Abstract
Gestational diabetes mellitus (GDM) is a common metabolic disease of pregnancy that threatens the health of several million women and their offspring. The highest prevalence of GDM is seen in women of low socioeconomic status. Women with GDM are at increased risk of adverse maternal outcomes, including increased rates of Cesarean section delivery, preeclampsia, perineal tears, and postpartum hemorrhage. However, of even greater concern is the increased risk to the fetus and long-term health of the child due to elevated glycemia during pregnancy. Although the use of continuous glucose monitoring (CGM) has been shown to reduce the incidence of maternal and fetal complications in pregnant women with type 1 diabetes and type 2 diabetes, most state Medicaid programs do not cover CGM for women with GDM. This article reviews current statistics relevant to the incidence and costs of GDM among Medicaid beneficiaries, summarizes key findings from pregnancy studies using CGM, and presents a rationale for expanding and standardizing CGM coverage for GDM within state Medicaid populations.
Keywords: gestational diabetes, GDM, Medicaid, continuous glucose monitoring, CGM, Centers for Medicare & Medicaid Services, insurance coverage, type 1 diabetes, type 2 diabetes
Introduction
Gestational diabetes mellitus (GDM) is a common metabolic disease of pregnancy that threatens the health of several million women and their offspring worldwide.1 -10 In 2020, the proportion of women with GDM in the United States was estimated at 7.8%, 11 a 13% increase from 6.9% in 2019. With the growing incidence of obesity in the United States, the number of women who develop GDM will continue to increase. 12
Gestational diabetes mellitus is defined as glucose intolerance diagnosed through formal testing during pregnancy of variable degree and is generally diagnosed between the 24th and 28th week of gestation. However, it can present earlier in pregnancy. 13 Upon receiving a diagnosis of GDM, women must immediately receive nutritional counseling, be warned about the risks of higher blood glucose levels, and informed that they will need to sustain even tighter glycemic targets than outside of pregnancy. This will require more frequent obstetrical visits and more stringent fetal monitoring. Although women with GDM are at increased risk of adverse maternal outcomes, of even greater concern is the increased risk to the immediate and long-term health of the child due to elevated glycemia during pregnancy.7,14,15
Importantly, the incidence and complications of GDM are disproportionally represented across racial/ethnic populations within the Medicaid population. The proportion of women with GDM who require Cesarean section (C-section) delivery is notably higher among black women (45.5%) than among white (41.3%) and Hispanic (40.6%) women. 16 Similar disparities exist in the proportion of black women (17.5%) whose newborns require neonatal intensive care unit (NICU) care compared with white (13.1%) and Hispanic (12.8%) women. Many of the women with GDM are covered by Medicaid, the public health insurance program for people with low income in the United States. As reported by the Kaiser Family Foundation, approximately one in five Americans are covered by Medicaid.17,18 Black (32.0%) and Hispanic (30.0%) beneficiaries comprise the largest percentage of the Medicaid population. 18
Frequent glucose monitoring of both fasting and postprandial glucose is essential for GDM management and avoiding these complications.19 -21 However, the reliability of and adherence to traditional blood glucose monitoring (BGM) are often challenging. As demonstrated in a study of women with GDM, 23.1% of participants had <90% matched values in their diary and meter memory, and 38.5% were considered nonadherent to their prescribed testing regimen. 22 Investigators found that poor adherence compared with good adherence (≥80% of required postprandial testing) was associated with a higher incidence of preeclampsia (12.2 vs 1.9%, respectively, P = .049), and inadequate postprandial test timing with a greater maternal hyperglycemia level as demonstrated by a higher glycated hemoglobin (HbA1c) level at delivery (5.4 ± 0.4% vs 5.0 ± 0.3%, P < .01) despite more frequent insulin treatment. This supports the importance of the need for complete maternal glucose information to help both clinicians and patients to achieve the most optimal pregnancy outcomes.
Over the past five years, a growing number of individuals with type 1 diabetes (T1D) and type 2 diabetes (T2D) have transitioned from BGM to continuous glucose monitoring (CGM) as their preferred method for daily glucose monitoring and management. As demonstrated in recent studies, the use of CGM has been shown to lower HbA1c,23 -34 reduce severe hypoglycemia events,29,30,35 increase time within the target glucose range,25 -27,33,36,37 and reduce time below range (hypoglycemia).26,27,38,39
Unlike BGM, the use of CGM presents a continuous stream of glucose data in real time and graphical formats, providing a more comprehensive overview of glucose excursions that enable users to make more informed therapy decisions. Importantly, current CGM systems display arrows indicating the rate of change, direction, and velocity of glucose excursions. This feature enables users to mitigate or prevent acute hypoglycemia and alter lifestyle and medications to reduce acute and chronic hyperglycemia.
Emerging evidence supports the efficacy and safety of CGM in improving GDM outcomes.40 -44 However, most state Medicaid programs do not provide CGM coverage for women with GDM. In this article, we review some of the literature supporting the use of CGM in pregnancy complicated by T1D, T2D, and GDM and discuss the complications and cost outcomes associated with GDM among Medicaid beneficiaries and explore how CGM coverage can result in improvements in clinical outcomes and cost reductions within this population.
CGM Use in T1D and Insulin-Treated T2D
A recent systematic review and meta-analysis by Chang et al 45 assessed the effects of CGM on maternal and neonatal outcomes in women with diabetes. A total of 10 randomized controlled trials, involving 1358 perinatal participants, were included in the analysis. Results showed that that CGM significantly improved HbA1c levels, lowered C-section rates, and reduced neonatal birth weight when compared to the BGM.
The CONCEPTT study was a multicenter, open-label, randomized, controlled trial that randomly assigned 325 women (pregnant n = 215; planning pregnancy, n = 110) to CGM or BGM and followed up the participants throughout their pregnancy. 46 A small but statistically significant between-group difference in HbA1c was observed (−0.19%, P = .0207), and CGM versus BGM users spent more time in the target range (68% vs 61%, P = .034) and less time above (27% vs 32%, P = .0279) and below the range (3% vs 4%, P = .10). Among pregnant patients (CGM, n = 100; BGM, n = 102), investigators observed significant improvements. Despite what might be considered a modest glycemic benefit in the mother, neonatal outcomes were substantially improved. The use of CGM versus BGM resulted in a lower incidence of large for gestational age (LGA) (53 vs 69, odds ratio [OR] 0.51, P = .0210), fewer incidences of neonatal hypoglycemia (15 vs 28, OR 0.45, P = .0250), fewer neonatal intensive care admissions lasting more than 24 hours (27 vs 43, OR 0.45, P = .0157), and 1-day shorter length of hospital stay (P = .0091). The number of C-section deliveries within the CGM was less than that observed in the BGM group but was not statistically significant (63 vs 74, P = .18).
Using maternal CGM results and neonatal outcome data combined for singleton pregnancies from both CONCEPTT 46 and the Swedish T1D Observational Trial, 47 benchmarks were established for CGM mean blood glucose level and time above the pregnancy range that prevented LGA births. 48 An earlier randomized trial by Murphy et al 49 assessed the use of CGM versus BGM during pregnancy in 71 women with T1D (n = 46) or T2D (n = 25). The intervention group used CGM intermittently for up to seven days at intervals of four to six weeks between 8 and 32 weeks of gestation. The use of CGM versus BGM was associated with significantly lower HbA1c levels (5.8% vs 6.4%, P = .007), notably lower birth weight (3340 g vs 3630 g, P = .07), and incidence of macrosomia (35 vs 60, P = .05). In a more recent randomized controlled trial involving pregnant women with T1D, Murphy et al 50 showed a reduction in NICU admission (27% vs 43%, P = .016) and the mean length of NICU stay (6.6 vs 9 days) in women using CGM compared with BGM. Analysis of the cost of CGM during pregnancy in women with T1D has shown a reduction in pregnancy-related costs based on the cost savings from improved fetal outcomes.50,51
CGM Use in GDM
Although the use of CGM in women with GDM has not been well studied, emerging evidence supports the efficacy and safety of CGM in improving glycemic control, 40 controlling maternal weight gain, 41 reducing the maternal and fetal complications of GDM, 40 and identifying patients who would benefit from closer glucose monitoring42,43 and provides insights regarding glycemic variability and nocturnal hyperglycemia. 44
In a randomized controlled trial, Wei et al 41 investigated the effects of CGM versus BGM in 106 women with GDM on maternal and neonatal outcomes. Following initial randomization to BGM or CGM, patients in the CGM group were then allocated to early and late subgroups in which patients in the early subgroup wore their CGM devices during gestational weeks 24 to 28, and patients in the late subgroup wore their devices during gestational weeks 28 to 36. Table 1 presents key findings from the study.
Table 1.
Major Outcomes Reported: CGM Versus BGM. 41
| Outcome | BGM | CGM | P value |
|---|---|---|---|
| C-section delivery, n (%) | 38 (69) | 31 (60) | .370 |
| Birth weight (g) | 3451.09 ± 514.05 | 3275.88 ± 519.72 | .084 |
| Apgar 5 minutes | 9.49 ± 0.50 | 9.40 ± 0.56 | .39 |
| Macrosomia, n (%) | 7 (12.7) | 4 (7.8) | .410 |
| LGA, n (%) | 29 (52.7) | 18 (35.3) | .071 |
| Neonatal hypoglycemia, n (%) | 7 (12.7) | 4 (7.8) | .410 |
| Gestational weight gain, kg (SD) | 13.56 ± 2.81 | 14.75 ± 2.91 | .004 |
Abbreviations: CGM, continuous glucose monitoring; BGM, blood glucose monitoring; LGA, large for gestational age (≥90th percentile).
Although notable clinical improvements in neonatal outcomes were observed, particularly in fewer C-section deliveries, fewer LGA births, and less gestational weight gain, the between-group differences did not reach statistical significance. This may be due to the relatively short duration of CGM use (four weeks in each CGM subgroup), which could be considered a limitation of the study. Given that glycemic variability was significantly higher in the late CGM group compared with that in the early CGM group (P = .046), it is reasonable to suggest that if CGM had been initiated when GDM was first diagnosed and used throughout the remaining gestational period without interruption, both clinically and statistically significant differences in neonatal outcomes would have been observed.
In a prospective cohort study of 340 women with GDM, Chinese pregnant women were allocated to either routine care with BGM (n = 190) or the CGM (n = 150). 40 Continuous glucose monitoring was performed during the first and fifth week of the study. Investigators reported significantly better glycemic control in CGM versus BGM users during week five, with lower glycemic variability as assessed by the mean amplitude of glycemic excursions (MAGE: 1.8 ± 0.6 mmol/L vs 2.4 ± 0.9, P < .001). Importantly, glucose monitoring with CGM resulted in a reduction in primary cesarean delivery compared to BGM (34.7% vs 46.6%, P = .028), with reduced risk of preeclampsia, LGA, and macrosomia (all P < .05). The MAGE score was found to be an independent factor for preeclampsia and composite neonatal outcomes.
In a recent randomized trial by Zhang et al, 52 investigators assessed the impact of CGM versus BGM in a cohort of 110 GDM patients. Patients were randomized to CGM in combination with BGM (CGM + BGM) or BGM alone. 52 The incidence of hypoglycemia, the qualified rate of weight gain at the end of pregnancy, compliance with BGM, and health behavior patterns were compared between the two groups. A total of three cases (5.45%) of hypoglycemia were detected with CGM + BGM, while there were 12 cases (21.82%) with BGM alone, P = .012. Significantly more CGM + BGM users achieved the qualified weight gain than BGM-alone users (90.91% vs 70.91%, P = .008), and compliance with glucose monitoring was significantly higher with CGM + BGM than with BGM alone (94.55% vs 74.55%, P = .004). Importantly, CGM + BGM users demonstrated significantly better overall improvements in health behaviors (eg, glucose monitoring, diet control, weight, exercise, obstetric checkups) than BGM-alone users: P = .000, P = .008, P = .002, P = .006, and P = .019, respectively.
A possible explanation for the less robust benefit of CGM in GDM than in other types of diabetes is that the pregnant women enrolled in the studies were overall well controlled and adherent to fingerstick testing, and adverse events were low. Additionally, because of the short duration of CGM use during pregnancy (four-eight weeks), it is likely that the women included in the studies lacked prior experience using CGM; achieving optimal outcomes requires patients to correctly interpret and act upon sensor values. Nevertheless, results from the studies strongly suggest significant benefits of CGM use in reducing both maternal and neonatal adverse events, consistent with demonstrated benefits in other forms of diabetes during pregnancy.
Neonatal Risks With GDM Do Not Differ From Other Types of Diabetes
Although differences in etiologies and treatment of T1D, T2D, and GDM impact the challenges associated with achieving and maintaining optimal glycemic control within these populations, many of the maternal outcomes associated with hyperglycemia are relatively similar. For example, although similar rates of C-section delivery and NICU admissions have been reported in T1D and GDM women (31.7% 53 vs 35.3% 54 and 8.7% 55 vs 9.1%, 56 respectively), severe hypoglycemia in T1D women has been reported as high as 45% 57 but rarely in women with GDM.
However, the neonatal complications resulting from elevated glycemia during pregnancy are universal. 58 Although women with GDM have an increased risk of numerous complications of pregnancy, including an immediate increased risk of C-section delivery, preeclampsia, instrumental delivery, perineal tears, shoulder dystocia, and postpartum hemorrhage,3 -10,14,15,59 -61 the neonatal outcomes attributed to maternal hyperglycemia can be even more severe and life-threatening.59 -61 These include but not limited to macrosomia, increased NICU stays, polyhydramnios, delayed fetal lung maturity, hyperbilirubinemia, and most tragically, an increased risk of neonatal demise. As reported in a recent systematic review and meta-analysis of 156 studies, numerous adverse outcomes were identified (Table 2).
Table 2.
| Short-term risks | Marker of enhanced long-term risk | |
|---|---|---|
| Neonatal | Stillbirth/neonatal death | Metabolic syndrome |
| Congenital malformations if hyperglycemia present in first trimester | Hyperinsulinemia | |
| Macrosomia | Childhood obesity | |
| Cardiomyopathy | Possible earlier-onset cardiovascular disease | |
| Birth trauma | Autism spectrum disorder | |
| Neonatal hypoglycemia | ||
| Respiratory distress syndrome | ||
| Hyperbilirubinemia |
Clinical Impact and Cost of GDM Within the Medicaid Population
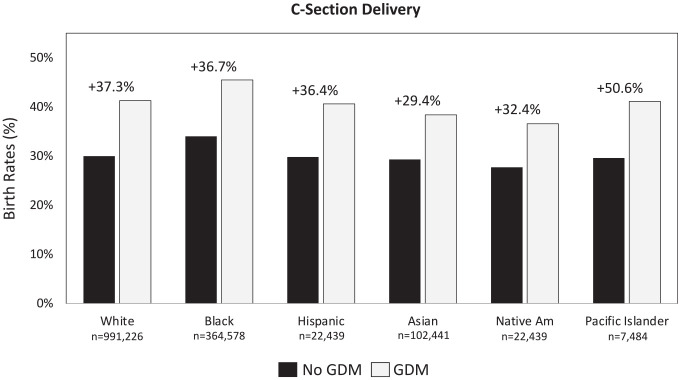
In 2020, 42% of all births in the United States were covered by Medicaid. 62 Although there are fewer women in the Medicaid population with GDM (n = 122 463) than without GDM (n = 1 365 281), the incidence of C-section deliveries is 35.7% greater among those with GDM (42.0% vs 30.9%, respectively), with the greatest increase among Pacific Islanders/Native Hawaiians. 16 A breakout of the incidence rates by race/ethnicity is presented in Figure 1.
Figure 1.
Rates of C-section deliveries by race/ethnicity. 16 Abbreviation: GDM, gestational diabetes mellitus.
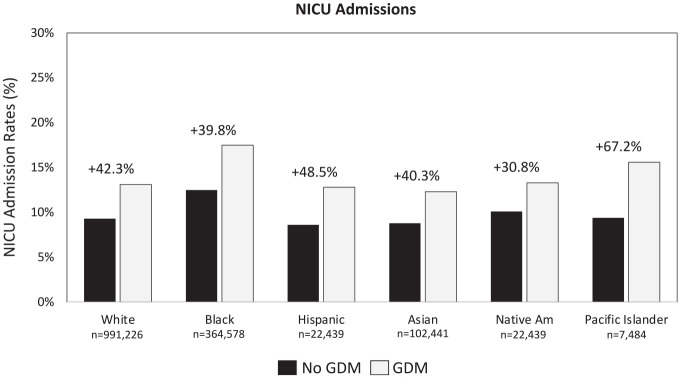
The overall incidence of NICU admissions among GDM beneficiaries is 38.8% higher than that among non-GDM beneficiaries (14.0% vs 10.1%, respectively), with the greatest relative increase among Pacific Islanders/Native Hawaiians 16 (Figure 2).
Figure 2.
Rates of NICU admissions by race/ethnicity. 14 Abbreviations: NICU, neonatal intensive care unit; GDM, gestational diabetes mellitus.
The percentage of neonates requiring assisted ventilation is notably higher among women with GDM than that among non-GDM women (7.34% vs 5.14, respectively). The difference is even more significant comparing GDM neonates requiring >6 hours of assisted ventilation with those with no GDM (2.69% vs 1.86%, respectively). The percentage of intensive care unit admissions among GDM women is also notably higher than that among non-GDM women (27% vs 19%).
The Cost of GDM Complications
Medicaid payments were approximately 50% higher for C-section deliveries than for vaginal births 63 (Table 3). Payments for NICU admissions were notably higher for infants delivered by C-section than by vaginal delivery.
Table 3.
Medicaid Payments for GDM C-Section Deliveries and NICU Admissions. 63
| Delivery method | Vaginal delivery | C-section delivery | Difference |
|---|---|---|---|
| Medicaid, $ | $10 493.13 | $15 617.31 | $5124.18 |
| Neonatal care | No NICU | NICU | Difference |
| Medicaid, $ | $5451.69 | $7361.80 | $1910.11 |
All payments are based on 2010 dollars adjusted for inflation measured via the Medicare Economic Index (MEI).
Abbreviations: NICU, neonatal intensive care unit; GDM, gestational diabetes mellitus.
Rationale for CGM Use in All Pregnancies Complicated by Diabetes
In order to reduce the excess costs of pregnancies in GDM, it is critical to focus on improving gestational glycemia to improve neonatal outcomes, including LGA, which are associated with increased C-section delivery rates and birth trauma risk. Recent meta-analyses and systematic reviews have found associations between elevated glycemia and neonatal outcomes in all pregnancies complicated by diabetes, regardless of diabetes type.15,64,65 The only difference observed in maternal outcomes was worsening of diabetes-related microvascular complications in women with T1D and T2D. 64 Therefore, given that the use of CGM has been shown to improve glycemic outcomes in women with GDM, 40 it is reasonable to assume that appropriate use of CGM can be beneficial within the larger overall GDM population in addition to reducing self-care burden.
Recognition of CGM Benefits and Utility in Diabetes-Complication Pregnancy
Current recommendations
Because the use of CGM during pregnancy in T1D pregestational diabetes improves maternal and fetal outcomes,43,46,48 the use of CGM during pregnancy in women with T1D is now advised by the American Diabetes Association. 66 The American Association of Clinical Endocrinologists recommends CGM for pregnant individuals with T1D and T2D treated with intensive insulin therapy and for women with GDM on insulin therapy and states CGM may be recommended for women with GDM who are not on insulin therapy. 67
Based on a technology appraisal from the UK National Health Service Wales, which showed that CGM use improves glycemic control, reduces the incidence of preeclampsia, reduces neonatal hypoglycemia, and reduces admission to and duration of stay in neonatal intensive care, 68 the National Institute for Health and Clinical Excellence recommends that CGM be offered to all pregnant women with T1D and that clinicians should consider CGM use in women with problematic hypoglycemia and/or unstable glycemia who are treated with insulin but do not have T1D. 69
Estimated cost savings associated with CGM
An increasing number of private insurers now provide CGM coverage for all pregnancies complicated by diabetes, including GDM (eg, United Health).47,48 However, most state Medicaid programs do not provide coverage for women with GDM despite the maternal and neonatal benefits demonstrated in this population. 18 Unfortunately, these states do not consider the potential cost savings associated with CGM use. The calculations presented in Table 4 are based on the following inputs: 3 605 201 total births in the United States, 2020; 70 7.8% GDM pregnancies; 11 35% C-section rate in GDM; 54 9.0% NICU admission rate; 56 and costs per service (C-section, $15 617.31; NICU admission, $7361.80). 63
Table 4.
Potential Cost Savings Associated With Reduction in C-Section Deliveries and NICU Admissions.
| Total GDM births in the United States, n = 281 206 | C-section | NICU |
|---|---|---|
| Rate (%) in GDM | 35% of the GDM population | 6.7%-11.7%, an average 9.2% in the GDM population |
| Estimated number of cases in GDM, n | 281 206 × 0.35 = 98 422 | 281 206 × 0.09 = 25 309 |
| Expected number of cases with monitoring (considering 5% reduction), n | 93 501 | 24 044 |
| Cost per service** | $15 617.31 | $7361.80 |
| Cost of the estimated number of cases | $1 537 086 884.82 | $186 319 796.20 |
| Cost of the expected number of cases (5% reduction) | $1 460 234 102.31 | $177 007 119.20 |
| Estimated cost savings | $76 852 782.51 | $9 312 677.00 |
Abbreviations: NICU, neonatal intensive care unit; GDM, gestational diabetes mellitus. ** Costs derived from Medicaid data.
As shown here, a 5% reduction in C-section delivery and NICU admissions could result in a total cost savings of $86 165 459.51 based on Medicaid payments. However, there are several limitations that must be noted. Our analysis does not factor in the incremental costs associated with CGM versus BGM, which our investigations found to be as high as $635.00 per GDM patient over an average of 10 weeks of sensor wear.71,72 This would result in a net cost increase of $92 400 350 with CGM use. While this appears to negate any cost advantage over BGM, it does not take into account other factors that would impact cost. For example, we know that adherence to BGM among many GDM patients is suboptimal, 22 which is a key driver of poor maternal and neonatal outcomes because neither patients nor clinicians have adequate glucose data to effectively manage glycemia. Although additional studies are needed to determine the efficacy of CGM use in GDM, we do know that the use of this technology does provide the necessary data for patients and their clinicians to make informed therapy decisions. As such, we can infer that outcomes would improve without quantifying these improvements.
Additionally, our analysis does not take into consideration the potential savings that would be realized by reducing other acute complications of poor glycemic control. Nor can we determine the degree to which CGM would reduce the risk of long-term complications, which include recurrence of GDM, increased risk of T2D in the mother and child, cardiovascular disease, chronic kidney disease, and nonalcoholic fatty liver disease.59 -61 Despite these limitations, our analysis demonstrates that an investment in CGM as a standard-of-care option for managing GDM is expected to result in major incremental clinical and quality-of-life benefits and a reduction in the costs of disease complications.
Discussion
Numerous studies have shown strong associations between suboptimal glycemic control and adverse maternal and neonatal outcomes in all pregnancies complicated by diabetes.3 -10,14,15,59 -61,64,65 Although the neonatal outcomes of suboptimal glycemic control are universal,15,59 -61 the maternal outcomes can vary due to differences in etiologies of the various types of diabetes. However, the increased frequency of C-section delivery, often due to macrosomia, is common to all types. 73
Frequent glucose monitoring is essential to avoid these complications.19 -21 However, adherence to prescribed glucose monitoring with traditional fingerstick testing is often suboptimal. 22 During the past five years, increasing numbers of individuals with T1D and T2D have adopted the use of CGM for their daily self-management. Findings from early and recent studies support the use of CGM in pregnancy-complicated diabetes cases within these populations,45,46,49 and a growing body of evidence is demonstrating the efficacy and safety of CGM use in women with GDM.40,41,52
Although the use of CGM is now recommended in pregnant women with T1D,66,68,69 most state Medicaid programs do not provide CGM coverage for women with GDM. 18 This lack of coverage imposes not only significant health risks on affected beneficiaries and their offspring but places additional economic burden on public insurers.
As reported here, the incidence of C-section deliveries and rates of NICU admissions are significantly higher among Medicaid beneficiaries with GDM, especially among black women, 16 with a significant increase (~50%) in associated costs 63 compared with beneficiaries with no GDM. Given the notable reductions in C-section deliveries observed in both the CONCEPTT trial 46 and the study by Wei et al 41 (14% and 13%, respectively), the potential cost savings of providing CGM coverage for GDM within the Medicaid population should not be discounted.
In 2020, the overall rate of GDM in the United States was 7.8 per 100 births, an increase of 30% from 2016. 11 The prevalence of GDM is highest among women with low socioeconomic status regardless of race/ethnicity. 74 According to birth certificate data from the Centers for Disease Control and Prevention, Medicaid covered approximately 50% of births nationwide in 2020, with significant variability among states. 16
Given the costs associated with poorly controlled GDM and the demonstrated efficacy of CGM in improving glycemic control, 40 reducing the risk of preeclampsia and C-section delivery, 40 and lowering the incidence of macrosomia, 43 with less maternal weight gain, 41 it is both ethical and fiscally responsible that state Medicaid programs provide and standardize CGM coverage for all women with diabetes who are at risk of poor clinical outcomes. From a policy perspective, state Medicaid programs should pay particular attention to the racial disparities in maternal and neonatal complications and costs. Large randomized controlled trials that include a cost-benefit analysis are needed to more fully elucidate the clinical and economic impacts of CGM use in GDM patients.
Acknowledgments
The authors wish to thank Hamza Alshannaq, MD, MPH, Greg Norman, PhD, and Sabrina Ilham, PharmD, all employees of Dexcom, Inc., for their thoughtful work in developing the cost savings analysis.
Footnotes
Abbreviations: BGM, blood glucose monitoring; CGM, continuous glucose monitoring; GDM, gestational diabetes mellitus; HbA1c, glycated hemoglobin; LGA, large for gestational age; MAGE, mean amplitude of glucose excursion; NICU, neonatal intensive care unit; OR, odds ratio; T1D; type 1 diabetes; T2D, type 2 diabetes.
Author Contributions: C.J.L., R.J.G., C.G.P., and N.B.A. wrote the manuscript. J.G. performed the statistical analysis. All authors reviewed the draft, provided input on the final draft, and approved its submission.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: R.J.G. has received unrestricted research support to Emory for investigator-initiated studies from Novo Nordisk and Dexcom, Inc. and consulting fees from Abbott Diabetes Care, Sanofi, Novo Nordisk, Eli Lilly, BI, and Valeritas. R.J.G. is partially supported by research grants from NIH/NIDDK P30DK11102 and 1K23DK123384-01. C.J.L. has received research support from Abbott Diabetes, Dexcom, Insulet, Senseonics, Insulet, Tandem, NIH, JDRF, and Helmsley Foundation paid to her institution; nonfinancial device support from Dexcom and Abbott Diabetes; and service as a consultant for Sanofi, Eli Lilly, and Dexcom. J.G. worked as a paid intern at Dexcom when this manuscript was developed. N.B.A. has consulted or been on advisory boards for Eli Lilly Diabetes, Dexcom, DiabeLoop, ConvaTec, and Senseonics and served on the speakers’ bureaus for Boehringer-Ingelheim, Dexcom, Eli Lilly Diabetes, MannKind, Novo Nordisk, Xeris, and Zealand Pharmaceuticals.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dexcom, Inc., provided funding for editorial assistance in developing this article.
ORCID iDs: Rodolfo J. Galindo  https://orcid.org/0000-0002-9295-3225
https://orcid.org/0000-0002-9295-3225
Christopher G. Parkin  https://orcid.org/0000-0001-6838-5355
https://orcid.org/0000-0001-6838-5355
Nicholas B. Argento  https://orcid.org/0000-0002-1207-3077
https://orcid.org/0000-0002-1207-3077
References
- 1. Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16:7. doi: 10.1007/s11892-015-0699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saravanan P; Diabetes in Pregnancy Working Group, Maternal Medicine Clinical Study Group, Royal College of Obstetricians and Gynaecologists, UK. Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 2020;8:793-800. doi: 10.1016/S2213-8587(20)30161-3. [DOI] [PubMed] [Google Scholar]
- 3. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5:47. doi: 10.1038/s41572-019-0098-8. [DOI] [PubMed] [Google Scholar]
- 4. Metzger BE, Lowe LP, Dyer AR, et al. ; HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 5. Persson M, Norman M, Hanson U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: a large, population-based study. Diabetes Care. 2009;32(11):2005-2009. doi: 10.2337/dc09-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Balsells M, García-Patterson A, Gich I, Corcoy R. Maternal and fetal outcome in women with type 2 versus type 1 diabetes mellitus: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2009;94(11):4284-4291. doi: 10.1210/jc.2009-1231. [DOI] [PubMed] [Google Scholar]
- 7. Farrar D, Simmonds M, Bryant M, et al. Hyperglycaemia and risk of adverse perinatal outcomes: systematic review and meta-analysis. Obstet Anesthes Dig. 2017;37:64-65. doi: 10.1097/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murphy HR, Steel SA, Roland JM, et al. ; East Anglia Study Group for Improving Pregnancy Outcomes in Women with Diabetes. Obstetric and perinatal outcomes in pregnancies complicated by Type 1 and Type 2 diabetes: influences of glycaemic control, obesity and social disadvantage. Diabet Med. 2011;28(9):1060-1067. doi: 10.1111/j.1464-5491.2011.03333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu Y, Liu B, Sun Y, et al. Association of maternal prepregnancy diabetes and gestational diabetes mellitus with congenital anomalies of the newborn. Diabetes Care. 2020;43(12):2983-2990. doi: 10.2337/dc20-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hildén K, Magnuson A, Hanson U, Simmons D, Fadl H. Trends in pregnancy outcomes for women with gestational diabetes mellitus in Sweden 1998-2012: a nationwide cohort study. Diabet Med. 2020;37(12):2050-2057. doi: 10.1111/dme.14266. [DOI] [PubMed] [Google Scholar]
- 11. Gregory ECW, Ely DM. Trends and characteristics in gestational diabetes: United States, 2016-2020. Centers for Disease Control & Prevention, National Vital Statistics Reports. https://www.cdc.gov/nchs/data/nvsr/nvsr71/nvsr71-03.pdf. Published 2022. Accessed September 1, 2022. [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention. Overweight & obesity. Date unknown. https://www.cdc.gov/obesity/data/childhood.html. Accessed August 9, 2020.
- 13. McIntyre HD. Discovery, knowledge, and action—diabetes in pregnancy across the translational spectrum: the 2016 Norbert Freinkel Award Lecture. Diabetes Care. 2018;41(2):227-232. [DOI] [PubMed] [Google Scholar]
- 14. Farrar D. Hyperglycemia in pregnancy: prevalence, impact, and management challenges. Int J Womens Health. 2016;8:519-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ye W, Luo C, Haung J, et al. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2022;377:e067946. doi: 10.1136/bmj-2021-067946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention. CDC wonder. Date unknown. https://wonder.cdc.gov/controller/datarequest/D149. Accessed August 30, 2022.
- 17. The Kaiser Family Foundation State Health Facts. Total Monthly Medicaid and CHIP enrollment. https://www.kff.org/health-reform/state-indicator/total-monthly-medicaid-and-chip-enrollment/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Published 2018. Accessed July 14, 2021.
- 18. Kaiser Family Foundation. Medicaid coverage rates for the nonelderly by race/ethnicity. Date unknown. https://www.kff.org/medicaid/state-indicator/nonelderly-medicaid-rate-by-raceethnicity/?currentTimeframe=0&selectedRows=%7B%22states%22:%7B%22all%22:%7B%7D%7D,%22wrapups%22:%7B%22united-states%22:%7B%7D%7D%7D&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed May 12, 2021.
- 19. Nigam A, Varun N, Sharma S, Munjal YP, Prakash A. Glycaemic profile in the second and third trimesters of normal pregnancy compared to non-pregnant adult females. Obstet Med. 2020;13(1):30-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hawkins JS, Casey BM, Lo JY, et al. Weekly compared with daily blood glucose monitoring in women with diet-treated gestational diabetes. Obstet Gynecol. 2009;113:1307-1312. [DOI] [PubMed] [Google Scholar]
- 21. de Veciana M, Major CA, Morgan MA, et al. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med. 1995;333:1237-1241. [DOI] [PubMed] [Google Scholar]
- 22. Cosson E, Baz B, Gary F, et al. Poor reliability and poor adherence to self-monitoring of blood glucose are common in women with gestational diabetes mellitus and may be associated with poor pregnancy outcomes. Diabetes Care. 2017;40(9):1181-1186. doi: 10.2337/dc17-0369. [DOI] [PubMed] [Google Scholar]
- 23. Lind M, Polonsky W, Hirsch IB. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317(4):379-387. [DOI] [PubMed] [Google Scholar]
- 24. Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317:371-378. [DOI] [PubMed] [Google Scholar]
- 25. Ruedy KJ, Parkin CG, Riddlesworth TD, Graham C; for the DIAMOND Study Group. Continuous glucose monitoring in older adults with type 1 and type 2 diabetes using multiple daily injections of insulin: results from the DIAMOND trial. J Diabetes Sci Technol. 2017;11(6):1138-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Šoupal J, Petruželková L, Flekač M, et al. Comparison of different treatment modalities for type 1 diabetes, including sensor-augmented insulin regimens, in 52 weeks of follow-up: a COMISAIR study. Diabetes Technol Ther. 2016;18(9):532-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Šoupal J, Petruželková L, Grunberger G, et al. Glycemic outcomes in adults with T1D are impacted more by continuous glucose monitoring than by insulin delivery method: 3 years of follow-up from the COMISAIR study. Diabetes Care. 2020;43(1):37-43. [DOI] [PubMed] [Google Scholar]
- 28. Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11(1):279-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Charleer S, De Block C, Van Huffel L, et al. Quality of life and glucose control after 1 year of nationwide reimbursement of intermittently scanned continuous glucose monitoring in adults living with type 1 diabetes (FUTURE): a prospective observational real-world cohort study. Diabetes Care. 2020;43(2):389-397. [DOI] [PubMed] [Google Scholar]
- 30. Fokkert M, van Dijk P, Edens M, et al. Improved well-being and decreased disease burden after 1-year use of flash glucose monitoring (FLARE-NL4). BMJ Open Diabetes Res Care. 2019;7(1):e000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tyndall V, Stimson RH, Zammitt NN, et al. Marked improvement in HbA1c following commencement of flash glucose monitoring in people with type 1 diabetes. Diabetologia. 2019;62(8):1349-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paris I, Henry C, Pirard F, Gérard AC, Colin IM. The new FreeStyle libre flash glucose monitoring system improves the glycaemic control in a cohort of people with type 1 diabetes followed in real-life conditions over a period of one year. Endocrinol Diabetes Metab. 2018;1(3):e00023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6354746/. Accessed May 6, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365-374. [DOI] [PubMed] [Google Scholar]
- 34. Manning JP, Halford J, Sulik B, Sulik M, Parkin CG, Liljenquist DR. Use of continuous glucose monitoring is acceptable and potentially beneficial in older T2DM patients treated with basal insulin therapy: a pilot study. Infusystems USA. 2014;11(1):1-5. [Google Scholar]
- 35. Charleer S, Mathieu C, Nobels F, et al. Effect of continuous glucose monitoring on glycemic control, acute admissions, and quality of life: a real-world study. Clin Endocrinol Metab. 2018;103(3):1224-1232. [DOI] [PubMed] [Google Scholar]
- 36. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388:2254-2263. [DOI] [PubMed] [Google Scholar]
- 37. van Beers CA, DeVries JH, Kleijer SJ, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol. 2016;4(11):893-902. [DOI] [PubMed] [Google Scholar]
- 38. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Use of flash glucose sensing technology for 12 months as a replacement for blood glucose monitoring in insulin-treated type 2 diabetes. Diabetes Ther. 2017;8(3):573-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8(1):55-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yu F, Lv L, Liang Z, et al. Continuous glucose monitoring effects on maternal glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus: a prospective cohort study. J Clin Endocrinol Metab. 2014;99(12):4674-4682. [DOI] [PubMed] [Google Scholar]
- 41. Wei Q, Sun Z, Yang Y, Yu H, Ding H, Wang S. Effect of a CGMS and SMBG on maternal and neonatal outcomes in gestational diabetes mellitus: a randomized controlled trial. Sci Rep. 2016;6(1):19920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Márquez-Pardo R, Torres-Barea I, Córdoba-Doña JA, et al. Continuous glucose monitoring and glycemic patterns in pregnant women with gestational diabetes mellitus. Diabetes Technol Ther. 2020;22(4):271-277. doi: 10.1089/dia.2019.0319. [DOI] [PubMed] [Google Scholar]
- 43. Voormolen DN, DeVries JH, Sanson RME, et al. Continuous glucose monitoring during diabetic pregnancy (GlucoMOMS): a multicentre randomized controlled trial. Diabetes Obes Metab. 2018;20(8):1894-1902. doi: 10.1111/dom.13310. [DOI] [PubMed] [Google Scholar]
- 44. Zaharieva DP, Teng JH, Ong ML, et al. Continuous glucose monitoring versus self-monitoring of blood glucose to assess glycemia in gestational diabetes. Diabetes Technol Ther. 2020;22(11):822-827. doi: 10.1089/dia.2020.0073. [DOI] [PubMed] [Google Scholar]
- 45. Chang VYX, Tan YL, Ang WHD, Lau Y. Effects of continuous glucose monitoring on maternal and neonatal outcomes in perinatal women with diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2022;184:109192. doi: 10.1016/j.diabres.2022.109192. [DOI] [PubMed] [Google Scholar]
- 46. Feig DS, Donovan LE, Corcoy R, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet. 2017;390:2347-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kristensen K, Ögge LE, Sengpiel V, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes: an observational cohort study of 186 pregnancies. Diabetologia. 2019;62(7):1143-1153. doi: 10.1007/s00125-019-4850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Scott EM, Murphy HR, Kristensen KH. Continuous glucose monitoring metrics and birth weight: informing management of type 1 diabetes throughout pregnancy. Diabetes Care. 2022;45(8):1724-1734. doi: 10.2337/dc22-0078. [DOI] [PubMed] [Google Scholar]
- 49. Murphy HR, Rayman G, Lewis K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ. 2008;337:a1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Murphy HR, Feig DS, Sanchez JJ, de Portu S, Sale A; CONCEPTT Collaborative Group. Modelling potential cost savings from use of real-time continuous glucose monitoring in pregnant women with type 1 diabetes. Diabet Med. 2019;36(12):1652-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sekhon J, Graham D, Mehrotra C, Li I. Continuous glucose monitoring: a cost-effective tool to reduce pre-term birth rates in women with type one diabetes. Aust N Z J Obstet Gynaecol. 2022. Jul 14. doi: 10.1111/ajo.13581. [DOI] [PubMed] [Google Scholar]
- 52. Zhang X, Jiang D, Wang X. The effects of the instantaneous scanning glucose monitoring system on hypoglycemia, weight gain, and health behaviors in patients with gestational diabetes: a randomised trial. Ann Palliat Med. 2021;10(5):5714-5720. doi: 10.21037/apm-21-439. [DOI] [PubMed] [Google Scholar]
- 53. Begum M, Pilkington R, Chittleborough C, Lynch J, Penno M, Smithers L. Caesarean section and risk of type 1 diabetes: whole-of-population study. Diabet Med. 2019;36(12):1686-1693. doi: 10.1111/dme.14131. [DOI] [PubMed] [Google Scholar]
- 54. Goldman M, Kitzmiller JL, Abrams B, Cowan RM, Laros RK., Jr. Obstetric complications with GDM. Diabetes. 1991;40(suppl 2):79-82. [DOI] [PubMed] [Google Scholar]
- 55. Lemaitre M, Ternynck C, Bourry J, et al. Association between HbA1c levels on adverse pregnancy outcomes during pregnancy in patients with type 1 diabetes. J Clin Endocrinol Metab. 2022;107(3):e1117-e1125. doi: 10.1210/clinem/dgab769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim Y, Ganduglia-Cazaban C, Chan W, Lee M, Goodman DC. Trends in neonatal intensive care unit admissions by race/ethnicity in the United States, 2008-2018. Sci Rep. 2021;11(1):23795. doi: 10.1038/s41598-021-03183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nielsen LR, Pedersen-Bjergaard U, Thorsteinsson B, Johansen M, Damm P, Mathiesen ER. Hypoglycemia in pregnant women with type 1 diabetes: predictors and role of metabolic control. Diabetes Care. 2008;31(1):9-14. doi: 10.2337/dc07-1066. [DOI] [PubMed] [Google Scholar]
- 58. Abourawi F. Diabetes mellitus and pregnancy. Libyan J Med. 2006;1(1):28-41. doi: 10.4176/060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sweeting A, Wong J, Murphy HR, Ross GP. A clinical update on gestational diabetes mellitus. Endocr Rev. 2022;43:763-793. doi: 10.1210/endrev/bnac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Centers for Disease Control and Prevention. Pregnancy: type 1 or type 2 diabetes and pregnancy. Date unknown. https://www.cdc.gov/pregnancy/diabetes-types.html. Accessed August 26, 2022.
- 61. Centers for Disease Control and Prevention. Gestational diabetes and pregnancy. Date unknown. https://www.cdc.gov/pregnancy/diabetes-gestational.html. Accessed August 26, 2022.
- 62. March of Dimes PERISTATS. Health insurance/income. Date unknown. https://www.marchofdimes.org/peristats/data?reg=99&top=11&stop=154&lev=1&slev=1&obj=18. Accessed December 7, 2022.
- 63. Truven. The cost of having a baby in the United States: executive summary. Truven Health Analytics Marketscan® Study. https://www.nationalpartnership.org/our-work/resources/health-care/maternity/archive/the-cost-of-having-a-baby-in-the-us.pdf. Published 2013. Accessed August 28, 2022. [Google Scholar]
- 64. Relph S, Patel T, Delaney L, Sobhy S, Thangaratinam S. Adverse pregnancy outcomes in women with diabetes-related microvascular disease and risks of disease progression in pregnancy: a systematic review and meta-analysis. PLoS Med. 2021;18(11):e1003856. doi: 10.1371/journal.pmed.1003856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Law GR, Alnaji A, Alrefaii L, et al. Suboptimal nocturnal glucose control is associated with large for gestational age in treated gestational diabetes mellitus. Diabetes Care. 2019;42(5):810-815. [DOI] [PubMed] [Google Scholar]
- 66. American Diabetes Association Professional Practice Committee. 15. Management of diabetes in pregnancy: standards of medical care in diabetes - 2022. Diabetes Care. 2022;45(suppl 1):S232-S243. [DOI] [PubMed] [Google Scholar]
- 67. Grunberger G, Sherr J, Allende M, et al. American Association of Clinical Endocrinology Clinical Practice Guideline: the use of advanced technology in the management of persons with diabetes mellitus. Endocr Pract. 2021;27(6):505-537. [DOI] [PubMed] [Google Scholar]
- 68. National Health Service (NHS). Health Technology Wales (HTW) guidance 012 (September 2019): continuous glucose monitoring in pregnant women with type 1 diabetes. Date unknown. https://www.healthtechnology.wales/wp-content/uploads/2019/10/GUI012-Continuous-glucose-monitoring-in-pregnant-women-with-type-1-diabetes-English-3.pdf. Accessed March 1, 2023.
- 69. National Institute for Health and Clinical Excellence. Diabetes in Pregnancy: Management From Preconception to the Postnatal Period. London, England: National Institute for Health and Care Excellence; 2020. [PubMed] [Google Scholar]
- 70. Centers for Disease Control and Prevention, NVSS/Vital Statistics Rapid Release. Births: provisional data for 2020 (report number 012). https://www.cdc.gov/nchs/data/vsrr/vsrr012-508.pdf. Published 2021. Accessed January 28, 2023.
- 71. RapidRx. OneTouch. Date unknown. https://rapidrxusa.com/collections/onetouch. Accessed February 3, 2023.
- 72. RapidRx. Dexcom G6 sensor 1-pack. Date unknown. https://rapidrxusa.com/products/dexcom-g6-sensor-1?gclid=Cj0KCQiAofieBhDXARIsAHTTldrXlHdvRda1TOGwz-HCH3MShbxeDUHkDXphE5AGqFBKa6VZ5VpAAbcaAgYlEALw_wcB. Accessed February 3, 2023.
- 73. Negrato CA, Mattar R, Gomes MB. Adverse pregnancy outcomes in women with diabetes. Diabetol Metabol Syndr. 2012;4:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhou T, Du S, Sun D, et al. Prevalence and trends in gestational diabetes mellitus among women in the United States, 2006-2017: a population-based study. Front Endocrinol (Lausanne). 2022;13:868094. doi: 10.3389/fendo.2022.868094. [DOI] [PMC free article] [PubMed] [Google Scholar]