Abstract
Introduction
Fasciola hepatica, also known as the common liver fluke, is a globally distributed trematode parasite responsible for high economic losses in ruminants. Infection with F. hepatica occurs in Polish cattle and sheep; however, very little is known about its occurrence in goats. Therefore, a serological and coproscopic survey was carried out in Polish goats to determine the herd-level prevalence of F. hepatica infection in the goat population of Poland.
Material and Methods
Between 2014 and 2022, 33 randomly selected goat herds were serologically screened in the regions of Poland for which risk of F. hepatica infection was estimated as increased based on the spatial distribution model developed within the frame of the GLOWORM project. Virtually all adult goats (>1 year-old) were tested using a commercial MM3-SERO ELISA. Risk factors for seropositive herd status were analysed in contingency tables. Also, faecal samples from 214 goat herds monitored for gastrointestinal nematode infections and anthelmintic resistance were examined using a sedimentation method.
Results
At least one seropositive goat was detected in 11 of 33 herds, indicating herd-level seroprevalence of 33.3% (95% confidence interval (CI 95%): 19.7%–50.4%). At the animal level, only 17 of 1,464 tested goats were seropositive (1.2%, CI 95%: 0.7%–1.9%). The within-herd seroprevalence ranged from 0.8% to 11.1%. The serological status of the herd was not significantly associated with the characteristics of the herd or the extent of contact with sheep. In one herd, located in central Poland, a single positive faecal sample was found indicating a herd-level prevalence of F. hepatica infection of 0.5% (CI 95%: 0.1%–2.6%). The animal’s post-mortem examination revealed liver lesions typical of chronic fasciolosis.
Conclusion
F. hepatica infection occurs sporadically in Polish goat population and its prevalence is much lower than in cattle or sheep. Therefore, treatment or prevention of fasciolosis should only be considered if it has been reliably confirmed by an accurate diagnostic test. This applies also to goats inhabiting geographical areas where F. hepatica infection appears to be widespread in cattle and sheep, very likely due to the fact that goats avoid wet areas.
Keywords: coproscopy, liver fluke, MM3-SERO ELISA, sedimentation, seroprevalence
Introduction
Fasciola hepatica, also known as the common liver fluke (in contrast to the giant liver flukes – Fasciola gigantica and Fascioloides magna), is a globally distributed trematode parasite. It poses a risk to human and animal health, as well as contributes to losses in livestock production in Europe and globally (8, 27). A wide range of mammals may serve as definitive hosts of F. hepatica, however the disease plays a major role in domestic and wild ruminants (27). F. hepatica is a digenetic trematode of the Trematoda class and Digenea subclass with an indirect life cycle, in which the intermediate hosts are water snails of the Lymnaeoidea superfamily. The risk of disease depends on climatic factors such as optimal temperature (above 10°C) and high soil moisture which enable the intermediate hosts and free-living larvae of F. hepatica to thrive. Infection of the definitive host occurs via the oral route by ingestion of infective metacercariae – encysted larvae attached to plants growing near water pools. Fasciolosis may follow an acute, subacute (both mainly involving traumatic hepatitis), or chronic course (due to hyperplastic cholangitis) depending on the rate of parasite ingestion. Contrary to sheep and cattle, goats are unlikely to suffer from an acute form of the disease, as their browsing habits preclude massive ingestion of metacercariae in a short period of time. However, chronic infection by a low number of trematodes may lead to production losses because of reduced growth rates, fertility, milk yield, and carcass weight and inferior carcass composition (45, 51).
Traditionally, the diagnosis of fasciolosis was based on direct detection of fluke eggs in fresh faeces using the faecal sedimentation method (coproscopy). The method is considered to have very high diagnostic specificity (Sp) but low diagnostic sensitivity (Se) due to the 8–12-week prepatent period of fasciolosis, the paucity of eggs present frequently in a faecal sample and the intermittency of egg shedding (26). Several molecular diagnostic methods detecting Fasciola DNA have recently been developed; however, their reproducibility is still limited (hence their use is restricted to reference laboratories) and they have not overcome the main problem of the long prepatent period because Fasciola eggs are the source of DNA to be detected (6). Immunoenzymatic assays (sandwich ELISAs) detecting in feces metabolites released by late immature and adult flukes into the bile (coproantigen) currently ensure higher Se (4, 24, 28, 29, 49). Also, ELISAs detecting specific antibodies against the common liver fluke in the serum or milk overcome the problem of false negative results during the prepatent period, because antibodies against F. hepatica are detectable in serum as early as 3–4 weeks post infection and remain detectable for a very long time (1, 28, 31, 38, 44, 48). Therefore, serological ELISAs appear to be the most optimal diagnostic tools for large-scale surveys.
The prevalence of F. hepatica infection in Polish cattle has been thoroughly studied over the previous 20 years (17, 18, 21, 22, 23, 32, 47), and it is known to vary between geographical regions and seasons within a range from 7% to 80%. Data regarding fasciolosis in small ruminants are limited, not only in Poland but generally in Europe (5), although they suffice to suggest that the prevalence differs considerably between geographical regions (42). Very few local studies conducted on F. hepatica infection in sheep and goats in Poland have shown that prevalence is below 10% (15, 40); however, data from large-scale epidemiological surveys are lacking. Therefore, the aim of the study was to determine the herd-level prevalence of F. hepatica infection in the goat population of Poland by serological and coproscopic examinations.
Material and Methods
Serological survey
A serological survey was conducted in the years 2014–2022. The herds were randomly selected for the study (according to simple random method) in the regions of Poland for which the risk of F. hepatica infection was estimated as increased based on the spatial distribution model developed within the frame of the GLOWORM project (12). The minimum number of herds to be tested was set at 25, so that it was representative for the goat population assuming the expected herd-level seroprevalence of 50%, expected precision of ±20%, and the 95% level of confidence (16). Goat herds were categorised as small (≤30 adult goats), medium (31–100 adult goats) or large (>100 adult goats).
Blood samples were collected from all adult goats (>1 year-old) kept in the herd at the moment of the visit of the scientific team. Blood collection was a part of caprine arthritis-encephalitis control programme described elsewhere. Blood sampling was approved by the Local Ethics Committee in Warsaw (approval nos 31/2013 and WAW2/048/2021). Blood was collected from the jugular vein into 10 mL clot activator tubes (BD Vacutainer; Becton Dickinson, Plymouth, UK), left overnight at +4°C for clotting, and centrifuged at 3,000 rpm (1,390 × g) for 10 min. The serum was harvested to 2 mL Eppendorf tubes and stored frozen at −20°C until testing. Serum samples were tested using a commercial MM3-SERO ELISA (Monoscreen Ab-ELISA Fasciola hepatica indirect double wells kit; BioX Diagnostics, Rochefort, Belgium) performed according to the manufacturer’s instruction. The MM3-SERO ELISA is coated with highly specific proteins from a 7–40 kDa fraction (mainly cathepsins L1 and L2) from the excretory/secretory products of F. hepatica captured by the monoclonal antibody MM3 (31, 37). This ELISA yields results as the sample-to-positive control ratios (S/P%) and its interpretation is as follows: samples with S/P% <10% are considered negative, 10%–15% as inconclusive (doubtful) and ≥15% as positive, between 15% and 45% indicating weak infestation, 45%–75% indicating medium infestation, and >75% severe infestation. The MM3-SERO ELISA is considered to have a very high Se of 99.2% (CI 95%: 97.0%–99.9%) and virtually perfect Sp of 100% (CI 95%: 98.6%–100%) in cattle and sheep (9, 11, 28, 30, 31). Therefore, the herd was considered seropositive if at least one goat in this herd tested positive in serology (i.e. had S/P% ≥15% in the MM3-SERO ELISA). The herd-level seroprevalence was calculated as the proportion of seropositive herds. The within-herd seroprevalence was calculated as the proportion of seropositive goats in the herd.
Coproscopic survey
Coproscopic survey (based on faecal egg count) was carried out in the years 2015– 2023. Faecal samples were obtained within the frame of the voluntary monitoring of gastrointestinal nematode infections and anthelmintic resistance run by the Division of Veterinary Epidemiology and Economics at the Institute of Veterinary Medicine of the Warsaw University of Life Sciences (35). Therefore, the number of herds to be tested was based on the non-random convenience method (46) and the minimum number of herds was not predetermined.
Faecal samples were collected directly from the rectum or immediately after defecation by farmers or by the scientific team during veterinary consultations on goat farms. If collected by the owners of the goats, faecal samples were placed individually in zip-lock plastic bags. Samples were delivered to the parasitological laboratory within 24 h at room temperature and processed within the next 24 h so that the total time which had elapsed between sample collection and examination did not exceed 48 h. The sample pooling procedure was the same whether the owner or a researcher had collected the samples. Faecal samples in each herd were combined into pooled samples consisting of samples of five goats each. The number of pooled samples for each herd varied depending on the overall number of examined animals. In the parasitological laboratory, five 3-g portions of faeces, one from each of five goats, were mixed and subsequently 3 g of this pooled sample was combined with 10 mL of water. The faecal samples were examined by centrifugal sedimentation test (50). The faecal solution was placed in a 15 mL test tube with 3 mL of (R)-(+)-Limonene 97% (Sigma-Aldrich/Merck, Darmstadt, Germany), and the tube was capped. The filled test tube was shaken several times and centrifuged for 5 min at approximately 500 × g. After centrifugation, the sediment was resuspended in a few drops of water, and two drops of the sediment were placed on a slide, covered with a coverslip, and examined at 100× magnification under a light microscope (Eclipse E200; Nikon, Tokyo, Japan) (50). A sample was classified as positive when at least one egg was found in the sample. In the case of a positive result for the pooled sample, all individual samples constituting the pool were tested separately using the aforementioned method to identify an infected animal. The herd was considered positive if at least one goat from this herd tested positive in coproscopy. The herd-level prevalence was calculated as the proportion of herds positive in coproscopy.
Statistical analysis
Numerical variables (the numbers of goats in herds and prevalences) were not normally distributed (according to normal probability Q-Q plots and the Shapiro–Wilk W test); therefore, they were summarised using the median, interquartile range (IQR), and range. Categorical variables (serological and coproscopic status of herds and goats, geographic locations of herds, herd size categories, grazing on the pasture, access to water pools on the pasture and contact with sheep and cattle on the farm) were presented as counts and proportions. Associations between categorical variables were analysed using the maximum-likelihood G test or Fisher exact test (if the minimum expected value of the contingency table was <5), and the size of effect was presented as the odds ratio (OR). The Haldane– Anscombe correction for zero cells in the contingency table was used, if necessary. The 95% confidence intervals (CI 95%) for proportions were calculated using the Wilson score method (3). A significance level (α) was set at 0.05. The analysis was carried out in TIBCO Statistica 13.3 (TIBCO Software Inc., Palo Alto, CA, USA).
Results
The serological survey enrolled 33 randomly selected herds counting from 9 to 183 adult goats with the median (IQR) of 35 (23–61) goats. Twelve herds (36.4%) were small, 17 (51.5%) were medium, and 4 (12.1%) large. In 32 of 33 herds, between 1 and 34 male goats (median of 3) were kept. The herds were located in 9 of the 16 provinces of Poland (Table 1). Between 9 and 168 adult goats with the median (IQR) of 33 (19–49) were tested per herd, which amounted to 1,464 adult goats (134 males and 1,330 females) in total.
Table 1.
Geographical distribution of goat herds tested for Fasciola hepatica infection
| Province | Number of herds tested serologically (% of 33 herds) | Number of seropositive herds / Number of tested herds | Number of herds tested coproscopically (% of 214 herds) |
|---|---|---|---|
| Małopolskie | 2 (6.1) | 2 / 2 | 35 (16.4) |
| Mazowieckie | 1 (3.0) | 0 / 1 | 33 (15.4) |
| Wielkopolskie | - | - | 19 (8.9) |
| Warmińsko-Mazurskie | 14 (42.4) | 3 / 14 | 16 (7.5) |
| Podkarpackie | - | - | 15 (7.0) |
| Śląskie | 1 (3.0) | 0 / 1 | 12 (5.6) |
| Pomorskie | 1 (3.0) | 0 / 1 | 11 (5.1) |
| Lubelskie | 4 (12.1) | 1 / 4 | 11 (5.1) |
| Łódzkie | - | - | 11 (5.1) |
| Lubuskie | - | - | 9 (4.2) |
| Kujawsko-Pomorskie | - | - | 9 (4.2) |
| Dolnośląskie | - | - | 8 (3.7) |
| Podlaskie | 5 (15.2) | 2 / 5 | 8 (3.7) |
| Świętokrzyskie | 3 (9.1) | 2 / 3 | 6 (2.8)* |
| Zachodniopomorskie | 2 (6.1) | 1 / 2 | 6 (2.8) |
| Opolskie | - | 5 (2.3) |
– the herd in which a goat positive in the MM3-SERO ELISA and coproscopy was found
Seropositive goats were detected in 11 of 33 herds, which corresponded to a herd-level seroprevalence of 33.3% (CI 95%: 19.7%–50.4%). At the animal level, a positive result was found in only 17 of 1,464 tested goats (1 male and 16 females) (1.2%, CI 95%: 0.7%–1.9%). Two positive MM3-SERO ELISA results were indicative of a medium infestation and 15 positive results corresponded to a weak infestation. In 8 herds only a single goat was seropositive, while two, three and four goats were seropositive in the 3 remaining herds. The within-herd seroprevalence ranged from 0.8% to 11.1% with the median (IQR) of 2.3% (1.5%–4.6%) (Table 2). There were 14 inconclusive serological results, 12 of them in herds in which positive results were also found.
Table 2.
Herds in which at least one goat positive for Fasciola hepatica in the MM3-SERO ELISA was found
| Herd | Number of adult goats in the herd | Sample size (sample fraction (%)) | Number of seropositive goats (weak/medium/strong infestation) | Within-herd seroprevalence (95% confidence interval) (%) |
|---|---|---|---|---|
| 1 | 158 | 153 (96.8) | 2 (2/0/0) | 1.3 (0.4–4.6) |
| 2 | 66 | 66 (100) | 1 (1/0/0) | 1.5 (0.3–8.1) |
| 3 | 62 | 62 (100) | 4 (4/0/0) | 6.5 (2.5–15.5) |
| 4 | 27 | 27 (100) | 3 (2/1/0) | 11.1 (3.9–28.1) |
| 5 | 36 | 35 (97.2) | 1 (1/0/0) | 2.9 (0.5–14.5) |
| 6 | 61 | 51 (83.6) | 1 (1/0/0) | 2.0 (0.4–10.3) |
| 7 | 22 | 22 (100) | 1 (1/0/0) | 4.6 (0.8–21.8) |
| 8 | 33 | 33 (100) | 1 (1/0/0) | 3.0 (0.5–15.3) |
| 9 | 48 | 48 (100) | 1 (1/0/0) | 2.1 (0.4–10.9) |
| 10 | 127 | 127 (100) | 1 (1/0/0) | 0.8 (0.1–4.3) |
| 11 | 58 | 44 (75.9) | 1 (0/1/0) | 2.3 (0.4–11.8) |
The serological status of the herd was not significantly associated with its geographical location (with the province in which the farm was located) (P = 0.291) or with any other investigated risk factor (Table 3). The proportion of seropositive males (0.7%; 1/134) was not significantly different from the proportion of seropositive females (1.2%; 16/1330; P = 0.617).
Table 3.
The analysis of risk factors for herd-level seropositivity for Fasciola hepatica infection in the goat population of Poland
| Risk factor | Category | Number of seropositive herds/Number of herds in the category (seroprevalence (%)) | P-value | Odds ratio (95% confidence interval) |
|---|---|---|---|---|
| Herd size | Small | 2 / 12 (16.7) | 0.291 | - |
| Medium | 7 / 17 (41.2) | |||
| Large | 2 / 4 (50.0) | |||
| Grazing on the pasture | Yes | 9 / 31 (29.0) | 0.104 | 0.08 (0.01–1.9) |
| No | 2 / 2 (100) | |||
| Access to water pools on the pasture | Yes | 3 / 8 (37.5) | 0.999 | 1.28 (0.24–6.7) |
| No | 8 / 25 (32.0) | |||
| Contact with sheep on the farm | Yes | 4 / 10 (40.0) | 0.696 | 1.52 (0.33–7.2) |
| No | 7 / 23 (30.4) | |||
| Contact with cattle on the farm | Yes | 1 /6 (16.7) | 0.637 | 0.34 (0.04–3.3) |
| No | 10 / 27 (37.0) |
Faecal samples of 2,770 goats from 214 herds were tested, including 17 of 33 serologically tested herds (51.5%; 8 of 11 seropositive and 9 of 22 seronegative herds). Between 1 and 83 faecal samples (median (IQR) of 10 (5–15)) per herd were tested. The herds were located in all provinces of Poland (Table 1). The herd-level prevalence of F. hepatica infection according to coproscopy turned out to be 0.5% (CI 95%: 0.1%–2.6%), which was a significantly lower figure compared to the seroprevalence (P < 0.001) (Fig. 1).
Fig. 1.
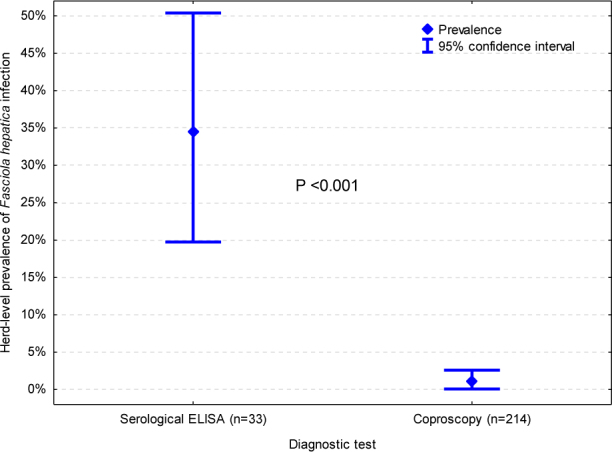
The herd-level prevalence of Fasciola hepatica infection in the goat population of Poland according to serological testing with an MM3-SERO ELISA and a coproscopic test
In only one herd, located in central Poland, a single positive faecal sample was found. This goat was also seropositive and its serological result indicated medium infestation. As the goat was emaciated, it was euthanised on the owner’s request and subjected to a necropsy. The post-mortem examination revealed liver lesions typical of chronic fasciolosis. The organ was fibrotic, with an irregular nodular surface and an opaque capsule. Upon cutting the thickened biliary ducts filled with numerous mature flukes were found.
Discussion
Our study shows that F. hepatica infection in goats in Poland occurs sporadically without any geographical pattern. Although seropositive goats were found in one third of tested herds, antibodies were detected only in single animals in each seropositive herd. Such a low number of seropositive results raises doubts as to the true status of these animals, given that coproscopy confirmed patent F. hepatica infection in only one seropositive animal. Only a test of a perfect Sp could ensure sufficiently high positive predictive value to draw reliable conclusions (45).
The MM3-SERO ELISA used in our study appears to be a very specific test according to studies so far carried out in sheep (31) and cattle (30). Such a high Sp is claimed to result from the use of a novel antigen captured by MM3 monoclonal antibodies and from controlling for potential unspecific cross-reactions by the use of additional wells on a plate which are coated solely by MM3 monoclonal antibodies. The very optimistic results presented in these studies should, however, be treated with great caution. They were all obtained by the same scientific team and have not been verified later by any other studies. Moreover, the MM3-SERO ELISA has never been validated on goats, although a large-scale cross-sectional study based on this ELISA was published for the goat population of Galicia, Spain (39). The MM3-SERO ELISA is based on anti-ruminant IgG1 conjugate; therefore the chance is low that it would work worse in goats than in sheep, but still an assumption that it is perfectly specific in goats should be made very cautiously.
Undoubtedly, our study shows that F. hepatica infection occurs in the Polish goat population, since the infection of one serologically positive goat was confirmed by the coproscopy and then by the post-mortem examination. A very low prevalence in coproscopy compared to seroprevalence has been observed in previous studies from other countries (13) and may be explained in two ways. First, it may be the consequence of the low Se of a single coproscopic examination. Coproscopy is known to detect F. hepatic infection from 8–9 weeks post infection at the earliest (2, 25), and only if a sufficient number of eggs is excreted in faeces. As a consequence, the Se of coproscopy is rarely estimated at more than 50% although few studies on the diagnostic accuracy of coproscopy in small ruminants have been published (25). The use of a coproantigen ELISA instead of coproscopy would have undoubtedly increased the Se, as it has been shown to detect even more positive goats than serological ELISAs (49). Secondly, it may result from past infections which have already been cleared by animals’ immune systems. As antibodies to F. hepatica are known to persist long beyond the period of active infection (31), the seroprevalence from our study may better correspond to the actual level of goat exposure to F. hepatica infection in Poland than do the estimates based on the coproscopy. The exposure to F. hepatica in goats is certainly very low compared to the situation observed in Polish cattle (19) and probably also in Polish sheep, although data on fasciolosis in sheep are rather scarce (15). The risk of fasciolosis is mostly determined by the presence and extensity of infected snails, in Europe mainly freshwater snails of the species Galba truncatula (10, 41, 43). According to previous studies, the prevalence of F. hepatica infection in Polish snails is quite high (20). Nevertheless, these gastropods can only survive in wet conditions, while goats tend to avoid wetlands and damp pastures. Moreover, goats prefer browsing to grazing, which is associated with frequent changes of place of feeding and precludes ingestion of large quantities of metacercariae even on highly contaminated pastures (45). According to our observations resulting from many years of veterinary service on Polish goat farms, goats rarely share pastures with reservoir hosts of F. hepatica such as sheep and cattle.
Moreover, the common use of benzimidazoles on goat farms in Poland may lead to unintentional and somehow accidental reduction of active F. hepatica infection in goats. Most antiparasitic treatments are implemented based on common opinions about the high risk which tapeworms or liver flukes pose for ruminants without any attempt to seek medical confirmation of a true infection prior to treatment (36). Our study indicates that such a practice is totally pointless in light of the extremely rare occurrence of F. hepatica infection in Poland. Furthermore, the unjustified use of anthelmintics stimulates the development of resistance of gastrointestinal nematodes to benzimidazoles, which is already a substantial problem of goat herds worldwide and also in Poland (33, 34, 35). This observation makes proper parasitological diagnostics in a goat herd crucial to maintain the effectiveness of anthelmintic drugs as well as animal health and welfare.
Conclusion
F. hepatica infection occurs sporadically in the Polish goat population and its prevalence is much lower than in cattle or sheep. Therefore, treatment or prevention against fasciolosis should only be considered if it has been reliably confirmed by a positive result of a highly accurate diagnostic test. This also applies to goats inhabiting geographical areas where F. hepatica infection appears to be widespread in cattle and sheep.
Footnotes
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement: The study was partially financed by a grant from the National Science Centre (decision no. DEC-2020/37/B/NZ6/00457) and a grant from the Ministry of Science and Higher Education of the Republic of Poland (decision no. 9506/E-385/R/2018). The latter grant provided financial support for the modernisation of the parasitological and serological laboratories in which the diagnostic procedures were performed. The research internship of one of the co-authors (Adrian-Valentin Potărniche) was supported by the Polish National Agency for Academic Exchange (NAWA) (decision no. BPN/ULM/2022/1/00087/U/00001). The funding bodies had no direct role in the design of the study, the collection, analysis or interpretation of the data or the writing of the manuscript.
Animal Rights Statement: Blood sampling was approved by the Local Ethics Committee in Warsaw (approval nos 31/2013 and WAW2/048/2021).
References
- 1.Ahumada M., Godino A., Guasconi L., Deheza C., Amaranto M., Pruzzo C.I., Vitulli-Moya G., Chiapello L., Carrizo M.E., Barra J.L., Cervi L. Antibody detection against Kunitz-type protein in Fasciola hepatica experimentally infected sheep using enzyme-linked immunosorbent assay (ELISA) Int J Vet Sci Med. 2023;11:126. doi: 10.1080/23144599.2023.2273678. : . , , –. , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almazán C., Avila G., Quiroz H., Ibarra F., Ochoa P. Effect of parasite burden on the detection of Fasciola hepatica antigens in sera and feces of experimentally infected sheep Vet Parasitol. 2001;97:101. doi: 10.1016/s0304-4017(01)00376-4. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 3.Altman D., Machin D., Bryant T., Gardner M. Statistics with Confidence: Confidence Intervals and Statistical Guidelines. Second Edition BMJ Books; London, UK: 2000. : , , , , . [Google Scholar]
- 4.Anderson N., Luong T.T., Vo N.G., Bui K.L., Smooker P.M., Spithill T.W. The sensitivity and specificity of two methods for detecting Fasciola infections in cattle Vet Parasitol. 1999;83:15. doi: 10.1016/s0304-4017(99)00026-6. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 5.Beesley N.J., Caminade C., Charlier J., Flynn R.J., Hodgkinson J.E., Martinez-Moreno A., Martinez-Valladares M., Perez J., Rinaldi L., Williams D.J.L. Fasciola and fasciolosis in ruminants in Europe: Identifying research needs Transbound Emerg Dis. 2018;65:S199. doi: 10.1111/tbed.12682. : . , –. , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvani N.E.D., George S.D., Windsor P.A., Bush R.D., Šlapeta J. Comparison of early detection of Fasciola hepatica in experimentally infected Merino sheep by real-time PCR, coproantigen ELISA and sedimentation Vet Parasitol. 2018;251:85. doi: 10.1016/j.vetpar.2018.01.004. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 7.Charlier J., Duchateau L., Claerebout E., Williams D., Vercruysse J. Associations between anti-Fasciola hepatica antibody levels in bulk-tank milk samples and production parameters in dairy herds Prev Vet Med. 2007;78:57. doi: 10.1016/j.prevetmed.2006.09.010. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 8.Charlier J., Rinaldi L., Musella V., Ploeger H.W., Chartier C., Vineer H.R., Hinney B., von Samson-Himmelstjerna G., Băcescu B., Mickiewicz M., Mateus T.L., Martinez-Valladares M., Quealy S., Azaizeh H., Sekovska B., Akkari H., Petkevicius S., Hektoen L., Höglund J., Morgan E.R., Bartley D.J., Claerebout E. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe Prev Vet Med. 2020;182:105103. doi: 10.1016/j.prevetmed.2020.105103. : . , , , doi: . [DOI] [PubMed] [Google Scholar]
- 9.Charlier J., Vercruysse J., Morgan E.R., van Dijk J., Williams D.J. Recent advances in the diagnosis, impact on production and prediction of Fasciola hepatica in cattle Parasitology. 2014;141:326. doi: 10.1017/S0031182013001662. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 10.Correa A.C., De Meeûs T., Dreyfuss G., Rondelaud D., Hurtrez-Boussès S. Galba truncatula and Fasciola hepatica: Genetic costructures and interactions with intermediate host dispersal Infect Genet Evol. 2017;55:186. doi: 10.1016/j.meegid.2017.09.012. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 11.Drescher G, de Vasconcelos T.C.B., Belo V.S., Pinto M.M.D.G., Rosa J.O., Morello L.G., Figueiredo F.B. Serological diagnosis of fasciolosis (Fasciola hepatica) in humans, cattle, and sheep: a meta-analysis Front Vet Sci. 2023;10:1252454. doi: 10.3389/fvets.2023.1252454. : . , , , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ducheyne E., Charlier J., Vercruysse J., Rinaldi L., Biggeri A., Demeler J., Brandt C., De Waal T., Selemetas N., Höglund J., Kaba J., Kowalczyk S.J., Hendrickx G. Modelling the spatial distribution of Fasciola hepatica in dairy cattle in Europe Geospat Health. 2015;9:261. doi: 10.4081/gh.2015.348. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 13.Ferre I., Ortega-Mora L.M., Rojo-Vázquez F.A. Seroprevalence of Fasciola hepatica infection in sheep in northwestern Spain Parasitol Res. 1995;81:137. doi: 10.1007/BF00931619. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 14.Fitzpatrick J.L. Global food security: the impact of veterinary parasites and parasitologists Vet Parasitol. 2013;195:233. doi: 10.1016/j.vetpar.2013.04.005. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 15.Górski P., Niżnikowski R., Popielarczyk D., Strzelec E., Gajewska A., Wedrychowicz H. Natural parasitic infections in various breeds of sheep in Poland Arch Anim Breed. 2004;47:50. : . , , –. . [Google Scholar]
- 16.Humphry R.W., Cameron A., Gunn G.J. A practical approach to calculate sample size for herd prevalence surveys Prev Vet Med. 2004;65:173. doi: 10.1016/j.prevetmed.2004.07.003. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 17.Kornaś S., Nowosad B., Skalska M., Wróbel A. Dairy cattle infection with Fasciola hepatica in small farms (in Polish) Med Weter. 2005;61:1368. : . , , –. . [Google Scholar]
- 18.Kowalczyk S.J., Czopowicz M., Weber C.N., Müller E., Kaba J. Accuracy of a diagnostic model based on serum biochemical parameters in detecting cows at an increased risk of chronic fascioliasis Vet Parasitol. 2018;254:15. doi: 10.1016/j.vetpar.2018.02.038. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 19.Kowalczyk S.J., Czopowicz M., Weber C.N., Müller E., Nalbert T., Bereznowski A., Kaba J. Herd-level seroprevalence of Fasciola hepatica and Ostertagia ostertagi infection in dairy cattle population in the central and northeastern Poland BMC Vet Res. 2018;14:131. doi: 10.1186/s12917-018-1455-7. : . , , , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozak M., Wędrychowicz H. The performance of a PCR assay for field studies on the prevalence of Fasciola hepatica infection in Galba truncatula intermediate host snails Vet Parasitol. 2010;168:25. doi: 10.1016/j.vetpar.2009.10.014. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 21.Kozak-Cieszczyk M. The occurrence of Fasciola hepatica in chosen regions of Poland based on molecular and serological methods (in Polish) Wiad Parazytol. 2006;52:137. : . , , –. . [PubMed] [Google Scholar]
- 22.Kozłowska-Łój J. Prevalence of Fasciola hepatica L. infection in cattle in the Lublin province (Poland) in the years 2005–2008 Wiad Parazytol. 2011;57:127. : . , , –. . [PubMed] [Google Scholar]
- 23.Kozłowska-Łój J., Łój-Maczulska A. The prevalence of Fasciola hepatica L. infection in cattle in the Lublin province in the years 2009–2012 Ann Parasitol. 2013;59:207. : . , , –. . [PubMed] [Google Scholar]
- 24.Kuerpick B., Schnieder T., Strube C. Evaluation of a recombinant cathepsin L1 ELISA and comparison with the Pourquier and ES ELISA for the detection of antibodies against Fasciola hepatica Vet Parasitol. 2013;193:206. doi: 10.1016/j.vetpar.2012.11.021. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 25.Martínez-Pérez J.M., Robles-Pérez D., Rojo-Vázquez F.A., Martínez-Valladares M. Comparison of three different techniques to diagnose Fasciola hepatica infection in experimentally and naturally infected sheep Vet Parasitol. 2012;190:80. doi: 10.1016/j.vetpar.2012.06.002. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 26.Mazeri S., Sargison N., Kelly R.F., Bronsvoort B.M., Handel I. Evaluation of the Performance of Five Diagnostic Tests for Fasciola hepatica Infection in Naturally Infected Cattle Using a Bayesian No Gold Standard Approach PLoS One. 2016;11:e0161621. doi: 10.1371/journal.pone.0161621. : . , , , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehmood K., Zhang H., Sabir A.J., Abbas R.Z., Ijaz M., Durrani A.Z., Saleem M.H., Ur Rehman M., Iqbal M.K., Wang Y., Ahmad H.I., Abbas T., Hussain R., Ghori M.T., Ali S., Khan A.U., Li J. A review on epidemiology, global prevalence and economical losses of fasciolosis in ruminants Microb Pathog. 2017;109:253. doi: 10.1016/j.micpath.2017.06.006. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 28.Mezo M., González-Warleta M., Carro C., Ubeira F.M. An ultrasensitive capture ELISA for detection of Fasciola hepatica coproantigens in sheep and cattle using a new monoclonal antibody (MM3) J Parasitol. 2004;90:845. doi: 10.1645/GE-192R. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 29.Mezo M., González-Warleta M., Castro-Hermida J.A., Martínez-Sernández V., Ubeira F.M. Field evaluation of the enhanced MM3-COPRO ELISA test for the diagnosis of Fasciola hepatica infection in sheep PLoS One. 2022;17:e0265569. doi: 10.1371/journal.pone.0265569. : . , , , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mezo M., González-Warleta M., Castro-Hermida J.A., Muiño L., Ubeira F.M. Field evaluation of the MM3-SERO ELISA for detection of anti-Fasciola IgG antibodies in milk samples from individual cows and bulk milk tanks Parasitol Int. 2010;59:610. doi: 10.1016/j.parint.2010.09.001. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 31.Mezo M., González-Warleta M., Ubeira F.M. The use of MM3 monoclonal antibody for the early immunodiagnosis of ovine fasciolosis J Parasitol. 2007;93:65. doi: 10.1645/GE-925R.1. : . , , –. , doi: [DOI] [PubMed] [Google Scholar]
- 32.Michalski M., Romaniuk K. Liver Fluke (Fasciola hepatica L.) in dairy cows in North-East Poland (in Polish) Med Weter. 2000;56:182. : . , , –. . [Google Scholar]
- 33.Mickiewicz M., Czopowicz M., Górski P., Kaba J. The first reported case of resistance of gastrointestinal nematodes to benzimidazole anthelmintic in goats in Poland Ann Parasitol. 2017;63:317. doi: 10.17420/ap6304.118. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 34.Mickiewicz M., Czopowicz M., Kawecka-Grochocka E., Moroz A., Szaluś-Jordanow O., Várady M., Königová A., Spinu M., Górski P., Bagnicka E., Kaba J. The first report of multidrug resistance in gastrointestinal nematodes in goat population in Poland BMC Vet Res. 2020;16:270. doi: 10.1186/s12917-020-02501-5. : . , , , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mickiewicz M., Czopowicz M., Moroz A., Potărniche A.V., Szaluś-Jordanow O., Spinu M., Górski P., Markowska-Daniel I., Várady M., Kaba J. Prevalence of anthelmintic resistance of gastrointestinal nematodes in Polish goat herds assessed by the larval development test BMC Vet Res. 2021;17:19. doi: 10.1186/s12917-020-02721-9. : . , , , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mickiewicz M., Czopowicz M., Moroz A., Witkowski L., Szaluś-Jordanow O., Nalbert T., Markowska-Daniel I., Górski P., Kaba J. Common endoparasitic infections in goats in Poland – diagnostics and treatment (in Polish) Życie Weter. 2017;92:665. : . , , –. . [Google Scholar]
- 37.Muiño L., Perteguer M.J., Gárate T., Martínez-Sernández V., Beltrán A., Romarís F., Mezo M., González-Warleta M., Ubeira F.M. Molecular and immunological characterization of Fasciola antigens recognized by the MM3 monoclonal antibody Mol Biochem Parasitol. 2011;179:80. doi: 10.1016/j.molbiopara.2011.06.003. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 38.Nur Hafizah S., Noor Izani N.J., Ahmad Najib M., Wan-Nor-Amilah W.A.W. Immunodiagnosis of Fascioliasis in Ruminants by ELISA Method: A Mini-Review Malays J Med Sci. 2023;30:25. doi: 10.21315/mjms2023.30.4.3. : . , , –. , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pérez-Creo A., Díaz P., López C., Béjar J.P., Martínez-Sernández V., Panadero R., Díez-Baños P., Ubeira F.M., Morrondo P. Fasciola hepatica in goats from north-western Spain: Risk factor analysis using a capture ELISA Vet J. 2016;208:104. doi: 10.1016/j.tvjl.2015.07.033. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 40.Pilarczyk B., Tomza-Marciniak A., Pilarczyk R., Bombik E., Seremak B., Udała J., Sadowska N. A Comparison of the Prevalence of the Parasites of the Digestive Tract in Goats from Organic and Conventional Farms Animals. 2021;11:2581. doi: 10.3390/ani11092581. : . , , , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Relf V., Good B., Hanrahan J.P., McCarthy E., Forbes A.B., deWaal T. Temporal studies on Fasciola hepatica in Galba truncatula in the west of Ireland Vet Parasitol. 2011;175:287. doi: 10.1016/j.vetpar.2010.10.010. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 42.Rinaldi L., Biggeri A., Musella V., De Waal T., Hertzberg H., Mavrot F., Torgerson P.R., Selemetas N., Coll T., Bosco A., Grisotto L., Cringoli G., Catelan D. Sheep and Fasciola hepatica in Europe: the GLOWORM experience Geospat Health. 2015;9:309. doi: 10.4081/gh.2015.353. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 43.Roldán C., Begovoeva M., López-Olvera J.R., Velarde R., Cabezón Ó., Molinar Min A.R., Pizzato F., Pasquetti M., Fernández Aguilar X., Mentaberre G., Serrano E., Puig Ribas M., Espunyes J., Castillo-Contreras R., Estruch J., Rossi L. Endemic occurrence of Fasciola hepatica in an alpine ecosystem, Pyrenees, Northeastern Spain Transbound Emerg Dis. 2021;68:2589. doi: 10.1111/tbed.13865. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 44.Sánchez-Andrade R., Paz-Silva A., Suárez J.L., Panadero R., Pedreira J., Díez-Baños P., Morrondo P. Effect of fasciolicides on the antigenaemia in sheep naturally infected with Fasciola hepatica Parasitol Res. 2001;87:609. doi: 10.1007/s004360100425. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 45.Smith M.C., Sherman D.M. Goat medicine. Third Edition John Wiley & Sons; Hoboken, NJ, USA: 2023. : , , , , . [Google Scholar]
- 46.Thrusfield M. Veterinary epidemiology. Fourth Edition Wiley-Blackwell; Hoboken, NJ, USA: 2018. pp. 272–277. : , , , , –. . [Google Scholar]
- 47.Tomczuk K., Szczepaniak K., Demkowska-Kutrzepa M., Roczeń-Karczmarz M., Junkuszew A., Gruszecki T., Drozd L., Karpiński M., Studzińska M. Occurrence of internal parasites in cattle in various management systems in South-East Poland (in Polish) Med Weter. 2018;74:501. doi: 10.21521/mw.6105. : . , , –. , doi: . [DOI] [Google Scholar]
- 48.Valero M.A., Ubeira F.M., Khoubbane M., Artigas P., Muiño L., Mezo M., Pérez-Crespo I., Periago M.V., Mas-Coma S. MM3-ELISA evaluation of coproantigen release and serum antibody production in sheep experimentally infected with Fasciola hepatica and F. gigantica Vet Parasitol. 2009;159:77. doi: 10.1016/j.vetpar.2008.10.014. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 49.Villa-Mancera A., Molina-Mendoza P., Hernández-Guzmán K., Olivares-Pérez J., Sarracent-Pérez J., Zumaquero-Ríos J. Comparative Diagnosis of Serum IgG1 and Coproantigen ELISA for Fasciolosis Detection of Goats in Mexico Biomed Res Int. 2016;2016:3860928. doi: 10.1155/2016/3860928. : . , , , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zajac A.M., Conboy G.A. Veterinary Clinical Parasitology. Seventh Edition Blackwell Publishing; Ames, IA, USA: 2012. Chapter 1, Fecal Examination for the diagnosis of parasitism; p. 14. : , , , , , p. . [Google Scholar]
- 51.Zerna G., Spithill T.W., Beddoe T. Current Status for Controlling the Overlooked Caprine Fasciolosis Animals. 2021;11:1819. doi: 10.3390/ani11061819. : . , , , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]


