Abstract
Introduction:
Mechanical thrombectomy (MT) is the standard treatment for acute ischemic stroke (AIS) due to anterior large vessel occlusion (LVO). Despite successful recanalization, some patients remain disabled after 3 months. Mechanisms that can cause futile recanalization (FR) are still largely unknown. We investigated if stress hyperglycemia might be associated with FR.
Patients and methods:
This is a retrospective analysis of consecutive patients with successful recanalization treated in four participating centers between January 2021 and December 2022. According to the modified Rankin scale (mRS) status at 3 months, patients were divided into two groups: FR, if mRS score >2, and useful recanalization (UR), if mRS score ⩽2. Stress hyperglycemia was estimated by the glucose-to-glycated hemoglobin ratio (GAR) index.
Results:
A total of 691 subjects were included. At 3 months, 403 patients (58.3%) were included in the FR group, while the remaining 288 patients (41.7%) were included in the UR group. At the multivariate analysis, variables independently associated with FR were the following: age (OR 1.04, 95% CI 1.02–1.06, p < 0.001), GAR index (OR 1.08, 95% CI 1.03–1.14, p = 0.003), NIHSS at admission (OR 1.16, 95% CI 1.11–1.22; p < 0.001), and procedure length (OR 1.01, 95% CI 1.00–1.02; p = 0.009). We observed that the model combining age, GAR index, NIHSS at admission, and procedure length had good predictive accuracy (AUC 0.78, 95% CI 0.74–0.81).
Conclusions:
Stress hyperglycemia predicts FR in patients with successful recanalization after MT. Further studies should explore if managing stress hyperglycemia may reduce futile recanalization. Additionally, we recommend paying close attention to AIS patients with a GAR index greater than 24.8 who exhibit a high risk of FR.
Keywords: Stress hyperglycemia, GAR index, acute ischemic stroke, outcome, mechanical thrombectomy
Graphical abstract.
Introduction
Hyperglycemia can impair clinical outcome of patients with acute ischemic stroke (AIS) treated with mechanical thrombectomy (MT) for large vessel occlusion (LVO). 1 A meta-analysis showed that high blood glucose levels at admission increase the risk of disability, symptomatic intracranial hemorrhage, and mortality in this specific population. 2 Despite several studies reporting a negative impact of glucose on functional and radiological outcomes, 3 trials implementing intensive glucose control in people with AIS were neutral,4,5 supporting the concept that hyperglycemia alone may not fully represent the disease course. Additionally, aggressive glycemic control may be dangerous due to its association with a high rate of hypoglycemic episodes. 6 Therefore, stress hyperglycemia, which weights glucose level for baseline glucose control, was investigated as a possible marker of poor outcomes in patients undergoing MT. 7
Stress hyperglycemia is a physiological response to a sudden clinical event that causes an increase in blood glucose levels. This increase typically returns to baseline following the acute phase. 8 However, stress hyperglycemia may induce damage after AIS through several mechanisms. These mechanisms include direct toxic damage to the ischemic brain due to accumulation of lactate and intracellular acidosis, increased circulating free fatty acids impairing endothelium-dependent vasodilation and promoting intracellular calcium overload, and reperfusion injury due to increased oxidative stress and inflammation. 7 Numerous studies show that patients with stress hyperglycemia after MT have worse clinical outcomes.9–12
Modern thrombectomy devices and techniques allow a successful recanalization in more than 80% of AIS patients undergoing MT.13,14 Unfortunately, many of them are disabled 3 months after stroke.15,16 Mechanisms that can cause this futile recanalization (FR) are still largely unknown. 17
Building on a previous report from our single-center experience of the negative impact of stress hyperglycemia on functional outcome, 18 this multicenter prospective observational study aimed at identifying the impact of stress hyperglycemia on functional outcome after successful MT for LVO-related AIS.
Patients and methods
Study design and participants
This is a multicenter retrospective analysis of prospectively collected data. The study was performed at four comprehensive stroke centers: Udine University Hospital, Udine (Italy); Bufalini Hospital, AUSL Romagna, Cesena, (Italy); Avezzano Hospital, L’Aquila (Italy); Charing Cross Hospital, Imperial College Healthcare NHS Trust, London (UK). From January 2021 to December 2022, consecutive AIS patients undergoing MT for LVO were included according to the following inclusion criteria: (1) occlusion in the anterior circulation, including intracranial internal carotid artery and proximal middle cerebral artery segments (M1 and proximal M2); (2) Alberta Stroke Program Early CT Score (ASPECTS) ⩾6 19 ; (3) pre-stroke modified Rankin scale (mRS) 0–2; (4) onset of symptoms within 6 h; (5) successful recanalization after MT. The angiographic result of the procedure was assessed on the final digital subtraction angiography image series and was classified according to the modified version of the modified TICI score (mTICI). Successful recanalization was defined as grade 2b, 2c, or 3 of reperfusion. 20 According to international guidelines, alteplase was used to treat AIS patients with onset of symptoms within 4.5 h and without contraindications. 21 Patients were included regardless of their pre-stroke glycemic status. We excluded patients who had moderate to severe pre-stroke disability (mRS > 2). Patients with missing fasting plasma glucose and HbA1c data were also excluded.
The study conformed to the Declaration of Helsinki of the World Medical Association and was approved by the local ethics committee of Udine University Hospital (Ref. No. CEUR-2020-Os-173), which was the coordinating center. Written informed consent was obtained from all patients or their representatives.
Data collection
All information was prospectively collected, including demographic characteristics (age and sex), medical history (previous transient ischemic attack or stroke, cardiovascular disease, hypertension, atrial fibrillation, diabetes mellitus, and hypercholesterolemia), previous antithrombotic treatment, admission systolic blood pressure, and laboratory findings with stress hyperglycemia. Stress hyperglycemia was estimated by the glucose-to-glycated hemoglobin ratio (GAR) index, which was calculated using the following formula: fasting plasma glucose (mg/dl)/HbA1c (%). 7 The coordinating center (Udine) has largely used this index to measure stress hyperglycemia in AIS patients treated with reperfusion therapy, including MT. 11 The fasting venous blood samples within 24 h after hospitalization were drawn during the morning hours (06.00–08:00) after an overnight fast (at least 12 h) to measure fasting plasma glucose and HbA1c. Raw data on fasting plasma glucose and HbA1c were collected in the coordinating center (Udine). After that, two trained physicians, independent and blinded to the patient’s outcome, calculated the GAR index for each patient. In case of any conflict between the two physicians, a senior physician, who was also blinded to the patient’s outcome, was involved as a third party. Based on the GAR index, patients were stratified into four groups according to quartiles of GAR (Q1–Q4). The higher the GAR index, the more severe the stress hyperglycemia was considered. Stroke severity was quantified at admission using the National Institutes of Health Stroke Scale (NIHSS) score. Trained and certified vascular neurologists conducted NIHSS and mRS scoring in every center. The degree of previous functional disability was calculated at admission, based on pre-stroke disability, and 3 months after stroke using the mRS. The mRS score after discharge was evaluated during an in-person consultation or through telemedicine/telephone interviews with patients or their immediate caregivers. ASPECTS was used for grading early ischemic changes within the MCA territory on a native scan. 19 The Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria were adopted for classifying ischemic strokes into different subtypes based on etiology. 22 The following information regarding endovascular procedure was collected: site of occlusion, type of device used for the MT procedure, number of retrieval attempts, presence of secondary embolization, time from onset of symptoms to groin puncture, time from hospital arrival to groin puncture (door-to-groin time), and procedure duration. For patients receiving alteplase, information was collected on the time from onset of symptoms and from hospital arrival to alteplase administration (door-to-needle time). During MT, patients received conscious sedation or general anesthesia with endotracheal intubation at the discretion of the anesthesiologists.
Definition of futile recanalization
We divided the sample into two groups, FR and useful recanalization (UR), according to their disability status 3 months after stroke. The FR group included all patients with a 3-month mRS score >2, whereas patients were allocated to the UR group if they had a 3-month mRS score ⩽2.
Statistical analysis
Statistical analysis was done using IBM SPSS Statistics, version 22.0 (IBM Corp., Armonk, NY, USA) and MedCalc, version 22.021, for Windows. Statistical comparisons were performed using the chi-square test or Fisher’s exact test, when appropriate, for categorical variables. The Mantel–Haenszel test for linear association was used on categorical data where appropriate to test for trends. Differences between the two groups were assessed using the Student t-test for independent samples when variables had a normal distribution and the Mann–Whitney U-test when variables had an abnormal distribution. The Kolmogorov–Smirnov test with Lilliefors significant correction was performed to test the normality of the variables. Binary logistic regression was used for detecting variables independently associated with FR. We performed two distinct models, one with the GAR index as a continuous variable and the other with it as a categorical variable. The models were adjusted for all the variables with a probability value of <0.05 in univariate analysis. The diagnostic values of factors, solely or in combination, to predict FR were tested with area under the receiver operating characteristic curves (AUC-ROC). The predictive model included the variables independently associated with FR after the multivariate analysis. No prespecified variables were added to the ROC analysis to enhance the model’s accuracy. ROC curves were compared using the DeLong test. Data are displayed in tables as median and interquartile range (IQR) unless otherwise specified. All probability values were two-tailed. Statistical significance was set at a p-value of <0.05.
Data availability
Anonymized study data can be made available upon request from a qualified researcher.
Results
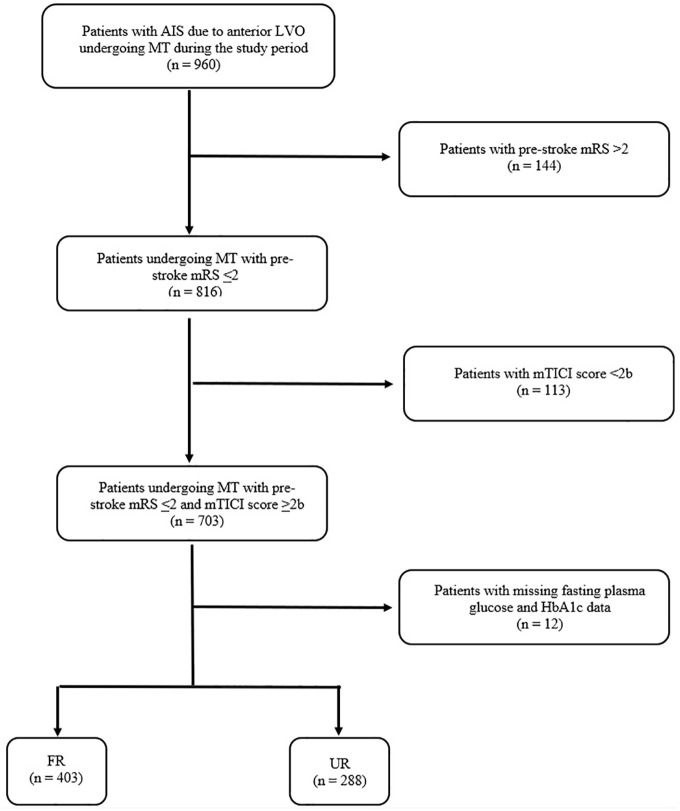
A total of 691 patients with successful recanalization after MT for anterior LVO were included in the study, all with mRS 0–2 at baseline. At 3 months, 403 patients (58.3%) had an mRS score >2 and were included in the FR group. The remaining 288 patients (41.7%) were included in the UR group having no or slight disability. The flow diagram of the study is reported in Figure 1.
Figure 1.
Flow diagram of the study.
AIS: acute ischemic stroke; LVO: large vessel occlusion; mTICI score: modified thrombolysis in cerebral infarction score; FR: futile recanalization; UR: useful recanalization.
The general characteristics of the two groups are reported in Table 1. Patients with FR were found to be older (p < 0.001) and more frequently female (p = 0.045) than those in the UR group. A history of hypertension (p = 0.008) and atrial fibrillation (p < 0.001) was significantly associated with FR. In addition, subjects with FR took more anticoagulants (p = 0.033) and had lower levels of Hb (p < 0.001). Stroke severity at admission (p < 0.001), measured by NIHSS, and baseline early ischemic change (p = 0.007), measured by ASPECTS, were found to be associated with FR.
Table 1.
General characteristics of the patients included in the futile recanalization (FR) and useful recanalization (UR) groups.
| FR (n = 403) | UR (n = 288) | p | |
|---|---|---|---|
| Demographic data | |||
| Age, years | 78 (71–84) | 72 (62–80) | 0.001 |
| Females, n (%) | 220 (54.6) | 135 (46.9) | 0.045 |
| Vascular risk factors | |||
| Previous transient ischemic attack/stroke, n (%) | 38 (9.4) | 26 (9) | 0.858 |
| Cardiovascular disease, n (%) | 49 (12.2) | 27 (9.4) | 0.249 |
| Hypertension, n (%) | 294 (73) | 183 (63.5) | 0.008 |
| Atrial fibrillation, n (%) | 125 (31) | 55 (19.1) | 0.001 |
| Diabetes mellitus, n (%) | 62 (15.4) | 35 (12.2) | 0.675 |
| Hypercholesterolemia, n (%) | 139 (34.5) | 82 (28.5) | 0.094 |
| Previous antithrombotic treatment | 0.033 | ||
| Antiplatelets, n (%) | 112 (29.3) | 74 (25.7) | |
| Anticoagulants, n (%) | 81 (20.1) | 41 (14.2) | |
| Systolic blood pressure at admission, mmHg | 154 (137.5–170) | 150 (135–170) | 0.171 |
| Laboratory findings | |||
| Hb, g/dl | 12.2 (10.8–13.3) | 12.8 (11.7–14) | 0.001 |
| Platelets, 103/mmc | 190 (153–237) | 199 (161–249) | 0.092 |
| Creatinine, mg/dl | 0.90 (0.74–1.08) | 0.87 (0.75–1.04) | 0.242 |
| Stroke subtypes based on TOAST classification | 0.326 | ||
| Large arterial atherosclerosis, n (%) | 66 (16.4) | 57 (19.8) | |
| Cardioembolism, n (%) | 206 (51.1) | 127 (44.1) | |
| Other determined etiology, n (%) | 25 (6.2) | 20 (6.9) | |
| Undetermined etiology, n (%) | 106 (26.3) | 84 (29.2) | |
| Baseline clinical characteristics | |||
| NIHSS score at admission, median (IQR) | 18 (15–22) | 14 (9–18) | 0.001 |
| Baseline ASPECTS, median (range) | 10 (8–10) | 10 (9–10) | 0.007 |
FR: futile recanalization; UR: useful recanalization; Hb: hemoglobin; INR: international normalized ratio; TOAST: Trial of ORG 10172 in Acute Stroke Treatment; NIHSS: National Institute of Health Stroke Scale; ASPECTS: Alberta Stroke Program Early CT Score.
Bold values denote statistical significance at the p < 0.05 level.
Figure 2 reports the GAR index in the two groups. Patients with FR had significantly higher values of the GAR index than those in the UR group (p < 0.001). Patients included in the first GAR quartile (Q1) had values lower than 25th percentile, that is, lower than 17.0; those in the second GAR quartile (Q2) had values within 25th to 50th percentile, that is, comprised between 17.0 and 20.0; those in the third GAR quartile (Q3) had values within 50th to 75th percentile, that is, comprised between 20.1 and 24.8; those in the fourth GAR quartile (Q4) had values higher than 75th percentile, that is, higher than 24.8. The Mantel–Haenszel test for linear association shows a significant trend between the four quartiles of GAR (p < 0.001). The prevalence of FR was 49.4%, 52.9%, 57.8%, and 73.8% across the first to fourth quartiles of GAR, respectively (see Figure 3).
Figure 2.

GAR index in patients with useful (UR) and futile (FR) recanalization.
GAR: glucose-to-glycated hemoglobin ratio.
Figure 3.

Number of patients with useful (UR) and futile (FR) recanalization across the four GAR quartiles.
GAR: glucose-to-glycated hemoglobin ratio; Q1: first GAR quartile; Q2: second GAR quartile; Q3: third GAR quartile; Q4: fourth GAR quartile.
Table 2 reports information related to endovascular procedures. FR was significantly associated with the presence of a tandem occlusion (p = 0.004), retrieval attempts >3 (p = 0.002), and a longer MT duration (p < 0.001). Over half of our sample (n = 423, 61.2%) received intravenous thrombolysis. Using alteplase before MT increased the probability of experiencing UR (p = 0.030).
Table 2.
Information on thrombectomy procedure of the patients included in the futile recanalization (FR) and useful recanalization (UR) groups.
| FR (n = 403) | UR (n = 288) | p | |
|---|---|---|---|
| Site of occlusion | 0.004 | ||
| MCA, n (%) | 297 (73.7) | 239 (74.4) | |
| Tandem, n (%) | 106 (26.3) | 49 (17) | |
| Type of device used for MT | 0.065 | ||
| Thromboaspiration, n (%) | 158 (39.2) | 137 (47.6) | |
| Stent retriever, n (%) | 40 (9.9) | 31 (10.8) | |
| Combined technique, n (%) | 165 (40.9) | 193 (35.8) | |
| Permanent stenting, n (%) | 40 (9.9) | 17 (5.9) | |
| Other information on recanalization therapy | |||
| Number of retrieval attempts > 3, n (%) a | 54 (22.7) | 21 (11.2) | 0.002 |
| Secondary embolization, n (%) b | 11 (5.6) | 22 (7.1) | 0.522 |
| Time from symptoms onset to MT, min | 210 (150–258) | 210 (160–253.2) | 0.689 |
| Door-to-groin time, min | 113 (68–157) | 115.5 (74–147.2) | 0.768 |
| Procedure length, min | 60 (45–95) | 50 (39–76.5) | 0.001 |
| Alteplase use before MT, n (%) | 233 (57.8) | 190 (66) | 0.030 |
| Time from symptoms onset to alteplase, min | 125 (99–153) | 126.5 (104.7–167.2) | 0.350 |
| Door-to-needle time, min | 50 (40.7–66) | 51.5 (42–63) | 0.796 |
| Type of anesthesia c | 0.172 | ||
| Conscious sedation, n (%) | 273 (81) | 208 (76.5) | |
| General anesthesia, n (%) | 64 (19) | 63 (23.5) | |
FR: futile recanalization; UR: useful recanalization; MCA: middle cerebral artery; MT: mechanical thrombectomy.
Bold values denote statistical significance at the p < 0.05 level.
Data available for 425 patients.
Data available for 502 patients.
Data available for 609 patients.
Both multivariate models demonstrated that age, NIHSS at admission, and procedure length are independently associated with FR. Regarding stress hyperglycemia, as measured by the GAR index, it seems to favor the occurrence of FR, particularly in patients with the highest GAR levels, that is, subjects in the fourth quartile having a GAR index greater than 24.8 (see Tables 3 and 4).
Table 3.
Odds ratio (95% confidence intervals) for futile recanalization using the GAR index as continuous variable.
| Futile recanalization | OR | 95% CI | p |
|---|---|---|---|
| Age | 1.04 | 1.02–1.06 | 0.001 |
| Females | 0.94 | 0.58–1.54 | 0.822 |
| Hypertension | 0.97 | 0.57–1.67 | 0.922 |
| Atrial fibrillation | 0.85 | 0.36–2.04 | 0.724 |
| Anticoagulants use | 1.16 | 0.45–2.97 | 0.759 |
| GAR index | 1.08 | 1.03–1.14 | 0.003 |
| NIHSS at admission | 1.16 | 1.11–1.22 | 0.001 |
| ASPECTS | 0.86 | 0.69–1.07 | 0.167 |
| Site of occlusion | |||
| MCA | 1 | ||
| Tandem | 1.69 | 0.91–3.14 | 0.096 |
| Number of retrieval attempts >3 | 1.09 | 0.51–2.33 | 0.829 |
| Procedure length | 1.01 | 1.00–1.02 | 0.009 |
| Alteplase use before MT | 0.65 | 0.37–1.16 | 0.146 |
GAR: glucose-to-glycated hemoglobin ratio; NIHSS: National Institute of Health Stroke Scale; ASPECTS: Alberta Stroke Program Early CT Score; MCA: middle cerebral artery; MT: mechanical thrombectomy.
Bold values denote statistical significance at the p < 0.05 level.
Table 4.
Odds ratio (95% confidence intervals) for futile recanalization using the GAR index as categorial variable, that is, GAR quartiles.
| Futile recanalization | OR | 95% CI | p |
|---|---|---|---|
| Age | 1.04 | 1.02–1.06 | 0.001 |
| Females | 0.95 | 0.58–1.55 | 0.832 |
| Hypertension | 0.94 | 0.54–1.62 | 0.813 |
| Atrial fibrillation | 0.86 | 0.36–2.08 | 0.747 |
| Anticoagulants use | 1.14 | 0.44–2.95 | 0.784 |
| GAR quartiles | |||
| Q1 | 1 | ||
| Q2 | 1.10 | 0.59–2.05 | 0.765 |
| Q3 | 1.61 | 0.83–3.12 | 0.155 |
| Q4 | 3.58 | 1.59–8.07 | 0.002 |
| NIHSS at admission | 1.16 | 1.11–1.22 | 0.001 |
| ASPECTS | 0.85 | 0.68–1.06 | 0.159 |
| Site of occlusion | |||
| MCA | 1 | ||
| Tandem | 1.69 | 0.91–3.13 | 0.099 |
| Number of retrieval attempts >3 | 1.10 | 0.51–2.36 | 0.814 |
| Procedure length | 1.01 | 1.00–1.02 | 0.008 |
| Alteplase use before MT | 0.65 | 0.37–1.15 | 0.141 |
GAR: glucose-to-glycated hemoglobin ratio; Q1: first GAR quartile; Q2: second GAR quartile; Q3: third GAR quartile; Q4: fourth GAR quartile; NIHSS: National Institute of Health Stroke Scale; ASPECTS: Alberta Stroke Program Early CT Score; MCA: middle cerebral artery; MT: mechanical thrombectomy.
Bold values denote statistical significance at the p < 0.05 level.
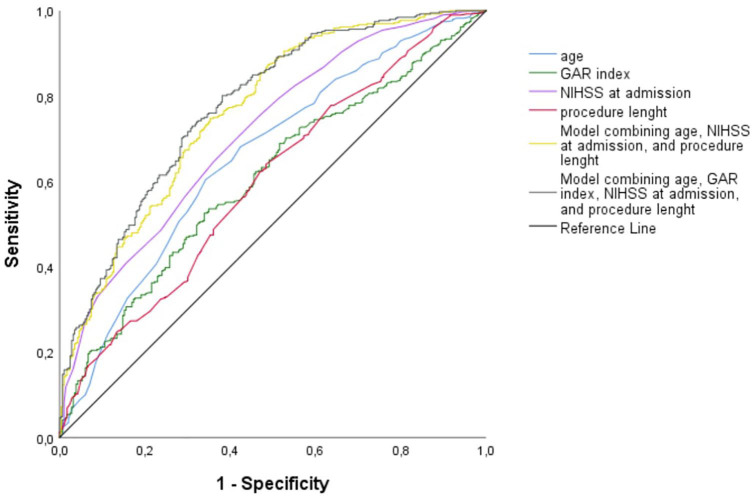
Using the ROC curves from the logistic regression analysis, we identified the predictive accuracy of age, GAR index, NIHSS at admission, procedure length, and combined models for predicting FR (see Figure 4). The AUC for age, GAR index NIHSS at admission, and procedure length were 0.66 (95% CI 0.61–0.70), 0.60 (95% CI 0.56–0.64), 0.61 (95% CI 0.56–0.65), and 0.71 (95% CI 0.67–0.75), and, respectively. The model combining age, NIHSS at admission, and procedure length had an AUC of 0.76 (95% CI 0.72–0.79). Including the GAR index in the previous model, we obtained the highest AUC (0.78, 95% CI 0.74–0.81). According to DeLong’s test, this latest model performed significantly better in predicting FR as compared to every single variable, that is, age (p < 0.001), GAR index (p < 0.001), NIHSS at admission (p < 0.001), and procedure length (p < 0.001). It also outperformed the model combining age, NIHSS at admission, and procedure length (p = 0.04).
Figure 4.
Receiver operating characteristics curves for predicting futile recanalization after mechanical thrombectomy.
GAR: glucose-to-glycated hemoglobin ratio; NIHSS: National Institute of Health Stroke Scale.
Discussion
This large multicenter study confirmed that FR is common among AIS patients with anterior LVO and successful recanalization. Stress hyperglycemia, in addition to older age, NIHSS at admission, and procedure length, is independently associated with FR.
Nie et al. 17 recently conducted a review with a focus on FR. The authors underlined that several non-modifiable and modifiable factors can predict clinically ineffective recanalization. Obviously, it is essential to pay close attention to modifiable predictors and treat them with individualized management strategies to increase the chance of favorable outcomes. In our sample, we identified four independent variables associated with FR. Two of these variables were non-modifiable, namely age and baseline NIHSS. The other two variables were modifiable: the length of the MT procedure and stress hyperglycemia.
Several previous studies reported that age plays a major role in favoring FR. The proportion of patients with FR after MT increased with age, peaking at 53% in the highest age quartile (77–94 years) in the study of Singer et al. 23 In a Chinese multicenter study, Xu et al. 24 observed that patients older than 74 had more than double the odds of FR compared to younger patients. The recent meta-analysis by Deng et al. 25 confirmed that older age is an independent predictor of FR in patients with LVO (mean difference 5.81, 95% CI 4.16–7.46, p < 0.00001). Our results are in line with the literature. We found that for every year of age increase, the odds ratio for FR was 1.04 (95% CI 1.02–1.06). Although advancing age predisposes to a higher likelihood of FR, substantial evidence supports the favorable impact of MT in patients aged 80 years and above.15,26
The severity of the stroke, measured with the NIHSS score at admission, is a non-modifiable, well-known predictor of FR in AIS patients with LVO. Shi et al. 27 analyzed data from the Multi MERCI, TREVO, and TREVO 2 trials. They observed that the odds ratio for FR increased to 1.08 (95% CI 1.02–1.15) with each one-point increase in NIHSS score at admission. Despite a slightly higher probability of FR (OR 1.16, 95% CI 1.11–1.22), our results support the findings of Shi et al. regarding baseline NIHSS. Although a severe stroke favors FR, it should not preclude MT. The therapeutic benefit of endovascular treatment increases as the stroke severity worsens. According to Lee et al., 28 patients with an NIHSS score of ⩽5 had a benefit of 0.1%, those with a score an NIHSS score of 11–20 had a benefit of 28.7%, and those with an NIHSS score of >20 had a benefit of 34.3%.
The term “golden hour” was coined by Spiotta et al. 29 to describe patients who underwent recanalization within 60 min. These patients had a significantly higher likelihood of achieving a good outcome than those with longer recanalization time (53.6% vs 30.8%; p = 0.009). Further studies confirmed the importance of reducing the duration of MT procedure to improve functional outcomes in patients with LVO.30,31 Procedural time impacts the occurrence of FR after successful recanalization in patients treated with MT. Xu et al. 24 reported a median puncture-to-recanalization time of 70 and 93 minutes in patients with favorable and futile recanalization, respectively (p < 0.001). Their logistic regression analysis revealed that subjects with a procedural time exceeding 80 min had more than double the risk of FR compared to those with a shorter duration. 24 Although with shorter procedural lengths, we confirmed that timing of recanalization is independently associated with FR. Efforts should be made to lead to faster recanalization times. The operator’s skill is essential, but standardized protocols can assist neuro-interventional physicians in shortening procedures. The protocol suggested by Frei et al. 32 reduced the puncture to recanalization time, with a significantly larger proportion of patients recanalized within 30 min of femoral artery puncture. Before implementation, 25% of patients were recanalized within 30 min of femoral artery puncture, while after implementation, 37% of patients were recanalized within 30 min (p = 0.04). One of the key actions outlined in the protocol was providing a stroke kit, which includes a micropuncture access kit, syringes, flush lines, sterile gauze, arterial line, and monitoring lines. This kit must always be readily available in the angiography suite. In addition, conscious sedation was preferred instead of general anesthesia, and neuro-interventionists adopted standardized steps for performing thrombectomy procedures.
Stress hyperglycemia is an increased blood glucose due to a sudden clinical event that returns to baseline following the acute phase. 8 Stroke patients, even those with no history of diabetes mellitus, commonly have stress hyperglycemia. 33 Although stress hyperglycemia represents a physiological phenomenon primarily driven by the hypothalamic-pituitary-adrenal axis, acute hyperglycemia, irrespective of a prior diagnosis of diabetes, may lead to an adverse prognosis after stroke. 7 The GAR index, which is one of the possible indexes for measuring stress hyperglycemia, has been associated with a poor prognosis in AIS patients who were treated with intravenous alteplase or MT.11,34 Only a small monocentric study reported a significant association between stress hyperglycemia and FR in patients with LVO. 18 This large, multicenter study confirmed that high levels of the GAR index put patients with anterior LVO at risk of clinically ineffective recanalization. This is particularly true for patients in the fourth quartile with a GAR index higher than 24.8. They have more than three times the risk of FR compared to those in the lowest quartile. Since stress hyperglycemia impairs the clinical outcome of these patients, pharmacological treatment would have a rationale for individuals with the highest levels of stress hyperglycemia. Unfortunately, randomized trials failed to demonstrate a positive effect of intensive glucose therapy on stroke prognosis. 7 The TEXAIS trial, which randomized 350 subjects with AIS to exenatide, a glucagon-like peptide-1, or standard care, has been recently published. Although exenatide significantly reduced the frequency of hyperglycemic events without causing hypoglycemia, it did not improve neurological impairment in AIS patients. 35 We wonder if the failure of all these trials might be attributed to focusing on absolute glycemic levels instead of considering stress hyperglycemia levels or glycemic variability. 36 Our results suggest adopting a new approach to monitor and manage glycemic status in AIS patients treated with MT. Vascular physicians should pay more attention to the levels of stress hyperglycemia of their patients. Furthermore, these subjects should receive pharmacological treatment not to lower the absolute glucose levels but to control high levels of stress hyperglycemia. Future clinical trials should investigate whether treating stress hyperglycemia with insulin or other drugs with a lower risk of hypoglycemic episodes can improve outcomes for AIS patients undergoing MT and potentially all patients with cerebrovascular disorders.
While our study has significant strengths, including systematic and prospective data ascertainment, a large patient cohort, and a multicenter design, it also has some limitations that must be acknowledged. The study’s retrospective nature could introduce systematic errors and biases, and the observed correlations do not necessarily prove causality.
Conclusion
FR is a common issue among patients with anterior LVO undergoing MT. Stress hyperglycemia, measured by the GAR index, predicts FR. Adding GAR to other known factors associated with FR may improve prediction accuracy. We suggest paying close attention to patients undergoing MT with a GAR index greater than 24.8, as they are at the highest risk of FR. The present study’s findings suggest investigating if managing stress hyperglycemia may improve the outcome of patients treated with MT.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from all patients or their representatives.
Ethical approval: The study conformed to the Declaration of Helsinki of the World Medical Association and was approved by the local ethics committee of Udine University Hospital (Ref. No. CEUR-2020-Os-173), which was the coordinating center.
Guarantor: GLG.
Contributorship: Conceptualization: GM; methodology: GM, MR, RO, MF, LD’A.; software: CDR, FT, AM; validation: GM, MR, RO, MF, LD’A; formal analysis: GM; investigation: FC, AT, ML, YT, SL, CG, FJ; resources: MS; data curation: GM, MR, RO, MF, LD’A; writing—original draft preparation: GM; writing—review and editing: GM; visualization: MS; supervision: SB, SS, GLG, MV.
ORCID iDs: Giovanni Merlino  https://orcid.org/0000-0002-6525-9529
https://orcid.org/0000-0002-6525-9529
Michele Romoli  https://orcid.org/0000-0001-8009-8543
https://orcid.org/0000-0001-8009-8543
Simona Sacco  https://orcid.org/0000-0003-0651-1939
https://orcid.org/0000-0003-0651-1939
Availability of data and material: The corresponding author can provide the datasets upon reasonable request.
References
- 1. Merlino G, Smeralda C, Sponza M, et al. Dynamic hyperglycemic patterns predict adverse outcomes in patients with acute ischemic stroke undergoing mechanical thrombectomy. J Clin Med 2020; 9: 1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perez-Vega C, Domingo RA, Tripathi S, et al. Influence of glucose levels on clinical outcome after mechanical thrombectomy for large-vessel occlusion: a systematic review and meta-analysis. J Neurointerv Surg 2022; 14: neurintsurg-2021-017771. [DOI] [PubMed] [Google Scholar]
- 3. Ahmed N, Dávalos A, Eriksson N, et al.; SITS Investigators. Association of admission blood glucose and outcome in patients treated with intravenous thrombolysis: results from the Safe Implementation of Treatments in Stroke International Stroke Thrombolysis Register (SITS-ISTR). Arch Neurol 2010; 67: 1123–1130. [DOI] [PubMed] [Google Scholar]
- 4. Gray CS, Hildreth AJ, Sandercock PA, et al. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK). Lancet Neurol 2007; 6: 397–406. [DOI] [PubMed] [Google Scholar]
- 5. Johnston KC, Bruno A, Pauls Q, et al. Intensive vs standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: the SHINE randomized clinical trial. JAMA 2019; 322: 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Palaiodimou L, Lioutas VA, Lambadiari V, et al. Glycemia management in acute ischemic stroke: current concepts and novel therapeutic targets. Postgrad Med 2019. ; 131: 423–437. [DOI] [PubMed] [Google Scholar]
- 7. Yao M, Hao Y, Wang T, et al. A review of stress-induced hyperglycaemia in the context of acute ischaemic stroke: definition, underlying mechanisms, and the status of insulin therapy. Front Neurol 2023; 14: 1149671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet 2009; 373: 1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang L, Zhou Z, Tian X, et al.; ACTUAL investigators. Impact of relative blood glucose changes on mortality risk of patient with acute ischemic stroke and treated with mechanical thrombectomy. J Stroke Cerebrovasc Dis 2019; 28: 213–219. [DOI] [PubMed] [Google Scholar]
- 10. Chen X, Liu Z, Miao J, et al. High stress hyperglycemia ratio predicts poor outcome after mechanical thrombectomy for ischemic stroke. J Stroke Cerebrovasc Dis 2019; 28: 1668–1673. [DOI] [PubMed] [Google Scholar]
- 11. Merlino G, Pez S, Gigli GL, et al. Stress hyperglycemia in patients with acute ischemic stroke due to large vessel occlusion undergoing mechanical thrombectomy. Front Neurol 2021; 12: 725002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang J, Dong D, Zeng Y, et al. The association between stress hyperglycemia and unfavorable outcomes in patients with anterior circulation stroke after mechanical thrombectomy. Front Aging Neurosci 2023; 14: 1071377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Happi Ngankou E, Gory B, Marnat G, et al. ; ETIS Registry Investigators. Thrombectomy complications in large vessel occlusions: incidence, predictors, and clinical impact in the ETIS registry. Stroke 2021; 52: e764–e768. [DOI] [PubMed] [Google Scholar]
- 14. Jia B, Ren Z, Mokin M, et al. ; ANGEL-ACT Study Group. Current status of endovascular treatment for acute large vessel occlusion in China: a real-world nationwide registry. Stroke 2021; 52: 1203–1212. [DOI] [PubMed] [Google Scholar]
- 15. Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 16. Jovin TG, Nogueira RG, Lansberg MG, et al. Thrombectomy for anterior circulation stroke beyond 6 h from time last known well (AURORA): a systematic review and individual patient data meta-analysis. Lancet 2022; 399: 249–258. [DOI] [PubMed] [Google Scholar]
- 17. Nie X, Leng X, Miao Z, et al. Clinically ineffective reperfusion after endovascular therapy in acute ischemic stroke. Stroke 2023; 54: 873–881. [DOI] [PubMed] [Google Scholar]
- 18. Merlino G, Pez S, Sartor R, et al. Stress hyperglycemia as a modifiable predictor of futile recanalization in patients undergoing mechanical thrombectomy for acute ischemic stroke. Front Neurol 2023; 14: 1170215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pexman JH, Barber PA, Hill MD, et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol 2001; 22: 1534–1542. [PMC free article] [PubMed] [Google Scholar]
- 20. Almekhlafi MA, Mishra S, Desai JA, et al. Not all “successful” angiographic reperfusion patients are an equal validation of a modified TICI scoring system. Interv Neuroradiol 2014; 20: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 22. Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 23. Singer OC, Haring HP, Trenkler J, et al. Age dependency of successful recanalization in anterior circulation stroke: the ENDOSTROKE study. Cerebrovasc Dis 2013; 36: 437–445. [DOI] [PubMed] [Google Scholar]
- 24. Xu H, Jia B, Huo X, et al. Predictors of futile recanalization after endovascular treatment in patients with acute ischemic stroke in a multicenter registry study. J Stroke Cerebrovasc Dis 2020; 29: 105067. [DOI] [PubMed] [Google Scholar]
- 25. Deng G, Xiao J, Yu H, et al. Predictors of futile recanalization after endovascular treatment in acute ischemic stroke: a meta-analysis. J Neurointerv Surg 2022; 14: 881–885. [DOI] [PubMed] [Google Scholar]
- 26. Meyer L, Alexandrou M, Flottmann F, et al. ; German stroke registry-endovascular treatment (GSR-ET). Endovascular treatment of very elderly patients aged ⩾90 with acute ischemic stroke. J Am Heart Assoc 2020; 9: e014447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi ZS, Liebeskind DS, Xiang B, et al. ; Multi MERCI, TREVO, and TREVO 2 Investigators. Predictors of functional dependence despite successful revascularization in large-vessel occlusion strokes. Stroke 2014; 45: 1977–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee SH, Kim BJ, Han MK, et al. Futile reperfusion and predicted therapeutic benefits after successful endovascular treatment according to initial stroke severity. BMC Neurol 2019; 19: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spiotta AM, Vargas J, Turner R, et al. The golden hour of stroke intervention: effect of thrombectomy procedural time in acute ischemic stroke on outcome. J Neurointerv Surg 2014; 6: 511–516. [DOI] [PubMed] [Google Scholar]
- 30. Wessell AP, Carvalho HDP, Le E, et al. A critical assessment of the golden hour and the impact of procedural timing in stroke thrombectomy. AJNR Am J Neuroradiol 2020; 41: 822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hassan AE, Shariff U, Saver JL, et al. Impact of procedural time on clinical and angiographic outcomes in patients with acute ischemic stroke receiving endovascular treatment. J Neurointerv Surg 2019; 11: 984–988. [DOI] [PubMed] [Google Scholar]
- 32. Frei D, McGraw C, McCarthy K, et al. A standardized neurointerventional thrombectomy protocol leads to faster recanalization times. J Neurointerv Surg 2017; 9: 1035–1040. [DOI] [PubMed] [Google Scholar]
- 33. Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 2001; 32: 2426–2432. [DOI] [PubMed] [Google Scholar]
- 34. Merlino G, Smeralda C, Gigli GL, et al. Stress hyperglycemia is predictive of worse outcome in patients with acute ischemic stroke undergoing intravenous thrombolysis. J Thromb Thrombolysis 2021; 51: 789–797. [DOI] [PubMed] [Google Scholar]
- 35. Bladin CF, Wah Cheung N, Dewey HM, et al. ; TEXAIS Investigators. Management of poststroke hyperglycemia: results of the TEXAIS randomized clinical trial. Stroke 2023; 54: 2962–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Palaiodimou L, Lioutas VA, Lambadiari V, et al. Glycemic variability of acute stroke patients and clinical outcomes: a continuous glucose monitoring study. Ther Adv Neurol Disord 2021; 14: 17562864211045876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized study data can be made available upon request from a qualified researcher.