Abstract
Background:
This analysis reports the findings from a predefined exploratory cohort (cohort B) from the ADAPT (ADvanced Hybrid Closed Loop Study in Adult Population with Type 1 Diabetes) study. Adults with type 1 diabetes (T1D) with suboptimal glucose control were randomly allocated to an advanced hybrid closed-loop (AHCL) system or multiple daily injections of insulin (MDI) plus real-time continuous glucose monitoring (RT-CGM).
Methods:
In this prospective, multicenter, exploratory, open-label, randomized controlled trial, 13 participants using MDI + RT-CGM and with HbA1c ≥8.0% were randomized to switch to AHCL (n = 8) or continue with MDI + RT-CGM (n = 5) for six months. Prespecified endpoints included the between-group difference in mean change from baseline in HbA1c, CGM-derived measures of glycemic control, and safety.
Results:
The mean HbA1c level decreased by 1.70 percentage points in the AHCL group versus a 0.60 percentage point decrease in the MDI + RT-CGM group, with a model-based treatment effect of −1.08 percentage points (95% confidence interval [CI] = −2.17 to 0.00 percentage points; P = .0508) in favor of AHCL. The percentage of time spent with sensor glucose levels between 70 and 180 mg/dL in the study phase was 73.6% in the AHCL group and 46.4% in the MDI + RT-CGM group; model-based between-group difference of 28.8 percentage points (95% CI = 12.3 to 45.3 percentage points; P = .0035). No diabetic ketoacidosis or severe hypoglycemia occurred in either group.
Conclusions:
In people with T1D with HbA1c ≥8.0%, the use of AHCL resulted in improved glycemic control relative to MDI + RT-CGM. The scale of improvement suggests that AHCL should be considered as an option for people not achieving good glycemic control on MDI + RT-CGM.
Keywords: automated insulin delivery, closed-loop system, diabetes, HbA1c, hypoglycemia, hyperglycemia, time in range
Introduction
The ADAPT (ADvanced Hybrid Closed Loop Study in Adult Population with Type 1 Diabetes) study was conducted in three countries (the United Kingdom, Germany, and France) and investigated the effect of switching people with type 1 diabetes (T1D) and HbA1c ≥8% from their current continuous glucose monitoring (CGM)-based therapy to an advanced hybrid closed-loop (AHCL) system (similar to the MiniMedTM 780G system) versus remaining on their current therapy. 1 For those participants using intermittently scanned CGM (IS-CGM) (cohort A), recently published findings demonstrated an overall between-group difference in reduction in HbA1c of 1.42 percentage points (P < .0001) and an increase in time in range (TIR) of 27.6 percentage points (P < .0001) in favor of AHCL over the 6-month study period. 2 While no participant reached defined therapeutic goals 3 at baseline, in the AHCL group specifically, 27.8% of participants achieved HbA1c <7% at 6 months, while 58.3% achieved a glucose management indicator (GMI) <7%, and 52.8% achieved TIR >70%. As these achievements were reached without compromising safety, these findings led the authors to conclude by recommending the use of automated insulin delivery systems at an early stage in the therapeutic pathway in the treatment of T1D. These findings, therefore, challenge the current recommendations from the United Kingdom and the United States, which advocate the integration of technological solutions in a stepwise manner.4,5
The MiniMed™ 780G AHCL system algorithm incorporates several advanced features such as auto basal and auto corrections, which adjust basal insulin delivery and provide corrections as required every five minutes, respectively, as well as several predictive modules for safe administration of manual meal and correction boluses. In addition to the ADAPT trial results, data from real-world settings have been extensively analyzed6,7 and have demonstrated consistent outcomes, with >70% of users reaching therapeutic goals.
The landscape of CGM devices is evolving rapidly, and while the most prevalent CGM device during the study period was IS-CGM, there is an increase in the use of real-time CGM (RT-CGM).8,9 Real-time CGM provides more streaming information as well as alerts for out-of-range glucose levels and predictive alerts based on simple extrapolation of past values. While increasing the user’s burden, it has been shown that RT-CGM has several advantages over IS-CGM, especially with regard to reducing hypoglycemia10,11 as well as a slight increase in TIR percentage points (+6.8%) and a 0.3 percentage point reduction in HbA1c. 11
To evaluate whether the use of RT-CGM by the control group would provide similar outcomes to those seen in cohort A, the ADAPT study statistical analysis plan (SAP) predefined a cohort of RT-CGM users (cohort B) to follow the same protocol but to be analyzed separately from the main confirmatory cohort of IS-CGM users (cohort A) as exploratory analyses. The outcomes from the RT-CGM exploratory cohort are presented here and are compared with the outcomes from the main confirmatory cohort.
Methods
Study Design and Oversight
The ADAPT study design is described in detail by de Portu et al. 1 In summary, it was a prospective, multicenter, open-label, randomized controlled trial consisting of a two-week run-in phase and a six-month study phase, followed by a six-month continuation phase. Two separate predefined cohorts were planned with a separate randomization for each. Cohort A was the confirmatory cohort and consisted of participants randomly allocated to continue with multiple daily injections of insulin (MDI) + IS-CGM or initiate AHCL. The primary endpoint in cohort A was the between-group difference in the mean HbA1c change from baseline to six months. The analysis of cohort A was powered for the primary endpoint (see de Portu et al 1 ). Cohort B was an independent exploratory cohort and consisted of participants randomly allocated to either continue with MDI + RT-CGM or initiate AHCL. Endpoints in cohort B were prespecified, but the analysis in cohort B was not powered to detect significant between-group differences. The protocol followed by cohort B was identical to that followed by cohort A. The findings presented here pertain exclusively to the 6-month study phase for cohort B; results for cohort A have been published separately. 2
The ADAPT study (inclusive of cohort A and cohort B) was conducted in 14 centers across three European countries (France, Germany, and the United Kingdom) and was conducted in line with the principles of the Declaration of Helsinki, good clinical practice, and local legislation in all three countries. For cohort B specifically, study participants were enrolled from a total of five participating centers across two countries (Germany [n = 11 participants] and the United Kingdom [n = 2 participants]). Approval from competent authorities and ethics committees was obtained for all study centers, and all participants provided written informed consent. The ADAPT study was registered in clinicaltrials.gov with ID NCT04235504. All authors have contributed to and reviewed the manuscript, and editorial assistance was provided by a medical writer funded by the study sponsor.
Study Participants
For inclusion, study participants were required to be aged ≥18 years, diagnosed with T1D for at least two years, in receipt of MDI for at least two years at the time of the screening visit, and have a baseline HbA1c ≥8.0% (64 mmol/mol). For cohort B specifically, participants were required to have been using RT-CGM for at least three months previously with sensor readings available for >70% of the time in the month prior to screening. Key exclusion criteria included the use of pramlintide, dipeptidyl-peptidase-4, inhibitors, glucagon-like peptide-1 agonists or mimetics, metformin, or sodium-glucose cotransporter-2 inhibitors at screening. Women of childbearing potential who were either pregnant at screening or planning to become pregnant during the study period were also excluded as were people with a history of hearing or visual impairment that would hinder the perception of glucose display and alarms and people with any unresolved skin conditions around the area of sensor placement. A full list of inclusion and exclusion criteria is provided in the article by de Portu et al. 1
Procedures
The study included a two-week run-in phase, during which participants were required to demonstrate tolerance to wearing the Guardian Sensor 3 and compliance with the blinded CGM procedure, which was performed using the Guardian Sensor 3 attached to a Guardian Link 3 transmitter (Medtronic, Northridge, CA, USA) acting as a recorder. Participants with a satisfactory run-in phase (ie, sensor worn for >70% of the time and no local reaction to the sensor) were randomly allocated (1:1 ratio) to either AHCL therapy or continuation with MDI + RT-CGM for the six-month study phase. For CGM-derived outcome measures, two-week blinded CGM periods were repeated at three and six months for those in the MDI + RT-CGM group. During these two-week periods, participants in the RT-CGM group were required to wear the Guardian Sensor 3 for the blinded data collection in addition to their usual RT-CGM device. The AHCL system used in the study was the MiniMed 670G version 4.0, an investigational system with an AHCL algorithm equivalent to that of the MiniMed 780G system, which does not include Bluetooth connectivity or have a glucose target of 110 mg/dL.
Randomization was performed electronically via case report forms and using an investigator-blinded block randomization procedure that utilized blocks of different sizes according to a sequence prepared by the study statistician. Due to the nature of the intervention, it was not possible to mask participants and treating clinicians to group assignment.
Endpoints
Prespecified endpoints in this exploratory study included the between-group difference in the mean change in HbA1c from baseline to six months; the mean percentage of time spent in the hyperglycemic (>180 mg/dL and >250 mg/dL), euglycemic (70-180 mg/dL), and hypoglycemic ranges (<70 mg/dL and <54 mg/dL); sensor glucose (SG) levels; standard deviation (SD) of SG; coefficient of variation; percentages of users reaching recommended glucose targets 3 ; sensor use and change in weight in both treatment groups; and the mean time spent in AHCL and frequency of self-monitoring of blood glucose (SMBG) in the AHCL group. Prespecified safety endpoints included the number of severe hypoglycemic (SH), diabetic ketoacidosis (DKA), serious adverse events (SAEs), device deficiencies, and (unanticipated) Serious Adverse Device Effects.
Statistical Analysis
HbA1c was measured at a centralized laboratory at three time points: baseline and at the end of months 3 and 6 and was analyzed using a repeated-measures random effects model with study group and period as factors. Sensor glucose-based endpoints were calculated at baseline and for each of the two 2-week measurement periods at the end of months 3 and 6 and analyzed with a similar repeated-measures random effects model adjusted by the baseline value of the variable associated with the endpoint as a covariate. The method of White and Thompson 12 was used to adjust for incomplete baseline measurements. Treatment effects were referred to as model-based estimates. The repeated-measures random effects model used all available data and accounted for missing data.
In this exploratory study, the between-group difference in endpoints was assessed by means of model-based 95% confidence intervals (CIs) with no adjustment for multiple statistical comparisons. P values are presented in the context of an exploratory analysis only. All effectiveness analyses were performed on an intent-to-treat basis, which included all randomized participants. All statistical analyses were performed using SAS® software version 9.4 (SAS Institute, Cary, NC, USA).
Results
Study Participants
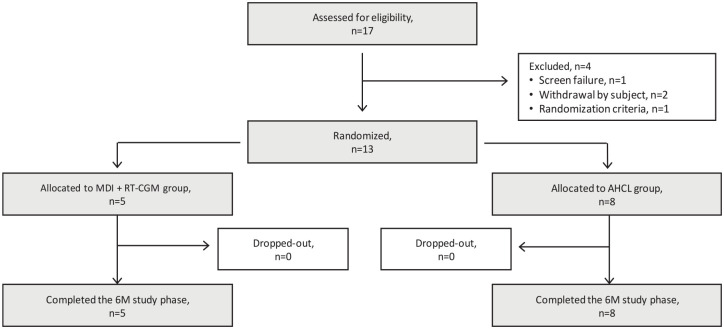
The participant flow chart including treatment allocation is shown in Figure 1. A total of 17 participants were assessed for eligibility between July 13, 2020, and March 12, 2021, 13 of whom were randomized. Eight participants were randomly allocated to the AHCL group, and five to the MDI + RT-CGM group. All participants completed the six-month study phase.
Figure 1.
Participant flow diagram.
Abbreviations: AHCL, advanced hybrid closed loop; MDI + RT-CGM, multiple daily injections of insulin + real-time continuous glucose monitoring.
Baseline characteristics according to the treatment group are summarized in Table 1. At baseline, randomized participants had a mean (SD) age of 41.8 (14.4) years, HbA1c of 9.12% (0.6%), weight of 79.4 (24.3) kg, and duration of T1D of 15.9 (9.6) years.
Table 1.
Summary of Baseline Characteristics for Randomized Participants.
| Characteristic | MDI + RT-CGM group, N = 5 | AHCL group, N = 8 | ||
|---|---|---|---|---|
| N | Value | N | Value | |
| Age (years) | ||||
| Mean | 5 | 36.2 ± 15.8 | 8 | 45.4 ± 13.3 |
| Range | 5 | 18-59 | 8 | 18-61 |
| Male, n (%) | 5 | 2 (40.0) | 8 | 5 (62.5) |
| Duration of T1D, years | 5 | 13.4 ± 8.6 | 8 | 17.4 ± 10.4 |
| Height, cm | 5 | 170.8 ± 11.3 | 7 | 174.1 ± 8.6 |
| Weight, kg | 5 | 78.8 ± 24.4 | 7 | 79.8 ± 26.2 |
| BMI, kg/m2 | 5 | 26.6 ± 5.6 | 7 | 26.1 ± 7.6 |
| HbA1c, % | 5 | 9.5 ± 0.6 | 8 | 8.9 0 ± 0.5 |
| HbA1c, mmol/mol | 5 | 79.9 ± 6.7 | 8 | 73.8 ± 5.0 |
| Insulin total daily dose, units | 5 | 79.2 ± 47.1 | 8 | 52.8 ± 29.1 |
| Sensor readings last month, % | 5 | 90.2 ± 10.1 | 8 | 93.1 ± 6.8 |
Values are mean ± SD unless otherwise stated.
Abbreviations: AHCL, advanced hybrid closed loop; BMI, body mass index; HbA1c, glycated hemoglobin; MDI + RT-CGM, multiple daily injections of insulin + real-time continuous glucose monitoring; T1D, type 1 diabetes.
During the study phase, participants who were randomly allocated to the AHCL group used the sensor for 90.3% of the time, spent 93.5% of the time in AHCL (the difference between sensor use and time in AHCL is due to the fact that algorithm-driven automation can continue for a limited time without CGM input), experienced a mean of 1.0 AHCL exits per week, and performed a mean of 3.3 SMBG measurements per day. Investigators selected the glucose target of 100 mg/dL (5.5 mmol/L) or 120 mg/dL (6.7 mmol/L) for 77.5% and 22.5% of the time, respectively, and an active insulin time of 2 hours, >2 to 3 hours, >3 to 4 hours, or >4 hours for 62.6%, 16.7%, 19.0%, and 1.7% of the time, respectively. For the MDI + RT-CGM group, participants used the sensor for a mean of 95.4% of the time during the study phase.
Efficacy
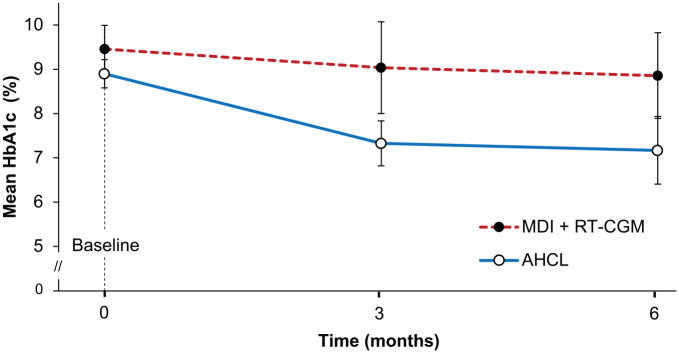
The model-based treatment effects are shown in Table 2. At six months, the mean (SD) change from baseline in HbA1c was −1.70 percentage points (1.04 percentage points) in the AHCL group and −0.60 percentage points (1.26 percentage points) in the MDI + RT-CGM group, resulting in a model-based treatment effect of −1.08 percentage points (95% CI = −2.17 to 0.00 percentage points, P = .0508) in favor of the AHCL group (Figure 2). In addition, 43% (three out of seven) of participants in the AHCL group achieved an HbA1c <7.0% at six months compared with 0% (zero out of five) in the MDI + RT-CGM group.
Table 2.
HbA1c and CGM-Derived Endpoints of Glycemic Control.
| MDI+RT-CGM group | AHCL group | Model-based treatment effect | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Run-in | Study | Run-in | Study | ||||||
| N | Value | N | Value | N | Value | N | Value | ||
| HbA1c, % | 5 | 9.46 ± 0.6 | 5 | 8.9 ± 1.1 | 8 | 8.9 ± 0.5 | 7 | 7.2 ± 1.0 | — |
| Change in HbA1c from baseline, percentage points | - | - | 5 | −0.6 ± 1.3 | - | - | 7 | −1.7 ± 1.0 | −1.1 (−2.2 to 0.0; P = .0508) |
| TIR, % | |||||||||
| >250 mg/dL (13.9 mmol/L) a | 4 | 20.7 ± 6.1 | 5 | 21.6 ± 9.3 | 7 | 22.8 ± 11.7 | 8 | 6.3 ± 5.2 | −16.0 (−26.0 to −6.2; P = .0051) |
| >180 mg/dL (>10.0 mmol/L) a | 4 | 53.9 ± 9.9 | 5 | 50.9 ± 15.6 | 7 | 55.2 ± 15.8 | 8 | 24.0 ± 13.5 | −28.5 (−45.3 to −11.6; P = .0043) |
| 70-180 mg/dL (3.9-10.0 mmol/L) a | 4 | 43.9 ± 9.6 | 5 | 46.4 ± 12.5 | 7 | 42.1± 15.3 | 8 | 73.6 ± 13.7 | 28.8 (12.3-45.3; P = .0035) |
| <70 mg/dL (3.9 mmol/L) b | 4 | 2.2 ± 2.5 | 5 | 2.7 ± 3.1 | 7 | 2.7 ± 3.0 | 8 | 2.4 ± 2.0 | −0.6 (−2.6 to 1.4; noninferiority met) |
| <54 mg/dL (3.0 mmol/L) c | 4 | 0.3 ± 0.4 | 5 | 1.0 ± 1.6 | 7 | 0.9 ± 1.4 | 8 | 0.5 ± 0.7 | −0.7 (−2.0 to 0.6; non-inferiority met) |
| Mean of SG, mg/dL | 4 | 191.8 ± 14.6 | 5 | 191.1 ± 26.4 | 7 | 194.6 ± 25.8 | 8 | 147.3 ± 20.8 | −46.9 (−73.8 to −20.0; P = .0034) |
| Standard deviation of SG, mg/dL | 4 | 70.1 ± 5.3 | 5 | 68.9 ± 7.4 | 7 | 67.2 ± 8.2 | 8 | 52.6 ± 12.7 | −16.2 (−31.1 to −1.2; P = .0372) |
| Users achieving HbA1c <7.0%, % (n/N) d | 5 | 0.0 (0/5) | 5 | 0.0 (0/5) | 8 | 0.0 (0/8) | 7 | 42.9 (3/7) | 0.0 (0.0 to 3.18; P = .2045) |
| Users achieving GMI <7.0%, % (n/N) d | 4 | 0.0 (0/4) | 5 | 20.0 (1/5) | 7 | 0.0 (0/7) | 8 | 62.5 (5/8) | 0.18 (0.0 to 3.0; P = .2657) |
| Users achieving TIR >70%, % (n/N) d | 4 | 0.0 (0/4) | 5 | 0.0 (0/5) | 7 | 0.0 (0/7) | 8 | 62.5 (5/8) | 0.0 (0.0 to 1.31; P = .0754) |
| Users achieving TBR <4%, % (n/N) d | 4 | 75.0 (3/4) | 5 | 80.0 (4/5) | 7 | 71.4 (5/7) | 8 | 75.0 (6/8) | 1.3 (0.0 to 98.0; P = .9999) |
Values are mean ± SD unless otherwise stated. The model-based treatment effect is the estimated treatment effect (AHCL group minus MDI + RT-CGM group) from the repeated-measures model.
Abbreviations: AHCL, advanced hybrid closed loop; CI, confidence interval; GMI, glucose management indicator; HbA1c, glycated hemoglobin; MDI + RT-CGM, multiple daily injections of insulin + real-time continuous glucose monitoring; SG, sensor glucose; TBR, time spent below range; TIR, time in range.
Model-based treatment effect (95% CI; P value for superiority test).
Model-based treatment effect (97.5% confidence limit; noninferiority met), noninferiority is met when the 97.5% upper confidence limit is less than the noninferiority margin of 5%.
Model-based treatment effect (97.5% confidence limit; noninferiority met), noninferiority is met when the 97.5% upper confidence limit is less than the noninferiority margin of 2%.
Post hoc analysis with odds ratio treatment effect estimates based on the Fisher exact test.
Figure 2.
Mean change in HbA1c. Error bars are 95% CIs. Figure based on available data.
Abbreviations: AHCL, advanced hybrid closed loop; CI, confidence interval; HbA1c, glycated hemoglobin; MDI + RT-CGM, multiple daily injections of insulin + real-time continuous glucose monitoring.
Participants in the AHCL group spent a significantly greater percentage of time with SG levels between 70 and 180 mg/dL (3.9-10.0 mmol/L) than those in the MDI + RT-CGM group (TIR: 73.6% and 46.4%, respectively; model-based treatment effect = 28.8 percentage points; 95% CI = 12.3 to 45.3 percentage points; P = .0035). In addition, 62.5% of participants in the AHCL group achieved a TIR >70% at six months, compared with 0% in the MDI + RT-CGM group. The mean percentage of time spent in the hyperglycemic range (time above range) was significantly lower in the AHCL group, while the mean percentage of time spent below range (TBR) was noninferior, compared with the MDI + RT-CGM group. A TBR70 of <4% was achieved by 75% of participants in the AHCL group compared with 80% in the MDI + RT-CGM group. These findings were largely replicated when the analysis was performed specifically for daytime (06:00 to 23:59) and nighttime (00:00 to 05:59), with the between-group differences being more pronounced at nighttime (Supplementary Table 1).
Mean SG levels were 147.3 mg/dL for the AHCL group compared with 191.1 mg/dL for the MDI + RT-CGM group (model-based treatment effect = −46.9 mg/dL; 95% CI = −73.8 to −20.0 mg/dL; P = .0034), corresponding to a GMI of 6.8% (0.5%) and 7.9% (0.6%), respectively (model-based treatment effect = −1.12 percentage points, 95% CI = −1.77 to −0.48 percentage points). In addition, 62.5% of participants in the AHCL group achieved a GMI <7.0% at six months, compared with 20% in the MDI + RT-CGM group.
Participants in the AHCL group had a weight increase of 0.5 kg from baseline compared with an increase of 3.1 kg in the MDI + RT-CGM group (model-based treatment effect = −2.58 kg; 95% CI = −5.5 to 0.4 kg; P = .0818). The total daily insulin dose increased by 1.7 units from baseline in the AHCL group and decreased by 0.4 units in the MDI + RT-CGM group with no between-group difference (95% CI = −12.6 to 16.8 units).
Safety
No SH or DKA events occurred in either group during the study period. A total of two SAEs occurred, of which one occurred in the run-in phase and the other during the study phase (AHCL group). Both SAEs occurred in the same participant and were related to each other but not to the device. Safety data are summarized in Supplementary Table 2.
Discussion
The findings from the exploratory cohort (cohort B) of the ADAPT study, which included users of RT-CGM, have reaffirmed the results demonstrated in the confirmatory cohort (cohort A, users of IS-CGM), by providing a reduction in HbA1c of −1.70 percentage points from baseline in the treatment group. The findings from cohort B, however, did demonstrate a lower between-group difference in mean change in HbA1c from baseline to six months compared with cohort A (−1.08 percentage points vs −1.42 percentage points), which was due to the slight reduction in HbA1c in the comparator group, not witnessed in cohort A. This may be a chance occurrence due to the small size of the cohort, although it may be secondary to the fact that RT-CGM has advantages over IS-CGM 11 and that these advantages may have been enhanced during the study phase, as a Hawthorne effect. 13 Nevertheless, irrespective of the HbA1c decrease in the control group, the between-group difference in mean change in HbA1c from baseline to six months was substantial at 1.08 percentage points; however, this was not statistically significant (95% CI = −2.17 to 0.00 percentage points; P = .0508) due to the low number of participants.
Interestingly, CGM data showed a between-group difference in TIR of 28.8 percentage points in favor of AHCL, which achieved statistical significance, even in this small number of participants. This is an equivalent of greater than six hours per day more within the target range. Time below ranges were not increased when compared with the control group and were well within international consensus targets.
Overall, we observed similar trends in improvement in glycemic control in those previously on MDI + RT-CGM and those previously on MDI + IS-CGM (cohort A). These improvements in glycemic control could potentially have important long-term effects on diabetes-related complications,14,15 as well as health economic implications, which will be discussed in a separate publication.
The analysis of cohort B was included in the SAP to compare, through an exploratory analysis, outcomes in the context of a likely situation in the future wherein a greater number of people with diabetes will be using RT-CGM. As new technologies in the treatment of diabetes continue to be introduced rapidly, 16 the ADAPT study was designed to use the comparator that was considered to be the most widely used therapy at the time (IS-CGM) while also exploring outcomes in an exploratory cohort of users of RT-CGM.
A key limitation of the study is that the cohort was small, consisting only of 13 randomized participants, and mostly limited to German study sites; therefore, the generalizability to other settings and robustness of the findings may be limited. The number of participants in cohort B was also lower than that anticipated (originally estimated at a total enrollment of 40 participants and 34 completing the study period) as enrollment for ADAPT was closed after the target sample size for cohort A was achieved. Furthermore, the study was not powered to show HbA1c reduction in a statistically significant manner. Although the between-group difference in HbA1c at the end of the study period was in excess of one percentage point, the small size of the study precludes the drawing of any definitive conclusions, and further large-scale studies are needed to confirm the findings reported here.
Conclusions
The findings from this exploratory cohort of the ADAPT study suggest that, for people with T1D, with a baseline HbA1c of ≥8.0% while on MDI + RT-CGM, the use of AHCL may confer benefits in terms of increasing TIR beyond those that can be achieved with MDI + RT-CGM. In terms of HbA1c reduction, the overall trends seen in cohort B were similar to those reported in cohort A. These findings suggest that for some people with T1D who are unable to achieve target glucose levels while on MDI + RT-CGM, the initiation of AHCL may potentially lead to improved outcomes and that the initiation of AHCL should not be delayed in people who are struggling to achieve good glycemic control on MDI + RT-CGM. Further studies are needed to confirm the findings of this exploratory small-scale study.
Supplemental Material
Supplemental material, sj-docx-1-dst-10.1177_19322968231161320 for Advanced Hybrid Closed Loop in Adult Population With Type 1 Diabetes: A Substudy From the ADAPT Randomized Controlled Trial in Users of Real-Time Continuous Glucose Monitoring by Tim van den Heuvel, Ralf Kolassa, Winfried Keuthage, Jens Kroeger, Roseline Ré, Simona de Portu, Linda Vorrink, John Shin, Javier Castañeda, Robert Vigersky and Ohad Cohen in Journal of Diabetes Science and Technology
Footnotes
Abbreviations: AHCL, advanced hybrid closed loop; AID, automated insulin delivery; CGM, continuous glucose monitoring; CI, confidence interval; GMI, glucose management indicator; IS-CGM, intermittently scanned continuous glucose monitoring; MDI, multiple daily injections of insulin; RT-CGM, real-time continuous glucose monitoring; SAP, statistical analysis plan; SD, standard deviation; SG, sensor glucose; SMBG, self-monitoring of blood glucose; T1D, type 1 diabetes; TAR, time above range; TBR, time below range; TIR, time in range.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: WK has received speaker fees from Medtronic and support for travel and attending ADAPT study meetings from Medtronic. JK has received payment and honoraria for lectures and presentations from Medtronic. RR, SdP, LV, JS, JC, TvdH, RV and OC are current employees and shareholders of Medtronic. RK declares no competing interests.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this study was provided by Medtronic International Trading Sàrl. The study was funded by Medtronic (Medtronic International Trading Sàrl [Tolochenaz, Switzerland]). Editorial support was provided by Jayne Smith-Palmer from Covalence Research Ltd and funded by Medtronic. ClinicalTrials.gov number NCT04235504.
ORCID iDs: Tim van den Heuvel  https://orcid.org/0000-0002-3907-6876
https://orcid.org/0000-0002-3907-6876
Robert Vigersky  https://orcid.org/0000-0002-3546-3385
https://orcid.org/0000-0002-3546-3385
Supplemental Material: Supplemental material for this article is available online.
References
- 1. de Portu S, Vorrink L, Re R, et al. Randomised controlled trial of advanced hybrid closed loop in an adult population with type 1 diabetes (ADAPT): study protocol and rationale. BMJ Open. 2022;12(2):e050635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Choudhary P, Kolassa R, Keuthage W, et al. ; ADAPT study Group. Advanced hybrid closed loop therapy versus conventional treatment in adults with type 1 diabetes (ADAPT): a randomised controlled study. Lancet Diabetes Endocrinol. 2022;10:720-731. [DOI] [PubMed] [Google Scholar]
- 3. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choudhary P, Campbell F, Joule N, Kar P; Diabetes UK. A Type 1 diabetes technology pathway: consensus statement for the use of technology in Type 1 diabetes. Diabet Med. 2019;36(5):531-538. [DOI] [PubMed] [Google Scholar]
- 5. Grunberger G, Sherr J, Allende M, et al. American Association of Clinical endocrinology clinical practice guideline: the use of advanced technology in the management of persons with diabetes mellitus. Endocr Pract. 2021;27(6):505-537. [DOI] [PubMed] [Google Scholar]
- 6. Silva JD, Lepore G, Battelino T, et al. Real-world performance of the MiniMed™ 780G system: first report of outcomes from 4120 users. Diabetes Technol Ther. 2022;24(2):113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castañeda J, Mathieu C, Aanstoot HJ, et al. Predictors of time in target glucose range in real-world users of the MiniMed 780G system. Diabetes Obes Metab. 2022;24:2212-2221. doi: 10.1111/dom.14807. [DOI] [PubMed] [Google Scholar]
- 8. The continuous glucose monitors market grows rapidly due to fast customer adoption. Medical Device Network. November 18, 2021. https://www.medicaldevice-network.com/comment/continuous-glucose-monitors-market-customer-adoption/. Accessed September 20, 2022.
- 9. Bruttomesso D, Laviola L, Avogaro A, et al. ; of the Italian Diabetes Society (SID). The use of real time continuous glucose monitoring or flash glucose monitoring in the management of diabetes: a consensus view of Italian diabetes experts using the Delphi method. Nutr Metab Cardiovasc Dis. 2019;29(5):421-431. [DOI] [PubMed] [Google Scholar]
- 10. Reddy M, Jugnee N, El Laboudi A, Spanudakis E, Anantharaja S, Oliver N. A randomized controlled pilot study of continuous glucose monitoring and flash glucose monitoring in people with Type 1 diabetes and impaired awareness of hypoglycaemia. Diabet Med. 2018;35(4):483-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Visser MM, Charleer S, Fieuws S, et al. Comparing real-time and intermittently scanned continuous glucose monitoring in adults with type 1 diabetes (ALERTT1): a 6-month, prospective, multicentre, randomised controlled trial. Lancet. 2021;397(10291):2275-2283. [DOI] [PubMed] [Google Scholar]
- 12. White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med. 2005;24(7):993-1007. [DOI] [PubMed] [Google Scholar]
- 13. McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67(3):267-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care. 2016;39(5):686-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boyko EJ, Zelnick LR, Braffett BH, et al. Risk of foot ulcer and lower-extremity amputation among participants in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2022;45(2):357-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zimmerman C, Albanese-O’Neill A, Haller MJ. Advances in type 1 diabetes technology over the last decade. Eur Endocrinol. 2019;15(2):70-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dst-10.1177_19322968231161320 for Advanced Hybrid Closed Loop in Adult Population With Type 1 Diabetes: A Substudy From the ADAPT Randomized Controlled Trial in Users of Real-Time Continuous Glucose Monitoring by Tim van den Heuvel, Ralf Kolassa, Winfried Keuthage, Jens Kroeger, Roseline Ré, Simona de Portu, Linda Vorrink, John Shin, Javier Castañeda, Robert Vigersky and Ohad Cohen in Journal of Diabetes Science and Technology