res. 2021;9(10):1111–1124. doi: 10.1158/2326-6066.CIR-20-1019. [DOI] [PubMed] [Google Scholar]
- 39.Derosa L, Routy B, Thomas AM, Iebba V, Zalcman G, Friard S, Mazieres J, Audigier-Valette C, Moro-Sibilot D, Goldwasser F, et al. Intestinal akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med. 2022;28(2):315–324. doi: 10.1038/s41591-021-01655-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newsome RC, Gharaibeh RZ, Pierce CM, da Silva WV, Paul S, Hogue SR, Yu Q, Antonia S, Conejo-Garcia JR, Robinson LA, et al. Interaction of bacterial genera associated with therapeutic response to immune checkpoint PD-1 blockade in a United States cohort. Genome Med. 2022;14(1):35. doi: 10.1186/s13073-022-01037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Z, Cai J, Hou W, Xu K, Wu X, Song Y, Bai C, Mo Y-Y, Zhang Z. Microbiome and spatially resolved metabolomics analysis reveal the anticancer role of gut akkermansia muciniphila by crosstalk with intratumoral microbiota and reprogramming tumoral metabolism in mice. Gut Microbes. 2023;15(1):2166700. doi: 10.1080/19490976.2023.2166700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Tang L, Feng Y, Zhao S, Han M, Zhang C, Yuan G, Zhu J, Cao S, Wu Q, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8 + T cells in mice. Gut. 2020;69(11):1988–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang Y, Xu Y, Zheng C, Ye L, Jiang P, Malik S, Xu G, Zhou Q, Zhang M. Acetyltransferase from Akkermansia muciniphila blunts colorectal tumourigenesis by reprogramming tumour microenvironment. Gut. 2023;72(7):1308–1318. [DOI] [PubMed] [Google Scholar]
- 44.Shi L, Sheng J, Chen G, Zhu P, Shi C, Li B, Park C, Wang J, Zhang B, Liu Z, et al. Combining IL-2-based immunotherapy with commensal probiotics produces enhanced antitumor immune response and tumor clearance. J Immunother Cancer. 2020; 8(2. doi: 10.1136/jitc-2020-000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michalovich D, Rodriguez-Perez N, Smolinska S, Pirozynski M, Mayhew D, Uddin S, Van Horn S, Sokolowska M, Altunbulakli C, Eljaszewicz A, et al. Obesity and disease severity magnify disturbed microbiome-immune interactions in asthma patients. Nat Commun. 2019;10(1):5711. doi: 10.1038/s41467-019-13751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rana S, Singh P, Bhardwaj T, Somvanshi P. A comprehensive metagenome study identifies distinct biological pathways in asthma patients: an in-silico approach. Biochem Genet. 2024; doi: 10.1007/s10528-023-10635-y. [DOI] [PubMed] [Google Scholar]
- 47.Yoon SA, Lim Y, Byeon HR, Jung J, Ma S, Hong M-G, Kim D, Song E-J, Nam Y-D, Seo J-G, et al. Heat-killed akkermansia muciniphila ameliorates allergic airway inflammation in mice. Front Microbiol. 2024;15:1386428. doi: 10.3389/fmicb.2024.1386428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie S, Li J, Lyu F, Xiong Q, Gu P, Chen Y, Chen M, Bao J, Zhang X, Wei R, et al. Novel tripeptide RKH derived from Akkermansia muciniphila protects against lethal sepsis. Gut. 2023;73(1):78–91. [DOI] [PubMed] [Google Scholar]
- 49.Cruz-Aguliar RM, Wantia N, Clavel T, Vehreschild MGT, Buch T, Bajbouj M, Haller D, Busch D, Schmid R, Stein-Thoeringer C, et al. An open-labeled study on fecal microbiota transfer in irritable bowel syndrome patients reveals improvement in abdominal pain associated with the relative abundance of Akkermansia Muciniphila. Digestion. 2019;100(2):127–138. doi: 10.1159/000494252. [DOI] [PubMed] [Google Scholar]
- 50.Hamazaki M, Sawada T, Yamamura T, Maeda K, Mizutani Y, Ishikawa E, Furune S, Yamamoto K, Ishikawa T, Kakushima N. et al. Fecal microbiota transplantation in the treatment of irritable bowel syndrome: a single-center prospective study in Japan. BMC Gastroenterol. 2022;22(1):342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meynier M, Daugey V, Mallaret G, Gervason S, Meleine M, Barbier J, Aissouni Y, Lolignier S, Bonnet M, Ardid D. et al. Pasteurized akkermansia muciniphila improves irritable bowel syndrome-like symptoms and related behavioral disorders in mice. Gut Microbes. 2024;16(1):2298026. doi: 10.1080/19490976.2023.2298026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016;21(6):786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 53.Li Z, Lai J, Zhang P, Ding J, Jiang J, Liu C, Huang H, Zhen H, Xi C, Sun Y, et al. Multi-omics analyses of serum metabolome, gut microbiome and brain function reveal dysregulated microbiota-gut-brain axis in bipolar depression. Mol Psychiatry. 2022;27(10):4123–4135. doi: 10.1038/s41380-022-01569-9. [DOI] [PubMed] [Google Scholar]
- 54.Yu S, Wang L, Jing X, Wang Y, An C. Features of gut microbiota and short-chain fatty acids in patients with first-episode depression and their relationship with the clinical symptoms. Front Psychol. 2023;14:1088268. doi: 10.3389/fpsyg.2023.1088268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Y, Le D, Xu J, Jin P, Zhang Y, Liao Z. Gut microbiota dysbiosis and inflammation dysfunction in late-life depression: an observational Cross-sectional analysis. Neuropsychiatr Dis Treat. 2024;20:399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X, Hou Y, Li Y, Wei W, Cai X, Shao H, Yuan Y, Zheng X. Taxonomic and metabolic signatures of gut microbiota for assessing the severity of depression and anxiety in Major depressive disorder patients. Neuroscience. 2022;496:179–189. doi: 10.1016/j.neuroscience.2022.06.024. [DOI] [PubMed] [Google Scholar]
- 57.Busch A, Roy S, Helbing DL, Colic L, Opel N, Besteher B, Walter M, Bauer M, Refisch A. Gut microbiome in atypical depression. J Affect Disord. 2024;349:277–285. doi: 10.1016/j.jad.2024.01.060. [DOI] [PubMed] [Google Scholar]
- 58.McGaughey KD, Yilmaz-Swenson T, Elsayed NM, Cruz DA, Rodriguiz RM, Kritzer MD, Peterchev AV, Roach J, Wetsel WC, Williamson DE, et al. Relative abundance of Akkermansia spp. And other bacterial phylotypes correlates with anxiety- and depressive-like behavior following social defeat in mice. Sci Rep. 2019;9(1):3281. doi: 10.1038/s41598-019-40140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen M, Wang L, Lou Y, Huang Z. Effects of chronic unpredictable mild stress on gut microbiota and fecal amino acid and short-chain fatty acid pathways in mice. Behav Brain Res. 2024;464:114930. doi: 10.1016/j.bbr.2024.114930. [DOI] [PubMed] [Google Scholar]
- 60.Guo H, Liu X, Chen T, Wang X, Zhang X. Akkermansia muciniphila improves depressive-like symptoms by modulating the level of 5-HT neurotransmitters in the gut and brain of mice. Mol Neurobiol. 2024;61(2):821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun Y, Zhu H, Cheng R, Tang Z, Zhang M. Outer membrane protein Amuc_1100 of akkermansia muciniphila alleviates antibiotic-induced anxiety and depression-like behavior in mice. Physiol Behav. 2023;258:114023. doi: 10.1016/j.physbeh.2022.114023. [DOI] [PubMed] [Google Scholar]
- 62.Choi J, Kwon H, Kim Y-K, Han P-L. Extracellular vesicles from gram-positive and gram-negative probiotics remediate stress-induced depressive behavior in mice. Mol Neurobiol. 2022;59(5):2715–2728. doi: 10.1007/s12035-021-02655-9. [DOI] [PubMed] [Google Scholar]
- 63.Guo D, Park C, Li Y, Li B, Yang Q, Deng Y, Gao NL, Li R, Wang X, Yi L, et al. Akkermansia muciniphila ameliorates depressive disorders in a murine alcohol-lps (mALPS) model. Food Funct. 2022;13(24):12766–12776. doi: 10.1039/D2FO01478E. [DOI] [PubMed] [Google Scholar]
- 64.Cheng R, Zhu H, Sun Y, Hang T, Zhang M. The modified outer membrane protein Amuc_1100 of akkermansia muciniphila improves chronic stress-induced anxiety and depression-like behavior in mice. Food Funct. 2022;13(20):10748–10758. doi: 10.1039/D2FO01198K. [DOI] [PubMed] [Google Scholar]
- 65.Chen T, Wang R, Duan Z, Yuan X, Ding Y, Feng Z, Bu F, Liu L, Wang Q, Zhou J, et al. Akkermansia muciniphila protects against psychological disorder-induced gut microbiota-mediated colonic mucosal barrier damage and aggravation of colitis. Front Cell Infect Microbiol. 2021;11:723856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ding Y, Bu F, Chen T, Shi G, Yuan X, Feng Z, Duan Z, Wang R, Zhang S, Wang Q, et al. A next-generation probiotic: akkermansia muciniphila ameliorates chronic stress–induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl Microbiol Biotechnol. 2021;105(21–22):8411–8426. doi: 10.1007/s00253-021-11622-2. [DOI] [PubMed] [Google Scholar]
- 67.Cheng R, Xu W, Wang J, Tang Z, Zhang M. The outer membrane protein Amuc_1100 of akkermansia muciniphila alleviates the depression-like behavior of depressed mice induced by chronic stress. Biochem Biophys Res Commun. 2021;566:170–176. doi: 10.1016/j.bbrc.2021.06.018. [DOI] [PubMed] [Google Scholar]
- 68.Wang J, Xu W, Wang R, Cheng R, Tang Z, Zhang M. The outer membrane protein Amuc_1100 of akkermansia muciniphila promotes intestinal 5-HT biosynthesis and extracellular availability through TLR2 signalling. Food Funct. 2021;12(8):3597–3610. doi: 10.1039/D1FO00115A. [DOI] [PubMed] [Google Scholar]
- 69.Wu F, Guo X, Zhang M, Ou Z, Wu D, Deng L, Lu Z, Zhang J, Deng G, Chen S, et al. An akkermansia muciniphila subtype alleviates high-fat diet-induced metabolic disorders and inhibits the neurodegenerative process in mice. Anaerobe. 2020;61:102138. doi: 10.1016/j.anaerobe.2019.102138. [DOI] [PubMed] [Google Scholar]
- 70.Keshavarz Azizi Raftar S, Hoseini Tavassol Z, Amiri M, Ejtahed H-S, Zangeneh M, Sadeghi S, Ashrafian F, Kariman A, Khatami S, Siadat SD, et al. Assessment of fecal akkermansia muciniphila in patients with osteoporosis and osteopenia: a pilot study. J Diabetes Metab Disord. 2021;20(1):279–284. doi: 10.1007/s40200-021-00742-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qin Q, Yan S, Yang Y, Chen J, Yan H, Li T, Gao X, Wang Y, Li A, Wang S. et al. The relationship between osteoporosis and intestinal microbes in the Henan Province of China. Front Cell Dev Biol. 2021;9:752990. doi: 10.3389/fcell.2021.752990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang N, Ma S, Fu L. Gut microbiota feature of senile osteoporosis by shallow shotgun sequencing using aged rats Model. Genes (Basel). 2022;13(4). doi: 10.3390/genes13040619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lawenius L, Scheffler JM, Gustafsson KL, Henning P, Nilsson KH, Colldén H, Islander U, Plovier H, Cani PD, de Vos WM, et al. Pasteurized akkermansia muciniphila protects from fat mass gain but not from bone loss. Am J Physiol Endocrinol Metab. 2020;318(4):E480–E491. doi: 10.1152/ajpendo.00425.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu JH, Chen C-Y, Liu Z-Z, Luo Z-W, Rao S-S, Jin L, Wan T-F, Yue T, Tan Y-J, Yin H, et al. Extracellular vesicles from child gut microbiota enter into bone to preserve bone Mass and strength. Adv Sci (Weinh). 2021;8(9):2004831. doi: 10.1002/advs.202004831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grander C, Adolph TE, Wieser V, Lowe P, Wrzosek L, Gyongyosi B, Ward DV, Grabherr F, Gerner RR, Pfister A, et al. Recovery of ethanol-induced akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018;67(5):891–901. [DOI] [PubMed] [Google Scholar]
- 76.Grander C, Grabherr F, Spadoni I, Enrich B, Oberhuber G, Rescigno M, Tilg H. The role of gut vascular barrier in experimental alcoholic liver disease and A. muciniphila supplementation. Gut Microbes. 2020;12(1):1851986. doi: 10.1080/19490976.2020.1851986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li F, Wang M, Wang J, Li R, Zhang Y. Alterations to the gut microbiota and their correlation with inflammatory factors in chronic kidney disease. Front Cell Infect Microbiol. 2019;9:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pei T, Hu R, Wang F, Yang S, Feng H, Li Q, Zhang J, Yan S, Ju L, He Z, et al. Akkermansia muciniphila ameliorates chronic kidney disease interstitial fibrosis via the gut-renal axis. Microb Pathog. 2023;174:105891. [DOI] [PubMed] [Google Scholar]
- 79.Chen L, Zhang G, Li G, Wang W, Ge Z, Yang Y, He X, Liu Z, Zhang Z, Mai Q, et al. Ifnar gene variants influence gut microbial production of palmitoleic acid and host immune responses to tuberculosis. Nat Metab. 2022;4(3):359–373. doi: 10.1038/s42255-022-00547-3. [DOI] [PubMed] [Google Scholar]
- 80.Jenkins TP, Rathnayaka Y, Perera PK, Peachey LE, Nolan MJ, Krause L, Rajakaruna RS, Cantacessi C. Infections by human gastrointestinal helminths are associated with changes in faecal microbiota diversity and composition. PLOS ONE. 2017;12(9):e0184719. doi: 10.1371/journal.pone.0184719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Su C, Su L, Li Y, Long SR, Chang J, Zhang W, Walker WA, Xavier RJ, Cherayil BJ, Shi HN. et al. Helminth-induced alterations of the gut microbiota exacerbate bacterial colitis. Mucosal Immunol. 2018;11(1):144–157. doi: 10.1038/mi.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jin X, Liu Y, Wang J, Wang X, Tang B, Liu M, Liu X. β-glucan-triggered akkermansia muciniphila expansion facilitates the expulsion of intestinal helminth via TLR2 in mice. Carbohydr Polym. 2022;275:118719. doi: 10.1016/j.carbpol.2021.118719. [DOI] [PubMed] [Google Scholar]
- 83.Hu X, Zhao Y, Yang Y, Gong W, Sun X, Yang L, Zhang Q, Jin M. Akkermansia muciniphila improves Host defense against influenza virus infection. Front Microbiol. 2020;11:586476. doi: 10.3389/fmicb.2020.586476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xie J, Li H, Zhang X, Yang T, Yue M, Zhang Y, Chen S, Cui N, Yuan C, Li J, et al. Akkermansia muciniphila protects mice against an emerging tick-borne viral pathogen. Nat Microbiol. 2023;8(1):91–106. doi: 10.1038/s41564-022-01279-6. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y, Li L, Zhao X, Sui S, Wang Q, Shi G, Xu H, Zhang X, He Y, Gu J, et al. Intestinal microflora changes in patients with mild Alzheimer’s disease in a Chinese cohort. J Alzheimers Dis. 2022;88(2):563–575. doi: 10.3233/JAD-220076. [DOI] [PubMed] [Google Scholar]
- 86.Khedr EM, Omeran N, Karam-Allah Ramadan H, Ahmed GK, Abdelwarith AM. Alteration of gut microbiota in Alzheimer’s disease and their relation to the cognitive impairment. J Alzheimers Dis. 2022;88(3):1103–1114. doi: 10.3233/JAD-220176. [DOI] [PubMed] [Google Scholar]
- 87.Kaiyrlykyzy A. et al. Study of gut microbiota alterations in Alzheimer’s dementia patients from Kazakhstan. Sci Rep. 2022;12(1):15115. doi: 10.1038/s41598-022-19393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ling Z, Zhu M, Yan X, Cheng Y, Shao L, Liu X, Jiang R, Wu S. Structural and functional dysbiosis of fecal microbiota in Chinese patients with Alzheimer’s disease. Front Cell Dev Biol. 2020;8:634069. doi: 10.3389/fcell.2020.634069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ou Z, Deng L, Lu Z, Wu F, Liu W, Huang D, Peng Y. Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer’s disease. Nutr. Diabetes. 2020;10(1):12. doi: 10.1038/s41387-020-0115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.He X, Yan C, Zhao S, Huang R, Li Y. The preventive effects of probiotic akkermansia muciniphila on D-galactose/AlCl3 mediated Alzheimer’s disease-like rats. Exp Gerontol. 2022;170:111959. doi: 10.1016/j.exger.2022.111959. [DOI] [PubMed] [Google Scholar]
- 91.Qu L, Liu F, Fang Y, Wang L, Chen H, Yang Q, Dong H, Jin L, Wu W, Sun D, et al. Improvement in zebrafish with diabetes and Alzheimer’s disease treated with pasteurized akkermansia muciniphila. Microbiol Spectr. 2023;11(3):e0084923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim HS, Son J, Lee D, Tsai J, Wang D, Chocron ES, Jeong S, Kittrell P, Murchison CF, Kennedy RE. et al. Gut- and oral-dysbiosis differentially impact spinal- and bulbar-onset ALS, predicting ALS severity and potentially determining the location of disease onset. BMC Neurol. 2022;22(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, Kleimeyer C, Moresi C, Harnik Y, Zur M, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019;572(7770):474–480. doi: 10.1038/s41586-019-1443-5. [DOI] [PubMed] [Google Scholar]
- 94.Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Low relative abundances of the mucolytic bacterium akkermansia muciniphila and bifidobacterium spp. In feces of children with autism. Appl Environ Microbiol. 2011;77(18):6718–6721. doi: 10.1128/AEM.05212-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zou R, Xu F, Wang Y, Duan M, Guo M, Zhang Q, Zhao H, Zheng H. Changes in the Gut microbiota of children with autism spectrum disorder. Autism Res. 2020;13(9):1614–1625. doi: 10.1002/aur.2358. [DOI] [PubMed] [Google Scholar]
- 96.Zurita MF, Cárdenas PA, Sandoval ME, Peña MC, Fornasini M, Flores N, Monaco MH, Berding K, Donovan SM, Kuntz T. et al. Analysis of gut microbiome, nutrition and immune status in autism spectrum disorder: a case-control study in Ecuador. Gut Microbes. 2020;11(3):453–464. doi: 10.1080/19490976.2019.1662260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu X, Cui Y, Zhang Y, Xiang G, Yu M, Wang X, Qiu B, Li X-G, Liu W, Zhang D. et al. Rescue of social deficits by early-life melatonin supplementation through modulation of gut microbiota in a murine model of autism. Biomed Pharmacother. 2022;156:113949. doi: 10.1016/j.biopha.2022.113949. [DOI] [PubMed] [Google Scholar]
- 98.Huang C, Li Y, Feng X, Li D, Li X, Ouyang Q, Dai W, Wu G, Zhou Q, Wang P, et al. Distinct gut microbiota composition and functional category in children with cerebral palsy and epilepsy. Front Pediatr. 2019;7:394. doi: 10.3389/fped.2019.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173(7):1728–1741 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, Kanner R, Bencosme Y, Lee YK, Hauser SL. et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci USA. 2017;114(40):10713–10718. doi: 10.1073/pnas.1711235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, Patel B, Mazzola MA, Liu S, Glanz BL, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7(1):12015. doi: 10.1038/ncomms12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cox LM, Maghzi AH, Liu S, Tankou SK, Dhang FH, Willocq V, Song A, Wasén C, Tauhid S, Chu R, et al. Gut microbiome in progressive multiple sclerosis. Ann Neurol. 2021;89(6):1195–1211. doi: 10.1002/ana.26084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vallino A, Dos Santos A, Mathé CV, Garcia A, Morille J, Dugast E, Shah SP, Héry-Arnaud G, Guilloux C-A, Gleeson PJ, et al. Gut bacteria Akkermansia elicit a specific IgG response in CSF of patients with MS. Neurol Neuroimmunol Neuroinflamm. 2020; 7(3. doi: 10.1212/NXI.0000000000000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu S, Rezende RM, Moreira TG, Tankou SK, Cox LM, Wu M, Song A, Dhang FH, Wei Z, Costamagna G, et al. Oral administration of miR-30d from feces of MS patients suppresses ms-like symptoms in mice by expanding akkermansia muciniphila. Cell Host Microbe. 2019;26(6):779–794 e8. doi: 10.1016/j.chom.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ahrens AP, Hyötyläinen T, Petrone JR, Igelström K, George CD, Garrett TJ, Orešič M, Triplett EW, Ludvigsson J. Infant microbes and metabolites point to childhood neurodevelopmental disorders. Cell. 2024;187(8):1853–1873 e15. doi: 10.1016/j.cell.2024.02.035. [DOI] [PubMed] [Google Scholar]
- 106.Zhang T, Li P, Wu X, Lu G, Marcella C, Ji X, Ji G, Zhang F. Alterations of akkermansia muciniphila in the inflammatory bowel disease patients with washed microbiota transplantation. Appl Microbiol Biotechnol. 2020;104(23):10203–10215. doi: 10.1007/s00253-020-10948-7. [DOI] [PubMed] [Google Scholar]
- 107.Lopez-Siles M, Enrich-Capó N, Aldeguer X, Sabat-Mir M, Duncan SH, Garcia-Gil LJ, Martinez-Medina M. Alterations in the abundance and Co-occurrence of Akkermansia muciniphila and faecalibacterium prausnitzii in the colonic mucosa of inflammatory bowel disease subjects. Front Cell Infect Microbiol. 2018;8:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Franzosa EA, Sirota-Madi A, Avila-Pacheco J, Fornelos N, Haiser HJ, Reinker S, Vatanen T, Hall AB, Mallick H, McIver LJ, et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol. 2019;4(2):293–305. doi: 10.1038/s41564-018-0306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lloyd-Price J, Arze C, Ananthakrishnan AN, Schirmer M, Avila-Pacheco J, Poon TW, Andrews E, Ajami NJ, Bonham KS, Brislawn CJ, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569(7758):655–662. doi: 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Seregin SS, Golovchenko N, Schaf B, Chen J, Pudlo NA, Mitchell J, Baxter NT, Zhao L, Schloss PD, Martens EC, et al. NLRP6 protects Il10(-/-) mice from colitis by limiting colonization of Akkermansia muciniphila. Cell Rep. 2017;19(4):733–745. doi: 10.1016/j.celrep.2017.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhai R, Xue X, Zhang L, Yang X, Zhao L, Zhang C. Strain-specific anti-inflammatory properties of two Akkermansia muciniphila strains on chronic colitis in mice. Front Cell Infect Microbiol. 2019;9:239. doi: 10.3389/fcimb.2019.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kang CS, Ban M, Choi E-J, Moon H-G, Jeon J-S, Kim D-K, Park S-K, Jeon SG, Roh T-Y, Myung S-J, et al. Extracellular vesicles derived from gut microbiota, especially akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS One. 2013;8(10):e76520. doi: 10.1371/journal.pone.0076520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gaifem J, Mendes-Frias A, Wolter M, Steimle A, Garzón MJ, Ubeda C, Nobre C, González A, Pinho SS, Cunha C, et al. Akkermansia muciniphila and parabacteroides distasonis synergistically protect from colitis by promoting ILC3 in the gut. mBio. 2024;15(4):e0007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zapala B, Stefura T, Wójcik-Pędziwiatr M, Kabut R, Bałajewicz-Nowak M, Milewicz T, Dudek A, Stój A, Rudzińska-Bar M. Differences in the composition of Gut microbiota between patients with Parkinson’s disease and healthy controls: a cohort study. J Clin Med. 2021;10(23). doi: 10.3390/jcm10235698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baldini F, Hertel J, Sandt E, Thinnes CC, Neuberger-Castillo L, Pavelka L, Betsou F, Krüger R, Thiele I, Aguayo G, et al. Parkinson’s disease-associated alterations of the gut microbiome predict disease-relevant changes in metabolic functions. BMC Biol. 2020;18(1):62. doi: 10.1186/s12915-020-00775-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cirstea MS, Yu AC, Golz E, Sundvick K, Kliger D, Radisavljevic N, Foulger LH, Mackenzie M, Huan T, Finlay BB, et al. Microbiota composition and metabolism are associated with gut function in Parkinson’s disease. Mov Disord. 2020;35(7):1208–1217. [DOI] [PubMed] [Google Scholar]
- 117.Bedarf JR, Hildebrand F, Coelho LP, Sunagawa S, Bahram M, Goeser F, Bork P, Wüllner U. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson’s disease patients. Genome Med. 2017;9(1):39. doi: 10.1186/s13073-017-0428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Heintz-Buschart A, Pandey U, Wicke T, Sixel‐Döring F, Janzen A, Sittig‐Wiegand E, Trenkwalder C, Oertel WH, Mollenhauer B, Wilmes P. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord. 2018;33(1):88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Amorim Neto DP, Bosque BP, Pereira de Godoy JV, Rodrigues PV, Meneses DD, Tostes K, Costa Tonoli CC, Faustino de Carvalho H, González-Billault C, de Castro Fonseca M. Akkermansia muciniphila induces mitochondrial calcium overload and α -synuclein aggregation in an enteroendocrine cell line. iScience. 2022;25(3):103908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schwabkey ZI, Wiesnoski DH, Chang C-C, Tsai W-B, Pham D, Ahmed SS, Hayase T, Ortega Turrubiates MR, El-Himri RK, Sanchez CA, et al. Diet-derived metabolites and mucus link the gut microbiome to fever after cytotoxic cancer treatment. Sci Transl Med. 2022;14(671):eabo3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gleeson PJ, Benech N, Chemouny J, Metallinou E, Berthelot L, da Silva J, Bex-Coudrat J, Boedec E, Canesi F, Bounaix C, et al. The gut microbiota posttranslationally modifies IgA1 in autoimmune glomerulonephritis. Sci Transl Med. 2024;16(740):eadl6149. [DOI] [PubMed] [Google Scholar]
- 122.Derosa L, Iebba V, Silva CAC, Piccinno G, Wu G, Lordello L, Routy B, Zhao N, Thelemaque C, Birebent R, et al. Custom scoring based on ecological topology of gut microbiota associated with cancer immunotherapy outcome. Cell. 2024;187(13):3373–3389 e16. [DOI] [PubMed] [Google Scholar]
- 123.Glover JS, Ticer TD, Engevik MA. Characterizing the mucin-degrading capacity of the human gut microbiota. Sci Rep. 2022;12(1):8456. doi: 10.1038/s41598-022-11819-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McGuckin MA, Lindén SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. 2011;9(4):265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 125.Luis AS, Jin C, Pereira GV, Glowacki RWP, Gugel SR, Singh S, Byrne DP, Pudlo NA, London JA, Baslé A, et al. A single sulfatase is required to access colonic mucin by a gut bacterium. Nature. 2021;598(7880):332–337. doi: 10.1038/s41586-021-03967-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Davey LE, Malkus PN, Villa M, Dolat L, Holmes ZC, Letourneau J, Ansaldo E, David LA, Barton GM, Valdivia RH. A genetic system for Akkermansia muciniphila reveals a role for mucin foraging in gut colonization and host sterol biosynthesis gene expression. Nat Microbiol. 2023;8(8):1450–1467. doi: 10.1038/s41564-023-01407-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.You HJ, Si J, Kim J, Yoon S, Cha KH, Yoon HS, Lee G, Yu J, Choi J-S, Jung M, et al. Bacteroides vulgatus SNUG 40005 restores Akkermansia depletion by metabolite modulation. Gastroenterology. 2023;164(1):103–116. doi: 10.1053/j.gastro.2022.09.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no research data in this paper.


